
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 05 June 2019
Sec. Stroke
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00563
Background: Overweight contributes to type 2 diabetes mellitus (T2DM) development. Although the obesity paradox has been suggested in many vascular diseases, little information is available about stroke patients with T2DM. We investigated whether body mass index (BMI) has a differential impact on the incidence of major adverse cardiovascular events (MACE) in patients with ischemic stroke and T2DM.
Methods: This retrospective study used a prospective cohort of patients with acute ischemic stroke and included consecutive patients with T2DM after excluding those with active cancer or who died within 1 month of an index stroke. We investigated the long-term risk of MACE (stroke, myocardial infarction, unstable angina, coronary revascularization procedure, and death) according to BMI.
Results: Among the 1,338 patients, MACE occurred in 415 patients (31.1%) during a median follow-up of 3.6 years. Compared to the normal weight group, MACE occurred more frequently in the underweight group [adjusted hazard ratio (HR) 1.55, 95% confidence interval (CI): 1.01–2.38], but less frequently in the overweight group (adjusted HR: 0.87, 95% CI: 0.70–1.08) and obese group (adjusted HR: 0.58, 95% CI: 0.41–0.86) group. In analyses of association between BMI and each component of MACE, stroke and cardiovascular mortality indicated an L- and a U-shaped pattern, respectively. However, fatal or non-fatal stroke showed an inverse pattern, and fatal or non-fatal cardiovascular events showed a reversed J-shaped pattern.
Discussions: This study showed the overall presence of the obesity paradox in stroke patients with T2DM. However, obese patients had different risks of cardiovascular events and stroke.
Obesity and diabetes mellitus are established risk factors for vascular disease (1). However, leaner adults have higher mortality rates than obese or overweight adults (2–4). This phenomenon has been called the obesity paradox and has been implicated in vascular diseases, including stroke and cardiovascular disease (CVD), as well as chronic diseases, such as hypertension, end stage renal disease, chronic obstructive pulmonary disease, and peripheral artery disease (5–9).
Many patients with type 2 diabetes mellitus (T2DM) are overweight or obese. Overweight is associated with impaired glucose tolerance and insulin resistance, which contribute to the development of T2DM. Due to the pathophysiological relationship between overweight and T2DM, several studies have investigated the association between body mass index (BMI) and mortality in patients with T2DM. Meta-analyses and large cohort studies in patients with T2DM alone showed a U-shaped or J-shaped relationship between BMI and long-term mortality (10–16). In contrast to these T2DM patients, BMI was inversely correlated with mortality in patients with T2DM and acute heart failure (HF) (17). In a prospective cohort study of T2DM patients without known CVD at baseline, overweight or obese patients had a higher rate of cardiac events, such as acute coronary syndrome and HF than those with normal BMI. In the same population, however, the risk of mortality was lower in overweight patients than those with normal BMI (12). These findings suggest that the relationship of BMI with the adverse effects of T2DM may differ depending on the specific disease group. In this study, we investigated whether the incidence of major adverse cardiovascular events (MACE) differs according to BMI in patients with ischemic stroke and T2DM.
This was a retrospective, observational study of prospectively registered patients with ischemic stroke and T2DM included in the Yonsei Stroke Cohort (18). Within 7 days of symptom onset, the cohort enrolled consecutive patients with acute ischemic stroke that have been admitted to the Severance Stroke Center of Yonsei University in South Korea. All patients underwent brain magnetic resonance imaging and/or computerized tomography and cerebral angiography (magnetic resonance angiography, computerized tomography angiography, or digital subtraction angiography). Routine evaluations of patients included standard blood tests, 12-lead electrocardiography, chest radiography, echocardiography, and continuous electrocardiographic monitoring while in the stroke unit or being Holter monitored. Glycated hemoglobin level was routinely assessed in patients with fasting blood sugar ≥ 5.55 mmol/L. All patients were managed according to a standardized protocol and care pathway, which were based on the guidelines.
T2DM was defined based on the American Diabetes Association criteria (19), which include a history of T2DM, current use of hypoglycemic medications or insulin, glycated hemoglobin ≥6.5%, fasting blood glucose level ≥7 mmol/L, or random blood glucose level ≥11.1 mmol/L with typical diabetic symptoms, and no signs of type 1 diabetes. This study was approved by the Institutional Review Board of Yonsei University Health System with a waiver of patients' informed consent due to the retrospective nature of the study.
We collected data for demographics, vascular risk factors for and previous history of stroke, coronary artery occlusive disease, peripheral artery occlusive disease, and chronic kidney disease. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or any history of anti-hypertensive agents. Hyperlipidemia was defined as serum total cholesterol ≥6.21 mmol/L, low-density lipoprotein cholesterol ≥4.14 mmol/L, or history of using lipid-lowering drugs after the diagnosis of hyperlipidemia. Current smoking was defined as a history of smoking any cigarettes within 1 year before admission. We also obtained a history of previously used medication at admission, which included anticoagulants, anti-platelet agents, anti-hypertensive agents, and statins. Laboratory data were also obtained for complete blood counts, lipid profile, initial blood glucose, glycated hemoglobin, blood urea nitrogen, and creatinine. The severity of stroke was assessed using the National Institutes of Health Stroke Scale (NIHSS) at admission. BMI at admission was calculated as weight in kilograms divided by height in meters squared (kg/m2).
After discharge, we regularly followed up patients at 3 months, 1 year, and every year thereafter. At each follow-up visit, we collected medical data on any cases of cardiovascular events or mortality via face-to-face interviews with neurologists or clinical research associates in an outpatient clinic. When the patients missed the visit, we obtained the information from the patient or their families via telephone interviews based on a structured questionnaire (20). We obtained necessary data by reviewing the medical records, if available. In addition, we also obtained mortality data from the Korean National Statistical Office (http://www.kostat.go.kr) using death certificates.
The primary outcome was the composite rate of MACE, which include non-fatal or fatal stroke, non-fatal or fatal myocardial infarction (MI), unstable angina, coronary revascularization procedure, and any death. Cardiovascular mortality was defined as any mortality due to MI, other cardiac diseases, or sudden death. Censoring date was December 31, 2013.
The patients were categorized into four groups according to BMI for the Asian population (underweight: BMI < 18.5 kg/m2; normal weight: 18.5 kg/m2 ≤ BMI < 23 kg/m2; overweight: 23 kg/m2 ≤ BMI < 27.5 kg/m2; and obese: BMI ≥ 27.5 kg/m2) provided by the World Health Organization Expert Consultation panel for appropriate BMI (21). The data were presented as mean ± standard deviation, median (interquartile range), or as number (%), as appropriate. The Shapiro–Wilk test was done to test normality of the continuous variables. The differences between the groups were compared using Kruskal–Wallis test for the continuous variable and a chi-square test or Fisher's exact test for the categorical variable. Post-hoc analyses were used for assessing the magnitude of the differences.
The survival curves were determined and plotted using the Kaplan–Meier method, and the group differences in survival time were analyzed using a log-rank test. To determine the independent predictor for MACE, the Cox proportional hazards regression analysis was used and summarized as hazard ratio (HR) and 95% confidential interval (CI). For multivariate analysis, age, sex, initial NIHSS, and variables with p < 0.1 in the univariate analyses were entered as covariates. The continuous measure of BMI was used to fit a smooth spline curve to obtain a representation of log HR for each component of MACE and mortality by adjusting the variables that were entered into the multivariate analysis. All tests were two-sided and p < 0.05 was considered as statistically significant. R software, 3.3.2 version (R foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses.
Between January 2007 and July 2013, a total of 3,727 consecutive patients with ischemic stroke were registered. After excluding 2,296 patients without T2DM, we excluded further 59 patients with active cancer (diagnosed or receiving antimitotic treatment within the past 6 months, recurrent, metastatic, or inoperable) (22). Then, we excluded 34 patients who died within 1 month from index stroke because their deaths may have been directly related to the index stroke. Finally, a total of 1,338 patients were included for this study (Figure 1).
The mean age of the patients was 66.6 ± 10.9 years, and 813 were men (60.8%). There were 40 (2.9%) underweight patients, 421 patients (31.5%) with normal weight, 698 overweight patients (52.2%), and 179 obese patients (13.4%). Several variables were different between groups, including age, sex, diastolic blood pressures, initial NIHSS, hemoglobin, blood urea nitrogen, total cholesterol, triglyceride, a history of hypertension, smoking, and chronic kidney disease (Table 1).
During the mean follow-up for 3.6 ± 1.8 years, MACE occurred in 415 patients (31.1%). All-cause death occurred in 351 [26.2%, fatal stroke in 100 (7.5%), fatal MI in 28 (2.1%), and other fatal events in 223 (16.7%)]. Mean annual event rates for MACE were 39.2% in the underweight, 18.0% in the normal weight, 12.0% in the overweight, and 7.5% in the obese group.
Kaplan–Meier curve analysis showed that MACE occurred more frequently in the normal weight group than the overweight or the obese group, but less frequently than the underweight group (log rank, p < 0.001) (Figure 2). After adjustment for age, sex, initial NIHSS, and variables with p < 0.10 in the univariate analysis, the obese group showed a significantly lower risk for MACE than the normal weight group (adjusted HR: 0.58, 95% CI: 0.41–0.86, p < 0.05) (Table 2). The risk for MACE was significantly higher in the underweight group than the normal weight group (adjusted HR: 1.55, 95% CI: 1.01–2.38, p < 0.05) (Table 2).
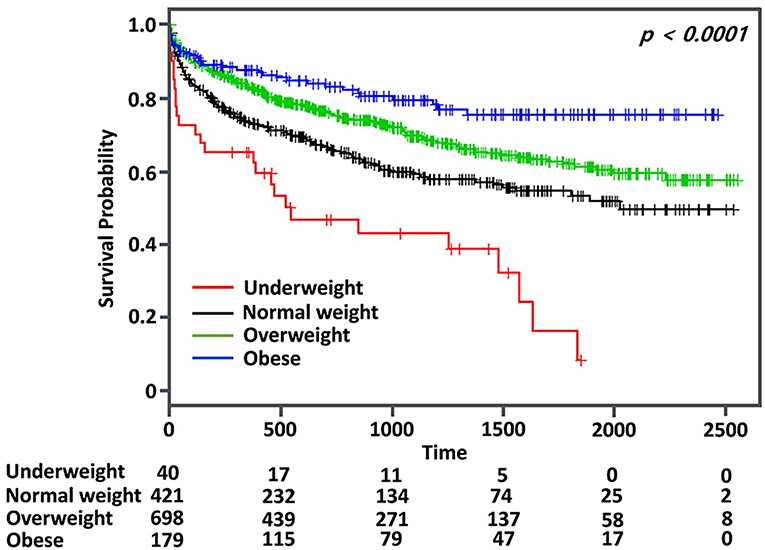
Figure 2. Kaplan–Meier survival analysis for major adverse cardiovascular event (MACE) according to body mass index in patients with ischemic stroke and type 2 diabetes mellitus.
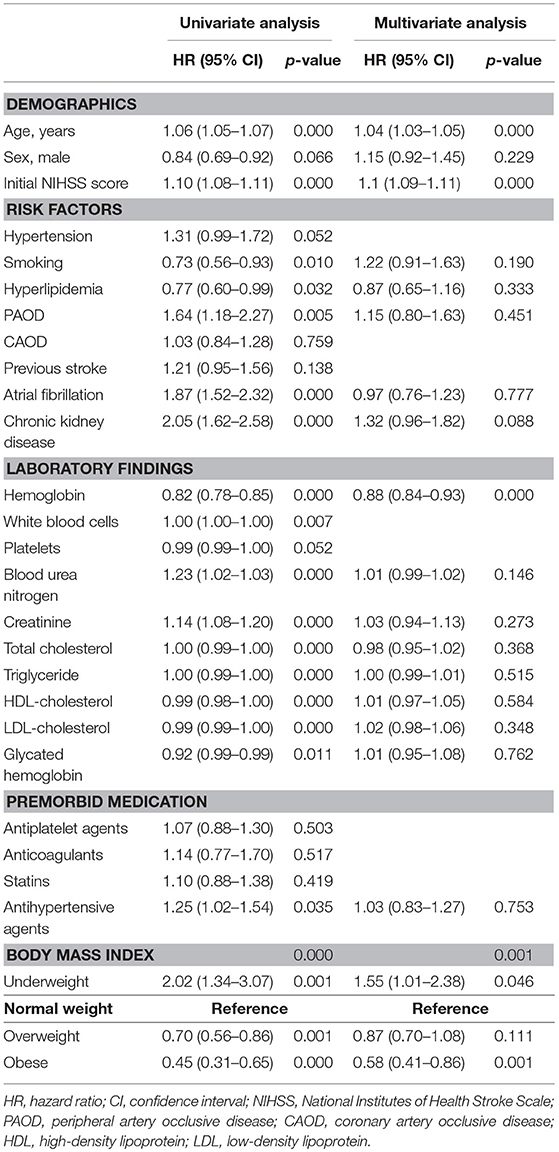
Table 2. Unadjusted and adjusted hazard ratio for MACE of type 2 diabetes patients with acute ischemic stroke according to BMI.
We determined the association of BMI with the risk for each component of MACE and mortality using the spline curve of log HR according to BMI as a continuous variable. While the all-cause mortality and cardiovascular mortality showed a U-shaped or a reversed J-shaped pattern, stroke mortality showed an L-shaped pattern. Other-cause mortality (death due to conditions other than CVD or stroke) showed an inverse relationship with BMI (Figure 3). A stroke (fatal or non-fatal stroke) showed a pattern of inverse relationship with BMI. However, any cardiovascular event (fatal or non-fatal event) showed a reversed J-shaped pattern in that the slope of the curve was sharply increased in body weight range belonging to the obese group (Figure 4).
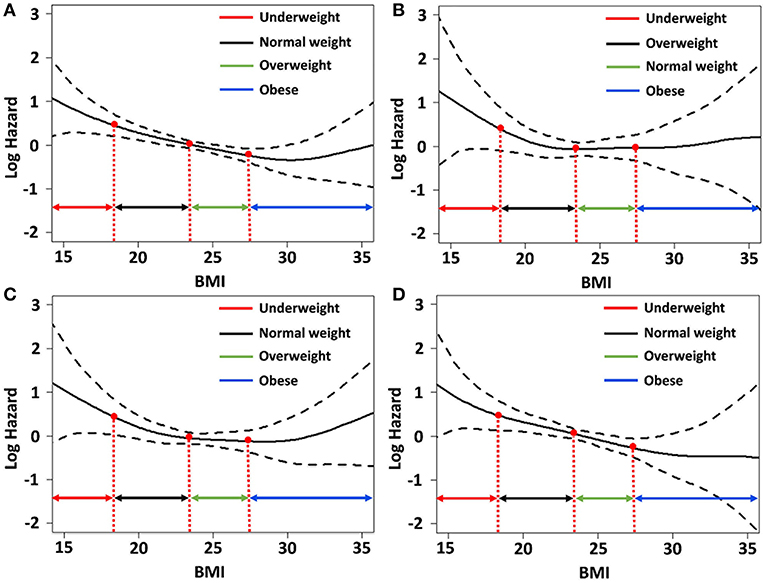
Figure 3. Relative hazards of body mass index (BMI) on (A) all-cause mortality; (B) fatal stroke; (C) fatal cardiovascular events; and (D) other-cause mortality after adjusting variables p < 0.1 in the univariate Cox regression analysis.
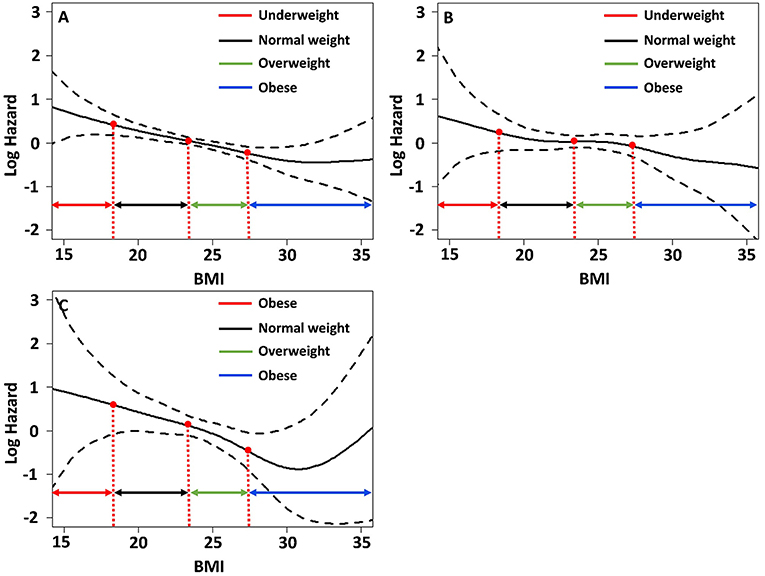
Figure 4. Relative hazards of BMI on (A) major adverse cardiovascular events; (B) any stroke events; (C) any cardiovascular events after adjusting variables p < 0.1 in the univariate Cox regression analysis.
This study showed an inverse relationship between BMI and occurrence of MACE in patients with acute stroke and T2DM. However, the risk for each component of MACE differed according to the BMI. While BMI had an inverse association with other-cause mortality, it had an L-shaped association with stroke mortality, and a U-shaped association with cardiovascular mortality.
Most studies in patients with T2DM alone showed the U-shaped association of BMI with all-cause and cardiovascular mortality (10, 11, 13, 14, 23). However, studies in T2DM patients with comorbidity suggested heterogeneity in mortality according to the specific comorbidity. The J-shaped pattern of mortality was observed in T2DM patients who were free of CVD and cancer (16). In patients with acute MI and T2DM, there were no survival advantages from an elevated BMI (24). While there was U-shaped association in patients with chronic HF and T2DM, there was an inverse association in those with acute HF and T2DM (17). This study also showed that the risk of cardiovascular mortality was higher in obese patients than in overweight patients. However, stroke mortality was not higher in obese patients, which supports the heterogeneity of mortality in T2DM patients according to the comorbidity.
While a majority of previous studies on the obesity paradox have focused on mortality, overweight or obesity may be a risk for vascular events. Therefore, we investigated the occurrence of non-fatal stroke and cardiovascular events as well as fatal events. This analysis showed that the risk for any stroke (fatal or non-fatal) in obese patients decreased and showed an inverse association with BMI. However, the risk of any cardiovascular events (fatal or non-fatal) sharply increased in obese patients and showed a reversed J-shaped pattern. These findings suggest that the risk of stroke and cardiovascular events is different in stroke patients with T2DM and obesity, in that the risk of cardiovascular events may increase and that the risk of stroke may not increase. The reason for the discrepancy in stroke incidences and cardiovascular events in obese patients is uncertain. However, while obesity is a common risk factor for both cardiovascular events and stroke, the attributable risk of obesity is higher for cardiovascular events than stroke (25).
In contrast to obese patients, underweight patients with T2DM and stroke consistently showed poor outcomes in this study. Previous studies also showed poor outcomes in underweight T2DM patients. The patients who have a greater genetic susceptibility to T2DM have a greater chance of developing T2DM at lower BMI, which will consequently lead to a poor prognosis (12). In a study that assessed weight loss after stroke, weight loss > 3 kg was associated with poor outcomes after stroke (26). Muscle power is crucial for mobilization and effective rehabilitation after stroke. Immobilization is associated with various complications and poor outcomes (27, 28). Lean muscle mass in underweight patients might negatively impact their mobility, which could contribute to poor outcome.
This study in stroke patients with T2DM yielded the best outcomes in terms of mortality and MACE in the overweight. However, it remains uncertain whether an intervention for body weight modification should be targeted to remain overweight because our findings and others' are based on observational studies. Current guidelines for the secondary prevention of ischemic stroke recommend weight loss and aerobic exercise (29, 30). Strength training is also beneficial in patients with T2DM as it increases glucose uptake and insulin signaling in skeletal muscles (31). Thus, instead of a uniform weight loss recommendation for ischemic stroke patients with T2DM, an individualized approach depending on weight status may be needed.
There are limitations in our study. First, this study is a retrospective, single-center study in the single ethnic population, which limits the generalization of findings. Second, we simply categorized the patients according to BMI at admission, which does not reflect changes in body weight over time. In addition, this study did not assess the muscle mass, which may be an important factor for prognosis. Third, while it is known that each category of diabetic drug may affect body weight (32), information about diabetic medications before admission was not collected. Lastly, this study may be subjected to the selection bias because a significant portion of screened patients excluded according to the inclusion criteria.
This study showed the overall presence of the obesity paradox in stroke patients with T2DM. However, obese patients had different risks of cardiovascular events and stroke. Since the risk of cardiovascular events sharply increased in obese patients, we may have to be more attentive to these patients to prevent these events. This could be done by assessing for asymptomatic coronary artery disease, considering that approximately one-third of stroke patients have asymptomatic significant (≥50%) coronary artery disease, and these patients have poor long-term outcomes (20, 33).
The datasets for this manuscript are not publicly available because This is the registry including patients' information. Although this information was collected after informed consent, this information cannot be publicly available because it contained individuals' information. Requests to access the datasets should be directed to aGpwYXJrMjA5MDQyQGdtYWlsLmNvbQ==.
HP, HWL, JY, HSN, YDK, and JH designed the study, collected data, and drafted the manuscript. HP and HSL performed the statistical analysis. All authors interpreted the data and revised the manuscript.
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), which was funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HC15C1056), and the Basic Science Research Program through the National Research Foundation of Korea (NRF-2018R1A2A3074996).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. (2013) 2013:653789. doi: 10.1155/2013/653789
2. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. (2018) 61:142–50. doi: 10.1016/j.pcad.2018.07.003
3. Lavie CJ, De Schutter A, Milani RV. Healthy obese versus unhealthy lean: the obesity paradox. Nat Rev Endocrinol. (2015) 11:55–62. doi: 10.1038/nrendo.2014.165
4. Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF, et al. Reprint of: Healthy weight and obesity prevention. JACC Health Promotion Series. (2018) 72:3027–52. doi: 10.1016/j.jacc.2018.10.024
5. Vemmos K, Ntaios G, Spengos K, Savvarl P, Vemmou A, Pappa T, et al. Association between obesity and mortality after acute first-ever stroke: the obesity–stroke paradox. Stroke. (2011) 42:30–6. doi: 10.1161/STROKEAHA.110.593434
6. Kim BJ, Lee S-H, Ryu W-S, Kim CK, Lee J, Yoon B-W. Paradoxical longevity in obese patients with intracerebral hemorrhage. Neurology. (2011) 76:567–73. doi: 10.1212/WNL.0b013e31820b7667
7. Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-Dehoff RM, Zhou Q, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. (2007) 120:863–70. doi: 10.1016/j.amjmed.2007.05.011
8. Yamauchi Y, Hasegawa W, Yasunaga H, Sunhora M, Jo T, Takami K, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis. (2014) 9:1337–46. doi: 10.2147/COPD.S75175
9. Golledge J, Cronin O, Iyer V, Bradshaw B, Moxon JV, Cunningham MA. Body mass index is inversely associated with mortality in patients with peripheral vascular disease. Atherosclerosis. (2013) 229:549–55. doi: 10.1016/j.atherosclerosis.2013.04.030
10. Zaccardi F, Dhalwani NN, Papamargaritis D, Webb DR, Murphy GJ, Davies MJ, et al. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: a systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia. (2017) 60:240–8. doi: 10.1017/s00125-016-4162-6
11. Kwon Y, Kim HJ, Park S, Park Y-G, Cho K-H. Body mass index-related mortality in patients with type 2 diabetes and heterogeneity in obesity paradox studies: a dose–response meta-analysis. PLoS ONE. (2017) 12:e0168247. doi: 10.1371/journal.opne.0168247
12. Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Klipatrick ES, et al. The obesity paradox in type 2 diabetes mellitus: Relationship of body mass index to prognosis: a cohort study. Ann Intern Med. (2015) 162:610–8. doi: 10.7326/M14-1551
13. Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Li W, Johnson J, et al. Body mass index and the risk of all-cause mortality among patients with type 2 diabetes. Circulation. (2014) 130:2143–51. doi: 10.1161/CIRCULATIONAHA.114.009098
14. Logue J, Walker JJ, Leese G, Linsay R, McKnight J, Morris A, et al. Association between BMI measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care. (2013) 36:887–93. doi: 10.2337/dc12-0944
15. Carnethon MR, De Chavez PJD, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. (2012) 308:581–90. doi: 10.1001/jama.2012.9282
16. Tobias DK, Pan A, Jackson CL, O'Reilly EJ, ding EL, Willet WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. (2014) 370:233–44. doi: 10.1056/NEJMOa1304051
17. Khalil CA, Sulaiman K, Singh R, Jayyousi A, Asaad N, Alhabib KF, et al. BMI is inversely correlated to the risk of mortality in patients with type 2 diabetes hospitalized for acute heart failure: Findings from the Gulf aCute heArt failuRE (Gulf-CARE) registry. Int J Cardiol. (2017) 241:262–9. doi: 10.1016/j.ijcard.2017.02.119
18. Lee BI, Nam HS, Heo JH, Kim DI. Yonsei stroke registry. Cerebrovasc Dis. (2001) 12:145–51. doi: 10.1159/000047697
19. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes−2018. Diabetes Care. (2018) 41:S13–27. doi: 10.2337/dc18-S002
20. Yoo J, Song D, Baek J-H, Kim K, Song T-J, Lee HS, et al. Poor long-term outcomes in stroke patients with asymptomatic coronary artery disease in heart CT. Atherosclerosis. (2017) 265:7–13. doi: 10.1016/j.atherosclerosis.2017.07.029
21. WHO EC. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
22. Seok JM, Kim SG, Kim JW, Chung CS, Kim GW, Lee KH, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. (2010) 68:213–9. doi: 10.1002/ana.22050a
23. Edqvist J, Rawshani A, Adiels M, Björck L, Lind M, Svensson AM, et al. BMI and mortality in patients with new-onset type 2 diabetes: a comparison with age- and sex-matched control subjects from the general population. Diabetes Care. (2018) 3:485–93. doi: 10.2337/dc17-1309
24. Colombo MG, Meisinger C, Amann U, Heiher M, von Scheidt W, Kuch B, et al. Association of obesity and long-term mortality in patients with acute myocardial infarction with and without diabetes mellitus: results from the MONICA/KORA myocardial infarction registry. Cardiovasc Diabetol. (2015) 14:24. doi: 10.1186/s12933-015-0189-0
25. Ndumele CE, Matsushita K, Lazo M, Bello N, Bluementhal RS, Gerstenblith G, et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. (2016) 5:e003921. doi: 10.1161/JAHA.116.003921
26. Jönsson A-C, Lindgren I, Norrving B, Lindgren A. Weight loss after stroke: a population-based study from the Lund Stroke Register. Stroke. (2008) 39:918–23. doi: 10.1161/STROKEAHA.107.497602
27. Rinde LB, Smabrekke B, Mathiesen EB, Løchen ML, Njølstad I, Hald EM, et al. Ischemic stroke and risk of venous thromboembolism in the general population: the tromso study. J Am Heart Assoc. (2016) 5:e004311. doi: 10.1161/JAHA.116.004311
28. Poole KE, Reeve J, Warburton EA. Falls, fractures, and osteoporosis after stroke: time to think about protection? Stroke. (2002) 33:1432–6. doi: 10.1161/01.STR.0000014510.48897.7D
29. Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2532–53. doi: 10.1161/STR.0000000000000022
30. Tsivgoulis G, Safouris A, Kim D-E, Alexandrov AV. Recent advances in primary and secondary prevention of atherosclerotic stroke. J Stroke. (2018) 20:145–66. doi: 10.5853/jos.2018.00773.e1
31. Di Meo S, Iossa S, Venditti P. Improvement of obesity-linked skeletal muscle insulin resistance by strength and endurance training. J Endocrinol. (2017) 234:R159–81. doi: 10.1530/JOE-17-0186
32. Kocarnik BM, Moore KP, Smith NL, Boyko EJ. Weight change after initiation of oral hypoglycemic monotherapy for diabetes predicts 5-year mortality: an observational study. Diabetes Res Clin Pract. (2017) 123:181–91. doi: 10.10161/j.diabres.2016.11.025
Keywords: ischemic stroke, diabetes mellitus, obesity, body mass index, major adverse cardiac event, mortality
Citation: Park H, Lee HW, Yoo J, Lee HS, Nam HS, Kim YD and Heo JH (2019) Body Mass Index and Prognosis in Ischemic Stroke Patients With Type 2 Diabetes Mellitus. Front. Neurol. 10:563. doi: 10.3389/fneur.2019.00563
Received: 18 March 2019; Accepted: 10 May 2019;
Published: 05 June 2019.
Edited by:
Raimund Helbok, Innsbruck Medical University, AustriaReviewed by:
Carl J. Lavie, Ochsner Medical Center, United StatesCopyright © 2019 Park, Lee, Yoo, Lee, Nam, Kim and Heo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Hoe Heo, amhoZW9AeXVocy5hYw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.