- 1Programa de Pós-Graduação em Ciências Médicas, Universidade Federal de Santa Catarina, Florianópolis, Brazil
- 2Laboratório de Fisiopatologia Experimental, Universidade do Extremo Sul Catarinense, Criciúma, Brazil
- 3Departamento de Bioquímica, Centro de Estudos em Estresse Oxidativo, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
- 4Instituto Estadual do Cérebro Paulo Niemeyer, Rio de Janeiro, Brazil
- 5Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
- 6Grupo de Pesquisa em Gestão do Cuidado, Integralidade e Educação na Saúde (GECIES) – Programa de Pós-Graduação em Saúde Coletiva, Universidade do Extremo Sul Catarinense, Criciúma, Brazil
- 7Instituto D'Or de Pesquisa e Ensino (IDOR), Rua Diniz Cordeiro, Rio de Janeiro, Brazil
- 8Serviço de Neurologia, Departamento de Clínica Médica, Centro de Cirurgia de Epilepsia de Santa Catarina (CEPESC), Centro de Neurociências Aplicadas (CeNAp), Hospital Universitário (HU), Universidade Federal de Santa Catarina (UFSC), Florianópolis, Brazil
- 9Hospital São José, Criciúma, Brazil
The presence of autoantibodies against neuronal cell surface or synaptic proteins and their relationship to autoimmune encephalitis have recently been characterized. These autoantibodies have been also reported in other pathologic conditions; however, their role during sepsis is not known. This study detected the presence of autoantibodies against neuronal cell surface or synaptic proteins in the serum of septic patients and determined their relationship to the occurrence of brain dysfunction and mortality. This prospective, observational cohort study was performed in four Brazilian intensive care units (ICUs). Sixty patients with community-acquired severe sepsis or septic shock admitted to the ICU were included. Blood samples were collected from patients within 24 h of ICU admission. Antibodies to six neuronal proteins were assessed, including glutamate receptors (types NMDA, AMPA1, and AMPA2); voltage-gated potassium channel complex (VGKC) proteins, leucine-rich glioma-inactivated protein 1 (LGI1), and contactin-associated protein-2 (Caspr2), as well as the GABAB1 receptor. There was no independent association between any of the measured autoantibodies and the occurrence of brain dysfunction (delirium or coma). However, there was an independent and significant relationship between anti-NMDAR fluorescence intensity and hospital mortality. In conclusion, anti-NMDAR was independently associated with hospital mortality but none of the measured antibodies were associated with brain dysfunction in septic patients.
Introduction
The presence of autoantibodies against neuronal cell surface or synaptic proteins and their relationship with autoimmune encephalitis have recently been characterized (1). Anti-N-methyl-D-aspartate (NMDA) receptor (NMDAR) was the first to be reported; since then, different types of autoantibodies against neuronal cell surface or synaptic proteins have been described (2). There are currently 16 known disorders with autoantibodies against cell surface or synaptic proteins (2). These autoantibodies were originally described in association with different tumors, mainly in young women (3–5).
These autoantibodies have been also reported in other pathologic conditions such as systemic lupus erythematous, epilepsy, stroke, mania, and schizophrenia in up to 30% of patients (6–12). Additionally, the presence of anti-NMDAR antibodies had been associated with worse neurologic outcomes after cardiac surgery (13). The functional consequences of anti-NMDAR have been characterized (14–17). They include the internalization of NMDARs in both excitatory and inhibitory hippocampal neurons and the induction of reduced NMDAR-mediated synaptic currents (14–17). Mice infused with cerebrospinal fluid (CSF) from auto-immune encephalitis patients showed memory deficits and depressive behaviors (18), as well as alterations in long-term potentiation (19), which are compatible with the proposed modifications on NMDA-mediated synaptic currents. Additionally, these autoantibodies also activate microglial cells (20) and their effects upon brain function appear to be related to alterations in the permeability of the blood-brain barrier (BBB) (21).
The induction of autoantibodies occurs rapidly in sepsis and in some cases remained elevated for several weeks (22). Antibodies have been identified against a spectrum of autoantigens including potassium channel regulator, gastric ATPase, glutamic decarboxylase-65, and several cytokines (22). In this context, therefore, autoantibodies against neuronal cell surface or synaptic proteins could be prevalent in septic patients, contributing to sepsis-associated brain dysfunction. Thus, the aim of the present study was to detect the presence of different autoantibodies against neuronal cell surface or synaptic proteins in serum of septic patients and to determine their relationship with the occurrence of brain dysfunction and mortality.
Methods
This study was conducted according to the principles of the Declaration of Helsinki; in addition, the Ethics Committee of D'Or Institute, Rio de Janeiro and São José Hospital, Criciúma approved the study and all patients or their proxies provided written informed consent.
From August 2015 to May 2016, all consecutive adult (between 18 and 85 years of age) patients admitted to four different intensive care units (ICUs) (three in Rio de Janeiro and one in Criciúma, Brazil) with a diagnosis of community-acquired severe sepsis or septic shock were prospectively followed. Severe sepsis and septic shock were defined according to the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference (23). Moribund patients (expected to die within 48 h) or those admitted to the ICU for palliative care, patients expected to stay in the ICU for <48 h, deafness, and an inability to speak Portuguese, as well as patients with a history of neurologic disorders, were excluded. No patient had a clinical suspicion or laboratory abnormalities associated with autoimmune encephalitis.
A random sample of the original cohort (40 patients from Rio de Janeiro and 20 patients from Criciúma) was analyzed. Blood was collected from these patients within 24 h of ICU admission to measure the levels of serum autoantibodies. Autoantibody levels were also measured in four young, healthy individuals.
Definitions, Participant Selection, and Data Collection
Demographic, clinical, and laboratory data were collected using standardized case report forms that included the main diagnosis for ICU admission, comorbidities, Simplified Acute Physiology Score (SAPS) II (24), and Sequential Organ Failure Assessment (SOFA) score (25). The duration of mechanical ventilation (MV) and the use of sedatives were also obtained. Patients were assessed for delirium twice daily by a trained investigator during the first 14 days of ICU stay. The level of arousal was measured using the Richmond Agitation Sedation Scale (RASS) (26). Coma was defined as RASS scores of−4 (responsive only to physical stimulus) or−5 (unresponsive to physical stimulus) (27). Delirium was diagnosed using the Confusion Assessment Method (CAM)-ICU (28). Brain dysfunction was defined as the presence of coma and/or delirium. All ICUs had a protocol for the daily suspension of sedation in order to perform RASS and CAM-ICU assessments.
Autoantibody Detection
Serum levels of antibodies to the following six neuronal proteins were assessed using the Autoimmune Encephalitis Mosaic 1 kit (EUROIMMUN, Luebeck, Germany): glutamate receptors (type NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid - AMPA1, and AMPA2); voltage-gated potassium channel complex proteins -leucine-rich glioma-inactivated protein 1 (LGI1), and contactin-associated protein-2 (Caspr2); as well as gamma-aminobutyric acid (GABA) B1 receptor. Briefly, the diluted serum samples were applied to the reaction fields and the reaction started by fitting the BIOCHIP slides into the reagent tray. The slides were then incubated with labeled antibody, drops of embedding medium were added to the BIOCHIP slides, and a cover glass was fitted. Images were acquired using a Microscopy EVOS® FL Auto Imaging System (AMAFD1000—Thermo Fisher Scientific; MA, USA) at 20x magnification. Fluorescence quantification was performed using ImageJ software (ImageJ v1.49, National Institutes of Health, USA). The fluorescence intensity was measured and expressed in arbitrary units.
Data Processing and Statistical analysis
Standard descriptive statistics were produced and continuous variables were reported as means and standard deviation (SD). Comparisons between groups were performed using two-tailed Student's t- or Mann-Whitney U tests for continuous variables according to the data distributions. Fisher's exact and chi-square tests were used for comparisons between categorical variables, as appropriate.
Due to the small number of events, the independent association between autoantibodies and brain dysfunction was analyzed in a regression model that included only variables with p < 0.05 in univariate analysis. Since age is incorporated in the SAPS II score, models including SAPS II did not also include patient age. This was also true of the SOFA score since several aspects of the score are incorporated in the SAPS II; thus, when SAPS II was included in the regression, the SOFA was not. Since there is a co-linearity between sedation use and mechanical ventilation, only sedation use was incorporated in the model.
Regression models were also constructed to evaluate the association between autoantibodies and hospital mortality. The same exclusions cited to the brain dysfunction model also applied in the mortality model. However, the mortality models also considered the co-linearity between sedation, mechanical ventilation, and brain dysfunction, and included only brain dysfunction in the model.
Statistical significance was set at p < 0.05. All analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA).
Results
The majority of patients included in the current study presented septic shock and predominant infection site was the lung. As expected the prevalence of brain dysfunction was high in this population. Sixty-eight percent of the population presented brain dysfunction (coma and/or delirium) during their ICU stay. Half of the included patients experienced coma and 52% experienced delirium. No healthy volunteer (n = 4) was positive for any of the measured autoantibodies. Supplementary Figure 1 shows representative images of the fluorescence of the autoantibodies.
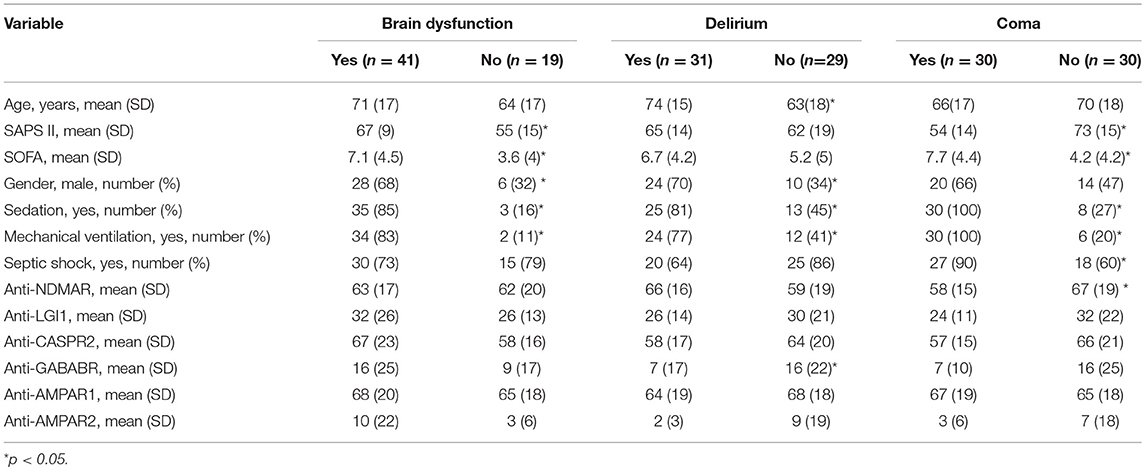
The association between brain dysfunction and autoantibodies is shown in Table 1. There was no consistent correlation between the fluorescence intensity of the of autoantibodies and brain dysfunction in septic patients, except for a lower intensity of anti-NMDAR in comatose patients and a lower intensity of anti-GABAR in delirium patients. However, the regression analysis revealed no independent association between the intensity of autoantibodies fluorescence and brain dysfunction.
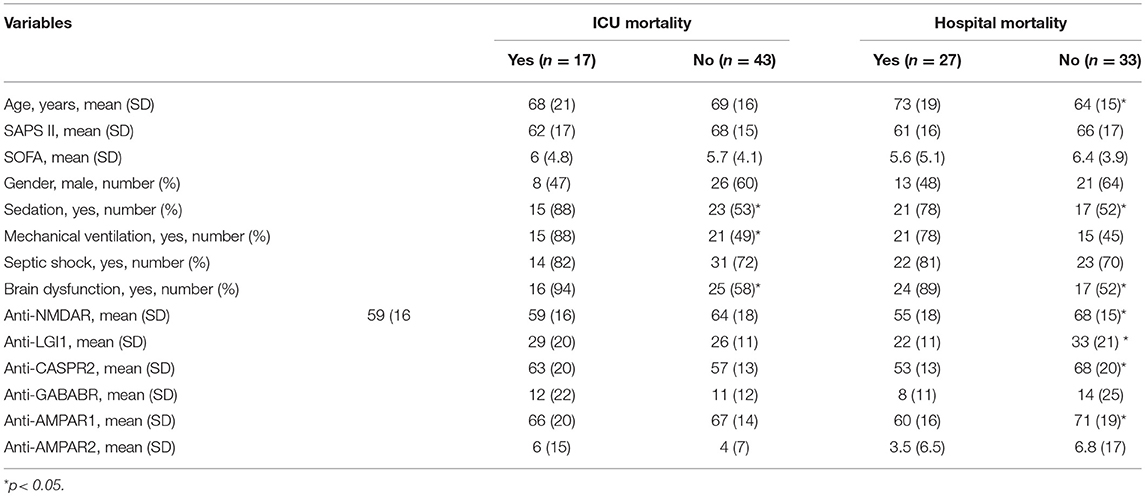
The association between autoantibodies and ICU and hospital mortality is shown in Table 2. There was no significant association between autoantibodies and ICU mortality. However, there was a significant association between the fluorescence of NMDAR, LGI1, CASPR2, and AMPAR1 and hospital mortality. From all these antibodies, only anti-NMDAR was independently associated with hospital mortality in the regression analysis. A lower fluorescence intensity was associated with hospital mortality.
Discussion
The results of the present study demonstrated that septic critically ill patients had autoantibodies against neuronal cell surface or synaptic proteins. Anti-NMDAR had an unexpected inverse association with hospital mortality but not with brain dysfunction.
The presence of autoantibodies against neuronal cell surface or synaptic proteins is probably secondary to the release of specific antigens by dying cells and its processing by memory B cells (1, 29). Izykenova et al. (30) reported that rats with induced cerebral ischemia exhibited increased plasma concentrations of NMDAR peptide fragments and antibodies to these receptor fragments (30). In humans, anti-NMDAR has been detected after ischemic stroke (8). Since these autoantibodies can have several pathophysiologic consequences, they may be not only biomarkers but may also participate in the development of a number of symptoms (4, 6, 13, 14, 17–21).
In the case of encephalitis, these autoantibodies, or B cells, cross the BBB to induce the prolonged synthesis of antibodies within the central nervous system (CNS) (1). The systemically-produced antibodies may reach the brain through a disrupted BBB (7). Outside of the content of critical illness, a synergism between anti-NMDAR and systemic lupus erythematosus-associated impaired cognition has been reported (31). Anti-NMDAR1 has been detected in individuals with slow cognitive impairment; the presence of this autoantibody affected synaptic protein expression and decreased NMDAR-mediated currents (32). Steiner et al. reported a higher prevalence of anti-NMDAR in schizophrenic patients (10). A seroprevalence of >10% was reported in a large-scale systematic screening for anti-NMDAR in the serum of healthy and neuropsychiatrically diseased subjects, many of whom were completely asymptomatic (33). In the context of critically ill patients, preoperative anti-NMDAR serum concentrations were predictive of severe neurological adverse events (delirium, transitory ischemic attack or stroke) after cardiac surgery with cardiopulmonary pass (13). It is uncertain if the persistence of these autoantibodies has any long-term clinical significance and should be followed-up, or if these patients are more prone to develop auto-immune encephalitis. None of the above cited studies presented a long-term follow-up to address this issue, and this should be evaluated further.
Brain dysfunction is a highly prevalent complication of sepsis (34) and is independently associated with increased short and long-term morbidity and mortality (35, 36). Additionally, BBB dysfunction is a hallmark of the development of sepsis-associated encephalopathy (37); thus, it is reasonable to suppose that autoantibodies are systemically produced during sepsis and could participate in the development of brain dysfunction. However, our results do not support this hypothesis. Additionally, the influence of autoantibodies on the function of organs other than the brain is not known. Since neurotransmitters are relevant not only to brain function in the context of sepsis (38–42), autoantibodies could also play a role in organ failure and mortality. Additionally, multiple organ failure and infections are one of the main causes of death in autoimmune encephalitis (43–45). Since some of these patients required ICU admission it is not possible to determine a causal link between the presence of autoantibodies and organ dysfunction. We did not observe a significant correlation between any of the measured autoantibodies and sepsis severity, organ dysfunction scores, and ICU mortality, suggesting that their presence did not have a direct pathophysiological role in sepsis progression. However, after adjusting for potential confounders, the fluorescence intensity of anti-NMDAR was inversely associated with hospital mortality. This is an intriguing factor that deserves further study. It is possible that their presence could be a surrogate to the general immune dysfunction, mainly T cell dysfunction, that occurs during sepsis development. Around 45% of septic patients were previously shown to have immunoreactivity to self-proteins, from KCNRG, a protein highly expressed in the lung and to autoantibodies associated with neurological targets (AQP-4 and GAD65) (22). The levels of some of these antibodies showed sustained elevation at days 20 to 28 after sepsis diagnosis. However, the clinical consequences of this observation are unclear. Additionally, due to our small sample size small changes in the number of events or non-events would have resulted in the loss of significance.
Several questions remain unanswered and the present study had several limitations. First, although none of the included patients had a clinical suspicion or laboratory abnormalities associated with autoimmune encephalitis, its presence cannot be completely excluded. Second, the titer of the antibodies was not defined, only the intensity of fluorescence, and a second technique such as enzyme-linked immunosorbent assay (ELISA) was not performed to confirm sample positivity. Third, in some conditions, such as after hospitalization for mania (12), there is a decrease in serum antibody positivity; we did not provide a time-dependent analysis of autoantibody levels after sepsis resolution. Fourth, the CSF levels of autoantibodies or BBB dysfunction were not assessed; this information could help to inform the relationship between autoantibodies and brain dysfunction in septic patients. Fifth, the relationship between autoantibodies and long-term cognitive impairment was not evaluated. This is a relevant topic for future study since, as seen in schizophrenic individuals, affected individuals with well-documented histories of birth complications and brain trauma show more severe neurological abnormalities when carrying anti-NMDAR (21). These findings strengthen the hypothesis of BBB involvement in these conditions and the long-term relationship between BBB dysfunction and cognitive impairment. Sixth, the number of measured events precluded the addition of more variables in the regression model to provide a more robust association between autoantibodies and outcomes.
In conclusion, anti-NMDAR was independently associated with hospital mortality but none of the measured antibodies were associated with brain dysfunction in septic patients. Studies including larger sample sizes are needed to confirm these results.
Author Contributions
HM: protocol design, statistical analysis, manuscript preparation. IS: analysis of samples, statistical analysis, manuscript revision. JG: analysis of samples, manuscript revision. CaR and CT: collection of samples, manuscript revision. DG, FB, RW, FD-P, and CrR: protocol design, statistical analysis, and manuscript revision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Laboratory of Experimental Pathophysiology is a member of the Centre of Excellence in Applied Neurosciences of Santa Catarina (NENASC). Its research is supported by grants from the National Council for Scientific and Technological Development (CNPq) (FD-P and CrR), the Foundation for Research and Innovation of the State of Santa Catarina (FAPESC) (FD-P), and UNESC (FD-P and CrR).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.00221/full#supplementary-material
References
1. Dalmau J. NMDA recepto encephalitis and other antibody-mediated disorders of the synapse: the 2016 Cotzias Lecture. Neurology. (2016) 87:2471–82. doi: 10.1212/WNL.0000000000003414
2. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
3. Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. (2007) 61:25–36. doi: 10.1002/ana.21050
4. Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. (2013) 70:1133–9. doi: 10.1001/jamaneurol.2013.3216
5. Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. (2010) 133:1655–67. doi: 10.1093/brain/awq113
6. Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Clarke A, et al. Autoantibodies as biomarkers for prediction of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. (2011) 70:1726–32. doi: 10.1136/ard.2010.148502
7. Levite M. Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy. J Neural Transm. (2014) 121:1029–75. doi: 10.1007/s00702-014-1193-3
8. Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. (2003) 49:1752–62. doi: 10.1373/49.10.1752
9. Dambinova SA, Izykenova GA, Burov SV, Grigorenko EV, Gromov SA. The presence of autoantibodies to N-terminus domain of GluR1 subunit of AMPA receptor in the blood serum of patients with epilepsy. J Neurol Sci. (1997) 152:93–7. doi: 10.1016/S0022-510X(97)00150-0
10. Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein HG, Vielhaber S, et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. (2013) 70:271–8. doi: 10.1001/2013.jamapsychiatry.86
11. Weissman JD, Khunteev GA, Heath R, Dambinova SA. NR2 antibodies: risk assessment of transient ischemic attack (TIA)/stroke in patients with history of isolated and multiple cerebrovascular events. J Neurol Sci. (2011) 300:97–102. doi: 10.1016/j.jns.2010.09.023
12. Dickerson F, Stallings C, Vaughan C, Origoni A, Khushalani S, Yolken R. Antibodies to the glutamate receptor in mania. Bipolar Disord. (2012) 14:547–53. doi: 10.1111/j.1399-5618.2012.01028.x
13. Bokesch PM, Izykenova GA, Justice JB, Easley KA, Dambinova SA. NMDA receptor antibodies predict adverse neurological outcome after cardiac surgery in high risk patients. Stroke. (2006) 37:1432–6. doi: 10.1161/01.STR.0000221295.14547.c8
14. Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. (2010) 30:5866–75. doi: 10.1523/JNEUROSCI.0167-10.2010
15. Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. (2014) 76:108–19. doi: 10.1002/ana.24195
16. Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. (2008) 7:1091–8. doi: 10.1016/S1474-4422(08)70224-2
17. Masdeu JC, Dalmau J, Berman KF. NMDA receptor internalization by autoantibodies: a reversible mechanism underlying psychosis? Trends Neurosci. (2016) 39:300–10. doi: 10.1016/j.tins.2016.02.006
18. Planagumà J, Leypoldt F, Mannara F, Gutiérrez-Cuesta J, Martín-García E, Aguilar E, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. (2015) 138:94–109. doi: 10.1093/brain/awu310
19. Planagumà J, Haselmann H, Mannara F, Petit-Pedrol M, Grünewald B, Aguilar E, et al. Ephrin-B2 prevents NMDA receptor antibody effects on memory and neuroplasticity. Ann Neurol. (2016) 80:388–400. doi: 10.1002/ana.24721
20. Kannan G, Crawford JA, Yang C, Gressitt KL, Ihenatu C, Krasnova IN, et al. Anti-NMDA receptor autoantibodies and associated neurobehavioral pathology in mice are dependent on age of first exposure to Toxoplasma gondii. Neurobiol Dis. (2016) 91:307–14. doi: 10.1016/j.nbd.2016.03.005
21. Kannan G, Gressitt KL, Yang S, Stallings CR, Katsafanas E, Schweinfurth LA, et al. Pathogen-mediated NMDA receptor autoimmunity and cellular barrier dysfunction in schizofrenia. Transl Psychiatry. (2017) 7:e1186. doi: 10.1038/tp.2017.162
22. Burbelo PD, Seam N, Groot S, Ching KH, Han BL, Meduri GU, et al. Rapid induction of autoantibodies during ARDS and septic shock. J Transl Med. (2010) 8:97. doi: 10.1186/1479-5876-8-97
23. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. (2003) 31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B
24. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. (1993) 270:2957–63. doi: 10.1001/jama.1993.03510240069035
25. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
26. Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. (2003) 289:2983–91. doi: 10.1001/jama.289.22.2983
27. Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. (2007) 298:2644–53. doi: 10.1001/jama.298.22.2644
28. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. (2001) 286:2703–10. doi: 10.1001/jama.286.21.2703
29. Dambinova SA, Bettermann K, Glynn T, Tews M, Olson D, Weissman JD, et al. Diagnostic potential of the NMDA receptor peptide assay for acute ischemic stroke. PLoS ONE. (2012) 7:e42362. doi: 10.1371/journal.pone.0042362
30. Gappoeva MU, Izykenova GA, Granstrem OK, Dambinova SA. Expression of NMDA neuroreceptors in experimental ischemia. Biochemistry. (2003) 68:696–702. doi: 10.1023/A:1024678112357
31. Gerosa M, Poletti B, Pregnolato F, Castellino G, Lafronza A, Silani V, et al. Antiglutamate receptor antibodies and cognitive impairment in primary antiphospholipd syndrome and systemic lups erythematosus. Front Immunol. (2016) 7:5. doi: 10.3389/fimmu.2016.00005
32. Prüss H, Höltje M, Maier N, Gomez A, Buchert R, Harms L, et al. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. (2012) 78:1743–53. doi: 10.1212/WNL.0b013e318258300d
33. Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. (2014) 19:1143–9. doi: 10.1038/mp.2013.110
34. Mazeraud A, Pascal Q, Verdonk F, Heming N, Chrétien F, Sharshar T. Neuroanatomy and physiology of brain dysfunction in sepsis. Clin Chest Med. (2016) 37:333–45. doi: 10.1016/j.ccm.2016.01.013
35. Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. (2012) 8:557–66. doi: 10.1038/nrneurol.2012.183
36. Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med. (2015) 3:61–9. doi: 10.1016/S2213-2600(14)70246-2
37. Danielski LG, Giustina AD, Badawy M, Barichello T, Quevedo J, Dal-Pizzol F, et al. Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol. (2017) 55:1045–53. doi: 10.1007/s12035-016-0356-7
38. da Cunha AA, Pauli V, Saciura VC, Pires MG, Constantino LC, de Souza B, et al. N-methyl-D-aspartate glutamate receptor blockade attenuates lung injury associated with experimental sepsis. Chest. (2010) 137:297–302. doi: 10.1378/chest.09-1570
39. Lin CS, Hung SF, Huang HS, Ma MC. Blockade of the N-methyl-D-aspartate glutamate receptor ameliorates lipopolysaccharide-induced renal insufficiency. PLoS ONE. (2015) 10:e0132204. doi: 10.1371/journal.pone.0132204
40. Bai W, Zhu WL, Ning YL, Li P, Zhao Y, Yang N, et al. Dramatic increases in blood glutamate concentrations are closely related to traumatic brain injury-induced acute lung injury. Sci Rep. (2017) 7:5380. doi: 10.1038/s41598-017-05574-9
41. Zhan LY, Du L, Xia ZY, Li WL, Zhao B. Study of the expression of1 GABA(A) receptor in rats during acute lung injury caused by endotoxin. Genet Mol Res. (2015) 14:13312–9. doi: 10.4238/2015.October.26.27
42. Jin S, Merchant ML, Ritzenthaler JD, McLeish KR, Lederer ED, Torres-Gonzalez E, et al. Baclofen, a GABABR agonist, ameliorates immune-complex mediated acute lung injury by modulating pro-infalammatory mediators. PLoS ONE. (2015) 10:e0121637. doi: 10.1371/journal.pone.0121637
43. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
44. Chi X, Wang W, Huang C, Wu M, Zhang L, Li J, et al. Risk factors for mortality in patients with anti-NMDA encephalitis. Acta Neurol Scand. (2017) 136:298–304. doi: 10.1111/ane.12723
Keywords: delirium, brain dysfunction, sepsis, neuronal autoantibodies, ICU
Citation: Malfussi H, Santana IV, Gasparotto J, Righy C, Tomasi CD, Gelain DP, Bozza FA, Walz R, Dal-Pizzol F and Ritter C (2019) Anti-NMDA Receptor Autoantibody Is an Independent Predictor of Hospital Mortality but Not Brain Dysfunction in Septic Patients. Front. Neurol. 10:221. doi: 10.3389/fneur.2019.00221
Received: 29 June 2018; Accepted: 20 February 2019;
Published: 15 March 2019.
Edited by:
Bryan G. Young, London Health Sciences Centre, CanadaReviewed by:
Jeanne Teitelbaum, McGill University, CanadaChristoph Stretz, School of Medicine, Yale University, United States
Copyright © 2019 Malfussi, Santana, Gasparotto, Righy, Tomasi, Gelain, Bozza, Walz, Dal-Pizzol and Ritter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristiane Ritter, Y3Jpc3RpYW5lLnJpdHRlcjFAZ21haWwuY29t
 Hamilton Malfussi
Hamilton Malfussi Iara Vidigal Santana2
Iara Vidigal Santana2 Juciano Gasparotto
Juciano Gasparotto Cristiane Damiani Tomasi
Cristiane Damiani Tomasi Daniel Pens Gelain
Daniel Pens Gelain Fernando A. Bozza
Fernando A. Bozza Roger Walz
Roger Walz Felipe Dal-Pizzol
Felipe Dal-Pizzol Cristiane Ritter
Cristiane Ritter