
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 07 June 2019
Sec. Movement Disorders
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00537
 Jonathan H. Drucker1,2
Jonathan H. Drucker1,2 K. Sathian1,2,3†
K. Sathian1,2,3† Bruce Crosson1,2,4,5
Bruce Crosson1,2,4,5 Venkatagiri Krishnamurthy1,2
Venkatagiri Krishnamurthy1,2 Keith M. McGregor1,2
Keith M. McGregor1,2 Ariyana Bozzorg1
Ariyana Bozzorg1 Kaundinya Gopinath6
Kaundinya Gopinath6 Lisa C. Krishnamurthy1,7
Lisa C. Krishnamurthy1,7 Steven L. Wolf1,8,9,10
Steven L. Wolf1,8,9,10 Ariel R. Hart10
Ariel R. Hart10 Marian Evatt2
Marian Evatt2 Daniel M. Corcos11
Daniel M. Corcos11 Madeleine E. Hackney1,8,10*
Madeleine E. Hackney1,8,10*Background: Externally guided (EG) and internally guided (IG) movements are postulated to recruit two parallel neural circuits, in which motor cortical neurons interact with either the cerebellum or striatum via distinct thalamic nuclei. Research suggests EG movements rely more heavily on the cerebello-thalamo-cortical circuit, whereas IG movements rely more on the striato-pallido-thalamo-cortical circuit (1). Because Parkinson's (PD) involves striatal dysfunction, individuals with PD have difficulty generating IG movements (2).
Objectives: Determine whether individuals with PD would employ a compensatory mechanism favoring the cerebellum over the striatum during IG lower limb movements.
Methods: 22 older adults with mild-moderate PD, who had abstained at least 12 h from anti-PD medications, and 19 age-matched controls performed EG and IG rhythmic foot-tapping during functional magnetic resonance imaging. Participants with PD tapped with their right (more affected) foot. External guidance was paced by a researcher tapping participants' ipsilateral 3rd metacarpal in a pattern with 0.5 to 1 s intervals, while internal guidance was based on pre-scan training in the same pattern. BOLD activation was compared between tasks (EG vs. IG) and groups (PD vs. control).
Results: Both groups recruited the putamen and cerebellar regions. The PD group demonstrated less activation in the striatum and motor cortex than controls. A task (EG vs. IG) by group (PD vs. control) interaction was observed in the cerebellum with increased activation for the IG condition in the PD group.
Conclusions: These findings support the hypothesized compensatory shift in which the dysfunctional striatum is assisted by the less affected cerebellum to accomplish IG lower limb movement in individuals with mild-moderate PD. These findings are of relevance for temporal gait dysfunction and freezing of gait problems frequently noted in many people with PD and may have implications for future therapeutic application.
Parkinson's disease (PD) is a neurodegenerative disorder leading to motor symptoms including impaired lower limb control, bradykinesia, freezing, and postural instability, as well as cognitive impairment and other non-motor symptoms. Mobility programs [e.g., mobility training, partnered dance (e.g., tango) and non-partnered dance (e.g., Dance for PD), tandem biking, tai chi] are effective for improving motor function in people with PD (3–7). These programs use a mix of externally guided (EG) and internally guided (IG) (1) movement strategies, both of which have evidence supporting their use in rehabilitative scenarios. However, little is known about whether EG or IG approaches are more effective in PD, and the relevant neural processes are not clearly understood.
In healthy people, functional magnetic resonance imaging (fMRI) studies have suggested distinct neural pathways for upper limb IG vs. EG movements in a variety of contexts (1, 8–14). PD affects both IG and EG movements. However, due to dysfunction of the striato-pallido-thalamo-cortical (STC) circuit, people with PD have particular difficulty with IG tasks (2, 15, 16). Because EG movements likely rely on relatively spared brain regions, at least in early stages of PD, the patterns of neural activation during EG tasks differ little between those with PD and controls, whereas there are distinct differences between groups during IG task performance (17). Proper completion of IG movements relies on efficient function of subcortical loops involving the basal ganglia (18, 19). The most immediate impact of dysfunction in IG movement in PD relates to impaired cortico-striatal communication during movement initiation. One behavioral manifestation of this disruption is the presence of akinesia in PD during IG movement (20). Cortical initiation of IG movements relies on modulation and signal augmentation that is highly sensitive to disruption when filtered through dysfunction of the basal ganglia (21). Under IG conditions, the cerebello-thalamo-cortical (CTC) pathway may be recruited more in people with PD than in neurotypical subjects, possibly denoting recruitment of EG circuitry, which is more robust to deterioration in PD than dopamine-dependent STC pathways (22). In keeping with the idea of increased compensatory activity/connectivity of cerebellar circuits during IG tasks, STC connections are weaker in individuals with PD than in controls, whereas CTC connections are stronger (2).
Visual, auditory, tactile, and attentional cues have all been used experimentally to examine the effects of external guidance in PD. Overwhelming evidence demonstrates benefits of visual and auditory cueing in behavioral studies for people with PD (23), and such cues are often incorporated in physical therapy (24). Tactile cues have been studied less; yet they may be processed faster and more efficiently, with less attentional demand, than visual and auditory cues (25–28). Somatosensory integration for prioritizing tactile and proprioceptive feedback is not disrupted by PD (26). Somatosensory cueing can supersede visual distractors (28). Using haptic speed cues from a moving handrail, people with PD increased stride length (25). Tactile cues decreased timing errors during a dual task (27). Rhythmic somatosensory cues increase turning speed and may be more effective than visual cues (29). Interestingly, humans can abstract a pattern of beats from tactile rhythms as efficiently as from auditory patterns (30).
Using fMRI, differences have been observed in the neural control of upper and lower extremity movement, including greater lateralization for upper compared to lower limb movements (14, 31, 32). However, studies specifically investigating neural correlates of movements of the lower extremity, particularly in the context of IG vs. EG control, are less common, especially in populations with neurological pathology like PD. Schwingenschuh and co-workers, using a simple EG ankle dorsiflexion task, found that both people with PD and healthy controls activated lobules I-V in the ipsilateral cerebellum during EG movement (33). Spraker et al. further noted increased recruitment of cerebellar structures and pathways with disease progression in PD (34, 35).
Knowledge about the effect of PD on neural circuits with respect to rhythmic EG or IG movements of the lower extremity is incomplete. The purpose of this study was to investigate neural correlates of IG and EG movements using rhythmic foot tapping in people with PD vs. age-matched neurotypical (NT) controls. We developed a task that is related to the foot-tapping test used in clinical practice from the Unified Parkinson's Disease Rating Scale (UPDRS), and which we adapted for fMRI to specifically assess lower limb rhythmic motor control. We used a marginally more complex rhythmic task than previous studies for use in future rehabilitative scenarios. We hypothesized that: (1) like the upper limb literature, the striatum would be less active in PD than controls, and (2) the cerebellum would be adaptively recruited for IG tasks by people with PD.
The institutional review board at Emory University School of Medicine and the Research and Development Committee of the Atlanta VA Health Care System approved this work. Participants provided written informed consent before participating.
Forty-one individuals (18 men, 23 women) were recruited. Inclusion criteria required participants be ≥40 years old and able to walk ≥3 meters with or without assistance. PD participants were recruited through the VA Informatics and Computing Infrastructure database, as well as support groups, educational meetings, newsletters, physician referrals, word of mouth, outreach events and research websites. Nineteen NT participants, age-matched to PD patients (6 men, 13 women; M age 64.7, SD = 10.2), had no history of neurological or psychiatric disorders. Twenty-two PD participants (12 men, 10 women; M age 67.7, SD = 9.9) were recruited. All participants were right handed as verified by the Edinburgh handedness survey. No participants had contraindications to undergo MRI. All PD participants were clinically diagnosed with PD by a movement disorders specialist based on the United Kingdom PD Society Brain Bank diagnostic criteria (36, 37). These patients had unilateral onset of symptoms, displayed clear symptomatic benefit from antiparkinsonian medications, e.g., levodopa (38), and were in Hoehn and Yahr stages I-III. Patients who had a tremor score >1 in either lower limb and/or moderate-severe head tremor were excluded. PD participants were tested in the OFF state, i.e., ≥12 h after their last dose of anti-parkinsonian medication. Participants' Levodopa Equivalent Daily Dosage was calculated with standardized procedures (39). Twenty-two participants were on carbidopa/levodopa including two individuals who were additionally taking an extended release version of this medication at night before bed. The most common formulation was ER 25/100. Three participants were taking Amantadine, and three people were taking Azilect. Two people were taking Mirapex, Ropinirole HCL, or Trihexyphenidyl. One person was taking Comtan, and one person was taking Entacapone. Twelve participants reported taking more than one PD medication.
Participants were evaluated for general health and ability to perform activities of daily living (ADLs) with the Composite Physical Function index (CPF) (40) Participants scored ≥19 on the Montreal Cognitive Assessment (41), excluding individuals with dementia (42). The Beck Depression Inventory-II (BDI-II) assessed depression (43). A score of ≥30, indicating severe depression, was a cutoff for the BDI-II. Focusing on lower-limb function, participants were administered mobility measures including Dynamic Gait Index (DGI) (44), the Single Timed Up and Go Test (TUG) (45) with cognitive (TUG-c, counting backwards by 3 s) and manual (TUG-m, carrying a full glass of water) conditions, and preferred and fast gait (46). PD participants also completed the Freezing of Gait questionnaire and were administered the Fullerton Advanced Balance Scale (47) and the Movement Disorders Society UPDRS (MDS-UPDRS) (48) parts I-IV to derive a total score and a subscale III score. PD and NT group characteristics are found in Table 1.
Laying supine, participants performed a foot-tapping task with an orthopedic wedge under the knees to maintain a 90° angle and isolate ankle movement (Figure 1A). They wore a custom-made instrumented ankle orthotic on the leg more affected by PD based on leg agility and foot tapping items of the MDS-UPDRS-III. For equal bilateral rating, patient-reported side of parkinsonian symptom onset was factored. We included only PD participants most affected on their right side. Controls tapped with their right foot. Participants were told to dorsiflex, then plantarflex till they reached a stop. The arc between stops, largest possible range of motion (ROM) was 20 degrees. The orthotic affixed to the lower extremity incorporated position measurement, and sampled foot position continuously (2,000 Hz). Custom software (BioMaq) built with LabView (National Instruments, Austin, TX) acquired data.
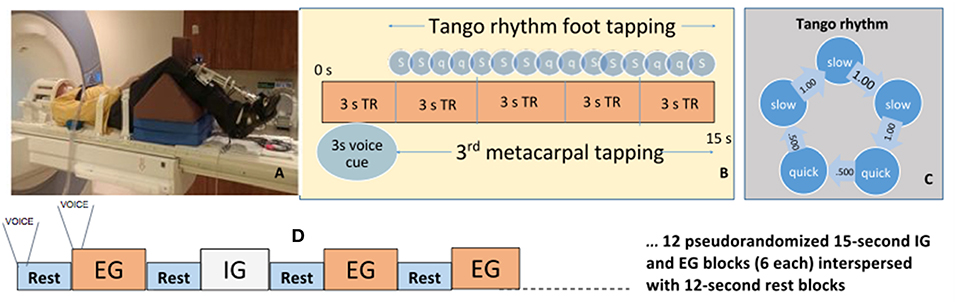
Figure 1. Activation paradigm and participant fMRI setup. (A) Participant prepped for scanner. (B) Ideal foot-tapping sequence within each active block. (C) The slow, slow, quick, quick, slow tango rhythm was used as the target rhythm for participants. (D) Activation paradigm for internally and externally guided blocks. Voice, voice cue.
Before scanning, participants learned a 4 s rhythmic sequence designed to approximate a basic rhythm of the Argentine tango dance: slow, slow, quick, quick, slow (Figure 1B). The tango rhythm was chosen to present a more complex rhythm than previous studies and for its current and future use in rehabilitation, e.g., with adapted tango therapies (49). Intervals between taps were 1 s, 1 s, 0.5 s, 0.5 s, 1 s (Figure 1C). All participants were trained ~20 min on the task while lying on the scanner table. Examiners verified that the participant fully understood the task and could perform the EG, IG, and Rest conditions proficiently and as instructed before beginning the scanning session. EG condition blocks: participants tapped their foot immediately after detecting a finger tap on their 3rd metacarpal head from an examiner listening to an audible version of the rhythm via headset. The examiner was placed just outside the scanner bore next to the patient table on the right side of the participant. The examiner ensured that they could comfortably reach the hand of the participant prior to the scan. This individual was extensively trained on delivering the taps to the participant such that the delivered taps matched the auditory timing provided with the headset. IG condition blocks: participants tapped the rhythm as practiced prior to the scan without prompting tactile cues on their hand. The examiner instead tapped the 3rd metacarpal head after each participant foot-tap to control for the experience of tactile sensation in the EG and Rest conditions. Participants were told to not pay attention to the taps on their hand during the IG condition. Rest condition: Examiners tapped the participants' 3rd metacarpal head at 1 Hz in order to control for the experience of the tactile sensation during the IG and EG conditions. There were twenty-four 15 s blocks of EG, and twenty-four 15s blocks of IG tapping across four runs. Block order was randomized in each functional run. Throughout the first 3 s of each block, a voice cue indicated whether the participant would experience an EG or IG trial. With 12 s rest blocks interposed between task, tapping lasted about 25 min (Figure 1D). Sequence order was counterbalanced across participants.
We performed a sound check prior to scanning and then checked in through the microphone with the participants during the scan if they did not appear to be compliant with the task. The voice cue was provided because we wanted the participants to keep their eyes closed throughout the scan so they would focus their attention on the tactile cues and sensation of these cues. The 3 s voice cue of the task instructions were included as a regressor of non-interest in the model.
Notes were taken by an observer in the scanning console room, regarding the task performance of participants.
Using a priori determined timestamps, each block consisted of three cycles of the rhythm (Figure 1) and was analyzed to identify peaks in the foot-tapper angle time-course, indicating discrete taps.
Within each block of three cycles, the standard deviation of amplitude measured performance consistency. We produced a standardized value of amplitude, reflecting differences between participants' maximum ROM, i.e., its height relative to the adjacent local minima. We divided the participant timecourse by the second-largest range achieved within a single block (to avoid referencing to occasional amplitude spikes) and multiplied by 20 degrees. This calibration was performed independently for every functional run.
This metric was defined on a 0–100 point scale. Observed foot-tap timing was compared against one ideal tap cycle (Figure 1C). The best cycle in the observed taps was identified by computing the linear regression, in a sliding window, of five consecutive observed taps against the ideal cycle. The cycle with the highest R2 for its regression (i.e., the best rate-invariant correspondence with the ideal cycle) was noted and removed. The process was repeated until all qualifying observed cycles had been identified. R2 values were summed and divided by 3, for three perfectly-executed cycles, to calculate timing accuracy. If participants completed a fourth cycle in a block, a denominator of four was used.
Neuroimaging data were collected on a 3T Siemens Trio scanner using a 12-channel head coil for functional and anatomical runs. In functional runs, 114 T2*-weighted echoplanar image volumes measuring BOLD contrast were collected using parallel imaging with an iPAT acceleration factor of two. Scan sequence parameters were: 55 contiguous, 3 mm slices in the axial plane, interleaved slice acquisition, repetition time (TR) = 3,000 ms, echo time (TE) = 24 ms, flip angle = 90°, bandwidth = 2,632 Hz/pixel, field of view (FOV) = 230 mm, matrix = 76 × 76, voxel size = 3.0 × 3.0 × 3.0 mm. At the beginning of the run, the scanner acquired 3 TRs which were discarded automatically. An anatomical image was collected using a high resolution MPRAGE scan sequence with 176 contiguous slices in the sagittal plane, single-shot acquisition, TR = 2,300 ms, TE = 2.89 ms, flip angle = 8°, FOV = 256 mm, matrix = 256 × 256, bandwidth = 140 Hz/pixel, voxel size = 1.0 × 1.0 × 1.0 mm.
Care was taken to ensure that the whole of the cerebellum was covered by the bounding box of the fMRI data acquisition for all subjects. The MR imaging parameters of the EPI sequence employed during fMRI contained 55 axial slices with 3 mm slice thickness. Coverage afforded by these parameters were enough to obtain whole brain fMRI data from all subjects.
Image preprocessing and statistical analyses were conducted with AFNI (50) and FSL 5.0 (51) software. Slice-time correction and motion correction was performed on the functional volumes using the AFNI function 3dvolreg. The six resulting motion vectors were incorporated into baseline GLM analyses. Artifacts due to head motion, circulatory activity, and other noise sources were removed using FIX (52). The anatomical image was skull-stripped using optiBET (53), corrected for intensity bias introduced by magnetic field inhomogeneity, and then transformed to MNI space using the FNIRT procedure, producing a non-linear transformation based on local spatial properties. Functional data were aligned to the anatomical image and transformed using the non-linear transformation and smoothed using an isotropic, 3D, 6 mm full-width-half-maximum (FWHM) Gaussian kernel. Signal intensities in each volume were scaled with z-transformation excluding the first six volumes from calculation of the mean and standard deviation, avoiding pre-steady-state outliers. Analyses were performed on scaled data.
TRs with head movement exceeding 0.3 mm were excluded for NT participants, and 0.5 mm for the clinical population.
Descriptive statistics were calculated. Independent-samples t-tests compared groups on continuous measures. Chi-square tests compared categorical variables.
Two trial-level statistics, amplitude variability, and timing accuracy, were calculated for active blocks. Pearson's r determined correlations between foot-tapping metrics and performance on mobility tests to ascertain ecological validity of the foot-tapping task. Participant means for each condition were calculated and submitted to a 2 condition (EG vs. IG foot-tapping) × 2 group (NT vs. PD) factorial ANOVA. Contrasts are reported for each dependent variable and the 2 × 2 interaction: EG vs. IG within each group, and NT vs. PD with each condition and Bonferroni corrected.
Individual-level regression was performed by fitting a general linear model to the scaled time series. Stimulus onsets were convolved with AFNI's “block” kernel, to model the hemodynamic responses for the EG and IG task conditions, and the rest periods. The auditory presentations of instructions were modeled using a canonical gamma variate kernel, modeling the hemodynamic responses to transient events. Six regressors obtained from motion correction during volume registration were included in the baseline model to remove residual signal change correlated with movement. Scanner drift was modeled by finding the best-fitting polynomial function correlated with the preprocessed timecourse data. A generalized least squares time series fit estimated temporal autocorrelation. The general linear model included three contrasts: EG vs. Rest, IG vs. Rest, and EG vs. IG. Beta coefficients and associated t-statistics from these contrasts from each participant's regression were entered into a mixed effects group-level analysis, accounting for individual estimates and their variability (54). Familywise error (FWE) corrected inferences were obtained through Monte Carlo (MC) simulation of the process of image generation, estimated spatial correlation of voxels, cluster detection thresholds and cluster identification (55) through the ClustSim program implemented in AFNI. This program assumes the underlying spatial correlation of the second-level analysis residuals is Gaussian (56, 57). The Gaussian spatial correlation employed by MC simulations was quantified by the average FWHM of first-level GLM analysis residuals across all participants (FWHMav = 8 mm). We acknowledge that the use of cluster size for statistical power has received some attention in related literature. A study by (58) reported that a cluster-based threshold of p < 0.001, when using a Gaussian kernel, may lead to false positives (58). In consideration of this report, we used an updated version of AFNI's 3dClustSim (Compile Date: 08/2016), using voxel padding to address edge effects associated with random field cluster generation. Recent studies have shown that the selected voxel cluster threshold using this technique avoid introduction of false positives as reported by (58) [see (55–57, 59)]. Voxels passing the cluster detection threshold, which was a family wise error-corrected cluster-level of p < 0.05, were considered to form a cluster if they shared a face.
For an ROI based analysis of the task × group interaction in the cerebellum, an adaptive Bonferroni method was used. For significance criterion α = 0.05 and k = 5 tests of interest (PD-IG vs. PD-EG, NT-IG vs. NT-EG, PD-IG vs. NT-IG, PD-EG vs. NT-EG, and the interaction), the smallest p-value was α/k = 0.01. The smallest remaining p-value was α/(k-1) = 0.0125, followed by α/(k-2) = 0.0133 and further until either a test failed to meet the criterion or all tests met the criterion.
Foot-tapping performance measures were correlated with preferred and fast gait speed, and the DGI for EG and IG conditions (r = 0.31 to 0.49).
The foot tapping test was shown to have validity with measures of gait and balance in people with PD, as Table 3 shows. Preferred and fast gait speed, the Dynamic Gait Index (DGI), and the Fullerton Advanced Balance (FAB) test were significantly correlated with timing and amplitude of the foot tapping task during EG and IG conditions. Timing during IG and EG conditions was significantly correlated with the Timed Up & Go (TUG) and the TUG-dual cognitive (TUG-c, counting backward by serial 3 s) measures; whereas only amplitude during IG was significantly correlated with TUG and TUG-c (Table 3).
ANOVAs revealed a significant task-by-group interaction in amplitude variability (F = 7.35, p = 0.009). Pairwise comparisons subjected to a Bonferroni correction (p = 0.0125) revealed that NT participants were less variable than PD participants in the IG condition [NT: M (SEM): 2.8 (0.22); PD: 3.6 (0.12); p < 0.001]. No significant differences in amplitude variability between groups in the EG condition [NT: 3.2(0.20); PD: 3.4 (0.14); p = 0.3] kept with the hypothesis that PD patients can perform EG tasks similarly to controls (10). The PD group was less accurate in timing for EG [NT: 57.1 (3.4)%; PD: 45.2 (3.4)%; p = 0.016] and IG tasks [NT:74.5(3.0)%; PD: 65.2(3.4)%; p = 0.044], but differences did not survive Bonferroni correction. Timing accuracy was greater for both groups during the IG task (p < 0.001).
Field notes taken during the scans regarding the performance of participants revealed that overall participants performed the task as directed. Two individuals who reported weekly freezing of gait and exhibited some freezing of gait during the UPDRS-III exam performed the task with several errors; however, one participant with frequent freezing of gait and who exhibited FOG during straight walking performed the task with no errors. The level of freezing in the group is shown in Table 1.
Comparing groups on EG and IG foot-tapping tasks against the resting baseline revealed robust activation throughout a motor network, including bilateral sensorimotor cortex, premotor cortex, the striatum (particularly putamen) (Figures 2A,B) and the cerebellum (Tables 2A,B). The left putamen was preferentially recruited by NT participants for EG (Table 2A) and IG tasks (Table 2B). For the EG task, clusters in the supplementary motor area (SMA), the caudate head, and the precuneus were preferentially activated for PD participants (Table 2A). For the IG task, activation for the PD group was more extensive in the caudate head and a left-lateralized set of frontal and parietal areas (Table 2B, Figures 2A,B).
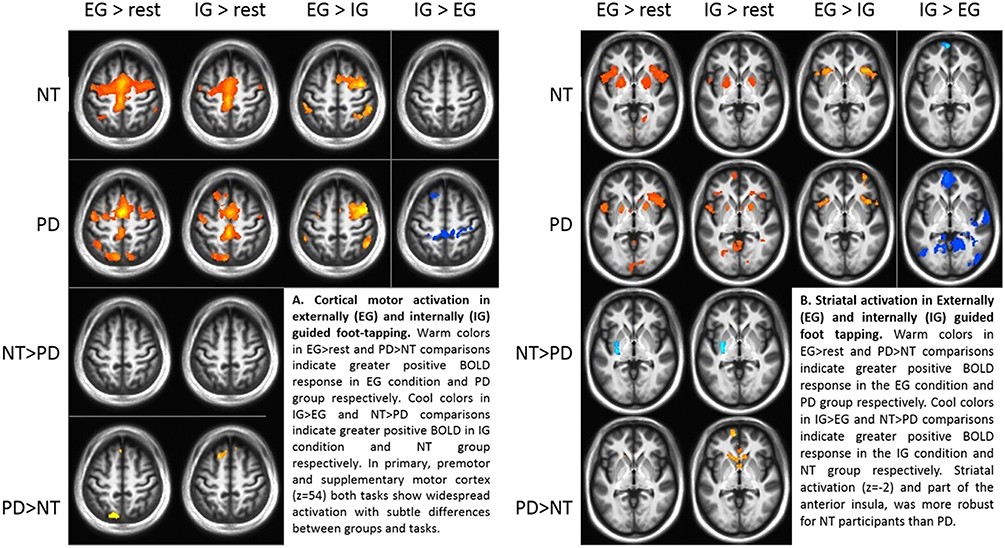
Figure 2. (A) Cortical motor activation during externally (EG) and internally (IG) guided foot-tapping. Warm colors in the EG>rest and PD>NT comparisons indicate greater positive BOLD response in EG condition and PD group respectively. Cool colors in IG>EG and NT>PD comparisons indicate greater positive BOLD in IG condition and NT group, respectively. In primary, premotor and supplementary motor cortex (z = 54) both tasks show widespread activation with subtle differences between groups and tasks. (B) Striatal activation for EG and IG guided foot tapping. Warm colors in the EG>rest and PD>NT comparisons indicate greater positive BOLD response in the EG condition and PD group, respectively. Cool colors in IG>EG and NT>PD comparisons indicate greater positive BOLD response in the IG condition and NT group, respectively. Activation in the striatum (z = −2) as well as part of the anterior insula, was more robust for NT participants than those with PD.
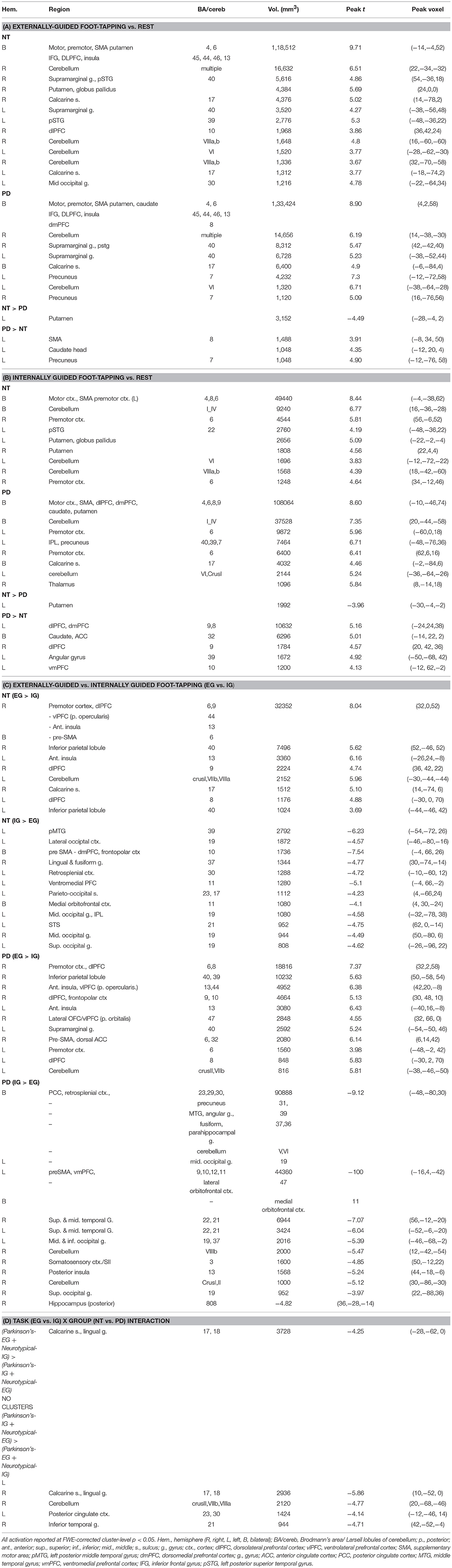
Table 2. EG and IG foot-tapping vs. Rest and EG vs. IG foot-tapping in Neurotypical and PD Participants.
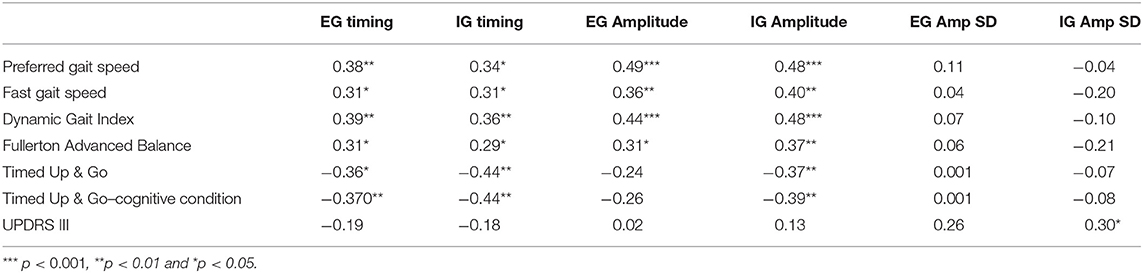
Table 3. Pearson's r correlations indicate the foottapping task has validity with balance and gait measures in participants with PD.
Both groups' left cerebellar activation was stronger for the EG condition. Conversely, PD participants showed greater activity for IG than EG in the right (ipsilateral) cerebellum (Table 2C). Both groups' ipsilateral premotor activation was more robust in the EG condition (Figure 2A), as was activation bilaterally in the inferior frontal gyrus and intraparietal sulcus. The putamen was not preferentially activated during IG tapping for either group, but a set of occipital and medial cortical regions was activated for both groups. The extent of the activation clusters was greater for the PD group (Figure 2B: 2nd row, 4th column).
We observed a task-by-group interaction in the right cerebellum (Figures 3A,B). The 265-voxel cluster spanned portions of right crus II and lobules VIIb and VIIIa. Both groups activated the ipsilateral cerebellar cluster equivalently for the EG task (Figure 3B). During the IG task, the NT group was near baseline whereas the PD group recruited this cluster to a greater extent than during the EG task. Additional clusters significant for task-by-group interaction included loci in bilateral occipital and temporal cortices, and the left posterior cingulate (Table 2D). The cluster was subjected to pairwise contrasts of interest with respect to the hypotheses (Figure 3B). Observed betas for each condition for each subject were averaged across the cluster. Differences between each pair of conditions were calculated. An adaptive Bonferroni approach was used to correct for multiple comparisons as described in section 2.6.3. Three contrasts were statistically significant: EG-IG within PD (p = 0.0002, criterion α/k = 0.0125); EG-IG within NT (p = 0.0010, α/k = 0.0133); and NT-PD within IG (p = 0.0077, α/k = 0.0250), whereas NT-PD within EG was not significant (p = 0.8838, α/k = 0.0500). Activity during IG was significantly greater than EG for PD participants. Activity during EG was significantly greater than IG for NT participants. The IG condition showed significantly greater activity for PD participants. There were no group differences for the EG condition.
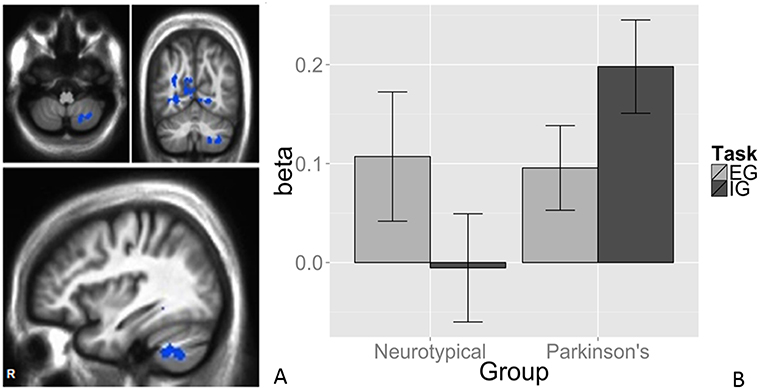
Figure 3. Task × group interaction in the cerebellum. Color contrasts illustrate group-by-task interaction in magnitude of BOLD response. Cool colors represent increased activation during the IG condition in the PD group, or during the EG condition in the NT group (A). The significant interactions were driven by activation during the IG condition in the PD group (B). Cerebellar differences between tasks were smaller in PD, suggesting an EG-like compensatory strategy for IG movement in participants with PD. The interaction was significant in Larsell Lobules, VIIb, VIIIa, and Crus II. MNI coordinates (36, −62, −50).
To assess the relationship between performance on the task and the observed neural effects, correlations were examined in four regions of interest, determined a priori and defined functionally. These correlations were performed for completeness in verifying or refuting the hypotheses. The left and right putamen were reliably activated in all four conditions vs. rest. The left cerebellum ROI was in a different location for each group, so the relevant EG > IG cluster was used for each group's analysis. Finally, the right cerebellum ROI was the one found significant in the task by group interaction.
For each ROI, correlation coefficients were calculated across subjects within each group (NT and PD), such that both performance metrics (timing accuracy and amplitude variability) were correlated against GLM contrast betas for each of the two tasks (EG and IG) vs. resting baseline (Table 4). None of these comparisons survived a Bonferroni correction.
This study is the first to demonstrate a task by disease state interaction in the cerebellum, providing evidence for a possible compensatory mechanism by which older adults with PD may partially circumvent cortico-striato-thalamic circuitry impaired by dopaminergic depletion during lower limb movement. Using a task based on a complex rhythm provides ecological validity not possible with the more commonly used simple isometrical beat timing task and suggests a therapeutic avenue. Tasks using complex rhythms can potentially be used to evaluate the effects of therapies involving such rhythms, e.g., music-movement training and dance-based therapies. It was surprising that the EG task had worse kinematic performance than the IG task in terms of timing. This finding is unexpected given the abundance of literature that suggests that EG movement, including timing and amplitude is facilitated by cues. However, previous studies have used very simple external cues in terms of rhythm. This task is one of the first to use a tactile cue with a more complex rhythm than previous studies. Importantly, in addition to interesting cortical involvement during the tasks, our findings lend additional support to research showing the cerebellum serves in a compensatory role to assist the dysfunctional basal ganglia during IG movements in people with mild-moderate PD.
Structures activated more during EG conditions in NT adults (the dlPFC, the anterior insula, pars opercularis of the inferior frontal gyrus, the IPL and the contralateral cerebellum) are related to sensory guidance of movement, beat perception, and using salience for attention-demanding tasks. Given task demands, these functions are plausibly recruited by NT adults.
These activation areas are like areas noted during EG tasks by other studies of activation during foot movement, including Sauvage et al. (60), Schwingenschuh et al. (33), and Trinastic et al. (61), and Ciccarelli et al. (62). Suggesting the participants may have been imagining the experimenter as he or she cued their EG movements, this task activated the dlPFC and areas activated during motor imagery, rather than motor execution in other work (60). Other studies showed anterior insular activation in subjects hearing a musical piece after 30 min of actively learning a rhythmic melody (63), like the rhythmic task our participants learned before scanning.
The structures comparatively activated more during IG conditions in NT adults [the middle temporal gyrus (MTG), the occipital gyri, the lingual and fusiform gyri, the retrosplenial cortex, the IPL and superior temporal sulcus, and the SMA] relate to mental imagery, planning complex behaviors, and extinction of conditioned responses. Participants needed to recall and perform a practiced task according to internal timing in IG tasks, meaning these functions are plausible. These activated areas are similar to areas noted during IG tasks by others (64, 65), and are common with areas recruited in motor imagery (66) (i.e., activations in the bilateral middle occipital gyrus and the lingual gyrus). Consistent with the present findings, prior research has found that movement paradigms where participants are free to choose their actions elicit activation in the preSMA, not the SMA (49, 67). Conversely EG movement paradigms have been shown to implicate the premotor cortex, a finding that was also replicated here (Table 2C).
We noted the cerebellum was activated more during EG in NT participants, but in the contralateral cerebellum (left side). However, within the NT group, the EG vs. IG contrasts did not reveal a task effect in the putamen despite prior findings suggesting a favored role in IG movements (68, 69). Our task was more rhythmically complex than the continuation task used elsewhere (22, 68), potentially contributing to this discrepancy. Coordination to a more complex external cue could also have led to poorer performance, as reflected in the poorer behavioral performance on the EG task as compared to the IG task.
During EG tasks, both groups activated similar frontal, premotor, and cerebellar areas; additionally, the PD group activated bilateral PMC, the supramarginal gyrus, the dorsal ACC, contralateral cerebellum (crus II), and the orbitofrontal and frontopolar cortices. Previous reports showed PD patients off-medication activated the supramarginal gyrus, the ACC, motor cortex, the SMA and cerebellum. Individuals with PD engage more frontal areas and additional cerebellar activation during EG foot movement (33). A similar pattern was noted in PD participants (tested both on and off anti-parkinsonian medication) vs. controls in an EG visually cued force task. Off-medication PD participants recruited additional areas of the bilateral cerebellum and primary motor cortex not seen in on-medication PD participants and controls (70). Cerasa et al. (22) reported PD patients and controls engaged similar neural networks in EG and IG movement during visually-cued finger tapping, yet the PD group showed greater activity in sensory and associative cortices.
In the IG task, both groups activated the retrosplenial cortex, the MTG, the fusiform, the middle and superior occipital gyrus, the vmPFC, the medial orbitofrontal cortex, and the pre-SMA; the PD group additionally activated the superior and MTG more extensively, the cerebellum (V, VI, VIIIb, Crus I, II), secondary somatosensory cortex (SII), the posterior insula, and the posterior hippocampus. These PD-unique regions in the IG task (apart from the cerebellum) are related to memory, sound processing (STG), and touch (posterior insula). For IG tasks, SII and cerebellar pathways are recruited (1, 10). During EG movements PD patients activate cerebellar circuitry similarly to controls, confirmed by NT group results. However, in PD, cerebellar and striatal circuits are activated during IG tasks (1). PD patients could have greater recruitment of the hippocampus and engage in more robust replay of auditory and somatosensory cues encountered during training.
Rhythmic EG and IG movements engage generally similar neural networks in PD and healthy controls (22). However, within IG and EG tasks, the putamen was activated more for NT participants (1) Loss of dopaminergic innervation to the basal ganglia hinders processing in cortico-striatal circuits (71). The PD group preferentially recruited SMA, caudate, and precuneus relative to NT participants in EG tasks, similar to previous findings (10), and dlPFC, SMA, angular gyrus, caudate, ACC and vmPFC in IG tasks. Cortical midline regions, e.g., medial prefrontal cortex, the precuneus, and the left angular gyrus, comprise the default mode network; their activation may imply PD participant difficulty attending to challenging IG tasks.
The left cerebellum activation pattern was enhanced for EG tasks vs. IG tasks in both groups (Table 2), supporting the proposed preferential role of the cerebellum in EG motor tasks and consistent with recruitment of the CTC circuit for IG tasks in the PD group (1, 10, 72). Cerebellar recruitment is preferential for, but not exclusive to, EG movement in controls. The task-generality of cerebellar circuits may explain the disease-related plasticity described below.
The task-by-group interaction was observed in the right (i.e., ipsilateral) cerebellum, the part of the cerebellum most directly relevant to right-sided foot-tapping. Both groups showed activation during the EG task relative to rest, as expected in a structure spared in early, mild to moderate PD and implicated in EG movement (Figure 3). The IG task did not recruit this region for the NT group, but did for the PD group. The direction of the relationship was reversed and the IG task recruited the right cerebellar cluster more than the EG task. Spraker et al. noted increased recruitment of cerebellar structures and pathways with PD progression (34), possibly becoming pathological over time (73).
The double dissociation between task and group suggests a compensatory role for the right cerebellum in PD. As the IG rhythmic motor task relies on the contribution of a STC mechanism that is compromised in PD, a parallel CTC circuit is co-opted. Prior work demonstrated a compensatory role of cerebellar motor circuits in PD (2, 10, 22, 72). Findings suggest the compensatory shift is specific to IG motor tasks, posing implications for PD patients frequently impaired in IG tasks (2, 15, 16).
This study had several limitations that restrict the generalizability of the study.
One of the main limitations is that the task performed in the scanner included only movements of the right foot while lying supine. Therefore, conclusions regarding actual walking are very limited, even though the task outcomes are correlated with gait activities. Human movement performed during daily activities is rarely purely internally or externally guided. The IG and EG conditions we presented to our participants are only proxies for purely internally generated and externally generated movements. However, there was a key difference between the IG condition and the EG condition. We asked participants to keenly follow the timing of the tapper during EG but told them explicitly to disregard the tapping they felt during the IG condition and instead to perform the task as practiced prior to entering the scanner. The attention to cue for timing vs. non-attention to cue resulted in an IG vs. EG comparison that approximates internally vs. externally guided conditions. Both conditions likely induced error monitoring. Neither task was trivially easy, and both produced mistakes. To control for the sensory experience of tapping during the EG condition, during the IG condition, participants received a tap to their hand immediately after their own foot taps. As such, internally generated foot taps did produce feedback. However, this feedback was the same every time and therefore predictable and did not provide any indication regarding the quality of the tap in terms of the performance variables, timing and amplitude.
It must be acknowledged that the EG condition included an element of reaction time, because participants were asked to tap the foot immediately after sensing a tap on their hand. Further, it is likely that all movements have an aspect of IG movement, but in this case, participants were told to pay strong attention to the cue on their hand to elicit performance, which is similar to prior studies that provided auditory, or tactile stimuli with an inherent rhythm that stimulated movement in the individual with PD.
Although the pathways considered here have been relatively well-researched in the upper limb, they have not been much studied in the lower limb. For a condition like Parkinson's, which gravely affects the lower limb motor system in a plurality of patients, leading to falls and associated sequelae, it is vital to have enhanced understanding of lower limb motor control. This understanding can only be gained by conducting careful studies of neural pathways associated with lower limb behavior. Studying lower limb movement in the scanner is inherently challenging but the methods adopted in this study made evaluating a moderately complex rhythm in the scanner possible. Adapting this complex rhythm for foot movement in the scanner is therefore novel. MRI does not lend itself to ecologically realistic motor tasks, thus techniques like functional near-infrared spectroscopy (fNIRS) and electroencephalography (EEG) could measure neural activity in freely-moving participants in real time (74). However, these surface measures are not sensitive to putaminal or other subcortical structure activities. Performance of the research assistant conducting the response to IG, and prompt for EG, movements was not measured, possibly introducing a confound if their performance varied systematically between the tasks and/or participants. We did not measure the performance of the research assistant themselves, i.e., we do not know to what degree their tapping deviated from the recorded tapping which they heard and were to approximate. As such, we also do not know to what extent any performance inconsistencies in examiner tapping affected the behavioral outcome measures. While we did not record the examiner delivery, the task was controlled and practiced, and every researcher achieved a level of proficiency in matching their timing to the external auditory source, similarly to what is capable of drummers, musicians in an orchestra, precision dancing, marching bands, etc. Given our interest specifically in tactile cues as a form of external guidance, it was important to have a human perform the tapping, rather than an automated, vibrotactile machine. Recording the performance of the human would have increased the validity of the study.
The groups were not gender matched, although there was not a statistically significant difference in sex distribution (Chi square statistic = 2.18, p = 0.14). However, the difference in numbers of males vs. females in each group could have affected the findings in unknown ways. That said, we are reasonably confident that the sex distribution in the current study represents the population well-given that the prevalence of males to females in the PD population is 60/40 (75) and our present sample is ~55%/45%. The study would have been better controlled if truly matched for gender, and certainly some work shows differences in neural control (e.g., visuospatial processing) based on sex or aging (76), but we do not see any reason that women and men would have performed the foot tapping task differently from one another in any systematic way, given that the amount of foot movement that is required is no more than that needed to drive or to use a piano pedal. Among classical musicians who play the piano, women and men are now considered to be equally able to take on all the most difficult repertory. Therefore, we believe there is little reason to suppose that the performance of this particular task would have differed for any reasons based on sex.
The sample size ultimately limits our ability to make definitive conclusions.
As we hypothesized, similarly to finger-tapping tasks (1, 10, 35), this study shows IG and EG foot movement tasks are mediated by neural mechanisms involving the striatum and cerebellum, respectively, in healthy individuals without PD. However, PD may influence circuits involving the striatum or cerebellum on a context- and/or task-specific basis. Additional regions, e.g., the ipsilateral cerebellum, were recruited by PD participants to perform the IG tasks. The internally guided and externally guided conditions of the task investigated here provides insight into the breadth of neural structures involved in complex, rhythmic movement in individuals with and without Parkinson's disease.
This study was carried out in accordance with the recommendations of the Emory University IRB and the VA R&D Review committees with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Emory University IRB and the VA R&D Review committees.
The research project concept was defined by MH, KS, BC, DC, SW, JD, and ME. MH, KM, VK, and KG organized the project. The research project was executed by MH, JD, KM, AB, AH, and ME. MH, JD, VK, KM, and LK designed the statistical analysis plan. The statistical analysis plan was executed by JD and MH and received critiques from KS, BC, VK, SW, DC, KG, and ME. JD and MH wrote the first draft of the manuscript. KS, BC, VK, SW, DC, KG, AH, and ME reviewed and critiqued the manuscript.
Department of Veterans Affairs R&D Service Career Development Award IK2RX000870 supported this work and ME Hackney. The National Science Foundation Emerging Frontiers in Research Initiatives (EFRI) award number 1137229 and the Atlanta Center for Visual and Neurocognitive Rehabilitation supported JD. Support for KS contribution to this work was provided by the Atlanta VAMC. We acknowledge the Emory Center for Health in Aging and the Emory University Center for Systems Imaging. Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported in part by funding from a Shared Instrumentation Grant (S10) grant 1S10OD016413-01 to the Emory University Center for Systems Imaging Core and by the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1TR000454.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the participants.
1. Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, McKeown MJ, et al. Task specific influences of Parkinson's disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience. (2007) 147:224–35. doi: 10.1016/j.neuroscience.2007.04.006
2. Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage. (2011) 55:204–15. doi: 10.1016/j.neuroimage.2010.11.074
3. Earhart GM. Dance as therapy for individuals with Parkinson disease. Eur J Phys Rehabil Med. (2009) 45:231–8.
4. Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait Posture. (2008) 28:456–60. doi: 10.1016/j.gaitpost.2008.02.005
5. Kadivar Z, Corcos DM, Foto J, Hondzinski JM. Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neurorehabilit Neural Repair. (2011) 25:626–35. doi: 10.1177/1545968311401627
6. Morris ME, Iansek R, Kirkwood B. A randomized controlled trial of movement strategies compared with exercise for people with Parkinson's disease. Mov Disord. (2009) 24:64–71. doi: 10.1002/mds.22295
7. Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabilit Neural Repair. (2009) 23:600–8. doi: 10.1177/1545968308328726
8. Gowen E, Miall RC. Differentiation between external and internal cuing: an fMRI study comparing tracing with drawing. Neuroimage. (2007) 36:396–410. doi: 10.1016/j.neuroimage.2007.03.005
9. Ogawa K, Inui T, Sugio T. Separating brain regions involved in internally guided and visual feedback control of moving effectors: an event-related fMRI study. Neuroimage. (2006) 32:1760–70. doi: 10.1016/j.neuroimage.2006.05.012
10. Sen S, Kawaguchi A, Truong Y, Lewis MM, Huang X. Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson's disease. Neuroscience. (2010) 166:712–9. doi: 10.1016/j.neuroscience.2009.12.036
11. Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol. (2003) 90:3330–40. doi: 10.1152/jn.00394.2003
12. Lee MY, Chang PH, Kwon YH, Jang SH. Differences of the frontal activation patterns by finger and toe movements: a functional MRI study. Neurosci Lett. (2013) 533:7–10. doi: 10.1016/j.neulet.2012.11.041
13. Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. (2004) 21:568–75. doi: 10.1016/j.neuroimage.2003.09.065
14. Volz LJ, Eickhoff SB, Pool EM, Fink GR, Grefkes C. Differential modulation of motor network connectivity during movements of the upper and lower limbs. Neuroimage. (2015) 119:44–53. doi: 10.1016/j.neuroimage.2015.05.101
15. Eckert T, Peschel T, Heinze HJ, Rotte M. Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J Neurol. (2006) 253:199–207. doi: 10.1007/s00415-005-0956-z
16. Low KA, Miller J, Vierck E. Response slowing in Parkinson's disease: a psychophysiological analysis of premotor and motor processes. Brain. (2002) 125:1980–94. doi: 10.1093/brain/awf206
17. Jahanshahi M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. 1. An investigation using measurement of regional cerebral blood-flow with PET and movement-related potentials in normal and Parkinsons-disease subjects. Brain. (1995) 118:913–33. doi: 10.1093/brain/118.4.913
18. Alexander GE, Delong MR, Strick PL. Parallel Organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. (1986) 9:357–81. doi: 10.1146/annurev.ne.09.030186.002041
19. Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. (1998) 121:1437–49. doi: 10.1093/brain/121.8.1437
20. Berardelli A, Rothwell JC, Thompson PD, Hallet M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. (2001) 124:2131–46. doi: 10.1093/brain/124.11.2131
21. Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson's disease: FDG-PET and network analysis. Hum Brain Mapp. (2004) 22:236–45. doi: 10.1002/hbm.20033
22. Cerasa A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC, Costa A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res Bull. (2006) 71:259–69. doi: 10.1016/j.brainresbull.2006.09.014
23. Rocha PA, Porfirio GM, Ferraz HB, Trevisani VF. Effects of external cues on gait parameters of Parkinson's disease patients: a systematic review. Clin Neurol Neurosurg. (2014) 124:127–34. doi: 10.1016/j.clineuro.2014.06.026
24. O'Sullivan SB, Schmitz TJ, Fulk GD. Physical Rehabilitation. Philadelphia, PA: F.A. Davis Co. (2014).
25. Rabin E, Demin A, Pirrotta S, Chen J, Patel H, Bhambri A, et al. Parkinsonian gait ameliorated with a moving handrail, not with a banister. Arch Phys Med Rehabil. (2015) 96:735–41. doi: 10.1016/j.apmr.2014.07.427
26. Rabin E, Muratori L, Svokos K, Gordon A. Tactile/proprioceptive integration during arm localization is intact in individuals with Parkinson's disease. Neurosci Lett. (2010) 470:38–42. doi: 10.1016/j.neulet.2009.12.051
27. Ivkovic V, Fisher S, Paloski WH. Smartphone-based tactile cueing improves motor performance in Parkinson's disease. Parkinsonism Relat Disord. (2016) 22:42–7. doi: 10.1016/j.parkreldis.2015.11.006
28. van Wegen E, de Goede C, Lim I, Rietberg M, Nieuwboer A, Willems A, et al. The effect of rhythmic somatosensory cueing on gait in patients with Parkinson's disease. J Neurol Sci. (2006) 248:210–4. doi: 10.1016/j.jns.2006.05.034
29. Nieuwboer A, Baker K, Willems AM, Jones D, Spildooren J, Lim I, et al. The short-term effects of different cueing modalities on turn speed in people with Parkinson's disease. Neurorehabil Neural Repair. (2009) 23:831–6. doi: 10.1177/1545968309337136
30. Brochard R, Touzalin P, Despres O, Dufour A. Evidence of beat perception via purely tactile stimulation. Brain Res. (2008) 1223:59–64. doi: 10.1016/j.brainres.2008.05.050
31. Harirchian MH, Oghabian MA, Rezvanizadeh A, Bolandzadeh N. Comparison of laterality index of upper and lower limb movement using brain activated fMRI. In: Proceedings SPIE 6916, Medical Imaging 2008: Physiology, Function, and Structure from Medical Images, 69161Q (San Diego, CA), (2008). doi: 10.1117/12.769776
32. Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Strimpakos N, Gouliamos A, et al. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage. (2006) 32:1709–21. doi: 10.1016/j.neuroimage.2006.05.043
33. Schwingenschuh P, Katschnig P, Jehna M, Koegl-Wallner M, Seiler S, Wenzel K, et al. Levodopa changes brain motor network function during ankle movements in Parkinson's disease. J Neural Transm. (2013) 120:423–33. doi: 10.1007/s00702-012-0896-6
34. Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. (2007) 98:821–34. doi: 10.1152/jn.00239.2007
35. Taniwaki T, Okayama A, Yoshiura T, Togao O, Nakamura Y, Yamasaki T, et al. Functional network of the basal ganglia and cerebellar motor loops in vivo: different activation patterns between self-initiated and externally triggered movements. Neuroimage. (2006) 31:745–53. doi: 10.1016/j.neuroimage.2005.12.032
36. Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. (1992) 42:1142–6. doi: 10.1212/WNL.42.6.1142
37. Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. (1992) 32(Suppl.):S125–7. doi: 10.1002/ana.410320721
38. Kempster PA, Williams DR, Selikhova M, Holton J, Revesz T, Lees AJ. Patterns of levodopa response in Parkinson's disease: a clinico-pathological study. Brain. (2007) 130:2123–8. doi: 10.1093/brain/awm142
39. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
40. Jones CJ, Rikli RE. The application of Fullerton's functional fitness test for older adults in a group setting. Sci Sport. (2000) 15:194–7. doi: 10.1016/S0765-1597(00)80005-2
41. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
42. Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. (2009) 73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47
43. Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, et al. Depression rating scales in Parkinson's disease: critique and recommendations. Mov Disord. (2007) 22:1077–92. doi: 10.1002/mds.21333
44. Shumway-Cook A, Woollacott MH. Motor Control: Theory and Practical Applications. Baltimore, MD: Williams & Wilkins (1995).
45. Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the timed “Up & Go” test in people with Parkinson disease. Phys Ther. (2001) 81:810–8. doi: 10.1093/ptj/81.2.810
46. Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. (1997) 26:15–9. doi: 10.1093/ageing/26.1.15
47. Hernandez D, Rose DJ. Predicting which older adults will or will not fall using the Fullerton Advanced Balance scale. Archiv Phys Med Rehabilit. (2008) 89:2309–15. doi: 10.1016/j.apmr.2008.05.020
48. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale. (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
49. Hackney ME, Lee HL, Battisto J, Crosson B, McGregor KM. Context-dependent neural activation: internally and externally guided rhythmic lower limb movement in individuals with and without neurodegenerative disease. Front Neurol. (2015) 6:251. doi: 10.3389/fneur.2015.00251
50. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. (1996) 29:162–73. doi: 10.1006/cbmr.1996.0014
51. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. (2012) 62:782–90. doi: 10.1016/j.neuroimage.2011.09.015
52. Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. (2014) 90:449–68. doi: 10.1016/j.neuroimage.2013.11.046
53. Lutkenhoff ES, Rosenberg M, Chiang J, Zhang K, Pickard JD, Owen AM, et al. Optimized brain extraction for pathological brains (optiBET). PLoS ONE. (2014) 9:e115551. doi: 10.1371/journal.pone.0115551
54. Chen G, Saad ZS, Nath AR, Beauchamp MS, Cox RW. FMRI group analysis combining effect estimates and their variances. Neuroimage. (2012) 60:747–65. doi: 10.1016/j.neuroimage.2011.12.060
55. Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. (2017) 7:152–71. doi: 10.1089/brain.2016.0475
56. Gopinath K, Krishnamurthy V, Sathian K. Accounting for non-Gaussian sources of spatial correlation in parametric functional magnetic resonance imaging paradigms I: revisiting cluster-based inferences. Brain Connect. (2018) 8:1–9. doi: 10.1089/brain.2017.0521
57. Gopinath K, Krishnamurthy V, Lacey S, Sathian K. Accounting for non-Gaussian sources of spatial correlation in parametric functional magnetic resonance imaging paradigms II: a method to obtain first-level analysis residuals with uniform and Gaussian spatial autocorrelation function and independent and identically distributed time-series. Brain Connect. (2018) 8:10–21. doi: 10.1089/brain.2017.0522
58. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. (2016) 113:7900–5. doi: 10.1073/pnas.1602413113
59. Bansal R, Peterson BS. Cluster-level statistical inference in fMRI datasets: the unexpected behavior of random fields in high dimensions. Magn Reson Imaging. (2018) 49:101–15. doi: 10.1016/j.mri.2018.01.004
60. Sauvage C, Jissendi P, Seignan S, Manto M, Habas C. Brain areas involved in the control of speed during a motor sequence of the foot: real movement versus mental imagery. J Neuroradiol. (2013) 40:267–80. doi: 10.1016/j.neurad.2012.10.001
61. Trinastic JP, Kautz SA, McGregor K, Gregory C, Bowden M, Benjamin MB, et al. An fMRI study of the differences in brain activity during active ankle dorsiflexion and plantarflexion. Brain Imaging Behav. (2010) 4:121–31. doi: 10.1007/s11682-010-9091-2
62. Ciccarelli O, Toosy AT, Marsden JF, Wheeler-Kingshott CM, Sahyoun C, Matthews PM, et al. Identifying brain regions for integrative sensorimotor processing with ankle movements. Exp Brain Res. (2005) 166:31–42. doi: 10.1007/s00221-005-2335-5
63. Mutschler I, Schulze-Bonhage A, Glauche V, Demandt E, Speck O, Ball T. A rapid sound-action association effect in human insular cortex. PLoS ONE. (2007) 2:e259. doi: 10.1371/journal.pone.0000259
64. Christensen MS, Lundbye-Jensen J, Petersen N, Geertsen SS, Paulson OB, Nielsen JB. Watching your foot move–an fMRI study of visuomotor interactions during foot movement. Cereb Cortex. (2007) 17:1906–17. doi: 10.1093/cercor/bhl101
65. Cunningham DA, Machado A, Yue GH, Carey JR, Plow EB. Functional somatotopy revealed across multiple cortical regions using a model of complex motor task. Brain Res. (2013) 1531:25–36. doi: 10.1016/j.brainres.2013.07.050
66. Wai YY, Wang JJ, Weng YH, Lin WY, Ma HK, Ng SH, et al. Cortical involvement in a gait-related imagery task: comparison between Parkinson's disease and normal aging. Parkinsonism Relat Disord. (2012) 18:537–42. doi: 10.1016/j.parkreldis.2012.02.004
67. Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage. (2008) 39:1215–26. doi: 10.1016/j.neuroimage.2007.10.002
68. Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. (1997) 17:5528–35. doi: 10.1523/JNEUROSCI.17-14-05528.1997
69. Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. (1998) 18:1085–95. doi: 10.1523/JNEUROSCI.18-03-01085.1998
70. Palmer SJ, Ng B, Abugharbieh R, Eigenraam L, McKeown MJ. Motor reserve and novel area recruitment: amplitude and spatial characteristics of compensation in Parkinson's disease. Eur J Neurosci. (2009) 29:2187–96. doi: 10.1111/j.1460-9568.2009.06753.x
71. Tsai ST, Lin SH, Chou YC, Pan YH, Hung HY, Li CW, et al. Prognostic factors of subthalamic stimulation in Parkinson's disease: a comparative study between short- and long-term effects. Stereotact Funct Neurosurg. (2009) 87:241–8. doi: 10.1159/000225977
72. Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. (2007) 35:222–33. doi: 10.1016/j.neuroimage.2006.11.047
73. Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS ONE. (2014) 9:e100291. doi: 10.1371/journal.pone.0100291
74. Piper SK, Krueger A, Koch SP, Mehnert J, Habermehl C, Steinbrink J, et al. A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. Neuroimage. (2014) 85(Pt 1):64–71. doi: 10.1016/j.neuroimage.2013.06.062
75. Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. (2004) 75:637–9. doi: 10.1136/jnnp.2003.020982
Keywords: Parkinson's, putamen, cerebellum, lower limb, internally guided, externally guided, striatum, fMRI
Citation: Drucker JH, Sathian K, Crosson B, Krishnamurthy V, McGregor KM, Bozzorg A, Gopinath K, Krishnamurthy LC, Wolf SL, Hart AR, Evatt M, Corcos DM and Hackney ME (2019) Internally Guided Lower Limb Movement Recruits Compensatory Cerebellar Activity in People With Parkinson's Disease. Front. Neurol. 10:537. doi: 10.3389/fneur.2019.00537
Received: 30 December 2018; Accepted: 03 May 2019;
Published: 07 June 2019.
Edited by:
Martina Mancini, Oregon Health & Science University, United StatesReviewed by:
Brett W. Fling, Colorado State University, United StatesCopyright © 2019 Drucker, Sathian, Crosson, Krishnamurthy, McGregor, Bozzorg, Gopinath, Krishnamurthy, Wolf, Hart, Evatt, Corcos and Hackney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madeleine E. Hackney, bWVoYWNrbkBlbW9yeS5lZHU=; bWFkZWxlaW5lLmhhY2tuZXlAZ21haWwuY29t
†Present Address: K. Sathian, Department of Neurology, Milton S. Hershey Medical Center and Penn State College of Medicine, Hershey, PA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.