- 1Department of Physical Medicine and Rehabilitation, Harvard Medical School, Boston, MA, United States
- 2Spaulding Research Institute, Spaulding Rehabilitation Hospital, Charlestown, MA, United States
- 3MassGeneral Hospital for Children Sport Concussion Program, Boston, MA, United States
- 4Home Base, A Red Sox Foundation and Massachusetts General Hospital Program, Charlestown, MA, United States
Some people experience persistent symptoms following a mild traumatic brain injury (MTBI), and the etiology of those symptoms has been debated for generations. Post-concussion-like symptoms are caused by many factors both before and after MTBI, and this non-specificity is the bedrock of the conundrum regarding the existence of the post-concussion syndrome. A latent model or common cause theory for the syndrome is inconsistent with the prevailing biopsychosocial conceptualization. It is the thesis of this paper that adopting a network perspective for persistent symptoms following MTBI, including the post-concussion syndrome, could lead to new insights and targeted treatment and rehabilitation strategies. The network perspective posits that symptoms co-occur because they are strongly inter-related, activating, amplifying, and mutually reinforcing, not because they arise from a common latent disease entity. This approach requires a conceptual shift away from thinking that symptoms reflect an underlying disease or disorder toward viewing inter-related symptoms as constituting the syndrome or disorder. The symptoms do not arise from an underlying syndrome—the symptoms are the syndrome. A network analysis approach allows us to embrace heterogeneity and comorbidity, and it might lead to the identification of new approaches to sequenced care. The promise of precision rehabilitation requires us to better understand the interconnections among symptoms and problems so that we can produce more individualized and effective treatment and rehabilitation.
Introduction
A substantial minority of people report persistent symptoms following a mild traumatic brain injury (MTBI) for several months and sometimes years (1–9). Whether these symptoms represent a “post-concussion syndrome” has been controversial for generations. For decades, researchers, and clinicians have questioned whether this diagnosis is a true syndrome, disorder, or disease entity [e.g., (10–15)], and the etiology of the syndrome has never been agreed upon [see (16–21) for reviews]. Many have suggested that the etiology of persistent symptoms is due to the biological effects of a MTBI, psychological factors, psychosocial factors (broadly defined), chronic pain, depression, or a combination of factors (22–30). Regardless of etiology, persistent symptoms after MTBI are associated with high levels of disability and health care service utilization, and lower health-related quality of life (9, 31–40).
The International Classification of Diseases 10th edition (ICD-10) specific research criteria for the post-concussional syndrome require symptoms to be present for more than 1 month and the person must have symptoms and problems in three or more of the following domains (1) complaints of unpleasant sensations and pains, such as headache, dizziness (usually lacking the features of true vertigo), general malaise and excessive fatigue, or noise intolerance; (2) emotional changes, such as irritability, emotional lability, both easily provoked or exacerbated by emotional excitement or stress, or some degree of depression and/or anxiety; (3) subjective complaints of difficulty in concentration and in performing mental tasks, and of memory complaints, without clear objective evidence (e.g., psychological tests) of marked impairment; (4) insomnia; (5) reduced tolerance to alcohol; and/or (6) preoccupation with the above symptoms and fear of permanent brain damage, to the extent of hypochondriacal over-valued ideas and adoption of a sick role (41). The Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) (42) included research criteria for the post-concussional disorder that differed from the ICD-10 criteria in a several ways, such as including somewhat different symptoms and requiring the presence of objectively measured cognitive deficits. For the 5th Edition (i.e., the DSM-5), published in 2013 (43), the post-concussional disorder was dropped and problems relating to MTBI can be coded as “mild neurocognitive disorder,” but this diagnosis does not include post-concussion symptoms—it is based on objective evidence of a decline in cognitive functioning.
A fundamental challenge in defining the syndrome is the non-specificity of the symptoms. Post-concussion-like symptoms are common in healthy children and adults in their daily lives (44–51). They are also common in people seen for psychological treatment (52), outpatients seen for minor medical problems (53), personal injury claimants (53, 54), and people with post-traumatic stress disorder (PTSD) (55), orthopedic injuries (11), chronic pain (42, 56–59), whiplash (60), anxiety (61, 62), and depression (63). Biopsychosocial conceptualizations of the symptoms and syndrome (64–66) emphasize a diverse range of personality and social psychological factors that contribute to how symptoms are perceived, experienced, and reported, such as expectations and misattributions (47, 67–71), coping and illness perceptions (72), “good-old-days” bias (47, 73–79), cognitive hypochondriasis (80), fear avoidance (81, 82) cogniphobia (83, 84), nocebo effect (85, 86), perceived injustice (87), iatrogenesis (17, 27), resilience (88, 89), Type D personality (90, 91), and other personality characteristics, particularly compulsive, histrionic, dependent, and narcissistic traits (21, 61). A multidimensional model for conceptualizing the post-concussion syndrome suggests that setbacks in several aspects of a person's life (physical, emotional, cognitive, psychosocial, vocational, financial, and recreational) serve as cumulative stressors that interact with personality and pre-morbid physical and mental health factors, resulting in the syndrome (21, 92, 93). Clearly, post-concussion-like symptoms are caused by many factors both before and after a mild injury to the brain. This non-specificity problem incumbers the development of new and innovative approaches to conceptualizing the etiology of symptoms and developing new treatment and rehabilitation strategies.
As seen in Figure 1, a diverse array of physical, psychological, and cognitive symptoms and problems can be amplifying and mutually reinforcing in people who have experienced a mild TBI. Some of those symptoms might be caused directly or indirectly by injuries to the brain, head, peripheral vestibular system, or body, and some symptoms might be caused, amplified, or maintained by a diverse range of other factors. It is essential to appreciate that these diverse symptoms and problems occur within a personal biopsychosocial context, as seen in Figure 1. Individuals have unique pre-injury vulnerability factors, current environmental stressors, social psychological reactionary factors, and personality characteristics. As such, an individual's symptoms and problems (from Figure 1) occur within a unique personal context. The symptoms that a person experiences, their underlying causes, and the biopsychosocial context in which the person lives all are subject to change over time, from the initial days following injury to weeks and months later.
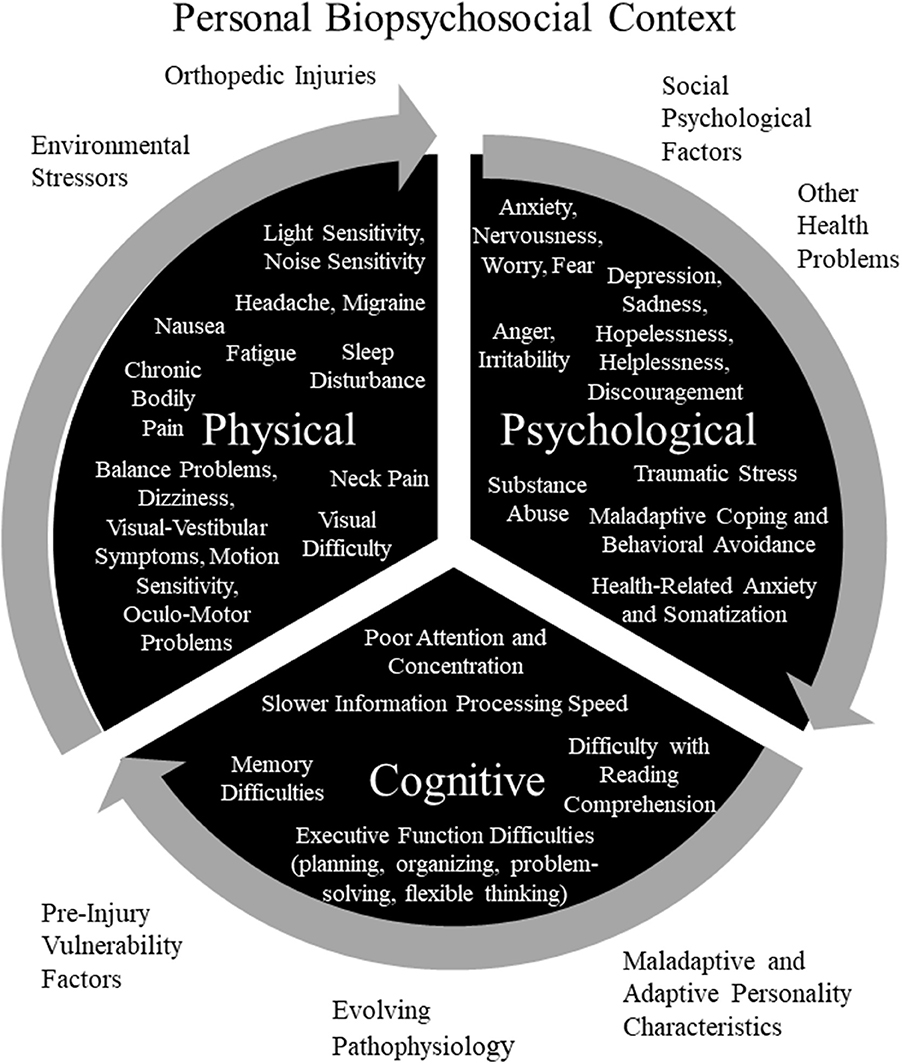
Figure 1. Potentially Amplifying and Reinforcing Persistent Symptoms and Problems and Personal Biopsychosocial Context for Experiencing Persistent Symptoms and Problems. Pre-Injury Vulnerability Factors: personal or family history of mental health problems and associated genetic and environmental vulnerability (childhood abuse or neglect, depression, anxiety, or traumatic stress); prior brain injuries; personal history of, or vulnerability to, migraine or other headache disorder; and history of motion sickness or other visual-vestibular vulnerability factor. Environmental Stressors: financial/occupational stress; academic stress; marital, family, or relationship problems; and litigation, compensation-seeking or maintaining, or other secondary gain issues. Social Psychological Factors: maladaptive coping, catastrophizing, expectations, “good-old-days” bias (tendency to view oneself as healthier in the past and underestimate past problems), nocebo effect, diagnosis threat, cognitive hypochondriasis and preoccupation, lifestyle and family dynamics changes, avoidance behavior, cogniphobia (fear and avoidance of mental exertion out of concern for developing or exacerbating a headache), reinforced illness behavior, anger, bitterness, perceived injustice, justification/entitlement, or iatrogenesis. Personality Characteristics or Disorders: neuroticism (a personality trait characterized by a strong tendency to experience negative emotions such as anxiety, depression, anger, and self-consciousness. Individuals with this trait have considerable difficulty coping with stress), anxiety sensitivity (a trait comprised of physical, psychological, and social pre-occupations and concerns, is characterized by fear of anxiety-related bodily sensations), alexithymia (a cluster of traits characterized by difficulty identifying feelings, difficulty describing feelings to others, externally oriented thinking, and limited capacity for imaginal thinking), perfectionism, egocentrism, Type D personality (personality pattern is characterized by two stable personality traits: negative affectivity and social inhibition), disagreeableness (a personality trait characterized by antagonism, skepticism, and egocentrism), unconscientiousness (a trait characterized by reduced self-discipline and ambition, disorganization, and a more lackadaisical approach to life), narcissistic, dependent, histrionic, or passive-aggressive. Adaptive Personality Characteristics: resilience, grit (passion and perseverance toward long-term goals), and psychological hardiness (personality characteristic consisting of three psychological attitudes and beliefs: commitment, challenge, and control). Copyright © 2019, Grant L. Iverson, Ph.D., Used with Permission.
The biopsychosocial heterogeneity and complexity associated with outcome from MTBI is illustrated further in an interesting review by Kenzie et al. (94). They propose a conceptual framework, involving multiple nested scales, based on a complex systems theoretical approach. The four nested scales are cellular (e.g., axonal injury, neuroinflammation, and synaptic changes), network (e.g., intrinsic connectivity and neuronal population dynamics), experiential (e.g., physical, cognitive, and psychological symptoms), and social (e.g., access to healthcare, social support, work or school pressures)—and each of these interacting scales can be influenced by a diverse range of personal characteristics and external environmental factors [see Figure 1 in Kenzie et al. (94)]. Given the complexity of persistent symptoms following a mild injury to the brain, as reflected in the conceptual model of Kenzie and colleagues and in Figure 1, it is not surprising that there is no unified latent disease model etiology for the post-concussion syndrome.
Network Analysis and Persistent Post-Concussion Symptoms
It is the thesis of this paper that adopting a network perspective for persistent symptoms following MTBI, including the post-concussion syndrome, could lead to new insights and targeted treatment and rehabilitation strategies. To my knowledge, there are no published studies applying network analysis to persistent symptoms and problems following MTBI. Network theory and analysis (95–100) posits that mental disorders can be viewed as a set of interacting symptoms. Conceptually and philosophically, the network approach does not require that the post-concussion syndrome, or syndromes, have a single underlying cause (e.g., brain injury) that is independent of the symptoms. Instead, the presence of the interacting and inter-related symptoms constitutes the syndrome. A syndrome may occur when a requisite number of symptoms become activated for a sufficient period of time. The network approach, applied to the post-concussion syndrome, posits that symptoms co-occur because they are strongly inter-related, activating, amplifying, and mutually reinforcing, not because they arise from a common latent disease entity. This approach requires a conceptual shift away from thinking that symptoms reflect an underlying disease or disorder toward viewing inter-related symptoms as constituting the syndrome or disorder. That is, the symptoms are the syndrome.
Network Analysis in Psychiatry and Psychology
Network analysis is a statistical and psychometric methodology for studying the interrelationships among symptoms. A number of articles describe the methodology of network analysis (96, 101, 102). A network, graphically represented, is comprised of nodes and edges. For the purpose of this paper, a node is a symptom or clinical feature of PCS, and the edges are connections between symptoms. The edges represent the statistical associations between symptoms. When represented as a figure, symptoms (i.e., nodes) that activate each other are connected by lines (i.e., edges). The interrelations among the symptoms represent the network. Specific symptoms within the network can be influenced by things in the “external field.” The external field is outside the symptom network, but not necessarily outside the person. The external field is comprised of intrinsic (e.g., microstructural brain injury, cervical injury, or peripheral vestibular injury) and extrinsic factors (e.g., life stress). The external field also includes the social and environmental context for the person, including being involved in personal injury litigation or a worker's compensation claim.
A network of symptoms is represented graphically, in a two-dimensional figure, by having circles representing symptoms and lines connecting circles representing the association (i.e., correlation) between the symptoms. These graphic depictions arise from statistical psychometric analyses of large databases, not from theory. The lines can be unweighted, which means that all statistically significant correlations are shown with the same thickness of lines, or they can be weighted, meaning that thicker lines represent stronger correlations. Line colors can also be used, such as green lines representing positive associations and red lines representing negative associations. The lines connecting the symptoms can be undirected (no arrows) or directed (one arrow). A directed line (i.e., edge) shows the hypothetical direction of the association between symptoms (e.g., symptom A activates symptom B). See Figure 2 for a hypothetical network of nine symptoms in slow to recover high school and college students with pre-existing anxiety problems. Symptom centrality is an important concept in the network analysis. Central symptoms are those that are most important in the network, and there are several was to measure centrality including the degree, strength, expected influence, closeness, and betweenness. It is important to appreciate that a limitation of network analysis diagrams is that they can lead to the impression that inter-related and interacting between symptoms are static, when in fact they might be temporally sequenced and dynamic.
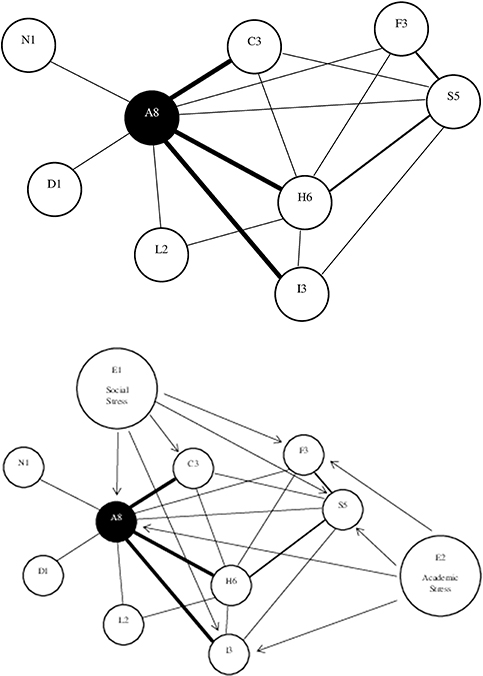
Figure 2. Hypothetical network of nine symptoms in slow to recover high school and college students with pre-existing anxiety problems. The top figure shows that anxiety (A8) has the greatest degree (eight connections to other symptoms) and strength (three heavier lines) of centrality, followed by headaches (H6) and sleep (S5). Three symptoms are connected to three other symptoms (nodes): irritability (I3), concentration problems (C3), and fatigue (F3). Light sensitivity (L2) is connected to two other symptoms, and nausea (N1) and dizziness (D1) are connected to only one other symptom. The bottom figure illustrates the role of external factors, in the external field, that are amplifying the network of symptoms, such as social stress (E1) and academic stress (E2).
Fried and colleagues reviewed the literature on network analysis in psychology and psychiatry (95). Network analysis has been used to better understand the structure of emotional and behavioral problems in children (103), the central symptoms and syndromic pathways of traumatic stress in children and adolescents (104–106), longitudinal developmental associations between symptoms of depression and anxiety (107), and the associations between internalizing and externalizing psychopathology in the transition from childhood to adolescence (107). In adults, syndromic pathways between social anxiety, perceived stress, and problematic alcohol use have been identified (108). In fact, network analysis has been used to simultaneously study 12 major psychiatric diagnoses in a sample of more than 34,000 adults, with the resulting network illustrating differential associations between symptoms within the same diagnosis and strong connections with symptoms from other diagnoses, illuminating the complexity of psychopathology and psychiatric comorbidity (100). Because depression and PTSD are so common in civilians, military service members, and veterans who have sustained MTBIs and who report long-term symptoms and problems, some recent advances in those areas, based on a network empirical and theoretical perspective, are discussed in the sections below.
Depression
Pre-injury mental health problems, such as depression and anxiety, are a risk factor for persistent symptoms following MTBI (21, 36, 109–112). Depression is common following TBIs of all severities (113–115). Depression is also common in people with chronic pain (116–119), chronic headaches (120–123), PTSD (55, 124–126), and substance abuse problems (127–131). Primary depression can mimic the post-concussion syndrome (132), and depression has a very large effect on post-concussion-like symptom reporting (48, 63, 132–134). Moreover, post-injury worry, stress, and anxiety are thought to be central features of long-term symptom reporting (17, 27, 41, 61).
Network analysis is leading to important new insights in depression (135–139). Depression can be viewed as a complex dynamic system of interacting symptoms, some of which are core syndromal symptoms of depression (e.g., sadness and anhedonia) and some of which are not (e.g., anxiety and sympathetic arousal) (137). It is well established in psychiatry that depression and anxiety are comorbid in many people (140), and cross-sectional network analysis studies have illustrated how major depressive disorder and generalized anxiety disorder are interconnected, entangled, and amplifying (141–143). Moreover, chronic pain and depression often co-occur (116–119), and new studies are examining associations between symptom networks in chronic pain and depression (144), and how self-efficacy, fear avoidance, and perceived disability might link the pain experience with affective disorder symptoms (145).
Network analysis has been used to better understand the course of illness and the probability of relapse. People in remission from a prior episode of depression are at increased risk for developing future depression, and resilience is central and important for successfully coping with stressors and maintaining good mental health (146). Moreover, transitional states from being healthy to being depressed are not well-understood. “Critical slowing” (147) is a phenomenon in depression characterized by dynamic networks of symptoms taking increasingly longer to adapt or recover to perturbations, eventually leading to a tipping point into the development of a syndrome. The concept of critical slowing is applicable to a pathway by which a person might develop persistent symptoms following an MTBI.
Post-traumatic Stress Disorder
Traumatic stress is fairly common in both civilians and military personnel who sustain MTBIs (148, 149). People with PTSD report symptoms that overlap with the post-concussion syndrome, such as irritability, cognitive problems, and sleep disturbance (55), and PTSD might have an amplifying or additive effect on symptom reporting following MTBI (150, 151). Network analysis has been used in diverse studies of PTSD (152–157). Researchers have used network analysis to examine (i) how specific combinations of symptoms might drive the development of PTSD in trauma-exposed adults (154); (ii) whether traumatic stress symptom presentations vary in association with different types of index traumas (158); (iii) the symptom connectivity and associations in combat veterans with PTSD and subthreshold PTSD (159, 160), and the interactions among traumatic stress symptoms, suicidal ideation, depression, and quality of life in veterans (153); (iv) the association between PTSD and alcohol use disorders (161); (v) the identification of central symptoms and bridging symptoms relating to the comorbidity of PTSD and major depressive disorder (162), and (vi) the comorbidity of GAD, depression, and PTSD (163). It is believed that better understanding of which symptoms of traumatic stress are more central and strongly interconnected than others may have implications for targeting clinical interventions.
Conclusions and Directions for Future Research
It has long been believed by some researchers that no central underlying disease mechanism for the post-concussion syndrome has ever been found because it does not exist. The longstanding challenge for conceptualizing the post-concussion syndrome is that the constellation of symptoms comprising the syndrome are non-specific. Therefore, it is difficult to accept that post-concussion symptoms cohere as a syndrome because they share a single latent underlying cause, such as brain damage or a mental disorder. Multiple social psychological factors, such as expectations and misattributions (47, 67–71), “good-old-days” bias (47, 73–79), perceived injustice (87), fear avoidance (81, 82), socio-environmental factors such as compensation seeking (164–166), vulnerability factors such as pre-injury mental health problems (21, 36, 109–111), and neurological factors, such as microstructural changes to white matter (167), have been shown to be associated with persistent post-concussion symptoms. However, none have emerged as a latent common cause, and most in the field accept that post-concussion symptoms are multifactorial in causation. A latent model or common cause theory for the syndrome is inconsistent with a biopsychosocial conceptualization (64–66).
A network perspective makes it possible to study the architecture of persistent symptoms and problems following MTBI, allowing the identification of symptoms that are more central and strongly interconnected. A network perspective allows us to embrace the challenges of heterogeneity, non-specificity, comorbidity, and the latent disease model that have plagued the field of mild neurotrauma. The network perspective eschews the idea that a single latent dimension is the underlying cause of both symptom emergence and coherence. Symptoms can be causally connected through diverse biopsychosocial mechanisms. Network theory is agnostic with regard to how causal relations among symptoms are exemplified. The direct causal associations between some symptoms might be predominately biological, whereas other symptoms might have associations that are more strongly psychological. Symptoms can form amplification and self-sustaining feedback loops. If the inter-relations among the symptoms are strong enough, the symptoms become entrenched, self-sustaining, and in combination they comprise and represent a syndrome. In this context, a post-concussion syndrome does not exist separately from the symptoms that constitute it. In fact, a network perspective might identify multiple syndromes, or at least clusters of prominent symptoms, that might occur in subgroups of people following MTBI.
Adopting a network perspective in clinical research might help us identify single, paired, or small clusters of strongly interconnected symptoms that could be initial targets for treatment and rehabilitation (168). In the hypothetical example set out in Figure 2, an aggressive treatment and rehabilitation strategy targeting the two most central symptoms, anxiety, and headaches, might dampen the amplifying inter-relations among multiple symptoms leading to improvement across the entire network of symptoms. In theory, and of particular relevance to sequenced care following MTBI, targeting one or two symptoms with a high degree of strength of centrality might dampen or even ameliorate other symptoms in the acute or subacute period following injury, potentially preventing entrenchment and persistence of symptoms. It might also help us better understand complex comorbidities, such as depression, anxiety, PTSD, chronic pain, peripheral vestibular problems, and substance abuse, how they are inter-related, and how they might bridge and amplify post-concussion-like symptoms. Future research using network analysis might reveal syndrome profiles and inter-relations with comorbidities that could be targets for time-sequenced precision rehabilitation—leading to more personalized health care. Precision rehabilitation requires us to better understand the interconnections among symptoms and problems so that we can produce more effective treatment and rehabilitation for them.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
The author acknowledges unrestricted philanthropic support from the Mooney-Reed Charitable Foundation, Heinz Family Foundation, ImPACT® Applications, Inc., and the Spaulding Research Institute.
Conflict of Interest Statement
The author has been reimbursed by the government, professional scientific bodies, and commercial organizations for discussing or presenting research relating to MTBI and sport-related concussion at meetings, scientific conferences, and symposiums. He has a clinical practice in forensic neuropsychology involving individuals who have sustained mild TBIs (including athletes). He has received honorariums for serving on research panels that provide scientific peer review of programs. He is a co-investigator, collaborator, or consultant on grants relating to mild TBI funded by the federal government and other organizations. He has received research support from test publishing companies in the past, including ImPACT® Applications Systems, Psychological Assessment Resources, and CNS Vital Signs. He has received grant funding from the National Football League and salary support from the Harvard Integrated Program to Protect and Improve the Health of NFLPA Members. He serves as a scientific advisor for BioDirection, Inc., SWAY Operations, LLC, and Highmark, Inc.
References
1. Sigurdardottir S, Andelic N, Roe C, Jerstad T, Schanke AK. Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: a prospective study. Brain Injury. (2009) 23:489–97. doi: 10.1080/02699050902926309
2. Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma. (2009) 66:289–96; discussion 96–7. doi: 10.1097/TA.0b013e3181961da2
3. Roe C, Sveen U, Alvsaker K, Bautz-Holter E. Post-concussion symptoms after mild traumatic brain injury: influence of demographic factors and injury severity in a 1-year cohort study. Disabil Rehabil. (2009) 31:1235–43. doi: 10.1080/09638280802532720
4. Kraus J, Hsu P, Schaffer K, Vaca F, Ayers K, Kennedy F, et al. Preinjury factors and 3-month outcomes following emergency department diagnosis of mild traumatic brain injury. J Head Trauma Rehabil. (2009) 24:344–54. doi: 10.1097/HTR.0b013e3181ae35fd
5. McCauley SR, Boake C, Pedroza C, Brown SA, Levin HS, Goodman HS, et al. Postconcussional disorder: are the DSM-IV criteria an improvement over the ICD-10? J Nerv Ment Dis. (2005) 193:540–50. doi: 10.1097/01.nmd.0000172592.05801.71
6. Ingebrigtsen T, Waterloo K, Marup-Jensen S, Attner E, Romner B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol. (1998) 245:609–12. doi: 10.1007/s004150050254
7. Dikmen S, Machamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. J Int Neuropsychol Soc. (2010) 16:401–11. doi: 10.1017/S1355617710000196
8. Theadom A, Parag V, Dowell T, McPherson K, Starkey N, Barker-Collo S, et al. Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br J Gen Pract. (2016) 66:e16–23. doi: 10.3399/bjgp16X683161
9. McMahon P, Hricik A, Yue JK, Puccio AM, Inoue T, Lingsma HF, et al. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma. (2014) 31:26–33. doi: 10.1089/neu.2013.2984
10. Cook JB. The post-concussional syndrome and factors influencing recovery after minor head injury admitted to hospital. Scand J Rehabil Med. (1972) 4:27–30.
11. Mickeviciene D, Schrader H, Obelieniene D, Surkiene D, Kunickas R, Stovner LJ, et al. A controlled prospective inception cohort study on the post-concussion syndrome outside the medicolegal context. Eur J Neurol. (2004) 11:411–9. doi: 10.1111/j.1468-1331.2004.00816.x
12. Rutherford WH, Merrett JD, McDonald JR. Symptoms at one year following concussion from minor head injuries. Injury. (1979) 10:225–30. doi: 10.1016/0020-1383(79)90015-9
13. Satz PS, Alfano MS, Light RF, Morgenstern HF, Zaucha KF, Asarnow RF, et al. Persistent Post-Concussive Syndrome: a proposed methodology and literature review to determine the effects, if any, of mild head and other bodily injury. J Clin Exp Neuropsychol. (1999) 21:620–8. doi: 10.1076/jcen.21.5.620.870
14. Mickeviciene D, Schrader H, Nestvold K, Surkiene D, Kunickas R, Stovner LJ, et al. A controlled historical cohort study on the post-concussion syndrome. Eur J Neurol. (2002) 9:581–7. doi: 10.1046/j.1468-1331.2002.00497.x
15. Lees-Haley PR, Fox DD, Courtney JC. A comparison of complaints by mild brain injury claimants and other claimants describing subjective experiences immediately following their injury. Arch Clin Neuropsychol. (2001) 16:689–95. doi: 10.1093/arclin/16.7.689
16. Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry. (2005) 18:301–17. doi: 10.1097/01.yco.0000165601.29047.ae
17. Wood RL. Understanding the ‘miserable minority': a diasthesis-stress paradigm for post-concussional syndrome. Brain Inj. (2004) 18:1135–53. doi: 10.1080/02699050410001675906
18. Ryan LM, Warden DL. Post concussion syndrome. Int Rev Psychiatry. (2003) 15:310–6. doi: 10.1080/09540260310001606692
19. Polinder S, Cnossen MC, Real RGL, Covic A, Gorbunova A, Voormolen DC, et al. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front Neurol. (2018) 9:1113. doi: 10.3389/fneur.2018.01113
20. Evans RW. Persistent post-traumatic headache, postconcussion syndrome, and whiplash injuries: the evidence for a non-traumatic basis with an historical review. Headache. (2010) 50:716–24. doi: 10.1111/j.1526-4610.2010.01645.x
21. Evered L, Ruff R, Baldo J, Isomura A. Emotional risk factors and postconcussional disorder. Assessment. (2003) 10:420–7. doi: 10.1177/1073191103259539
22. Binder LM. Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J Clin Exp Neuropsychol. (1986) 8:323–46. doi: 10.1080/01688638608401325
23. Brown SJ, Fann JR, Grant I. Postconcussional disorder: time to acknowledge a common source of neurobehavioral morbidity. J Neuropsychiatry Clin Neurosci. (1994) 6:15–22. doi: 10.1176/jnp.6.1.15
24. Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil. (1995) 10:1–17. doi: 10.1097/00001199-199510030-00002
25. Heilbronner RL. Factors associated with postconcussion syndrome: neurological, psychological, or legal? Trial Diplomacy J. (1993) 16:161–7.
26. Youngjohn JR, Burrows L, Erdal K. Brain damage or compensation neurosis? The controversial post-concussion syndrome. Clin Neuropsychol. (1995) 9:112–23. doi: 10.1080/13854049508401593
27. Lishman WA. Physiogenisis and psychogenisis in the ‘post-concussional syndrome'. Br J Psychiatry. (1988) 153:460–9. doi: 10.1192/bjp.153.4.460
28. Mittenberg W, Strauman S. Diagnosis of mild head injury and the postconcussion syndrome. J Head Trauma Rehabil. (2000) 15:783–91. doi: 10.1097/00001199-200004000-00003
29. Larrabee GJ. Neuropsychological outcome, post concussion symptoms, and forensic considerations in mild closed head trauma. Semin Clin Neuropsychiatry. (1997) 2:196–206.
30. Bijur PE, Haslum M, Golding J. Cognitive and behavioral sequelae of mild head injury in children. Pediatrics. (1990) 86:337–44.
31. Vanderploeg RD, Curtiss G, Duchnick JJ, Luis CA. Demographic, medical, and psychiatric factors in work and marital status after mild head injury. J Head Trauma Rehabil. (2003) 18:148–63. doi: 10.1097/00001199-200303000-00006
32. Wrightson P, Gronwall D. Time off work and symptoms after minor head injury. Injury. (1981) 12:445–54. doi: 10.1016/0020-1383(81)90161-3
33. Kraus J, Schaffer K, Ayers K, Stenehjem J, Shen H, Afifi AA. Physical complaints, medical service use, and social and employment changes following mild traumatic brain injury: a 6-month longitudinal study. J Head Trauma Rehabil. (2005) 20:239–56. doi: 10.1097/00001199-200505000-00007
34. Drake AI, Gray N, Yoder S, Pramuka M, Llewellyn M. Factors predicting return to work following mild traumatic brain injury: a discriminant analysis. J Head Trauma Rehabil. (2000) 15:1103–12. doi: 10.1097/00001199-200010000-00004
35. King NS, Kirwilliam S. Permanent post-concussion symptoms after mild head injury. Brain Injury. (2011) 25:462–70. doi: 10.3109/02699052.2011.558042
36. Mooney G, Speed J, Sheppard S. Factors related to recovery after mild traumatic brain injury. Brain Inj. (2005) 19:975–87. doi: 10.1080/02699050500110264
37. de Koning ME, Scheenen ME, van der Horn HJ, Hageman G, Roks G, Spikman JM, et al. Non-hospitalized patients with mild traumatic brain injury: the forgotten minority. J Neurotrauma. (2017) 34:257–61. doi: 10.1089/neu.2015.4377
38. Kristman VL, Cote P, Yang X, Hogg-Johnson S, Vidmar M, Rezai M. Health care utilization of workers' compensation claimants associated with mild traumatic brain injury: a historical population-based cohort study of workers injured in 1997–1998. Arch Phys Med Rehabil. (2014) 95:S295–302. doi: 10.1016/j.apmr.2013.08.296
39. Kirsch NL, de Leon MB, Maio RF, Millis SR, Tan-Schriner CU, Frederiksen S. Characteristics of a mild head injury subgroup with extreme, persisting distress on the Rivermead Postconcussion Symptoms questionnaire. Arch Phys Med Rehabil. (2010) 91:35–42. doi: 10.1016/j.apmr.2009.09.019
40. Voormolen DC, Polinder S, von Steinbuechel N, Vos PE, Cnossen MC, Haagsma JA. The association between post-concussion symptoms and health-related quality of life in patients with mild traumatic brain injury. Injury. (2018) doi: 10.1016/j.injury.2018.12.002. [Epub ahead of print].
41. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization (1992).
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association (1994).
43. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing (2013). doi: 10.1176/appi.books.9780890425596
44. Gouvier WD, Uddo-Crane M, Brown LM. Base rates of post-concussional symptoms. Arch Clin Neuropsychol. (1988) 3:273–8. doi: 10.1093/arclin/3.3.273
45. Machulda MM, Bergquist TF, Ito V, Chew S. Relationship between stress, coping, and post concussion symptoms in a healthy adult population. Arch Clin Neuropsychol. (1998) 13:415–24. doi: 10.1093/arclin/13.5.415
46. Iverson GL, Lange RT. Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. (2003) 10:137–44. doi: 10.1207/S15324826AN1003_02
47. Mittenberg W, DiGiulio DV, Perrin S, Bass AE. Symptoms following mild head injury: expectation as aetiology. J Neurol Neurosurg Psychiatry. (1992) 55:200–4. doi: 10.1136/jnnp.55.3.200
48. Trahan DE, Ross CE, Trahan SL. Relationships among postconcussional-type symptoms, depression, and anxiety in neurologically normal young adults and victims of brain injury. Arch Clin Neuropsychol. (2001) 16:435–45. doi: 10.1093/arclin/16.5.435
49. Sawchyn JM, Brulot MM, Strauss E. Note on the use of the postconcussion syndrome checklist. Arch Clin Neuropsychol. (2000) 15:1–8. doi: 10.1093/arclin/15.1.1
50. Wong JL, Regennitter RP, Barrios F. Base rate and simulated symptoms of mild head injury among normals. Arch Clin Neuropsychol. (1994) 9:411–25. doi: 10.1093/arclin/9.5.411
51. Iverson GL, Silverberg ND, Mannix R, Maxwell BA, Atkins JE, Zafonte R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. (2015) 169:1132–40. doi: 10.1001/jamapediatrics.2015.2374
52. Fox DD, Lees-Haley PR, Ernest K, Dolezal-Wood S. Post-concussive symptoms: base rates and etiology in psychiatric patients. Clin Neuropsychol. (1995) 9:89–92. doi: 10.1080/13854049508402064
53. Lees-Haley PR, Brown RS. Neuropsychological complaint base rates of 170 personal injury claimants. Arch Clin Neuropsychol. (1993) 8:203–9. doi: 10.1016/0887-6177(93)90036-Z
54. Dunn JT, Lees-Haley PR, Brown RS, Williams CW, English LT. Neurotoxic complaint base rates of personal injury claimants: implications for neuropsychological assessment. J Clin Psychol. (1995) doi: 10.1002/1097-4679(199507)51:4<;577::AID-JCLP2270510418>3.0.CO;2-E
55. Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychol Assessment. (1997) 9:445–51. doi: 10.1037/1040-3590.9.4.445
56. Smith-Seemiller L, Fow NR, Kant R, Franzen MD. Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Injury. (2003) 17:199–206. doi: 10.1080/0269905021000030823
57. Radanov BP, Dvorak J, Valach L. Cognitive deficits in patients after soft tissue injury of the cervical spine. Spine. (1992) 17:127–31. doi: 10.1097/00007632-199202000-00001
58. Iverson GL, McCracken LM. ‘Postconcussive' symptoms in persons with chronic pain. Brain Injury. (1997) 11:783–90. doi: 10.1080/026990597122990
59. Gasquoine PG. Postconcussional symptoms in chronic back pain. Appl Neuropsychol. (2000) 7:83–9. doi: 10.1207/S15324826AN0702_3
60. Sullivan MJ, Hall E, Bartolacci R, Sullivan ME, Adams H. Perceived cognitive deficits, emotional distress and disability following whiplash injury. Pain Res Manag. (2002) 7:120–6. doi: 10.1155/2002/502984
61. Kay T, Newman B, Cavallo M, Ezrachi O, Resnick M. Toward a neuropsychological model of functional disability after mild traumatic brain injury. Neuropsychology. (1992) 6:371–84. doi: 10.1037/0894-4105.6.4.371
62. Bay E, de-Leon MB. Chronic stress and fatigue-related quality of life after mild to moderate traumatic brain injury. J Head Trauma Rehabil. (2011) 26:355–63. doi: 10.1097/HTR.0b013e3181f20146
63. Lange RT, Iverson GL, Rose A. Depression strongly influences postconcussion symptom reporting following mild traumatic brain injury. J Head Trauma Rehabil. (2011) 26:127–37. doi: 10.1097/HTR.0b013e3181e4622a
64. Silverberg ND, Iverson GL. Etiology of the post-concussion syndrome: physiogenesis and psychogenesis revisited. Neurorehabilitation. (2011) 29:317–29. doi: 10.3233/NRE-2011-0708
65. Iverson GL. A biopsychosocial conceptualization of poor outcome from mild traumatic brain injury. In: Bryant R, Keane T, editors. PTSD and Mild Traumatic Brain Injury. New York, NY: Guilford Press (2012). p. 37–60.
66. Iverson GL, Silverberg N, Lange RT, Zasler N. Conceptualizing outcome from mild traumatic brain injury. In: Zasler ND, Katz RD, Zafonte RD, editors. Brain Injury Medicine: Principles and Practice. 2nd ed. New York, NY: Demos Medical Publishing (2012). p. 470–97.
67. Suhr JA, Gunstad J. “Diagnosis Threat”: the effect of negative expectations on cognitive performance in head injury. J Clin Exp Neuropsychol. (2002) 24:448–57. doi: 10.1076/jcen.24.4.448.1039
68. Mittenberg W, Tremont G, Zielinski RE, Fichera S, Rayls KR. Cognitive-behavioral prevention of postconcussion syndrome. Arch Clin Neuropsychol. (1996) 11:139–45. doi: 10.1093/arclin/11.2.139
69. Snell DL, Siegert RJ, Hay-Smith EJ, Surgenor LJ. Factor structure of the brief COPE in people with mild traumatic brain injury. J Head Trauma Rehabil. (2011) 26:468–77. doi: 10.1097/HTR.0b013e3181fc5e1e
70. Whittaker R, Kemp S, House A. Illness perceptions and outcome in mild head injury: a longitudinal study. J Neurol Neurosurg Psychiatry. (2007) 78:644–6. doi: 10.1136/jnnp.2006.101105
71. Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosur Psychiatry. (2012) 83:217–23. doi: 10.1136/jnnp-2011-300767
72. Anderson JFI, Fitzgerald P. Associations between coping style, illness perceptions and self-reported symptoms after mild traumatic brain injury in prospectively studied pre-morbidly healthy individuals. Neuropsychol Rehabil. (2018) doi: 10.1080/09602011.2018.1556706. [Epub ahead of print].
73. Davis CH. Self-perception in mild traumatic brain injury. Am J Phys Med Rehabil. (2002) 81:609–21. doi: 10.1097/00002060-200208000-00009
74. Gunstad J, Suhr JA. “Expectation as etiology” versus “the good old days”: postconcussion syndrome symptom reporting in athletes, headache sufferers, and depressed individuals. J Int Neuropsychol Soc. (2001) 7:323–33. doi: 10.1017/S1355617701733061
75. Gunstad J, Suhr JA. Cognitive factors in postconcussion syndrome symptom report. Arch Clin Neuropsychol. (2004) 19:391–405. doi: 10.1016/S0887-6177(03)00073-8
76. Hilsabeck RC, Gouvier WD, Bolter JF. Reconstructive memory bias in recall of neuropsychological symptomatology. J Clin Exp Neuropsychol. (1998) 20:328–38. doi: 10.1076/jcen.20.3.328.813
77. Lange RT, Iverson GL, Rose A. Post-concussion symptom reporting and the “good-old-days” bias following mild traumatic brain injury. Arch Clin Neuropsychol. (2010) 25:442–50. doi: 10.1093/arclin/acq031
78. Iverson GL, Lange RT, Brooks BL, Rennison VL. “Good old days” bias following mild traumatic brain injury. Clin Neuropsychol. (2010) 24:17–37. doi: 10.1080/13854040903190797
79. Lees-Haley PR, Williams CW, Zasler ND, Marguilies S, English LT, Stevens KB. Response bias in plaintiffs' histories. Brain Injury. (1997) 11:791–9. doi: 10.1080/026990597123007
80. Delis DC, Wetter SR. Cogniform disorder and cogniform condition: proposed diagnoses for excessive cognitive symptoms. Arch Clin Neuropsychol. (2007) 22:589–604. doi: 10.1016/j.acn.2007.04.001
81. Silverberg ND, Panenka W, Iverson GL. Fear avoidance and clinical outcomes from mild traumatic brain injury. J Neurotrauma. (2018) 35:1864–73. doi: 10.1089/neu.2018.5662
82. Wijenberg MLM, Stapert SZ, Verbunt JA, Ponsford JL, Van Heugten CM. Does the fear avoidance model explain persistent symptoms after traumatic brain injury? Brain Inj. (2017) 31:1597–604. doi: 10.1080/02699052.2017.1366551
83. Silverberg ND, Martin P, Panenka WJ. Headache trigger sensitivity and avoidance after mild traumatic brain injury. J Neurotrauma. (2019). doi: 10.1089/neu.2018.6025. [Epub ahead of print].
84. Silverberg ND, Iverson GL, Panenka W. Cogniphobia in mild traumatic brain injury. J Neurotrauma. (2017) 34:2141–6. doi: 10.1089/neu.2016.4719
85. Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. (1997) 26:607–11. doi: 10.1006/pmed.1996.0124
87. Iverson GL, Terry DP, Karr JE, Panenka WJ, Silverberg ND. Perceived injustice and its correlates after mild traumatic brain injury. J Neurotrauma. (2018) 35:1156–66. doi: 10.1089/neu.2017.5402
88. Reid MW, Cooper DB, Lu LH, Iverson GL, Kennedy JE. Adversity and resilience are associated with outcome after mild traumatic brain injury in military service members. J Neurotrauma. (2018) 35:1146–55. doi: 10.1089/neu.2017.5424
89. Losoi H, Silverberg ND, Waljas M, Turunen S, Rosti-Otajarvi E, Helminen M, et al. Resilience is associated with outcome from mild traumatic brain injury. J Neurotrauma. (2015) 32:942–9. doi: 10.1089/neu.2014.3799
90. Stulemeijer M, Vos PE, Bleijenberg G, van der Werf SP. Cognitive complaints after mild traumatic brain injury: things are not always what they seem. J Psychosom Res. (2007) 63:637–45. doi: 10.1016/j.jpsychores.2007.06.023
91. Stulemeijer M, Andriessen TM, Brauer JM, Vos PE, Van Der Werf S. Cognitive performance after mild traumatic brain injury: the impact of poor effort on test results and its relation to distress, personality and litigation. Brain Injury. (2007) 21:309–18. doi: 10.1080/02699050701209980
92. Ruff RM, Camenzuli L, Mueller J. Miserable minority: emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Injury. (1996) 10:551–65. doi: 10.1080/026990596124124
93. Ruff RM, Richardson AM. Mild traumatic brian injury. In: Sweet JJ, editor. Forensic Neuropsychology: Fundamentals and Practice Studies on Neuropsychology, Development, and Cognition. Bristol, PA: Swets & Zeitlinger (1999). p. 315–38.
94. Kenzie ES, Parks EL, Bigler ED, Lim MM, Chesnutt JC, Wakeland W. Concussion as a multi-scale complex system: an interdisciplinary synthesis of current knowledge. Front Neurol. (2017) 8:513. doi: 10.3389/fneur.2017.00513
95. Fried EI, van Borkulo CD, Cramer AO, Boschloo L, Schoevers RA, Borsboom D. Mental disorders as networks of problems: a review of recent insights. Soc Psychiatry Psychiatr Epidemiol. (2017) 52:1–10. doi: 10.1007/s00127-016-1319-z
96. Borsboom D, Rhemtulla M, Cramer AO, van der Maas HL, Scheffer M, Dolan CV. Kinds versus continua: a review of psychometric approaches to uncover the structure of psychiatric constructs. Psychol Med. (2016) 46:1567–79. doi: 10.1017/S0033291715001944
97. Bringmann LF, Vissers N, Wichers M, Geschwind N, Kuppens P, Peeters F, et al. A network approach to psychopathology: new insights into clinical longitudinal data. PLoS ONE. (2013) 8:e60188. doi: 10.1371/journal.pone.0060188
98. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. (2013) 9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608
99. Borsboom D. A network theory of mental disorders. World Psychiatry. (2017) 16:5–13. doi: 10.1002/wps.20375
100. Boschloo L, van Borkulo CD, Rhemtulla M, Keyes KM, Borsboom D, Schoevers RA. The network structure of symptoms of the diagnostic and statistical manual of mental disorders. PLoS ONE. (2015) 10:e0137621. doi: 10.1371/journal.pone.0137621
101. Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychol Methods. (2018) 23:617–34. doi: 10.1037/met0000167
102. Dalege J, Borsboom D, van Harreveld F, van der Maas HLJ. Network analysis on attitudes: a brief tutorial. Soc Psychol Personal Sci. (2017) 8:528–37. doi: 10.1177/1948550617709827
103. Boschloo L, Schoevers RA, van Borkulo CD, Borsboom D, Oldehinkel AJ. The network structure of psychopathology in a community sample of preadolescents. J Abnorm Psychol. (2016) 125:599–606. doi: 10.1037/abn0000150
104. Cao X, Wang L, Cao C, Fang R, Chen C, Hall BJ, et al. Sex differences in global and local connectivity of adolescent posttraumatic stress disorder symptoms. J Child Psychol Psychiatry. (2019) 60:216–24. doi: 10.1111/jcpp.12963
105. Bartels L, Berliner L, Holt T, Jensen T, Jungbluth N, Plener P, et al. The importance of the DSM-5 posttraumatic stress disorder symptoms of cognitions and mood in traumatized children and adolescents: two network approaches. J Child Psychol Psychiatry. (2019) 60:545–54. doi: 10.1111/jcpp.13009
106. Russell JD, Neill EL, Carrion VG, Weems CF. The network structure of posttraumatic stress symptoms in children and adolescents exposed to disasters. J Am Acad Child Adolesc Psychiatry. (2017) 56:669–77.e5. doi: 10.1016/j.jaac.2017.05.021
107. McElroy E, Fearon P, Belsky J, Fonagy P, Patalay P. Networks of depression and anxiety symptoms across development. J Am Acad Child Adolesc Psychiatry. (2018) 57:964–73. doi: 10.1016/j.jaac.2018.05.027
108. Anker JJ, Kummerfeld E, Rix A, Burwell SJ, Kushner MG. Causal network modeling of the determinants of drinking behavior in comorbid alcohol use and anxiety disorder. Alcohol Clin Exp Res. (2019) 43:91–7. doi: 10.1111/acer.13914
109. Greiffenstein FM, Baker JW. Comparison of premorbid and postinjury mmpi-2 profiles in late postconcussion claimants. Clin Neuropsychol. (2001) 15:162–70. doi: 10.1076/clin.15.2.162.1895
110. Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology. (2011) 25:454–65. doi: 10.1037/a0022580
111. Ponsford J, Nguyen S, Downing M, Bosch M, McKenzie JE, Turner S, et al. Factors associated with persistent post-concussion symptoms following mild traumatic brain injury in adults. J Rehabil Med. (2019) 51:32–9. doi: 10.2340/16501977-2492
112. Legarreta AD, Brett BL, Solomon GS, Zuckerman SL. The role of family and personal psychiatric history in postconcussion syndrome following sport-related concussion: a story of compounding risk. J Neurosurg Pediatr. (2018) 22:238–43. doi: 10.3171/2018.3.PEDS1850
113. Kreutzer JS, Seel RT, Gourley E. The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Injury. (2001) 15:563–76. doi: 10.1080/02699050010009108
114. Seel RT, Kreutzer JS, Rosenthal M, Hammond FM, Corrigan JD, Black K. Depression after traumatic brain injury: a National Institute on Disability and Rehabilitation Research Model Systems multicenter investigation. Archiv Phys Med Rehabilit. (2003) 84:177–84. doi: 10.1053/apmr.2003.50106
115. Singh R, Mason S, Lecky F, Dawson J. Prevalence of depression after TBI in a prospective cohort: the SHEFBIT study. Brain Inj. (2018) 32:84–90. doi: 10.1080/02699052.2017.1376756
116. Atkinson JH, Slater MA, Patterson TL, Grant I, Garfin SR. Prevalence, onset, and risk of psychiatric disorders in men with chronic low back pain: a controlled study. Pain. (1991) 45:111–21. doi: 10.1016/0304-3959(91)90175-W
117. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. (2003) 54:399–409. doi: 10.1016/S0006-3223(03)00545-6
118. Wilson KG, Eriksson MY, D'Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain. (2002) 18:77–83. doi: 10.1097/00002508-200203000-00002
119. Ericsson M, Poston WS, Linder J, Taylor JE, Haddock CK, Foreyt JP. Depression predicts disability in long-term chronic pain patients. Disability Rehabilit. (2002) 24:334–40. doi: 10.1080/09638280110096241
120. Hung CI, Liu CY, Fuh JL, Juang YY, Wang SJ. Comorbid migraine is associated with a negative impact on quality of life in patients with major depression. Cephalalgia. (2006) 26:26–32. doi: 10.1111/j.1468-2982.2005.00985.x
121. Breslau N, Schultz LR, Stewart WF, Lipton RB, Lucia VC, Welch KM. Headache and major depression: is the association specific to migraine? Neurology. (2000) 54:308–13. doi: 10.1212/WNL.54.2.308
122. Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KM. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology. (2003) 60:1308–12. doi: 10.1212/01.WNL.0000058907.41080.54
123. Sheftell FD, Atlas SJ. Migraine and psychiatric comorbidity: from theory and hypotheses to clinical application. Headache. (2002) 42:934–44. doi: 10.1046/j.1526-4610.2002.02217.x
124. Franklin CL, Zimmerman M. Posttraumatic stress disorder and major depressive disorder: investigating the role of overlapping symptoms in diagnostic comorbidity. J Nerv Ment Dis. (2001) 189:548–51. doi: 10.1097/00005053-200108000-00008
125. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. (1995) 52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012
126. Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, et al. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. (1998) 155:630–7. doi: 10.1176/ajp.155.5.630
127. Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. (2004) 291:1887–96. doi: 10.1001/jama.291.15.1887
128. Grothues J, Bischof G, Reinhardt S, Hapke U, Meyer C, John U, et al. Intention to change drinking behaviour in general practice patients with problematic drinking and comorbid depression or anxiety. Alcohol Alcohol. (2005) 40:394–400. doi: 10.1093/alcalc/agh182
129. Frisher M, Crome I, Macleod J, Millson D, Croft P. Substance misuse and psychiatric illness: prospective observational study using the general practice research database. J Epidemiol Community Health. (2005) 59:847–50. doi: 10.1136/jech.2004.030833
130. Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. (2004) 61:807–16. doi: 10.1001/archpsyc.61.8.807
131. Brady KT, Verduin ML. Pharmacotherapy of comorbid mood, anxiety, and substance use disorders. Subst Use Misuse. (2005) 40:2021–41, 43–8. doi: 10.1080/10826080500294924
132. Iverson GL. Misdiagnosis of the persistent postconcussion syndrome in patients with depression. Arch Clin Neuropsychol. (2006) 21:303–10. doi: 10.1016/j.acn.2005.12.008
133. Garden N, Sullivan KA. An examination of the base rates of post-concussion symptoms: the influence of demographics and depression. Appl Neuropsychol. (2010) 17:1–7. doi: 10.1080/09084280903297495
134. Suhr JA, Gunstad J. Postconcussive symptom report: the relative influence of head injury and depression. J Clin Exp Neuropsychol. (2002) 24:981–93. doi: 10.1076/jcen.24.8.981.8372
135. van Loo HM, Van Borkulo CD, Peterson RE, Fried EI, Aggen SH, Borsboom D, et al. Robust symptom networks in recurrent major depression across different levels of genetic and environmental risk. J Affect Disord. (2018) 227:313–22. doi: 10.1016/j.jad.2017.10.038
136. Cramer AO, van Borkulo CD, Giltay EJ, van der Maas HL, Kendler KS, Scheffer M, et al. Major depression as a complex dynamic system. PLoS ONE. (2016) 11:e0167490. doi: 10.1371/journal.pone.0167490
137. Fried EI, Epskamp S, Nesse RM, Tuerlinckx F, Borsboom D. What are ‘good' depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. J Affect Disord. (2016) 189:314–20. doi: 10.1016/j.jad.2015.09.005
138. van Borkulo C, Boschloo L, Borsboom D, Penninx BW, Waldorp LJ, Schoevers RA. Association of symptom network structure with the course of [corrected] depression. JAMA Psychiatry. (2015) 72:1219–26. doi: 10.1001/jamapsychiatry.2015.2079
139. Cramer AO, Borsboom D, Aggen SH, Kendler KS. The pathoplasticity of dysphoric episodes: differential impact of stressful life events on the pattern of depressive symptom inter-correlations. Psychol Med. (2012) 42:957–65. doi: 10.1017/S003329171100211X
140. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. (2003) 289:3095–105. doi: 10.1001/jama.289.23.3095
141. Cramer AO, Waldorp LJ, van der Maas HL, Borsboom D. Comorbidity: a network perspective. Behav Brain Sci. (2010) 33:137–50; discussion: 50–93. doi: 10.1017/S0140525X09991567
142. Beard C, Millner AJ, Forgeard MJ, Fried EI, Hsu KJ, Treadway MT, et al. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol Med. (2016) 46:3359–69. doi: 10.1017/S0033291716002300
143. Curtiss J, Klemanski DH. Taxonicity and network structure of generalized anxiety disorder and major depressive disorder: an admixture analysis and complex network analysis. J Affect Disord. (2016) 199:99–105. doi: 10.1016/j.jad.2016.04.007
144. McWilliams LA, Sarty G, Kowal J, Wilson KG. A network analysis of depressive symptoms in individuals seeking treatment for chronic pain. Clin J Pain. (2017) 33:899–904. doi: 10.1097/AJP.0000000000000477
145. Thompson EL, Broadbent J, Fuller-Tyszkiewicz M, Bertino MD, Staiger PK. A network analysis of the links between chronic pain symptoms and affective disorder symptoms. Int J Behav Med. (2018) 26:59–68. doi: 10.1007/s12529-018-9754-8
146. Hoorelbeke K, Marchetti I, De Schryver M, Koster EH. The interplay between cognitive risk and resilience factors in remitted depression: a network analysis. J Affect Disord. (2016) 195:96–104. doi: 10.1016/j.jad.2016.02.001
147. van de Leemput IA, Wichers M, Cramer AO, Borsboom D, Tuerlinckx F, Kuppens P, et al. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci USA. (2014) 111:87–92. doi: 10.1073/pnas.1312114110
148. Carlson KF, Kehle SM, Meis LA, Greer N, Macdonald R, Rutks I, et al. Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J Head Trauma Rehabilit. (2011) 26:103–15. doi: 10.1097/HTR.0b013e3181e50ef1
149. Hoffman JM, Dikmen S, Temkin N, Bell KR. Development of posttraumatic stress disorder after mild traumatic brain injury. Arch Phys Med Rehabilit. (2012) 93:287–92. doi: 10.1016/j.apmr.2011.08.041
150. Brenner LA, Ivins BJ, Schwab K, Warden D, Nelson LA, Jaffee M, et al. Traumatic brain injury, posttraumatic stress disorder, and postconcussive symptom reporting among troops returning from iraq. J Head Trauma Rehabilit. (2010) 25:307–12. doi: 10.1097/HTR.0b013e3181cada03
151. Jamora CW, Young A, Ruff RM. Comparison of subjective cognitive complaints with neuropsychological tests in individuals with mild vs more severe traumatic brain injuries. Brain Injury. (2012) 26:36–47. doi: 10.3109/02699052.2011.635352
152. Bryant RA, Creamer M, O'Donnell M, Forbes D, McFarlane AC, Silove D, et al. Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: a network analysis. JAMA Psychiatry. (2017) 74:135–42. doi: 10.1001/jamapsychiatry.2016.3470
153. Armour C, Fried EI, Deserno MK, Tsai J, Pietrzak RH. A network analysis of DSM-5 posttraumatic stress disorder symptoms and correlates in U.S. military veterans. J Anxiety Disord. (2017) 45:49–59. doi: 10.1016/j.janxdis.2016.11.008
154. Greene T, Gelkopf M, Epskamp S, Fried E. Dynamic networks of PTSD symptoms during conflict. Psychol Med. (2018) 48:2409–17. doi: 10.1017/S0033291718000351
155. Sullivan CP, Smith AJ, Lewis M, Jones RT. Network analysis of PTSD symptoms following mass violence. Psychol Trauma. (2018) 10:58–66. doi: 10.1037/tra0000237
156. Gluck TM, Knefel M, Lueger-Schuster B. A network analysis of anger, shame, proposed ICD-11 post-traumatic stress disorder, and different types of childhood trauma in foster care settings in a sample of adult survivors. Eur J Psychotraumatol. (2017) 8:1372543. doi: 10.1080/20008198.2017.1372543
157. Knefel M, Karatzias T, Ben-Ezra M, Cloitre M, Lueger-Schuster B, Maercker A. The replicability of ICD-11 complex post-traumatic stress disorder symptom networks in adults. Br J Psychiatry. (2019) doi: 10.1192/bjp.2018.286. [Epub ahead of print].
158. Benfer N, Bardeen JR, Cero I, Kramer LB, Whiteman SE, Rogers TA, et al. Network models of posttraumatic stress symptoms across trauma types. J Anxiety Disord. (2018) 58:70–7. doi: 10.1016/j.janxdis.2018.07.004
159. Phillips RD, Wilson SM, Sun D, Workgroup VAM-AM, Morey R. Posttraumatic stress disorder symptom network analysis in U.S. Military Veterans: examining the impact of combat exposure. Front Psychiatry. (2018) 9:608. doi: 10.3389/fpsyt.2018.00608
160. Ross J, Murphy D, Armour C. A network analysis of DSM-5 posttraumatic stress disorder and functional impairment in UK treatment-seeking veterans. J Anxiety Disord. (2018) 57:7–15. doi: 10.1016/j.janxdis.2018.05.007
161. Afzali MH, Sunderland M, Batterham PJ, Carragher N, Calear A, Slade T. Network approach to the symptom-level association between alcohol use disorder and posttraumatic stress disorder. Soc Psychiatry Psychiatr Epidemiol. (2017) 52:329–39. doi: 10.1007/s00127-016-1331-3
162. Afzali MH, Sunderland M, Teesson M, Carragher N, Mills K, Slade T. A network approach to the comorbidity between posttraumatic stress disorder and major depressive disorder: the role of overlapping symptoms. J Affect Disord. (2017) 208:490–6. doi: 10.1016/j.jad.2016.10.037
163. Price M, Legrand AC, Brier ZMF, Hebert-Dufresne L. The symptoms at the center: examining the comorbidity of posttraumatic stress disorder, generalized anxiety disorder, and depression with network analysis. J Psychiatr Res. (2019) 109:52–8. doi: 10.1016/j.jpsychires.2018.11.016
164. Hanks RA, Rapport LJ, Seagly K, Millis SR, Scott C, Pearson C. Outcomes after concussion recovery education: effects of litigation and disability status on maintenance of symptoms. J Neurotrauma. (2019) 36:554–8. doi: 10.1089/neu.2018.5873
165. Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg RD. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc. (2005) 11:215–27. doi: 10.1017/S1355617705050277
166. Binder LM, Rohling ML. Money matters: a meta-analytic review of the effects of financial incentives on recovery after closed-head injury. Am J Psychiatry. (1996) 153:7–10. doi: 10.1097/00001199-199608000-00012
167. Khong E, Odenwald N, Hashim E, Cusimano MD. Diffusion tensor imaging findings in post-concussion syndrome patients after mild traumatic brain injury: a systematic review. Front Neurol. (2016) 7:156. doi: 10.3389/fneur.2016.00156
168. Collins MW, Kontos AP, Okonkwo DO, Almquist J, Bailes J, Barisa M, et al. Statements of agreement from the targeted evaluation and active management (TEAM) approaches to treating concussion meeting held in Pittsburgh, October 15–16, 2015. Neurosurgery. (2016) 79:912–29. doi: 10.1227/NEU.0000000000001447
Keywords: concussion, traumatic brain injury, rehabilitation, post-concussional syndrome, depression
Citation: Iverson GL (2019) Network Analysis and Precision Rehabilitation for the Post-concussion Syndrome. Front. Neurol. 10:489. doi: 10.3389/fneur.2019.00489
Received: 16 February 2019; Accepted: 23 April 2019;
Published: 29 May 2019.
Edited by:
Karen M. Barlow, University of Queensland, AustraliaReviewed by:
Brad G. Kurowski, Cincinnati Children's Hospital Medical Center, United StatesAlessandro Giustini, Consultant, Arezzo, Italy
Copyright © 2019 Iverson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grant L. Iverson, Z2l2ZXJzb25AbWdoLmhhcnZhcmQuZWR1
 Grant L. Iverson
Grant L. Iverson