- 1Department of Rehabilitation Sciences, Cyprus University of Technology, Limassol, Cyprus
- 2Center for Applied Neuroscience, University of Cyprus, Nicosia, Cyprus
- 3Department of Psychology, University of Cyprus, Nicosia, Cyprus
- 4Division of Anaesthesia, University of Cambridge, Cambridge, United Kingdom
- 5Medical Physics Laboratory, Medical School, Democritus University of Thrace, Alexandroupoli, Greece
TBI results in significant cognitive impairments and in altered brain functional connectivity. However, no studies explored so far, the relationship between global functional connectivity and cognitive outcome in chronic moderate-severe TBI. This proof of principle study employed the intrinsic connectivity contrast, an objective voxel-based metric of global functional connectivity, in a small sample of chronic moderate-severe TBI participants and a group of healthy controls matched on gender (males), age, and education. Cognitive tests assessing executive functions, verbal memory, visual memory, attention/organization, and cognitive reserve were administered. Group differences in terms of global functional connectivity maps were assessed and the association between performance on the cognitive measures and global functional connectivity was examined. Next, we investigated the spatial extent of functional connectivity in the brain regions found to be associated with cognitive performance, using traditional seed-based analyses. Global functional connectivity of the TBI group was altered, compared to the controls. Moreover, the strength of global functional connectivity in affected brain areas was associated with cognitive outcome. These findings indicate that impaired global functional connectivity is a significant consequence of TBI suggesting that cognitive impairments following TBI may be partly attributed to altered functional connectivity between brain areas involved in the specific cognitive functions.
Introduction
Traumatic brain injury (TBI) represents a major medical, public health and socioeconomic problem worldwide (1–3). According to the World Health Organization (WHO), TBI will surpass many diseases as the major cause of death and disability by the year 2020. Indeed, it has been estimated that globally at least 10 million people per year sustain a TBI that is serious enough to result in death or hospitalization. The number of people who have been hospitalized with at least one TBI has been estimated at 57 million, but the proportion of those living with TBI-related disability is still unknown (2).
Approximately 10% of patients with mild TBI, 66% of patients with moderate TBI and all patients with severe TBI will require extensive and costly rehabilitation services due to the effects of their injury (4). In general, TBI is more common in young adults which places a high burden on society because of many life years lost due to disability. TBI results in the greatest number of years lived with a disability due to trauma in Europe (5, 6); the annual cost of traumatic brain injuries is estimated at approximately US$400 billion (7).
Much research has documented that patients with TBI have reduced capacity for activities including functional independence, studying, employment, leisure activities, as well as for personal and social relationships (8–13). Perhaps most importantly, TBI results in significant cognitive dysfunctions, such as attention deficits, memory impairments, and executive functions problems, which relate to mental slowness and reduced processing speed (14–18). Such impairments are thought to be the result of structural brain damage that occurs during the early stage of TBI but importantly, as we now know, continues during the chronic stages of the disease (19, 20).
Recent work indicates that patients with moderate-severe TBI exhibit significant alterations in gray matter volume that are associated with cognitive deficits (21). These effects were found to persist during the chronic stages and at several years post injury supporting the notion that TBI is a long-term condition with chronic implications rather than a static condition following a short recovery phase. Other studies using diffusion tensor imaging (DTI) and/or functional magnetic resonance imaging (fMRI) demonstrated that TBI damages white matter tracts altering structural brain connectivity, which, in turn, may impact functional brain connectivity (22–27).
Evidence of impaired white matter tracts connecting distant brain regions that comprise structural brain networks raises the question as to how TBI affects the functional connectivity of brain areas critical for cognitive functions. Indeed, several recent studies, demonstrated that these abnormalities correlate with the cognitive impairment of patients with mild TBI and are predictive of the cognitive recovery of these patients following a rehabilitation period (23, 28–33).
Despite such plethora of evidence demonstrating altered brain functional connectivity in mild TBI, evidence examining the relationship between cognitive impairment and functional connectivity in patients with moderate-severe chronic TBI is scarce. Previous studies on moderate-severe TBI focused mainly on investigating long-distance interactions between remote brain regions that produce distributed brain networks with distinct functions, termed intrinsic connectivity networks (ICNs). In traditional seed-based analyses, brain areas within ICNs show highly consistent interactions in a pattern that reflects the underlying anatomical structure of white matter connections. A number of ICNs have been identified with abnormalities in TBI patients, including the default mode network [DMN; V. (34–38)], the motor network (39), the thalamic network (40, 41), the executive control network (39, 42), as well as interhemispheric functional connectivity (43).
However, one limitation of this approach is that it restricts analyses due to the requirement to a priori identify ROIs in seed-based analyses. Instead, the intrinsic connectivity contrast [ICC; (44)] has been recently suggested as an approach which allows identification of brain areas with altered functional connectivity without any a priori information that can subsequently be used as seeds in traditional seed-based analyses. Once this is done, the relationship between brain areas with altered functional connectivity and cognitive performance can be examined and contrasted with healthy controls. Our purpose in this proof-of-principle study was to investigate whether this approach can be useful in identifying the relationship between brain areas with altered functional connectivity and their relationship to cognitive outcome in participants with moderate-severe TBI.
We collected resting-state fMRI data and employed the ICC index, a whole-brain voxel-based measure that produces global functional connectivity maps. The ICC index relies on network theory representing the connectivity of each single voxel to the rest of the gray matter voxels in the brain based on the presence of functional connections and the strength of such connections. As mentioned above, in contrast to the traditional ROI methods that require a priori knowledge for the choice and the selection of the ROIs, this index does not require any a priori information or assumptions (44, 45). Our first main aim was to calculate the within-group functional connectivity maps of the participants with TBI and the matched healthy control groups. We computed the ICC relationship with the cognitive measures of executive functions, verbal memory, visual memory, attention/organization, and cognitive reserve, reflecting areas of deficits documented in the chronic TBI literature (15, 21, 46). Following this step, we examined between-group differences in the spatial extent of functional connectivity in the brain regions found to be associated with cognitive performance, using traditional seed-based analyses.
This proof of principle study can contribute toward understanding the neural correlates of cognitive impairments in patients with brain injury. We hypothesized that the global functional connectivity patterns of participants with TBI as indexed by the ICC would be impaired (compared to healthy controls), and that local deficits would include areas associated with the cognitive outcome of the participants with TBI.
Materials and Methods
Participants
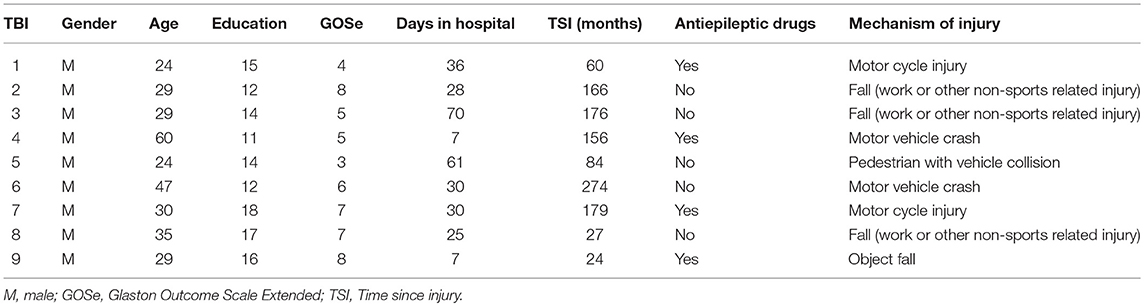
Eleven participants with TBI were matched to eleven neurologically healthy participants on age, gender and education. All participants were right-handed. Two participants with TBI were subsequently excluded from the analysis due to technical difficulties (one participant was claustrophobic and could not enter the bore of the MRI scanner and the MR images of another participant with TBI were presented with significant motion artifacts rendering the imaging data unusable). The participants with TBI were referred from collaborating physicians using a rolling admission process. Table 1 provides the demographics, time since injury (time lapse between TBI and MRI scan), and injury causes for the remaining nine participants with TBI. Primary causes of TBI were as follows: 44% of the participants (4 participants with TBI) were injured in motor vehicle accidents and another 33% (3 participants with TBI) were injured as a result of work-related falls. The remaining 22% (2 participants with TBI) were injured as a result of falling objects and pedestrian-vehicle collision. All of the TBI participants showed microbleeds on the fluid-attenuated inversion recovery (FLAIR) images. None of the healthy participants showed any microbleeds.
In the present study we recruited only male participants with TBI who had not received any systematic post-acute rehabilitation, which allowed us to avoid the confounding effects of sex and systematic post-injury rehabilitation. Previous research has shown that systematic post-injury rehabilitation results in significant improvements in cognitive functioning of people with TBI (15, 47, 48), whereas research on the effects of sex on cognitive outcome in people with TBI is very limited and often contradictory (49–51).
The inclusion/exclusion criteria included a primary diagnosis of a moderate-to-severe head injury which was determined by at least three of the following indices: (1) initial Glasgow Coma Scale score of <12, (2) abnormal initial computed tomography (CT) or MRI findings indicating acute central nervous system pathology, (3) length of impaired consciousness >20 min as specified by the emergency records, (4) length of post-traumatic amnesia >24 h as specified in the acute hospital/emergency records, (5) length of acute hospital stay >3 days, (5) abnormal neurological examination on hospital admission and discharge indicating focal sensory and motor deficits, or changes in mental status attributed to brain injury, (6) medical complications secondary to the brain injury, and (7) head injury classification as moderate-severe according to hospital records. Other inclusion criteria consisted of the Rancho Los Amigos Scale Level VI or higher (which indicates appropriate, goal-oriented behavior, and post-traumatic amnesia resolution). Additionally, time since injury was at least 24 months prior to the study recruitment (mean = 127.33, SD = 83.68 months in the studied group). All participants were native speakers of the Greek language with an age range of 24 to 60 years old (M = 34.11, SD = 11.92), whilst their education ranged from 11 to 18 years (M = 14.12, SD = 2.40). None of the participants had received systematic and comprehensive post-acute rehabilitation in the past or at the time of study recruitment. Some of the participants received inpatient rehabilitation services and fragmented individualized outpatient treatment during the acute phase of their recovery. All participants were residing at home at the time of study participation.
The exclusion criteria consisted of the presence of a penetrating head injury, a diagnosis of a stroke at the time of injury, a premorbid central nervous system disorder or learning disability, a premorbid major depression or other significant psychiatric disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), (52) an active or current alcohol, drug or other controlled substance abuse that would interfere with participation in the study and presence of aphasia with the exception of mild to moderate word finding problems.
All participants in the healthy control (HC) group were Greek-speaking males with no history of a neurological condition or brain trauma, documented psychiatric history, learning disability, or substance abuse. The HC group had an age range of 23 to 60 years old (M = 36.55, SD = 11.18) and education ranged from 8 to 17 years (M = 13.36, SD = 2.98).
Each participant gave written informed consent prior to his participation in the study, as approved by the Cyprus National Bioethics committee.
Neuropsychological Measures
Neuropsychological performance was assessed with a battery of neuropsychological tests which comprised the following conceptually motivated constructs: executive functions, verbal memory, visual memory, attention/organization and cognitive reserve.
The executive functions construct included the Symbol Digits Modalities Test (53), the Trail Making Tests A and B (54) and the Control Oral Word Association Test [Animal naming and words from letter F; (55)].
The Verbal Memory construct included the Greek adaptation of the Auditory Verbal Learning Test [total score in trials 1-5, difference score between trial 5 and trial 1, short delay free recall, long delay free recall, and list A true positive recognition score; (46)] the Digit Span Forward and Backwards total score [adapted Wechsler Memory Scale-Revised, WMS-R (56)], and the Greek adaptation of the paragraphs from the WMS-R Logical Memory I and II [sum of the score of the free recall and the sum of the delayed recall; (56)].
The Visual Memory construct included the Rey Complex Figure Test [immediate recall, delayed recall, recognition total score (57)], the Visual Span Forward and Backwards [from the WMS-R (56)], the spatial visual short-term memory (VSTM) capacity estimate, and the object VSTM capacity estimate [adapted from (21)]. For the spatial and object VSTM capacity tasks, each participant's capacity was assessed using a staircase procedure that estimates the number of spatial locations and the number of objects that a participant can keep in VSTM.
The Attention/Organization construct included the Rey Complex Figure Test [copy and time to copy (57)], and the Distractibility index and the mean reaction time (RT) in a response competition task (21, 58).
The Cognitive Reserve construct included the Pseudowords test [adapted from the Wechsler Individual Achievement Test Second Edition; WIAT-II (59)], and the Peabody Picture Vocabulary Test [Greek adaptation from the PPVT-4; (60, 61)].
Standard Score Transformation
We followed a standard procedure for calculating composite scores by combining scores from the various tests into the conceptually motived constructs [e.g., (21, 62)], although the validity and reliability of this approach was not tested herein. This approach greatly facilitates examining the association between performance on the cognitive measures and global functional connectivity. Performance scores from each of the relevant cognitive and experimental tests are combined into composite scores representing the conceptually motivated constructs of executive functions, verbal memory, visual memory, attention/organization, and cognitive reserve. Each participant's score from each of the individual measures was transformed into a standard score (z-score) based on the mean and the standard deviation of the HC group, in order to allow group comparisons. For this reason, the mean score of the HC group in Table 2 is omitted.
Standard scores from tasks where a higher score indicated worst performance (e.g., response times) were transformed by being multiplied with minus one such that higher scores in all tasks indicated better performance. The resulting standard scores from each of the measures were then averaged together to derive a score for each of the constructed measures.
MRI Data Acquisition and Analysis
MR images were acquired with a 3.0-T scanner (Achieva, Philips Medical Systems, Best, The Netherlands). The built-in quadrature RF body coil and a phased array 8-channel head coil were used for proton excitation and signal detection, respectively. The scanning session included other standard pulse sequences [e.g., T1-weighted rapid acquisition gradient-echo, T2-weighted turbo spin echo, diffusion weighted imaging, diffusion tensor imaging, fluid-attenuated inversion recovery (FLAIR) and susceptibility-weighted imaging (SWI)] to exclude significant brain pathology of a different etiology.
fMRI assessment involved a resting state scan with series of 160 volumes for which participants were instructed to not think of anything in particular and to keep their eyes open. After the scanning session, participants confirmed they had kept their eyes open during the scan and had not fallen asleep. None of the participants underwent an MRI under general anesthesia or sedation. Data were acquired using a spin echo EPI (echo-planar imaging) pulse sequence with the following parameters: TR = 3,000 ms, TE = 70 ms, flip angle = 90°, acquisition voxel size = 2.4 × 2.4 × 4.0 mm, reconstruction voxel size = 1.8 × 1.8 × 4.0 mm, slices per volume = 28.
Prior to preprocessing, the first five fMRI volumes were removed to eliminate saturation effects and achieve steady-state magnetization. Images were preprocessed using Statistical Parametric Mapping 12 (SPM12) (Wellcome Trust Center for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) implemented in MatLab R2015 (Mathworks, Natick, MA, USA). Images were slice time corrected, realigned and unwarped, coregistered (without reslice) to the individual participant's morphological scan, spatially smoothed with a narrow Gaussian kernel of 8 mm at full width half maximum FWHM, and spatially normalized to a standard EPI template in the Montreal Neurological Institute (MNI) space. Visual inspection after every step was performed to ensure appropriate quality of preprocessing. The CONN fMRI functional connectivity toolbox (63) was used to calculate the intrinsic connectivity contrast (ICC) of each participant. Before the ICC calculation we used CompCor, a strict noise reduction method, to remove data components attributable to the signal from white matter and cerebrospinal fluid (64). The method is based on white matter and cerebrospinal fluid masks from the T1-weighted segmented images and eliminates the need for global signal normalization (65, 66). The demographic factors of Age and Education, and the six subject-specific realignment parameters with their first order derivatives were also factored-out before calculating the ICC index (67). A temporal filter of 0.009 and 0.08 Hz was applied to focus on low-frequency fluctuations (68).
Anatomical brain regions were found using SPM Anatomy toolbox (69, 70) and MRIcroN (71).
Intrinsic Connectivity Contrast Analyses
The Intrinsic Connectivity Contrast (ICC) is a voxel-wise index (a single number for each voxel) that represents how well-connected each voxel is to the rest of the gray matter in the brain (44). Following the calculation of the resting state ICC map for each participant, the images were entered into a single multiple regression analysis in SPM12. The design matrix of the model included the following regressors: group (TBI vs. HC) and one regressor for each of the cognitive measures (i.e., the composite scores of executive function, verbal memory, visual memory, attention/organization and cognitive reserve). The design matrix also included age, years of education, and time since injury as regressors of no interest. To capture any motion-related artifacts, motion parameters were also included in the model. Sex was not included in the design matrix since all participants were males. Automatic orthogonalization in SPM was applied to address the problem of collinear regressors in the model. Following false discovery rate (FDR) correction for multiple comparisons across the whole brain, a statistical threshold of p < 0.05 was used.
Associations between the estimated neuropsychological measures and ICCs were tested using a voxel–wise approach within the general linear model framework. Specifically, we assessed the whole brain correlation between each neuropsychological measure and the voxel level ICC of each group, as well as the interaction between the neuropsychological measure and the variable Group (participants with TBI vs. HC) in the multiple regression model described above.
Seed-Based Analyses
The ICC index is a global functional connectivity index and does not provide information on the spatial extent of functional connectivity. For this reason and, in order to calculate within-group functional connectivity maps of the regions found to be associated with each of the neuropsychological measures, we employed seed-based analysis using the CONN fMRI toolbox. This analysis also allowed us to examine between-group (participants with TBI vs. HC) spatial differences in network integrity.
Results
Demographics and MRI
Two-tailed, two-sample t-tests revealed that the groups with TBI and HC participants were very similar in terms of age and education (all t < 1). Any significant differences in the following comparisons between the two groups cannot, thus, be attributed to sample differences in terms of gender, age or education.
Structural and morphological imaging findings in TBI participants included atrophy, impaired white mater integrity, gliosis, and haemosiderin depositions (demonstrated in SWI images). All of the above findings are compatible with chronic head trauma. None of the healthy participants showed any microbleeds. Neither superficial siderosis nor other significant pathology was detected in any participant.
Cognitive Measures
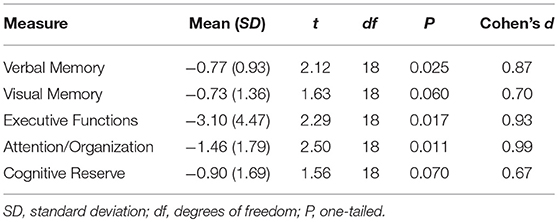
One-tailed independent samples t-tests were conducted in order to compare the performance of the two groups on the constructed measures of Verbal Memory, Visual Memory, Executive Functions, Attention/Organization, and Cognitive Reserve. As shown in Table 2, compared to the non-injured HC participants, the performance of participants with TBI was significantly lower on the neuropsychological constructed measures of Verbal Memory, Attention, and Executive Functions. The performance on the remaining two measures of Visual Memory (p = 0.06) and Cognitive Reserve (p = 0.07) did not differ significantly between the two groups.
Intrinsic Connectivity Contrast
Figure 1 depicts the within-group ICC maps for the TBI and the HC groups showing that HC brains appear to be more connected than TBI patients' brains. Specifically, in both groups, within-group high ICC was observed in posterior regions including the lateral occipital cortex, the angular gyrus, the occipital pole, the supramarginal gyrus, the superior parietal lobule, and the precuneus. High ICC maps were also observed in the frontal pole, the middle frontal gyrus, and the superior frontal gyrus.
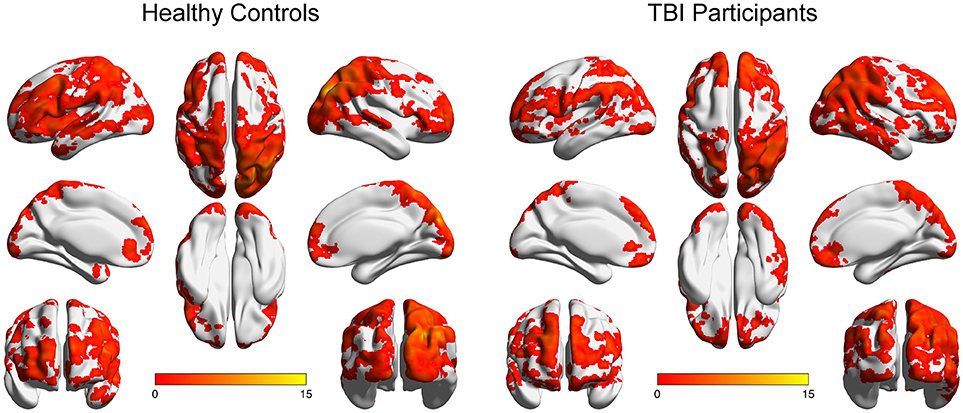
Figure 1. Within-group ICC maps for both groups. Results are presented on inflated brains created using the BrainNet tool (72). Images are displayed in neurological convention (left is left). Color scale represents t-score values.
However, of main interest here are specific areas that correlate with the cognitive measures. Several brain areas in the group with TBI, described in Table 3, exhibited significant correlations between the cognitive constructed measures and the corresponding within-group ICC maps. Specifically, the within-group ICC maps at the left medial Temporal pole, exhibited a significant positive correlation with Executive Functions scores, whereas the left Putamen exhibited a significant negative relationship. The within-group ICC maps at the left medial temporal pole, left superior parietal lobule, right inferior frontal gyrus, and the superior part of the right medial frontal gyrus exhibited a significant positive correlation with the Verbal Memory scores, whereas the right precuneus and right angular gyrus exhibited a significant negative relationship with the Verbal Memory scores. The left middle temporal gyrus exhibited a significant positive correlation with Visual Memory scores, whereas the left putamen, left superior orbital gyrus, left hippocampus, left pallidum, and right anterior cingulate cortex (ACC), exhibited a significant negative correlation with Visual Memory scores. The left superior temporal gyrus exhibited a significant positive correlation with Attention scores, whereas the left putamen and right ACC exhibited a significant negative correlation with Attention scores. The left superior temporal gyrus exhibited a significant positive correlation with Cognitive Reserve scores.
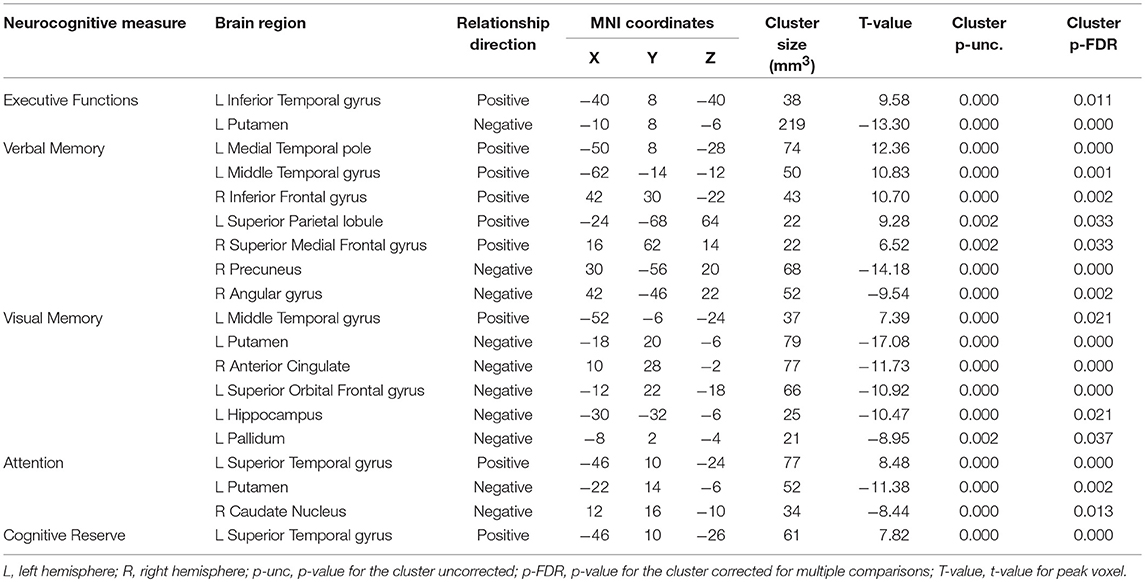
Table 3. Within-group correlations between ICC and cognitive measures (p-FDR < 0.05 corrected) for the TBI patients.
A positive correlation between the ICC score and the score on a composite measure in the above analyses indicates that global connectivity in a specific brain area and the corresponding cognitive function move in the same direction, that is, increased global connectivity of that specific brain area is associated with better ability to exercise the corresponding cognitive function or reduced connectivity is associated with impaired cognitive ability. On the contrary, a negative correlation indicates that global connectivity at a specific brain area and the corresponding cognitive function move in opposite directions, where either increased connectivity is associated with better cognitive ability or reduced connectivity is associated with impaired cognitive ability.
Significant between-group interactions of ICC correlations with cognitive measures are shown in Figure 2. All significant correlations were strong as indicated by all r>0.68. Specifically, the correlation between ICC in the right middle temporal gyrus and Executive Functions was negative in the healthy controls and positive in the participants with TBI (Figure 2A); between ICC in the right inferior temporal gyrus and Verbal Memory was positive in the healthy controls and negative in the participants with TBI (Figure 2B); between ICC in the right middle frontal gyrus and Cognitive Reserve was positive in the healthy controls and negative in the participants with TBI (Figure 2C); between ICC in the left temporal pole and Cognitive Reserve was negative in the healthy controls and positive in the participants with TBI (Figure 2D); between ICC in the left subcallosal cortex and Cognitive Reserve was negative in the healthy controls and positive in the participants with TBI (Figure 2E).
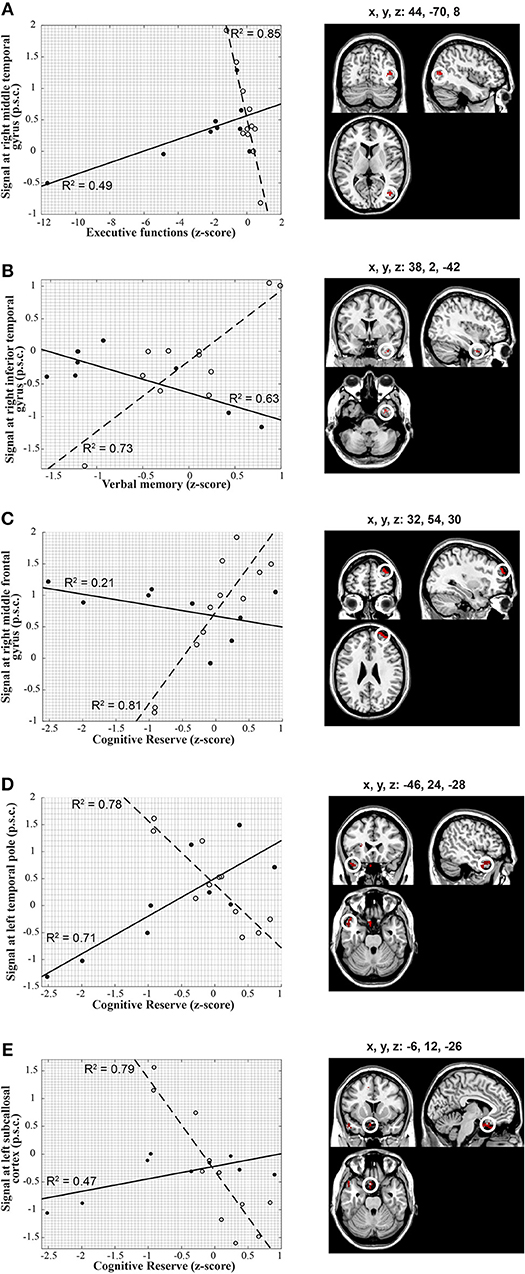
Figure 2. Voxel-wise correlations between ICC extracted from brain regions depicted on the right-hand panels and individual scores in the cognitive constructed measures along with the corresponding scatter plots (left-hand panels). The scatter plots on the left show in detail the relationship between ICC and cognitive measures in each of the statistical peaks for both groups (TBI: solid lines, closed circles, HC: dashed lines, open circles). Results are overlaid onto a standard single subject T1-weighted MR-image (ch2-template) in the MRICroN software (71). (A) Correlation between the cognitive measure of executive function and right middle Temporal gyrus in both groups. The correlation of ICC with executive function scores was positive in participants with TBI [r(6) = 0.70; p = 0.04] and negative in the healthy controls [r(9) = −0.92; p < 0.001] for the right middle temporal gyrus [peak at x, y, z = 44, −70, 8; t(1, 16) = 6.66; p-FDR < 0.05]. (B) Correlation between verbal memory and right inferior Temporal gyrus in both groups. The correlation of ICC with verbal memory scores was negative in participants with TBI [r(6) = 0.79; p = 0.01] and positive in the healthy controls [r(9) = 0.85; p = 0.001] for the right inferior Temporal gyrus [peak at x, y, z = 38, 2, −42; t(1, 16) = 6.01; p-FDR = 0.04]. (C) Correlation between cognitive reserve and right middle Frontal gyrus in both groups. The correlation of ICC with cognitive reserve scores exhibited a negative trend in participants with TBI but did not reach statistical significance [r(6) = −0.46; p = 0.21], whereas it was positive in the healthy controls [r(9) = 0.90; p < 0.001] for the right middle frontal gyrus [peak at x, y, z = 32, 54, 30; t(1, 16) = 6.13; p-FDR < 0.001]. (D) Correlation between cognitive reserve and left Temporal pole in both groups. The correlation of ICC with cognitive reserve scores was positive in participants with TBI [r(6) = 0.84; p = 0.005] and negative in the healthy controls [r(9) = −0.88; p < 0.001) for the left temporal pole [peak at x, y, z = −46, 24, −28; t(1, 16) = 6.46; p-FDR < 0.001). (E) Correlation between cognitive reserve and left Subcallosal cortex in both groups. The correlation of ICC with cognitive reserve scores was positive in participants with TBI [r(6) = 0.68; p < 0.05] and negative in the healthy controls [r(9) = −0.89; p < 0.001] for the left subcallosal cortex [peak at x, y, z = −6, 12, −26; t(1, 16) = 6.80; p-FDR < 0.001]. Percent signal change, p.s.c.
Seed-Based Analyses
Figure 3 depicts the within-group functional connectivity maps of each group calculated using as seeds the regions that exhibited a significant relationship between the TBI within-group ICC maps and each of the neuropsychological measures (see Table 3). Figure 4 depicts the within-group ICC maps calculated from seed areas that displayed a significant between-group interaction with the cognitive measures.
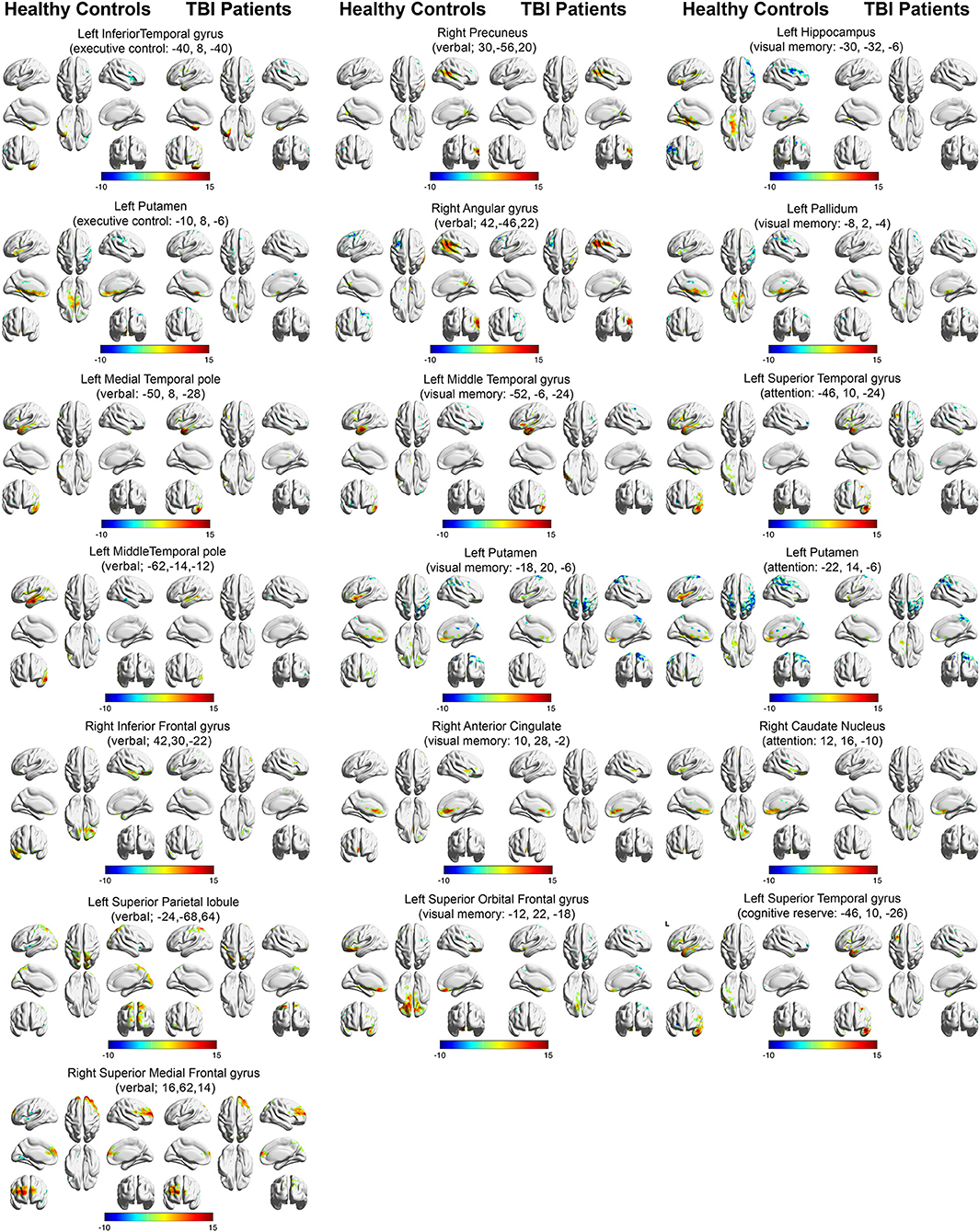
Figure 3. Within-group functional connectivity maps calculated from seed areas showing significant correlations with the TBI participants. Voxels showing significant positive functional connectivity are shown in red–yellow color scale, and voxels showing significant negative functional connectivity are shown in green-blue color scale.
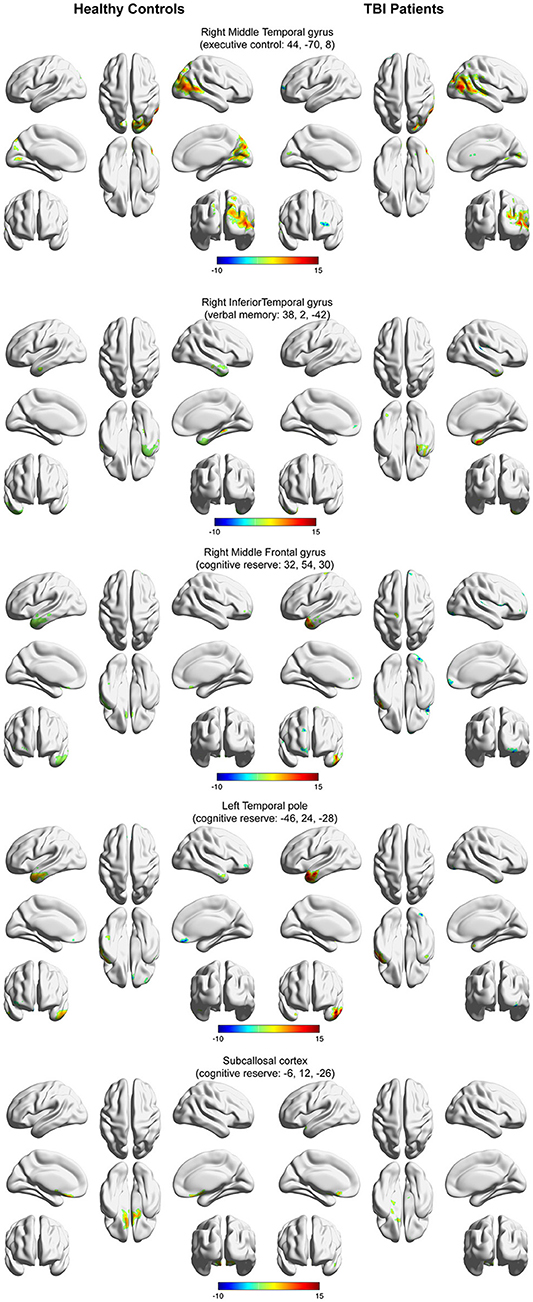
Figure 4. Within-group functional connectivity maps calculated from seed areas showing a significant interaction between the healthy controls and the TBI participants. Voxels showing significant positive functional connectivity are shown in red–yellow color scale, and voxels showing significant negative functional connectivity are shown in green-blue color scale.
Figure 5 shows the between-group functional connectivity differences in the TBI and healthy control groups using as seeds the brain areas with significant relationships between the TBI within-group ICC maps and each of the neuropsychological measures. This analysis revealed that compared to the healthy control group, participants with TBI showed greater functional connectivity between the left middle temporal pole seed and a region in left middle frontal sulcus [peak at x, y, z = −22, 8, 48; t(1, 16) = 3.69; pDFR = 0.022; Figure 5A], the left putamen seed and a region in right postcentral gyrus [peak at x, y, z = 56, −8, 36; t(1, 18) = 3.61; pDFR = 0.036; Figure 5B], and the right angular gyrus seed and the left precuneus [peak at x, y, z = −10, −80, 42; t(1, 17) = 3.65; pDFR = 0.001; Figure 5K]. Moreover, participants with TBI showed reduced functional connectivity (compared to the healthy control group) between the left pallidum seed and a region in left superior medial frontal gyrus [peak at x, y, z = 6, 72, 10; t(1, 18) = 3.61; pDFR = 0.001; Figure 5C], the left hippocampus seed and the right middle frontal sulcus [peak at x, y, z = 30, 38, 26; t(1, 18) = 3.61; pDFR = 0.003; Figure 5D], the right precuneus [peak at x, y, z = 16, −78, 48; t(1, 18) = 3.61; pDFR = 0.039; Figure 5E], the right precentral sulcus [peak at x, y, z = 42, −12, 36; t(1, 18) = 3.61; pDFR = 0.039; Figure 5F], the left superior occipital lobule [peak at x, y, z = −10, −80, 42; t(1, 18) = 3.61; pDFR = 0.047; Figure 5G], the right precentral sulcus [peak at x, y, z = 42, 2, 34; t(1, 18) = 3.61; pDFR = 0.047; Figure 5H], the left superior orbito-frontal gyrus seed with the left calcarine sulcus [peak at x, y, z = −14, −80, 10; t(1, 18) = 3.61; pDFR = 0.048; Figure 5I] and the left inferior orbito-frontal gyrus [peak at x, y, z = −14, 14, −26; t(1, 18) = 3.61; pDFR = 0.048; Figure 5J], and the left middle temporal gyrus seed and the left anterior cingulum [peak at x, y, z = 0, 42, 0; t(1, 18) = 3.61; pDFR = 0.017; Figure 5L].
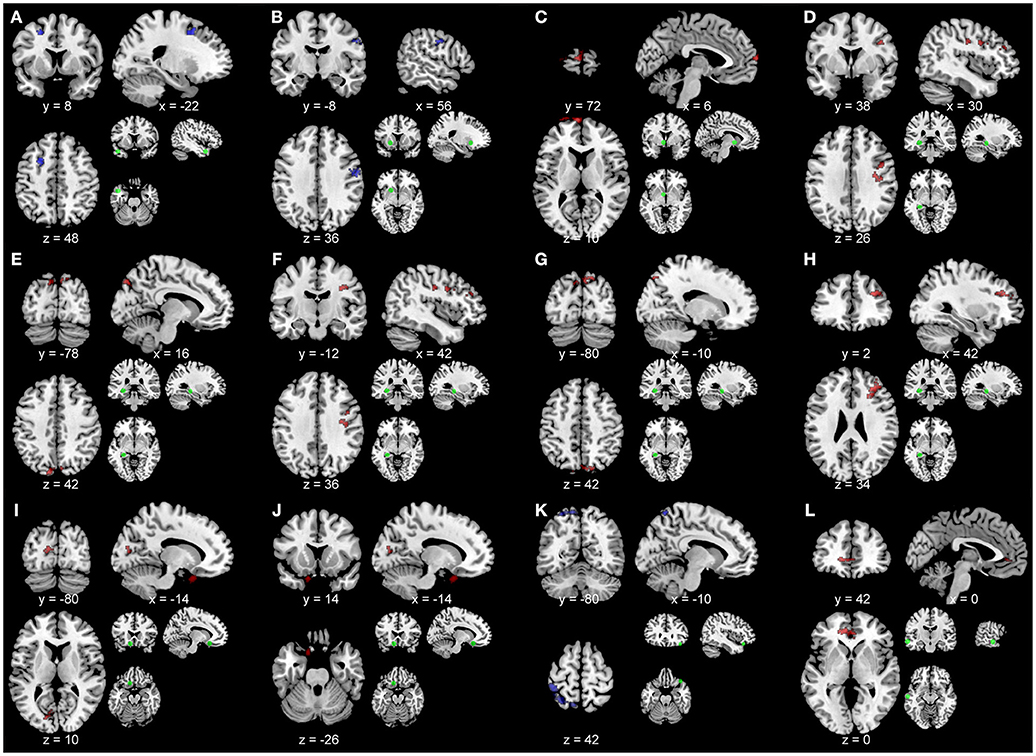
Figure 5. Results of the between-group comparisons of the seed-based analyses overlaid on a template brain. Blue represents greater functional connectivity in the TBI participants compared to the healthy controls. Red color represents greater functional connectivity in the healthy controls compared to the TBI participants. Small brain images with green circles represent the seed area. Results are overlaid onto a standard single subject T1-weighted MR-image (ch2bet-template) in the MRICroN software (71). Images are displayed in neurological convention (left is left). (A) Seed, left middle temporal pole. Peak, left middle frontal sulcus at x, y, z = −22, 8, 48; t(1, 16) = 3.69; pDFR = 0.022. (B) Seed, left putamen. Peak, right postcentral gyrus at x, y, z = 56, −8, 36; t(1, 18) = 3.61; pDFR = 0.036. (C) Seed, left pallidum. Peak, left superior medial frontal gyrus at x, y, z = 6, 72, 10; t(1, 18) = 3.61; pDFR = 0.001. (D) Seed, left hippocampus. Peak, right middle frontal sulcus at x, y, z = 30, 38, 26; t(1, 18) = 3.61; pDFR = 0.003. (E) Seed, left hippocampus. Peak, right precuneus at x, y, z = 16, −78, 48; t(1, 18) = 3.61; pDFR = 0.039. (F) Seed, left hippocampus. Peak, right precentral sulcus at x, y, z = 42, −12, 36; t(1, 18) = 3.61; pDFR = 0.039. (G) Seed, left hippocampus. Peak, left superior occipital lobule [peak at x, y, z = −10, −80, 42; t(1, 18) = 3.61; pDFR = 0.047]. (H) Seed, left hippocampus. Peak, right precentral sulcus at x, y, z = 42, 2, 34; t(1, 18) = 3.61; pDFR = 0.047. (I) Seed, left superior orbito-frontal gyrus. Peak, left calcarine sulcus [peak at x, y, z = −14, −80, 10; t(1, 18) = 3.61; pDFR = 0.048]. (J) Seed, left superior orbito-frontal gyrus. Peak, left inferior orbito-frontal gyrus at x, y, z = −14, 14, −26; t(1, 18) = 3.61; pDFR = 0.048. (K) Seed, right angular gyrus. Peak, left precuneus at x, y, z = −10, −80, 42; t(1, 17) = 3.65; pDFR = 0.001. (L) Seed, left middle temporal gyrus. Peak, left anterior cingulum at x, y, z = 0, 42, 0; t(1, 18) = 3.61; pDFR = 0.017.
Discussion
The main aim of this proof of principle study was to utilize the ICC index to investigate whether changes in functional brain connectivity patterns in moderate-severe TBI are related to cognitive outcome. Although TBI has been previously associated with altered functional brain connectivity and with impaired cognitive functioning, the relationship between these two variables has not been explored. Due to the high prevalence of TBI among males, we focused on a homogeneous group of male participants with moderate-severe chronic TBI that had not received any post-acute systematic comprehensive rehabilitation. Studying this homogeneous group allowed us to gain a more precise understanding of the true relationship between functional connectivity and cognitive outcome in males by avoiding the confounding effects of sex and rehabilitation (15, 47–51). We utilized a novel index (ICC) to determine the global functional connectivity at the level of individual voxels, (73) followed by seed-based analyses to characterize the spatial extend of functional connectivity changes. Our main finding was that the brain areas exhibiting altered integrity of functional brain connectivity at rest in participants with TBI, compared to matched healthy controls, were associated with outcomes in cognitive measures of executive function, verbal memory, visual memory, attention/organization and cognitive reserve.
Within-group maps of the ICC were obtained in posterior cortical areas (e.g., lateral and medial occipital areas), parietal areas (e.g., angular gyrus and superior parietal lobe), frontal lobe areas (including lateral, superior and medial regions), and lateral and inferior temporal lobe areas, for both groups. However, close visual inspection of the within-group functional connectivity maps of participants with TBI and the corresponding maps of healthy controls revealed that participants with TBI exhibited similar maps albeit with more limited spatial extend. This finding demonstrates that the strength of global connectivity between several brain areas and the rest of the brain is altered in chronic moderate-severe TBI, indicating that the impaired global connectivity is a significant consequence of brain injury.
Similar reduced resting-state functional connectivity maps have also been found in patients with mild cognitive impairment and Alzheimer's disease (74). In these neurodegenerative diseases, reduced functional connectivity at rest reflects loss of neurons that consequently affects connectivity and results in breakdown of brain networks. It is suggested, therefore, that the reduced structural brain connectivity [e.g., (75)] and reduced brain volume due to the brain injury [e.g. (21)] that have been observed previously may be responsible for the results of altered global functional connectivity observed in this study. Taken together with earlier findings on the impaired cognitive functioning of the participants with TBI (compared to the healthy controls), this study utilizing the ICC index provides a new line of evidence for the suggestion that the injured brain remains affected for many years after the initial insult.
The findings of the present study, using a similar methodology to a recent report by Moreno-López et al. (73) that characterized the association between depressive symptomatology and global functional connectivity in TBI, complement previous reports in highlighting that altered functional connectivity in chronic TBI may be predictive of cognitive outcome (34–42, 76). Current findings indicate for the first time that the strength of global functional connectivity is related to the cognitive outcome of people with moderate-severe TBI, demonstrating that the ICC index could potentially prove to be a useful imaging biomarker in characterizing and monitoring the TBI impact on cognitive performance.
An additional contribution of the current study may lay in the fact that through combining the ICC index analysis with more traditional seed-based analyses, our findings contribute in the understanding of the relationship between functional connectivity and cognitive processing in chronic moderate-severe TBI, confirming well-established links between executive functioning, verbal memory, visual memory, and cognitive reserve, with frontal, temporal, parietal, cerebellar, and subcortical areas.
Specifically, the regions associated here with performance on executive functions, including the putamen, the left medial temporal pole and the right middle temporal gyrus, have been previously implicated in executive functions, including goal-directed behavior (77), executive control during performance in visuospatial tasks (78), cognitive control during goal-directed mental simulation (79), performance in a planning task (80), and in a divided attention task (81), interference control (82), mental rotation (83), mental updating (84), and task switching and resolution of competition between potentially relevant tasks (85).
With regards to verbal memory, the finding that performance in verbal memory is associated with functional connectivity in temporal lobe regions is consistent with models in which medial temporal cortex is involved in semantic processing across a range of input modalities [including verbal, visual, and tactile; (86–88)]. Moreover, the right inferior frontal gyrus was previously found to be active during reading, semantic, and phonological decision tasks (89) and during deep processing of verbal information in memory (90). The left superior parietal lobule showed significant activity during memory retrieval of verbal information (91) and increased fMRI activity in a letter delayed recognition task (92). These findings support the view that the short-term retention of verbal information is supported by regions that carry out relatively early stages of acoustic, lexical, phonological, and speech-based processing indicating that these brain regions may have a dual function, the short-term maintenance in addition to the precise encoding of this information (93).
Performance in the visual memory, attention/organization and cognitive reserve was related to global functional connectivity in brain areas that were previously shown to exhibit activity during corresponding experimental tasks, confirming well-established links between cognitive performance and brain function. For instance, the left medial temporal gyrus has been involved in delay-period activity in a delayed match to sample short-term memory task for visual information (94), and activity in this area during a similar visual short-term memory task was found to be specifically related only to memory probes indicating that it is specifically involved in visual memory recognition (95). Brain regions with functional connectivity related to attention/organization were found to exhibit activity during attention tasks. For example, in a selective attention task of car experts, the left superior temporal gyrus showed fMRI activity that was specific to the attended condition (96). The left Putamen showed significantly enhanced activity associated with cued attention shifts during an auditory selective attention task (97), and activity in this brain area was specific to covert attention shifts, specifically when participants performed a covert visual assessment of a peripheral stimulus in the absence of any saccades (98). The right Caudate nucleus showed fMRI activity related to the attended condition in a selective attention task of car experts (96). Taken together, these findings demonstrate that impaired global functional connectivity is associated with cognitive outcome and it constitutes an important consequence of brain injury.
Comparing functional connectivity between the two groups revealed that the direction of relationship between global functional connectivity (as measured with the ICC index) and measures of executive functions, verbal memory, and cognitive reserve was found to depend on whether the participants were TBI survivors or healthy controls. These findings indicate that perhaps the reduced structural brain connectivity and reduced brain volume due to the brain injury (19) result in reduced global functional connectivity in some brain areas but also could produce increased global functional connectivity in other brain areas, presumably in a way that compensates for the loss of brain volume and structural brain connectivity. Furthermore, reductions in cognitive performance in chronic moderate-severe TBI have been associated with reductions in white matter and gray matter volume that was not widespread, but followed a fronto-thalamic pattern (21).
The small sample size of the TBI group constitutes the main limitation of the current study. Nevertheless, the validity of the present results is enhanced by the strict inclusion/exclusion criteria adopted, and by the homogeneity of the participants included. Specifically, most previous studies investigating the effects of TBI on brain structure and function are affected by the confounding factors of comprehensive post-injury rehabilitation and sex. Comprehensive post-injury rehabilitation has been shown to improve cognitive functioning in TBI (15, 47, 48). For example, Till et al. (99) showed that the best predictor of cognitive outcome at 2-5 years post-injury was the amount of hours of rehabilitation at 5 months postinjury, independent of injury severity or the initial severity of cognitive impairment. The present study avoided the confounding effects of rehabilitation by recruiting a group of participants that had not received comprehensive post-injury rehabilitation, thus gaining a more accurate understanding of the true effects of chronic TBI on brain connectivity.
Sex constitutes another confounding factor of previous research investigating the chronic effects of TBI on the brain. For example, in animal models of TBI, females demonstrate better outcomes compared to males, supporting the idea that in TBI gonadal steroids (i.e., estrogen and progesterone) may induce neuroprotective effects in TBI (100–103). However, the findings on the effects of sex on cognitive outcome due to TBI in humans are still very limited and with contradictory findings (49, 50, 103, 104). In order to circumvent possible confounding effects of sex and due to the higher prevalence of TBI among males, compared to females (105), the current study focused on a homogeneous group of male TBI participants.
Future work should focus on dissociating the effects of sex and better understand the effects of rehabilitation on brain connectivity. Moreover, future work should investigate the temporal pattern of the functional connectome changes related to moderate-severe TBI and should investigate if the ICC index can be used as a surrogate imaging biomarker for prognosis, treatment planning and prediction of cognitive outcome in TBI. Changes to the functionally related neural networks in the resting state could also be studied during the recovery phase before and after the application of state-of-the-art neurorehabilitative interventions to assess their effectiveness. Additionally, future studies may find it useful to employ ICC maps along with DTI and brain volumetric data in order to provide a more accurate characterization of the underlying neurophysiological and structural sequalae in chronic moderate-severe TBI.
Conclusion
In conclusion, this study demonstrated that cognitive impairments of participants with TBI associated with outcomes in cognitive measures of executive functions, verbal memory, visual memory, attention/organization, and cognitive reserve are related to altered integrity of global functional brain connectivity at rest. These findings, which are associated with differences in network connectivity in frontal and temporal cortical and subcortical networks and persist for several years after the injury, may account for part of the unaccounted variance regarding the neurophysiological substrates of cognitive deficits in chronic TBI. Larger studies are warranted to validate the above findings across the severity continuum, link ICC with anatomical connectivity patterns (e.g., using DTI) and further explore the utility of the ICC as an index of neuropathology following TBI.
Author Contributions
NK collected neuropsychological data, collected MRI data, conducted the MRI data processing, statistical analyses, and drafted the initial manuscript. EP recruited participants, collected neuropsychological data and conducted the neuropsychological data analyses. ES advised on MRI data analyses and manuscript review. IS collected MRI data and advised on MRI data analyses, data interpretation and manuscript review. FC recruited participants, advised on data interpretation and manuscript review.
Funding
This work was supported by the Cyprus Research Promotion Foundation through a grant co-funded by the Cyprus Government and the European Regional Development Fund (FC, PI; NEW INFRASTRUCTURE/STRATEGIC/0309/37).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the volunteers who participated in the study.
References
1. Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation (2007) 22:341–53.
2. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury a brief overview. J Head Trauma Rehabil. (2006) 21:375–8. doi: 10.1097/00001199-200609000-00001
3. Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health (2016) 1:e76–e83. doi: 10.1016/S2468-2667(16)30017-2
4. Bennett ER, Reuter-Rice K, Laskowitz DT. Genetic Influences in Traumatic Brain Injury. Translational Research in Traumatic Brain Injury. Boca Raton, FL: CRC Press; Taylor and Francis Group (2016). Available online at: http://europepmc.org/books/NBK326717
5. Polinder S, Meerding WJ, van Baar ME, Toet H, Mulder S, van Beeck EF. Cost estimation of injury-related hospital admissions in 10 European countries. J Trauma Injury Infect Crit Care (2005) 59:1283–91. doi: 10.1097/01.ta.0000195998.11304.5b
6. Polinder S, Meerding WJ, Mulder S, Petridou E, Van Beeck E, Bauer R, et al. Assessing the burden of injury in six European countries. Bull World Health Organ. (2007) 85:27–34. doi: 10.2471/BLT.06.030973
7. Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. (2017)16:987–1048. doi: 10.1016/S1474-4422(17)30371-X
8. Dikmen SS, Ross BL, Machamer JE, Temkin NR. One year psychosocial outcome in head injury. J Int Neuropsychol Soc. (1995) 1:67–77. doi: 10.1017/S1355617700000126
9. Lehtonen S, Stringer AY, Millis SR, Boake C, Englander J, Hart T, et al. Neuropsychological outcome and community re-integration following traumatic brain injury: the impact of frontal and non-frontal lesions. Brain Injury (2005) 19:239–56. doi: 10.1080/0269905040004310
10. Novack TA, Bush BA, Meythaler JM, Canupp K. Outcome after traumatic brain injury: Pathway analysis of contributions from premorbid, injury severity, and recovery variables. Arch Phys Med Rehabil. (2001) 82:300–5. doi: 10.1053/apmr.2001.18222
11. Oddy M, Coughlan T, Tyerman A, Jenkins D. Social adjustment after closed head injury: a further follow-up seven years after injury. J Neurol Neurosurg Psychiatry (1985) 48:564–8. doi: 10.1136/jnnp.48.6.564
12. Olver JH, Ponsford JL, Curran CA. Outcome following traumatic brain injury: A comparison between 2 and 5 years after injury. Brain Injury (1996) 10:841–8. doi: 10.1080/026990596123945
13. Tate RL, Lulham JM, Broe GA, Strettles B, Pfaff A. Psychosocial outcome for the survivors of severe blunt head injury: the results from a consecutive series of 100 patients. J Neurol Neurosurg Psychiatry (1989) 52:1128–34. doi: 10.1136/jnnp.52.10.1128
14. Christensen BK, Colella B, Inness E, Hebert D, Monette G, Bayley M, et al. Recovery of cognitive function after traumatic brain injury: a multilevel modeling analysis of canadian outcomes. Arch Phys Med Rehabil. (2008) 89(12 Suppl.):S3–15. doi: 10.1016/j.apmr.2008.10.002
15. Constantinidou F, Thomas RD, Robinson L. Benefits of categorization training in patients with traumatic brain injury during post–acute rehabilitation. J Head Trauma Rehabil. (2008) 23:312–28. doi: 10.1097/01.HTR.0000336844.99079.2c
16. Spikman JM, Zomeren AH, van and Deelman BG. Deficits of attention after closed-head injury: slowness only? J Clin Exp Neuropsychol. (1996) 18:755–67. doi: 10.1080/01688639608408298
17. Wilson JTL, Pettigrew LEL, Teasdale GM. Emotional and cognitive consequences of head injury in relation to the Glasgow outcome scale. J Neurol Neurosurg Psychiatry (2000) 69:204–9. doi: 10.1136/jnnp.69.2.204
18. Wood RL, Rutterford NA. Demographic and cognitive predictors of long-term psychosocial outcome following traumatic brain injury. J Int Neuropsychol Soc. (2006) 12:350–8. doi: 10.1017/S1355617706060498
19. Green REA, Colella B, Maller JJ, Bayley M, Glazer J, Mikulis DJ. Scale and pattern of atrophy in the chronic stages of moderate-severe TBI. Front Hum Neurosci. (2014) 8:67. doi: 10.3389/fnhum.2014.00067
20. Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma (2010) 27:1529–40. doi: 10.1089/neu.2010.1358
21. Konstantinou N, Pettemeridou E, Seimenis I, Eracleous E, Papacostas SS, Papanicolaou AC, et al. Assessing the relationship between neurocognitive performance and brain volume in chronic moderate-severe traumatic brain injury. Front Neurol. (2016) 7:29. doi: 10.3389/fneur.2016.00029
22. Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. Am J Neuroradiol. (2013) 34:2064. doi: 10.3174/ajnr.A3395
23. Kasahara M, Menon DK, Salmond CH, Outtrim JG, Tavares JVT, Carpenter TA, et al. Traumatic brain injury alters the functional brain network mediating working memory. Brain Injury (2011) 25:1170–87. doi: 10.3109/02699052.2011.608210
24. Palacios EM, Sala-Llonch R, Junque C, Fernandez-Espejo D, Roig T, Tormos JM, et al. (2013). Long-term declarative memory deficits in diffuse TBI: correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex 49:646–57. doi: 10.1016/j.cortex.2012.02.011
25. Sharp DJ, Ham TE. Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol. (2011) 24:558–63. doi: 10.1097/WCO.0b013e32834cd523
26. Warner MA, Youn TS, Davis T, Chandra A, Marquez De La Plata C, Moore C, et al. Regionally selective atrophy after traumatic axonal injury. Arch Neurol. (2010) 67:1336–44. doi: 10.1001/archneurol.2010.149
27. Xiao H, Yang Y, Xi J, Chen Z. Structural and functional connectivity in traumatic brain injury. Neural Regener Res. (2015) 10:2062. doi: 10.4103/1673-5374.172328
28. Caeyenberghs K, Leemans A, Leunissen I, Gooijers J, Michiels K, Sunaert S, et al. Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct Funct. (2014) 219:193–209. doi: 10.1007/s00429-012-0494-2
29. Caeyenberghs K, Siugzdaite R, Drijkoningen D, Marinazzo D, Swinnen SP. Functional connectivity density and balance in young patients with traumatic axonal injury. Brain Connect. (2015) 5:423–32. doi: 10.1089/brain.2014.0293
30. Castellanos NP, Paúl N, Ordóñez VE, Demuynck O, Bajo R, Campo P, et al. Reorganization of functional connectivity as a correlate of cognitive recovery in acquired brain injury. Brain (2010) 133:2365–81. doi: 10.1093/brain/awq174
31. Falletta Caravasso C, de Pasquale F, Ciurli P, Catani S, Formisano R, Sabatini U. The default mode network connectivity predicts cognitive recovery in severe acquired brain injured patients: a longitudinal study. J Neurotrauma (2016) 33:1247–62. doi: 10.1089/neu.2015.4003
32. Nakamura T, Hillary FG, Biswal BB. Resting network plasticity following brain injury. PLoS ONE (2009) 4:e8220. doi: 10.1371/journal.pone.0008220
33. Palacios EM, Yuh EL, Chang YS, Yue JK, Schnyer DM, Okonkwo DO, et al. Resting-state functional connectivity alterations associated with six-month outcomes in mild traumatic brain injury. J Neurotrauma (2017) 34:1546–57. doi: 10.1089/neu.2016.4752
34. Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. (2011) 31:13442–51. doi: 10.1523/JNEUROSCI.1163-11.2011
35. Messé A, Caplain S, Pélégrini-Issac M, Blancho S, Lévy R, Aghakhani N, et al. Specific and evolving resting-state network alterations in post-concussion syndrome following mild traumatic brain injury. PloS ONE (2013) 8:e65470. doi: 10.1371/journal.pone.0065470
36. Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain (2011) 134:2233–47. doi: 10.1093/brain/awr175
37. Stevens MC, Lovejoy D, Kim J, Oakes H, Kureshi I, Witt ST. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging and Behavior (2012) 6:293–318. doi: 10.1007/s11682-012-9157-4
38. Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, et al. Default-mode network disruption in mild traumatic brain injury. Radiology (2012) 265:882–92. doi: 10.1148/radiol.12120748
39. Shumskaya E, Andriessen TMJC, Norris DG, Vos PE. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology (2012) 79:175–82. doi: 10.1212/WNL.0b013e31825f04fb
40. Tang L, Ge Y, Sodickson DK, Miles L, Zhou Y, Reaume J, et al. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology (2011) 260:831–40. doi: 10.1148/radiol.11110014
41. Zhou Y, Lui YW, Zuo X-N, Milham MP, Reaume J, Grossman RI, et al. Characterization of thalamo-cortical association using amplitude and connectivity of functional MRI in mild traumatic brain injury. J Magn Resonan Imagin. (2014) 39:1558–68. doi: 10.1002/jmri.24310
42. Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Map. (2011) 32:1825–35. doi: 10.1002/hbm.21151
43. de la Plata CDM, Garces J, Kojori ES, Grinnan J, Krishnan K, Pidikiti R, et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol. (2011) 68:74–84. doi: 10.1001/archneurol.2010.342
44. Martuzzi R, Ramani R, Qiu M, Shen X, Papademetris X, Constable RT. A whole-brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. NeuroImage (2011) 58:1044–50. doi: 10.1016/j.neuroimage.2011.06.075
45. Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. (2009) 29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009
46. Constantinidou F, Evripidou C. Stimulus modality and working memory performance in Greek children with reading disabilities: additional evidence for the pictorial superiority hypothesis. Child Neuropsychol. (2012) 18:256–80. doi: 10.1080/09297049.2011.602013
47. Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. (2011) 92:519–30. doi: 10.1016/j.apmr.2010.11.015
48. Tsaousides T, Gordon WA. Cognitive rehabilitation following traumatic brain injury: assessment to treatment. Mt Sinai J Med. (2009) 76:173–81. doi: 10.1002/msj.20099
49. Chase S, Ratcliff G, Vernich L, Al-Sukhni E, Yasseen B, Colantonio A. Preventive health practices and behavioral risk factors in women surviving traumatic brain injury. Health Care Women Int. (2012) 33:631–45. doi: 10.1080/07399332.2012.673652
50. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. (2012) 40:1303–12. doi: 10.1177/0363546512444554
51. Harris JE, Colantonio A, Bushnik T, Constantinidou F, Dawson D, Goldin-Lauretta Y, et al. Advancing the health and quality-of-life of girls and women after traumatic brain injury: workshop summary and recommendations. Brain Injury (2012) 26:177–82. doi: 10.3109/02699052.2011.635361
52. American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders V. Arlington. American Psychiatric Pub. doi: 10.1176/appi.books.9780890425596
53. Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services (1982) p. 22.
54. Zalonis I, Kararizou E, Triantafyllou NI, Kapaki E, Papageorgiou S, Sgouropoulos P, et al. A normative study of the trail making test A and B in Greek adults. Clin Neuropsychol. (2007) 22:842–50. doi: 10.1080/13854040701629301
55. Kosmidis MH, Vlahou CH, Panagiotaki P, Kiosseoglou G. The verbal fluency task in the Greek population: normative data, and clustering and switching strategies. J Int Neuropsychol Soc. (2004) 10:164–72. doi: 10.1017/S1355617704102014
56. Wechsler D. Wechsler Adult Intelligence Scale: Administration and Scoring Manual. 3rd ed. San Antonio, TX: Psychological Corporation (1997).
57. Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial Professional Manual. Psychological Assessment Resources (1995).
58. Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Attent Percept Psychophys. (1974) 16:143–9. doi: 10.3758/BF03203267
59. Simos PG, Sideridis GD, Kasselimis D, Mouzaki A. Reading fluency estimates of current intellectual function: demographic factors and effects of type of stimuli. Journal of the International Neuropsychological Society (2013) 19:355–61. doi: 10.1017/S1355617712001518
61. Simos PG, Kasselimis D, Mouzaki A. Age, gender, and education effects on vocabulary measures in Greek. Aphasiology (2011) 25:475–91. doi: 10.1080/02687038.2010.512118
62. Rojas DC, Bennett TL. Single versus composite score discriminative validity with the Halstead-Reitan Battery and the Stroop Test in mild brain injury. Arch Clin Neuropsychol. (1995) 10:101–10. doi: 10.1093/arclin/10.2.101
63. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. (2012) 2:125–41. doi: 10.1089/brain.2012.0073
64. Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage (2007) 37:90–101. doi: 10.1016/j.neuroimage.2007.04.042
65. Chai XJ, Castañón AN, Öngür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage (2012) 59:1420–8. doi: 10.1016/j.neuroimage.2011.08.048
66. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage (2009) 44:893–905. doi: 10.1016/j.neuroimage.2008.09.036
67. Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. (2007) 104:13507–12. doi: 10.1073/pnas.0705843104
68. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. From The Cover: The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. (2005) 102:9673–8. doi: 10.1073/pnas.0504136102
69. Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage (2005) 25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034
70. Zilles K, Amunts K. Centenary of Brodmann's map conception and fate. Nat Rev Neurosci. (2010) 11:139–45. doi: 10.1038/nrn2776
71. Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. (2000) 12:191–200. doi: 10.1155/2000/421719
72. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE (2013) 8:e68910. doi: 10.1371/journal.pone.0068910
73. Moreno-López L, Sahakian BJ, Manktelow A, Menon DK, Stamatakis EA. Depression following traumatic brain injury: a functional connectivity perspective. Brain Injury (2016) 30:1319–28. doi: 10.1080/02699052.2016.1186839
74. Wang Z, Yan C, Zhao C, Qi Z, Zhou W, Lu J, et al. Spatial patterns of intrinsic brain activity in mild cognitive impairment and alzheimer's disease: a resting-state functional MRI study. Hum Brain Map. (2011) 32:1720–40. doi: 10.1002/hbm.21140
75. Greenberg G, Mikulis DJ, Ng K, Desouza D, Green RE. Use of diffusion tensor imaging to examine subacute white matter injury progression in moderate to severe traumatic brain injury. Arch Phys Med Rehabil. (2008) 89:S45–50. doi: 10.1016/j.apmr.2008.08.211
76. Venkatesan UM, Dennis NA, Hillary FG. Chronology and chronicity of altered resting-state functional connectivity after traumatic brain injury. J Neurotrauma (2015) 32:252–64. doi: 10.1089/neu.2013.3318
77. Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Gjedde A. Cognitive and emotional modulation of brain default operation. J Cogn Neurosci. (2009) 21:1065–80. doi: 10.1162/jocn.2009.21086
78. Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage (2010) 53:303–17. doi: 10.1016/j.neuroimage.2010.06.016
79. Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. NeuroImage (2011) 55:1816–24. doi: 10.1016/j.neuroimage.2011.01.030
80. van den Heuvel OA, Van Gorsel HC, Veltman DJ, Van Der Werf YD. Impairment of executive performance after transcranial magnetic modulation of the left dorsal frontal-striatal circuit. Hum Brain Map. (2013) 34:347–55. doi: 10.1002/hbm.21443
81. Clément F, Gauthier S, Belleville S. Executive functions in mild cognitive impairment: emergence and breakdown of neural plasticity. Cortex (2013) 49:1268–79. doi: 10.1016/j.cortex.2012.06.004
82. Persson J, Larsson A, Reuter-Lorenz PA. Imaging fatigue of interference control reveals the neural basis of executive resource depletion. J Cogn Neurosci. (2013) 25:338–51. doi: 10.1162/jocn_a_00321
83. Seurinck R, Vingerhoets G, Vandemaele P, Deblaere K, Achten E. Trial pacing in mental rotation tasks. NeuroImage (2005) 25:1187–96. doi: 10.1016/j.neuroimage.2005.01.010
84. Chen Q, Marshall JC, Weidner R, Fink GR. Zooming in and zooming out of the attentional focus: an fMRI study. Cerebr Cortex (2009) 19:805–19. doi: 10.1093/cercor/bhn128
85. Wylie GR, Javitt DC, Foxe JJ. Don't think of a white bear: an fMRI investigation of the effects of sequential instructional sets on cortical activity in a task-switching paradigm. Hum Brain Map. (2004) 21:279–97. doi: 10.1002/hbm.20003
86. Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. (2000) 4:131–8. doi: 10.1016/S1364-6613(00)01463-7
87. Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition (2004) 92:67–99. doi: 10.1016/j.cognition.2003.10.011
88. Price CJ. Tha anatomy of language:contributions from functional neuroimaging. J Anat. (2000) 197:335–59. doi: 10.1046/j.1469-7580.2000.19730335.x
89. Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. (2003) 18:176–85. doi: 10.1002/hbm.10091
90. Strandberg M, Elfgren C, Mannfolk P, Olsrud J, Stenberg L, van Westen D, et al. FMRI memory assessment in healthy subjects: a new approach to view lateralization data at an individual level. Brain Imagin Behav. (2011) 5:1–11. doi: 10.1007/s11682-010-9106-z
91. Sakai K, Passingham RE. Prefrontal selection and medial temporal lobe reactivation in retrieval of short-term verbal information. Cerebr Cortex (2004) 14:914–21. doi: 10.1093/cercor/bhh050
92. Feredoes E, Tononi G, Postle BR. The neural bases of the short-term storage of verbal information are anatomically variable across individuals. J Neurosci. (2007) 27:11003–8. doi: 10.1523/JNEUROSCI.1573-07.2007
93. Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nat Rev Neurosci. (2005) 6:97–107. doi: 10.1038/nrn1637
94. Postle BR, Hamidi M. Nonvisual codes and nonvisual brain areas support visual working memory. Cerebr Cortex (2007) 17:2151–62. doi: 10.1093/cercor/bhl123
95. Rahm B, Kaiser J, Unterrainer JM, Simon J, Bledowski C. NeuroImage fMRI characterization of visual working memory recognition. NeuroImage (2014) 90:413–22. doi: 10.1016/j.neuroimage.2013.12.017
96. McGugin RW, Van Gulick AE, Tamber-Rosenau BJ, Ross DA, Gauthier I. Expertise effects in face-selective areas are robust to clutter and diverted attention, but not to competition. Cerebr Cortex (2015) 25:2610–22. doi: 10.1093/cercor/bhu060
97. Alho K, Salmi J, Koistinen S, Salonen O. Top-down controlled and bottom-up triggered orienting of auditory attention to pitch activate overlapping brain networks. Brain Res. (2015) 1626:136–45. doi: 10.1016/j.brainres.2014.12.050
98. Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention. Brain (2000) 123:2273–88. doi: 10.1093/brain/123.11.2273
99. Till C, Colella B, Verwegen J, Green RE. Postrecovery cognitive decline in adults with traumatic brain injury. Arch Phys Med Rehabil. (2008) 89:S25–34. doi: 10.1016/j.apmr.2008.07.004
100. Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive de cits a er a contusion of the rat pre-frontal cortex. Neuroscience (2004) 123:349–59. doi: 10.1016/j.neuroscience.2003.09.023
101. Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma (2005) 22:106–18. doi: 10.1089/neu.2005.22.106
102. Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. (1994) 129:64–9. doi: 10.1006/exnr.1994.1147
103. Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. (2003) 6:13–22. doi: 10.1080/1363849031000095279
104. Munivenkatappa A, Agrawal A, Shukla DP, Kumaraswamy D, Devi BI. Traumatic brain injury: does gender influence outcomes? Int J Crit Illness Inj Sci. (2016) 6:70–3. doi: 10.4103/2229-5151.183024
Keywords: traumatic brain injury, resting state, functional connectivity, cognitive outcome, intrinsic connectivity contrast
Citation: Konstantinou N, Pettemeridou E, Stamatakis EA, Seimenis I and Constantinidou F (2019) Altered Resting Functional Connectivity Is Related to Cognitive Outcome in Males With Moderate-Severe Traumatic Brain Injury. Front. Neurol. 9:1163. doi: 10.3389/fneur.2018.01163
Received: 18 July 2018; Accepted: 17 December 2018;
Published: 10 January 2019.
Edited by:
Elham Rostami, Academic Hospital, SwedenReviewed by:
Vincenzo Paolo Senese, Università degli Studi della Campania Luigi Vanvitelli Caserta, ItalyCarlo Augusto Mallio, Campus Bio-Medico University, Italy
Copyright © 2019 Konstantinou, Pettemeridou, Stamatakis, Seimenis and Constantinidou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikos Konstantinou, bmlrb3Mua29uc3RhbnRpbm91QGN1dC5hYy5jeQ==
 Nikos Konstantinou
Nikos Konstantinou Eva Pettemeridou
Eva Pettemeridou Emmanuel A. Stamatakis
Emmanuel A. Stamatakis Ioannis Seimenis
Ioannis Seimenis Fofi Constantinidou
Fofi Constantinidou