- 1Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, Netherlands
- 2Department of Primary and Community Care, Radboud university medical center, Nijmegen, Netherlands
- 3Departments of Neurology and Anesthesiology, Pain and Palliative Care, Radboud university medical center, Nijmegen, Netherlands
- 4Department of Neurology, Donders Institute for Brain, Cognition and Behaviour, Radboud university medical center, Nijmegen, Netherlands
- 5Groenhuysen Organisation, Roosendaal, Netherlands
- 6Dutch Parkinson's Disease Association, Bunnik, Netherlands
- 7Department of Anesthesiology, Pain and Palliative Care/Expertise Center for Palliative Care, Radboud university medical center, Nijmegen, Netherlands
- 8Radboudumc Alzheimer Center, Nijmegen, Netherlands
- 9De Waalboog “Joachim en Anna,” Center for Specialized Geriatric Care, Nijmegen, Netherlands
Dementia and Parkinson's disease are incurable neurological conditions. Patients often experience specific, complex, and varying needs along their disease trajectory. Current management typically employs a multidisciplinary team approach. Recognition is growing that this team approach should also address palliative care issues to optimize quality of life for patient and family caregivers, but it remains unclear how palliative care is best delivered. To inspire future service development and research, we compare the trajectories and conceptualization of palliative care between dementia and Parkinson's disease. Both Parkinson's disease and dementia are characterized by a protracted course, with progressive but fairly insidious development of disability. However, patients with Parkinson's disease may experience relatively stable periods initially but with time, a wide range of debilitating symptoms develops, many of which do not respond well to treatment. Eventually, dementia develops in most Parkinson patients, while motor disability develops in many dementia patients. In both diseases, symptoms such as pain, apathy, sleeping problems, falls, and a high caregiver burden are prevalent. Advance care planning has benefits in terms of being prepared before the disease progresses into a stage with communication problems or severe cognitive impairment. However, for both conditions, the protracted disease trajectories complicate conceptualization of palliative care through different stages of the disease, with pertinent questions such as when to offer what interventions pro-actively. Given the similarities and differences, we should develop palliative approaches that are partially generic and partially disease-specific. These should be integrated seamlessly with disease-specific care. Substantial research is already being performed on dementia palliative care. This may also inform the further development of palliative care for Parkinson's disease, including an evaluation of palliative interventions and services.
Introduction
Palliative care has been developed to improve quality of life, mostly for patients with incurable cancer (1, 2). However, equity of access to palliative care involves access on the same footing for patients with other incurable diseases. This does not mean that palliative care is, or should be, the same across these diseases. On the contrary, to optimally tailor care to individuals, the contents of palliative care, and how, when and where it is delivered, can and in fact should differ between diseases.
Along the trajectory of chronic-progressive and incurable neurological diseases such as dementia or Parkinson's disease (PD), various complex needs arise, some of which are disease-specific. Palliative care promotes quality of life in the face of any life-threatening, or progressive, incurable illness (3, 4). To optimize it for individuals, however, a good understanding of disease-specific aspects of palliative care is helpful, i.e., a conceptualization of what palliative care entails exactly for a specific disease.
Epidemiology of Dementia and Parkinson's Disease (PD)
Dementia and PD are both diagnosed frequently and increase mortality (5, 6). Perhaps dementia is perceived more so as a memory problem and a disease of old age, but the incidence of dementia and PD in younger age is similar. In the Netherlands, for dementia, the incidence per 1,000 person-years is 0.4 among those aged 60–64 (7), and for PD, it is 0.3 (ages 55–65) (8, 9). Dementia incidence patterns, however, show a much steeper increase with age; mounting to 27 per 1,000 person-years for those 85 and over, compared to 4 for PD over 85. In view of similar mortality (6), therefore, the prevalence of dementia in the general population is much higher than prevalence of PD (8–11). However, adjusted for age and other factors, 6-year mortality in PD is higher than in Alzheimer's dementia (6). Age adjustment is relevant also as it shows that comorbid disease may be equally prevalent for Alzheimer's–a main type of dementia–and PD across the same age groups (12).
Comparing Trajectories and Conceptualization of Palliative Care for Dementia and PD
The two disease trajectories may overlap partly as dementia is a frequent manifestation of PD. Mild cognitive impairment may already present upon diagnosis of PD (13). Importantly, it is independently associated with lower quality of life (14). Across studies, typically about a quarter of patients with PD have dementia (15, 16), but ultimately, most develop dementia (15–17).
A clear conceptualization of palliative care in chronic-progressive diseases is important for the development of healthcare systems that facilitate the integration of a palliative approach (18). Therefore, in this article we compare the disease trajectories of dementia and PD in as far as relevant for the conceptualizations of palliative care. We do not include atypical Parkinsonian disorders such as multiple system atrophy because these warrant a special approach with earlier palliative care (19). We first provide background on where we are by describing how palliative care for dementia and PD developed.
Palliative Care in Dementia
The first evaluated palliative care program specific to dementia was described in 1986 (20). The volume of research has grown exponential after 2000 (21, 22). There are few randomized controlled trials, and therefore, there is still little evidence on effectiveness (23, 24). However, many western countries have funded observational studies resulting in numerous publications describing patient, family and professional caregiver needs (25, 26).
Research specific to dementia is important because the course of the disease is highly variable and uncertain. Because of the progressive dementia, patients themselves often cannot remain involved in decision making. Also, health services and changes such as transfer to a hospice, do not necessarily represent optimal care for people with dementia (27). Palliative care in dementia needed a clear conceptualization, and the European Association for Palliative Care (EAPC) along with experts agreed to a distinct concept in terms of eleven domains, different from “usual” palliative care (28).
Palliative Care in Parkinson's Disease
Palliative care for people with PD and their caregivers has progressed over the last 10 years but it is still an upcoming field. Evidence of effects is limited (17, 29, 30) but trials are underway (31). Qualitative studies on palliative care needs (32–34) and natural history studies (35–37) have indicated that the needs of people with advanced PD are complex. Awareness of the potential benefit of palliative care is growing, but we know little about useful components (17, 29). To the best of our knowledge, there is no clear conceptualization of the specifics of palliative care in PD.
Differences and Similarities
We highlight key similarities and differences between the trajectories and perceptions of the disease, and treatment and care for people with dementia and PD based on recent literature. The comparator is a population without the disease, sometimes matched or adjusted for differences such as age or co-morbidities.
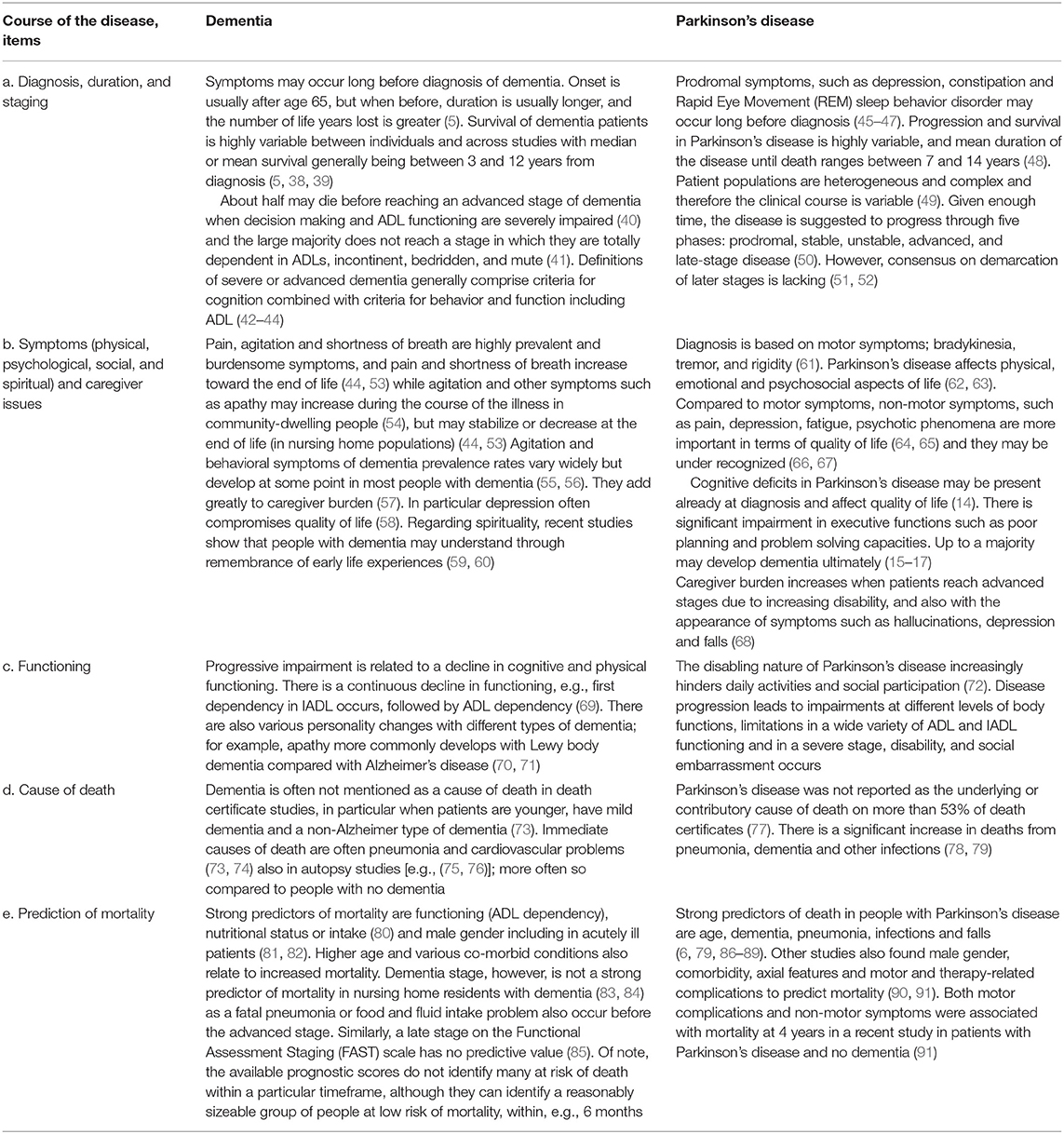
The Disease Trajectories
With both diseases, the diagnosis may be delayed due to gradual onset with a-specific symptoms after which burdensome symptoms develop, while the disease duration is highly variable (Table 1, items a and b). Burdensome symptoms that decrease quality of life often include rather unspecific symptoms such as pain and depression. Clearly, PD is distinct from dementia as it is characterized by its motor symptoms, such as bradykinesia, rigidity, and tremor. Symptomatic treatments are available and with the right therapeutic approach, the course of PD typically includes an initially relatively stable phase.
For PD patients, loss of functional ability (item c) occurs with symptoms that are largely unresponsive to treatment speech problems, postural imbalance and cognitive deterioration; (51, 92) or with symptoms that may worsen due to treatment (psychosis, orthostatic hypotension) (51). Other reasons for functional deterioration are age-related comorbid disease (92) and under-treatment of symptoms (93, 94), which also happen with dementia. With dementia, loss of motor or functional ability often relates to progressive cognitive dysfunction.
Defining a severe or advanced stage of the disease (item a) has been recognized as important for palliative care in case of dementia (42–44). For PD, the most widely used measure to define disease stage is the Hoehn and Yahr scale (51, 52, 95). However, it selectively focuses on motor function. A recent consensus study defined key factors for diagnosing advanced PD including, for example, ADL impairment, and dementia (96).
The stage of dementia is often being perceived as relevant for palliative care although there is no consensus how exactly (28). Also, it is not a particularly strong predictor of mortality among those with moderate or severe dementia, despite sensitive measures; this may be related to uncertainty as to in what stage acute problems such as pneumonia develop or different resilience among long-term survivors (Table 1, items d and e) (74, 83, 84). Similarly, a late stage on the Functional Assessment Staging (FAST) scale has shown no predictive value (85), but practice lags behind, still promoting it for prognostication in dementia (97). ADL dependency, on the other hand, is a strong predictor of mortality in dementia (44, 80, 81). In contrast, it may not predict mortality in PD well (98). In PD, dementia or cognitive impairment independently predicts mortality (6, 35, 99).
Pneumonia is a relatively frequent cause of death in dementia and in PD (74, 78, 81). However, well-known problems in coding practice include dementia being grossly underreported on the death certificate (73), also in those with PD (78). Similarly, PD often goes unreported (79).
The overlap between PD and dementia is significant as up to a majority of patients with PD eventually develop dementia (9, 16, 17), due to spreading of Lewy bodies. Because of initial stability and uncertainty as to whether patients develop severe cognitive problems or die before, PD may be perceived as an even more protracted disease course than the dementias.
Conceptualization of the Diseases, Needs, and Interventions
Both dementia and PD are incurable and progressive diseases with often complex problems and needs, for which tailored interventions are available (Table 2, items a–d). For dementia, experts agree that “recognizing its eventual terminal nature is the basis for anticipating future problems and an impetus to the provision of adequate palliative care” (28). Some advocate advanced dementia to be a terminal disease to support eligibility for palliative care. However, as about half of dementia patients never reach an advanced stage (Table 1); (40), it may be a late trigger to initiate palliative care. There is no consensus, however, at which stage palliative care in dementia should start (153, 154).
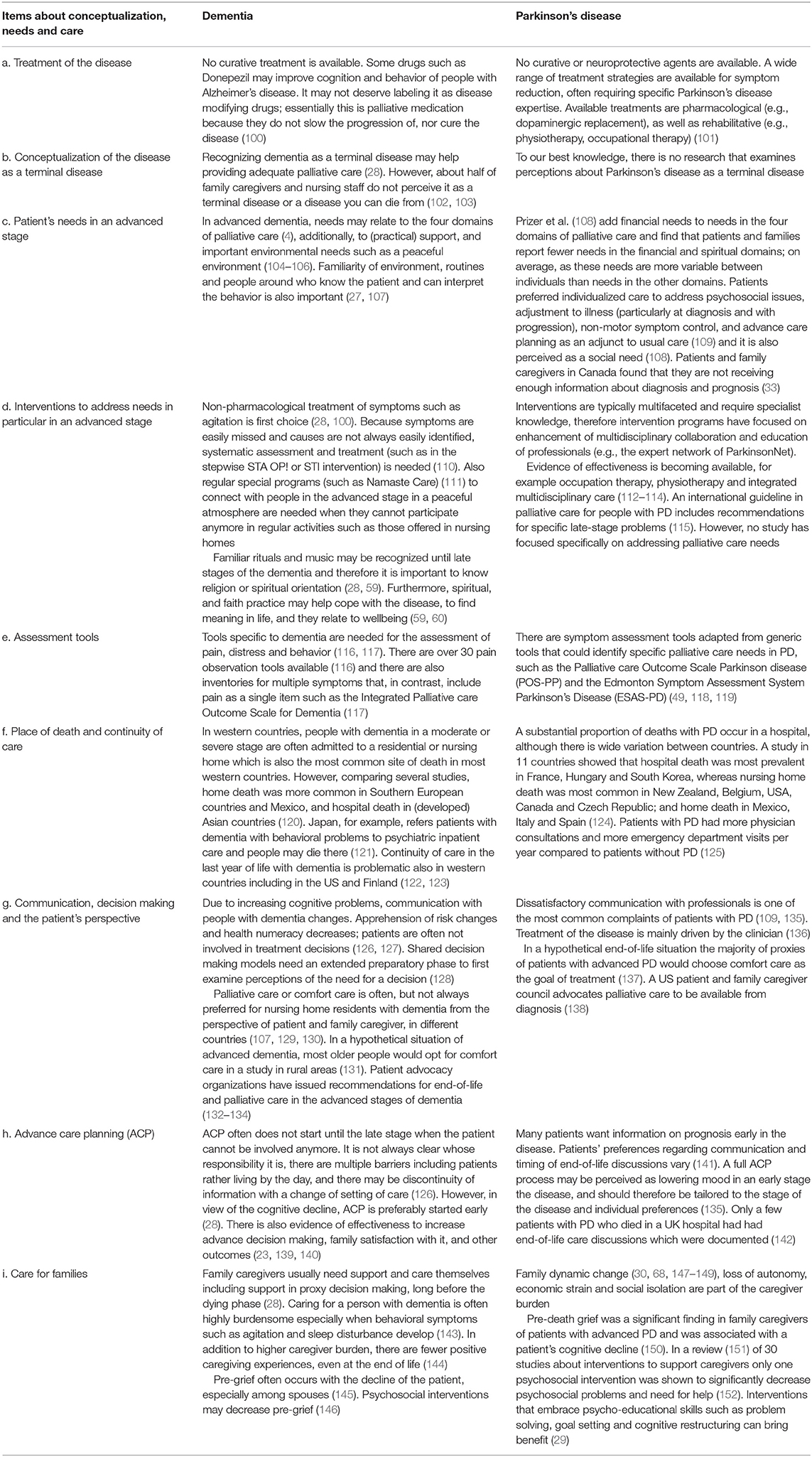
Table 2. Conceptualization of the disease, needs of patients and family caregivers, and interventions.
For PD there are no curative treatments either, but the success of dopaminergic replacement therapy and deep brain stimulation has enabled the majority of patients to live independently with a relatively low symptom burden for the first 10 years after diagnosis-when they live up to a decade (48). This may contribute to PD generally not being recognized as an illness for which a palliative approach may be helpful (155, 156). A US patient and caregivers council recommends palliative care to be available from diagnosis of PD (138). This is also the ideal of the European Parkinson's Disease Association (EPDA) (157) although they emphasize that when to start palliative care is an individual decision.
Patients with dementia may have a number of needs in the four domains of palliative care (physical, psychological, social and spiritual) in addition to specific needs for a peaceful, familiar environment, and practical support (104–106). Typically, complex, multifaceted interventions could address needs. Psychosocial needs may be pronounced in young-onset dementia (onset under age 65) (158, 159). Patient advocacy organizations recognize the importance of high-quality end-of-life and palliative care in the advanced stages (132–134).
To measure symptom burden, specific tools are available (Table 2, item e). For dementia, these typically involve proxy (caregiver) report. Quality of care and dying assessment tools specifically developed for dementia show the best psychometric properties (129). For PD, there are adapted versions of generic tools (118, 160). Regardless, effective use of tools and implementation of complex interventions requires multidisciplinary communication and team work (161).
Place of death varies by country (Table 2, item f). Patterns are similar for dementia (120, 121) and PD (124), with dying in nursing homes being common in many western countries except Southern European countries with more frequent home death, while hospital death is more common in Asian countries, France and Hungary. In the UK, a trend of decreasing hospital death and increasing nursing home death has been observed in dementia (162). However, continuity of care may be problematic across countries, with nursing home or hospital admissions at the end of life in people with dementia and PD (122–124, 163). Also, with PD, specific knowledge of the disease is often suboptimal among nursing staff in nursing homes, while upon admission, neurologists often stop seeing patients with PD, with communication of neurologists with primary care being suboptimal too (93, 94).
“Person-centered care, communication and shared decision making” was among the most important domains of palliative care in dementia according to experts around the world, and it was prioritized for research (28). Advance care planning (ACP) is a special form of ongoing communication about preferred future health care (Table 2, items g and h). Researchers and policy makers are increasingly interested in researching and implementing ACP, and some beneficial effects have been documented in dementia (23, 139, 140). However, there are numerous barriers such as patient and family expecting the physician to start it while physicians may not prioritize such anticipatory care. Also, while many appreciate discussions, not all would like to decide about future treatment (126, 164). Similar barriers might exist in PD (141). In a synthesis of studies, interview studies have shown physicians to be hesitant in discussing progression of the disease in early disease stages as they fear to diminish hope (17). Also patients indicate a conflicted need as they reported both a wish for more information on disease progression and death, and fear to receive the information (“an information tension”) (17).
Family caregivers usually have information and support needs in regards to proxy decision making and how to best care for their loved one (28, 165) (Table 2, item i). They may experience a magnitude of stress in caregiving and pre-grief in dementia (145) and PD caregiving (150). In dementia, anticipatory grief in response to compounded serial losses is common, and stress in caregiving preceding physical death may be equal to or greater than stress in bereavement. Roland et al. (166) found caregiving experiences and stressors to be similar between caregivers care for a patient with dementia, PD, and PD and dementia.
Disease-Specific Palliative Care and Practice
Disease-specific palliative care is needed; services and tools taken uncritically from cancer palliative care have shown to not fit well with dementia and require adaptation or even redevelopment from scratch (27, 167, 168). Palliative care specialists, however, may not know enough about the specifics of dementia and PD. In addition to suboptimal access to palliative care (17) access to disease-specific multidisciplinary care for PD may be suboptimal (93, 169, 170).
Clearly, better integration of disease-specific and palliative care expertise is needed. To establish dementia-specific palliative care, the EAPC therefore recommends collaboration between disease-specific (dementia) and palliative care (28). In the UK there are initiatives for outreach with specialist dementia palliative care to support the familiar care team (27, 171, 172).
Regarding PD, the provision of palliative care is widely advocated (17, 29, 32, 173). A special task force of the International Parkinson & Movement Disease Society is dedicated to improving palliative care in PD (29, 174). A mapping exercise in the UK showed service provision to vary across regions, and services for PD were not well-integrated with palliative care (175). There are some patchy examples of integration of expertise from a palliative care department with a neurology department (176). Patients and family caregivers found they lacked knowledge about palliative care services. Only few patients received care from a palliative care service and coordination of care was poor (33, 147, 148). The need for palliative care, including early in the disease trajectory, has been emphasized by a collaborative statement from the EAPC and the European Academy of Neurology (EAN) (177).
Among dementia care specialists, providing palliative care early is controversial (154, 171). Soon after diagnosis, palliative care can start in the form of ACP if patient and family caregiver are willing to talk about the future (28). Waiting until an advanced stage means many will never receive palliative care, mortality having been predicted inaccurately so patients die well before palliative care issues could be addressed. Establishing criteria to restrict access to US hospice care to those closest to the end of life has been subject of considerable research [e.g., (82, 85)]. Prediction research consistently shows we can identify those likely to survive accurately, but not, or very few of those likely to die. Needs may differ with more advanced dementia though, and ideally, a needs-based approach is adopted (176). Similarly, for people with PD, triggers for palliative care (98) and access to US hospice care have been sought using a mortality prediction approach (178). For example, a BMI less than 18.5, accelerated weight loss and reduction of dopaminergic medications was suggested for referral to US hospice care (178).
There is a lack of awareness about palliative care being applicable to dementia both among the general public and health care professionals, and this is perceived as a major barrier to improve palliative care, for example by Dutch and British physicians (179). Nursing staff may feel that they lack competencies to deliver high-quality palliative dementia care (Bolt et al., under review). Also, in PD, professionals may feel uncertain about the palliative care they deliver and often experience a lack of education and competence in this field (155, 156, 180, 181).
Conclusion
Substantial research is being performed on dementia palliative care. Much has happened since early descriptive research in dementia compared symptoms and treatment with cancer [e.g., (182)], and introduced a hospice model of care (20). The research has culminated into a clearer definition of what palliative care should entail with dementia and into some understanding of its effects. Comparisons with other diseases are now available regarding a variety of aspects (e.g., a higher caregiver burden compared with cancer (183), symptoms compared with various other chronic-progressive diseases (184), or specific problems described in subgroups with both dementia and cancer (185–188). Nevertheless, it is still unclear what is important at what stage and how to best incorporate individual preferences, for example regarding discussions about future care.
PD follows an even more protracted course which complicates a clear definition and there is no agreed-upon, evidence-based, disease-specific conceptualization of palliative care. Even more varied multidisciplinary expertise may be needed including also dementia care expertise (in addition to PD disease-specific care, palliative care and generic long-term care for older people). More specific tools may also be needed, for example, application of pain observation tools in PD dementia should consider that facial expressions indicating pain are distinct [e.g., less eye narrowing but similar upper lip raising (189)], to avoid possible underreporting in Parkinson's disease compared with Alzheimer's disease (190).
The combining of various expertise requires clear roles and inter-professional collaboration which is challenging in the face of uncertain disease trajectories (191). However, integration of palliative care has shown to improve process outcomes and patient and caregiver outcomes in cancer and chronic-progressive disease (192). Integration should take place at the clinical (patient) level but also, for example, through relationships between professionals and between organizations and in the wider system (193). For this, multidisciplinary networking and teams sharing expertise is important, supported more formally by shared guidelines and pathways (194).
Sawatzky et al. (18) describe three reasons how a clear conceptualization of palliative care in chronic-progressive disease may be helpful: (1) earlier recognition (“upstream”) of needs, (2) to promote adaptation of palliative care knowledge and expertise for unique disease profiles, (3) to operationalize a palliative approach through integration into systems and models of care that do not specialize in palliative care. Such conceptualization is promoted by looking at similarities with other diseases with more established palliative care models, but also taking a closer look at divergences, such as the initial stable phase in PD and how this should affect palliative care services. It raises the question whether ACP is an integral part of palliative care, or could precede it, also for dementia considering ambiguity around early palliative care. This resonates with recommendations of Temel et al. (195) for “early” palliative care to depend on the type of cancer; with low symptom burden, waiting until a change in health status or emergency room admission may be a reasonable approach.
For palliative dementia care, it has been helpful to also consider how it differs from “usual” dementia care, for example, by a highly proactive approach. Such understanding of what needs to be changed in practice facilitates the integration of a palliative approach in dementia care so that ultimately, the integrated care becomes the standard (196). With Kluger et al. (29), we believe that palliative care in PD will benefit from a clearer conceptualization.
Although probably not directly suitable as entry criteria for palliative care, research on prognostic factors in PD may be helpful. For example, ADL dependency strongly predicts mortality in older people and in dementia (80, 81, 85), probably covering cognitive and physical impairments and other risk factors. In contrast, it may not predict mortality in PD well (98), perhaps because motor function declines early; some dependency therefore occurs earlier than in dementia. Further, we agree that more work is needed regarding assessment of needs–including spiritual needs, development of assessment and educational tools and interventions for patient and caregiver support (29, 108).
Although we could not compare directly, we hope that our review contributes to an emerging understanding as to what elements of palliative care with neurological conditions are disease-specific and which are more general. We acknowledge that the brevity of the review did not allow more depth regarding aspects of the disease and care in different types of the diseases or forms of parkinsonism which need further research. More could be written about possible implications such as how to organize disease-specific palliative care in a cost-effective manner. We recognize a need for basic guidance for clinical practice which we offer in Box 1, awaiting high-quality trials and other research we need to build refined evidence-based practices that optimally serve individuals with neurological disease.
Box 1. Basic recommendations for practice of palliative care based on similarities and divergences between progressive neurological diseases and available evidence*.
1) Do not wait with bringing palliative care to the table until a late or terminal stage of the disease. Although it seems an obvious and safe choice to limit to a late stage, it may be too late to involve the patient or to implement a palliative care treatment plan. With PD, there is often opportunity to speak about palliative care when cognitive problems are still mild or absent. With dementia, it is difficult to predict who will die already before the late stage, while many will, with unmet palliative care needs. Therefore, discussion of palliative care before a moderate stage is recommended.
2) Improving awareness, among all involved, of the progressive course of the disease supports a shared understanding of the disease, implications for death and dying and what it means for the individuals involved. This will be helpful in identifying and addressing palliative care needs.
3) Common causes of hospital admissions include pneumonia, sepsis, and falls. Physicians could discuss these scenarios as a starting point to establish patients' views and preferences regarding invasive therapies and the benefits of a palliative care approach.
4) Elicit preferences of patient and family and the preferred style with regard to talking about future care and end-of-life scenarios. Address any information needs, and a step-wise approach with discussions continued later on may avoid feelings of being overwhelmed.
5) The course of the disease is uncertain, whereas change is. All members of a care team should help identify and discuss subtle changes in symptom and caregiver burden early.
6) Palliative care is an approach in which intervening is still possible even if active treatment of the disease or its complications is not possible or when other treatment is being withheld. It can be a potent adjunct to usual care but it should be well-integrated, also at a system level. As opposed to being uniform, straightforward, hassle free fix, “multi” is the important term in this: multifaceted interventions targeted to the individual in a context of multidisciplinary collaboration between generalists and disease and palliative care specialists.
7) Tools to identify needs and a change in the patient's condition (physical, psychosocial, spiritual, caregiver needs) should be sufficiently specific to the disease while a context or setting specific system should be in place to support its continued use (for example, a systematic approach to managing pain, behavioral symptoms, autonomic dysfunction, sleep dysfunction or motor fluctuations/dyskinesias sustainably implemented in long-term or acute care).
8) Pre-grief with progressive decline of the patient and prolonged social isolation are common. Psychosocial support is needed in different phases to empower patients and family caregivers to cope with both chronic stressors and crises.
*We inferred this general and more disease-specific guidance from the items on course of the diseases and conceptualizations in Tables 1, 2, acknowledging that these are only a couple of key recommendations and that evidence is limited. Also, this guidance should be refined and fit the local context when implemented. For more detailed guidance for clinical practice, we refer to the recommendations as part of the European Association for Palliative Care (EAPC) dementia white paper (28) and guidelines from the Irish Palliative Care in Parkinson's Disease Group (115).
Author Contributions
JS, HL, and DH: manuscript development, manuscript writing, and manuscript authorization; BA, MG, JH, BB, RK: manuscript writing and manuscript authorization.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Leiden University Medical Center, Leiden, The Netherlands, and Radboud university medical center, Nijmegen, The Netherlands.
References
1. Clark D. ‘Total pain', disciplinary power and the body in the work of Cicely Saunders, 1958–1967. Soc Sci Med. (1999) 49:727–36.
2. Saunders C. The evolution of palliative care. J R Soc Med. (2001) 94:430–2. doi: 10.1007/978-1-4471-3069-7_1
3. EAPC. White Paper on standards and norms for hospice and palliative care in Europe: part 1 Recommendations from the European Association for Palliative Care. Eur J Palliat Care. (2010) 17:278–89.
4. WHO Definition of Palliative Care,. (2002). Available online at: http://www.who.int/cancer/palliative/definition/en/ (Accessed July 20, 2018).
5. Brodaty H, Seeher K, Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr. (2012) 24:1034–45. doi: 10.1017/S1041610211002924
6. Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch Neurol. (2012) 69:601–7. doi: 10.1001/archneurol.2011.2370
7. van Bussel EF, Richard E, Arts DL, Nooyens AC, Coloma PM, de Waal MW, et al. Dementia incidence trend over 1992–2014 in the Netherlands: analysis of primary care data. PLoS Med. (2017) 14:e1002235. doi: 10.1371/journal.pmed.1002235
8. de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. (2004) 63:1240–4. doi: 10.1212/01.wnl.0000140706.52798.be
9. van de Vijver DA, Roos RA, Jansen PA, Porsius AJ, de Boer A. Estimation of incidence and prevalence of Parkinson's disease in the elderly using pharmacy records. Pharmacoepidemiol Drug Saf. (2001) 10:549–54. doi: 10.1002/pds.624
11. Nussbaum RL. Alzheimer's disease and Parkinson's disease. N Engl J Med. (2003) 348:1356. doi: 10.1056/NEJM2003ra020003
12. Santos García D, Suárez Castro E, Expósito I, de Deus T, Tuñas C, Aneiros A, et al. Comorbid conditions associated with Parkinson's disease: a longitudinal and comparative study with Alzheimer disease and control subjects. J Neurol Sci. (2017) 15:210–15. doi: 10.1016/j.jns.2016.12.046
13. Meireles J, Massano J. Cognitive impairment and dementia in Parkinson's disease: clinical features, diagnosis, and management. Front Neurol. (2012) 3:88. doi: 10.3389/fneur.2012.00088
14. Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, Barker RA, et al. Severity of mild cognitive impairment in early Parkinson's disease contributes to poorer quality of life. Parkinson Relat Disord. (2014) 20:1071–5. doi: 10.1016/j.parkreldis.2014.07.004
15. Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord. (2005) 20:1255–63. doi: 10.1002/mds.20527
16. Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson's disease. Brain Pathol. (2010) 20:633–9. doi: 10.1111/j.1750-3639.2009.00369.x
17. Richfield EW, Jones EJ, Alty JE. Palliative care for Parkinson's disease: a summary of the evidence and future directions. Palliat Med. (2013) 27:805–10. doi: 10.1177/0269216313495287
18. Sawatzky R, Porterfield P, Lee J, Dixon D, Lounsbury K, Pesut B, et al. Conceptual foundations of a palliative approach: a knowledge synthesis. BMC Palliat Care. (2016) 15:5. doi: 10.1186/s12904-016-0076-9
19. Wiblin L, Lee M, Burn D. Palliative care and its emerging role in Multiple System Atrophy and Progressive Supranuclear Palsy. Parkinson Relat Disord. (2017) 34:7–14. doi: 10.1016/j.parkreldis.2016.10.013
20. Volicer L, Rheaume Y, Brown J, Fabiszewski K, Brady R. Hospice approach to the treatment of patients with advanced dementia of the Alzheimer type. JAMA. (1986) 256:2210–3.
21. van der Steen JT, Sternberg S, Volicer L. Palliative care in dementia 1986–2016: progress and remaining challenges. J Am Med Dir Assoc. (2017) 18:190–1. doi: 10.1016/j.jamda.2016.11.009
22. van der Steen JT. Dying with dementia: what we know after more than a decade of research. J Alzheimer Dis. (2010) 22:37–55. doi: 10.3233/JAD-2010-100744
23. Murphy E, Froggatt K, Connolly S, O'Shea E, Sampson EL, Casey D, et al. Palliative care interventions in advanced dementia. Cochrane Database Syst Rev. (2016) 12:CD011513. doi: 10.1002/14651858.CD011513.pub2
24. Singer AE, Goebel JR, Kim YS, Dy SM, Ahluwalia SC, Clifford M, et al. Populations and interventions for palliative and end-of-life care: a systematic review. J Palliat Med. (2016) 19:995–1008. doi: 10.1089/jpm.2015.0367
25. Hughes JC, Volicer L, van der Steen JT. Complexity and gaps: the high-hanging fruit of dementia and palliative care research. Palliat Med. (2018) 32:591–3. doi: 10.1177/0269216318755280
26. van der Steen JT, Goodman C. What research we no longer need in neurodegenerative disease at the end of life: the case of research in dementia. Palliat Med. (2015) 29:189–92. doi: 10.1177/0269216315569998
27. van der Steen JT, Lemos Dekker N, Gijsberts MHE, Vermeulen LH, Mahler MM, The BA. Palliative care for people with dementia in the terminal phase: a mixed-methods qualitative study to inform service development. BMC Palliat Care. (2017) 16:28. doi: 10.1186/s12904-017-0201-4
28. van der Steen JT, Radbruch L, Hertogh CM, de Boer ME, Hughes JC, Larkin P, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. (2014) 28:197–209. doi: 10.1177/0269216313493685
29. Kluger BM, Fox S, Timmons S, Katz M, Galifianakis NB, Subramanian I, et al. Palliative care and Parkinson's disease: meeting summary and recommendations for clinical research. Parkinson Relat Disord. (2017) 37:19–26. doi: 10.1016/j.parkreldis.2017.01.008
30. Miyasaki JM, Kluger B. Palliative care for Parkinson's disease: has the time come? Curr Neurol Neurosci Rep. (2015) 15:26. doi: 10.1007/s11910-015-0542-4
31. Does Outpatient Palliative Care Improve Patient-Centered Outcomes in Parkinson's Disease? ClinicalTrials,.gov (2018). Available online at: https://clinicaltrials.gov/ct2/show/study/NCT02533921 (Accessed July 20, 2018).
32. Hudson PL, Toye C, Kristjanson LJ. Would people with Parkinson's disease benefit from palliative care? Palliat Med. (2006) 20:87–94. doi: 10.1191/0269216306pm1108oa
33. Giles S, Miyasaki J. Palliative stage Parkinson's disease: patient and family experiences of health-care services. Palliat Med. (2009) 23:120–5. doi: 10.1177/0269216308100773
34. Goy ER, Carter JH, Ganzini L. Parkinson disease at the end of life: caregiver perspectives. Neurology. (2007) 69:611–2. doi: 10.1212/01.wnl.0000266665.82754.61
35. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. (2008) 23:837–44. doi: 10.1002/mds.21956
36. Hoehn MM. The natural history of Parkinson's disease in the pre-levodopa and post-levodopa eras. Neuro Clin. (1992) 10:331–9.
37. Reid WG, Hely MA, Morris JG, Loy C, Halliday GM. Dementia in Parkinson's disease: a 20-year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry. (2011) 82:1033–7. doi: 10.1136/jnnp.2010.232678
38. Kua EH, Ho E, Tan HH, Tsoi C, Thng C, Mahendran R. The natural history of dementia. Psychogeriatrics. (2014) 14:196–201. doi: 10.1111/psyg.12053
39. Wolfson C, Wolfson DB, Asgharian M, M'Lan CE, Ostbye T, Rockwood K, et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med. (2001) 344:1111–6. doi: 10.1056/NEJM200104123441501
40. van der Steen JT, Ribbe MW, Deliens L, Gutschow G, Onwuteaka-Philipsen BD. Retrospective and prospective data collection compared in the Dutch End Of Life in Dementia (DEOLD) study. Alzheimer Dis Assoc Disord. (2014) 28:88–94. doi: 10.1097/WAD.0b013e318293b380
41. Koopmans RT, Ekkerink JL, van Weel C. Survival to late dementia in Dutch nursing home patients. J Am Geriatr Soc. (2003) 51:184–7. doi: 10.1046/j.1532-5415.2003.51056.x
42. Byrne EJ, Benoit M, Lopez Arrieta JM, Geraldi C, Koopmans R, Rolland Y, et al. For whom and for what the definition of severe dementia is useful: an EDCON consensus. J Nutr Health Aging. (2008) 12:714–9.
43. van der Steen JT, Volicer L, Gerritsen DL, Kruse RL, Ribbe MW, Mehr DR. Defining severe dementia with the Minimum Data Set. Int J Geriatr Psychiatry. (2006) 21:1099–106. doi: 10.1002/gps.1618
44. Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med. (2009) 361:1529–38. doi: 10.1056/NEJMoa0902234
45. Gaenslen A, Swid I, Liepelt-Scarfone I, Godau J, Berg D. The patients' perception of prodromal symptoms before the initial diagnosis of Parkinson's disease. Mov Disord. (2011) 26:653–8. doi: 10.1002/mds.23499
46. Gonera EG, van't Hof M, Berger HJ, van Weel C, Horstink MW. Symptoms and duration of the prodromal phase in Parkinson's disease. Mov Disord. (1997) 12:871–6. doi: 10.1002/mds.870120607
47. Oertel WH. Recent advances in treating Parkinson's disease. F1000 Res. (2017) 6:260. doi: 10.12688/f1000research.10100.1
48. Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2014) 29:1615–22. doi: 10.1002/mds.25898
49. Saleem TZ, Higginson IJ, Chaudhuri KR, Martin A, Burman R, Leigh PN. Symptom prevalence, severity and palliative care needs assessment using the Palliative Outcome Scale: a cross-sectional study of patients with Parkinson's disease and related neurological conditions. Palliat Med. (2013) 27:722–31. doi: 10.1177/0269216312465783
50. Bouca-Machado R, Titova N, Chaudhuri KR, Bloem BR, Ferreira JJ. Palliative care for patients and families with Parkinson's Disease. Int Rev Neurobiol. (2017) 132:475–509. doi: 10.1016/bs.irn.2017.02.017
51. Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. (2012) 8:435–42. doi: 10.1038/nrneurol.2012.126
52. Titova N, Martinez-Martin P, Katunina E, Chaudhuri KR. Advanced Parkinson's or “complex phase” Parkinson's disease? Re-evaluation is needed. J Neural Transm. (2017) 124:1529–37. doi: 10.1007/s00702-017-1799-3
53. Hendriks SA, Smalbrugge M, Galindo-Garre F, Hertogh CM, van der Steen JT. From admission to death: prevalence and course of pain, agitation, and shortness of breath, and treatment of these symptoms in nursing home residents with dementia. J Am Med Dir Assoc. (2015) 16:475–81. doi: 10.1016/j.jamda.2014.12.016
54. Borsje P, Wetzels RB, Lucassen PL, Pot AM, Koopmans RT. The course of neuropsychiatric symptoms in community-dwelling patients with dementia: a systematic review. Int Psychogeriatr. (2015) 27:385–405. doi: 10.1017/S1041610214002282
55. van der Linde RM, Dening T, Stephan BC, Prina AM, Evans E, Brayne C. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry. (2016) 209:366–77. doi: 10.1192/bjp.bp.114.148403
56. Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta-analysis. J Affect Disord. (2016) 190:264–71. doi: 10.1016/j.jad.2015.09.069
57. van der Lee J, Bakker TJ, Duivenvoorden HJ, Droes RM. Multivariate models of subjective caregiver burden in dementia: a systematic review. Ageing Res Rev. (2014) 15:76–93. doi: 10.1016/j.arr.2014.03.003
58. Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry. (2009) 24:15–24. doi: 10.1002/gps.2090
59. Daly L, Fahey-McCarthy E, Timmins F. The experience of spirituality from the perspective of people living with dementia: a systematic review and meta-synthesis. Dementia. (2016). doi: 10.1177/1471301216680425. [Epub ahead of print].
60. Agli O, Bailly N, Ferrand C. Spirituality and religion in older adults with dementia: a systematic review. Int Psychogeriatr. (2015) 27:715–25. doi: 10.1017/S1041610214001665
61. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
62. Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. (2000) 15:1112–8. doi: 10.1002/1531-8257(200011)15:6 < 1112::aid-mds1008>3.0.co;2-a
63. Martinez- Martin P, Rodriguez-Blazquez C, João Forjaz M. Quality of life and burden in caregivers for patients with Parkinson's disease: concepts, assessment and related factors. Rev Pharmacoecon Outcomes Res. (2012) 12:221–30. doi: 10.1586/erp.11.106
64. Skorvanek M, Rosenberger J, Minar M, Grofik M, Han V, Groothoff JW, et al. Relationship between the non-motor items of the MDS-UPDRS and Quality of Life in patients with Parkinson's disease. J Neurol Sci. (2015) 353:87–91. doi: 10.1016/j.jns.2015.04.013
65. Weerkamp NJ, Tissingh G, Poels PJ, Zuidema SU, Munneke M, Koopmans RT, et al. Nonmotor symptoms in nursing home residents with Parkinson's disease: prevalence and effect on quality of life. J Am Geriatr Soc. (2013) 61:1714–21. doi: 10.1111/jgs.12458
66. Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S, et al. The nondeclaration of nonmotor symptoms of Parkinson's disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord. (2010) 25:704–9. doi: 10.1002/mds.22868
67. Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinson Relat Disord. (2002) 8:193–7. doi: 10.1016/s1353-8020(01)00015-3
68. Schrag A, Hovris A, Morley DQuinn N, Jahanshahi M. Caregiver-burden in parkinson's disease is closely associated with psychiatric symptoms, falls, and disability. Parkinson Relat Disord. (2006) 12:35–41. doi: 10.1016/j.parkreldis.2005.06.011
69. Laver K, Dyer S, Whitehead C, Clemson L, Crotty M. Interventions to delay functional decline in people with dementia: a systematic review of systematic reviews. BMJ Open. (2016) 6:e010767. doi: 10.1136/bmjopen-2015-010767
70. Cipriani G, Borin G, Del Debbio A, Di Fiorino M. Personality and dementia. J Nerv Ment Dis. (2015) 203:210–4. doi: 10.1097/NMD.0000000000000264
71. Galvin JE, Malcom H, Johnson D, Morris JC. Personality traits distinguishing dementia with Lewy bodies from Alzheimer disease. Neurology. (2007) 68:1895–901. doi: 10.1212/01.wnl.0000263131.80945.ad
72. Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Mov Disord. (2008) 23:790–6. doi: 10.1002/mds.21879
73. Romero JP, Benito-Leon J, Louis ED, Bermejo-Pareja F. Under reporting of dementia deaths on death certificates: a systematic review of population-based cohort studies. J Alzheimer Dis. (2014) 41:213–21. doi: 10.3233/JAD-132765
74. Foley NC, Affoo RH, Martin RE. A systematic review and meta-analysis examining pneumonia-associated mortality in dementia. Dement Geriatr Cogn Disord. (2015) 39:52–67. doi: 10.1159/000367783
75. Keene J, Hope T, Fairburn CG, Jacoby R. Death and dementia. Int J Geriatr Psychiatry. (2001) 16:969–74. doi: 10.1002/gps.474
76. Attems J, Konig C, Huber M, Lintner F, Jellinger KA. Cause of death in demented and non-demented elderly inpatients; An autopsy study of 308 cases. J Alzheimers Dis. (2005) 8:57–62. doi: 10.3233/jad-2005-8107
77. Fall PA, Saleh A, Fredrickson M, Olsson JE, Granerus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson's disease: a 9-year follow-up. Mov Disord. (2003) 18:1312–6. doi: 10.1002/mds.10537
78. Hobson P, Meara J. Mortality and quality of death certification in a cohort of patients with Parkinson's disease and matched controls in North Wales, UK at 18 years: a community-based cohort study. BMJ Open. (2018) 8:e018969. doi: 10.1136/bmjopen-2017-018969
79. Beyer MK, Herlofson K, Arsland D, Larsen JP. Causes of death in a community-based study of Parkinson's disease. Acta Neurol Scand. (2001) 103:7–11. doi: 10.1034/j.1600-0404.2001.00191.x
80. Brown MA, Sampson EL, Jones L, Barron AM. Prognostic indicators of 6-month mortality in elderly people with advanced dementia: a systematic review. Palliat Med. (2013) 27:389–400. doi: 10.1177/0269216312465649
81. van der Steen JT, Mehr DR, Kruse RL, Sherman AK, Madsen RW, D'Agostino RB, et al. Predictors of mortality for lower respiratory infections in nursing home residents with dementia were validated transnationally. J Clin Epidemiol. (2006) 59:970–9. doi: 10.1016/j.jclinepi.2005.12.005
82. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. (2010) 304:1929–35. doi: 10.1001/jama.2010.1572
83. Hendriks SA, Smalbrugge M, van Gageldonk-Lafeber AB, Galindo-Garre F, Schipper M, Hertogh C, et al. Pneumonia, intake problems, and survival among nursing home residents with variable stages of dementia in the Netherlands: results from a prospective observational study. Alzheimer Dis Assoc Disord. (2017) 31:200–8. doi: 10.1097/WAD.0000000000000171
84. van der Steen JT, Ooms ME, Mehr DR, van der Wal G, Ribbe MW. Severe dementia and adverse outcomes of nursing home-acquired pneumonia: evidence for mediation by functional and pathophysiological decline. J Am Geriatr Soc. (2002) 50:439–48. doi: 10.1046/j.1532-5415.2002.50108.x
85. Mitchell SL, Miller SC, Teno JM, Davis RB, Shaffer ML. The advanced dementia prognostic tool: a risk score to estimate survival in nursing home residents with advanced dementia. J Pain Symptom Manage. (2010) 40:639–51. doi: 10.1016/j.jpainsymman.2010.02.014
86. Lethbridge L, Johnston GM, Turnbull G. Co-morbidities of persons dying of Parkinson's disease. Prog Palliat Care. (2013) 21:140–5. doi: 10.1179/1743291X12Y.0000000037
87. Huang YF, Cherng YG, Hsu SP, Yeh CC, Chou YC, Wu CH, et al. Risk and adverse outcomes of fractures in patients with Parkinson's disease: two nationwide studies. Osteoporos Int. (2015) 26:1723–32. doi: 10.1007/s00198-015-3052-y
88. Hussain J, Adams D, Campbell C. End-of-life care in neurodegenerative conditions: outcomes of a specialist palliative neurology service. Int J Palliat Nurs. (2013) 19:162–9. doi: 10.12968/ijpn.2013.19.4.162
89. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. (2006) 5:525–35. doi: 10.1016/S1474-4422(06)70471-9
90. Macleod AD, Dalen I, Tysnes OB, Larsen JP, Counsell CE. Development and validation of prognostic survival models in newly diagnosed Parkinson's disease. Mov Disord. (2018) 33:108–16. doi: 10.1002/mds.27177
91. Santos-Garcia D, Suarez-Castro E, Ernandez J, Exposito-Ruiz I, Tunas-Gesto C, Aneiros-Diaz M, et al. Predictors of mortality in nondemented patients with Parkinson Disease: motor symptoms versus nonmotor symptoms. J Geriatr Psychiatry Neurol. (2018) 31:19–26. doi: 10.1177/0891988717743589
92. Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. (2008) 70:2241–7. doi: 10.1212/01.wnl.0000313835.33830.80
93. van Rumund A, Weerkamp N, Tissingh G, Zuidema SU, Koopmans RT, Munneke M, et al. Perspectives on Parkinson disease care in Dutch nursing homes. J Am Med Dir Assoc. (2014) 15:732–7. doi: 10.1016/j.jamda.2014.05.009
94. Weerkamp NJ, Zuidema SU, Tissingh G, Poels PJ, Munneke M, Koopmans RT, et al. Motor profile and drug treatment of nursing home residents with Parkinson's disease. J Am Geriatr Soc. (2012) 60:2277–82. doi: 10.1111/jgs.12027
96. Luquin MR, Kulisevsky J, Martinez-Martin P, Mir P, Tolosa ES. Consensus on the definition of advanced Parkinson's Disease: a neurologists-based Delphi study (CEPA Study). Parkinson Dis. (2017) 2017:4047392. doi: 10.1155/2017/4047392
97. Healthcare Improvement Scotland. Palliative care identification tools comparator Scotland: Ihub living well communities (2018). Available online at: https://livingwellincommunities.com/2018/04/11/comparing-tools-that-can-help-to-identify-people-who-could-benefit-from-a-palliative-care-approach/
98. Hussain J, Allgar V, Oliver D. Palliative care triggers in progressive neurodegenerative conditions: an evaluation using a multi-centre retrospective case record review and principal component analysis. Palliat Med. (2018) 32:716–25. doi: 10.1177/0269216318755884
99. Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson's disease and its association with dementia and depression. Acta Neurol Scand. (2004) 110:118–23. doi: 10.1111/j.1600-0404.2004.00292.x
100. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. (2017) 390:2673–734. doi: 10.1016/S0140-673631363-6
101. Bloem BR, van Laar T, Keus SHJ, De Beer H, Poot E, Buskens E, et al. Multidisciplinaire richtlijn Parkinson 2006–2010. Multidisciplinaire richtlijn Ziekte van Parkinson. Alphen aan den Rijn: Van Zuiden Communications (2010).
102. Robinson A, Eccleston C, Annear M, Elliott KE, Andrews S, Stirling C, et al. Who knows, who cares? Dementia knowledge among nurses, care workers, and family members of people living with dementia. J Palliat Care. (2014) 30:158–65. doi: 10.1177/082585971403000305
103. van der Steen JT, Onwuteaka-Philipsen BD, Knol DL, Ribbe MW, Deliens L. Caregivers' understanding of dementia predicts patients' comfort at death: a prospective observational study. BMC Med. (2013) 11:105. doi: 10.1186/1741-7015-11-105
104. Perrar KM, Schmidt H, Eisenmann Y, Cremer B, Voltz R. Needs of people with severe dementia at the end-of-life: a systematic review. J Alzheimer Dis. (2015) 43:397–413. doi: 10.3233/JAD-140435
105. Fleming R, Kelly F, Stillfried G. 'I want to feel at home': establishing what aspects of environmental design are important to people with dementia nearing the end of life. BMC Palliat Care. (2015) 14:26. doi: 10.1186/s12904-015-0026-y
106. Schmidt H, Eisenmann Y, Golla H, Voltz R, Perrar KM. Needs of people with advanced dementia in their final phase of life: a multi-perspective qualitative study in nursing homes. Palliat Med. (2018) 32:657–67. doi: 10.1177/0269216317746571
107. Mulqueen K, Coffey A. Preferences of residents with dementia for end of life care. Nurs Older People. (2017) 29:26–30. doi: 10.7748/nop.2017.e862
108. Prizer LP, Gay JL, Wilson MG, Emerson KG, Glass AP, Miyasaki JM, et al. A mixed-methods approach to understanding the palliative needs of Parkinson's patients. J Appl Gerontol. (2018). doi: 10.1177/0733464818776794. [Epub ahead of print].
109. Boersma I, Jones J, Carter J, Bekelman D, Miyasaki J, Kutner J, et al. Parkinson disease patients' perspectives on palliative care needs: what are they telling us? Neurol Clin Pract. (2016) 6:209–19. doi: 10.1212/CPJ.0000000000000233
110. Pieper MJ, van der Steen JT, Francke AL, Scherder EJ, Twisk JW, Achterberg WP. Effects on pain of a stepwise multidisciplinary intervention (STA OP!) that targets pain and behavior in advanced dementia: a cluster randomized controlled trial. Palliat Med. (2018) 32:682–92. doi: 10.1177/0269216316689237
111. McNiel P, Westphal J. Namaste Care: a person-centered care approach for Alzheimer's and advanced dementia. West J Nurs Res. (2018) 40:37–51. doi: 10.1177/0193945916679631
112. Sturkenboom IH, Graff MJ, Hendriks JC, Veenhuizen Y, Munneke M, Bloem BR, et al. Efficacy of occupational therapy for patients with Parkinson's disease: a randomised controlled trial. Lancet Neurol. (2014) 13:557–66. doi: 10.1016/S1474-4422(14)70055-9
113. van der Marck MA, Munneke M, Mulleners W, Hoogerwaard EM, Borm GF, Overeem S, et al. Integrated multidisciplinary care in Parkinson's disease: a non-randomised, controlled trial (IMPACT). Lancet Neurol. (2013) 12:947–56. doi: 10.1016/S1474-4422(13)70196-0
114. Ypinga JHL, de Vries NM, Boonen L, Koolman X, Munneke M, Zwinderman AH, et al. Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: a retrospective analysis of medical claims data. Lancet Neurol. (2018) 17:153–61. doi: 10.1016/S1474-4422(17)30406-4
115. The Irish Palliative Care in Parkinson's Disease Group. Palliative Care in People with Parkinson's Disease: Guidelines for Professional Healthcare Workers on the Assessment and Management of Palliative Care Needs in Parkinson's Disease and Related Parkinsonian Syndromes. Cork: University College Cork (2016).
116. Lichtner V, Dowding D, Esterhuizen P, Closs SJ, Long AF, Corbett A, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. (2014) 14:138. doi: 10.1186/1471-2318-14-138
117. Ellis-Smith C, Evans CJ, Murtagh FE, Henson LA, Firth AM, Higginson IJ, et al. Development of a caregiver-reported measure to support systematic assessment of people with dementia in long-term care: the integrated palliative care outcome scale for dementia. Palliat Med. (2017) 31:651–60. doi: 10.1177/0269216316675096
118. Miyasaki JM, Long J, Mancini D, Moro E, Fox SH, Lang AE, et al. Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS-PD. Parkinson Relat Disord. (2012) 18 (Suppl. 3):S6–9. doi: 10.1016/j.parkreldis.2012.06.013
119. Hearn J, Higginson IJ. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care. (1999) 8:219–27.
120. Reyniers T, Deliens L, Pasman HR, Morin L, Addington-Hall J, Frova L, et al. International variation in place of death of older people who died from dementia in 14 European and non-European countries. J Am Med Dir Assoc. (2015) 16:165–71. doi: 10.1016/j.jamda.2014.11.003
121. Nakanishi M, Niimura J, Yamasaki S, Nishida A. Death of dementia patients in psychiatric hospitals and regional supply of psychiatric services: study of the national data from 1996 to 2014 in Japan. J Alzheimer Dis. (2017) 56:817–24. doi: 10.3233/JAD-160935
122. Amjad H, Carmichael D, Austin AM, Chang CH, Bynum JP. Continuity of care and health care utilization in older adults with dementia in fee-for-service Medicare. JAMA Intern Med. (2016) 176:1371–8. doi: 10.1001/jamainternmed.2016.3553
123. Aaltonen M, Raitanen J, Forma L, Pulkki J, Rissanen P, Jylha M. Burdensome transitions at the end of life among long-term care residents with dementia. J Am Med Direct Assoc. (2014) 15:643–8. doi: 10.1016/j.jamda.2014.04.018
124. Moens K, Houttekier D, Van den Block L, Harding R, Morin L, Marchetti S, et al. Place of death of people living with Parkinson's disease: a population-level study in 11 countries. BMC Palliat Care. (2015) 14:28. doi: 10.1186/s12904-015-0021-3
125. Parashos SA, Maraganore DM, O'Brien PC, Rocca WA. Medical services utilization and prognosis in Parkinson disease: a population-based study. Mayo Clin Proc. (2002) 77:918–25. doi: 10.4065/77.9.918
126. van der Steen JT, van Soest-Poortvliet MC, Hallie-Heierman M, Onwuteaka-Philipsen BD, Deliens L, de Boer ME, et al. Factors associated with initiation of advance care planning in dementia: a systematic review. J Alzheimers Dis. (2014) 40:743–57. doi: 10.3233/JAD-131967
127. Stevenson M, McDowell ME, Taylor BJ. Concepts for communication about risk in dementia care: a review of the literature. Dementia. (2018) 17:359–90. doi: 10.1177/1471301216647542
128. Groen-van de Ven L, Smits C, de Graaff F, Span M, Eefsting J, Jukema J, et al. Involvement of people with dementia in making decisions about their lives: a qualitative study that appraises shared decision-making concerning daycare. BMJ Open. (2017) 7:e018337. doi: 10.1136/bmjopen-2017-018337
129. van Soest-Poortvliet MC, van der Steen JT, de Vet HC, Hertogh CM, Onwuteaka-Philipsen BD, Deliens LH. Factors related to establishing a comfort care goal in nursing home patients with dementia: a cohort study among family and professional caregivers. J Palliat Med. (2014) 17:1317–27. doi: 10.1089/jpm.2014.0205
130. Mitchell SL, Palmer JA, Volandes AE, Hanson LC, Habtemariam D, Shaffer ML. Level of Care preferences among nursing home residents with advanced dementia. J Pain Symptom Manage. (2017) 54:340–5. doi: 10.1016/j.jpainsymman.2017.04.020
131. Volandes AE, Ferguson LA, Davis AD, Hull NC, Green MJ, Chang Y, et al. Assessing end-of-life preferences for advanced dementia in rural patients using an educational video: a randomized controlled trial. J Palliat Med. (2011) 14:169–77. doi: 10.1089/jpm.2010.0299
132. Gove D, Diaz-Ponce A, Georges J. Palliative care covers more than end-of-life issues: why is this not common practice in dementia care and what are the implications? Ann Palliat Med. (2017) 6:390–2. doi: 10.21037/apm.2017.06.25
133. Gove D, Sparr S, Dos Santos Bernardo AM, Cosgrave MP, Jansen S, Martensson B, et al. Recommendations on end-of-life care for people with dementia. J Nutr Health Aging. (2010) 14:136–9.
134. Tilly J, Fok A. Quality End of Life Care for Individuals with Dementia in Assisted Living and Nursing Homes and Public Policy Barriers to Delivering this Care. US Alzheimer's Association (2006).
135. Fox S, Cashell A, Kernohan WG, Lynch M, McGlade C, O'Brien T, et al. Palliative care for Parkinson's disease: patient and carer's perspectives explored through qualitative interview. Palliat Med. (2017) 31:634–41. doi: 10.1177/0269216316669922
136. Bloem BR, Stocchi F. Move for Change Part III: a European survey evaluating the impact of the EPDA Charter for People with Parkinson's Disease. Eur J Neurol. (2015) 22:133–41.e8–9. doi: 10.1111/ene.12544
137. Kwak J, Wallendal MS, Fritsch T, Leo G, Hyde T. Advance care planning and proxy decision making for patients with advanced Parkinson disease. South Med J. (2014) 107:178–85. doi: 10.1097/smj.0000000000000075
138. Hall K, Sumrall M, Thelen G, Kluger BM, Parkinson's Disease Foundation sponsored “Palliative C, Parkinson's Disease” Patient Advisory C. Palliative care for Parkinson's disease: suggestions from a council of patient and carepartners. NPJ Parkinson Dis. (2017) 3:16. doi: 10.1038/s41531-017-0016-2
139. Dixon J, Karagiannidou M, Knapp M. The effectiveness of advance care planning in improving end-of-life outcomes for people with dementia and their carers: a systematic review and critical discussion. J Pain Symptom Manage. (2018) 55:132–50.e1. doi: 10.1016/j.jpainsymman.2017.04.009
140. Brazil K, Carter G, Cardwell C, Clarke M, Hudson P, Froggatt K, et al. Effectiveness of advance care planning with family carers in dementia nursing homes: a paired cluster randomized controlled trial. Palliat Med. (2018) 32:603–12. doi: 10.1177/0269216317722413
141. Tuck KK, Brod L, Nutt J, Fromme EK. Preferences of patients with Parkinson's disease for communication about advanced care planning. Am J Hosp Palliat Care. (2015) 32:68–77. doi: 10.1177/1049909113504241
142. Walker RW, Churm D, Dewhurst F, Samuel M, Ramsell A, Lawrie C. Palliative care in people with idiopathic Parkinson's disease who die in hospital. BMJ Support Palliat Care. (2014) 4:412. doi: 10.1136/bmjspcare-2012-000412
143. Terum TM, Andersen JR, Rongve A, Aarsland D, Svendsboe EJ, Testad I. The relationship of specific items on the Neuropsychiatric Inventory to caregiver burden in dementia: a systematic review. Int J Geriatr Psychiatry. (2017) 32:703–17. doi: 10.1002/gps.4704
144. Boogaard JA, van der Steen JT, de Boer AH, Hertogh CMPM, Broese van Groenou MI. Informal end-of-life care for community-dwelling older persons with or without dementia: caregiver burden and positive experiences. In: European Journal of Palliative Care 2017. 15th World Congress of the European Association for Palliative Care (EAPC) (Madrid) (2017). p. 114. Available online at: http://www.eapc-2017.org/final-programme.html
145. Blandin K, Pepin R. Dementia grief: a theoretical model of a unique grief experience. Dementia. (2015) 16:67–78. doi: 10.1177/1471301215581081
146. Wilson S, Toye C, Aoun S, Slatyer S, Moyle W, Beattie E. Effectiveness of psychosocial interventions in reducing grief experienced by family carers of people with dementia: a systematic review. JBI Database System Rev Implement Rep. (2017) 15:809–39. doi: 10.11124/JBISRIR-2016-003017
147. Hasson F, Kernohan WG, McLaughlin M, Waldron M, McLaughlin D, Chambers H, et al. An exploration into the palliative and end-of-life experiences of carers of people with Parkinson's disease. Palliat Med. (2010) 24:731–6. doi: 10.1177/0269216310371414
148. Goy ER, Carter JH, Ganzini L. Needs and experiences of caregivers for family members dying with Parkinson disease. J Palliat Care. (2008) 24:69–75.
149. McLaughlin D, Hasson F, Kernohan WG, Waldron M, McLaughlin M, Cochrane B, et al. Living and coping with Parkinson's disease: perceptions of informal carers. Palliat Med. (2011) 25:177–82. doi: 10.1177/0269216310385604
150. Carter JH, Lyons KS, Lindauer A, Malcom J. Pre-death grief in Parkinson's caregivers: a pilot survey-based study. Parkinson Relat Disord. (2012) 18 (Suppl. 3):S15–8. doi: 10.1016/j.parkreldis.2012.06.015
151. Hempel S, Norman G, Golder S, Aguiar-Ibanez R, Eastwood A. Psychosocial interventions for non-professional carers of people with Parkinson's disease: a systematic scoping review. J Adv Nurs. (2008) 64:214–28. doi: 10.1111/j.1365-2648.2008.04806.x
152. A'Campo LE, Wekking EM, Spliethoff-Kamminga NG, Le Cessie S, Roos RA. The benefits of a standardized patient education program for patients with Parkinson's disease and their caregivers. Parkinson Relat Disord. (2010) 16:89–95. doi: 10.1016/j.parkreldis.2009.07.009
153. van Riet Paap J, Mariani E, Chattat R, Koopmans R, Kerherve H, Leppert W, et al. Identification of the palliative phase in people with dementia: a variety of opinions between healthcare professionals. BMC Palliat Care. (2015) 14:56. doi: 10.1186/s12904-015-0053-8
154. van der Steen JT, Radbruch L, de Boer ME, Junger S, Hughes JC, Larkin P, et al. Achieving consensus and controversy around applicability of palliative care to dementia. Int Psychogeriatr. (2016) 28:133–45. doi: 10.1017/S1041610215000824
155. Waldron M, Kernohan WG, Hasson F, Foster S, Cochrane B, Payne C. Allied health professional's views on palliative care for people with advanced Parkinson's disease. Int J Ther Rehabil. (2011) 18:48–57. doi: 10.12968/ijtr.2011.18.1.48
156. Fox S, Cashell A, Kernohan WG, Lynch M, McGlade C, O'Brien T, et al. Interviews with Irish healthcare workers from different disciplines about palliative care for people with Parkinson's disease: a definite role but uncertainty around terminology and timing. BMC Palliat Care. (2016) 15:1–9. doi: 10.1186/s12904-016-0087-6
157. EPDA European Parkinson's Disease Association. (2018). What is Palliative Care and When Should It Start? Available online at: http://www.epda.eu.com/about-parkinsons/later-in-life/what-is-palliative-care-and-when-should-it-start/ (Accessed July 20, 2018).
158. Richardson A, Pedley G, Pelone F, Akhtar F, Chang J, Muleya W, et al. Psychosocial interventions for people with young onset dementia and their carers: a systematic review. Int Psychogeriatr. (2016) 28:1441–54. doi: 10.1017/S1041610216000132
159. Koopmans RT, van der Steen JT, Bakker C. Palliative care in people with Young-Onset Dementia (YOD): an undiscovered area! J Am Med Dir Assoc. (2015) 16:1008–9. doi: 10.1016/j.jamda.2015.07.001
160. Higginson IJ, Gao W, Saleem TZ, Chaudhuri KR, Burman R, McCrone P, et al. Symptoms and quality of life in late stage Parkinson syndromes: a longitudinal community study of predictive factors. PLoS ONE. (2012) 7:e46327. doi: 10.1371/journal.pone.0046327
161. Goodman C. Dying in care homes, when advance care planning requires wraparound care. Palliat Med. (2018) 32:312–3. doi: 10.1177/0269216317751799
162. Sleeman KE, Ho YK, Verne J, Gao W, Higginson IJ, project GUC. Reversal of English trend towards hospital death in dementia: a population-based study of place of death and associated individual and regional factors, 2001–2010. BMC Neurol. (2014) 14:59. doi: 10.1186/1471-2377-14-59
163. Low V, Ben-Shlomo Y, Coward E, Fletcher S, Walker R, Clarke CE. Measuring the burden and mortality of hospitalisation in Parkinson's disease: a cross-sectional analysis of the English Hospital Episodes Statistics database 2009–2013. Parkinson Relat Disord. (2015) 21:449–54. doi: 10.1016/j.parkreldis.2015.01.017
164. Sampson EL, Jones L, Thune-Boyle IC, Kukkastenvehmas R, King M, Leurent B, et al. Palliative assessment and advance care planning in severe dementia: an exploratory randomized controlled trial of a complex intervention. Palliat Med. (2011) 25:197–209. doi: 10.1177/0269216310391691
165. A'Campo LE, Spliethoff-Kamminga NG, Macht M, Roos RA. Caregiver education in Parkinson's disease: formative evaluation of a standardized program in seven European countries. Qual Life Res. (2010) 19:55–64. doi: 10.1007/s11136-009-9559-y
166. Roland KP, Chappell NL. Caregiver experiences across three neurodegenerative diseases: Alzheimer's, Parkinson's, and Parkinson's with dementia. J Aging Health. (2017) 31:256–79. doi: 10.1177/0898264317729980
167. Lemos Dekker N, Gysels M, van der Steen JT. Professional caregivers' experiences with the Liverpool Care Pathway in dementia: an ethnographic study in a Dutch nursing home. Palliat Support Care. (2017) 16:479–86. doi: 10.1017/S1478951517000645
168. Husebo BS, Flo E, Engedal K. The Liverpool Care Pathway: discarded in cancer patients but good enough for dying nursing home patients? A systematic review. BMC Med Ethics. (2017) 18:48. doi: 10.1186/s12910-017-0205-x
169. Bloem BR, Stocchi F. Move for change part I: a European survey evaluating the impact of the EPDA charter for people with Parkinson's disease. Eur J Neurol. (2012) 19:402–10. doi: 10.1111/j.1468-1331.2011.03532.x
170. Stocchi F, Bloem BR. Move for Change Part II: a European survey evaluating the impact of the EPDA Ccarter for people with Parkinson's disease. Eur J Neurol. (2013) 20:461–72. doi: 10.1111/j.1468-1331.2012.03876.x
171. Annear MJ, Toye C, McInerney F, Eccleston C, Tranter B, Elliott KE, et al. What should we know about dementia in the 21st century? A Delphi consensus study. BMC Geriatr. (2015) 15:5. doi: 10.1186/s12877-015-0008-1
172. Amador S, Goodman C, Robinson L, Sampson EL, Team SR. UK end-of-life care services in dementia, initiatives and sustainability: results of a national online survey. BMJ Support Palliat Care. (2016) 8:424-7. doi: 10.1136/bmjspcare-2016-001138
173. Rudkins H, Thomas A. The importance of early consideration of palliative care in Parkinson's disease. Br J Neurosci Nurs. (2006) 2:10–6. doi: 10.12968/bjnn.2006.2.1.20497
174. MDS. Available online at: https://www.movementdisorders.org/MDS/About/CommitteesH-Other-Groups/MDS-Task-Forces/Task-Force-on-Palliative-Care.htm
175. van Vliet LM, Gao W, DiFrancesco D, Crosby V, Wilcock A, Byrne A, et al. How integrated are neurology and palliative care services? Results of a multicentre mapping exercise. BMC Neurol. (2016) 16:63. doi: 10.1186/s12883-016-0583-6
176. Ghoche R. The conceptual framework of palliative care applied to advanced Parkinson's disease. Parkinson Relat Disord. (2012) 18 (Suppl. 3):S2–5. doi: 10.1016/j.parkreldis.2012.06.012
177. Oliver DJ, Borasio GD, Caraceni A, de Visser M, Grisold W, Lorenzl S, et al. A consensus review on the development of palliative care for patients with chronic and progressive neurological disease. Eur J Neurol. (2016) 23:30–8. doi: 10.1111/ene.12889
178. Goy ER, Bohlig A, Carter J, Ganzini L. Identifying predictors of hospice eligibility in patients with Parkinson disease. Am J Hosp Palliat Care. (2015) 32:29–33. doi: 10.1177/1049909113502119
179. Brazil K, Galway K, Carter G, van der Steen JT. Providing optimal palliative care for persons living with dementia: a comparison of physician perceptions in the Netherlands and the United Kingdom. J Palliat Med. (2017) 20:473–7. doi: 10.1089/jpm.2015.0274
180. Waldron M, Kernohan WG, Hasson F, Foster S, Cochrane B. What do social workers think about the palliative care needs of people with Parkinson's disease? Br J Soc Work. (2013) 43:81–98. doi: 10.1093/bjsw/bcr157
181. Fox S, Gannon E, Cashell A, Kernohan WG, Lynch M, McGlade C, et al. Survey of health care workers suggests unmet palliative care needs in Parkinson's Disease. Mov Disord Clin Pract. (2015) 2:142–8. doi: 10.1002/mdc3.12133
182. McCarthy M, Addington-Hall J, Altmann D. The experience of dying with dementia: a retrospective study. Int J Geriatr Psychiatry. (1997) 12:404–9.
183. Harding R, Gao W, Jackson D, Pearson C, Murray J, Higginson IJ. Comparative analysis of informal caregiver burden in advanced cancer, dementia, and acquired brain injury. J Pain Symptom Manage. (2015). doi: 10.1016/j.jpainsymman.2015.04.005
184. Moens K, Higginson IJ, Harding R, Euro I. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. (2014) 48:660–77. doi: 10.1016/j.jpainsymman.2013.11.009
185. Huang HK, Hsieh JG, Hsieh CJ, Wang YW. Do cancer patients with dementia receive less aggressive treatment in end-of-life care? A nationwide population-based cohort study. Oncotarget. (2017) 8:63596–604. doi: 10.18632/oncotarget.18867
186. McWilliams L, Farrell C, Grande G, Keady J, Swarbrick C, Yorke J. A systematic review of the prevalence of comorbid cancer and dementia and its implications for cancer-related care. Aging Ment Health. (2017) 22:1254–71. doi: 10.1080/13607863.2017.1348476
187. Morin L, Beaussant Y, Aubry R, Fastbom J, Johnell K. Aggressiveness of end-of-life care for hospitalized individuals with cancer with and without dementia: a nationwide matched-cohort study in France. J Am Geriatr Soc. (2016) 64:1851–7. doi: 10.1111/jgs.14363
188. Monroe TB, Carter MA, Feldt KS, Dietrich MS, Cowan RL. Pain and hospice care in nursing home residents with dementia and terminal cancer. Geriatr Gerontol Int. (2013) 13:1018–25. doi: 10.1111/ggi.12049
189. Priebe JA, Kunz M, Morcinek C, Rieckmann P, Lautenbacher S. Does Parkinson's disease lead to alterations in the facial expression of pain? J Neurol Sci. (2015) 359:226–35. doi: 10.1016/j.jns.2015.10.056
190. Kutschar P, Lex K, Osterbrink J, Lorenzl S. [Parkinson's disease, Alzheimer's disease and oncological diseases in residential geriatric care: pain frequency and selected healthcare features in comparison]. Schmerz. (2018) 32:356–63. doi: 10.1007/s00482-018-0302-x
191. Oishi A, Murtagh FE. The challenges of uncertainty and interprofessional collaboration in palliative care for non-cancer patients in the community: a systematic review of views from patients, carers and health-care professionals. Palliat Med. (2014) 28:1081–98. doi: 10.1177/0269216314531999
192. Siouta N, Van Beek K, van der Eerden ME, Preston N, Hasselaar JG, Hughes S, et al. Integrated palliative care in Europe: a qualitative systematic literature review of empirically-tested models in cancer and chronic disease. BMC Palliat Care. (2016) 15:56. doi: 10.1186/s12904-016-0130-7
193. Valentijn PP, Boesveld IC, van der Klauw DM, Ruwaard D, Struijs JN, Molema JJ, et al. Towards a taxonomy for integrated care: a mixed-methods study. Int J Integr Care. (2015) 15:e003. doi: 10.5334/ijic.1513
194. den Herder-van der Eerden M, van Wijngaarden J, Payne S, Preston N, Linge-Dahl L, Radbruch L, et al. Integrated palliative care is about professional networking rather than standardisation of care: a qualitative study with healthcare professionals in 19 integrated palliative care initiatives in five European countries. Palliat Med. (2018) 32:1091–102. doi: 10.1177/0269216318758194
195. Temel J. What do we mean by early? In: 10th World Research Congress of the European Association for Palliative Care (EAPC). Bern (2018).
196. Gomez-Batiste X, Murray SA, Thomas K, Blay C, Boyd K, Moine S, et al. Comprehensive and integrated palliative care for people with advanced chronic conditions: an update from several european initiatives and recommendations for policy. J Pain Symptom Manage. (2017) 53:509–17. doi: 10.1016/j.jpainsymman.2016.10.361
Keywords: end of life care, hospice care, palliative care, health services, nervous system diseases
Citation: van der Steen JT, Lennaerts H, Hommel D, Augustijn B, Groot M, Hasselaar J, Bloem BR and Koopmans RTCM (2019) Dementia and Parkinson’s Disease: Similar and Divergent Challenges in Providing Palliative Care. Front. Neurol. 10:54. doi: 10.3389/fneur.2019.00054
Received: 22 July 2018; Accepted: 16 January 2019;
Published: 11 March 2019.
Edited by:
Marianne De Visser, University of Amsterdam, NetherlandsReviewed by:
Pratap Chand, Saint Louis University, United StatesPedro Chana, Universidad de Santiago de Chile, Chile
Mohamed Mosaad Salama, Mansoura University, Egypt
Copyright © 2019 van der Steen, Lennaerts, Hommel, Augustijn, Groot, Hasselaar, Bloem and Koopmans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jenny T. van der Steen, anR2YW5kZXJzdGVlbkBsdW1jLm5s
 Jenny T. van der Steen
Jenny T. van der Steen Herma Lennaerts
Herma Lennaerts Danny Hommel
Danny Hommel Bertie Augustijn
Bertie Augustijn Marieke Groot7
Marieke Groot7