- 1Department of Neurology, University Hospital Würzburg, Würzburg, Germany
- 2Department of Neurology, University Hospital Tübingen, Tubingen, Germany
- 3Department of Neurology, University Hospital Marburg, Marburg, Germany
Heterozygous mutations in the glucocerebrosidase gene (GBA1) represent the most common genetic risk factor for Parkinson's disease (PD) and are histopathologically associated with a widespread load of alpha-synuclein in the brain. Therefore, PD patients with GBA1 mutations are a cohort of high interest for clinical trials on disease-modifying therapies targeting alpha-synuclein. There is evidence that detection of phospho-alpha-synuclein (p-syn) in dermal nerve fibers might be a biomarker for the histopathological identification of PD patients even at premotor or very early stages of disease. It is so far unknown whether dermal p-syn deposition can also be found in PD patients with GBA1 mutations and may serve as a biomarker for PD in these patients. Skin biopsies of 10 PD patients with different GBA1 mutations (six N370S, three E326K, one L444P) were analyzed by double-immunofluorescence labeling with anti-p-syn and anti-protein gene product 9.5 (PGP9.5, axonal marker) to detect intraaxonal p-syn deposition. Four biopsy sites (distal, proximal leg, paravertebral Th10, and C7) per patient were studied. P-syn was found in six patients (three N370S, three E326K). P-syn deposition was mainly detected in autonomic nerve fibers, but also in somatosensory fibers and was not restricted to a certain GBA1 mutation. In summary, dermal p-syn in PD patients with GBA1 mutations seems to offer a similar distribution and frequency as observed in patients without a known mutation. Skin biopsy may be suitable to study p-syn deposition in these patients or even to identify premotor patients with GBA1 mutations.
Introduction
Pre-mortal diagnosis of Parkinson's disease (PD) is based on its clinical presentation with tremor, rigor, akinesia, and postural instability. Alpha-synuclein aggregates in neurons of the substantia nigra represent the histopathological hallmark of the disease and are not only considered as post-mortem disease marker but also offer insights into the pathogenesis of the disease.
In the last few years, focus has been set on the onset of PD-associated neurodegeneration and it is known that the disease starts many years before the onset of motor symptoms (1). Non-motor symptoms such as obstipation, hyposmia, depression, or rapid eye movement sleep behavior disorder (RBD) may occur during the prodromal phase of PD when the patients do not show any motor symptoms but alpha-synuclein deposition and neuronal loss can already be found in the brain (2). Major efforts of drug development focus on the deposition of alpha-synuclein as a probable pathogenic key event. Clinical trials of drugs targeting alpha-synuclein deposition require reliable identification of patients with primarily alpha-synuclein-driven neurodegeneration who are in the prodromal stage of the disease and in whom pre-mortem non-invasive monitoring of alpha-synuclein deposition is possible. Within a high-risk cohort for PD, skin biopsy might be a potential tool to identify individuals at the earliest stages of the disease and to monitor progression of alpha-synuclein deposition. One of such a high-risk PD cohort are patients with RBD (3) and it has already been shown that p-syn deposition can be found in skin biopsies of RBD patients, rendering skin biopsy a potential biomarker for prodromal PD (4, 5). Another risk factor for the development of PD are glucocerebrosidase gene (GBA1) mutations that are supposed to be found in 4–10% of all PD patients (6–8) and increase the risk of developing PD 20-fold (8). PD patients carrying GBA1 mutations are of special interest as a first clinical trial with a substrate reduction inhibitor, GZ/SAR402671, has already started in in this subgroup of PD. However, alpha-synuclein deposition in skin biopsy has not yet been tested in PD patients with GBA1 mutations.
In the present study, we aimed to evaluate the use of skin biopsy for the detection of p- syn in PD patients with GBA1 mutations and to evaluate potential differences of dermal p- syn deposition in patients with GBA1 mutation associated PD compared to results from former studies on patients with idiopathic PD.
Materials and Methods
Patients
Ten patients with a known GBA1 mutation were prospectively recruited at the University Hospital Tübingen (mean age 61.7 (±8.1) years. Initially, they had been recruited for the prospective observational MiGAP study (Markers in GBA1 associated Parkinson) funded by the DZNE (German Centre for Neurodegenerative Diseases, Site Tuebingen)1 Out of 100 patients of the MIGAP study, we randomly selected and asked 10 PD patients to take part in the present sub-study. Diagnosis of PD was based on the UK brain bank criteria (9). Stage of disease was assessed using the Hoehn&Yahr scale (10), motor function was evaluated by Unified Parkinson's Disease Ranking Scale part III (UPDRS-III) (11). The bradykinesia score and annual UPDRSIII progression were calculated as previously described (12). Ten age and gender matched healthy controls who were recruited for former studies (5) and whose biopsy material was stored at our department were also investigated. All patients and controls gave oral and written informed consent to participate. The study was approved by the Ethic's committee of the University of Würzburg. Demographic data of all patients and controls and the type of mutation are summarized in Table 1.
Skin Biopsy
Skin punch biopsies were taken from the distal and proximal leg, back (Th10), and neck (C7), fixed with paraformaldehyde and cryconserved until use as previously described (13). Twenty micrometer serial cryosections were cut. Double-immunofluorescence-labeling was performed using anti-PGP9.5 (axonal marker, Zytomed Systems, Berlin, Germany, 1:200) and anti-p-syn (Biolegend, San Diego, CA, United States, 1:500) and appropriate Cy3 and AlexaFluor488-conjugated secondary antibodies (Dianova, Hamburg, Germany, 1:100/1:400).
Microscopy
Double-immunofluorescence-labeling was assessed in a blinded manner using a fluorescence microscope with CARVII system (Ax10, Zeiss, Oberkochen, Germany/Visitron GmbH, Puchheim, Germany). All slides were scanned for p-syn-positive dermal nerve fibers. Nerve fibers were identified by staining with anti-PGP9.5 and only p-syn deposition within nerve fibers was considered “positive.” A biopsy was assessed “positive” if at least one dermal nerve fiber was immunoreactive for p-syn. P-syn-positive nerve fibers were categorized as sudomotor, vasomotor, pilomotor, or somatosensory (subepidermal plexus or intraepidermal) according to their location. Nerve fibers that could not be assigned to a certain skin structure were assessed as dermal nerve bundles. P-syn deposition was quantified as the number of skin structures that contained at least one p-syn-positive nerve fiber.
Statistical Evaluation
Statistical analysis was calculated using SPSS Statistics 23 software (IBM, New York, United States). Two-sided Pearson's correlation test was used for correlation analysis. A significance level of 5% was applied.
Results
P-syn deposition was found in 6/10 PD patients with GBA1 mutations, not in any healthy control (Figure 1). P-syn-positive nerve fibers were found in four biopsies of the distal leg, four of the proximal leg, two of the back, and two of the neck. Autonomic vasomotor fibers were affected in three cases, sudomotor fibers in one patient (Figure 1C), pilomotor in two (Figure 1A), and dermal nerve bundles in five cases. Somatosensory nerve fibers of the subepidermal plexus were found positive in two patients, in one of them, intraepidermal fibers were positive (Figure 1B). P-syn-positive fibers were not restricted to a certain mutation within GBA1 and were found in 3/6 patients with N370S mutation, 3/3 patients with E326K mutation, and 0/1 patient with L444P mutation. The number of p-syn positive dermal structures correlated with the duration of disease (p = 0.02, r = 0.71), but not with age at assessment. Correlation analysis between p-syn-positive structures and H&Y stage, bradykinesia score and annual UPDRSIII progression was not significant (H&Y: p = 0.06, r = 0.61, bradykinesia score: p = 0.37, r = 0.32, annual UPDRSIII progression: p = 0.08, r = −0.58).
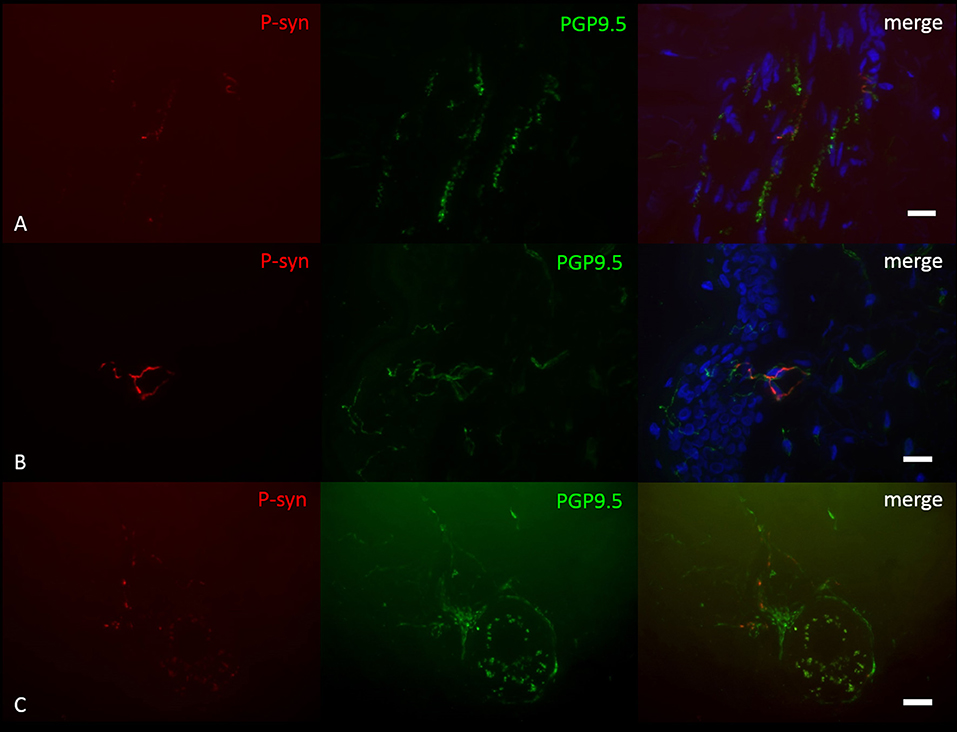
Figure 1. Photomicrographs of a double-immunofluorescence staining with anti-p-syn (red) and anti-PGP9.5 (green). Cell nuclei are stained with DAPI (blue). P-syn deposition is detectable in pilomotor fibers (A), intraepidermal fibers (B) and sudomotor fibers (C) of patients with GBA1 mutation-associated PD. Scale Bar = 10 μm.
Discussion
Here, we report p-syn deposition in dermal nerve fibers of PD patients carrying a mutation in GBA1.
The frequency of 60% in our study is comparable with former skin biopsy studies in idiopathic PD using similar protocols (5, 13, 14). Predominant autonomic involvement with vasomotor fibers as the mostly affected fibers is also in line with previous studies (13, 15). Our results indicate that dermal p-syn pathology of patients with GBA1 mutations does not differ from idiopathic PD. This corresponds to findings from brain autopsy studies that also did not show a clear difference except for some studies describing more extensive diffuse cortical Lewy bodies (6, 16) that could not be confirmed by others (17, 18). Dermal p-syn deposition was not restricted to a certain mutation and was also found in patients with the E326K mutation which is considered a rather “mild” mutation (19). Our results indicate that clinical and neuropathological similarity between patients with GBA1 mutations and without can be extended to the PNS, rendering skin biopsy a pre-mortem tool to investigate p-syn pathology in this patient group.
In recent studies no correlation between p-syn deposition and stage or duration of disease could be determined for PD (13, 15). Only in RBD, a potential prodromal stage of PD, a correlation between dermal p-syn and disease progression markers could be found, indicating a steady-state of dermal p-syn deposition during motor stages of disease. In the present study, p-syn positive structures correlated with duration of disease. This might indicate progressive p-syn deposition during the course of disease in patients with GBA1 mutations. This is of special interest as this subgroup of PD patients was shown to present a more rapid disease progression (20). The frequency of p-syn deposition in early or even prodromal stages of GBA1 associated PD needs to be investigated in future studies.
The exact underlying pathomechanism of p-syn deposition in patients with GBA1 mutations is still unclear, but there is evidence that impaired lysosomal function and endoplasmatic reticulum stress play a role (21) and that accumulation of alpha synuclein is promoted by glucocerebrosidase deficiency (22). Involvement of dermal nerve fibers in p-syn pathology in GBA1 mutation associated PD provides the opportunity for the use of skin biopsy as a pre-mortem easily accessible tissue for the investigation of p-syn pathology in this subgroup of PD.
In summary, this pilot study gives evidence that dermal nerve fibers are affected by p-syn pathology in PD with GBA1 mutations. A major limitation is the small sample size that does not allow a clear conclusion on the frequency and distribution of p-syn deposition in this subgroup compared to idiopathic PD. Detection of p-syn in skin biopsies of GBA1-associated PD is a basic prerequisite for future studies on prodromal GBA1-associated PD.
Author Contributions
KD planned and designed the study, performed, and analyzed skin biopsies and wrote the first draft of the manuscript. KB planned and designed the study, recruited and characterized patients, performed skin biopsies, and revised the manuscript. AS performed and analyzed skin biopsies. IW recruited and characterized patients. JV and WO were involved in the study design. CS was involved in study design and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Barbara Dekant and Barbara Reuter for expert technical assistance. The study was funded by Parkinson Fonds Deutschland. WO is Hertie Senior Research Professor supported by the charitable Hertie Foundation, Frankfurt/Main, Germany.
Abbreviations
GBA1, glucocerebrosidase gene; PD, Parkinson's disease; RBD, REM sleep behavior disorder; PGP9. 5, protein gene product 9.5; p-syn, phospho-alpha-synuclein.
Footnotes
References
1. Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. (2015) 30:1600–11. doi: 10.1002/mds.26431
2. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging (2003) 24:197–211. doi: 10.1016/S0197-4580(02)00065-9
3. Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. (2013) 12:443–53. doi: 10.1016/S1474-4422(13)70056-5
4. Antelmi E, Donadio V, Incensi A, Plazzi G, Liguori R. Skin nerve phosphorylated alpha-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology (2017) 88:2128–31. doi: 10.1212/WNL.0000000000003989
5. Doppler K, Jentschke HM, Schulmeyer L, Vadasz D, Janzen A, Luster M, et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol. (2017) 133:535–45. doi: 10.1007/s00401-017-1684-z
6. Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain (2009) 132:1783–94. doi: 10.1093/brain/awp044
7. Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. (2009) 361:1651–61. doi: 10.1056/NEJMoa0901281
8. Schapira AH. Glucocerebrosidase and Parkinson disease: recent advances. Mol Cell Neurosci. (2015) 66:37–42. doi: 10.1016/j.mcn.2015.03.013
9. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr. (1992) 55:181–4. doi: 10.1136/jnnp.55.3.181
10. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology (1967) 17:427–42. doi: 10.1212/WNL.17.5.427
11. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
12. Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Wade-Martins R, Talbot K, Ben-Shlomo Y, et al. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord. (2014) 20:99–105. doi: 10.1016/j.parkreldis.2013.09.025
13. Doppler K, Ebert S, Uceyler N, Trenkwalder C, Ebentheuer J, Volkmann J, et al. Cutaneous neuropathy in parkinson's disease: a window into brain pathology. Acta Neuropathol. (2014) 128:99–109. doi: 10.1007/s00401-014-1284-0
14. Doppler K, Weis J, Karl K, Ebert S, Ebentheuer J, Trenkwalder C, et al. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov Disord. (2015) 30:1688–92. doi: 10.1002/mds.26293
15. Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, et al. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology (2014) 82:1362–9. doi: 10.1212/WNL.0000000000000316
16. Clark LN, Kartsaklis LA, Wolf Gilbert R, Dorado B, Ross BM, Kisselev S, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. (2009) 66:578–83. doi: 10.1001/archneurol.2009.54
17. Parkkinen L, Neumann J, O'Sullivan SS, Holton JL, Revesz T, Hardy J, et al. Glucocerebrosidase mutations do not cause increased lewy body pathology in parkinson's disease. Mol Genet Metab. (2011) 103:410–2. doi: 10.1016/j.ymgme.2011.04.015
18. Adler CH, Beach TG, Shill HA, Caviness JN, Driver-Dunckley E, Sabbagh MN, et al. GBA mutations in Parkinson disease: earlier death but similar neuropathological features. Eur J Neurol. (2017) 24:1363–8. doi: 10.1111/ene.13395
19. Berge-Seidl V, Pihlstrom L, Maple-Grodem J, Forsgren L, Linder J, Larsen JP, et al. The GBA variant E326K is associated with Parkinson's disease and explains a genome-wide association signal. Neurosci Lett. (2017) 658:48–52. doi: 10.1016/j.neulet.2017.08.040
20. Brockmann K, Srulijes K, Pflederer S, Hauser AK, Schulte C, Maetzler W, et al. GBA-associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. (2015) 30:407–11. doi: 10.1002/mds.26071
21. Beavan MS, Schapira AH. Glucocerebrosidase mutations and the pathogenesis of Parkinson disease. Ann Med. (2013) 45:511–21. doi: 10.3109/07853890.2013.849003
Keywords: Parkinson's disease, glucocerebrosidase mutation, alpha-synuclein, skin biopsy, biomarker
Citation: Doppler K, Brockmann K, Sedghi A, Wurster I, Volkmann J, Oertel WH and Sommer C (2018) Dermal Phospho-Alpha-Synuclein Deposition in Patients With Parkinson's Disease and Mutation of the Glucocerebrosidase Gene. Front. Neurol. 9:1094. doi: 10.3389/fneur.2018.01094
Received: 06 October 2018; Accepted: 29 November 2018;
Published: 17 December 2018.
Edited by:
Tobias Warnecke, Universitätsklinikum Münster, GermanyReviewed by:
Tino Prell, Friedrich-Schiller-Universität Jena, GermanyFrancisco José Pan-Montojo, Ludwig Maximilian University of Munich, Germany
Copyright © 2018 Doppler, Brockmann, Sedghi, Wurster, Volkmann, Oertel and Sommer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathrin Doppler, RG9wcGxlcl9LQHVrdy5kZQ==
†These authors have contributed equally to this work
 Kathrin Doppler
Kathrin Doppler Kathrin Brockmann2†
Kathrin Brockmann2† Isabel Wurster
Isabel Wurster Jens Volkmann
Jens Volkmann Claudia Sommer
Claudia Sommer