- 1Department of Neurology, Jena University Hospital, Jena, Germany
- 2Center for Healthy Ageing, Jena University Hospital, Jena, Germany
Parkinson's disease is a common multisystem neurodegenerative disorder characterized by typical motor and non-motor symptoms. There is an urgent need for biomarkers for assessment of disease severity, complications and prognosis. In addition, biomarkers reporting the underlying pathophysiology assist in understanding the disease and developing neuroprotective therapies. Ultimately, biomarkers could be used to develop a more efficient personalized approach for clinical trials and treatment strategies. With the goal to improve quality of life in Parkinson's disease it is essential to understand and objectively monitor non-motor symptoms. This narrative review provides an overview of recent developments of biomarkers (biofluid samples and imaging) for three common neuropsychological syndromes in Parkinson's disease: dementia, fatigue, and depression.
Introduction
Parkinson's disease (PD) is now considered as progressive and multisystem α-synucleinopathy. Therefore, PD is characterized not only by motor symptoms, but also a broad range of non-motor symptoms (NMS) (1). NMS can aggravate disease burden and significantly contribute to worsening of quality of life (2). Biomarkers which are associated with worse motor performance as well as development of NMS are of special importance in PD. A biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (3). The ideal PD biomarkers should have a reasonable effect size, are reproducible across different cohorts and are ideally verified in neuropathological proven PD cases. Biomarkers in PD can include (i) biomarker for prodromal stage to identify PD before motor symptoms occur, (ii) biomarkers of susceptibility to identify persons who are at risk for PD, (iii) biomarkers for motor and non-motor burden to assess disease severity and monitor the efficacy of therapies. The last one can help to identify patients who are at risk to develop complications and may lead to individual optimization and prevention in health care. This review provides an update on recent advances in the development of biomarkers (biofluid samples and neuroimaging) for three common neuropsychological syndromes: dementia, fatigue and depression.
Cognitive Impairment
Cognitive deficits are common in PD and can present as mild dysfunction in the prodromal and early stages, or as dementia (PDD) in advanced stages (4). Approximately 20% of patients with de novo PD have mild cognitive impairment (MCI) (5). The concept of PD-MCI was introduced 2012 (MDS Task Force) and characterizes a cognitive decline that is assessed during neuropsychological testing but does not impair activities of daily living (6). MCI is considered an intermediate state of cognitive dysfunction in PD that may progress to PDD. Up to 75% of patients will develop dementia over the longterm disease course (7). However, the rate to PDD, the cognitive profile and severity of cognitive dysfunction show high interindividual variation. Given its high medical and social impact and its health-related costs, the identification of biomarkers for PDD is of high priority (8). Biomarkers reflecting cognitive decline can facilitate early diagnosis and may indicate response to therapeutic interventions.
Clinical factors, such as higher age, male sex, low level of education, longer disease duration, higher Hoehn & Yahr stage, axial impairment, excessive daytime sleepiness, cardiovascular autonomic dysfunction, REM sleep behavior disorder, hallucinations and PD-MCI were found to strongly predict the development of PDD (9–13). Moreover, impairment of memory and language (posterior-cortical dysfunction) seems to be linked to a higher risk of PDD (14, 15).
Given the neuropathology of PDD several studies aimed to identify biomarkers which reflect proteinopathy, neuronal loss, abnormal neurotransmitters, and structural and functional brain changes. Lewy bodies and amyloid plaques in the neocortex and limbic system are typical neuropathological features of Alzheimer's disease and PDD (16, 17). Hence, the majority of studies investigated amyloid-ß 1–42 (Aß), tau protein, and α-synuclein in the cerebrospinal fluid (CSF) of PD patients (Table 1). In many studies the level of Aß was reduced in PDD. Low CSF levels of Aß were found to be related to deterioration in attention, executive function, semantic fluency and memory (21, 38, 40, 45). One-half of PDD patients had the CSF biomarker signature of Alzheimer's disease (46) suggestive of an overlap with Alzheimer's disease pathology (47). Low baseline CSF Aβ was associated with more rapid cognitive decline later in disease. By contrast, the levels of total (t-tau) and phosphorylated tau (p-tau) were found to be increased or unchanged in PDD (Table 1). For clinicians it is highly relevant to know which biomarkers accurately predict the progression from MCI to PDD. Therefore, based on the data from cross-sectional and longitudinal studies one can assume that reduced Aß predicts cognitive decline in PD (40, 42, 48).
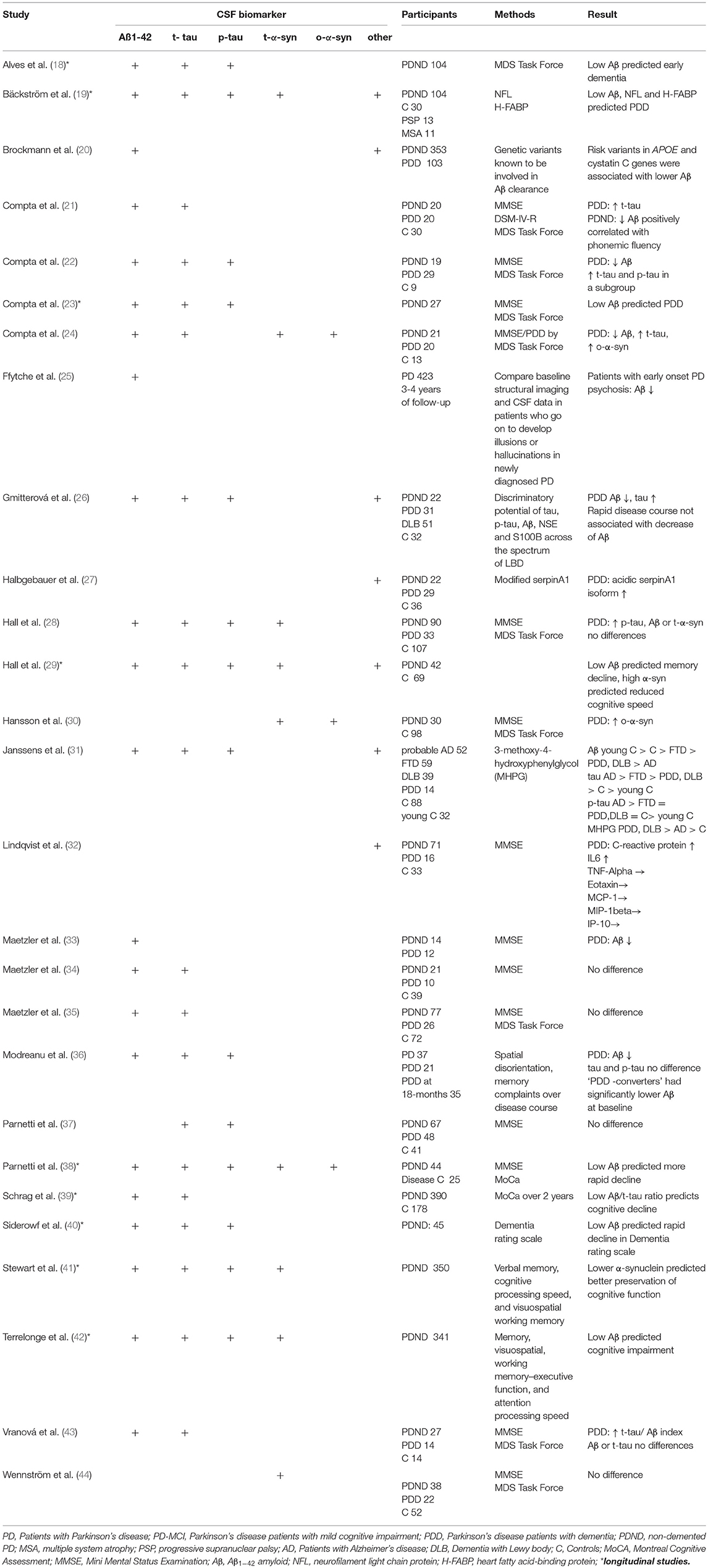
Table 1. Cerebrospinal-fluid (CSF) biomarkers of cognitive impairment and dementia in Parkinson's disease.
Several studies assessed the CSF levels of α-synuclein in PD. Meta-analyses demonstrated that total α-synuclein levels are lower in PD compared to controls (49, 50). However, in terms of α-synuclein and cognitive decline there are conflicting results with both low and high levels in the presence of cognitive impairment (29, 41, 48). In the DATATOP study with up to 8 years of follow-up, lower α-synuclein levels predicted better preservation of cognitive function (verbal learning and memory, visuospatial working memory) in early disease. Thus, α-synuclein may reflect changes in multiple cognitive domains and may predict cognitive decline in PD (29, 41, 48). On the other hand most studies of non-demented PD failed to find any association between α-synuclein levels and cognition (51, 52). It seems that CSF α-synuclein levels may increase with disease stage. This could explain why cognitive deficits in connection with high levels of α- synuclein were found in more advanced disease stages (53). Isoforms of α-synuclein (e.g., phosphorylated, ubiquitinated, oligomeric) are potentially more sensitive to cognitive decline than the total α-synuclein level (24, 30). Another study examining plasma levels of α-synuclein found higher levels in PDD and a correlation with mini mental state examination scores (54). This finding, however, requires further investigations.
In another longitudinal study, high neurofilament light chain protein, low Aβ and high heart fatty acid–binding protein at baseline were related to future PDD with a relatively high diagnostic accuracy (19). Also several serum proteins, such as C-reactive protein, interleukins, interferon-γ, tumor necrosis factor α, uric acid, and cystatin C were found to be associated with cognition in PD (55). In particular, low uric acid concentrations, low levels of epidermal growth factor (EGF) and insulin-like growth factor (ILGF) seems to have predictive value for deterioration of cognitive function in PD (56–61). In combination with clinical markers, a study of 390 patients from the Progression Markers Initiative study with newly diagnosed PD, the occurrence of cognitive impairment at 2 years follow-up could be predicted with good accuracy using a model combining information on age, non-motor assessments, DAT imaging, and CSF biomarkers. Here, the Montreal Cognitive Assessment (MoCA) scores and low CSF Aβ to t-tau ratio and DAT imaging results were the best predictors of cognitive impairment (39). Using data from the Parkinson's Progression Markers Initiative, Fereshtehnejad et al., identified distinct subgroups via a cluster analysis of a comprehensive dataset consisting of clinical characteristics, neuroimaging, biospecimen and genetic information. Here, the CSF biomarkers differed between these PD subtypes. Patients with diffuse malignant disease course and fast cognitive decline, showed an Alzheimer's disease-like CSF profile (low Aβ, low Aβ/t-tau ratio) (62).
Applying computerized neuroimaging analyses several MRI studies have found gray matter atrophy and disruptions of white matter integrity in PDD, although findings in non-demented PD and PD-MCI remain inconsistent (63) (Tables 2, 3). A longitudinal study using voxel-based morphometry (VBM) found neocortical volume reduction (temporo-occipital region, hippocampal and parahippocampal) as the most relevant finding in patients who develop PDD (97). Another study has identified a validated Alzheimer's disease pattern of brain atrophy as an independent predictor of cognitive impairment in PD (64). More specifically cortical thinning in the right precentral, frontal, and in the anterior cingulate cortex as well as gray matter atrophy (prefrontal, insula, caudate nucleus, hippocampal) predicted cognitive decline in PD (23, 66, 70, 76, 98). Cognitive impairment was also found to be associated with lower gray matter volume and increased mean diffusivity in the nucleus basalis of Meynert, compared to non-demented patients. Moreover, these changes were predictive for developing cognitive impairment in cognitively intact patients with PD, independent of other clinical and non-clinical markers of the disease (99). The nucleus basalis of Meynert and the pedunculopontine nucleus in the brainstem are important cholinergic projections in and post-mortem studies have shown that neuronal loss in in the nucleus basalis is an early phenomenon in PD (100, 101). Combining many modalities, Compta et al. (23) performed a longitudinal study in non-demented PD patients including CSF, neuropsychological and MRI studies at baseline and 18 months follow up. Here, a combination of lower CSF Aβ, reduced verbal learning, semantic fluency and visuoperceptual scores, as well as cortical thinning in superior-frontal/anterior cingulate and precentral regions were found to be predictive for PDD.
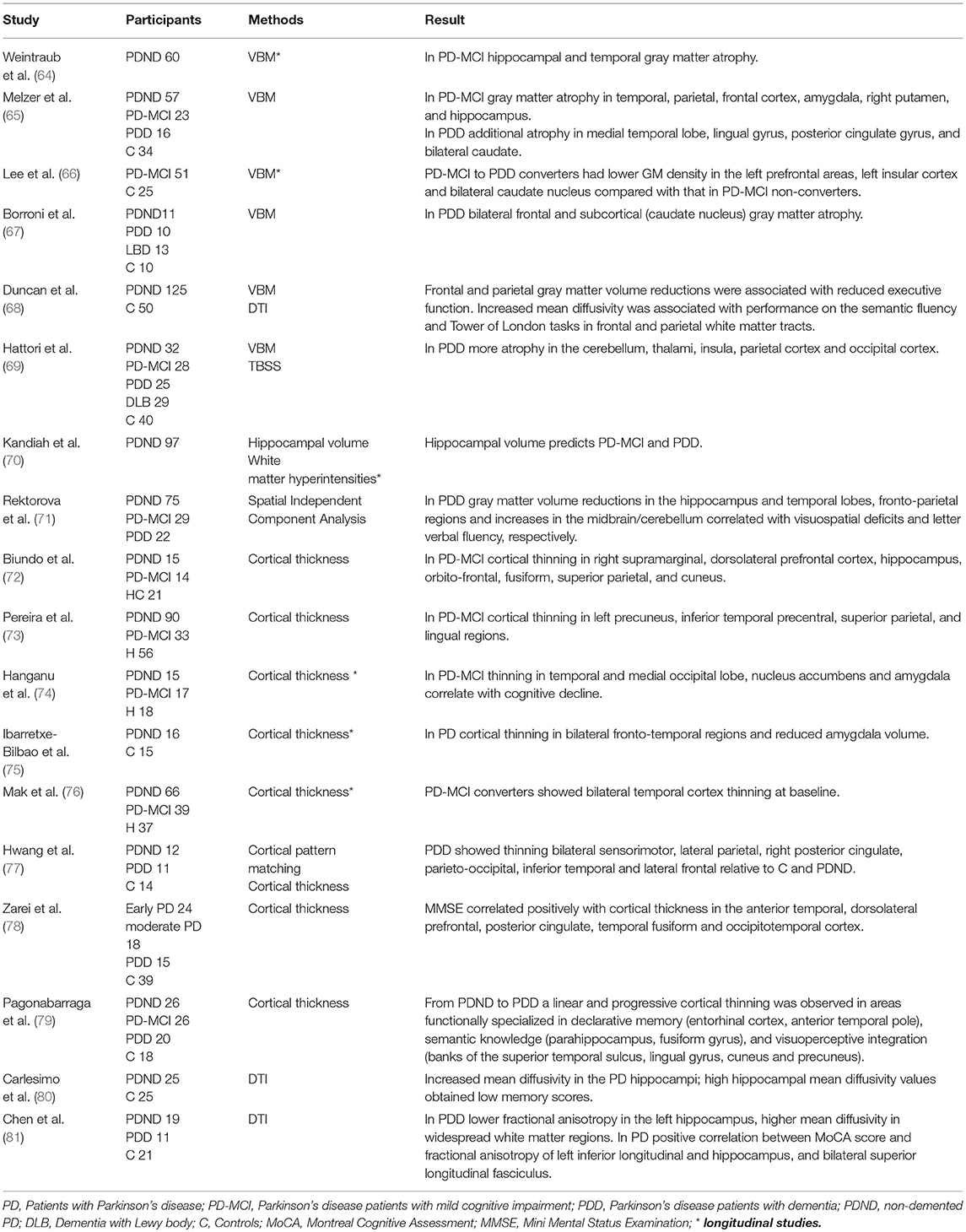
Table 2. Cortical and subcortical structural changes related to cognitive impairment and dementia in Parkinson's disease.
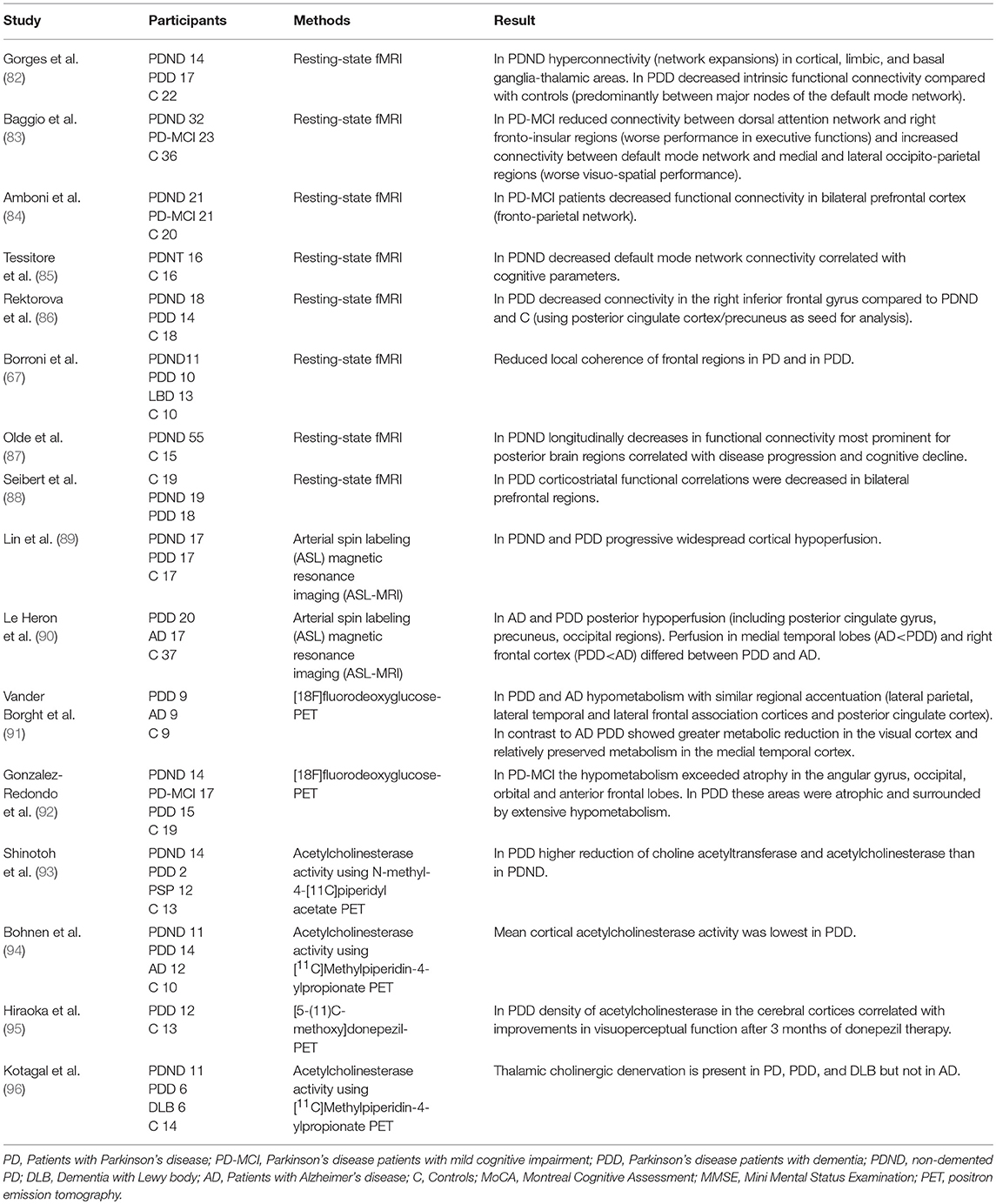
Table 3. Changes of function and connectivity related to cognitive impairment and dementia in Parkinson's disease.
For the assessment of white matter pathology using DTI and imaging of metabolites (Proton magnetic resonance spectroscopy) there is currently not enough longitudinal data available and the value of these techniques to predict cognitive decline has to be further explored. The existing studies indicate that microstructural changes, such as increased mean diffusivity or reduced fractional anisotropy in the hippocampus, the frontal and parietal white matter tracts are associated with cognitive decline in PD (68, 80, 81, 102–104). In particular, an increased mean diffusivity may be predictive for cognitive decline before fractional anisotropy decreases. However, these findings need further validation in longitudinal studies.
Fatigue
Fatigue is a common symptom that includes both mental and physical aspects. Up to 70% of individuals with PD experience fatigue every day (105). Fatigue dramatically impairs quality of life (106). It is a complex syndrome emerging from dysfunction in the nervous, endocrine and immune system (107). From a clinical point of view fatigue is frequently associated with other non-motor syndromes, like sleepiness, apathy, depression and autonomic dysfunction (105, 108). However, fatigue can also occur as an isolated syndrome; it is therefore important to understand that fatigue and sleepiness or depression is not the same condition (109, 110). Central fatigue is commonly measured through questionnaires, such as the Fatigue Severity Scale (111) which is recommended by the Movement Disorder Society (MDS) task force (112). Central fatigue can be described as a feeling of constant exhaustion and can occur in various chronic disorders. Peripheral fatigue is characterized by failure to sustain the force of muscle contraction and is more readily accessible to quantification (106, 113).
A key mechanism underlying fatigue is the activation of the inflammatory cytokine network (107, 114). Therefore, inflammatory markers serve as potential biomarkers of fatigue. In particular, higher serum levels of IL-6, IL1-Ra, sIL-2R, and VCAM-1 were associated with higher fatigue levels in patients with newly diagnosed, drug-naïve PD (115, 116). This neuroinflammatory processes may promote glutamate dysregulation and further influence neuronal activity and neuroplasticity, and impact neuronal circuits mediating distress and motivation in PD (117–119). Interestingly, higher serum uric acid levels were significantly associated with less fatigue (120).
In addition, dysfunction of the endocrine system, such as hypothalamic-pituitary-adrenal system which is connected to basal ganglia, amygdala, thalamus and frontal cortex, seems to contribute to the pathophysiology of fatigue (113). Although there are no neuropathological studies of PD-fatigue supporting this model so far, several neuroimaging studies showed that multiple brain areas are involved in fatigue in PD. These include frontal, temporal and parietal regions indicative of emotion, motivation and cognitive functions (121–126). In SPECT imaging with technetium-99 hexamethyl-propylene-amine-oxime PD-fatigue was associated with reduced perfusion in the frontal lobe (125). Others used PET with dopaminergic and serotonergic markers in fatigued vs. non-fatigued PD patients. Less serotonergic marker binding was found in striatal and limbic regions (thalamus, anterior cingulate, amygdala, insula) in PD-fatigue. The striatal 18F-dopa uptake was similar in fatigued and non-fatigued groups, but voxel-based analysis localized the reduced dopamine uptake to the caudate and insula in PD-fatigue (127). In addition the serotonin transporter (SERT) availability was significantly reduced in the striatum and thalamus of fatigued PD patients, suggesting that increasing the brain level of serotonin may improve PD-fatigue (127). The reduced serotonergic transmission suggests that a disturbed neurotransmitter balance within the basal ganglia and associated regions changes the integration of emotional and motor information in limbic regions, thus resulting in fatigue symptoms (128). With regard to striatal dopamine transporter uptake, results are conflicting. Two studies found no difference between fatigued and non-fatigued PD (127, 129). In the study by Chou et al., striatal dopamine transporter uptake was a significant predictor of fatigue in mild but not moderate-to-severe PD. They postulated that the lack of association between fatigue and nigrostriatal loss in advanced PD may reflect a denervation “floor” effect (130). Many of these studies have assessed advanced disease stages and patients on dopaminergic treatment. In contrast, Tessitore et al. studied fatigue in drug-naïve early PD using resting-state functional MRI (fMRI). Fatigue itself, and fatigue severity were associated with a decreased connectivity within the supplementary motor area and an increased connectivity within the default mode network (121). Importantly, these functional abnormalities occur independently from both dopamine-induced connectivity and structural changes. This study is in line with earlier neurophysiological studies suggesting that abnormal premotor and primary motor cortices connectivity correlate with fatigue (131, 132). Tessitore et al. hypothesized that the increased connectivity of the default mode network represents an initial cognitive compensatory response to the fatigue-related motor connectivity changes. In this sense fatigued PD-patients, when internally oriented, have to increase mental expenditure to maintain the same level of motor planning performance in order to switch more easily to externally oriented processing (121).
In summary, abnormalities in motivation of self-initiated tasks and motor function may play a significant role in the pathophysiology of fatigue (133). While non-dopaminergic basal ganglia pathways seem to be involved in PD-fatigue, the dopaminergic dysfunction may only play a role through extrastriatal projections.
Depression
PD patients are twice as likely to develop depression compared to healthy individuals (134). Depressive symptoms affect 40–50% of PD patients and significantly impact quality of life in PD (2). In particular, patients with cognitive impairment, longer disease duration, motor fluctuations, female gender, and higher doses of levodopa are at risk to develop depression (9).
Like other NMS, depression seems to be linked to inflammatory signaling. Increased inflammatory responses have been described both in the brain and peripheral blood of PD patients (135). Depression correlated with a high serum level of IL-10 (136) and IL-6 (137). High levels of both sIL-2R and TNF-α in blood samples from PD patients were significantly associated with more severe depression and anxiety (119). As reflection of CNS involvement, high CRP levels in CSF of PD patients were associated with more severe symptoms of depression (32). However, these findings are not specific for PD. Chronic inflammation in physically ill patients is often associated with symptoms of depression and also occurs in normal aging (138–140). Moreover, PD in general is characterized by elevated levels of inflammatory cytokines, such as IL-6, tumor necrosis factor, IL-1β, IL-2, IL-10, C-reactive protein, and RANTES (141).
Depression in PD is associated with several structural and functional changes in the limbic system. In particular, changes in the amygdala, hippocampus and orbitofrontal cortex were frequently reported in PD depression (142–151). The involvement of the serotonergic system was demonstrated in post-mortem tissue and validated in vivo by several PET imaging studies (152–155). Compared to controls the serotonin transporter binding in non-depressed PD was lower in the striatal region, the orbitofrontal cortex, and the dorsolateral pre-frontal cortex which is an area known to be involved in major depression (155). Using dopaminergic and serotonergic presynaptic transporter radioligands a prominent role of serotonergic degeneration in limbic regions such as the anterior cingulate cortex was demonstrated (156, 157). Other PET studies observed a higher availability of the serotonin transporter in the raphe nuclei and limbic regions of depressed PD patients (152, 153). Likewise, decreased plasma levels of serotonin were found to be correlated with severity of depression (158). However, studies of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in CSF from depressed and non-depressed PD patients, have yielded contradictory results (159), and serotonergic dysfunction alone may only explain vulnerability to depression in PD. Yet, symptoms of depression are also linked to mesolimbic dopaminergic degeneration (160, 161) which is in line with the clinical observation of improvement of depression by dopaminergic treatment (162).
Conclusion
From this overview emerges a comprehensive picture of recent fluid and imaging biomarkers which have been studied in a number of clearly defined and sizable cohorts of PD patients with PD. Especially longitudinal studies are necessary to make the biomarkers potentially useful for therapeutic or even clinical trial evaluation. A number of recent studies have provided ample evidence for specific predictive biomarkers across multiple domains combining clinical, biochemical, and neuroimaging information. Yet, at this stage a lack of standardized and comparable methods preclude clinical everyday use of these biomarkers beyond their value as diagnostic or prognostic tools in cohorts of patients. Thus, more research needs to be undertaken into finding reliable combinations of predictors of NMS in PD on an individual level, and standardization and harmonization of protocols in particular in CSF handling and neuroimaging has to be taken further.
Author Contributions
TP and JG: conception, collection of data, interpretation of data, drafting the work; OW: revising the work critically for important intellectual content.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Elena Huß for assistance of data collection.
References
1. Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. (2017) 18:509. doi: 10.1038/nrn.2017.91
2. van Uem JM, Marinus J, Canning C, van Lummel R, Dodel R, Liepelt-Scarfone I, et al. Health-related quality of life in patients with parkinson's disease–a systematic review based on the ICF model. Neurosci Biobehav Rev. (2016) 61:26–34. doi: 10.1016/j.neubiorev.2015.11.014
3. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. (2001) 69:89–95. doi: 10.1067/mcp.2001.113989
4. Fengler S, Liepelt-Scarfone I, Brockmann K, Schäffer E, Berg D, Kalbe E. Cognitive changes in prodromal Parkinson's disease: a review. Mov Disord. (2017) 32:1655–66. doi: 10.1002/mds.27135
5. Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study Group. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. (2009) 72:1121–6. doi: 10.1212/01.wnl.0000338632.00552.cb
6. Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement disorder society task force guidelines. Mov Disord. (2012) 27:349–56. doi: 10.1002/mds.24893
7. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. (2003) 60:387–92. doi: 10.1001/archneur.60.3.387
8. Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol. (2012) 11:697–707. doi: 10.1016/S1474-4422(12)70152-7
9. Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson's disease. Lancet Neurol. (2018) 17:559–68. doi: 10.1016/S1474-4422(18)30127-3
10. Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. (2014) 83:1253–60. doi: 10.1212/WNL.0000000000000842
11. Zhu K, van Hilten JJ, Marinus J. Predictors of dementia in Parkinson's dise findings from a 5-year prospective study using the SCOPA-COG. Parkinsonism Relat Disord. (2014) 20:980–5. doi: 10.1016/j.parkreldis.2014.06.006
12. Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, et al. MDS Task Force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Mov Disord. (2011) 26:1814–24. doi: 10.1002/mds.23823
13. Pagano G, De Micco R, Yousaf T, Wilson H, Chandra A, Politis M. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology. (2018) 91:e894–e905. doi: 10.1212/WNL.0000000000006134
14. Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. (2007) 130(Pt 7):1787–98. doi: 10.1093/brain/awm111
15. Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener Dis. (2013) 11:79–92. doi: 10.1159/000341998
16. Braak H, Rüb U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. (2005) 64:1404–10. doi: 10.1212/01.WNL.0000158422.41380.82
17. Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol. (2005) 58:773–6. doi: 10.1002/ana.20635
18. Alves G, Lange J, Blennow K, Zetterberg H, Andreasson U, Førland MG, et al. CSF Aβ42 predicts early-onset dementia in Parkinson disease. Neurology. (2014) 82:1784–90. doi: 10.1212/WNL.0000000000000425
19. Bäckström DC, Eriksson Domellöf M, Linder J, Olsson B, Öhrfelt A, Trupp M, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. (2015) 72:1175–82. doi: 10.1001/jamaneurol.2015.1449
20. Brockmann K, Lerche S, Dilger SS, Stirnkorb JG, Apel A, Hauser AK, et al. SNPs in Aβ clearance proteins: lower CSF Aβ1−42 levels and earlier onset of dementia in PD. Neurology. (2017) 89:2335–40. doi: 10.1212/WNL.0000000000004705
21. Compta Y, Martí MJ, Ibarretxe-Bilbao N, Junqué C, Valldeoriola F, Mu-oz E, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord. (2009) 24:2203–10. doi: 10.1002/mds.22594
22. Compta Y, Ezquerra M, Mu-oz E, Tolosa E, Valldeoriola F, Rios J, et al. High cerebrospinal tau levels are associated with the rs57 tau gene variant and low cerebrospinal β-amyloid in Parkinson disease. Neurosci Lett. (2011) 487:169–73. doi: 10.1016/j.neulet.2010.10.015
23. Compta Y, Pereira JB, Ríos J, Ibarretxe-Bilbao N, Junqué C, Bargalló N, et al. Combined dementia-risk biomarkers in Parkinson's disease: a prospective longitudinal study. Parkinsonism Relat Disord. (2013) 19:717–24. doi: 10.1016/j.parkreldis.2013.03.009
24. Compta Y, Valente T, Saura J, Segura B, Iranzo Á, Serradell M, et al. Correlates of cerebrospinal fluid levels of oligomeric- and total-α-synuclein in premotor, motor and dementia stages of Parkinson's disease. J Neurol. (2015) 262:294–306. doi: 10.1007/s00415-014-7560-z
25. Ffytche DH, Pereira JB, Ballard C, Chaudhuri KR, Weintraub D, Aarsland D. Risk factors for early psychosis in PD: insights from the Parkinson's progression markers initiative. J Neurol Neurosurg Psychiatry. (2017) 88:325–31. doi: 10.1136/jnnp-2016-314832
26. Gmitterová K, Gawinecka J, Llorens F, Varges D, Valkovic P, Zerr I. Cerebrospinal fluid markers analysis in the differential diagnosis of dementia with Lewy bodies and Parkinson's disease dementia. Eur Arch Psychiatry Clin Neurosci. (2018). doi: 10.1007/s00406-018-0928-9
27. Halbgebauer S, Nagl M, Klafki H, Haußmann U, Steinacker P, Oeckl P, et al. Modified serpinA1 as risk marker for Parkinson's disease dementia: analysis of baseline data. Sci Rep. (2016) 6:26145. doi: 10.1038/srep26145
28. Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. (2012) 69:1445–52. doi: 10.1001/archneurol.2012.1654
29. Hall S, Surova Y, Öhrfelt A, Zetterberg H, Lindqvist D, Hansson O. CSF biomarkers and clinical progression of Parkinson disease. Neurology. (2015) 84:57–63. doi: 10.1212/WNL.0000000000001098
30. Hansson O, Hall S, Ohrfelt A, Zetterberg H, Blennow K, Minthon L, et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson's disease with dementia and dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Res Ther. (2014) 6:25. doi: 10.1186/alzrt255
31. Janssens J, Vermeiren Y, Fransen E, Aerts T, Van Dam D, Engelborghs S, et al. Cerebrospinal fluid and serum MHPG improve Alzheimer's disease versus dementia with Lewy bodies differential diagnosis. Alzheimers Dement. (2018) 10:172–81. doi: 10.1016/j.dadm.2018.01.002
32. Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson's disease: associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. (2013) 33:183–9. doi: 10.1016/j.bbi.2013.07.007
33. Maetzler W, Liepelt I, Reimold M, Reischl G, Solbach C, Becker C, et al. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis. (2009) 34:107–12. doi: 10.1016/j.nbd.2008.12.008
34. Maetzler W, Stapf AK, Schulte C, Hauser AK, Lerche S, Wurster I, et al. Serum and cerebrospinal fluid uric acid levels in lewy body disorders: associations with disease occurrence and amyloid-β pathway. J Alzheimers Dis. (2011) 27:119–26. doi: 10.3233/JAD-2011-110587
35. Maetzler W, Tian Y, Baur SM, Gauger T, Odoj B, Schmid B, et al. Serum and cerebrospinal fluid levels of transthyretin in Lewy body disorders with and without dementia. PLoS ONE. (2012) 7:e48042. doi: 10.1371/journal.pone.0048042
36. Modreanu R, Cerquera SC, Martí MJ, Ríos J, Sánchez-Gómez A, Cámara A, et al. Cross-sectional and longitudinal associations of motor fluctuations and non-motor predominance with cerebrospinal τ and Aβ as well as dementia-risk in Parkinson's disease. J Neurol Sci. (2017) 373:223–9. doi: 10.1016/j.jns.2016.12.064
37. Parnetti L, Tiraboschi P, Lanari A, Peducci M, Padiglioni C, D'Amore C, et al. Cerebrospinal fluid biomarkers in Parkinson's disease with dementia and dementia with Lewy bodies. Biol Psychiatry. (2008) 64:850–5. doi: 10.1016/j.biopsych.2008.02.016
38. Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, Giannandrea D, et al. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson's Disease. Front Aging Neurosci. (2014) 6:53. doi: 10.3389/fnagi.2014.00053
39. Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol. (2017) 16:66–75. doi: 10.1016/S1474-4422(16)30328-3
40. Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, et al. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology. (2010) 75:1055–61. doi: 10.1212/WNL.0b013e3181f39a78
41. Stewart T, Liu C, Ginghina C, Cain KC, Auinger P, Cholerton B, et al. Parkinson Study Group DATATOP Investigators. Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol. (2014) 184:966–75. doi: 10.1016/j.ajpath.2013.12.007
42. Terrelonge M Jr, Marder KS, Weintraub D, Alcalay RN. CSF β-amyloid 1-42 predicts progression to cognitive impairment in newly diagnosed parkinson disease. J Mol Neurosci. (2016) 58:88–92. doi: 10.1007/s12031-015-0647-x
43. Vranová HP, Hényková E, Kaiserová M, Menšíková K, Vaštík M, Mareš J, et al. Tau protein, beta-amyloid1−42and clusterin CSF levels in the differential diagnosis of Parkinsonian syndrome with dementia. J Neurol Sci. (2014). 343:120–4. doi: 10.1016/j.jns.2014.05.052
44. Wennström M, Surova Y, Hall S, Nilsson C, Minthon L, Boström F, et al. Low CSF levels of both α-synuclein and the α-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS ONE. (2013) 8:e53250. doi: 10.1371/journal.pone.0053250
45. Liu C, Cholerton B, Shi M, Ginghina C, Cain KC, Auinger P, et al. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson's disease. Parkinson Relat Disord. (2015). 21:271–6. doi: 10.1016/j.parkreldis.2014.12.027
46. Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, Ginghina C, et al. CSF Aβ42 and tau in Parkinson's disease with cognitive impairment. Mov Disord. (2010) 25:2682–5. doi: 10.1002/mds.23287
47. Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. (2014) 71:1282–9. doi: 10.1001/jamaneurol.2014.1358
48. Skogseth RE, Bronnick K, Pereira JB, Mollenhauer B, Weintraub D, Fladby T, et al. Associations between cerebrospinal fluid biomarkers and cognition in early untreated Parkinson's disease. J Parkinsons Dis. (2015) 5:783–92. doi: 10.3233/JPD-150682
49. Zhou B, Wen M, Yu WF, Zhang CL, Jiao L. The diagnostic and differential diagnosis utility of cerebrospinal fluid α-synuclein levels in Parkinson's disease: a meta-analysis. Parkinsons Dis. (2015) 2015:567386 doi: 10.1155/2015/567386
50. Gao L, Tang H, Nie K, Wang L, Zhao J, Gan R, et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson's disease diagnosis: a systematic review and meta-analysis. Int J Neurosci. (2015) 125:645–54. doi: 10.3109/00207454.2014.961454
51. Stav AL, Aarsland D, Johansen KK, Hessen E, Auning E, Fladby T. Amyloid-β and α-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson's disease. Parkinsonism Relat Disord. (2015) 21:758–64. doi: 10.1016/j.parkreldis.2015.04.027
52. Buddhala C, Campbell MC, Perlmutter JS, Kotzbauer PT. Correlation between decreased CSF α-synuclein and Aβ1−42 in Parkinson disease Neurobiol Aging. (2015) 36:476–84. doi: 10.1016/j.neurobiolaging.2014.07.043
53. Hall S, Surova Y, Öhrfelt A, Swedish BioFINDER Study, Blennow K, Zetterberg H, et al. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson's Disease. Mov Disord. (2016) 31:898–905. doi: 10.1002/mds.26578
54. Lin CH, Yang SY, Horng HE, Yang CC, Chieh JJ, Chen HH, et al. Plasma α-synuclein predicts cognitive decline in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2017) 88:818–24. doi: 10.1136/jnnp-2016-314857
55. Delgado-Alvarado M, Gago B, Navalpotro-Gomez I, Jiménez-Urbieta H, Rodriguez-Oroz MC. Biomarkers for dementia and mild cognitive impairment in Parkinson's disease. Mov Disord. (2016) 31:861–81. doi: 10.1002/mds.26662
56. Annanmaki T, Pessala-Driver A, Hokkanen L, Murros K. Uric acid associates with cognition in Parkinson's disease. Parkinsonism Relat Disord. (2008) 14:576–8. doi: 10.1016/j.parkreldis.2007.11.001
57. Moccia M, Picillo M, Erro R, Vitale C, Longo K, Amboni M, et al. Is serum uric acid related to non-motor symptoms in de-novo Parkinson's disease patients? Parkinsonism Relat Disord. (2014) 20:772–5. doi: 10.1016/j.parkreldis.2014.03.016
58. Moccia M, Picillo M, Erro R, Vitale C, Longo K, Amboni M, et al. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson's disease. Eur J Neurol. (2015) 22:93–8. doi: 10.1111/ene.12533
59. Pellecchia MT, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson's disease patients. J Neurol. (2013) 260:438–44. doi: 10.1007/s00415-012-6648-6
60. Chen-Plotkin AS, Hu WT, Siderowf A, Weintraub D, Goldmann Gross R, Hurtig HI, et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol. (2011) 69:655–63. doi: 10.1002/ana.22271
61. Pellecchia MT, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, et al. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naïve Parkinson's disease. Eur J Neurol. (2014) 21:802–7. doi: 10.1111/ene.12137
62. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain. (2017) 140:1959–76. doi: 10.1093/brain/awx118
63. Prell T. Structural and functional brain patterns of non-motor syndromes in Parkinson's Disease. Front Neurol. (2018) 9:138. doi: 10.3389/fneur.2018.00138
64. Weintraub D, Dietz N, Duda JE, Wolk DA, Doshi J, Xie SX, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain. (2012). 135(Pt 1):170–180. doi: 10.1093/brain/awr277
65. Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry. (2012) 83:188–94. doi: 10.1136/jnnp-2011-300828
66. Lee JE, Cho KH, Song SK, Kim HJ, Lee HS, Sohn YH, et al. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2014) 85:7–16. doi: 10.1136/jnnp-2013-305062
67. Borroni B, Premi E, Formenti A, Turrone R, Alberici A, Cottini E, et al. Structural and functional imaging study in dementia with Lewy bodies and Parkinson's disease dementia. Parkinsonism Relat Disord. (2015) 21:1049–55. doi: 10.1016/j.parkreldis.2015.06.013
68. Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Brooks DJ, Barker RA, et al. Gray and white matter imaging: a biomarker for cognitive impairment in early Parkinson's disease? Mov Disord. (2016) 31:103–10. doi: 10.1002/mds.26312
69. Hattori T, Orimo S, Aoki S, Ito K, Abe O, Amano A, et al. Cognitive status correlates with white matter alteration in Parkinson's disease. Hum Brain Mapp. (2012) 33:727–39. doi: 10.1002/hbm.21245
70. Kandiah N, Zainal NH, Narasimhalu K, Chander RJ, Ng A, Mak E, et al. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson's disease. Parkinsonism Relat Disord. (2014) 20:1203–8. doi: 10.1016/j.parkreldis.2014.08.024
71. Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A. Grey matter changes in cognitively impaired Parkinson's disease patients. PLoS ONE. (2014) 9:e85595. doi: 10.1371/journal.pone.0085595
72. Biundo R, Calabrese M, Weis L, Facchini S, Ricchieri G, Gallo P, et al. Anatomical correlates of cognitive functions in early Parkinson's disease patients. PLoS ONE. (2013) 8:e64222. doi: 10.1371/journal.pone.0064222
73. Pereira JB, Svenningsson P, Weintraub D, Brønnick K, Lebedev A, Westman E, et al. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology. (2014) 82:2017–25. doi: 10.1212/WNL.0000000000000483
74. Hanganu A, Bedetti C, Degroot C, Mejia-Constain B, Lafontaine AL, Soland V, et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson's disease longitudinally. Brain. (2014) 137(Pt 4):1120–9. doi: 10.1093/brain/awu036
75. Ibarretxe-Bilbao N, Junque C, Segura B, Baggio HC, Marti MJ, Valldeoriola F, et al. Progression of cortical thinning in early Parkinson's disease. Mov Disord. (2012) 27:1746–53. doi: 10.1002/mds.25240
76. Mak E, Su L, Williams GB, Firbank MJ, Lawson RA, Yarnall AJ, et al. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain. (2015) 138(Pt 10):2974–86. doi: 10.1093/brain/awv211
77. Hwang KS, Beyer MK, Green AE, Chung C, Thompson PM, Janvin C, et al. Mapping cortical atrophy in Parkinson's disease patients with dementia. J Parkinsons Dis. (2013) 3:69–76. doi: 10.3233/JPD-120151
78. Zarei M, Ibarretxe-Bilbao N, Compta Y, Hough M, Junque C, Bargallo N, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2013) 84:875–81. doi: 10.1136/jnnp-2012-304126
79. Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, Llebaria G, García-Sánchez C, Pascual-Sedano B, et al. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson's disease. PLoS ONE. (2013) 8:e54980. doi: 10.1371/journal.pone.0054980
80. Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. (2012) 78:1939–45. doi: 10.1212/WNL.0b013e318259e1c5
81. Chen B, Fan GG, Liu H, Wang S. Changes in anatomical and functional connectivity of Parkinson's disease patients according to cognitive status. Eur J Radiol. (2015) 84:1318–24. doi: 10.1016/j.ejrad.2015.04.014
82. Gorges M, Müller HP, Lulé D; LANDSCAPE Consortium, Pinkhardt EH, Ludolph AC, et al. To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol Aging. (2015) 36:1727–35. doi: 10.1016/j.neurobiolaging.2014.12.026
83. Baggio HC, Segura B, Sala-Llonch R, Marti MJ, Valldeoriola F, Compta Y, et al. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Hum Brain Mapp. (2015) 36:199–212. doi: 10.1002/hbm.22622
84. Amboni M, Tessitore A, Esposito F, Santangelo G, Picillo M, Vitale C, et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson's disease. J Neurol. (2015) 262:425–34. doi: 10.1007/s00415-014-7591-5
85. Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. (2012) 79:2226–32. doi: 10.1212/WNL.0b013e31827689d6
86. Rektorova I, Krajcovicova L, Marecek R, Mikl M. Default mode network and extrastriate visual resting state network in patients with Parkinson's disease dementia. Neurodegener Dis. (2012) 10:232–7. doi: 10.1159/000334765
87. Olde Dubbelink KT, Schoonheim MM, Deijen JB, Twisk JW, Barkhof F, Berendse HW. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology. (2014) 83:2046–53. doi: 10.1212/WNL.0000000000001020
88. Seibert TM, Murphy EA, Kaestner EJ, Brewer JB. Interregional correlations in Parkinson disease and Parkinson-related dementia with resting functional MR imaging. Radiology. (2012) 263:226–34. doi: 10.1148/radiol.12111280
89. Lin WC, Chen PC, Huang YC, Tsai NW, Chen HL, Wang HC, et al. Dopaminergic therapy modulates cortical perfusion in parkinson disease with and without dementia according to arterial spin labeled perfusion magnetic resonance imaging. Medicine. (2016) 95:e2206. doi: 10.1097/MD.0000000000002206
90. Le Heron CJ, Wright SL, Melzer TR, Myall DJ, MacAskill MR, Livingston L, et al. Comparing cerebral perfusion in Alzheimer's disease and Parkinson's disease dementia: an ASL-MRI study. J Cereb Blood Flow Metab. (2014) 34:964–70. doi: 10.1038/jcbfm.2014.40
91. Vander Borght T, Minoshima S, Giordani B, Foster NL, Frey KA, Berent S, et al. Cerebral metabolic differences in Parkinson's and Alzheimer's diseases matched for dementia severity. J Nucl Med. (1997) 38:797–802.
92. González-Redondo R, García-García D, Clavero P, Gasca-Salas C, García-Eulate R, Zubieta JL, et al. Grey matter hypometabolism and atrophy in Parkinson's disease with cognitive impairment: a two-step process. Brain. (2014). 137(Pt 8):2356–67. doi: 10.1093/brain/awu159
93. Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka S, Iyo M, et al. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson's disease and progressive supranuclear palsy. Ann Neurol. (1999) 46:62–9. doi: 10.1002/1531-8249(199907)46:1<62::AID-ANA10>3.0.CO;2-P
94. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. (2003) 60:1745–8. doi: 10.1001/archneur.60.12.1745
95. Hiraoka K, Okamura N, Funaki Y, Hayashi A, Tashiro M, Hisanaga K, et al. Cholinergic deficit and response to donepezil therapy in Parkinson's disease with dementia. Eur Neurol. (2012) 68:137–143. doi: 10.1159/000338774
96. Kotagal V, Müller ML, Kaufer DI, Koeppe RA, Bohnen NI. Thalamic cholinergic innervation is spared in Alzheimer disease compared to parkinsonian disorders. Neurosci Lett. (2012) 514:169–72. doi: 10.1016/j.neulet.2012.02.083
97. Ramírez-Ruiz B, Martí MJ, Tolosa E, Bartrés-Faz D, Summerfield C, Salgado-Pineda P, et al. Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol. (2005) 252:1345–52. doi: 10.1007/s00415-005-0864-2
98. Morales DA, Vives-Gilabert Y, Gómez-Ansón B, Bengoetxea E, Larra-aga P, Bielza C, et al. Predicting dementia development in Parkinson's disease using bayesian network classifiers. Psychiatry Res. (2013) 213:92–8. doi: 10.1016/j.pscychresns.2012.06.001
99. Schulz J, Pagano G, Fernández Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson's disease. Brain. (2018) 141:1501–16. doi: 10.1093/brain/awy072
100. Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff's Disease. Acta Neuropathol. (1983) 61:101–8. doi: 10.1007/BF00697388
101. Candy JM, Perry RH, Perry EK, Irving D, Blessed G, Fairbairn AF, et al. Pathological changes in the nucleus of meynert in Alzheimer's and Parkinson's diseases. J Neurol Sci. (1983) 59:277–89. doi: 10.1016/0022-510X(83)90045-X
102. Agosta F, Canu E, Stefanova E, Sarro L, Tomić A, Špica V, et al. Mild cognitive impairment in Parkinson's disease is associated with a distributed pattern of brain white matter damage. Hum Brain Mapp. (2014) 35:1921–9. doi: 10.1002/hbm.22302
103. Theilmann RJ, Reed JD, Song DD, Huang MX, Lee RR, Litvan I, et al. White-matter changes correlate with cognitive functioning in Parkinson's disease. Front Neurol. (2013) 4:37. doi: 10.3389/fneur.2013.00037
104. Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson's disease. Hum Brain Mapp. (2014) 35:1325–33. doi: 10.1002/hbm.22256
105. Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, Lou JS, et al. (2007). Working Group on Fatigue in Parkinson's Disease.Fatigue in Parkinson's disease: a review. Mov Disord. 22:297–308. doi: 10.1002/mds.21240
106. Friedman JH, Beck JC, Chou KL, Clark G, Fagundes CP, Goetz CG, et al. Fatigue in Parkinson's disease: report from a mutidisciplinary symposium. NPJ Parkinsons Dis. (2016) 2:15025. doi: 10.1038/npjparkd.2015.25
107. Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain Behav Immun. (2012) 26:1202–10. doi: 10.1016/j.bbi.2012.06.006
108. Chou KL, Gilman S, Bohnen NI. Association between autonomic dysfunction and fatigue in Parkinson disease. J Neurol Sci. (2017) 377:190–2. doi: 10.1016/j.jns.2017.04.023
109. Alves G, Wentzel-Larsen T, Larsen JP. Is fatigue an independent and persistent symptom in patients with Parkinson disease? Neurology. (2004) 63:1908–11. doi: 10.1212/01.WNL.0000144277.06917.CC
110. van Hilten JJ, Weggeman M, van der Velde EA, Kerkhof GA, van Dijk JG, Roos RA. Sleep, excessive daytime sleepiness and fatigue in Parkinson's disease. J Neural Transm Park Dis Dement Sect. (1993) 5:235–44. doi: 10.1007/BF02257678
111. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. (1989) 46:1121–3. doi: 10.1001/archneur.1989.00520460115022
112. Kluger BM, Herlofson K, Chou KL, Lou JS, Goetz CG, Lang AE, et al. Parkinson's disease-related fatigue: A case definition and recommendations for clinical research. Mov Disord. (2016) 31:625–31. doi: 10.1002/mds.26511
113. Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. (2004) 363:978–88. doi: 10.1016/S0140-6736(04)15794-2
114. Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. (2007) 21:863–71. doi: 10.1016/j.bbi.2007.03.013
115. Herlofson K, Heijnen CJ, Lange J, Alves G, Tysnes OB, Friedman JH, et al. Inflammation and fatigue in early, untreated Parkinson's disease. Acta Neurol Scand. (2018) 138:394–9. doi: 10.1111/ane.12977
116. Pereira JR, Santos LVD, Santos RMS, Campos ALF, Pimenta AL, de Oliveira MS, et al. IL-6 serum levels are elevated in Parkinson's disease patients with fatigue compared to patients without fatigue. J Neurol Sci. (2016) 370:153–6. doi: 10.1016/j.jns.2016.09.030
117. Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. (2012) 37:1397–416. doi: 10.1016/j.psyneuen.2012.03.019
118. Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. (2013) 30:297–306. doi: 10.1002/da.22084
119. Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-motor symptoms in patients with Parkinson's disease - correlations with inflammatory cytokines in serum. PLoS ONE. (2012) 7:e47387. doi: 10.1371/journal.pone.0047387
120. Huang X, Ng SY, Chia NS, Acharyya S, Setiawan F, Lu ZH, et al. Serum uric acid level and its association with motor subtypes and non-motor symptoms in early Parkinson's disease: PALS study. Parkinsonism Relat Disord. (2018) 55:50–54. doi: 10.1016/j.parkreldis.2018.05.010
121. Tessitore A, Giordano A, De Micco R, Caiazzo G, Russo A, Cirillo M, et al. Functional connectivity underpinnings of fatigue in “Drug-Naïve” patients with Parkinson's disease. Mov Disord. (2016) 31:1497–505. doi: 10.1002/mds.26650
122. Zhang JJ, Ding J, Li JY, Wang M, Yuan YS, Zhang L, et al. Abnormal resting-state neural activity and connectivity of fatigue in Parkinson's disease. CNS Neurosci Ther. (2017) 23:241–7. doi: 10.1111/cns.12666
123. Li J, Yuan Y, Wang M, Zhang J, Zhang L, Jiang S, et al. Alterations in regional homogeneity of resting-state brain activity in fatigue of Parkinson's disease. J Neural Transm. (2017) 124:1187–95. doi: 10.1007/s00702-017-1748-1
124. Cho SS, Aminian K, Li C, Lang AE, Houle S, Strafella AP. Fatigue in Parkinson's disease: The contribution of cerebral metabolic changes. Hum Brain Mapp. (2017) 38:283–92. doi: 10.1002/hbm.23360
125. Abe K, Takanashi M, Yanagihara T. Fatigue in patients with Parkinson's disease. Behav Neurol. (2000) 12:103–6. doi: 10.1155/2000/580683
126. Zhang L, Li T, Yuan Y, Tong Q, Jiang S, Wang M, et al. Brain metabolic correlates of fatigue in Parkinson's disease: a PET study. Int J Neurosci. (2018) 128:330–6. doi: 10.1080/00207454.2017.1381093
127. Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain. (2010) 133:3434–43. doi: 10.1093/brain/awq268
128. Politis M, Loane C. Serotonergic dysfunction in Parkinson's disease and its relevance to disability. Scientific World Journal. (2011) 11:1726–34. doi: 10.1100/2011/172893
129. Schifitto G, Friedman JH, Oakes D, Shulman L, Comella CL, Marek K, et al. Investigators. Fatigue in levodopa-naive subjects with Parkinson disease. Neurology. (2008) 71:481–5. doi: 10.1212/01.wnl.0000324862.29733.69
130. Chou KL, Kotagal V, Bohnen NI. Neuroimaging and clinical predictors of fatigue in Parkinson disease. Parkinsonism Relat Disord. (2016) 23:45–9. doi: 10.1016/j.parkreldis.2015.11.029
131. Lou JS, Benice T, Kearns G, Sexton G, Nutt J. Levodopa normalizes exercise related cortico-motoneuron excitability abnormalities in Parkinson's disease. Clin Neurophysiol. (2003) 114:930–7. doi: 10.1016/S1388-2457(03)00040-3
132. Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. (2001) 124(Pt 11):2131–46. doi: 10.1093/brain/124.11.2131
133. Fabbrini G, Latorre A, Suppa A, Bloise M, Frontoni M, Berardelli A. Fatigue in Parkinson's disease: motor or non-motor symptom? Parkinsonism Relat Disord. (2013) 19:148–52. doi: 10.1016/j.parkreldis.2012.10.009
134. Becker C, Brobert GP, Johansson S, Jick SS, Meier CR. Risk of incident depression in patients with Parkinson disease in the UK. Eur J Neurol. (2011) 18:448–53. doi: 10.1111/j.1468-1331.2010.03176.x
135. Pessoa Rocha N, Reis HJ, Vanden Berghe P, Cirillo C. Depression and cognitive impairment in Parkinson's disease: a role for inflammation and immunomodulation? Neuroimmunomodulation. (2014) 21:88–94. doi: 10.1159/000356531
136. Karpenko MN, Vasilishina AA, Gromova EA, Muruzheva ZM, Bernadotte A. Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson's disease. Cell Immunol. (2018) 327:77–82. doi: 10.1016/j.cellimm.2018.02.011
137. Veselý B, Dufek M, Thon V, Brozman M, Királová S, Halászová T, et al. Interleukin 6 and complement serum level study in Parkinson's disease. J Neural Transm. (2018). 125:875–81. doi: 10.1007/s00702-018-1857-5
138. Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. (2001) 8:131–6. doi: 10.1097/00062752-200105000-00001
139. Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep. (2016) 13:3391–6. doi: 10.3892/mmr.2016.4948
140. Brites D, Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci. (2015) 9:476. doi: 10.3389/fncel.2015.00476
141. Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. (2016) 73:1316–24. doi: 10.1001/jamaneurol.2016.2742
142. Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, et al. Depression in Parkinson's disease. Diffusion tensor imaging study. J Neurol. (2007) 254:1170–3. doi: 10.1007/s00415-006-0236-6
143. Feldmann A, Illes Z, Kosztolanyi P, Illes E, Mike A, Kover F, et al. Morphometric changes of gray matter in Parkinson's disease with depression: a voxel-based morphometry study. Mov Disord. (2008) 23:42–6. doi: 10.1002/mds.21765
144. Kostić VS, Agosta F, Petrović I, Galantucci S, Spica V, Jecmenica-Lukic M, et al. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. (2010) 75:857–63. doi: 10.1212/WNL.0b013e3181f11c1d
145. Surdhar I, Gee M, Bouchard T, Coupland N, Malykhin N, Camicioli R. Intact limbic-prefrontal connections and reduced amygdala volumes in Parkinson's disease with mild depressive symptoms. Parkinsonism Relat Disord. (2012) 18:809–13. doi: 10.1016/j.parkreldis.2012.03.008
146. van Mierlo TJ, Chung C, Foncke EM, Berendse HW, van den Heuvel OA. Depressive symptoms in Parkinson's disease are related to decreased hippocampus and amygdala volume. Mov Disord. (2015) 30:245–52. doi: 10.1002/mds.26112
147. Huang C, Ravdin LD, Nirenberg MJ, Piboolnurak P, Severt L, Maniscalco JS, et al. Neuroimaging markers of motor and nonmotor features of Parkinson's disease: an 18f fluorodeoxyglucose positron emission computed tomography study. Dement Geriatr Cogn Disord. (2013) 35:183–96. doi: 10.1159/000345987
148. O'Callaghan C, Shine JM, Lewis SJ, Hornberger M. Neuropsychiatric symptoms in Parkinson's disease: fronto-striatal atrophy contributions. Parkinsonism Relat Disord. (2014) 20:867–72. doi: 10.1016/j.parkreldis.2014.04.027
149. Huang P, Lou Y, Xuan M, Gu Q, Guan X, Xu X, et al. Cortical abnormalities in Parkinson's disease patients and relationship to depression: A surface-based morphometry study. Psychiatry Res Neuroimag. (2016) 250:24–8. doi: 10.1016/j.pscychresns.2016.03.002
150. Cardoso EF, Maia FM, Fregni F, Myczkowski ML, Melo LM, Sato JR, et al. Depression in Parkinson's disease: convergence from voxel-based morphometry and functional magnetic resonance imaging in the limbic thalamus. Neuroimage. (2009) 47:467–72. doi: 10.1016/j.neuroimage.2009.04.059
151. Lou Y, Huang P, Li D, Cen Z, Wang B, Gao J, et al. Altered brain network centrality in depressed Parkinson's disease patients. Mov Disord. (2015) 30:1777–84. doi: 10.1002/mds.26321
152. Boileau I, Warsh JJ, Guttman M, Saint-Cyr JA, McCluskey T, Rusjan P, et al. Elevated serotonin transporter binding in depressed patients with Parkinson's disease: a preliminary PET study with [11C]DASB. Mov Disord. (2008) 23:1776–80. doi: 10.1002/mds.22212
153. Politis M, Wu K, Loane C, Turkheimer FE, Molloy S, Brooks DJ, et al. Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurology. (2010) 75:1920–7. doi: 10.1212/WNL.0b013e3181feb2ab
154. Ballanger B, Klinger H, Eche J, Lerond J, Vallet AE, Le Bars D, et al. Role of serotonergic 1A receptor dysfunction in depression associated with Parkinson's disease. Mov Disord. (2012) 27:84–9. doi: 10.1002/mds.23895
155. Guttman M, Boileau I, Warsh J, Saint-Cyr JA, Ginovart N, McCluskey T, et al. Brain serotonin transporter binding in non-depressed patients with Parkinson's disease. Eur J Neurol. (2007) 14:523–8. doi: 10.1111/j.1468-1331.2007.01727.x
156. Maillet A, Krack P, Lhommée E, Météreau E, Klinger H, Favre E, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain. (2016) 139(Pt 9):2486–502. doi: 10.1093/brain/aww162
157. Skidmore FM, Yang M, Baxter L, von Deneen K, Collingwood J, He G, et al. Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease. Neuroimage. (2013) 81:484–95. doi: 10.1016/j.neuroimage.2011.07.012
158. Tong Q, Zhang L, Yuan Y, Jiang S, Zhang R, Xu Q, et al. Reduced plasma serotonin and 5-hydroxyindoleacetic acid levels in Parkinson's disease are associated with nonmotor symptoms. Parkinsonism Relat Disord. (2015) 21:882–7. doi: 10.1016/j.parkreldis.2015.05.016
159. Svenningsson P, Pålhagen S, Mathé AA. Neuropeptide Y and calcitonin gene-related peptide in cerebrospinal fluid in parkinson's disease with comorbid depression versus patients with major depressive disorder. Front Psychiatry. (2017) 8:102. doi: 10.3389/fpsyt.2017.00102
160. Thobois S, Ardouin C, Lhommée E, Klinger H, Lagrange C, Xie J, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson's disease: predictors and underlying mesolimbic denervation. Brain. (2010) 133(Pt 4):1111–27. doi: 10.1093/brain/awq032
161. Koerts J, Leenders KL, Koning M, Portman AT, van Beilen M. Striatal dopaminergic activity (FDOPA-PET) associated with cognitive items of a depression scale (MADRS) in Parkinson's disease. Eur J Neurosci. (2007) 25:3132–6. doi: 10.1111/j.1460-9568.2007.05580.x
Keywords: Parkinson's disease, biomarker, non-motor syndromes, depression, fatigue, dementia
Citation: Prell T, Witte OW and Grosskreutz J (2019) Biomarkers for Dementia, Fatigue, and Depression in Parkinson's Disease. Front. Neurol. 10:195. doi: 10.3389/fneur.2019.00195
Received: 01 October 2018; Accepted: 15 February 2019;
Published: 08 March 2019.
Edited by:
Tobias Warnecke, University Hospital Münster, GermanyReviewed by:
Walter Maetzler, University of Kiel, GermanyGennaro Pagano, King's College London, United Kingdom
Copyright © 2019 Prell, Witte and Grosskreutz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tino Prell, dGluby5wcmVsbEBtZWQudW5pLWplbmEuZGU=
 Tino Prell
Tino Prell Otto W. Witte
Otto W. Witte Julian Grosskreutz
Julian Grosskreutz