
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 08 May 2019
Sec. Stroke
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00493
This article is part of the Research Topic Cutting-Edge Approaches for CNS Protection and Repair: Focus on Vascular and Degenerative Disorders View all 83 articles
Cerebral stroke is a leading cause of death and persistent disability of elderly in the world. Although stroke prevention by targeting several risk factors such as diabetes and hypertension has decreased the stroke incidence, the total number of strokes is increasing due to the population aging and new preventive therapies are needed. Moreover, post-stroke acute pharmacological strategies aimed to reduce stroke-induced brain injury have failed in clinical trials despite being effective in animal models. Finally, approximately 30% of surviving stroke patients do not recover from stroke and remain permanently dependent on supportive care in activities of daily living. Therefore, strategies to improve stroke recovery in the post-acute phase are highly needed. Linagliptin is a dipeptidyl peptidase-4 inhibitor which is clinically approved to reduce hyperglycemia in type 2 diabetes. The regulation of glycemia by dipeptidyl peptidase-4 inhibition is mainly achieved by preventing endogenous glucagon-like peptide-1 (GLP-1) degradation. Interestingly, linagliptin has also shown glycaemia-independent beneficial effects in animal models of stroke, Parkinson's disease and Alzheimer's disease. In some case the preclinical data have been supported with some clinical data. Although potentially very interesting for the development of new strategies against stroke and neurodegenerative disorders, the mode of action of linagliptin in the brain is still largely unknown and seems to occur in a GLP-1R-independent manner. The purpose of this mini-review is to summarize and discuss the recent experimental and clinical work regarding the effects of linagliptin in the central nervous system, with special emphasis on acute neuroprotection, stroke prevention and post-stroke recovery. We also highlight the main questions in this research field that need to be addressed in clinical perspective.
Stroke is a highly prevalent condition and a major cause of death and disabilities (1–5). Globally, 15 million people suffer a stroke every year with up to a 40% death rate (6). Of the surviving patients, up to 30% remain permanently disabled and require assistance in activities of daily living (5). In recent years, the incidence and mortality rates of stroke have significantly declined in high-income countries (4, 7). The decrease in stroke incidence is probably due to targeted intervention programs against major stroke risk factors such as type 2 diabetes (T2D), obesity, smoking, sedentary lifestyle, hypertension, and alcohol abuse (8), while the decrease in mortality rate and disability could be attributed to faster intervention by thrombolytics and/or clot removal surgery resulting in blood flow restoration which minimize stroke damage (9). However, there is a significant geographic variations of stroke burden (10), and the total number of strokes and associated disability burden have substantially increased due to the increase of global population and life expectancy (4, 7). For instance, in Europe the number of elderly is projected to increase by 35% by 2050 (11). Thus, the total number of stroke cases is unlikely to decrease, unless more advanced preventive and/or curative strategies will be developed.
Potential future approaches to reduce acute stroke damage that have been investigated over the last decades are therapeutic hypothermia and pharmacological neuroprotection (12, 13). However, neither of these strategies have seen successful translation into clinical practice. The major obstacle for this type of strategies is the rapid tissue death in ischemic core and the limited effective intervention time-window in the ischemic penumbra (14–16).
Pharmacological interventions of stroke aimed toward recovery to combat chronic post-stroke disabilities is also promising based on animal studies, although full translation from bench-to-bed remains to be achieved (17–20).
Recent research suggests that stroke therapeutics could be developed from diabetes research. In fact, several studies have shown that anti-diabetic drugs targeting the glucagon-like protein 1 receptor [(GLP-1-receptor agonists and dipeptidyl peptidase-4 inhibitors (DPP-4i)] can mediate anti-stroke efficacy in animal models, and has been suggested to decrease the incidence of stroke in some clinical studies [reviewed in (21, 22)]. These drugs are in clinical use for T2D and their robust safety profile suggest high potential for the possible repositioning into stroke therapies.
The aim of this review was to summarize the recent experimental and clinical data regarding the effects of DPP-4i (also named gliptins) in the central nervous system, with special emphasis on linagliptin and stroke. Specifically, we focused our discussion about the effects of DPP-4i in relation to stroke prevention, acute neuroprotection, and post-stroke recovery. We also highlighted the main gaps of knowledge that will need to be addressed in clinical perspective.
This review is based on a literature search in Pubmed, or at the scientific conference websites of major international cardiology (e.g., ESC, ESC HF, ACC, or AHA) or diabetes (i.e., EASD or ADA) societies until Jan 31st 2019. Pubmed was searched using free-text terms and medical subject heading. A uniform search strategy was applied to Pubmed to identify the reported studies. The primary MeSH terms and keywords used were as follows: dipeptidyl peptidase 4 inhibitor, DPP IV, gliptins, linagliptin, stroke, ischemia, neuroprotection, Parkinson's, Alzheimer's, dementia, neurogenesis, and neuroplasticity. Studies were screened by title, abstract and full text.
Linagliptin is a once daily oral DPP-4i launched in 2011 for the treatment of T2D. The IC50 of linagliptin on its primary target, DPP-4, is 1 nM, which makes it one of the most potent inhibitors within the class (23). The other DPP-4 inhibitors possess lower potency in the range of IC50 7–95 nM. Linagliptin shows high selectivity for DPP-4, over other dipeptidyl peptidases and related proteases (such as DPP-8 and 9) Chemically the drug is based on an optimized and unique xanthine scaffold (See Figure 1) possessing very slow dissociation from the human DPP-4 enzyme (koff < 0.00002 s−1). This extremely slow off-rate is the main factor for the high affinity (KD = 6.6 pM) and results in a prolonged drug-target residence time over several hours (24).
Following absorption, linagliptin is distributed into tissues with high DPP-4 expression, e.g., the kidney and liver. At low concentrations (< 1 nM), 99% of linagliptin is bound to soluble and circulating DPP-4 and elimination is low. In higher concentrations (>100 nM) plasma DPP-4 is saturated and protein binding decreases to 70–80%. That is one of the reasons why in contrast to other DPP-4 inhibitors with linear pharmacokinetics, linagliptin is unique in having non-linear pharmacokinetics in the therapeutic dose (5 mg, human dose) range. The linagliptin's binding characteristics were shown to be absent in DPP-4 deficient animals (25). Moreover, renal excretion of linagliptin at its therapeutic dose is < 7%, which is unique in the DPP-4 inhibitor class, that are primarily eliminated via the kidney (26). Linagliptin further shows low interaction with Cytochrom P450 and high stability in human cytosolic and microsomal compartment, however linagliptin is a P-gp (P-glycoprotein) substrate (27) limiting penetration across the blood brain barrier (bbb) under normal conditions (28).
The concept behind DPP-4i for therapeutic use in T2D is based on the prolongation of the half-life of the incretins GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), both secreted via specific endocrine gut cells following meal digestion. Incretins cause subsequently glucose-dependent insulin secretion for mainly postprandial glucose regulation. Further, DPP-4 inhibition suppresses glucagon secretion from α-cells. Numerous other substrates for DPP-4 have been described (29) such as SDF1α, GLP-2, NPY, substance P, which are activated or deactivated by the DPP-4 protease and play additional roles in inflammation, food intake, pain and vascular regulation, however less contribute to glucose control. Most clinically used DPP-4 inhibitors, like linagliptin, are once daily drugs because they show >80% inhibition of DPP-4 activity over 24 h. This is associated with the high increase of plasma GLP-1 and consistent reductions in elevated plasma glucose and HbA1c in various patient populations and across various background therapies (30). The recently completed cardiovascular outcome trial CARMELINA (CArdiovascular and Renal Microvascular outcomE study with LINAgliptin) confirmed the tolerability of linagliptin, and its cardiovascular (CV) safety, without any signal of heart failure (31). Due to an excretion primarily via the bile, linagliptin does not need dose adjustment, including in patients with T2D and impaired kidney function. CARMELINA proved safety in these renally impaired patients, and additionally showed a significant reduction in risk for progression albuminuria (32), which has not been assessed in a similar robust manner with the other members of the class of DPP-4 inhibitors.
Beside their glycemic properties, it has been recently reported that DPP-4 inhibitors can also affect the brain. For instance, studies have shown that DPP-4i exert neuroprotective actions in animal models of Parkinson's disease (PD) (33–35) and ongoing studies investigating the safety of intranasal delivery of the DPP-4i omarigliptin for the treatment of PD are ongoing (36). Moreover, D'Amico et al. (37) have shown that sitagliptin delayed AD-like pathology in a mouse model, and several studies confirmed these findings by using different DPP-4i inhibitors, including linagliptin [(38–41) and reviewed in (42)]. Other effects improving cognitive function, neuroplasticity and neurogenesis have been recently reported by employing vildagliptin (43), sitagliptin (44–48), and linagliptin (49). Furthermore, Hasegawa et al. (50) showed that linagliptin decreased hippocampal neuronal cell death and improved cognitive function in a model of aging. However, one study has also shown negative effects of sitagliptin in the brain, i.e., increased tau phosphorylation and insulin resistance (51). Finally, a recent study showed neuroprotection by sitagliptin in a model of brain trauma (52). In summary, although the passage of DPP-4i through the bbb in the damaged brain is undetermined and the mechanisms are unknown, the evidence of favorable effects of DPP-4i on brain complications is substantial.
It is difficult to study the potential efficacy of candidate drugs to reduce stroke risk using animal models. However, to determine the efficacy for acute neuroprotection, several animal models exist. A few studies have tested the potential efficacy of DPP-4i for acute neuroprotection and/or recovery after stroke. Moreover, acute ischemic stroke severity has been recently associated to changes in DPP-4 activity (53), suggesting that the regulation of this enzyme might have a therapeutic value.
Rohnert et al. (54) first showed that DPP-4 inhibition is neuroprotective in stroke via intracerebral administration of sitagliptin in the rat. We recently showed that 4 weeks per-oral pretreatment followed by 3 weeks post-stroke treatment with linagliptin reduced brain damage after stroke, in both normal and T2D/obese mice (55). Similar effects in non-diabetic rats were recently shown by Yang et al. (56) using alogliptin and by El-Sahar et al. (57) using vildagliptin. In the Yang et al. study, neuroprotection correlated to increased levels of brain BDNF. Moreover, DPP-4 inhibition by genistein resulted in similar findings (58). By using an experimental design consisting of chronic administration of linagliptin before and after stroke, Darsalia et al. (59) also showed that linagliptin-mediated neuroprotection against stroke occurred in correlation with increased neural stem cells proliferation (60) and, importantly, was not mediated by the GLP-1R.
A recent work by Ma et al. (61) has shown that the linagliptin treatment starting after stroke can decrease the stroke-induced brain damage in a rat model of transient cerebral ischemia induced by bilateral common carotid artery occlusion. Similar data using transient middle cerebral occlusion were reported by Chiazza et al. (62) who also showed that linagliptin improved functional recovery 3 days after stroke through the activation of the SDF-1α/CXCR4 pathway. Although one cannot rule out that the results of these two studies are due to the presence of linagliptin close to stroke time (suggesting acute neuroprotective effects), the data also suggest a pharmacological effect that goes beyond acute neuroprotection because the study design allowed extending the observation period from days to weeks after experimental stroke, thus evaluating the effects of linagliptin treatment in the post-stroke recovery phase. The likelihood of positive effects during the post-stroke recovery phase is also supported by the work of Darsalia et al. (59) showing that a single, acute bolus administration of linagliptin at stroke time was ineffective in reducing the brain damage. Furthermore, the potential role of DPP-4 inhibition in endogenous brain tissue remodeling and repair processes after stroke has been recently suggested by Wesley et al. (63).
Whether DPP-4i leads to neuroprotection and promotes recovery after stroke by directly acting on neurons remains to be determined, although a recent in vitro study suggested direct neuroprotection in neural cells (64). However, additional cellular mechanisms may be involved. Indeed, Mi et al. (65) showed that linagliptin increases the in vitro proliferation of rat brain microvascular endothelial cells via the SIRT1/HIF-1α/VEGF pathway. Furthermore, recent works have shown that linagliptin improves cerebrovascular dysfunction and remodeling in a rat model of T2D, independent of glycemic control (66, 67).
Admission hyperglycemia is per se a negative prognostic marker for patients suffering acute ischemic stroke. Interestingly, hyperglycemia in patients without previously known diabetes is associated with a greater risk for poor outcome compared to patients with identified diabetes prior the stroke (68–70). Remarkably most of the effects reviewed here occurred independently from the regulation of glycemia in both normal and diabetic models, suggesting that the potential efficacy of DPP-4i to increase acute neuroprotection and/or recovery may be clinically relevant not only for the diabetic population under a DPP-4i-mediated therapy.
The incidence of CV complications in T2D, including stroke, has declined substantially but it is still high (71). An increasing CV risk factor control attainment has probably contributed to this, and recently, it was demonstrated that maintaining control of glycated hemoglobin level, low-density lipoprotein cholesterol level, albuminuria, and blood pressure, as well as abstaining from smoking, was associated with no excess risk of CV death or stroke in T2D as compared to the general population (72). Interestingly, glycated hemoglobin was the strongest risk factor for stroke in this registry analysis.
Because, historically, there have been CV safety concerns regarding anti-diabetic drugs in the treatment of T2D, regulators in US and Europe focus specifically on this issue [(73) and http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf], and as a response, fifteen CV outcome trials assessing 3 novel classes of antihyperglycemic therapies (i.e., DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT-2 inhibitors) had been completed by end of 2018, of which none reported an increase in risk for major adverse CV events (MACE), whereas 6 agents have demonstrated CV benefits (74). Within the class of DPP-4i, four large CV outcome trials have been published till date for saxagliptin (75), sitagliptin (76), alogliptin (77), and linagliptin (32).
These trials have used the composite Major Adverse CV events (MACE); i.e., CV death, non-fatal myocardial infarction and stroke, with or without hospitalized unstable angina, as primary outcome. All trials have demonstrated CV safety, without incremental benefit for MACE, including no statistical significant difference in risk for non-fatal stroke. In the saxagliptin trial there was however a significantly higher numbers of patients hospitalized for heart failure (75), a signal also reported in the alogliptin trial (77). Due to this, the class has received a heart failure warning by the US Food and Drug Administration, especially if used in patients with high CV risk underlying heart and kidney disease (73). The linagliptin trial (CARMELINA®) (32) was designed to evaluate the CV safety and kidney outcomes of linagliptin in patients with T2D at high CV risk (75% of patients had prevalent kidney disease). Despite a frailer population, in comparison with the other DPP4i trials, linagliptin resulted in a non-inferior risk of MACE [compared to placebo added to standard care; hazard ratio 1.02 (95% CI: 0.89, 1.17)], including across a number of subgroups such as by sex and age, and did not affect the risk of heart failure [hazard ratio 0.90 (95% CI: 0.74, 1.08)] (31). Furthermore, the progression of albuminuria occurred less frequently in the linagliptin group [hazard ratio 0.86 (95% CI: 0.78, 0.95)] (32), but despite MACE safety, no significant protection for stroke [fatal/non-fatal stroke hazard ratio 0.91 (95% CI: 0.67, 1.23)] was observed. Nonetheless, their broad tolerability and the safety profile are today well-documented and a good choice for the pharmacology treatment of T2D. Importantly, it remains to be determined if these class of drugs can improve stroke outcome in the recovery phase (78) as it has been shown in several experimental studies. Interestingly, outcome trials with linagliptin (32, 79) will be exploring this question by determining the post-stroke functional outcome in a subgroup of patients hospitalized with stroke with T2D by using the modified rankin scale 3–6 months after stroke.
Cognitive impairment, including mild cognitive impairment (MCI) and dementia, is increasingly recognized as an important T2D complication (80). High HbA1C concentration and glucose variability are negatively associated with subtle cognitive changes but the association is weak and more randomized controlled trials are needed (81). The underlying processes of cognitive dysfunction in T2D are largely unknown and till date, no pharmacological intervention has proven efficacious (82). Given that incretin therapies have emerged as a potential therapeutic lead for vascular brain injury, studying effects of these on cognitive outcomes are of interest. This is further supported by findings, in an observational study in elderly patients with T2D, that increased plasma DPP-4 activity is associated with elevated risk of MCI (83) and some small, and hypothesis generating, and underpowered, clinical observational studies, reporting some benefits of DPP-4i on clinical cognitive outcomes (84–86).
Both in CARMELINA, and in CAROLINA (a recently completed head-to-head study of linagliptin vs. the sulfonylurea glimepiride) cognitive studies with linagliptin have been completed, but none yet published. The CARMELINA-cognition and the CAROLINA-cognition sub-studies were integral parts of CARMELINA (32) and CAROLINA trials (87), respectively. Both cognition sub-studies aimed to test whether linagliptin prevents accelerated cognitive decline by applying the mini-mental state examination, as a measure of global cognitive function. In addition, more domain-sensitive composite measure of attention & executive functioning, using two additional tests: the Trail Making Test and the verbal fluency test, have been applied. The results of these sub-studies will be informative for refining the research area within this emerging and highly developing field.
Preclinical studies showing favorable effects of DPP-4i in several CNS disorders and stroke, encourage further research aiming to clinically reposition these diabetic drugs as active CNS-centric drugs. Clinical studies mainly investigating the safety of DPP4i have shown that these drugs are safe albeit no effect to decrease stroke incidence has been shown.
The clinical efficacy of acute neuroprotection after stroke is largely dependent on timely intervention within a very short therapeutic window (few hours from stroke onset), which is difficult to achieve. However, if the neuroprotective substance is systemically present at stroke time, the chances of minimizing stroke-induced tissue loss are significantly greater. The success of this strategy could be achieved in T2D patients (at high stroke risk) who routinely take DPP-4i for the daily management of T2D. These patients could benefit from this treatment when suffering from stroke.
Another potential strategy to exploit the advantages of a DPP4i-based stroke therapy could be the promotion of stroke recovery and rehabilitation in the post-acute/chronic phase that could be theoretically applicable to all stroke patients. However, the potential of this approach has not yet been thoroughly investigated in animal studies and research in this field is highly needed, also in the clinical setting (78), including exploring whether there are differences in effects according to patient-characteristics; a field of emerging importance in the personalized medicine area. Indeed recent animal studies have shown pro-neurogenic (46), anti-inflammatory (54) and neuroplasticity (49) effects mediated by DPP-4i that could result beneficial in the post-stroke recovery phase and long-term clinical outcome.
Finally, studies are needed to understand the molecular mechanisms of DPP-4i in the brain, since the passage of DPP-4i through the bbb seems not to occur under normal conditions and after stroke is undetermined. Therefore, to study the mechanism of action of DPP4i in the brain substances with bbb permeability need to be synthesized in the future. Moreover, the systemic peripheral doses of GIP and GLP-1 after DPP-4i administration are low in comparison to GLP-1 agonists, e.g., in the pg range, and neuroprotection by linagliptin treatment has been shown to occur independently from GLP-1R (59). This suggest that alternative mechanisms are likely involved. Since the DPP-4 is an enzyme with over 40 known, biologically active substrates, many of which with proven CNS effects, the deeper understanding of how exactly DPP-4i regulate these substrates could lead to the identifications of new therapeutic targets in the CNS.
The demonstrated beneficial CNS effects in preclinical studies and the proven clinical safety of DPP-4i make them good candidates for their potential repositioning against stroke. While the data so far suggest that DPP-4i cannot reduce stroke risk, studies are needed to determine if T2D people who use them for daily T2D management could gain advantages in terms of reduced brain damage in the event of stroke. Both T2D and non-diabetic stroke patients could also benefit from the use of DPP-4i as post-stroke curative agents promoting recovery and rehabilitation in post-acute phase. However, more pre-clinical and clinical research is highly needed in this research field (See Figure 2 for the summary of DPP-4i-mediated effects in stroke and potential mechanisms of action).
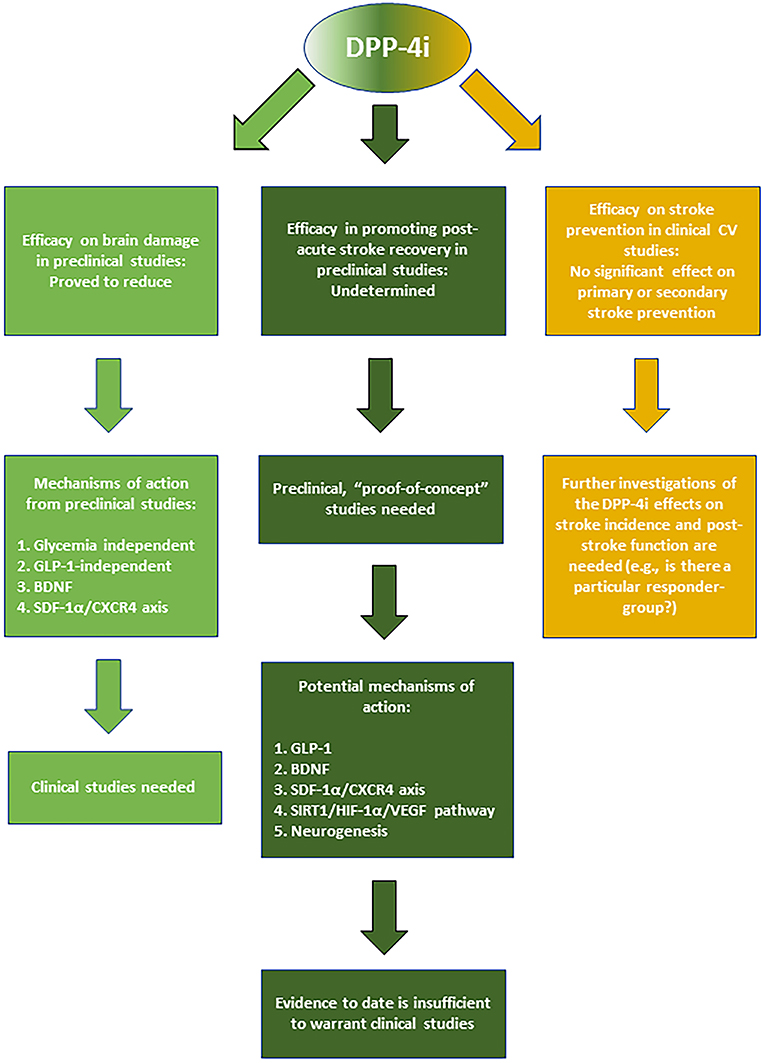
Figure 2. Summary of the reported effects and mechanisms mediated by DPP-4i in stroke prevention, acute neuroprotection, and post-stroke recovery.
VD conceived and wrote the review. OJ wrote the Linagliptin and Clinical Effects on Cognitive Outcomes section and edited the manuscript. GL wrote the Introduction section. TN wrote and reviewed the DPP-4 Inhibitors and Stroke Prevention With a Focus on CARMELINA, a Cardiorenal Outcome Trial With Linagliptin section. TK conceived the review and wrote The Pharmacology of Linagliptin and Linagliptin and the Treatment of T2D sections. CP conceived, wrote, and coordinated the review.
Financial support in our laboratory is provided by the European Foundation for the Study of Diabetes (EFSD), the Swedish Research Council (2018-02483), the Swedish Heart-Lung Foundation (20160511), the Novo Nordisk foundation (NNF17OC0026924), Karolinska Institutet (Foundation for Geriatric Diseases), KI Stiftelser och Fonder, Stohnes Stiftelse, O. E. och Edla Johanssons Stiftelse, STROKE Riksförbundet, Gamla Tjänarinnor Stiftelse, and by the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet. All foundations have provided funding that may also include publication costs.
Work in our laboratory is partly financed by Boehringer Ingelheim Pharma GmbH & Co. TK is employed at Boehringer Ingelheim Pharma GmbH & Co and Odd Erik Johansen by Boehringer Ingelheim Norway. TN has received unrestricted grants from AstraZenca and consultancy fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Merck, and Sanofi-Aventis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Thrift AG, Thayabaranathan T, Howard G, Howard VJ, Rothwell PM, Feigin VL, et al. Global stroke statistics. Int J Stroke. (2017) 12:13–32. doi: 10.1177/1747493016676285
2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-−2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–528. doi: 10.1161/CIR.0000000000000659
3. Bejot Y, Bailly H, Durier J, Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. (2016) 45:e391–8. doi: 10.1016/j.lpm.2016.10.003
4. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. (2017) 120:439–48. doi: 10.1161/CIRCRESAHA.116.308413
5. Ullberg T, Zia E, Petersson J, Norrving B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke. (2015) 46:389–94. doi: 10.1161/STROKEAHA.114.006538
6. WHO. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva (2011). Available online at: https://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ (accessed February, 2019).
7. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. (2014) 383:245–54. doi: 10.1016/S0140-6736(13)61953-4
8. Larsson SC, Akesson A, Wolk A. Primary prevention of stroke by a healthy lifestyle in a high-risk group. Neurology. (2015) 84:2224–8. doi: 10.1212/WNL.0000000000001637
9. Khandelwal P, Yavagal DR, Sacco RL. Acute ischemic stroke intervention. J Am Coll Cardiol. (2016) 67:2631–44. doi: 10.1016/j.jacc.2016.03.555
10. Kim AS, Johnston SC. Temporal and geographic trends in the global stroke epidemic. Stroke. (2013) 44:S123–5. doi: 10.1161/STROKEAHA.111.000067
11. United Nations DoEaSA. Population Division, World Population Ageing—Highlights (ST/ESA/SER.A/397). (2017). Available online at: http://www.un.org/en/development/desa/population/theme/ageing/WPA2017.shtml (accessed February, 2019).
12. Karnatovskaia LV, Wartenberg KE, Freeman WD. Therapeutic hypothermia for neuroprotection: history, mechanisms, risks, and clinical applications. Neurohospitalist. (2014) 4:153–63. doi: 10.1177/1941874413519802
13. Patel RAG, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis. (2017) 59:542–8. doi: 10.1016/j.pcad.2017.04.005
14. Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. (2007) 30:433–9. doi: 10.1016/j.tins.2007.06.009
15. Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. (2004) 1:36–45. doi: 10.1602/neurorx.1.1.36
16. Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: current status and future perspectives. Int J f Mol Sci. (2012) 13:11753–72. doi: 10.3390/ijms130911753
17. Roth S, Liesz A. Stroke research at the crossroads—where are we heading? Swiss Med Wkly. (2016) 146:w14329. doi: 10.4414/smw.2016.14329
18. Chollet F, Cramer SC, Stinear C, Kappelle LJ, Baron JC, Weiller C, et al. Pharmacological therapies in post stroke recovery: recommendations for future clinical trials. J Neurol. (2014) 261:1461–8. doi: 10.1007/s00415-013-7172-z
19. Viale L, Catoira NP, Di Girolamo G, Gonzalez CD. Pharmacotherapy and motor recovery after stroke. Expert Rev Neurother. (2018) 18:65–82. doi: 10.1080/14737175.2018.1400910
20. Yeo SH, Lim ZI, Mao J, Yau WP. Effects of central nervous system drugs on recovery after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Drug Investig. (2017) 37:901–28. doi: 10.1007/s40261-017-0558-4
21. Darsalia V, Klein T, Nystrom T, Patrone C. Glucagon-like receptor 1 agonists and DPP-4 inhibitors: anti-diabetic drugs with anti-stroke potential. Neuropharmacology. (2017) 136 (Pt B):280–6. doi: 10.1016/j.neuropharm.2017.08.022
22. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. (2017) 136:849–70. doi: 10.1161/CIRCULATIONAHA.117.028136
23. Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylm ethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther. (2008) 325:175–82. doi: 10.1124/jpet.107.135723
24. Schnapp G, Klein T, Hoevels Y, Bakker RA, Nar H. Comparative analysis of binding kinetics and thermodynamics of dipeptidyl peptidase-4 inhibitors and their relationship to structure. J Med Chem. (2016) 59:7466–77. doi: 10.1021/acs.jmedchem.6b00475
25. Retlich S, Withopf B, Greischel A, Staab A, Jaehde U, Fuchs H. Binding to dipeptidyl peptidase-4 determines the disposition of linagliptin (BI 1356)–investigations in DPP-4 deficient and wildtype rats. Biopharm Drug Dispos. (2009) 30:422–36. doi: 10.1002/bdd.676
26. Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. (2012) 51:501–14. doi: 10.1007/BF03261927
27. Ishiguro N, Shimizu H, Kishimoto W, Ebner T, Schaefer O. Evaluation and prediction of potential drug-drug interactions of linagliptin using in vitro cell culture methods. Drug Metab Dispos. (2013) 41:149–58. doi: 10.1124/dmd.112.048470
28. Fuchs H, Binder R, Greischel A. Tissue distribution of the novel DPP-4 inhibitor BI 1356 is dominated by saturable binding to its target in rats. Biopharm Drug Dispos. (2009) 30:229–40. doi: 10.1002/bdd.662
29. Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci. (2005) 108:277–92. doi: 10.1042/CS20040302
30. Deeks ED. Linagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. (2012) 72:1793–824. doi: 10.2165/11209570-000000000-00000
31. McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. (2019) 139:351–61. doi: 10.1161/CIRCULATIONAHA.118.038352
32. Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. (2018) 321:69–79. doi: 10.1001/jama.2018.18269
33. Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI. Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson's disease. Inflammopharmacology. (2017) 25:369–82. doi: 10.1007/s10787-017-0331-6
34. Abdelsalam RM, Safar MM. Neuroprotective effects of vildagliptin in rat rotenone Parkinson's disease model: role of RAGE-NFkappaB and Nrf2-antioxidant signaling pathways. J Neurochem. (2015) 133:700–7. doi: 10.1111/jnc.13087
35. Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM. Saxagliptin: a novel antiparkinsonian approach. Neuropharmacology. (2015) 89:308–17. doi: 10.1016/j.neuropharm.2014.10.007
36. Ayoub BM, Mowaka S, Safar MM, Ashoush N, Arafa MG, Michel HE, et al. Repositioning of omarigliptin as a once-weekly intranasal anti-parkinsonian agent. Sci Rep. (2018) 8:8959. doi: 10.1038/s41598-018-27395-0
37. D'Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer's prone mice. Exp Gerontol. (2010) 45:202–7. doi: 10.1016/j.exger.2009.12.004
38. Kosaraju J, Holsinger RM, Guo L, Tam KY. Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer's disease. Mol Neurobiol. (2016) 54:6074–84. doi: 10.1007/s12035-016-0125-7
39. Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, et al. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer's disease. J Pharm Pharmacol. (2013) 65:1773–84. doi: 10.1111/jphp.12148
40. Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, et al. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer's disease. Neuropharmacology. (2013) 72:291–300. doi: 10.1016/j.neuropharm.2013.04.008
41. Ma QH, Jiang LF, Mao JL, Xu WX, Huang M. Vildagliptin prevents cognitive deficits and neuronal apoptosis in a rat model of Alzheimer's disease. Mol Med Rep. (2018) 17:4113–9. doi: 10.3892/mmr.2017.8289
42. Angelopoulou E, Piperi C. DPP-4 inhibitors: a promising therapeutic approach against Alzheimer's disease. Ann Transl Med. (2018) 6:255. doi: 10.21037/atm.2018.04.41
43. Zhang DD, Shi N, Fang H, Ma L, Wu WP, Zhang YZ, et al. Vildagliptin, a DPP4 inhibitor, alleviates diabetes-associated cognitive deficits by decreasing the levels of apoptosis-related proteins in the rat hippocampus. Exp Ther Med. (2018) 15:5100–6. doi: 10.3892/etm.2018.6016
44. Dong Q, Teng SW, Wang Y, Qin F, Li Y, Ai LL, et al. Sitagliptin protects the cognition function of the Alzheimer's disease mice through activating glucagon-like peptide-1 and BDNF-TrkB signalings. Neurosci Lett. (2018) 696:184–90. doi: 10.1016/j.neulet.2018.12.041
45. Bachor TP, Marquioni-Ramella MD, Suburo AM. Sitagliptin protects proliferation of neural progenitor cells in diabetic mice. Metab Brain Dis. (2015) 30:885–93. doi: 10.1007/s11011-015-9656-2
46. Gault VA, Lennox R, Flatt PR. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diab Obes Metab. (2015) 17:403–13. doi: 10.1111/dom.12432
47. Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YY, et al. Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypo-perfusion through suppressing oxidative stress and inflammatory reaction. J Hypertens. (2015) 33:1001–13. doi: 10.1097/HJH.0000000000000529
48. Sakr HF. Effect of sitagliptin on the working memory and reference memory in type 2 diabetic Sprague-Dawley rats: possible role of adiponectin receptors 1. J Physiol Pharmacol. (2013) 64:613–23.
49. Lietzau G, Davidsson W, Ostenson CG, Chiazza F, Nathanson D, Pintana H, et al. Type 2 diabetes impairs odour detection, olfactory memory and olfactory neuroplasticity; effects partly reversed by the DPP-4 inhibitor Linagliptin. Acta Neuropathol Commun. (2018) 6:14. doi: 10.1186/s40478-018-0517-1
50. Hasegawa Y, Hayashi K, Takemoto Y, Cheng C, Takane K, Lin B, et al. DPP-4 inhibition with linagliptin ameliorates the progression of premature aging in klotho−/− mice. Cardiovasc Diabetol. (2017) 16:154. doi: 10.1186/s12933-017-0639-y
51. Kim DH, Huh JW, Jang M, Suh JH, Kim TW, Park JS, et al. Sitagliptin increases tau phosphorylation in the hippocampus of rats with type 2 diabetes and in primary neuron cultures. Neurobiol Dis. (2012) 46:52–8. doi: 10.1016/j.nbd.2011.12.043
52. DellaValle B, Brix GS, Brock B, Gejl M, Rungby J, Larsen A. Oral administration of sitagliptin activates CREB and is neuroprotective in murine model of brain trauma. Front Pharmacol. (2016) 7:450. doi: 10.3389/fphar.2016.00450
53. Baerts L, Brouns R, Kehoe K, Verkerk R, Engelborghs S, De Deyn PP, et al. Acute ischemic stroke severity, progression, and outcome relate to changes in dipeptidyl peptidase IV and fibroblast activation protein activity. Transl Stroke Res. (2017) 8:157–64. doi: 10.1007/s12975-016-0493-3
54. Rohnert P, Schmidt W, Emmerlich P, Goihl A, Wrenger S, Bank U, et al. Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN-like proteases in cerebral ischemia. J Neuroinflamm. (2012) 9:44. doi: 10.1186/1742-2094-9-44
55. Darsalia V, Ortsater H, Olverling A, Darlof E, Wolbert P, Nystrom T, et al. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: a comparison with glimepiride. Diabetes. (2013) 62:1289–96. doi: 10.2337/db12-0988
56. Yang D, Nakajo Y, Iihara K, Kataoka H, Yanamoto H. Alogliptin, a dipeptidylpeptidase-4 inhibitor, for patients with diabetes mellitus type 2, induces tolerance to focal cerebral ischemia in non-diabetic, normal mice. Brain Res. (2013) 1517:104–13. doi: 10.1016/j.brainres.2013.04.015
57. El-Sahar AE, Safar MM, Zaki HF, Attia AS, Ain-Shoka AA. Sitagliptin attenuates transient cerebral ischemia/reperfusion injury in diabetic rats: implication of the oxidative-inflammatory-apoptotic pathway. Life Sci. (2015) 126:81–6. doi: 10.1016/j.lfs.2015.01.030
58. Rajput MS, Sarkar PD, Nirmal NP. Inhibition of DPP-4 activity and neuronal atrophy with genistein attenuates neurological deficits induced by transient global cerebral ischemia and reperfusion in streptozotocin-induced diabetic mice. Inflammation. (2017) 40:623–35. doi: 10.1007/s10753-017-0509-5
59. Darsalia V, Larsson M, Lietzau G, Nathanson D, Nystrom T, Klein T, et al. Gliptins-mediated neuroprotection against stroke requires chronic pre-treatment and is glucagon-like peptide-1 receptor independent. Diab Obes Metab. (2016) 18:537–41. doi: 10.1111/dom.12641
60. Darsalia V, Olverling A, Larsson M, Mansouri S, Nathanson D, Nystrom T, et al. Linagliptin enhances neural stem cell proliferation after stroke in type 2 diabetic mice. Regul Pept. (2014) 190–1:25–31. doi: 10.1016/j.regpep.2014.05.001
61. Ma M, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, Nakagawa T, et al. DPP-4 inhibition with linagliptin ameliorates cognitive impairment and brain atrophy induced by transient cerebral ischemia in type 2 diabetic mice. Cardiovasc Diabetol. (2015) 14:54. doi: 10.1186/s12933-015-0218-z
62. Chiazza F, Tammen H, Pintana H, Lietzau G, Collino M, Nystrom T, et al. The effect of DPP-4 inhibition to improve functional outcome after stroke is mediated by the SDF-1alpha/CXCR4 pathway. Cardiovasc Diabetol. (2018) 17:60. doi: 10.1186/s12933-018-0702-3
63. Wesley UV, Hatcher JF, Ayvaci ER, Klemp A, Dempsey RJ. Regulation of dipeptidyl peptidase IV in the post-stroke rat brain and in vitro ischemia: implications for chemokine-mediated neural progenitor cell migration and angiogenesis. Mol Neurobiol. (2017) 54:4973–85. doi: 10.1007/s12035-016-0039-4
64. Kornelius E, Lin CL, Chang HH, Li HH, Huang WN, Yang YS, et al. DPP-4 inhibitor linagliptin attenuates abeta-induced cytotoxicity through activation of AMPK in neuronal cells. CNS Neurosci Ther. (2015) 21:549–57. doi: 10.1111/cns.12404
65. Mi DH, Fang HJ, Zheng GH, Liang XH, Ding YR, Liu X, et al. DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci Ther. (2018) 25:323–32. doi: 10.1111/cns.13042
66. Hardigan T, Yasir A, Abdelsaid M, Coucha M, El-Shaffey S, Li W, et al. Linagliptin treatment improves cerebrovascular function and remodeling and restores reduced cerebral perfusion in Type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. (2016) 311:R466–77. doi: 10.1152/ajpregu.00057.2016
67. Yasir Y, Hardigan T, Ergul A. Diabetes-mediated middle cerebral artery remodeling is restored by linagliptin: interaction with the vascular smooth muscle cell endothelin system. Life Sci. (2016) 159:76–82. doi: 10.1016/j.lfs.2016.02.096
68. Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diab Investig. (2018) 10:780–92. doi: 10.1111/jdi.12932
69. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. (2001) 32:2426–32. doi: 10.1161/hs1001.096194
70. Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long term follow up study. BMJ. (1997) 314:1303. doi: 10.1136/bmj.314.7090.1303
71. Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. (2017) 376:1407–18. doi: 10.1056/NEJMoa1608664
72. Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2018) 379:633–44. doi: 10.1056/NEJMoa1800256
73. Cefalu WT, Kaul S, Gerstein HC, Holman RR, Zinman B, Skyler JS, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors' expert forum. Diab Care. (2018) 41:14–31. doi: 10.2337/dci17-0057
74. McGuire DK, Marx N, Johansen OE, Inzucchi SE, Rosenstock J, George JT. FDA guidance on antihyperglycemic therapies for type 2 diabetes: one decade later. Diab Obes Metab. (2019) 21:1079–80. doi: 10.1111/dom.13645
75. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. (2013) 369:1317–26. doi: 10.1056/NEJMoa1307684
76. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2015) 373:232–42. doi: 10.1056/NEJMoa1501352
77. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. (2013) 369:1327–35. doi: 10.1056/NEJMoa1305889
78. Darsalia V, Larsson M, Klein T, Patrone C. The high need for trials assessing functional outcome after stroke rather than stroke prevention with GLP-1 agonists and DPP-4 inhibitors. Cardiovasc Diabetol. (2018) 17:32. doi: 10.1186/s12933-018-0674-3
79. Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA(R)). Diab Vasc Dis Res. (2015) 12:164–74. doi: 10.1177/1479164115570301
80. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. (2018) 14:591–604. doi: 10.1038/s41574-018-0048-7
81. Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diab Endocrinol. (2015) 3:75–89. doi: 10.1016/S2213-8587(14)70148-2
82. Fink HA, Jutkowitz E, McCarten JR, Hemmy LS, Butler M, Davila H, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical alzheimer-type dementia: a systematic review. Ann Intern Med. (2018) 168:39–51. doi: 10.7326/M17-1529
83. Zheng T, Qin L, Chen B, Hu X, Zhang X, Liu Y, et al. Association of plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: results from the GDMD study in China. Diab Care. (2016) 39:1594–601. doi: 10.2337/dc16-0316
84. Tasci I, Naharci MI, Bozoglu E, Safer U, Aydogdu A, Yilmaz BF, et al. Cognitive and functional influences of vildagliptin, a DPP-4 inhibitor, added to ongoing metformin therapy in elderly with type 2 diabetes. Endocr Metab Immune Disord Drug Targets. (2013) 13:256–63. doi: 10.2174/18715303113139990037
85. Kim YG, Jeon JY, Kim HJ, Kim DJ, Lee KW, Moon SY, et al. Risk of dementia in older patients with type 2 diabetes on dipeptidyl-peptidase IV inhibitors versus sulfonylureas: a real-world population-based cohort study. J Clin Med. (2018) 8:E28. doi: 10.3390/jcm8010028
86. Dumbrill JL, Moulton CD. Effects of incretin-based therapies on neurocognitive function in humans: a systematic review of the literature. Prim Care Diab. (2018) 12:51–8. doi: 10.1016/j.pcd.2017.06.009
87. Biessels GJ, Janssen J, van den Berg E, Zinman B, Espeland MA, Mattheus M, et al. Rationale and design of the CAROLINA(R)—cognition substudy: a randomised controlled trial on cognitive outcomes of linagliptin versus glimepiride in patients with type 2 diabetes mellitus. BMC Neurol. (2018) 18:7. doi: 10.1186/s12883-018-1014-7
Keywords: dipeptidyl peptidase-4 inhibitors, linagliptin, stroke, stroke recovery, neuroprotection
Citation: Darsalia V, Johansen OE, Lietzau G, Nyström T, Klein T and Patrone C (2019) Dipeptidyl Peptidase-4 Inhibitors for the Potential Treatment of Brain Disorders; A Mini-Review With Special Focus on Linagliptin and Stroke. Front. Neurol. 10:493. doi: 10.3389/fneur.2019.00493
Received: 12 March 2019; Accepted: 23 April 2019;
Published: 08 May 2019.
Edited by:
Gregory Jaye Bix, University of Kentucky, United StatesReviewed by:
Richard F. Keep, University of Michigan, United StatesCopyright © 2019 Darsalia, Johansen, Lietzau, Nyström, Klein and Patrone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vladimer Darsalia, dmxhZGltZXIuZGFyc2FsaWFAa2kuc2U= orcid.org/0000-0002-6693-934X
Cesare Patrone, Y2VzYXJlLnBhdHJvbmVAa2kuc2U= orcid.org/0000-0003-0470-4606
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.