
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 09 April 2019
Sec. Stroke
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.00336
 Yuze Cao1
Yuze Cao1 Mengyu Zhang1
Mengyu Zhang1 Lixin Zhou1
Lixin Zhou1 Ming Yao1
Ming Yao1 Bin Peng1
Bin Peng1 Yicheng Zhu1
Yicheng Zhu1 Jun Ni1*
Jun Ni1* Liying Cui1,2* on behalf of the SMART investigators
Liying Cui1,2* on behalf of the SMART investigatorsObjective: Lipohyalinosis or atherosclerosis might be responsible for single subcortical infarctions (SSIs); however, ways of differentiating between the two clinically remain uncertain. We aimed to investigate whether consecutive slides on axial view or transversal diameter is more effective to differentiate mechanisms by comparing their relationships with white matter hyperintensities (WMHs).
Methods: All the participants from the Standard Medical Management in Secondary Prevention of Ischemic stroke in China (SMART) cohort who had SSIs in the lenticulostriate artery territory were included and categorized according to consecutive slides on axial view (≥4 consecutive slices or not) and transversal diameter (≥15 mm or not). The associations between the severity of WMHs and the different categories were analyzed.
Results: Among the 3,821 patients of the SMART study, 281 had diffusion-weighted image-proven SSIs in the lenticulostriate artery territory. When classified by consecutive slides on axial view, SSIs on ≥4 slices were significantly associated with the severity of the WMHs, both in deep WMH (DWMH) (odds ratio [OR], 0.32; 95% confidence interval [CI], 0.11–0.97; p = 0.04) and periventricular hyperintensity (PVH) (OR, 0.37; 95% CI, 0.17–0.78; p = 0.01). No such association was found on the basis of the transversal diameter (p > 0.1).
Conclusion: Consecutive slides on axial view (≥4 consecutive slices) might be more effective than transversal diameter to identify the atherosclerotic mechanisms of SSIs in the lenticulostriate artery territory.
Clinical Trial Registration: http://www.clinicaltrials.gov. Unique identifier: NCT00664846
Single subcortical infarctions (SSIs) have been considered to be caused by lipohyalinosis degeneration in small artery disease, traditionally called lacunar infarct (1). However, atherosclerosis occurring in the parental artery blocking the orifice of the branch artery or atherosclerosis in the proximal branch artery can also contribute to the etiology of SSIs, which are termed as “branch atheromatous disease” (2, 3). Both the “lipohyalinotic” and “atheromatous” mechanisms of SSIs have been pathologically proved (4, 5), but the two are difficult to distinguish in vivo. Although computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) are used to detect atherosclerosis frequently, they cannot detect diffuse atheromatous wall involvement without focal stenosis. Current direct and conventional imaging techniques, including high-resolution magnetic resonance imaging (HRMRI) cannot identify the wall features of the branch artery effectively (6), which limits further research on the etiology of SSIs.
Clinically, identification of different etiologies is of great importance in guiding treatment and predicting functional prognosis (3, 7). For SSIs in the pontine, unilateral lesions extending to the ventral surface were considered an atherosclerotic mechanism. However, for SSIs in the lenticulostriate artery territory, methods to differentiate mechanisms are still controversial. One method is based on the diameter of the ischemic lesion, and another is based on the consecutive slides on axial view. Whether the consecutive slides on axial view or transversal diameter is more effective remains controversial. As white matter hyperintensities (WMHs) have been widely considered as the most frequently used imaging marker of cerebral small vessel disease, identifying the different etiological subtypes of SSIs by comparing their discrepancies in WMHs might be helpful. Given the anatomical features of the lenticulostriate artery, we hypothesized that consecutive slides on axial view would be more effective than transversal diameter to predict the mechanism of SSIs. To further clarify this issue, we examined the association between WMHs and the lesion characteristics of SSIs in a prospective multicenter cohort study, known as the Standard Medical Management in Secondary Prevention of Ischemic stroke in China (SMART) study (8).
Data were obtained from the SMART study, a large, multicenter, randomized controlled trial to assess the effectiveness of a guideline-based program in secondary stroke prevention in 47 hospitals in China. The detailed study protocol and main results of the SMART study have been published elsewhere (8, 9). Among the 3,821 participants enrolled in the SMART study between April 2008 and December 2010, 1,129 were proven to have acute ischemic stroke by using diffusion-weighted imaging (DWI). Of the patients, 281 with single subcortical infarctions located in the lenticulostriate artery territory were included. Patients with a probable etiology of cardioembolism or lack of complete images for review were excluded.
Demographic features and risk factors were reviewed from data collected from the database, including age, sex, hypertension, diabetes mellitus, hyperlipidemia, and smoking habit.
The SMART study was approved by the central ethics committee of the leading study center at Peking Union Medical College Hospital and the ethics committees of all participating institutions. Written informed consent was obtained from all the participants or their legal surrogates.
WMHs are hyperintense on T2-weighted sequences, isointense or hypointense on T1-weighted sequences, and isointense on diffusion-weighted images (DWI) (10). The burden and severity of WMHs were assessed on the basis of the Fazekas visual rating scale (11). Periventricular hyperintensity (PVH) and deep WMH (DWMH) were scored separately. DWMH was rated as follows: 0 = absence, 1 = punctate foci, 2 = beginning confluence of foci, and 3 = large confluent areas. PVH was rated as follows: 0 = absence, 1 = caps or pencil-thin lining, 2 = smooth halo, 3 = irregular PVH extending into the deep white matter. Scores of 2 and 3 were defined as severe WMHs. All WMHs were independently graded by 2 well-trained neurologists. The inter-rater reliability was assessed using a subset of 50 random study subjects. Kappa value for the inter-rater agreement was 0.85 for PVH and 0.89 for DWMH.
In this study, transversal and longitudinal features of single subcortical infarctions pertaining to the lenticulostriate artery terminations (single ischemic lesions located in the lentiform nucleus and/or corona radiate) were analyzed. In previous published studies, two-dimensional cutoffs of lesion features were widely used to differentiate the atheromatous mechanism of SSIs from the traditional lacune (3, 12, 13). Transversally, the lesion ≥15 mm in diameter on the axial picture, or longitudinally, the lesion observed in ≥4 axial pictures (Figure 1) were suggestive of atheromatous mechanisms. In our study, MRI scans were performed using 1.5 or 3.0 T MR imaging units. Slice thickness ranged from 5 to 6 mm in different centers. We defined the following findings on DWI to determine lesion features. “Transversal diameter” was measured as the maximal diameter of the lesion on DWI. “Consecutive slides on axial view” was defined as the number of lesion axial slices visible on DWI to evaluate the longitudinal extension of the lesion. All the SSI lesions were classified on the basis of the two criteria.
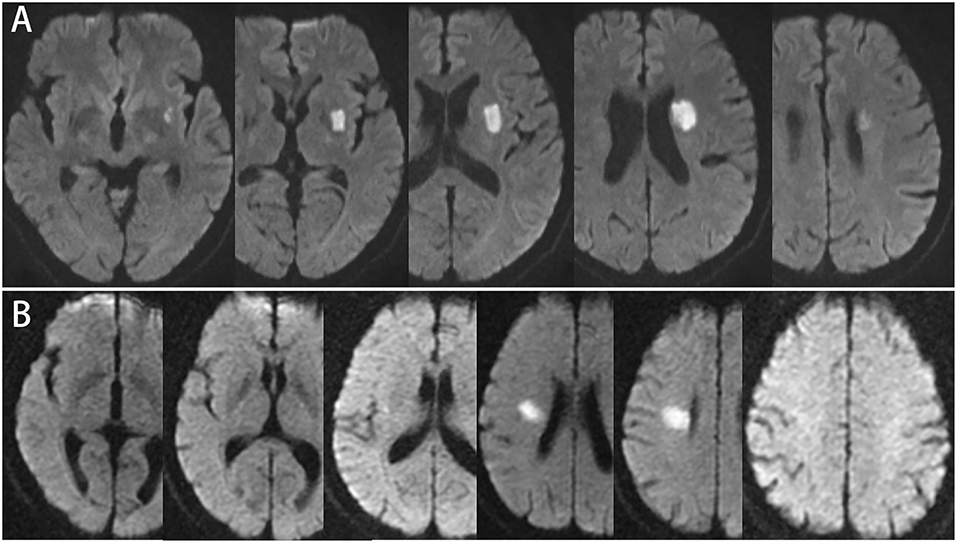
Figure 1. (A) 57-year-old male, consecutive slices DWI imaging: infarct lesion ≥4 slices, involving the base of basal ganglia, but the maximum axial diameter is 21.7 mm. (B) 52-year-old male, consecutive slices DWI imaging: infarction lesion ≤3 slices, involving radiation corona, and the maximum diameter of axis is 21.2 mm.
In the description of general clinical characteristics, continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range). The categorized variables were reported as percentage. To examine the association between the severity of WMHs and SSI features, binary logistic regression models, with adjustment for age, sex, and hypertension, were computed with dichotomized SSI lesion characteristics, using <4 consecutive slides on axial view or a transversal diameter of <15 mm as the reference category. P-values of <0.05 for the two-tailed test were considered significant. Statistical analyses were performed using IBM SPSS statistics version 19 (SPSS Inc., Chicago, IL, USA).
Of the 3,821 participants of the SMART study, 281 (7.35%) with DWI-proven SSIs were enrolled in the study. The general characteristics and risk factors of the patients in the two groups are summarized in Table 1. Seventy-four patients (26.33%) with SSIs had ≥4 consecutive slices, and 137 patients (53.94%) had a diameter of ≥15 mm.
When classified according to consecutive slides on axial view, SSIs of ≥4 slices were significantly negatively associated with both DWMH (odds ratio [OR], 0.32; 95% confidence interval [CI], 0.11–0.97; p = 0.04) and PVH (OR, 0.37; 95% CI, 0.17–0.78; p = 0.01) after adjustment for age, sex, and hypertension. By contrast, transversal SSI lesion feature, based on a transversal diameter of ≥15 mm, was not found to be statistically related to either DWMH or PVH (as shown in Table 2).
The present results obtained in a multicenter cohort with SSIs in the lenticulostriate artery territory showed that SSI lesions of ≥4 consecutive slices negatively correlated with the severity of WMHs, but no such relationship was found in SSI lesions with diameters of ≥15 mm as compared with those with diameters of < 15 mm. Our findings strongly suggest that consecutive slides on axial view might be more effective than transversal diameter for identifying the etiologies of SSIs in the lenticulostriate artery territory.
The etiologies and mechanisms of SSIs have been a research focus in recent years. The mechanisms could be classified as atherosclerotic and lipohyalinotic (6), and the former could be further categorized into three groups as follows: plaque of the parental artery obliterating the orifice of the branch artery, plaque from the parental artery extending into the branch artery, and microatheroma blocking the proximal portion of the branch artery (2). Differentiation of the atherosclerotic and lipohyalinotic mechanisms of SSIs is highly important because of their different prognoses and treatment strategies (3, 7, 12). This would be helpful to guide the treatment with dual or single antiplatelet therapies in the acute phase and secondary prevention. SSIs with atherosclerotic causes are more prone to early neurological deterioration (END) (7), and for these patients, dual antiplatelets and intensive statin therapy might be necessary.
As described previously, the conventional imaging technique cannot be used in the direct diagnosis of the etiology of SSIs, especially those in the proximal portion of the branch artery (6). HRMRI was found to be useful in detecting atherosclerotic plaques in medium-to-large vessels (14, 15). However, all the above-mentioned methods cannot visualize atherosclerosis in the proximal branch artery. Therefore, the mechanisms of SSIs cannot be identified effectively using direct and conventional imaging techniques, which limits clinical decision.
Previous studies of subtentorial SSIs mainly focused on pontine infarction and supratentorial SSIs on lenticulostriate artery territory (3). As for pontine infarctions, unilateral lesions extending to the ventral pontine surface were usually considered as atherosclerotic mechanisms (16–18). However, as for SSIs in the lenticulostriate artery territory, approaches to differentiate mechanisms still remain controversial. Previous studies suggested that the diameter of the SSI lesion could indicate the underlying mechanisms, as SSIs with atheromatous mechanisms are expected to be larger than lacunar infarcts (7, 12). However, others believe that consecutive slices on a transversal plane might differentiate the mechanism, as atheromatous lesions are located proximally along the branch artery (13, 19). The more appropriate method must be further clarified.
In the present study, SSIs with ≥4 consecutive slices, rather than a diameter of ≥15 mm, were found to negatively correlate with WMHs. As WMH is widely considered to be a strong imaging marker of small vessel disease (SVD), SSIs caused by atherosclerotic disease might have less severe WMHs than those caused by SVD. Thus, our findings suggest that classifying SSIs according to ≥4 consecutive slices might be more effective for identifying the atherosclerotic mechanisms of SSI. This could be explained by the anatomy of the branch artery. Findings from a recent 7-T MRI research showed that SSIs caused by lenticulostriate artery occlusion were more likely to extend from the corona radiata to the lower part of the basal ganglia along the longitudinal direction, which was compatible to the distribution of the lenticulostriate artery (20). More than 4 consecutive slices implies that the lesion involves the base of the basal ganglia and is closer to the orifice of the lenticulostriate branch artery. While atherosclerotic vascular lesions are located proximally along the branch artery in comparison with lipohyalinosis, the etiology of SSIs of ≥4 consecutive slices are more likely to be atherosclerotic at the orifice of the branch artery or its parent artery. Furthermore, considering the possible deviation of the diameter measurement, consecutive slides on axial view are easy to evaluate clinically.
The methodological strengths of our study include its prospective multicenter design and the large sample size of the SMART cohort, even though the subgroup analysis was retrospective. In addition, confirmation of different mechanisms requires autopsy, which is difficult to attain. In this study, we provided a new perspective by using WMHs to compare the effectiveness of different lesion features. However, our study has several limitations. First, the variability of the MRI parameter, including the field strength and thickness of the considered slice, among the 47 participating hospitals in the SMART study may have a potential influence on the description of ischemic lesions. However, the MRI slice thickness in most hospitals was 6 mm, which minimizes the difference among the centers. Second, the mechanism was not confirmed, as our findings provided only circumstantial rather than direct evidence for differentiating the etiologies and mechanisms of SSIs. Recently, 7-T MRI has been shown to be sensitive for identifying small branches (20), further research studies are needed to confirm this.
In summary, the present study suggests that consecutive slides on axial view (≥4 consecutive slices) might be more effective than transversal diameter for identifying the mechanisms of SSIs in the lenticulostriate artery territory. To establish the association between the lesion features and mechanisms of SSI, future investigation with a 7-T MRI device might be necessary.
YC and JN contributed to the writing of the article. MZ, MY, and YZ contributed to the collection of data. LZ and JN contributed to the rating of images. LC and BP contributed to revising the article. All authors read and approved the manuscript.
This study was supported by the National Key Research and Development Program of China (No.2016YFC1300500-5), National Natural Science Foundation of China (No.81801190), the key projects in the National Science and Technology Pillar Program in the Eleventh Five-year Plan Period (No.2006BAI01A10) and the Twelve Five-plan Periods (No.2011BAI08B03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Standard Medical Management in Secondary Prevention of Ischemic Stroke in China (SMART) investigators and centers for their participation in this trial. We thank all of the patients who participated in SMART study.
1. Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology. (1965) 15:774–84. doi: 10.1212/WNL.15.8.774
2. Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. (1989) 39:1246–50. doi: 10.1212/WNL.39.9.1246
3. Petrone L, Nannoni S, Del Bene A, Palumbo V, Inzitari D. Branch atheromatous disease: a clinically meaningful, yet unproven concept. Cerebrovasc Dis. (2016) 41:87–95. doi: 10.1159/000442577
4. Fisher CM, Caplan LR. Basilar artery branch occlusion: a cause of pontine infarction. Neurology. (1971) 21:900–5. doi: 10.1212/WNL.21.9.900
5. Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. (1979) 36:65–73. doi: 10.1001/archneur.1979.00500380035003
6. Kim JS, Yoon Y. Single subcortical infarction associated with parental arterial disease: important yet neglected sub-type of atherothrombotic stroke. Int J Stroke. (2013) 8:197–203. doi: 10.1111/j.1747-4949.2012.00816.x
7. Suto Y, Nakayasu H, Maeda M, Kusumi M, Kowa H, Awaki E, et al. Long-term prognosis of patients with large subcortical infarctions. Eur Neurol. (2009) 62:304–10. doi: 10.1159/000235943
8. Peng B, Zhu Y, Cui L, Ni J, Xu W, Zhou L, et al. Standard medical management in secondary prevention of ischemic stroke in China (SMART). Int J Stroke. (2011) 6:461–5. doi: 10.1111/j.1747-4949.2011.00648.x
9. Peng B, Ni J, Anderson CS, Zhu Y, Wang Y, Pu C, et al. Implementation of a structured guideline-based program for the secondary prevention of ischemic stroke in China. Stroke. (2014) 45:515–9. doi: 10.1161/STROKEAHA.113.001424
10. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
11. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
12. Kwan MW, Mak W, Cheung RT, Ho SL. Ischemic stroke related to intracranial branch atheromatous disease and comparison with large and small artery diseases. J Neurol Sci. (2011) 303:80–4. doi: 10.1016/j.jns.2011.01.008
13. Yoon Y, Lee DH, Kang DW, Kwon SU, Suh DC, Bang OY, et al. Stroke recurrence patterns are predicted by the subtypes and mechanisms of the past, non-cardiogenic stroke. Eur J Neurol. (2013) 20:928–34. doi: 10.1111/ene.12101
14. Chung JW, Kim BJ, Sohn CH, Yoon BW, Lee SH. Branch atheromatous plaque: a major cause of lacunar infarction (high-resolution MRI study). Cerebrovasc Dis Extra. (2012) 2:36–44. doi: 10.1159/000341399
15. Miyaji Y, Kawabata Y, Joki H, Seki S, Mori K, Kamide T, et al. High-resolution magnetic resonance imaging findings of basilar artery plaque in a patient with branch atheromatous disease: a case report. J Med Case Rep. (2014) 8:395. doi: 10.1186/1752-1947-8-395
16. Men X, Wu A, Zhang B, Li H, Zhang L, Chen S, et al. Leukoaraiosis and NIHSS score help to differentiate subtypes of intracranial branch atheromatous disease in Southern Han Chinese patients with stroke. Neurol Sci. (2013) 34:1727–33. doi: 10.1007/s10072-013-1322-z
17. Nakase T, Yoshioka S, Sasaki M, Suzuki A. Clinical evaluation of lacunar infarction and branch atheromatous disease. J Stroke Cerebrovasc Dis. (2013) 22:406–12. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.005
18. Zhou L, Yao M, Peng B, Zhu Y, Ni J, Cui L. Atherosclerosis might be responsible for branch artery disease: evidence from white matter hyperintensity burden in acute isolated pontine infarction. Front Neurol. (2018) 9:840. doi: 10.3389/fneur.2018.00840
19. Deguchi I, Hayashi T, Kato Y, Nagoya H, Ohe Y, Fukuoka T, et al. Treatment outcomes of tissue plasminogen activator infusion for branch atheromatous disease. J Stroke Cerebrovasc Dis. (2013) 22:e168–72. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.012
Keywords: single subcortical infarction, lenticulostriate artery territory, etiological categorization, white matter hyperintensity, SMART study
Citation: Cao Y, Zhang M, Zhou L, Yao M, Peng B, Zhu Y, Ni J and Cui L (2019) Consecutive Slides on Axial View Is More Effective Than Transversal Diameter to Differentiate Mechanisms of Single Subcortical Infarctions in the Lenticulostriate Artery Territory. Front. Neurol. 10:336. doi: 10.3389/fneur.2019.00336
Received: 25 January 2019; Accepted: 19 March 2019;
Published: 09 April 2019.
Edited by:
Eric Jouvent, Université Sorbonne Paris Cité, FranceReviewed by:
Dominique Hervé, Groupe Hospitalier Lariboisière Fernand Widal, FranceCopyright © 2019 Cao, Zhang, Zhou, Yao, Peng, Zhu, Ni and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Ni, cHVtY2huaWp1bkAxNjMuY29t
Liying Cui, cHVtY2hjdWlseUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.