Abstract
Rheumatoid meningitis is a rare extra-articular manifestation of rheumatoid arthritis, often with non-specific symptoms. In most cases brain MRI shows a patchy lepto- and pachymeningeal enhancement, but the diagnosis currently relies on examination of a meningeal biopsy with presence of plasma cells and rheumatoid noduli. Presence of IgM rheumatic factor (RF) has been found in several cases and recently four cases have shown high titer anti-cyclic citrullinated peptide (anti-CCP) in CSF, suggesting this as a potential marker for rheumatoid meningitis. We present a 62 year-old woman with sero-positive (IgM RF and anti-CCP) rheumatoid arthritis, presenting with headache and gait impairment. Brain MRI revealed the classical patchy meningeal enhancement and the diagnosis of rheumatoid meningitis was confirmed by neuropathological examination of a meningeal biopsy. Analysis of the CSF revealed positive IgM RF (92.7 IU/mL) and strongly positive anti-CCP (19,600 IU/mL) and CXCL-13 (>500 ng/L). After treatment with high-dose steroid and Rituximab the clinical symptoms resolved. A 6 month follow-up analysis of CSF showed a dramatic decrease in all these markers with negative IgM RF and a decrease in both anti-CCP (64 IU/mL) and CXCL-13 (<10 ng/L). Our case further underlines the potential use of CSF anti-CCP and IgM RF in the diagnosis of RM and the use of these markers and CXCL-13 in evaluation of treatment response. A case review of 48 cases of rheumatoid meningitis published since 2010, including, symptoms, serum, and CSF findings, treatment, and outcome is provided.
Background
Rheumatoid meningitis (RM) is a rare but potentially aggressive extra-articular manifestation of rheumatoid arthritis (RA) involving both pachy- and leptomeninges (1, 2). It can occur at all disease stages, and manifestations are often non-specific, mimicking a variety of neurological disorders, malignancies, or infections (1–6). Brain MRI with patchy leptomeningeal contrast enhancement and cerebrospinal fluid (CSF) rheumatoid factor (RF) are useful to guide, but diagnosis still relies on pathological examination of a meningeal biopsy often showing unspecific inflammation, rheumatic noduli, and in some cases vasculitis (2, 7–11). Four recent cases have shown presence of CSF anti-cyclic citrullinated peptide (anti- CCP) in patients with RM (12–15). Here, we describe a patient with RM with strongly positive anti-CCP, IgM RF, and chemokine (C-X-C motif) ligand 13 (CXCL13) levels in CSF that normalized after treatment suggesting a potential use of these markers in both diagnosis and treatment management of RM. Furthermore, we review 48 cases of RM published in the English literature since 2010 focusing on symptoms, serum and CSF findings, treatment, and outcome.
Case Presentation
A 62 year-old woman was admitted after 4 months history of intermittent frontal headache, nausea, and gait and balance disturbances. She had a 3 year history of IgM-RF and anti-CCP positive RA, with a previously episode of pleuritis. Within the last year, she had been treated with Leflunomide, Infliximab, and was currently treated with Methotrexate and Salazopyrine entabs. Neurological examination was normal, except for a mild gait ataxia and her RA was well-controlled with no symptoms of active synovitis at time of admission.
Due to chronic headache a brain MRI was performed. This showed patchy interhemispheric pachy- and leptomenigeal enhancement adjacent to the parietal- and occipital lobes (Figure 1A). Blood tests revealed signs of inflammation with high levels of IgM RF (56 IU/mL), anti-CCP (>1,600 U/mL), Interleukin-2 receptor (ILR-2–1,065 kU/L) (Table 1), c-reactive protein (43 mg/L), and erythrocyte sedimentation rate (106 mm). Remaining systemic antibody examinations were negative (anti-DNA antibody, anti-nuclear antibody (ANA) IgG, anti-neutropil cytoplasmatic antibody (ANCA) IgG, Anti-Ro (SSA)/La (SSB), anti-cardiolipin antibody, phospholipid antibody, and lupus anticoagulant). Immunoglobulin A, G, and M levels were normal.
Figure 1
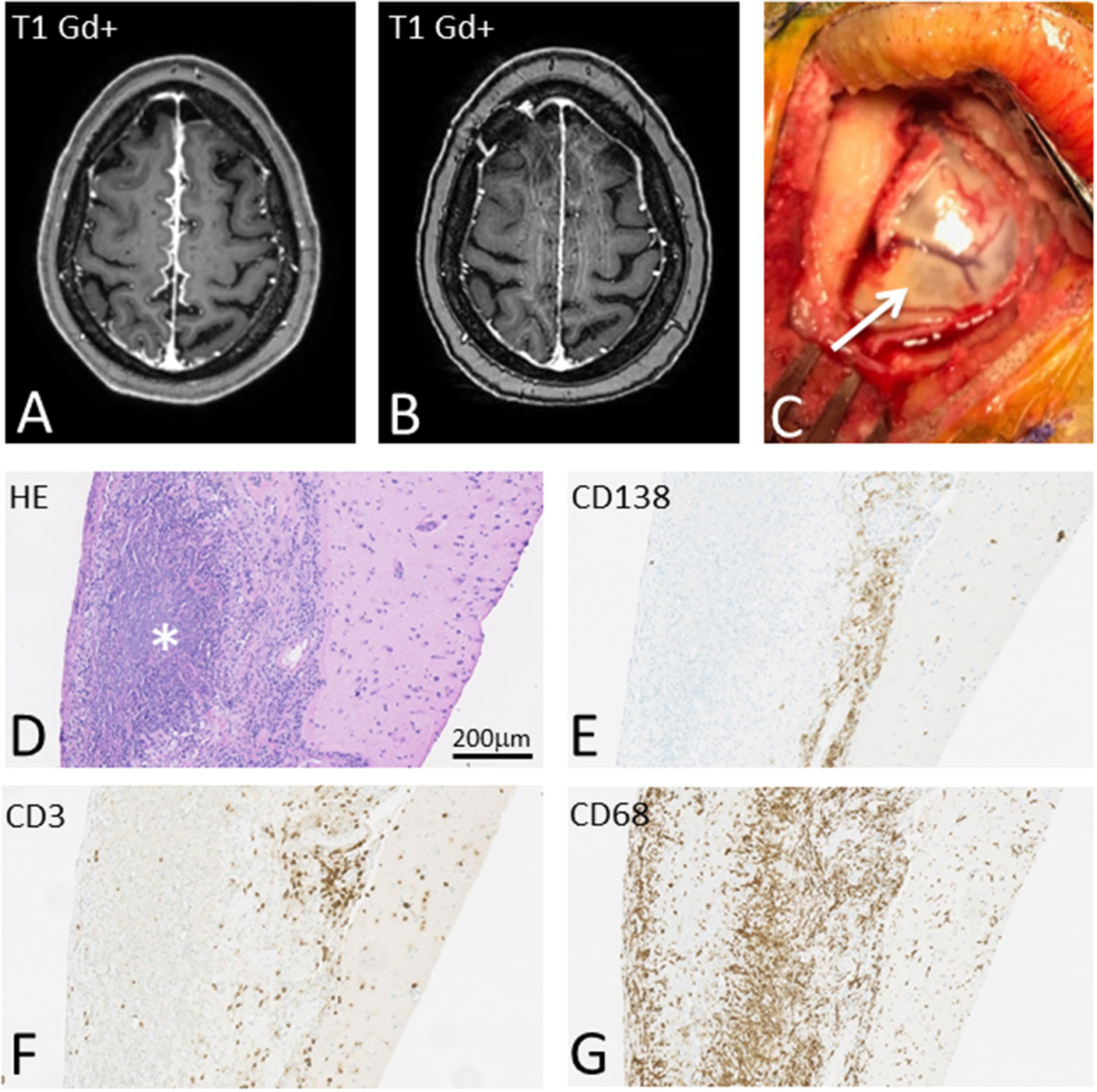
T1-weighted brain MRI showing interhemispheric leptomeningeal Gd+ enhancement before (A) and after (B) treatment with high dose steroids, Methotrexate and Rituximab. On gross inspection meninges appear severely inflamed (C) and pathological examination reveals massive meningeal granulomatous inflammation (D) with pre-dominant CD138 positive plasma cells (E), but also CD3 positive T cells (F). Massive infiltration with CD68 positive histiocytes with rheumatic granuloma formation was also seen (G).
Table 1
| Test/(range) | Pre-treatment | Post-treatment |
|---|---|---|
| Serum | ||
| IgM RF (<15 IU/mL) | 56 | 18 |
| Anti-CCP (<25 U/mL) | >1,600 | 706 |
| ILR-2 (158–623 kU/L) | 1,065 | N/A |
| CSF | ||
| Leukocytes (<5 E6/L) | 170 | <5 |
| Protein (0.40–0.70 g/L) | 1.16 | 0.28 |
| IgG index (<0,60) | 1.45 | 0.45 |
| Oligoclonal bands | Present | Absent |
| B lymfocytes (%) | 7.80 | – |
| Plasma cells (%) | 1.80 | – |
| RF IgM* (<15 IU/mL) | 92.7 | Negative |
| Anti-CCP* (<25 IU/mL) | 19,600 | 64 |
| CXCL-13 (<10 ng/L) | >500 | <10 |
Serum and CSF markers before and after treatment.
Range in serum; -, not performed.
Cerebrospinal Fluid (CSF) analysis revealed a mononuclear pleocytosis (170 E6/L) and elevated protein level (1.16 g/L). Due to the pleocytosis, intravenous ceftriaxone, and aciclovir were administered, to cover for bacterial meningitis and Herpes Simplex Virus (HSV) encephalitis. Subsequent CSF cultures revealed no growth of bacteria, no Borrelia antibodies, and viral/bacterial PCR (E. coli, hemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, hemolytic streptococcus, streptococcus pneumoniae, cytomegalovirus, enterovirus, herpes simplex virus, varicella zoster, Cryptococcus, and micromiome 16S/18S), and flowcytometry, and cytological analysis for malignancy were negative. Therefore, antiviral- and antibiotic- treatment was terminated.
The following days the patient displayed sporadic confusion, delusions, and fever (38.5°C). Subsequent tests, including HIV, syphilis, and tuberculosis were negative. Re-examination of CSF showed continuous mononuclear pleocytosis (130 E6/L), high IgG index (1.45) and presence of oligoclonal bands, suggestive of inflammation. Repeated cultures for bacteria were negative and cytological analysis showed an inflammatory pattern with an elevated number of B-lymphocytes (7.8 %) and plasma cells (1.8%, Table 1).
To investigate possible systemic inflammation or malignancy whole-body FDG-PET CT was performed. This showed hypermetabolism of the cerebral cortex, adjacent to the meningeal enhancement found on MRI, and a right medial lobe infiltrate of the lung. CT of thorax and abdomen confirmed an infiltrate, slight pleural effusion, and pleural thickening. Endobronchial ultrasound with biopsy was performed revealing no malignancy or infection.
On suspicion of RM, we performed analysis on undiluted CSF showing moderately positive IgM RF (92.7 IU/mL) and strongly positive anti-CCP (19,600 IU/mL) and CXCL-13 (>500 ng/L, Table 1).
Subsequent, biopsy of meninges (Figure 1C) confirmed chronic inflammation dominated by CD138 positive plasma cells and a limited number of CD3 positive T-lymphocytes with limited infiltration into the underlying gray matter (Figures 1E,F). Additionally, granulomatous inflammation with dense infiltration of CD68+ histiocytes and the presence of rheumatoid nodules were found (Figures 1D,G). Microbial stains, PCR, and cultures of biopsy tissue for fungi, parasites, acid-fast bacilli, HSV 1, HSV 2, CMV, SV40, M. tuberculosis, and toxoplasmosis were negative.
Based on the (i) MRI findings with patchy meningeal enhancement, (ii) high titer of IgM-RF and anti-CCP in CSF and (iii) histopathological chronic inflammation of meninges with plasma cells and rheumatic nodules, the diagnosis RM was established. Concurrently, the patient displayed extra articular manifestations of RA in her lungs.
Intravenous high dose methylprednisolone (750 + 1,000 + 1,000 mg on three consecutive days) followed by oral tapering was administered in addition to current treatment with methotrexate. Within days symptoms improved, but did not completely resolve. The following weeks, the patient received Rituximab (1,000 mg intravenous, repeated after 14 days). CSF levels of IgM RF, anti-CCP, and CXCL-13 decreased accordingly to the patient reporting significant treatment response (Table 1). A 6 month follow-up MRI showed regression of meningeal enhancement (Figure 1B) and follow-up FDG-PET CT showed almost complete regression of pulmonary findings. Neurological examination at 6 month follow up confirmed resolution of clinical symptoms.
Discussion
Meningitis in RA is a rare serious extra-articular complication (1, 2, 7, 16). Clinical neurological manifestations are often non-specific and duration and manifestations of RA is unreliable, as less than half of patients display active synovitis (2, 17). Sometimes CNS involvement even precedes the onset of arthritis (17–20). In cases published since 2010, 34% (13 of 38) had no history of RA before the diagnosis of RM (Table A1). CSF findings are variable but most often include a mild pleocytosis with elevated protein concentration and normal glucose (Table A1). Gadolinium enhanced MRI is often useful, showing asymmetrical pachy- or leptomeningeal enhancement (11, 18). Recently, a review of 29 cases of RM showed definite asymmetric meningeal involvement in 62% of patients, and most common neurological features were hemiparesis or hemisensory symptoms mimicking stroke or epilepsy related to localization of meningeal involvement (11). In comparison to this, we find that 70% (33 of 47) had transient or permanent weakness, sensory deficits, or speech disorders, whereas 36% (17 of 47) had seizures (Table A1) It is not uncommon that patients display other extra-articular manifestations of RA such as subcutaneous nodules or pulmonary manifestations, as seen in our case (2, 5, 21, 22).
Patients with RA are often treated with various immunosuppressants which increase the risk of aseptic meningitis or opportunistic infections. Therefore, it is important to rule out iatrogenic aseptic, septic, and fungal diseases before diagnosis of RM. Concurrently, autoimmune diseases, malignancies, other granulomatous diseases or IgG4-related disease can display a similar pattern of dural thickening, making them possible considerations in the differential diagnosis of RM (10, 11, 16).
Until now, there are no known RM biomarkers in CSF and meningeal biopsy is required for definite diagnosis. Biopsy shows thickening of meninges (Figure 1C) and histopathological features include pachy- and leptomeningeal inflammation with plasma cells and the presence of rheumatoid noduli, and in some cases vasculitis (1, 2, 17). Patients diagnosed at autopsy almost all display meningeal rheumatoid noduli, while patients diagnosed with meningeal biopsy most often show non-specific inflammation (2, 7). In some previous cases correlation between strongly elevated CSF RF and Il-6 and RM has been proposed (12, 13, 23, 24), however this still needs validation as a diagnostic tool.
No clear guideline for treatment of RM exists and cyclophosphamide, methotrexate, and azathioprine in combination with corticosteroids have all been described with improvement of symptoms (7, 17, 18, 25). In some cases, improvement on corticosteroid treatment alone has been described (5, 11, 12, 14, 20, 24, 26–31). In our case review 41% (18 of 44) were treated with corticosteroids alone, 2% (1 of 44) received no treatment, whereas the remaining received corticosteroids in combination with another therapy (Table A1). Seven patients (16 %) received rituximab. On these regimens only 1 case worsened (32), 8 (18%) had an incomplete improvement, whereas 80% improved (Table A1).
To our knowledge, anti-CCP in CSF has only been examined in four cases of RM and found to be elevated in three of these (12–15). Serum anti-CCP antibodies help distinguish RA from other types of arthritis, can help to identify patients with a higher risk of severe disease and are rarely found in other autoimmune conditions (33). They are often used in combination with IgM RF in the diagnosis of RA. In this case, anti-CCP level in CSF was strongly positive and a crucial element in both diagnosing RM and monitoring treatment response. With this case, we show a novel clear response of anti-CCP to the treatment of RM. Moreover, in addition to CSF anti-CCP and IgM RF, we also find the B cell chemoattractant CXCL-13 levels associated with treatment response, which to our knowledge has not previously been investigated.
We propose using anti-CCP, IgM RF, and CXCL-13 in CSF as potential biomarkers not only for diagnosis of RM, but also in evaluation of treatment response. Further studies are needed to clarify their potential use.
Statements
Data availability statement
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Ethics statement
Clinical data in this case report was collected with the consent of the patient. A written informed consent was obtained from the patient for the publication of this case report.
Author contributions
MN and AN: design and draft of the manuscript and interpretation of data. JF and JM: draft of manuscript. MW and CB: acquisition of data and draft of manuscript. K-EB and TE: revised manuscript for intellectual content. MB: draft of manuscript, acquisition of data, and revised manuscript for intellectual content.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Pellerin D Wodkowski M Guiot MC AlDhukair H Blotsky A Karamchandani J et al . Rheumatoid meningitis presenting with acute parkinsonism and protracted non-convulsive seizures: an unusual case presentation and review of treatment strategies. Front Neurol. (2019) 10:163. 10.3389/fneur.2019.00163
2.
Bathon JM Moreland LW DiBartolomeo AG . Inflammatory central nervous system involvement in rheumatoid arthritis. Semin Arthritis Rheum. (1989) 18:258–66. 10.1016/0049-0172(89)90047-4
3.
Roy B Uphoff DF Silverman IE . Rheumatoid meningitis presenting with multiple strokelike episodes. JAMA Neurol. (2015) 72:1073-6. 10.1001/jamaneurol.2015.1105
4.
Hayashi Y Namekawa M Ohtani K Watanabe E Nakano I . Parkinsonism as an initial manifestation of rheumatoid meningitis. Neurol Sci. (2014) 35:1139–41. 10.1007/s10072-014-1699-3
5.
Krysl D Zamecnik J Senolt L Marusic P . Chronic repetitive nonprogressive epilepsia partialis continua due to rheumatoid meningitis. Seizure. (2013) 22:80–2. 10.1016/j.seizure.2012.10.006
6.
Rijkers K Postma A Riedl R Schijns O . Rheumatoid arthritis mimicking an intracranial malignancy. Acta Neurochir. (2014) 156:427–8. 10.1007/s00701-013-1936-1
7.
Kato T Hoshi K Sekijima Y Matsuda M Hashimoto T Otani M et al . Rheumatoid meningitis: an autopsy report and review of the literature. Clin Rheumatol. (2003) 22:475–80. 10.1007/s10067-003-0788-0
8.
Chowdhry V Kumar N Lachance DH Salomao DR Luthra HS . An unusual presentation of rheumatoid meningitis. J Neuroimaging. (2005) 15:286–8. 10.1111/j.1552-6569.2005.tb00325.x
9.
Yeaney GA Denby EL Jahromi BS Mangla R . Rheumatoid-associated meningitis and vasculopathy. Neurology. (2015) 84:1717–8. 10.1212/WNL.0000000000001498
10.
Gherghel N Stan A Stan H . Pearls & oy-sters: rheumatoid meningitis occurring during treatment with etanercept. Neurology. (2018) 91:806–8. 10.1212/WNL.0000000000006397
11.
Choi SJ Ho Park Y Kim JA Han JH Choe G Kim S . Pearls & oy-sters: asymmetric meningeal involvement is a common feature of rheumatoid meningitis. Neurology. (2017) 88:e108–10. 10.1212/WNL.0000000000003744
12.
Shibahara T Matsushita T Matsuo R Fukushima Y Fukuda K Sugimori H et al . Anti-cyclic citrullinated peptide antibody-positive meningoencephalitis in the preclinical period of rheumatoid arthritis. Case Rep Neurol. (2016) 8:156–60. 10.1159/000447627
13.
Akamatsu M Maki F Akiyama H Hara D Hoshino M Hasegawa Y . Rheumatoid meningitis presenting with a stroke-like attack treated with recombinant tissue plasminogen activator: a case presentation. BMC Neurol. (2018) 18:139. 10.1186/s12883-018-1143-z
14.
Schuster S Braass H Iking-Konert C Schnoor U Matschke J Gerloff C et al . Rheumatoid meningitis: A rare cause of aseptic meningitis with frequently stroke-like episodes. Neurol Clin Pract. (2018) 8:451–5. 10.1212/CPJ.0000000000000504
15.
Lubomski M Sy J Buckland M Lee AS Richards B Thompson E et al . Rheumatoid leptomeningitis presenting with an acute neuropsychiatric disorder. Pract Neurol. (2019) 19:68–71. 10.1136/practneurol-2018-001978
16.
DeQuattro K Imboden JB . Neurologic manifestations of rheumatoid arthritis. Rheum Dis Clin North Am. (2017) 43:561–71. 10.1016/j.rdc.2017.06.005
17.
Starosta MA Brandwein SR . Clinical manifestations and treatment of rheumatoid pachymeningitis. Neurology. (2007) 68:1079–80. 10.1212/01.wnl.0000257824.72457.91
18.
Jones SE Belsley NA McLoud TC Mullins ME . Rheumatoid meningitis: radiologic and pathologic correlation. AJR Am J Roentgenol. (2006) 186:1181–3. 10.2214/AJR.05.0859
19.
Finkelshtein V Lampl Y Lorberboym M Kanner A Ben-Ami Raichman D Dabby R et al . Self-limited rheumatoid meningitis as a presenting symptom of rheumatoid arthritis. Isr Med Assoc J. (2018) 20(4):262-4.
20.
Padjen I Mayer M Habek M Kolenc D Dotlic S . Redefining a diagnosis: from meningeal plasma cell granuloma to rheumatoid meningitis. Report of a patient follow-up. Neurol Sci. (2015) 36:1047–8. 10.1007/s10072-015-2075-7
21.
Matsuda S Yoshida S Takeuchi T Fujiki Y Yoshikawa A Makino S . Asymptomatic rheumatoid meningitis revealed by magnetic resonance imaging, followed by systemic rheumatic vasculitis: A case report and a review of the literature. Modern Rheumatol. (2019) 29:370–6. 10.1080/14397595.2016.1232333
22.
Duray MC Marchand E Gohy S Weynand B De Coene B Laloux P . Granulomatous meningitis due to rheumatoid arthritis. Acta Neurol Belg. (2012) 112:193–7. 10.1007/s13760-012-0021-5
23.
Oono M Fujita Y Uchida N Kawai U Fujita-Nakata M Nakanishi M et al . Rheumatoid meningitis developed in patient with stable rheumatoid arthritis and myasthenia gravis-detailed analysis of intracranial inflammation using flow cytometry. J Neuroinflammation. (2018) 15:151. 10.1186/s12974-018-1196-3
24.
Matsushima M Yaguchi H Niino M Akimoto-Tsuji S Yabe I Onishi K et al . MRI and pathological findings of rheumatoid meningitis. J Clin Neurosci. (2010) 17:129–32. 10.1016/j.jocn.2009.01.033
25.
Shimada K Matsui T Kawakami M Hayakawa H Futami H Michishita K et al . Diffuse chronic leptomeningitis with seropositive rheumatoid arthritis: report of a case successfully treated as rheumatoid leptomeningitis. Modern Rheumatol. (2009) 19:556–62. 10.3109/s10165-009-0186-9
26.
Kim HY Park JH Oh HE Han HJ Shin DI Kim MH . A case of rheumatoid meningitis: pathologic and magnetic resonance imaging findings. Neurol Sci. (2011) 32:1191–4. 10.1007/s10072-011-0727-9
27.
Lu L Chwalisz B Pfannl R Narayanaswami P . Rheumatoid meningitis: a rare complication of rheumatoid arthritis. BMJ Case Rep. (2015) 2015:bcr2014208745. 10.1136/bcr-2014-208745
28.
Magaki S Chang E Hammond RR Yang I Mackenzie IR Chou BT et al . Two cases of rheumatoid meningitis. Neuropathology. (2016) 36:93–102. 10.1111/neup.12238
29.
Seago S Stroberg E Metting A . Rheumatoid meningitis associated with infliximab. Proc (Bayl Univ Med Cent). (2016) 29:204–6. 10.1080/08998280.2016.11929419
30.
McKenna MC Vaughan D Bermingham N Cronin S . Rheumatoid arthritis presenting as rheumatoid meningitis. BMJ Case Rep. (2019) 12:bcr-2018-226649. 10.1136/bcr-2018-226649
31.
Lee Ching C Kenyon L Berk M Park C . Rheumatoid meningitis sine arthritis. J Neuroimmunol. (2019) 328:73–5. 10.1016/j.jneuroim.2018.12.001
32.
Cianfoni A Falcone C Faustini F Lauriola L Imbesi S Della Marca G et al . Rheumatoid leptomeningitis: magnetic resonance imaging and pathologic findings–a case report. J Neuroimaging. (2010) 20:192–4. 10.1111/j.1552-6569.2008.00299.x
33.
Niewold TB Harrison MJ Paget SA . Anti-CCP antibody testing as a diagnostic and prognostic tool in rheumatoid arthritis. QJM. (2007) 100:193–201. 10.1093/qjmed/hcm015
34.
Inan AS Masatlioglu S Ozyurek SC Engin D Erdem I . Unusual central nervous system involvement of rheumatoid arthritis: successful treatment with steroid and azathioprine. Rheumatol Int. (2011) 31:1383–5. 10.1007/s00296-009-1266-z
35.
Aguilar-Amat MJ Abenza-Abildua MJ Vivancos F Rodriguez de Rivera FJ Morales-Bastos C Gandia-Gonzalez ML et al . Rheumatoid meningitis mimicking progressive supranuclear palsy. Neurologist. (2011) 17:136–40. 10.1097/NRL.0b013e31821735ad
36.
Servioli MJ Chugh C Lee JM Biller J . Rheumatoid meningitis. Front Neurol. (2011) 2:84. 10.3389/fneur.2011.00084
37.
Hasiloglu ZI Asik M Erer B Dikici AS Altintas A Albayram S . Magnetic resonance imaging of rheumatoid meningitis: a case report and literature review. Rheumatol Int. (2012) 32:3679–81. 10.1007/s00296-011-2105-6
38.
Huys AC Guerne PA Horvath J . Rheumatoid meningitis occurring during adalimumab and methotrexate treatment. Joint Bone Spine. (2012) 79:90–2. 10.1016/j.jbspin.2011.07.008
39.
Roques M Tanchoux F Calviere L Cuinat L Lubrano V Uro-Coste E et al . MRI with DWI helps in depicting rheumatoid meningitis. J Neuroradiol. (2014) 41:275–7. 10.1016/j.neurad.2013.10.005
40.
Bourgeois P Rivest J Bocti C . Rheumatoid meningitis presenting with stroke-like episodes. Neurology. (2014) 82:1564–5. 10.1212/WNL.0000000000000366
41.
Nihat A Chinthapalli K Bridges L Johns P Sofat N Moynihan B . Rheumatoid meningitis. Pract Neurol. (2016) 16:312–4. 10.1136/practneurol-2015-001306
42.
Moeyersoons A Verschueren P Tousseyn T De Langhe E . Rheumatoid granulomatous disease and pachymeningitis successfully treated with rituximab. Acta Clin Belg. (2018) 73:307–12. 10.1080/17843286.2017.1375193
43.
Tsuzaki K Nakamura T Okumura H Tachibana N Hamano T . Rheumatoid Meningitis occurring during etanercept treatment. Case Rep Neurol Med. (2017) 2017:7638539. 10.1155/2017/7638539
44.
Degboe Y Fajadet B Laurent C Cantagrel A Constantin A Ruyssen-Witrand A . A rare case of rheumatoid pachyleptomeningitis successfully treated with rituximab. Rheumatology. (2017) 56:1238-40. 10.1093/rheumatology/kex059
45.
Jessee R T Keenan R . Rheumatoid arthritis presenting as rheumatoid meningitis: a case report. BMJ. (2017) 12:17 p. 10.5430/crim.v4n3p17
46.
Alexander SK Di Cicco M Pohl U Cifelli A . Rheumatoid disease: an unusual cause of relapsing meningoencephalitis. BMJ Case Rep. (2018) 2018:bcr2017–222587. 10.1136/bcr-2017-222587
47.
Parsons AM Zuniga LA Hoxworth JM Lyons M Aslam F Goodman BP . Rheumatoid meningitis: a case review. Neurologist. (2018) 23:83–5. 10.1097/NRL.0000000000000158
48.
Harrison NS Kishore S Majithia V . Rheumatoid meningitis: successful remission with rituximab. BMJ Case Rep. (2018) 11:e226642. 10.1136/bcr-2018-226642
49.
Grose D Linger M Tinni S Sahathevan R . Rheumatoid meningitis: a rare cause of unilateral pachymeningitis. BMJ Case Rep. (2019) 12:e227905. 10.1136/bcr-2018-227905
50.
Scheitel M Ives ST Nasr R Nolan MW . When the plot thickens: a rare complication of rheumatoid arthritis. J community Hosp intern med perspect. (2019) 9:143–6. 10.1080/20009666.2019.1593780
Appendix
Table A1
| References | Patient (Age, sex) | Years of RA | Treatment of RA | Symptoms of RM | Serum | CSF | MRI compatible with RM | Biopsi compatible with RM | Treatment | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM RF (IU/mL) | Anti-CCP (IU/mL) | Other | Cells/μl | Protein (mg/L) | Glucose (mg/dl) | IgM RF (U/mL) | Anti-CCP(IU/mL) | Other | |||||||||
| Cianfoni et al. (32) | 74, F | 5 | CS, MTX | Progressive left-side weakness and hypoesthesia | 506 | – | – | 65 | 0.43 | Normal | – | – | – | Yes | Yes | CS, IT MTX | Worsening |
| Matsushima et al. (24) | 80, F | 20 | CS, sulfasalazine, bucillamine, etanercept | Transient weakness and numbness right-side | Normal | – | – | 18 | 0.55 | – | – | – | IL-6 = 4.6 pg/ml | Yes | Yes | CS | Improvement |
| Inan et al. (34) | 70, F | 0 | None | Headache, nausea, vomiting, and confusion | 108 | – | ESR = 124 mm/h | 140 | 1.13 | 34 | 98 (after treatment <20) | – | – | Normal | Not performed | CS, AZA | Improvement |
| Aguilar-Amat et al. (35) | 71, F | 15 | NR | Seizures and PSP-like phenotype | 27.9 | – | – | Normal | Normal | Normal | – | – | – | Yes | Yes | CS, MTX | Improvement |
| Kim et al. (26) | 66, M | 0 | None | Seizures (SE) and left-sided weakness | High levels | 1,448 | ANA high | 11 | Normal | Normal | – | – | – | Yes | Yes | CS | Improvement |
| Servioli et al. (36) | 80, F | NR | CS, HCQ | Unsteady gait with falls. Progression to left-sided weakness | <20 | – | ESR = 35 mm/h | 2–7 | 0.75–0.77 | 60 | – | – | – | Yes | Yes | Not reported | Not reported |
| Hasiloglu et al. (37) | 62, F | 4 | CS, MTX | Headache, paresis, and paresthesia right UE | 351 | 120 | – | 40 | 0.40 | – | – | – | – | Yes | Not performed | CS, MTX | Improvement |
| Huys et al. (38) | 58, F | 9 month | MTX, Adalimumab | Headache and psychomotor retardation, seizures | – | – | – | 30 | 0.55 | – | – | – | – | Yes | Yes | CS, RTX, Leflunomide, MTX d/c, Adalimumab d/c | Improvement |
| Duray et al. (22) | 73, M | 1 | CS, MTX | Disorientation, apathy, and astenia, walking difficulty | 2,720 | >340 | – | 83–91 | 1.3–2.22 | 42–58 | – | – | – | First MRI normal, yes | Yes | CS, CYC | Improvement |
| Krysl et al. (5) | 62, M | 10 | HCQ | Epilepsia partialis continua right side | 1:320 | 760 | – | 0–32 | 0.245–0.345 | – | – | – | OCBs in one CSF sample | Yes | Yes (2 year after initial symtoms) | CS | Improvement |
| Roques et al. (39) | 60, M | NR | MTX | Transient right-sided paresis and hypoesthesia | – | – | – | Increased | Mild elevation | Normal | – | – | – | Yes | Yes | Not reported | Not reported |
| Hayashi et al. (4) | 60, M | 10 | CS | Parkinsonism not responsive to levo-dopa | – | – | – | 13 | 0.75 | Normal | – | – | – | Yes | Yes | CS | Incomplete improvement |
| Bourgeois et al. (40) | 70, M | NR | NR | Transient right hemiparesis, headache | Positive | – | – | 68 | 0.47 | 2,9 mmol /L | – | – | – | Yes | Yes | CS, HCQ, sulfasalazine | Improvement |
| Rijkers et al. (6) | 57, F | NR | NR | Tonic-clonic seizures | – | – | – | – | – | – | – | – | – | Yes | Yes | CS | Not reported |
| Yeaney et al. (9) | 63, M | 9 | NR | Headache and paresis | – | – | – | – | – | – | – | – | – | Yes | Yes | Not reported | Not reported |
| Padjen et al. (20) | 77, F | 0 | None | Seizures and right hemiparesis | 171.7 | 405.3 | – | Normal | Normal | Normal | – | – | – | Yes | Yes | CS | Improvement |
| Lu et al. (27) | 60, F | 23 | CS, Auranofin | Headache, photophobia, insomnia, panic attacks, hallucinations | >1:160 | Strongly positive | – | 2 | 0.26 | 58 | – | – | – | Yes | Yes | CS | Improvement |
| Roy et al. (3) | Late 50s, F | NR | MTX, sulfasalazine | Transient aphasia, confusion, headache right leg weakness, right facial drop | – | High | – | 12 | 0.55 | 58 | – | – | – | Yes | Yes | MTX, MMF, MTX d/c | Improvement |
| Magaki et al. (28) | 37, M | 0 | None | Headache, facial weakness, speech disorder, right hand dysfunction | 83 | >250 | – | 10–16 | 0.35–0.50 | 50–89 | – | – | – | Yes | Yes | CS | Improvement |
| Magaki et al. (28) | 62, F | 0 | None | Confusion and transient loss of consiousness, seizures, and lower limb weakness | Negative | – | – | – | – | – | – | – | – | Not reported | Yes | CS | Incomplete improvement |
| Nihat et al. (41) | 71, F | 6 | Adalimumab, MTX | Dysarthria, paresthesia left face and arm, difficulty walking, tremor, and headache | 7,900 U/L | 226 | ESR = 76 mm/h; ANA 1:80 | 50–80 | 0.46–0.67 | 2.4 mmol/L | – | – | – | Yes | Yes | CS, CYC, MTX | Improvement |
| Saego et al. (29) | 66, F | 12 | Infliximab | LE numbness, aphasia developing into headache, LE paralysis | – | – | – | 213–216 | 4.4–8.59 | 41–44 | RF elevated | – | – | Yes | Yes | CS | Improvement |
| Shibahara et al. (12) | 63, M | 0 | Headache, vertigo, confusion | 140 | 472 | ESR = 18 mm/h | 37 | 0.92 | Normal | – | 4.4–26.2 | IL-6 = 482 pg/ml | Yes | Not performed | CS | Improvement | |
| Matsuda et al. (21) | 66, M | 19 | CS, MTX, iguratimod | Falls | 160 | 310 | ESR = 38 mm/h; ANA 1:5120; SSA and SSB positive | 71 | 1.14 | 27 | – | – | Yes | Not performed | CS, MTX d/c | improvement | |
| Moeyersoons et al. (42) | 49, F | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Not performed | CS, RTX. adalimumab d/c, leflunomide d/c | Improvement |
| Tsuzaki et al. (43) | 65, M | 7 month | CS, MTX, Entanercept | Transient loss of consciousness, seizures, transient dysrthria, left leg weakness | 12 | 275 | sIL2R = 555 U/mL; ANA = 80; SSA 297 U/mL; SSB 18.6 U/mL | 12 | 0.32 | 55 | – | – | First normal, yes | Yes | CS, tocilizumab, etanercpt d/c | Improvement | |
| Choi et al. (11) | 65, F | 3 | CS, MTX, leflunomide | Headache, confusion, and recurrent left hemiparesis | 69.3 | 48.8 | – | 20 | 1.134 | 43 | RF 17.6 | – | – | Yes | Yes | CS | Improvement |
| Degboé et al. (44) | 59, M | 6 | MTX | Transient right-sided hypoesthesia and hemiparesis | – | – | – | 30 | 0.75 | 3.2 mmol/L | – | – | – | Yes | Yes | CS, MTX, RTX | Improvement |
| Jessee and Keenan(45) | 68, F | 0 | None | Confusion, right-sided weakness, and seizures | 208 | 95.8 | ANA 1:640 | 8 | 0.65 | 56 | – | – | Not done (pacemaker) | Yes | CS, MTX | Incomplete improvement | |
| Alexander et al. (46) | 73, M | NR | Leflunomide | Transient speech disorder, behavoiral change and seizure | 45 | >340 | – | 18–100 | 0.69–1.03 | 2.5–3.1 mmol/L | – | – | Yes | Yes | CS, RTX | Incomplete improvement | |
| Finkelshtein et al. (19) | 66, F | 0 | None | Headache, transient paresthesia left leg | 23–25 | 266 | – | – | – | – | – | – | – | Yes | Yes | None | Improvement |
| Parsons et al. (47) | 76, M | 30 | MTX | Transient left UE paresis, new onset seizures | Elevated | Elevated | ANA elevated | 239 | 0.39 | 51 | RF negative | – | – | Yes | Yes | CS, MTX | Improvement |
| Oono et al. (23) | 36, F | 13 | CS, MTX | Headache and transient sensory disturbance right face and UE | – | – | ESR = 56 mm/h; anti-RNP = 15 U/mL | 19 | 0.57 | 51 | – | IL−6 = 843 pg/ml, OCBs | Yes | Not performed | CS, MTX d/c | Improvement | |
| Akamatsu et al. (13) | 55, F | 6 month | MTX | Speech difficulty, left-sided hemiparesis, and spatial neglect | 85 U/L | 223.7 | 68 | 0.40 | 52 | 3.7 | IL-6 = 271 pg/mL | Yes | Not performed | CS | Incomplete improvement | ||
| Gherghel et al. (10) | 77, F | >9 year | Ethanercept, leflunomide | Recurrent speech disorder and left-sided paresthesia and hemparesis | 86 | 119 | ANA 1:160 | 5 | 0.49 | – | – | – | – | Yes | Yes | CS, etanercept d/c, leflunomide d/c | Incomplete improvement |
| Schuster et al. (14) | 48, M | 0 | None | Headache, recurrent left-sided weakness | 298 | >340 | – | 300 | 1.37 | – | – | >340 | – | Yes | Not performed | CS | Improvement |
| Schuster et al. (14) | 62, F | Not stated | NR | Recurrent tingling and weakness | 146 | 265 | – | Normal | – | – | – | – | Yes | Not performed | CS, MTX | Improvement | |
| Schuster et al. (14) | 72, M | 0 | None | Recurrent sensory motor deficit left-side | 133 | 154 | – | 51 | Normal | – | – | – | – | Yes | Yes | CS | Improvement |
| Schuster et al. (14) | 62, M | 11 | NR | Alexia, agraphia, acalculia, headache, seizures | 22.3 | 329 | – | Normal | Normal | – | – | – | – | Yes | Not performed | CS, tocilizumab | Improvement |
| Schuster et al. (14) | 65, F | 11 | NR | Recurrent sensory motor deficit left-side, speech disorder | 313 | 26 | – | 8 | 0.653 | – | – | – | – | Yes | Yes | CS, tocilizumab, leflunomide d/c; MTX d/c | Improvement |
| Schuster et al. (14) | 45, M | 30 | NR | Recurrent left-side hypoesthesia, headache, ataxia | 113 | 7 | – | 37 | 4.6 | – | – | – | – | Yes | Yes | CS, CYC, MTX, leflunomide d/c; HCQ d/c | Improvement |
| Ching et al. (31) | 72, F | 0 | None | Left-sided weakness, psychiatric symptoms, seizures | Negative | 197.5 | ESR = 39 mm/h | 12 | 0.25 | 58 | – | – | – | Yes | Yes | CS | Improvement |
| Harrison et al. (48) | 53, M | NR | CS, leflunomide, tofacitinib citrate | Headache, seizures, right LE paresis | 293 | 250 | ESR 46 mm/h | 7 | 0.64 | 48 | – | – | – | Yes | Yes | CS, RTX | Improvement |
| McKenna et al. (30) | 59, M | 0 | None | Headache and left-sided weakness, focal onset seizures | 88.2 | >340 | ACE = 70 U/L | Pleocytosis | 0.672 | 3.4 mmol/L | – | – | – | Yes | Yes | CS | Improvement |
| Pellerin et al. (1) | 74, M | 3–4 | CS, HCQ, MTX | Expressive aphasia, imbalance, potural tremor, parkinsonism, seizures | High | High | ACE 66 U/L, beta 2 mikroglobulin 4,6 mg/L | 6 | 0.86 | Normal | – | – | – | Yes | Yes | CS, CYC, MTX d/c | Incomplete improvement |
| Grose et al. (49) | 87, F | NR | None | UE weakness, confusion, hallucinations | 143 | >200 | ANA 1:640 | 104 | 1.55 | Normal | – | – | – | Yes | Not performed | CS | Incomplete improvement |
| Scheitel et al. (50) | 75, F | 9 | CS, leflunomide | UE paresthesia, weakness, headache, facial jerks, Rytmic jerks | High | High | ESR = 92 mm/h | 14 | 0.69 | – | – | – | – | Yes | Not performed | CS, RTX | Improvement |
| Lubomski et al. (15) | 49, M | 0 | None | Headache, deterioration in mental state, delusions | 8 | >600 | – | 1 | 0.39 | 3.4 mmol/l | Strongly positive | Yes | Yes | CS, RTX | Improvement | ||
Summary of RM cases from 2010 to present.
ACE, angiotensin converting enzyme; ANA, antinuclear antibodies; AZA, azathioprin; CS, corticosteroids; CYC, cyclophosphamide; d/c, discontinued; ESR, erythrocyte sedimentation rate; F, female; HCQ, Hydroxychloroquine; IL-6, interleukin-6; IT, intrathecal; LE, lower extremity; M, male; MTX, methotrexate; MMF, Mycophenolate mofetil; N/A, not avaliable; NR, not reported; RA, rheumatoid arthritis; RF, rheumatic factor; RM, rheumatoid meningitis; RNP, ribonucleoprotein; RTX, Rituximab; sIL2R, soluble interleukin-2 receptor; SSA, Anti-Sjögren's-syndrome-related antigen A; SSB, Sjögren's-syndrome-related antigen B; UE, upper extremity.
Summary
Keywords
rheumatoid meningitis, inflammation, anti-CCP, CXCL13, biomarker
Citation
Nissen MS, Nilsson AC, Forsberg J, Milthers J, Wirenfeldt M, Bonde C, Byg K-E, Ellingsen T and Blaabjerg M (2019) Use of Cerebrospinal Fluid Biomarkers in Diagnosis and Monitoring of Rheumatoid Meningitis. Front. Neurol. 10:666. doi: 10.3389/fneur.2019.00666
Received
28 March 2019
Accepted
06 June 2019
Published
26 June 2019
Volume
10 - 2019
Edited by
Thomas Skripuletz, Hannover Medical School, Germany
Reviewed by
Elena Bartoloni, University of Perugia, Italy; Geoffrey M. Thiele, University of Nebraska Medical Center, United States
Updates
Copyright
© 2019 Nissen, Nilsson, Forsberg, Milthers, Wirenfeldt, Bonde, Byg, Ellingsen and Blaabjerg.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mette Scheller Nissen mette.scheller.nissen2@rsyd.dk
This article was submitted to Multiple Sclerosis and Neuroimmunology, a section of the journal Frontiers in Neurology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.