- 1Department of Neurosurgery, Seirei-Hamamatsu General Hospital, Hamamatsu, Japan
- 2Comprehensive Epilepsy Center, Seirei-Hamamatsu General Hospital, Hamamatsu, Japan
- 3Department of Pathology, Seirei-Hamamatsu General Hospital, Hamamatsu, Japan
Neuropathological features in Alzheimer's disease (AD) are amyloid β (Aβ) deposits and neurofibrillary changes. AD is characterized by memory impairment and cognitive dysfunction, with some reports associating these impairments with hyperexcitability caused by Aβ in the medial temporal lobe. Epileptic seizures are known to be common in AD. We encountered a 65-year-old patient with cavernous malformation (CM) in the right temporal lobe who exhibited epileptic amnesia (EA) and AD-like symptoms. Scalp electroencephalography (EEG), including long-term video-EEG, showed no interictal discharges, but intraoperative subdural electrode (SE) recording from the right parahippocampal area showed frequent epileptiform discharges. Neuropathologically, senile plaques were found in the surrounding normal cortex of the CM. Postoperatively, the patient has remained free of EA and AD-like symptoms since total removal of the CM. This is the first surgical case report to confirm temporal lobe hyperexcitability associated with EA and AD-like symptoms.
Background
Alzheimer's disease (AD) is the most frequent neurodegenerative disease causing dementia in elderly people. This pathology is characterized by synapse loss in the cortex, as well as deposition of certain distinctive lesions such as senile plaques and cerebral amyloid angiopathy mainly comprising amyloid β (Aβ) and neurofibrillary tangles composed predominantly of hyperphosphorylated tau (1, 2). Clinically, memory impairment is conspicuous, along with progression of cognitive dysfunctions such as disorientation disorder, aphasia, alexia, or agnosia. On the one hand, incipient protein pathologies such as Aβ in the medial temporal lobe are widely accepted to instigate the loss of episodic memory in AD (3, 4). On the other hand, other investigators have associated these impairments in AD with hyperexcitability of the medial temporal lobe induced by Aβ (5, 6). Epileptic seizures are known to occur in AD (7, 8) and many elderly individuals exhibit temporal lobe epilepsy (9, 10). A link between AD and epilepsy has thus been suggested (11–13).
One report of two cases with AD and amnesia provided a theoretical explanation of amnesic episodes arising from medial temporal lobe epileptic seizures using data from foramen ovale electrodes (12). Here, we encountered a patient with cavernous malformation (CM) in the right temporal lobe who had been diagnosed with AD and who exhibited repetitive and progressive amnesic episodes without epileptiform discharges on scalp electroencephalography (EEG). We hypothesized that the temporal lobe CM had caused the amnesic episodes and AD-like symptoms.
Case Presentation
A 63-year-old, right-handed man exhibited transient episodes of amnesia. He also showed independent, short-duration loss of awareness with oral automatism. He had therefore visited a local hospital, where brain magnetic resonance imaging (MRI) had revealed CM in the right amygdala (Figure 1). As the frequency of loss of awareness and transient amnestic episodes increased and memory disturbances exacerbated over a period of years, he was diagnosed with early-stage AD concomitant with temporal lobe epilepsy at the hospital. He was therefore referred to our epilepsy center at 64 years old.
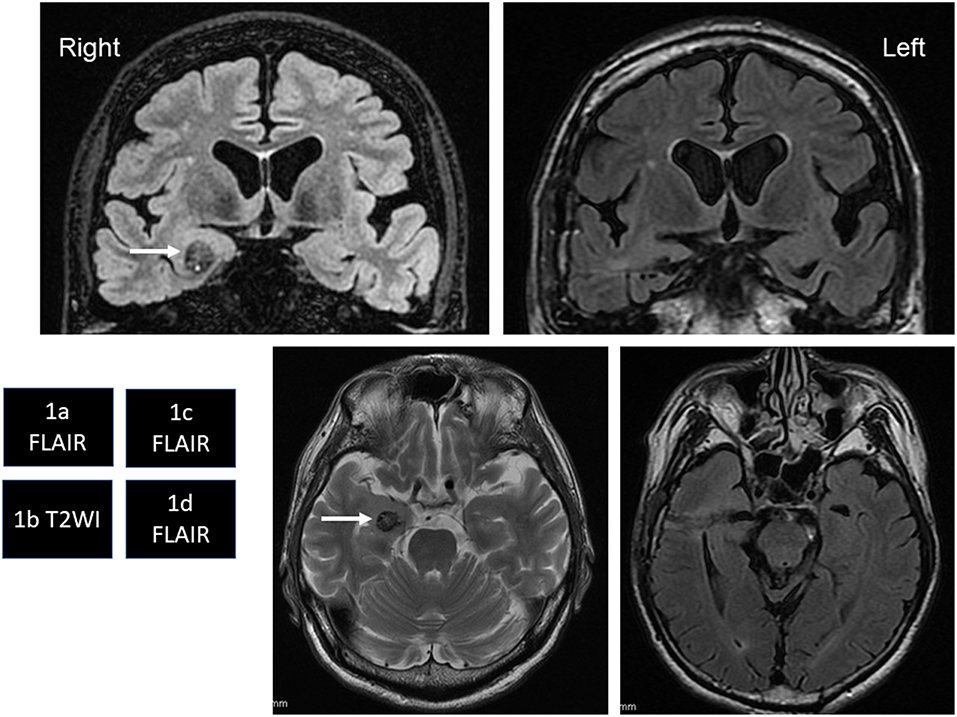
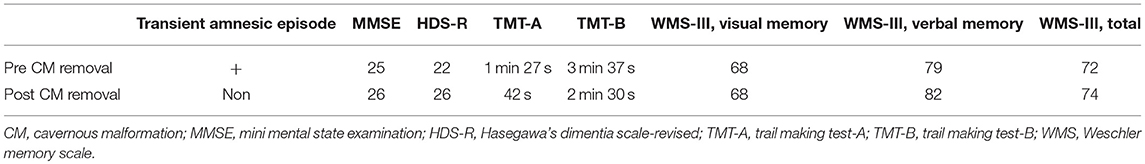
Figure 1. (a,b) White arrows in coronal fluid-attenuated inversion recovery (FLAIR) image (a) and axial T2-weighted image (b) show right temporal cavernous malformation (CM). Cortical atrophy and ventriculomegaly of the brain are also seen. (c,d) FLAIR images show total removal of the CM by a transcortical approach.
Seven-day scalp video-EEG performed at our epilepsy center captured no interictal epileptiform discharges, impaired awareness seizures, or oral automatisms. However, based on the clinical history and right amygdala CM, we diagnosed epileptic amnesia (EA) and prescribed levetiracetam. Levetiracetam mildly decreased the frequency of intermittent amnesic episodes, but did not resolve them completely. As we had started speculating that the CM in the right amygdala might have contributed to EA and cognitive deterioration, he underwent neuropsychological examinations: preoperative Mini-Mental State Examination (MMSE) score, 25/30; Hasegawa's dementia scale-revised (HDS-R), 22/30; Weschler memory scale (WMS)-III, verbal memory 79, visual memory 68, total memory 72; trail making test (TMT)-A, 1 min 27 s; TMT-B, 3 min 37 s. We planned minimally invasive intraoperative subdural electrode (SE) recording directly from the parahippocampal area via a small burr hole and small skin incision. We had decided in advance that if epileptiform discharges were obtained from the SE recording, we would proceed to remove the CM. If no epileptiform discharges were obtained, we would just withdraw the SE and close the incision.
Intraoperatively, the SE recording from the right parahippocampal area (Figure 2a) showed frequent epileptiform discharges (Figure 2b). We therefore selectively removed the CM under a transcortical approach at 65 years old. After removal of the CM, the SE recording showed no interictal discharges (Figure 2c).
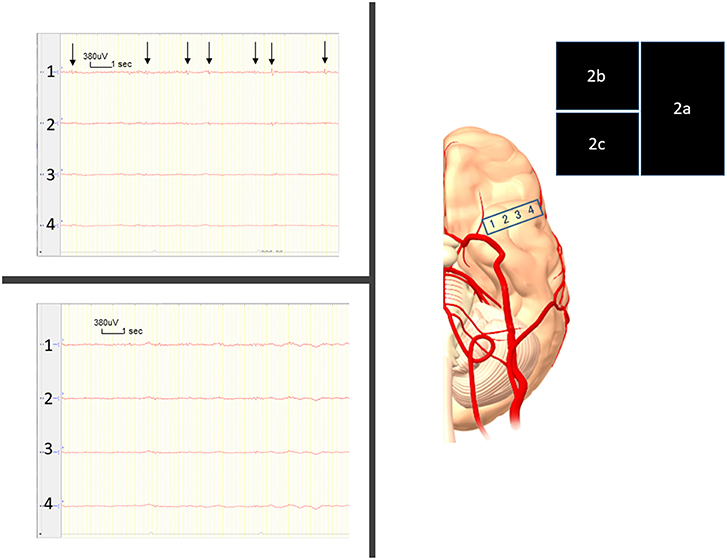
Figure 2. (a) Intraoperatively, we place the four-contact subdural strip on the parahippocampal area. (b) Frequent, very small-amplitude spikes and spike-waves are seen, mostly from contact 1 (arrows) before removal of the cavernous malformation (CM). (c) Right after CM removal, these epileptiform discharges have disappeared.
Postoperatively, the patient remained free from transient amnesic episodes and impaired awareness seizures. Four months after the surgery, neuropsychological examinations conducted without changing any medication showed: MMSE, 26/30; HDS-R, 26/30; WMS-R, verbal memory 82, visual memory 68, total memory 74; TMT-A, 42 s; and TMT-B, 2 min 30 s (Table 1). Postoperatively, the patient could walk, talk, and eat faster and became more sociable.
The neuropathological examination showed CM, with senile plaques found in the normal cortex surrounding the CM according to periodic acid methenamine silver staining and immunohistochemical staining (Figure 3).
Figure 3. (a) Hematoxylin and eosin (HE) staining shows cavernous malformation. Eosinophilic nodules are considered to represent organized thrombi (blue arrow). (b) HE staining shows brain tissue with senile plaque (yellow arrow). (c) Periodic acid methenamine silver (PAM) staining shows two lesions of filamentous material (red arrows), comprising senile plaque. (d) Immunohistochemical staining shows immunoreactive senile plaque (arrow).
Discussion
Hyperexcitability of the temporal lobe was directly recorded by the intraoperative SE. Removal of the right amygdala CM completely resolved the transient amnesic episodes and mildly improved patient cognitive function. Therefore, in this case we could say that the CM in the temporal lobe generated medial temporal lobe excitability, as confirmed by the SE recording and that this hyperexcitability produced the EA and AD-like symptoms. Although CM could occur as a de novo formation (14, 15), this lesion is generally considered congenital in nature or at least presents as a long-term process (16, 17) and many are found incidentally while the patient is asymptomatic (18–20). Brain hyperexcitability generated by adjacent to CM is widely recognized (21) and this hyperexcitability is associated with epileptogenicity (22, 23). This meant that the patient had been potentially in an epileptic state due to the CM for a long time, but had not exhibited clinical epileptic seizures. We did not regard CM as directly contributing to senile plaque formation, with other causes leading to accumulation of senile plaque in the brain. As senile plaque was found in the patient, the Aβ also added to any underlying hyperexcitability (5, 6, 24) in the potentially epileptic medial temporal lobe with the CM. As his brain MRI showed cortical atrophy and ventriculomegaly, this condition was also attributed to AD-like symptoms.
AD is a complex disorder in which synaptic loss, accumulation of senile plaque, neurofibrillary tangles, and microvascular amyloid is seen in meningocortical regions (2). Overly, this patient had multiple factors causing AD-like symptoms. He had started to develop impaired awareness seizures, EAs, and AD-like symptoms over a period of years. However, removal of the CM reduced the temporal lobe hyperexcitability, and consequently completely eliminated EA and impaired awareness seizures, and mildly improved AD-like symptoms.
We could say that: (1) EA and impaired awareness seizures were generated by temporal lobe hyperexcitability; (2) this temporal lobe hyperexcitability was somehow associated with AD-like symptoms, since the symptoms of EA and impaired awareness seizures completely resolved and symptoms of AD-like symptoms improved mildly after removal of the CM that generated hyperexcitability of the mesial temporal lobe as proven by direct recording.
Memantine works to improve AD as a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist (25) and its effectiveness against EA has been reported (25, 26). Levetiracetam might improve symptoms of AD (27). A link between epilepsy and dementia with Levy bodies has also been reported (28).
Diagnosing epilepsy in elderly individuals is very difficult, because focal onset impaired awareness seizures are also reportedly short in the elderly (10), while patients with temporal lobe epilepsy may exhibit recent verbal and non-verbal memory dysfunction, cognitive declines (29), and psychiatric symptoms such as fear and hallucinations (30, 31), which are also seen in AD and are hard to distinguish from each other. Moreover, transient EA might not have a phase of impaired awareness seizures (32). The sensitivity and specificity of scalp EEG are low (33, 34). The diagnosis of epilepsy might not be considered until the patient exhibits convulsive seizures.
As epileptic seizures are frequently seen in the earlier stages of AD, which is known as mild cognitive impairment (30), we could say that the patient was in the prodromal state of AD and we were able to cure most of his symptoms. However, we need to follow-up this patient over the long term. Furthermore, accumulation of data from more patients is needed to evaluate whether epilepsy treatment can prevent progression of AD.
As the incidence of epilepsy in elderly patients is increasing dramatically (35), the possibility must be considered that among the many forms of dementia, some underlying conditions associated with epilepsy (36–39) could be curable.
Data Availability
All datasets generated for this study are included in the manuscript and/or the supplementary files.
Ethics Statement
Written informed consent for publication of case details was obtained from our patient and his caregiver. This study was approved by the ethics committee at Seirei Hamamatsu General Hospital.
Author Contributions
YH and AF: acquisition of data. AF, KS, NI, TO, and HE: analysis and interpretation of EEG. AF and NI: surgery. YO: neuropathology. AF, TO, HE, and TT: advice on the paper.
Contribution to the Field
Cavernous malformation (CM) was found in the right medial temporal lobe (TL) of a 65-year-old man diagnosed with early-onset AD. TL hyperexcitability has been hypothesized as the cause of AD. We therefore thought that the CM might have been the cause of AD symptoms in this patient.
As TL hyperexcitability could not be measured by scalp electroencephalography, we directly measured TL activity near the CM and found epileptic discharges, which meant hyperexcitability in the TL. We therefore removed the CM.
Postoperatively, amnestic episodes disappeared, and AD symptoms were alleviated.
In pathological tissues, CM and senile plaque were observed.
The CM had potential hyperexcitability in the TL over a long period, and Aβ added further hyperexcitability in his old age to the TL, so TL hyperexcitability was created by both CM and Aβ, leading to the clinical symptoms. Among the many forms of dementia, some may involve underlying conditions associated with epilepsy.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. (2012) 123:1–11. doi: 10.1007/s00401-011-0910-3
2. Vinters HV. Emerging concepts in Alzheimer's disease. Annu Rev Pathol. (2015) 10:291–319. doi: 10.1146/annurev-pathol-020712-163927
3. Hyman BT, Van Hoesen GW, Damasio AR. Memory-related neural systems in Alzheimer's disease: an anatomic study. Neurology. (1990) 40:1721–30. doi: 10.1212/WNL.40.11.1721
4. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (1991) 82:239–59. doi: 10.1007/BF00308809
5. Noebels J. A perfect storm: converging paths of epilepsy and Alzheimer's dementia intersect in the hippocampal formation. Epilepsia. (2011) 52 (Suppl 1):39–46. doi: 10.1111/j.1528-1167.2010.02909.x
6. Tamano H, Ide K, Adlard PA, Bush AI, Takeda A. Involvement of hippocampal excitability in amyloid beta-induced behavioral and psychological symptoms of dementia. J Toxicol Sci. (2016) 41:449–57. doi: 10.2131/jts.41.449
7. Born HA. Seizures in Alzheimer's disease. Neuroscience. (2015) 286:251–63. doi: 10.1016/j.neuroscience.2014.11.051
8. Horvath A, Szucs A, Barcs G, Noebels JL, Kamondi A. Epileptic seizures in Alzheimer disease: a review. Alzheimer Dis Assoc Disord. (2016) 30:186–92. doi: 10.1097/WAD.0000000000000134
9. Tanaka A, Akamatsu N, Shouzaki T, Toyota T, Yamano M, Nakagawa M, et al. Clinical characteristics and treatment responses in new-onset epilepsy in the elderly. Seizure. (2013) 22:772–5. doi: 10.1016/j.seizure.2013.06.005
10. Fujimoto A, Okanishi T, Masuda Y, Sato K, Nishimura M, Baba S, et al. Short duration of focal onset awareness and impaired awareness seizure are characteristic features of epilepsy in elderly patients. Neuropsychiatr Dis Treat. (2018) 14:2879–87. doi: 10.2147/NDT.S179720
11. Costa C, Parnetti L, D'Amelio M, Tozzi A, Tantucci M, Romigi A, et al. Epilepsy, amyloid-beta, and D1 dopamine receptors: a possible pathogenetic link? Neurobiol Aging. (2016) 48:161–71. doi: 10.1016/j.neurobiolaging.2016.08.025
12. Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer's disease. Nat Med. (2017) 23:678–80. doi: 10.1038/nm.4330
13. Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer's disease: causes and clinical relevance. Lancet Neurol. (2017) 16:311–22. doi: 10.1016/S1474-4422(17)30044-3
14. Brinjikji W, El-Masri AE, Wald JT, Flemming KD, Lanzino G. Prevalence of cerebral cavernous malformations associated with developmental venous anomalies increases with age. Childs Nerv Syst. (2017) 33:1539–43. doi: 10.1007/s00381-017-3484-0
15. Cox EM, Bambakidis NC, Cohen ML. Pathology of cavernous malformations. Handb Clin Neurol. (2017) 143:267–77. doi: 10.1016/B978-0-444-63640-9.00025-4
16. Mooney MA, Zabramski JM. Developmental venous anomalies. Handb Clin Neurol. (2017) 143:279–82. doi: 10.1016/B978-0-444-63640-9.00026-6
17. Winkler EA, Rutledge C, Ward M, Tihan T, Sneed PK, Barbaro N, et al. Radiation-induced cavernous malformation as a late sequelae of stereotactic radiosurgery for epilepsy. Cureus. (2018) 10:e2308. doi: 10.7759/cureus.2308
18. Dalyai RT, Ghobrial G, Awad I, Tjoumakaris S, Gonzalez LF, Dumont AS, et al. Management of incidental cavernous malformations: a review. Neurosurg Focus. (2011) 31:E5. doi: 10.3171/2011.9.FOCUS11211
19. Akers A, Al-Shahi Salman R, Awad IA, Dahlem K, Flemming K, Hart B, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. (2017) 80:665–80. doi: 10.1093/neuros/nyx091
20. Velz J, Stienen MN, Neidert MC, Yang Y, Regli L, Bozinov O. Routinely performed serial follow-up imaging in asymptomatic patients with multiple cerebral cavernous malformations has no influence on surgical decision making. Front Neurol. (2018) 9:848. doi: 10.3389/fneur.2018.00848
21. Jehi LE, Palmini A, Aryal U, Coras R, Paglioli E. Cerebral cavernous malformations in the setting of focal epilepsies: pathological findings, clinical characteristics, and surgical treatment principles. Acta Neuropathol. (2014) 128:55–65. doi: 10.1007/s00401-014-1294-y
22. Upchurch K, Stern JM, Salamon N, Dewar S, Engel J Jr., Vinters HV, et al. Epileptogenic temporal cavernous malformations: operative strategies and postoperative seizure outcomes. Seizure. (2010) 19:120–8. doi: 10.1016/j.seizure.2009.11.006
23. Sevy A, Gavaret M, Trebuchon A, Vaugier L, Wendling F, Carron R, et al. Beyond the lesion: the epileptogenic networks around cavernous angiomas. Epilepsy Res. (2014) 108:701–8. doi: 10.1016/j.eplepsyres.2014.02.018
24. Sompol P, Furman JL, Pleiss MM, Kraner SD, Artiushin IA, Batten SR, et al. Calcineurin/NFAT signaling in activated astrocytes drives network hyperexcitability in abeta-bearing mice. J Neurosci. (2017) 37:6132–48. doi: 10.1523/JNEUROSCI.0877-17.2017
25. Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–too little activation is bad, too much is even worse. Neuropharmacology. (2007) 53:699–723. doi: 10.1016/j.neuropharm.2007.07.013
26. Leeman-Markowski BA, Meador KJ, Moo LR, Cole AJ, Hoch DB, Garcia E, et al. Does memantine improve memory in subjects with focal-onset epilepsy and memory dysfunction? A randomized, double-blind, placebo-controlled trial. Epilepsy Behav. (2018) 88:315–24. doi: 10.1016/j.yebeh.2018.06.047
27. Stockburger C, Miano D, Baeumlisberger M, Pallas T, Arrey TN, Karas M, et al. A mitochondrial role of SV2a protein in aging and Alzheimer's disease: studies with levetiracetam. J Alzheimers Dis. (2016) 50:201–15. doi: 10.3233/JAD-150687
28. Ukai K, Watanabe M. Transient epileptic amnesia accompanied by prodromal symptoms of dementia with Lewy bodies: the second case report in the literature. Psychogeriatrics. (2019). doi: 10.1111/psyg.12434 [Epub ahead of print].
29. Tai XY, Koepp M, Duncan JS, Fox N, Thompson P, Baxendale S, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain. (2016) 139:2441–55. doi: 10.1093/brain/aww187
30. Cretin B, Philippi N, Bousiges O, Dibitonto L, Sellal F, Martin-Hunyadi C, et al. Do we know how to diagnose epilepsy early in Alzheimer's disease? Rev Neurol. (2017) 173:374–80. doi: 10.1016/j.neurol.2017.03.028
31. Clancy MJ, Clarke MC, Connor DJ, Cannon M, Cotter DR. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. (2014) 14:75. doi: 10.1186/1471-244X-14-75
32. Ukai K, Watanabe M. Transient epileptic amnesia without epileptic seizures: proposal of a new entity. Psychogeriatrics. (2017) 17:491–2. doi: 10.1111/psyg.12249
33. Van Cott AC. Epilepsy and EEG in the elderly. Epilepsia. (2002) 43 (Suppl 3):94–102. doi: 10.1046/j.1528-1157.43.s.3.10.x
34. Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. (2005) 331:1317–22. doi: 10.1136/bmj.331.7528.1317
35. Hernandez-Ronquillo L, Adams S, Ballendine S, Tellez-Zenteno JF. Epilepsy in an elderly population: classification, etiology and drug resistance. Epilepsy Res. (2018) 140:90–4. doi: 10.1016/j.eplepsyres.2017.12.016
36. Cretin B, Blanc F, Gaultier C, Sellal F. Epileptic amnesic syndrome revealing Alzheimer's disease. Epilepsy Res. (2012) 102:206–9. doi: 10.1016/j.eplepsyres.2012.08.002
37. Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. (2013) 70:1158–66. doi: 10.1001/jamaneurol.2013.136
38. Cretin B, Philippi N, Sellal F, Dibitonto L, Martin-Hunyadi C, Blanc F. Can the syndrome of transient epileptic amnesia be the first feature of Alzheimer's disease? Seizure. (2014) 23:918–20. doi: 10.1016/j.seizure.2014.07.008
Keywords: cavernous malformation, epileptic amnesia, subdural electrode recording, Alzheimer disease-like symptoms, amyloid β, hyperexcitability of the medial temporal lobe
Citation: Hirokawa Y, Fujimoto A, Ichikawa N, Sato K, Tanaka T, Enoki H, Otsuki Y and Okanishi T (2019) Temporal Lobe Cavernous Malformation Caused Epileptic Amnesic Episodes and Mild Cognitive Impairment. Front. Neurol. 10:620. doi: 10.3389/fneur.2019.00620
Received: 28 March 2019; Accepted: 28 May 2019;
Published: 12 June 2019.
Edited by:
Jose F. Tellez-Zenteno, University of Saskatchewan, CanadaReviewed by:
Harry Vinters, UCLA Neuropathology Consultation Service, United StatesAna Luisa Velasco, Beilinson Hospital, Rabin Medical Center, Israel
Copyright © 2019 Hirokawa, Fujimoto, Ichikawa, Sato, Tanaka, Enoki, Otsuki and Okanishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayataka Fujimoto, YWZ1amltb3Rvc2NpZW5jZWFjYWRlbXlAZ21haWwuY29t
 Yusuke Hirokawa1
Yusuke Hirokawa1 Ayataka Fujimoto
Ayataka Fujimoto Tohru Okanishi
Tohru Okanishi