
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 15 May 2019
Sec. Neurotrauma
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00511
This article is part of the Research TopicDecompressive Craniectomy in the Management of Neurological EmergenciesView all 11 articles
 Raghunath Avanali1
Raghunath Avanali1 M. S. Gopalakrishnan2
M. S. Gopalakrishnan2 B. Indira Devi3,4*
B. Indira Devi3,4* Dhananjaya I. Bhat3
Dhananjaya I. Bhat3 Dhaval P. Shukla3
Dhaval P. Shukla3 Nagesh C. Shanbhag3
Nagesh C. Shanbhag3Cerebral venous sinus thrombosis (CVST) is a relatively uncommon cause of stroke more often affecting women and younger individuals. Blockage of the venous outflow rapidly causes edema and space-occupying venous infarctions and it seems intuitive that decompressive craniectomy (DC) can effectively reduce intracranial pressure just like it works for malignant middle cerebral artery infarcts and traumatic brain injury. But because of the relative rarity of this type of stroke, strong evidence from randomized controlled trials that DC is a life-saving procedure is not available unlike in the latter two conditions. There is a possibility that other forms of interventions like endovascular recanalization, thrombectomy, thrombolysis, and anticoagulation, which cannot be used in established middle cerebral artery infarcts and TBI, can reverse the ongoing pathology of increasing edema in CVST. Such interventions, although presently unproven, could theoretically obviate the need for DC when used in early stages. However, in the absence of such evidence, we recommend that DC be considered early as a life-saving measure whenever there are large hemorrhagic infarcts, expanding edema, radiological, and clinical features of impending herniation. This review gives an overview of the etiology and risk factors of CVST in different patient populations and examines the effectiveness of DC and other forms of interventions.
Cerebral venous sinus thrombosis (CVST) is a stroke caused by blockage of cortical veins and dural venous sinuses which leads to infarction of the draining zone brain parenchyma. It manifests as headache (in 75–95% of cases), seizures, papilledema, altered consciousness, and focal neurological deficits (1–4). CVST is the least common form of acute cerebrovascular disease, accounting for just 0.5% of all types of stroke (5–7). However, this figure rises to 15% of all young strokes in the Asian population (8, 9). The commonest site of origin of thrombosis is believed to be the junction of cerebral veins and larger sinuses (10). Several disorders can cause or predispose patients to CVST such as genetic and acquired prothrombotic disorders, cancer, hematological diseases, vasculitis, systemic inflammatory disorders, pregnancy, puerperium, and infections. In addition, there are a number of local causes such as brain tumors, arteriovenous malformations, basilar skull fracture, CNS infections, and extracranial infections like those arising from the ear, sinus, mouth, face, or neck (11–14). Medical or surgical conditions that increase the likelihood of deep vein thrombosis also increase the risk of intracranial venous thrombosis. In the international prospective study on the cerebral vein and dural sinus thrombosis (ISCVT), 44% of the patients had >1 risk factors. Congenital or genetic thrombophilia was present in 22% of patients. In about 13% of adult CVST patients, despite an extensive search, no underlying risk factors could be found (15). More than 90% of the CVST occurs in people below 60 years of age and it is more commonly seen in women between 20 and 35 years. Young women have a higher risk due to pregnancy, puerperium, and oral contraceptive usage (16–20). The incidence of CVST is estimated to be 1–13 cases per 100,000 per year (5, 16, 21). The incidence in neonates and children is 0.67 cases per 100,000 children, and that of perinatal CVST is 11.6 per 100,000 deliveries in pregnant women (16, 22, 23).
About 4% of the patients develop supratentorial parenchymal hemorrhagic lesions and cerebral edema severe enough to cause brain herniation and deterioration in neurological functions (8). The term “malignant CVST” is often used to designate this entity (24). Although anticoagulation to promote recanalization by preventing thrombosis progression is considered the mainstay of CVST treatment, it is insufficient to treat the ongoing mass effect of a malignant CVST (24). When aggressive medical management fails to control the raised intracranial pressure, DC is needed to mitigate the deleterious effects of cerebral herniation (Figures 1A–C).
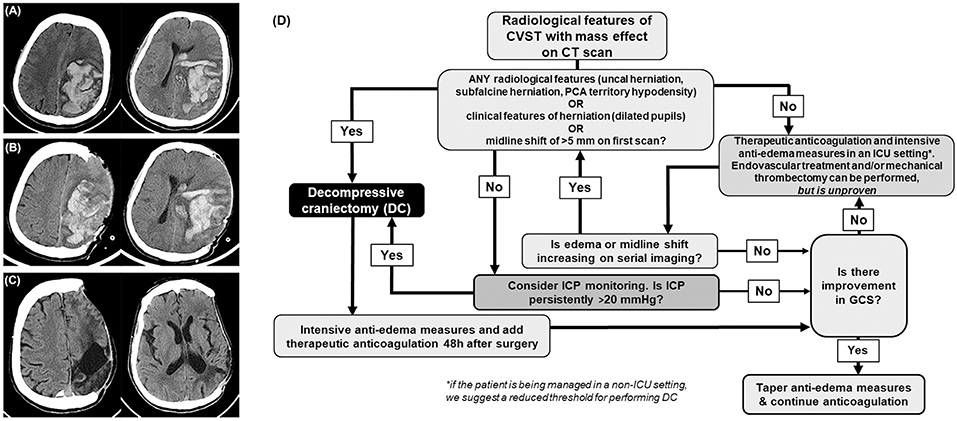
Figure 1. Cerebral venous sinus thrombosis (CVST). (A) Computed tomography depicts a confluence of blotchy areas of bleed typically seen in hemorrhagic CVST with mass effect. (B) Day 1, and (C) 8 months, post decompressive craniectomy. (D) Flowchart outlining the management of CVST. CT, computed tomography; ICU, intensive care unit. GCS, Glasgow coma scale; ICP, intracranial pressure.
We recommend that DC is offered as early as possible when the patients develop clinical signs (such as a third nerve palsy) and radiological features of herniation due to large or expanding hemorrhagic or edematous infarcts (Figure 1D). Radiological features that prompt consideration of DC are large uncal herniation, midline shift ≥5 mm, and herniation induced hypodensity of the posterior cerebral artery territory. When it is anticipated that aggressive medical management is likely to fail and if there is insufficient time for anticoagulation to act by facilitating recanalization, DC should be done. Such a policy helps reduce the chances of herniation induced irreversible brain stem damage and posterior cerebral artery infarcts which can occur without much warning. In less severe cases, where there is no gross evidence of herniation, a trial of standard intensive care management of raised ICP with ventricular CSF drainage, osmotic agents, and transient hyperventilation may be done. Where there is uncertainty, the decision can be guided by ICP monitoring, but the insertion of a parenchymal or ventricular device must be done with normal coagulation parameters. Persistent ICP levels above 20 cm of CSF despite conservative management should also prompt consideration for DC (Figure 1D).
A sufficiently large, unilateral hemicraniectomy, ideally centered on the site of the largest hematoma and venous infarct, allows expansive duraplasty with homologous or artificial material to reduce ICP. We believe that the recommendations for a large hemicraniectomy of 15 cm or greater for middle cerebral artery infarcts should also hold true for CVST since the reduction in ICP is the primary effect of DC. Infarcts affecting both anterior frontal lobes may be better dealt with using a bifrontal craniectomy, although there are no trials comparing both techniques. Evacuation of infarcted tissue is generally not recommended. However, spontaneous rupture of infarcts that typically occur at the site of dural opening warrants its removal. Medical management of cerebral edema should be continued in the postoperative period and may also be guided by ICP monitoring. There are as yet no definitive guidelines for ICP monitoring either before or after DC. The bone flap should be replaced once the brain swelling has subsided and this usually takes 3–6 months. We believe that cranioplasty should be done as early as reasonably possible to reduce the risk of complications of DC like subdural effusions, sunken skin flap syndrome, and hydrocephalus.
Since CVST is a rare cause of stroke and because of the ethical difficulties in delaying or not offering decompression when there is an obvious mass effect, large double-blinded randomized controlled studies seems difficult to conduct (25). Though the quality of evidence is low (Class IIb; Level of Evidence C), the intuitive need to perform DC in CVST is strong in select circumstances (6, 21). Meta-analysis and well-conducted systematic reviews combining data from multiple centers are useful when randomized controlled trials are unavailable. Unfortunately, even such studies evaluating the role of DC in CVST are sparse. Almost 10 years back, Coutinho et al. (26) and Lanterna et al. (27) independently published two reviews based on three previously published cases each where DC for large venous infarcts led to good outcomes. In the ISCVT study (28), the largest evaluation of its kind in CVST, 624 adult patients were registered. Most of the investigators of the trial were neurologists and only nine patients (1.4%) had a surgical intervention (29). Due to the low numbers of patients who underwent surgery, the role of DC was not analyzed. Seven years later, in 2011, the same investigators reviewed the role of DC with a combined retrospective registry and systematic review of 69 patients in 22 centers who had a surgical evacuation (30). During the last follow-up (median: 12 months), 15.9% of patients died and 5.8% of patients were severely dependent. The corresponding figures in their first report, wherein only 1.4% had undergone DC (n = 624) were 8.3 and 2.2%. Given the fact that only patients with malignant CVST underwent surgery (nine in ISCVT, 69 in the 2011 systematic review), the differences were comparable.
Over the years, multiple small observational studies suggest that surgery improves survival and produces acceptable outcomes even in patients with severe clinical conditions (8, 25, 30–32). The average death rate among patients treated with DC was 18.5%. The complete recovery rate was 30.7% and severe dependency rates were only 3.4% (21). The benefits are thought to be not only due to the prevention of progression of herniation but is also attributed to an improvement in the cortical venous collateral drainage that happens with the reduction in raised intracranial pressure. Unlike arterial infarcts, the variable patterns of apparent diffusion coefficient maps in MRI suggest that even large venous infarcts have a far better potential for recovery (24). Even in comatose patients and those with bilateral fixed pupils, DC seems beneficial, and leads to a good recovery in about one-third (8, 24) (Figure 1D).
Zuurbier et al. in a prospective cohort study of 10 DC patients reported a good clinical outcome in six patients, while two died (31). Aaron et al. (33), in a single center retrospective study on 44 patients undergoing DC reported a good outcome in 27 patients (61.4%) while nine patients died (20%). Theaudin et al. (24) retrospectively studied 12 patients with malignant cerebral edema out of 255 patients with CVST. All the four non-operated patients died, and all but one of the seven patient who underwent surgery survived and improved neurologically. The six survivors had a modified Rankin score (mRS) of 0 or 1 at 1 year. Authors also recommend that resection of infarcted tissue was not justified given the potential for recovery of venous infarction and suggests selective removal of large hematomas alone (24). Mohindra et al. in a retrospective study of 13 patients who underwent DC, reported a good outcome in all the 11 patients who survived. The two patients in their series who did not survive had a preoperative GCS <5 (34). In another retrospective study by Zhang et al. (32) of 58 patients who underwent DC, 46.6% had hemorrhage-dominated lesions and 56.9% had edema-dominated lesions. At 6 months, 56.9% of the patients attained a favorable outcome, while 13.8% died. Hemorrhage-dominated lesions and deep venous involvement cases had poorer outcomes.
We reviewed studies which were published after the last systematic review in 2011 by Ferro et al (30). Medline, PubMed, Google Scholar were used to identify studies reported in the English language with combinations of the following search terms: “cerebral sinus thrombosis,” “venous thrombosis,” and “craniectomy.” Only those studies which evaluated more than 15 patients who underwent DC and had follow-up assessment, were selected. Table 1 shows studies (2012 till the present) which evaluated the role of DC in CVST. Most of the single-center, high-volume studies were published from low-to-middle income countries, and includes two from the author's institution [see Table 1; (35, 37)] and it reflects the prevalence of uncorrected nutritional anemia and deficient perinatal care in general. Though endovascular services are available at the author's institution, DC was often required because the majority of the patients presented with impending herniation where the role of thrombolysis was limited. A total of 169 patients underwent DC for CVST in five studies and the mortality rate was 16.1%. A favorable outcome, defined as complete recovery or slight disability (mRS of 0–2, GOS of 5), could be calculated from three studies (n = 136) and such an outcome was achieved in 54.4% of the patients at the end of the follow-up period. Pre-operative ICP monitoring to guide the management plan was used only in one study but its effectiveness in decision making was not analyzed. Four studies favored DC in CVST with large hemorrhagic lesions causing midline shift and radiological features of intracerebral herniation. The results of our review are consistent with that by Ferro et al where mortality of 15.9% and a favorable outcome of 56.5% were reported. In addition to the obvious limitation that none of the studies had survival data from a control group who were managed conservatively, other shortcomings include the variations in the protocols employed, the myriad ways those protocols could have been escalated while the patients were being managed conservatively in different centers and the bias that is inherent to all retrospective uncontrolled studies. As mentioned before, the major strength of the present mini-review is that it included all the major studies till date which were published after the last systematic review in 2011.
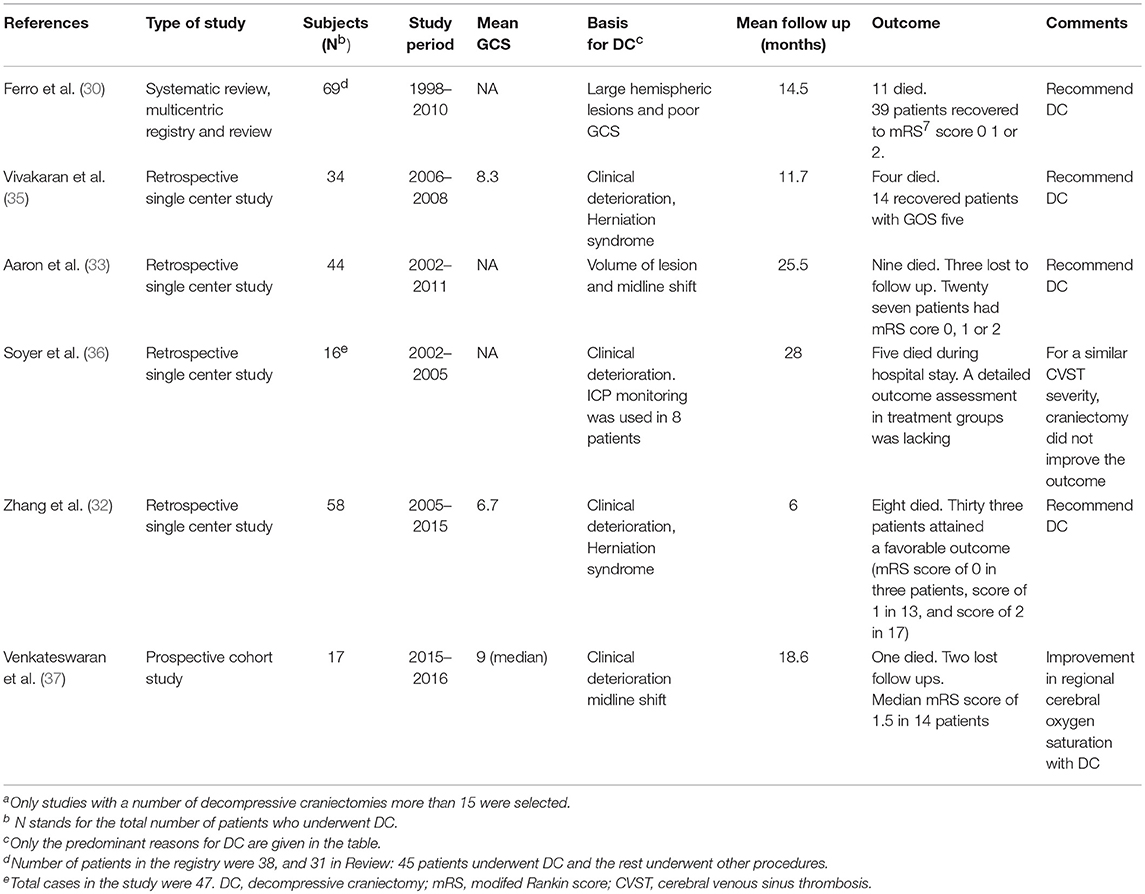
Table 1. Major studies evaluating the role of decompressive craniectomy in cerebral venous sinus thrombosis published after the systematic review in 2011a.
In 1941, Lyons reported the first successful use of unfractionated heparin in two cases of cavernous sinus thrombosis (9). Presently, anticoagulation with hydration is the first-line treatment for CVST. Anticoagulation prevents propagation of the thrombus, hastens its spontaneous resolution, and aids in the prevention of deep vein thrombosis and embolism, without adversely promoting intracranial hemorrhage (ICH) (2, 19, 25, 38). ICH is not considered a contraindication for anticoagulation (9). Unfractionated heparin has to be given intravenously and it requires a dose adjustment based on activated partial thromboplastin time. Low-molecular-weight heparin (LMWH) is advantageous in that it can be administered as a subcutaneous injection based on body weight, and it has a more predictable pharmacokinetic profile (21) (Table 2). However, its effects are only partially reversed with protamine sulfate. The quality of evidence is too low to choose between the agents (21). LMWH is associated with lesser risk of new hematomas and seems to have better outcomes in ISCVT trial and other studies (25, 39, 40). It can be given in patients with normal renal function and those who do not require neurosurgical intervention (16, 39).
The time to restart therapeutic anticoagulation after DC is not clear (41, 42). Previous studies suggest that anticoagulation can be restarted after 24 to 48 h and some authors prefer to start with half the dosage for a period of 72 h (25, 31–33). Permanent anticoagulation is needed in those with prothrombotic states or with recurrent venous thrombosis (43). Other patients can be treated with oral Vitamin K antagonists for a period of 3–12 months (21, 44). There is limited safety data for oral anticoagulants such as Apixaban (45).
Systemic administration of fibrinolytic agents such as urokinase to recanalize thrombosed pathways has been attempted but strong evidence regarding its safety and efficacy is lacking (9). Endovascular thrombolysis may also be considered in patients who are unresponsive and deteriorating, despite aggressive medical treatment (10). Siddiqui et al., in their systematic review assessing mechanical thrombectomy with or without intrasinus thrombolysis suggested that this approach is safe (46). The overall death or dependency rate was 16% in patients who underwent mechanical thrombectomy, even though 47% of patients in this series were comatose or stuporous. The rate is comparable to that in the ISCVT study and indicates the safety of the approach (46). In cases where DC has no reasonable immediate role due to the absence of a life-threatening mass effect, patients may benefit from endovascular interventions (28, 47). Thrombolysis or anticoagulation for Cerebral Venous Thrombosis (TO-ACT trial) (48) study which sought to evaluate the role of endovascular thrombolysis was prematurely terminated due to futility.
Only case reports are available which describe DC in pregnant women. Patients might require a cesarean section for the safe delivery and resuscitation of the neonate (49). Puerperal CVST is more common and can be severe enough to warrant DC as a life-saving measure. Most of the studies indicate that future pregnancies are not contraindicated in women with a previous history of CVST (50). The absolute risk of recurrent venous sinus thrombosis associated with pregnancy in women who had a previous episode of CVST seems to be low, although the relative risk is much higher than the rate in the general population (50). Regardless of antithrombotic prophylaxis, the pooled estimate for recurrent CVST and non-cerebral venous thromboembolism associated with pregnancy was 9 per 1,000 pregnancies and 27 per 1,000 pregnancies, respectively (51). The avoidance of oral contraceptives and the use of anticoagulation prophylaxis during pregnancy dramatically reduced the probability of thrombosis recurrence in women (52).
In a study based on a Canadian pediatric stroke registry, neonates comprised of 43% of the children diagnosed with CVST, and 54% were younger than 1 year old (23). The increased risk in the neonates is attributed to multiple reasons such as the damage sustained by dural venous sinuses secondary to the molding of the skull bones during delivery, general prothrombotic state and dehydration (25). Infection is a major cause for CVST in children, and hypoxia is also thought to play a significant role in neonates (6, 23). Treatment with anticoagulants is generally considered to be safe, although studies are few (25, 53). In children over 2 years of age, a duration 3 to 6 months of anticoagulation should be tailored according to the cause (54). The indications and risk-benefit analysis of DC in pediatric CVST are not clear. DC is generally thought to be risky in neonates and young infants but may be cautiously considered in older children (55).
CVST tends to be equally prevalent in older men and women. A headache as a presenting symptom is less common in the elderly (56). CVST should thus be added to the long list of disorders that cause depressed consciousness or mental changes in patients, and an extensive search must be done for such causes. In ISCVT, 8.2% of the patients were aged 65 years or older (22). The prognosis was worse with 49% of patients being dead or dependent at the end of the follow-up period. Due to an increased risk of thrombotic events, anticoagulation for more than 6 months may be warranted.
CVST has a favorable outcome when compared with other types of stroke. Due to increasingly early diagnosis and the widespread use of anticoagulation, the outcomes have been better than what existed half a decade ago (25, 57). The overall death rate is below 5% and about 80% of the patients make a complete recovery (mRS scores: 0–1) (21, 25, 38). However, mortality in severe cases with parenchymal lesions still remains as high as 35–50% (16, 50, 58). In ISCVT, 3.4% of patients died within the first month of thrombosis, 6.8% after 6 months and 8.3%, at the last follow up (median follow-up 16 months). Moderate to severe disability was reported in 5.1% of the patients (32). The role of recanalization of thrombosed veins in relation to the outcome is not very well established (39, 59, 60). In ISCVT, the main predictors of mortality within 30 days were male gender, age more than 37 years, seizure, mental status disturbance, GCS score <9, deep CVST, central nervous system infection, posterior fossa lesions and malignancy (8, 24). Patients older than 50 years, midline shift of more than 10 mm, total effacement of basilar cisterns, deep venous involvement, and bilateral lesions imply a poorer outcome in patients who underwent DC (8, 32).
We recommend DC in select patients with medically intractable mass effect and raised intracranial pressure where herniation is an immediate risk. In less severe cases, therapeutic anticoagulation with LMWH and medical management of raised ICP seems reasonable. A large decompressive flap like the one recommended for middle cerebral artery infarcts for predominantly unilateral lesions or a bifrontal craniectomy for bifrontal infarcts are the surgical options.
RA wrote, edited and reviewed the manuscript. MG wrote and edited the manuscript. BD edited, critically reviewed the manuscript. DB and DS reivewed the manuscript. NS edited, reviewed the manuscript and prepared figures. All authors approved the final version.
BD, DB, DS, and NS are supported by the NIHR Global Health Research Group on Neurotrauma that was commissioned by the NIHR using official development assistance (ODA) funding (Project 16/137/105). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, NIHR, or the Department of Health. Images provided were originally derived from the (NIMHANS) database. All authors consent for their publication as part of this mini-review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Agostoni E, Aliprandi A, Longoni M. Cerebral venous thrombosis. Expert Rev Neurother. (2009) 9:553–64. doi: 10.1586/ern.09.3
2. Masuhr F, Mehraein S, Einhaupl K. Cerebral venous and sinus thrombosis. J Neurol. (2004) 251:11–23. doi: 10.1007/s00415-004-03217
3. Steven A, Raghavan P, Altmeyer W, Gandhi D. Venous thrombosis: causes and imaging appearance. Hematol Oncol Clin North Am. (2016) 30:867–85. doi: 10.1016/j.hoc.2016.03.008
4. Mubbashir Shariff E, Alhameed M. Multiple cranial neuropathies in cerebral venous sinus thrombosis. Oxf Med Case Rep. (2014) 2014:21–23. doi: 10.1093/omcr/omu007
5. Ruiz-Sandoval JL, Chiquete E, Banuelos-Becerra LJ, Torres-Anguiano C, Gonzalez-Padilla C, Arauz A, et al. Cerebral venous thrombosis in a Mexican multicenter registry of acute cerebrovascular disease: the RENAMEVASC study. J Stroke Cerebrovasc Dis. (2012) 21:395–400. doi: 10.1016/j.jstrokecerebrovasdis.2011.01.001
6. Saposnik G, Barinagarrementeria F, Brown RD Jr., Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2011) 42:1158–92. doi: 10.1161/STR.0b013e31820a8364
7. Iqbal N, Sharma A. Cerebral venous thrombosis: a mimic of brain metastases in colorectal cancer associated with a better prognosis. Case Rep Oncol Med. (2013) 2013:109412. doi: 10.1155/2013/109412
8. Mahale R, Mehta A, Varma RG, Hegde AS, Acharya PT, Srinivasa R. Decompressive surgery in malignant cerebral venous sinus thrombosis: what predicts its outcome? J Thromb Thrombol. (2017) 43:530–9. doi: 10.1007/s11239-017-1489-x
9. Khan M, Kamal AK, Wasay M. Controversies of treatment modalities for cerebral venous thrombosis. Stroke Res Treat. (2010) 2010:956302. doi: 10.4061/2010/956302
10. Behrouzi R, Punter M. Diagnosis and management of cerebral venous thrombosis. Clin Med. (2018) 18:75–79. doi: 10.7861/clinmedicine.18-1-75
11. Lee SS, Baek SH, Shin DI. A case of cerebral venous thrombosis as the presenting sign of occult prostate cancer. J Clin Neurol. (2009) 5:195–7. doi: 10.3988/jcn.2009.5.4.195
12. Bansal H, Chaudhary A, Mahajan A, Paul B. Acute subdural hematoma secondary to cerebral venous sinus thrombosis: case report and review of literature. Asian J Neurosurg. (2016) 11:177. doi: 10.4103/1793-5482.175632
13. Zhao X, Rizzo A, Malek B, Fakhry S, Watson J. Basilar skull fracture: a risk factor for transverse/sigmoid venous sinus obstruction. J Neurotrauma. (2008) 25:104–11. doi: 10.1089/neu.2007.0351
14. Lindvall P, Koskinen LO. Intracranial hypertension due to cerebral venous sinus thrombosis following head trauma: a report of two cases. Case Rep Neurol. (2013) 5:168–74. doi: 10.1159/000355394
15. Ferro JM, Canhao P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. (2014) 16:523. doi: 10.1007/s11886-014-0523-2
16. Luo Y, Tian X, Wang X. Diagnosis and treatment of cerebral venous thrombosis: a review. Front Aging Neurosci. (2018) 10:2. doi: 10.3389/fnagi.2018.00002
17. Coutinho JM, Zuurbier SM, Gaartman AE, Dikstaal AA, Stam J, Middeldorp S, et al. Association between anemia and cerebral venous thrombosis: case-control study. Stroke. (2015) 46:2735–40. doi: 10.1161/STROKEAHA.115.009843
18. Ilkhchoui Y, Szabo EE, Gerstein NS, Jaime F. Cerebral venous thrombosis complicating severe preeclampsia in the postpartum period: a diagnostic challenge. J Clin Anesth. (2014) 26:143–146. doi: 10.1016/j.jclinane.2013.11.007
19. Dash D, Prasad K, Joseph L. Cerebral venous thrombosis: an Indian perspective. Neurol India. (2015) 63:318–28. doi: 10.4103/0028-3886.158191
20. Aaron S, Alexander M, Maya T, Mathew V, Goel M, Nair SC, et al. Underlying prothrombotic states in pregnancy associated cerebral venous thrombosis. Neurol India. (2010) 58:555–9. doi: 10.4103/0028-3886.68676
21. Ferro JM, Bousser MG, Canhao P, Coutinho JM, Crassard I, Dentali F, et al. European stroke organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur J Neurol. (2017) 24:1203–13. doi: 10.1111/ene.13381
22. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. (2007) 6:162–70. doi: 10.1016/S1474-4422(07)70029-7
23. deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. (2001) 345:417–23. doi: 10.1056/NEJM200108093450604
24. Theaudin M, Crassard I, Bresson D, Saliou G, Favrole P, Vahedi K, et al. Should decompressive surgery be performed in malignant cerebral venous thrombosis?: a series of 12 patients. Stroke. (2010) 41:727–31. doi: 10.1161/STROKEAHA.109.572909
25. Star M, Flaster M. Advances and controversies in the management of cerebral venous thrombosis. Neurol Clin. (2013) 31:765–83. doi: 10.1016/j.ncl.2013.03.013
26. Coutinho JM, Majoie CB, Coert BA, Stam J. Decompressive craniectomy in cerebral sinus thrombosis: consecutive case series and review of the literature. Stroke. (2009) 40:2233–5. doi: 10.1161/STROKEAHA.108.543421
27. Lanterna LA, Gritti P, Manara O, Grimod G, Bortolotti G, Biroli F. Decompressive surgery in malignant dural sinus thrombosis: report of 3 cases and review of the literature. Neurosurg Focus. (2009) 26:E5. doi: 10.3171/2009.3.FOCUS0910
28. Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F, Investigators I. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. (2004) 35:664–70. doi: 10.1161/01.STR.0000117571.76197.26
29. Guenther G, Arauz A. Cerebral venous thrombosis: a diagnostic and treatment update. Neurologia. (2011) 26:488–98. doi: 10.1016/j.nrleng.2010.09.002
30. Ferro JM, Crassard I, Coutinho JM, Canhao P, Barinagarrementeria F, Cucchiara B, et al. Decompressive surgery in cerebrovenous thrombosis: a multicenter registry and a systematic review of individual patient data. Stroke. (2011) 42:2825–31. doi: 10.1161/STROKEAHA.111.615393
31. Zuurbier SM, Coutinho JM, Majoie CB, Coert BA, van den Munckhof P, Stam J. Decompressive hemicraniectomy in severe cerebral venous thrombosis: a prospective case series. J Neurol. (2012) 259:1099–105. doi: 10.1007/s00415-011-6307-3
32. Zhang S, Zhao H, Li H, You C, Hui X. Decompressive craniectomy in hemorrhagic cerebral venous thrombosis: clinicoradiological features and risk factors. J Neurosurg. (2017) 127:709–15. doi: 10.3171/2016.8.JNS161112
33. Aaron S, Alexander M, Moorthy RK, Mani S, Mathew V, Patil AK, et al. Decompressive craniectomy in cerebral venous thrombosis: a single centre experience. J Neurol Neurosurg Psychiatry. (2013) 84:995–1000. doi: 10.1136/jnnp-2012-303356
34. Mohindra S, Umredkar A, Singla N, Bal A, Gupta SK. Decompressive craniectomy for malignant cerebral oedema of cortical venous thrombosis: an analysis of 13 patients. Br J Neurosurg. (2011) 25:422–429. doi: 10.3109/02688697.2011.578775
35. Rajan Vivakaran TT, Srinivas D, Kulkarni GB, Somanna S. The role of decompressive craniectomy in cerebral venous sinus thrombosis. J Neurosurg. (2012) 117:738–44. doi: 10.3171/2012.6.JNS11102
36. Soyer B, Rusca M, Lukaszewicz AC, Crassard I, Guichard JP, Bresson D, et al. Outcome of a cohort of severe cerebral venous thrombosis in intensive care. Ann Intensive Care. (2016) 6:29. doi: 10.1186/s13613-016-0209-6
37. Venkateswaran P, Sriganesh K, Chakrabarti D, Srinivas DB, Rao GSU. Regional cerebral oxygen saturation changes after decompressive craniectomy for malignant cerebral venous Thrombosis: a prospective cohort study. J Neurosurg Anesthesiol. (2018) 31:241–6. doi: 10.1097/ANA.0000000000000498
38. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. (2005) 352:1791–8. doi: 10.1056/NEJMra042354
39. Xu W, Gao L, Li T, Shao A, Zhang J. Efficacy and risks of anticoagulation for cerebral venous thrombosis. Medicine. (2018) 97:e10506. doi: 10.1097/MD.0000000000010506
40. Misra UK, Kalita J, Chandra S, Kumar B, Bansal V. Low molecular weight heparin versus unfractionated heparin in cerebral venous sinus thrombosis: a randomized controlled trial. Eur J Neurol. (2012) 19:1030–6. doi: 10.1111/j.1468-1331.2012.03690.x
41. Arauz A, Vargas-Gonzalez JC, Arguelles-Morales N, Barboza MA, Calleja J, Martinez-Jurado E, et al. Time to recanalisation in patients with cerebral venous thrombosis under anticoagulation therapy. J Neurol Neurosurg Psychiatry. (2016) 87:247–51. doi: 10.1136/jnnp-2014-310068
42. Pizzi MA, Alejos DA, Siegel JL, Kim BY, Miller DA, Freeman WD. Cerebral venous thrombosis associated with intracranial hemorrhage and timing of anticoagulation after hemicraniectomy. J Stroke Cerebrovasc Dis. (2016) 25:2312–6. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.025
43. Palazzo P, Agius P, Ingrand P, Ciron J, Lamy M, Berthomet A, et al. Venous thrombotic recurrence after cerebral venous thrombosis: a long-term follow-up study. Stroke. (2017) 48:321–6. doi: 10.1161/STROKEAHA.116.015294
44. Caprio F, Bernstein RA. Duration of anticoagulation after cerebral venous sinus thrombosis. Neurocrit Care. (2012) 16:335–42. doi: 10.1007/s12028-011-9661-1
45. Rao SK, Ibrahim M, Hanni CM, Suchdev K, Parker D, Rajamani K, et al. Apixaban for the treatment of cerebral venous thrombosis: a case series. J Neurol Sci. (2017) 381:318–20. doi: 10.1016/j.jns.2017.09.007
46. Siddiqui FM, Dandapat S, Banerjee C, Zuurbier SM, Johnson M, Stam J, et al. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke. (2015) 46:1263–8. doi: 10.1161/STROKEAHA.114.007465
47. Zhen Y, Zhang N, He L, Shen L, Yan K. Mechanical thrombectomy combined with recombinant tissue plasminogen activator thrombolysis in the venous sinus for the treatment of severe cerebral venous sinus thrombosis. Exp Ther Med. (2015) 9:1080–4. doi: 10.3892/etm.2015.2198
48. Coutinho JM, Ferro JM, Zuurbier SM, Mink MS, Canhao P, Crassard I, et al. Thrombolysis or anticoagulation for cerebral venous thrombosis: rationale and design of the TO-ACT trial. Int J Stroke. (2013) 8:135–40. doi: 10.1111/j.1747-4949.2011.00753.x
49. Ebke M, Jurgens KU, Tomandl B, Merten U, Kastrup A. Surgical treatment of space occupying edema and hemorrhage due to cerebral venous thrombosis during pregnancy. Neurocrit Care. (2011) 15:166–9. doi: 10.1007/s12028-010-9389-3
50. Mehraein S, Ortwein H, Busch M, Weih M, Einhaupl K, Masuhr F. Risk of recurrence of cerebral venous and sinus thrombosis during subsequent pregnancy and puerperium. J Neurol Neurosurg Psychiatry. (2003) 74:814–6. doi: 10.1136/jnnp.74.6.814
51. Aguiar de Sousa D, Canhao P, Ferro JM. Safety of pregnancy after cerebral venous thrombosis: a systematic review. Stroke. (2016) 47:713–8. doi: 10.1161/STROKEAHA.115.011955
52. Bender A, Schulte-Altedorneburg G, Mayer TE, Pfefferkorn T, Birnbaum T, Feddersen B, et al. Functional outcome after severe cerebral venous thrombosis. J Neurol. (2007) 254:465–70. doi: 10.1007/s00415-006-0391-9
53. Heller C, Heinecke A, Junker R, Knofler R, Kosch A, Kurnik K, et al. Cerebral venous thrombosis in children: a multifactorial origin. Circulation. (2003) 108:1362–7. doi: 10.1161/01.CIR.0000087598.05977.45
54. Kenet G, Kirkham F, Niederstadt T, Heinecke A, Saunders D, Stoll M, et al. Risk factors for recurrent venous thromboembolism in the European collaborative paediatric database on cerebral venous thrombosis: a multicentre cohort study. Lancet Neurol. (2007) 6:595–603. doi: 10.1016/S1474-4422(07)70131-X
55. Medlock MD, Olivero WC, Hanigan WC, Wright RM, Winek SJ. Children with cerebral venous thrombosis diagnosed with magnetic resonance imaging and magnetic resonance angiography. Neurosurgery. (1992) 31:870–6; discussion 6. doi: 10.1097/00006123-199211000-00007
56. Zuurbier SM, Hiltunen S, Lindgren E, Silvis SM, Jood K, Devasagayam S, et al. Cerebral venous thrombosis in older patients. Stroke. (2018) 49:197–200. doi: 10.1161/STROKEAHA.117.019483
57. Weimar C. Diagnosis and treatment of cerebral venous and sinus thrombosis. Curr Neurol Neurosci Rep. (2014) 14:417. doi: 10.1007/s11910-013-0417-5
58. Duman T, Uluduz D, Midi I, Bektas H, Kablan Y, Goksel BK, et al. A multicenter study of 1144 patients with cerebral venous thrombosis: The VENOST Study. J Stroke Cerebrovasc Dis. (2017) 26:1848–57. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.020
59. Gazioglu S, Eyuboglu I, Yildirim A, Aydin CO, Alioglu Z. Cerebral venous sinus thrombosis: clinical features, long-term outcome and recanalization. J Clin Neurosci. (2017) 45:248–251. doi: 10.1016/j.jocn.2017.07.028
Keywords: anticoagulation, cerebral venous sinus thrombosis, decompressive craniectomy, outcome, risk factors
Citation: Avanali R, Gopalakrishnan MS, Devi BI, Bhat DI, Shukla DP and Shanbhag NC (2019) Role of Decompressive Craniectomy in the Management of Cerebral Venous Sinus Thrombosis. Front. Neurol. 10:511. doi: 10.3389/fneur.2019.00511
Received: 08 August 2018; Accepted: 29 April 2019;
Published: 15 May 2019.
Edited by:
Stephen Honeybul, Sir Charles Gairdner Hospital, AustraliaReviewed by:
Eric Peter Thelin, Karolinska Institute (KI), SwedenCopyright © 2019 Avanali, Gopalakrishnan, Devi, Bhat, Shukla and Shanbhag. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: B. Indira Devi, YmlkZXZpZHJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.