- 1Neurology Unit, University Hospital of Verona, Verona, Italy
- 2Alzheimer's Disease Assessment Unit, Laboratory of Neuropsychology, IRCCS Mondino Foundation, Pavia, Italy
- 3Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy
- 4Neurorehabilitation Unit, Department of Rehabilitation, HABILITA, Bergamo, Italy
Background: Alzheimer's disease (AD) and dementia are chronic diseases with progressive deterioration of cognition, function, and behavior leading to severe disability and death. The prevalence of AD and dementia is constantly increasing because of the progressive aging of the population. These conditions represent a considerable challenge to patients, their family and caregivers, and the health system, because of the considerable need for resources allocation. There is no disease modifying intervention for AD and dementia, and the symptomatic pharmacological treatments has limited efficacy and considerable side effects. Non-pharmacological treatment (NPT), which includes a wide range of approaches and techniques, may play a role in the treatment of AD and dementia.
Aim: To review, with a narrative approach, current evidence on main NPTs for AD and dementia.
Methods: PubMed and the Cochrane database of systematic reviews were searched for studies written in English and published from 2000 to 2018. The bibliography of the main articles was checked to detect other relevant papers.
Results: The role of NPT has been largely explored in AD and dementia. The main NPT types, which were reviewed here, include exercise and motor rehabilitation, cognitive rehabilitation, NPT for behavioral and psychological symptoms of dementia, occupational therapy, psychological therapy, complementary and alternative medicine, and new technologies, including information and communication technologies, assistive technology and domotics, virtual reality, gaming, and telemedicine. We also summarized the role of NPT to address caregivers' burden.
Conclusions: Although NPT is often applied in the multidisciplinary approach to AD and dementia, supporting evidence for their use is still preliminary. Some studies showed statistically significant effect of NPT on some outcomes, but their clinical significance is uncertain. Well-designed randomized controlled trials with innovative designs are needed to explore the efficacy of NPT in AD and dementia. Further studies are required to offer robust neurobiological grounds for the effect of NPT, and to examine its cost-efficacy profile in patients with dementia.
Introduction
Dementia represents one of the major health problems in elderly individuals, with progressive deterioration of cognition, daily activity functioning and behavior that together lead to disability. Worldwide, approximately 40 million people over 65 years suffer from dementia, and 70% of them are affected by Alzheimer's disease (AD) that represents the most diffuse type of dementia. The incidence of dementia increases with age, which is the most important risk factor. Therefore, the prevalence of AD is expected to increase in the future, in parallel with the progressive aging of the population. In the European Union, the most reliable estimates forecast dementia cases to overcome 15 million in 2020 (1–3). Moreover, the improved life conditions in developing countries will lead to increased life expectancy, and therefore to higher incidence of dementia (4).
No disease-modifying treatment has been demonstrated to be effective in AD. Cholinesterase inhibitors and memantine, which are the only commercially available symptomatic drugs, may improve cognitive and behavioral outcomes, but their clinical impact remains modest and controversial (5–7). Despite research has been focused on the early [i.e., mild cognitive impairment (MCI) with biological evidence of AD] or preclinical stages of the disease, and new disease-modifying treatments have been tested to reduce the supposed pathophysiological changes of AD (i.e., amyloid deposition), no significant clinical effect has been achieved, to date (8).
Because of the limited efficacy of current pharmacological therapy and with the knowledge that caring for people with AD and dementia requires the involvement of a large number of professionals (e.g., psychologists, occupational therapists, etc.) and caregivers to offer a comprehensive and individualized management, research focused on non-pharmacological treatment (NPT). NPT may improve function, independence, and quality of life (QoL) in agreement with the International Classification of Functioning, Disability and Health (ICF) (9), is non-invasive, safe and has few side effects (10). NPT encompasses a wide range of interventions to improve patients' symptoms, reduce the stress of caregivers, and ameliorate the environment. NPT is based on different methodologies, ranging from simpler (e.g., environmental interventions) to complex approaches (e.g., virtual reality, home automation) (11). These interventions are not aimed to influence the underlying pathophysiological mechanisms but to maintain function and participation as long as possible, as the disease progresses, thus reducing disability and improving patient's and caregiver's QoL. However, a direct effect of NPT on cognition through neural plasticity/adaptation cannot be excluded in early disease stages.
The present narrative review summarized evidence on NPTs for AD and dementia. Because of the large bulk of data on this topic, we focused on the main NPT strategies. We did not report and discuss data on NPT in MCI, an intermediate state between intact cognition and dementia (12), because of the large number of studies that would have required a paper apart.
Search Strategy
PubMed and the Cochrane database of systematic reviews were searched for studies published from 2000 to 2018 with a search string including the following terms: Alzheimer's disease, dementia, behavioral and psychological symptoms, non-pharmacological, intervention, therapy, exercise, motor, rehabilitation, neurorehabilitation, cognitive, training, stimulation, occupational, psychological, psychotherapy, multicomponent, multidimensional, complementary and alternative medicine, aromatherapy, music therapy, art therapy, massage and touch, technology, information and communication, assistive device, domotics, virtual reality, gaming, serious games, telemedicine, related terms, and MeSH. Only papers written in English were considered. The bibliography of the main articles (reviews, systematic reviews, meta-analyses) was also checked to detect other relevant papers.
In consideration of the great heterogeneity of interventions that are classified as NPT and of the variety of outcome measures reported in the different studies, this work was not conceived as a systematic review of the literature, but as a narrative one to provide a useful tool for clinicians on the state of the art of NPT in dementia.
Exercise and Motor Rehabilitation
A growing amount of evidence suggests that a healthy lifestyle can reduce the risk of cardiovascular diseases (13, 14), osteoporosis, diabetes, and depression (15). A number of prospective cohort studies suggested that a regular physical activity may enhance cognitive function and reduce the risk of AD and other dementias and delay their onset or progression (16–24). A recent paper derived from the population-based Mayo Clinic study of aging on 280 MCI patients showed that moderate intensity midlife physical activity was significantly associated with a decreased risk of incident dementia (25). Regular physical exercise is recommended to all older adults, and indeed to those at increased risk of AD, as it may improve physical health, reduce frailty, lower the risk of depression, improve cognitive function in the short and long term (26–31).
Conversely, the evidence on the protecting role of short-term, single-component physical activity interventions seems to be largely insufficient (32).
Exercise has been documented to improve physical health and well-being (33), to reduce behavioral and psychological symptoms of dementia (BPSD) (34–36), and to enhance performance in the activities of daily living (ADL) (37, 38) in patients with AD or dementia.
A Cochrane review including 17 randomized controlled trials (RCTs) confirmed the positive effect of exercise programs in reducing the progression of dependence in ADL in people with dementia but showed limited evidence of benefit for the remaining outcomes (i.e., cognition, BPSD, QoL, depression, mortality, caregiver burden, and use of healthcare services) (39). This finding is of some relevance because preserving functional independence in ADL is critical for improving the QoL of people with dementia and their caregivers and delaying institutionalization.
The exercise interventions explored in dementia include a variety of training methods (e.g., aerobic exercise, resistance training or weightlifting, balance, and flexibility training), and the physical/motor outcome measures were quite different across studies (e.g., chair stand tests, timed up and go, timed walking tests). Comparative studies to explore the most effective exercise intervention, the amount of exercise, the most sensitive and significant outcome, and the role of physical activity in different patients' groups are needed.
According to animal models, a number of mechanisms may mediate the effects of exercise on brain and cognition (40). It has been suggested that exercise may improve vascular health by reducing blood pressure (41, 42), arterial stiffness (42), oxidative stress (43), systemic inflammation (44), and enhance endothelial dysfunction (45), all of which are associated with better cerebral perfusion (46, 47). Exercise may also preserve neurons and promote neurogenesis, synaptogenesis, (48–50), and neuronal plasticity (51–55).
Imaging studies in aging people confirmed the positive association between physical activity and gray matter volume. Both aerobic fitness and coordinative exercises was associated with reduced brain atrophy in the frontal and temporal regions and the hippocampus, all areas that are critical for higher cognitive function (56–58). A recent review on the association between fitness, physical activity and gray matter volume showed that both cross-sectional studies and randomized interventions consistently suggested a change in the size of prefrontal cortex and hippocampus after moderate intensity exercise in elderly people (59).
Neurotrophins, in particular the brain-derived neurotrophic factor, may be the common pathway of these mechanisms, may mediate the exercise-driven brain responses and can drive neuronal plasticity, improve cognition, and protect neurons against insult (60–63).
Future studies aimed to identify lifestyle, behavioral and/or biologic factors that may increase the likelihood of successful brain aging would help implementing preventive intervention to reduce age-related brain atrophy and consequently the risk for cognitive decline.
Conversely, the effect of physical exercise on other biological measures of AD pathology, such as cerebrospinal fluid biomarkers is still unclear (64, 65), and further studies are needed on this topic.
Cognitive Intervention
Cognitive intervention is currently the NPT that has been better explored in dementia and represents the most robust alternative and/or complement to pharmacological treatment. Cognitive intervention is typically classified as cognitive stimulation (CS), cognitive training (CT), and cognitive rehabilitation (CR) (66). CS refers to a broad range of activities (i.e., reality orientation therapy, reminiscence therapy) aimed to enhance the general cognitive and social functioning of the individual (67). While CS consists in a global approach to arouse all cognitive domains, CT focuses on a particular cognitive function (e.g., attention, memory, executive functions, language) through a set of standard tasks to improve or maintain—as is the case for neurodegenerative diseases—its normal functioning as long as possible. CR refers to a tailor-made approach which sets realistic goals to help patients and their families in everyday life. To achieve these aims, the CR therapist may choose a restorative or a compensative approach (68).
Even though the distinction between CS, CT, and CR is clear, the literature on cognitive interventions is, to some extent, confused. These terms are often used interchangeably, even if they refer to distinct approaches with a different rationale. Moreover, CR has been rarely used in dementia, because it may be difficult to apply in this condition. For these reasons, our review will focus on studies exploring CS or CT for people with dementia.
CS is currently the intervention with the most robust evidence. Several studies reported an improvement of general cognitive functioning in patients with mild-to-moderate dementia after CS sessions of variable length (69–74). A recent meta-analysis showed that CS has a moderate effect size on the Mini Mental State Examination (75) and a small effect size on the Alzheimer's Disease Assessment Scale-cognitive subscale (76), which represent two commonly-used general cognitive functioning outcomes, in patients with dementia (73). There are still few data on the efficacy of CS on other outcomes, such as mood, behavioral symptoms, QoL, and well-being. However, preliminary results indicate that CS may improve QoL and well-being of caregivers of people with AD and dementia (69, 70, 74).
The evidence on CT for people with dementia is less robust. A recent systematic review indicated that CT may improve patient's performance in trained tasks and similar exercises but has no consistent effect on everyday functioning (77). Most RCTs showed no effect of CT on other outcomes, but their low quality impede any solid conclusion (70–72, 74, 77).
Some studies documented the association of CS and CT did not result in better outcomes than single interventions (73, 74).
The Cognitive Stimulation Therapy (CST) was proposed as a structured and methodologically sound CS protocol (78, 79). CST is currently considered the evidence-based NPT of choice for the cognitive symptoms of mild-to-moderate dementia of any etiology, including vascular one (80–83). Moreover, CST is at present, the only one for which international RCTs consistently demonstrated significant improvements to cognition and QoL, using the same CST program which has been culturally adapted using standardized guidelines. CST involves 14 or more sessions of themed activities, usually related to tasks including money use, word games, word association, object categorization, current affairs and famous faces, and is typically run twice weekly (78). Each CST session is aimed to stimulate cognitive abilities and social relationships with other members and operators (78, 79). Several studies suggested the area of major CST efficacy in people with mild-to-moderate dementia is general cognitive functioning, but some cognitive areas, namely memory, orientation and language comprehension seems to be those with larger improvement following CST in an open study (84).
Several issues (e.g., health or mobility problems, services not offering this treatment) could undermine the participation of patients with dementia to CST groups (85) and the interest for individualized CST (iCST) is increasingly growing (85, 86). However, a recent RCT showed that a home-based iCST program had neither effect on cognition and QoL of patients with mild-to-moderate dementia, nor on QoL of their caregivers (87). The reasons for this negative finding might include the lack of additional stimulation from participation in a group social context and the difficulty for the caregiver to administer the iCST program in the proper way, being not professional, and because of the difficult relationship with the person with dementia.
In conclusion, while the CST program—a precise technique with a specific manual and training—has a robust evidence-based body of data, the high heterogeneity in terms of duration of treatment, length, and number of single sessions and severity of dementia impede any definite conclusion on the efficacy of other CS and CT interventions (73).
Non-pharmacological Management of Behavioral and Psychological Symptoms of Dementia
Behavioral and psychological symptoms of dementia, i.e., the so-called BPSD, are characterized by alterations of perception (hallucinations, misidentification), thinking (delusions), mood (depression, anxiety, apathy), and behavior (aggression, agitation, disinhibition, wandering, socially or sexually inappropriate behavior) (88), being highly prevalent in all types of dementia, i.e., 50–80% in AD, >80% in fronto-temporal dementia, 50–70% in Lewy body dementia and vascular dementia (89, 90). BPSD may occur in all stages of AD, and even in people with MCI, but there is no agreement on the possible relationship with disease evolution. Indeed, although some studies showed that the prevalence and severity of BPSD increase with dementia severity, other reports suggest they are more frequent in the moderate AD stages, and diminish with disease progression (89, 90). Factors that may influence BPSD occurrence include premorbid personality, younger age, poor education, depression, high burden, concurrent medical conditions, and drugs. Environmental factors such as noise, overstimulation, isolation, inadequate temperature, change of routine, as well as inappropriate relationship between patient and caregiver (e.g., communication style, body language) or the inability of the patient to express his needs, may all trigger BPSD (91).
BPSD represent the most frequent cause of institutionalization and drug prescription, increase patient's disability, worsen patient's and caregiver's QoL, contribute to increase costs, but their influence on cognition is unclear (92).
According to consensus papers based on expert opinion and systematic revision of RCTs, the general principles for BPSD management include the following steps: symptoms evaluation (i.e., type, entity, frequency), recognition and treatment of possible organic causes or triggering factors, NPT, pharmacological therapy, educational, and psychological support for the caregiver (93–96).
Considering the limited evidence and concerns on the safety of drug treatment (94–99), all guidelines agree that individualized and person-centered NPT are the first-line treatment for BPDS and the use of a pharmacological strategy should be considered only later, using the least harmful drug for the shortest time.
Some theoretical models have been proposed to explain the role of non-pharmacological approaches to BPSD (100). The progressively lowered stress threshold (PLST) model suggests that, with disease progression, people with dementia experience increasing vulnerability to stress and external stimuli because of the environmental demand. According to the PLST model, minimizing environmental demands that exceed functional capacity, and regulating activity and stimulation levels throughout the day can reduce agitation. According to the competence-environmental press (CEP) model, the highest individual functioning results from optimal combinations of environmental conditions and personal competencies. The CEP model suggests that the right balance between individual ability and external environmental demands results in adaptive behaviors, while excessive or reduced environmental activities may result in BPSD. Both models suggest that BPSD can be reduced or managed by modifying environmental factors that place too much demand or pressure on the patient.
A different approach is provided by the need-driven, dementia-compromised behavior (NDB) that conceptualized BPSD as the result of the combination between the inability of the caregiver to understand patient's needs and the inability of the patient to express them. Therefore, BPSD may represent patient's attempts to express physical or emotional distress due to unmet needs. According to this model, NDB are induced by both background (i.e., cognitive, neurological, health, and psychosocial variables) and proximal factors (i.e., environmental and situational issues such as pain, fatigue, and noise); while background factors are quite stable, proximal ones are modifiable and represent the target for the interventions (101, 102).
The number of reports and reviews on the efficacy of NPT of BPSD has increased in recent years. Most studies have been performed in nursing homes on patients with advanced dementia. The interventions range from sensorial to psychological and behavioral approaches, and include environmental redesign, validation therapy, reminiscence therapy, light therapy, aromatherapy, massage and other sensory-based strategies, acupuncture, music therapy, pleasant events and activity engagement, behavioral management techniques, caregiver training (70, 99, 103–107). Potential benefits of these interventions have been documented, but the strength of evidence is overall low and the great heterogeneity in terms of type of intervention, follow-up duration, type of outcome measures hamper comparison across studies. In particular, there are no conclusive results for validation and reminiscence therapy, the effectiveness of light therapy is not clear, aromatherapy is better than no treatment. The small body of evidence for other sensory interventions, such as massage and touch therapy, suggests they could be viable treatment options, especially given the ease of implementation and minimal training involved. No effect has been documented for acupuncture and transcutaneous electrical nerve stimulation. Music therapy and behavioral management techniques seem to cause a short-term reduction of BPSD, but long-term efficacy is unclear.
In general, no conclusions can be drawn on the safety of these NPT, because this feature is largely unexplored (103). Some reports indicate the NPT to BPSD to be potentially cost-effective (70, 99, 106), but further research is needed on this point.
Factors that may predict a better response to these interventions include a higher level of cognitive functioning, fewer difficulties in ADL and communication, and relative preservation of speech, while staff barriers and pain are associated to worse outcome (108).
Independently from the results, all studies underline the relevance of a tailored individualized intervention and a careful and close monitoring of the outcomes to ameliorate and modulate the treatment (109, 110). Since pain and discomfort may cause difficulties in communication and self-management, and contribute to BPSD, these factors should be carefully searched and treated (111, 112).
In our opinion, the type of setting of interventions is an important point, as it may influence aims of the treatment and outcomes. The majority of studies on BPSD have been performed in nursing homes, where different health professionals (i.e., physicians, psychologists, nurses) are involved, and they may interact to offer a better outcome (111). In contrast, as caregiver is often the only figure that manage patients' BPSD at home, future studies should address the role of NPT for BPSD in non-institutionalized people with dementia (106).
Occupational Therapy
Disease progression reduces the ability to participate in occupations—mainly ADL, IADL, leisure and social activities—in patients with dementia, leading to a negative impact on QoL and well-being of patients and caregivers (113–117). Therefore, improving and preserving ADLs is one of the most important patient-related outcome in dementia (118).
To increase functional abilities and enhance independence, occupational therapy (OT) uses a combined approach including activity simplification, environmental modification, adaptive aids, problem-solving strategies, skill training, and caregiver education (119, 120), according to the bio-psycho-social model of health proposed by the ICF (116). An RCT on patients with mild-to-moderate dementia living in the community showed that a multimodal OT approach, including cognitive and behavioral interventions to train patients to compensate for cognitive impairment and caregivers to cope with patients' abnormal behavior, was effective in improving ADL and in reducing caregiver's burden with effects lasting for up to 12 weeks (121). A review including seven RCTs showed that OT interventions may maintain physical function and ADL in community-dwelling persons with dementia (105, 122).
OT seems also effective in reducing BPSD, increasing patient's participation, improving physical performance, and QoL and reducing negative communication (123–129). These positive results have been confirmed in a recent review that showed OT interventions based on sensory stimulation, environmental modification and functionally oriented tasks, to be effective in improving BPSD and depression in dementia (130). Among environment-based interventions, ambient music, aromatherapy, and Snoezelen (i.e., a soothing and stimulating multisensory environment) were modestly effective in reducing agitation, but they did not have long-term effects, while the evidence to support bright light therapy for mood and sleep disorders was only preliminary (131).
An evidence-based review suggested that patient-centered leisure activities that involve social interaction may enhance caregiver satisfaction with visits for residential patients, while interventions focused on ADL and IADL may ameliorate patients' well-being and QoL. Social participation interventions, mainly involving people in the early or middle stage of the disease when verbal competence is still fairly spared, were shown to have at least short-term positive effect on patient's well-being (132).
Gitlin and collaborators proposed a 4-month structured home occupational therapy intervention, the Tailored Activity Program (TAP), that provided persons with dementia with activities tailored to their abilities, while caregivers were trained to effectively use these activities in daily life and to generalize them to different settings (133). Besides being effective in reducing caregiver burden, in that caregiver had significantly more free time, TAP was demonstrated to be highly cost-effective (123).
Some general considerations have been issued on OT: (a) programs should be tailored to elicit the patient's highest level of retained skill and interest; (b) cues for assisting people with AD to complete tasks should be short and provide clear direction; (c) compensatory strategies in the form of environmental modifications and simple adaptive equipment should be specifically tailored to the specific needs of single patients; (d) caregiver training and involvement are essential in implementing individualized programs (134).
OT is recommended by several guidelines for dementia management (135–137). The recently published clinical practice guidelines and principles of care for people with dementia in Australia reported important evidence-based recommendations for occupational therapists, based on the most updated high-quality evidence (138). However, well designed RCTs are lacking on this topic and often the studies use different approach, lacking specificity. Therefore, more research is needed to identify specific OT components and the optimal dosage of the intervention and to evaluate the long-term effects and the contraindications of OT in patients with dementia.
Subtle functional changes in highly cognitively demanding activities have been reported in individuals in the early stage of the disease, suggesting that, similarly to cognition, function declines along a continuum from healthy aging to dementia (139–141). Incorporating more challenging and high-level activities into the assessment of patients with early dementia may increase the sensitivity of functional measures, thus enabling early detection of impairment and expanding the window for timely OT interventions (142). These points may represent directions for future research on this topic.
Psychological Therapy
In recent years, the growing awareness of the deep emotional impact of the diagnosis of dementia and of the need of a holistic and individualized approach to the disease, has led to a rising interest on the psychological care for persons with dementia (143).
Actually, experiencing depression and anxiety is very common in people with dementia, even if there is a lack of consensus on their prevalence rates as diagnostic criteria may vary among studies (144–147). All studies agree that depression and anxiety may have significant functional impact. Consistent evidence suggests that, in people with dementia, the severity of depression increases the severity of neurological impairment, institutionalization (148, 149), and caregiver burden (150), while anxiety is associated with decreased independence and increased risk of nursing home placement (151).
Although different from each other, depression often co-occurs with apathy. Apathy, which is defined as a loss or diminution of goal-directed behavior, cognition or emotion (152) is one the of the most common neuropsychiatric symptom in AD, with a prevalence ranging from 19 to 88% (153). Even if symptoms like social withdrawal or reduced initiation and motivation may occur both in apathy and depression, true apathy is usually not associated with depressive symptoms, such as sadness, guilt, or hopelessness (154). The presence of apathy seems to be related to greater caregiver burden, faster functional impairment, reduced QoL and increased morbidity, being a reliable longitudinal predictor of death in people with dementia (155–157).
Many recommendations underscored that the treatment of anxiety and depressive symptoms should be an essential part of the management of AD and other dementias (93, 152, 153) and suggested that psychological interventions could be effective for these patients (158). However, empirical evidence supporting the efficacy of psychological interventions is rather sparse (159), mainly because a broad group of interventions that are termed as psychological ones have been included in reviews (160, 161), but actually are not based on well-defined theory-driven psychological interventions.
According to the World Health Organization, cognitive behavioral therapy, psychodynamic therapy, interpersonal therapy, and supportive counseling (i.e., Rogersian person-centered therapy) are the main psychotherapeutic approaches with evidence of effectiveness in treating depression and anxiety in adults (162). Studies that have adopted one of these approaches have been included in a recent systematic review on psychological interventions for anxiety and depression in dementia or MCI (six RCTs, psychological treatment: n = 216 patients, usual care or placebo intervention: n = 223) (163). The Authors concluded that there is evidence that psychological interventions added to usual care can reduce symptoms of depression and clinician-rated anxiety in dementia and that psychological interventions have the potential to improve patient's well-being, while no effect was found on ADL, self- and caregiver-rated patient's QoL, BPSD, cognition, or caregivers' self-reported depressive symptoms (163).
A recent systematic review concluded that the strongest evidence supported the use of short-term group therapy soon after dementia diagnosis to reduce levels of depression and enhance QoL, while tailored and multi-component interventions seemed to reduce inappropriate behaviors in patients living in nursing homes with mild-to-moderate levels of impairment (164).
Systematic reviews indicated a variety of NPTs (e.g., one-to-one activities, activity programs tailored on patients' preferences, individualized cognitive rehabilitation, music therapy, art therapy) as potentially effective and safe in reducing apathy, although none of them is strictly a psychological intervention (165, 166).
Overall, the proposed psychological approaches were methodologically heterogeneous, some of them lacked specificity and conceptual clarity and often the description of the interventions was quite generic. Moreover, most studies included patients in the first stage of dementia, because cognitive decline prevents the understanding and feasibility of psychological therapy in advanced stages.
In conclusion, the current evidence for psychological support and psychotherapy in AD and dementia is limited and not conclusive. Therefore, further studies are needed to demonstrate which psychological interventions may lead to better outcome and to identify common factors across different approaches that may be effective in enhancing well-being of persons with dementia.
Multicomponent and Multidimensional Strategies
The proposal of multidimensional protocols of treatment is based on the hypothesis that combining different approaches may be the most suitable intervention to improve different outcomes in AD. An Italian group developed the Multidimensional Stimulation Therapy (MST), a multidimensional approach that combined cognitive stimulation, recreational activities, OT and physical exercises, and demonstrated that MST was more effective than a cognitive-specific intervention in improving both behavioral and functional outcomes in AD patients (167, 168). A recent fMRI study suggested restoration of neural functioning as the mechanism underlying the positive effects of MST (169).
Similarly, the Meeting Centers Support Program (MCSP), a supportive person-centered approach for mild-to-moderate dementia patients living in the community and their caregivers was implemented in the Netherlands. MCSP is based on the combination of recreational activities and psychomotor therapy for patients, psico-educational and support groups for caregivers, social activities for both, and regular center meetings (170). MCSP yielded promising results in terms of user satisfaction, reduction of patients' behavioral and emotional disturbances, decrease of caregivers' burden and delayed institutionalization (170–173). A study exploring the dissemination and implementation of MCSP to different European countries is currently ongoing (174).
Actually, some systematic reviews demonstrated that multicomponent interventions were more effective than single activities in improving general well-being of both persons with dementia and their caregivers (70, 175), and in hospitalized older adults with cognitive impairment (176, 177).
As dementia represents a complex disease with cognitive, psychological, functional and behavioral symptoms, the adoption of multicomponent approaches may represent a winning strategy, in agreement with the biopsychosocial model of care, upon which any rehabilitative intervention is founded. The encouraging results obtained so far suggest further studies in this area of research.
Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) has become more commonly used over the last decade and has been applied to a wide range of health problems, including dementia. We will report evidence on the main CAM types, i.e., aromatherapy, music therapy, art therapy, massage, and touch. These NPTs are part of the main category of sensorial stimulation techniques, i.e., interventions aimed to promote the arousal of the patients' senses (178).
Aromatherapy
Among CAM, aromatherapy is probably the most familiar to consumers. Aromatherapy is part of phytotherapy and is based on the use of pure essential oils from fragrant plants to relieve health problems and improve QoL. The potential effects of essential oils are varied, including promotion of relaxation and sleep, relief of pain (179), reduction of depressive symptoms. Aromatherapy might be a useful intervention for people with cognitive disturbances, who are confused, or for whom verbal interaction is difficult and current conventional medicine provides only partial benefit (180). Indeed, aromatherapy has been used to address BPSD (181–186) and sleep disturbances (187–189) and to stimulate motivational behavior (190). A meta-analysis identified only seven RCTs and concluded that the benefits of aromatherapy for people with dementia are equivocal, mainly because of severe methodological problems (191). Therefore, the effects of aromatherapy in this condition should be better explored in high-quality RCTs.
Music Therapy
Music therapy (MT) can be defined as an NPT that uses music or sound as a non-verbal communication tool to induce educational, rehabilitative or therapeutic effects in several disease, including dementia. Music-based interventions can be conducted in single patients or in group, and they most consist of singing, listening, improvising or playing along on musical instruments (192). We can therefore classify MT in active and passive interventions. Since most studies on MT included both active and passive techniques, which type of intervention can be more effective in dementia is still unclear (193).
There is substantive literature reporting the importance and benefits of music and music therapy programs for older adults, and more specifically for those with dementia (194–199). Neurophysiologic and psychological data provide the theoretical basis for the use of music therapy. Studies showed that sound can exert a significant action on the brain (200–204) by activating a large number of cortical and subcortical areas, and, particularly, the limbic and paralimbic areas devoted to emotional processing (205). From a psychological point of view, music facilitates communicative and relational processes and emotional expression (206). Moreover, since music can stimulate both motor and cognitive functions, it can be an efficient tool for rehabilitation (207). A growing amount of evidence shows that patients with dementia can enjoy music, and their ability to respond to music is potentially preserved, even in the late to severe stages of disease when verbal communication may have ceased. Several studies reported the efficacy of music to treat BPSD (208–213), to enhance communications, relational processes and participation (199, 214–216), and to maintain or increase cognitive functions (217–222). However, reviews on this topic pointed out the methodological limitations and the scarce quality of previous studies and questioned the specificity of the effect of music that was found to be no more beneficial than other pleasant activities (223, 224).
A recent meta-analysis concluded that a music-based therapeutic intervention probably reduces depressive symptoms but has little or no effect on agitation or aggression, emotional well-being or QoL, overall behavioral problems and cognition in dementia, while effects on anxiety or social behavior, and long-term effects are unclear (192).
A recent RCT found that music therapy added to pharmacotherapy yielded no further benefits for language and verbal communication in comparison to pharmacotherapy alone, but this integrated approach could improve the psycho-behavioral profile (225).
In conclusion, the question whether music may have specific effects in patients with dementia is still open. Future studies should employ larger sample sizes, include all important outcomes, in particular positive ones, such as emotional and social well-being, and examine the duration of the effect in relation to the overall duration of the treatment and the number of sessions (192, 226).
Art Therapy
The British Association of Art Therapists defines art therapy as “a form of psychotherapy that uses art media as its primary mode of communication” and underscored that (a) clients who are referred to an art therapist need not have experience or skill in art; (b) the art therapist is not primarily concerned with making an aesthetic or diagnostic assessment of the client's image; (c) the overall aim of its practitioners is to enable a client to change and grow on a personal level through the use of art materials in a safe and facilitating environment” (227).
Whether art therapy can be useful and may represent an effective rehabilitative strategy for people suffering from dementia is a debated issue (228, 110). It has been suggested that patients with dementia can produce and appreciate visual art and that aesthetic preference can remain stable, despite cognitive decline (229).
Case reports, observational studies, small trials and anecdotal evidence suggest that art therapy can engage attention, provide pleasure, and improve BPSD, social behavior, and self-esteem, contributing to the individual sense of well-being in dementia, and that these effects may be long-lasting (230–243). However, few high-quality studies have been performed so far on this topic, and there is too little empirical evidence to prove the effectiveness of this type of CAM. Therefore, RCTs with adequate methods and appropriate outcomes are needed to support the efficacy and define optimal conditions for the use of art therapy in dementia.
Massage and Touch
Massage and touch have been suggested as a CAM or supplement to other treatments to reduce a range of conditions associated with dementia. Evidence suggests that massage and touch may relax older people with dementia, reduce some BPSD, such as pacing, wandering and resisting care, improve appetite, sleep and communication problems, and, to some extent, counteract cognitive decline (244–250).
Massage seems to produce different physiological effects such as a decrease in pulse and respiration and an increase in body temperature (251). Physiological models suggest that the effects of massage may be mediated by the production of oxytocin that can reduce discomfort, agitation, blood pressure and heart rate, and improve mood. On the other hand, psychological models suggest that massage can help people with dementia to retain a sense of meaningful and reassuring communication even when words fail and may help to activate memories (252).
A recent meta-analysis of 11 RCTs showed that massage and touch interventions may have significant effect on physical non-aggressive, verbal aggressive and non-aggressive behavior, but the relatively small sample size and the low quality of the included studies do not allow to draw a firm conclusion on the effect on BPSD or any useful suggestion for clinical practice (253).
As for other CAM types, RCTs of better methodological quality are needed to provide evidence supporting the benefit of massage and touch in dementia.
New Technologies
The availability of new technologies, their diffusion and, for some of them, the relatively cheap price, resulted in a progressively larger number of studies exploring their role in dementia. We will briefly summarize evidence on the application of information and communication technologies (ICT), assistive devices, domotics, virtual reality, gaming and telemedicine to monitor, and treat people with dementia.
ICT, Assistive Devices and Domotics
Recent technological advances in ICT, including computational devices, robotics, machine learning algorithms, miniaturization of sensors and technologies, as well as the reduced costs of these devices increased the availability of intelligent devices and technology-embedded environments to support the daily life of persons with dementia and their caregivers, and to assess disease severity and progression (254–256). Nowadays, devices and sensors can be integrated into the patient's environment as part of a “smart” or automated home, or in the patient's clothes to offer healthcare professionals a much clearer view of the patient's condition and activity than that available from short periods of monitoring in a clinical setting (257). In particular, ICT allows continuous monitoring of patient's performance and actions in real time and real-life situations (258–260).
Moving freely outdoors is considered an important ADL outcome associated with the ability to maintain independence and participation. The development of technologies, which allow people with dementia to stay outdoors in a safe and secure way, is considered a priority research area. Therefore, tracking technologies that record the position of the patient based on global positioning system (GPS), and can localize him/her if lost is the most developed area of ICT research in dementia (261–265). Tracking technology devices can promote perceived safety and security among caregivers and relatives of people with dementia (266), in that they can locate the patient at any moment and represent a precious support in everyday life (263, 267). Many studies in this area focused on the perspective of relatives and/or healthcare staff, but only a limited number of them explored the experience of people when wearing a GPS tracking technology (259, 262, 268, 269).
Another application of ICT is represented by devices that can be used for surveillance and management of high-risk behaviors and may replace more severe forms of physical restraints (270–272). Acoustic sensors placed in the living room or bedroom can record noise, or patient's movement. Cameras or GPS devices can also be used to allow caregivers or staff to take actions when the patient is in a potentially dangerous condition, without restricting freedom of movement. Furthermore, patients can wear a chip in their clothes to give them access to certain parts of the building, while preventing them to move to other forbidden or dangerous areas (266).
However, the application of surveillance technologies to the care of people with dementia led to considerable ethical debate. Indeed, opponents of this approach claim that the continuous monitoring may represent a breach of patient's privacy and freedom and argue that tracking devices may diminish human contact between patients and the environment (273). A systematic review on the ethical and practical concerns of surveillance technologies in residential care for people with dementia concluded that there is no consensus and underlined the need for clearer policies on this topic (274). A recent descriptive review showed that a significant portion of current intelligent assistive technology is designed in the absence of explicit ethical considerations, and this may represent a structural limitation in the translation from designing labs to bedside (275). The Authors called for a coordinated effort to proactively incorporate ethical considerations early in the design and development of new ICT products (275).
ICT has also been applied to monitoring and prevention of falls in dementia. Body-worn gyroscopes measuring angular velocity, sensors to measure the acceleration of the trunk, and inertial sensors may detect when a fall occurs, along with the causes of the fall (276–279). Among non-wearable systems cameras, motion sensors, microphones and floor sensors have been applied to this aim (280, 281). Information obtained by accelerometers contained in a wristband may result in vibratory and visual feedback to increase the patient's awareness of their risk of falling (282). Similarly, a shoe insole that delivers vibration may prevent falls and improve gait and balance. Research projects are currently exploring how exoskeletons can correct and assist postural balance and improve overall function. Technology will hopefully provide many more options for assessment, prevention, and intervention in this area of health care (283).
Video and audio analysis techniques, computerized testing and actigraphy, besides being useful to promote patients' safety, may represent promising tools to improve functional and cognitive assessment of patients with AD (256). For instance, automatic speech analysis techniques through computerized speech recognition interfaces could represent a non-invasive and cheap method to collect information on verbal communication impairment in patients with cognitive disorders (284). Video processing technologies can be adopted to obtain pragmatic, ecological, and objective measures to improve assessment of ADL, and to provide additional information that cannot be gathered in a clinical setting (285).
ICT can be used for the assessment of agitation, one of the most challenging symptoms to manage for caregivers (286, 287). A core feature of agitation is excessive motor activity, and accelerometers can objectively measure abnormal motor activity, speech analyses may assess verbal aggression, and automated video analysis and activity recognition techniques can detect activities and movement sequences that underline physical aggression (288). Sensors measuring electrodermal activity could early detect agitation in persons with dementia. These devices could theoretically support nursing staff in recognizing early warning signs of BPSD, but further studies in a clinical setting are needed to confirm this hypothesis (289).
Overall, this is a challenging domain for clinicians, who should define the clinically relevant markers, and for ICT engineers, who should adapt the technical constraints to the clinical requirements.
Assistive technologies can support and facilitate disabled people in ADL (290). For instance, external cueing systems can assist people with cognitive disabilities by reminding them to perform a task at the appropriate time (e.g., take their pills), or by providing guidance through a task (291–293), and sensors recording a patient's position in space may help in navigation through environment (294). Moreover, assistive technologies may help to support decision-making in vocational and personal health domains (295), to read (296), or use the telephone (297).
Domotics is another important field of research, because nearly three quarters of older adults with dementia are cared for in their own homes (298), and there is a strong correlation between home environment and disability-related outcomes (299). Dementia-specific adapted home environment and related assistive devices may be helpful for both the patient and the caregiver (300–302). Changes to home environment may result in lack of familiarity for the patient, and earlier home modifications might enable individuals with dementia to adjust to their adapted environment (303).
Future smart homes will incorporate intelligent interfaces embedded in everyday objects, such as furniture, clothes, vehicles, and smart materials to observe the residents and provide proactive services (304). One of the key-supporting features of a smart home is the ability to monitor the ADL and safety of people, and to detect changes in their daily routines. With the availability of inexpensive low-power sensors, radios, and embedded processors, smart homes may be equipped with large amounts of networked sensors, which collaboratively process and make deductions from the acquired data on the state of the home, and the activity and behavior of its residents (305–310). Therefore, the smart home technology seems to be a promising and cost-effective way of improving home care for people with dementia in a non-obtrusive way, allowing greater independence, maintaining good health, preventing social isolation, and relieving the workload from family caregivers and health providers (311).
The data on domotics are encouraging but some factors, e.g., the accessibility and affordability of these devices and the limited caregiver's awareness of their availability and utility, may prevent a more widespread use of these technologies (312).
In conclusion, ICT applications are rapidly expanding to support assistive and clinical tasks in people with dementia, but structural limitations to successful adoption include partial lack of clinical validation and insufficient focus on patient's needs. Clinicians and stakeholders involved in the implementation and management of dementia care across ICT applications should collaborate to facilitate the translation of medical engineering research into clinical practice (313).
Virtual Reality and Gaming
Virtual reality (VR) allows a user to navigate through, and interact with, a virtual environment that is a 3D digital space generated by computing technology and consisting of objects or entities seemingly “real” because they share at least one attribute of the real thing, without sharing all of its physical characteristics (e.g., volume, weight, surface friction) (314). The potential applications of VR systems to assess and train AD patients has been reported by several studies (315–320). Cognitively impaired patients can be exposed to computer-generated virtual environments, where they can safely perform quasi-naturalistic real-life activities and tasks to assess and/or treat cognitive and motor deficits under a range of conditions that are not easily controllable and quantifiable in the real world (321). The most specific domains for VR diagnostic and training purposes in AD patients include attention (322), executive functions (323–326), memory (327–333), orientation (322, 327, 334–336), and ADL (337, 338). Research is currently focusing on the design of VR based tests aimed to be more ecological and sensitive compared to common pen-and-paper tests. A recent study proposed a novel AD screening tests based on virtual environment and game principles to evaluate memory loss for common objects and recent events, and language changes (339).
The advantages of VR in comparison to traditional assessment and training tools are represented by the naturalistic situations, the safety from potential dangers that could occur in real-life situations and the enhancement of motivation because of its gaming, interactive, and immersive quality. For these reasons, computer-driven simulations of real-life environments can bridge the gap between traditional test outcomes and compensatory strategies that are transferable to daily life.
Since most of the evidence on the role of VR come from single case reports or to restricted samples, the debate on its role in patients with dementia is still open.
In this scenario, serious games (SG), i.e., interactive virtual simulations to represent real situations, otherwise hardly reproducible, allow the player to act in a real-life-like environment, or a fictitious scenario that serves as a gym for deliberately decontextualized learning. SG are widely recognized as promising non-pharmacological tools to assess patient's cognition and function (340, 341), and to treat, stimulate, and improve patient's well-being (342). Boosted by the publication of a Nature letter showing that video game training can enhance cognitive control in older adults (343), SG specifically adapted to people with AD have been developed and employed to train physical and cognitive abilities, showing preliminary but encouraging results (341, 344–348).
A recent literature review concluded that game-based interventions can positively affect both physical health by improving balance, gait and voluntary motor control and cognitive function by enhancing attention, memory, and visuo-spatial abilities in older adults and in patients with dementia (349). Both physical and cognitive games seem to have a positive impact on social and emotional function, improving mood and increasing positive affect and sociability (350).
Since the use of SG in neurodegenerative disorders is expanding, recent studies have provided recommendations in terms of ergonomic choices for their design, and to create customize SG specific to the target users (351). Considering that the actual recommendations are gathered from a relatively small group of experts working in the domain of SG for health, further work is needed to verify if they hold true for a wider expert population, and their applicability to patients.
On the basis of these preliminary data, clear evidence on the effectiveness of VR and SG for the assessment and training of people with AD requires further research that should adopt methodologies based upon best practices from clinical trials and incorporate behavioral and neurophysiological data.
Telemedicine
Technology provides new opportunities for interventions to improve quality and access to health care. Since dementia is a chronic disease, with issues related to continuous long-term care and limited accessibility to medical service, telemedicine could be a valuable mean to provide assessment and care to dementia patients at home (352). Indeed, telemedicine can provide follow-up care to help people with dementia to maintain their independence and continue living in their own homes, improve their safety, (353, 354), ameliorate clinical outcomes, and reduce access to health services (355, 356). Moreover, likely because of the reduction of the discomfort due to travel, telemedicine was found to be an independent predictor for increased treatment duration (357). Telemedicine appears to be very promising and effective in the context of dementia assessment, especially in countries, such as the USA, where there are several rural retirement communities that are still medically underserved. A recent study conducted in a Southern California community showed not only that telemedicine was able to improve access and quality of dementia care, but also that most of the patients preferred the telemedicine consult compared to the traditional one (358).
Several studies confirmed the feasibility to perform cognitive assessment via telemedicine in elderly subjects with dementia and showed a good agreement with conventional face-to-face testing, and a high level of user satisfaction (359–362). However, since data are still preliminary, there is no consensus on the best practices for conducting neuropsychological assessment by telemedicine (362).
Besides the diagnostic evaluation and follow-up monitoring, telemedicine interventions for dementia include internet-based information and support groups, robotic companions, the use of smartphones to report symptoms (258, 363, 364), and cognitive rehabilitation training (365).
Telemedicine has been applied to support caregivers of people with dementia through in-home video monitoring and recording and feedback to help managing difficult situations (366). Telephone-based support groups is a promising way to provide support for caregivers, but research is only at the beginning (367). A systematic review on technology-driven telemedicine interventions, such as online training and support programs, for caregivers of persons with dementia concluded that included studies yielded positive findings in terms of mental health outcomes, but their marked heterogeneity impeded robust conclusions (368).
Overall, establishing systems for the care of dementia patients and their caregivers using telemedicine technologies seems to be feasible, but the evidence on clinical benefits is still scarce, cost-effectiveness data are lacking, and there is very little evidence of widespread practical applications (369).
Limitations of the Study
The main limitation of the present review is its narrative design, but a systematic one would have not been feasible because of the larger number of studies on NPT in AD and dementia. Another limitation is the use of two search engines, only (i.e., PubMed and the Cochrane database of systematic reviews). Searching in other databases (e.g., Embase, PsycINFO, Web of Science) would have yielded more pieces of literature, but it has been suggested that MEDLINE, which is completely covered by PubMed, may produce a sufficiently extensive coverage (370). A study comparing 15 databases for RCTs on CAM showed low overlap between databases and indicated that comprehensive CAM literature searches will require multiple databases (371) but this appear a minor problem in the present review because of the overall low quality of studies on CAM interventions.
Conclusions
Appropriate management of patients with AD and dementia is a significant public health concern, given the limited effectiveness of pharmacological therapies combined with their potentially life-threatening side effects. The development of effective NPT for these conditions is of paramount importance, and a large number of interventions has been proposed.
The interventions reviewed in this paper show different level of evidence of efficacy on different outcomes that are summarized in Table 1. Some of them share methodological problems that are common to all non-pharmacological studies, which are typically practice-oriented. They include small number of high-quality studies or double-blind RCTs, small sample sizes, heterogeneity in terms of study design, type of intervention and outcomes, uncertainty about the clinical significance of outcomes. The landscape of clinical trials has changed since the introduction of randomization 80 years ago, and new challenges in their design, conduction, and interpretation have emerged. Evidence-grading systems are biased toward RCTs, and they may lead to understatement of findings from non-RCT studies (372). The direct transposition of the traditional methods of drug RCTs to the rehabilitation and NPT scenarios raises some issues, and innovative trial designs would be helpful in these settings. Moreover, cost-effectiveness studies are needed to better address the role of NPT for dementia.
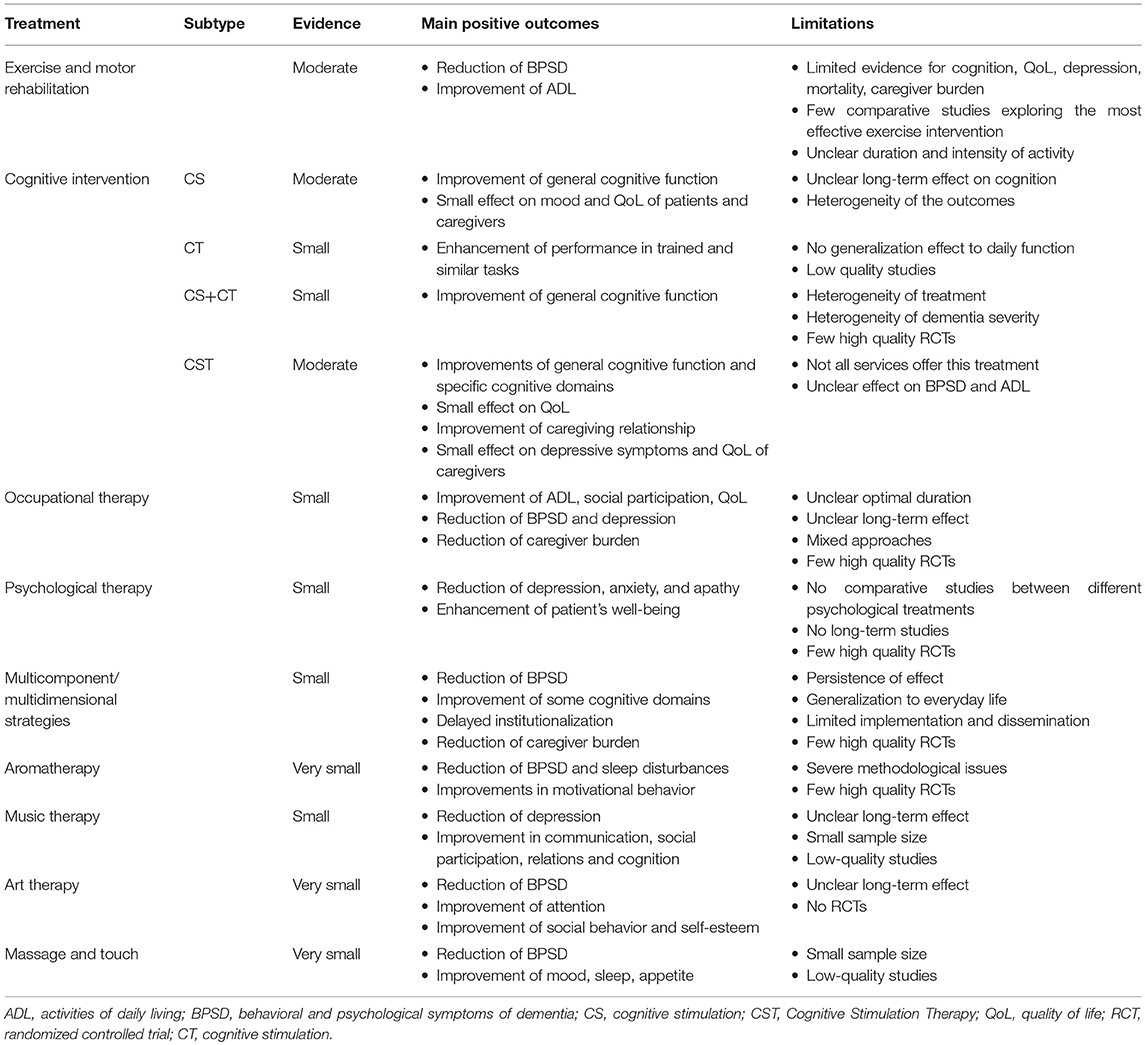
Table 1. Summary of the level of evidence, main positive outcomes, and limitations of the non-pharmacological treatment for Alzheimer's disease and dementia.
Author Contributions
CZ, ES, and MB conceived and designed the study. All authors collected, analyzed, and interpreted the data. CZ, ES, AF, EM, SB, and MB drafted the manuscript. ST and RC revised critically the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Alzheimer Report 2015. The Global Impact of Dementia. An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer Disease International, 2015.
2. Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer's disease. Arch Med Res. (2012) 43:600–8. doi: 10.1016/j.arcmed.2012.11.003
3. Brodaty H, Breteler MM, Dekosky ST, Dorenlot P, Fratiglioni L, Hock C, et al. The world of dementia beyond 2020. J Am Geriatr Soc. (2011) 59:923–927. doi: 10.1111/j.1532-5415.2011.03365.x
4. Catindig JA, Venketasubramanian N, Ikram MK, Chen C. Epidemiology of dementia in Asia: insights on prevalence, trends and novel risk factors. J Neurol Sci. (2012) 321:11–6. doi: 10.1016/j.jns.2012.07.023
5. Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. (2006) 1:CD005593. doi: 10.1002/14651858.CD005593
6. Birks JS, Grimley Evans J. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev. (2015) 4:CD001191. doi: 10.1002/14651858.CD001191.pub3
7. Wong CW. Pharmacotherapy for dementia: a practical approach to the use of cholinesterase inhibitors and memantine. Drugs Aging (2016) 33:451–60. doi: 10.1007/s40266-016-0372-3
8. Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet (2016) 388:505–17. doi: 10.1016/S0140-6736(15)01124-1
9. World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva: WHO (2001).
10. Poulos CJ, Bayer A, Beaupre L, Clare L, Poulos RG, Wang RH, et al. A comprehensive approach to reablement in dementia. Alzheimers Dement. (2017) 3:450–8. doi: 10.1016/j.trci.2017.06.005
11. McDermott O, Charlesworth G, Hogervorst E, Stoner C, Moniz-Cook E, Spector A, et al. Psychosocial interventions for people with dementia: a synthesis of systematic reviews. Aging Ment Health (2018) 17:1–11. doi: 10.1080/13607863.2017.1423031
12. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer Dement. (2011) 7:270–9. doi: 10.1016/j.jalz.2011.03.008
13. NIH. Physical activity and cardiovascular health. NIH consensus development panel on physical activity and cardiovascular health. JAMA (1996) 276:241–6. doi: 10.1001/jama.1996.03540030075036
14. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (Subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity). Circulation (2003) 107:3109–16. doi: 10.1161/01.CIR.0000075572.40158.77
15. World Health Organization. Chronic Disease Information Sheets: Physical Activity. Geneva: WHO (2006).
16. Lautenschlager NT, Cox K, Kurz AF. Physical activity and mild cognitive impairment and Alzheimer's disease. Curr Neurol Neurosci Rep. (2010) 10:352–8. doi: 10.1007/s11910-010-0121-7
17. Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. (2001) 161:1703–8. doi: 10.1001/archinte.161.14.1703
18. Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the cardiovascular health cognition study. Am J Epidemiol. (2005) 161:639–51. doi: 10.1093/aje/kwi092
19. Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K, et al. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. (2008) 56:1658–64. doi: 10.1111/j.1532-5415.2008.01841.x
20. Middleton LE, Mitnitski A, Fallah N, Kirkland SA, Rockwood K. Changes in cognition and mortality in relation to exercise in late life: a population based study. PLoS ONE (2008) 3:e3124. doi: 10.1371/journal.pone.0003124
21. Brown BM, Pfeiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer's disease? Mol Psychiatry (2013) 18:864–74. doi: 10.1038/mp.2012.162
22. Hamer M, Muniz Terrera G, Demakakos P. Physical activity and trajectories in cognitive function: English longitudinal study of ageing. J Epidemiol Community Health (2018) 72:477–83. doi: 10.1136/jech-2017-210228
23. Loprinzi PD, Frith E, Edwards MK, Sng E, Ashpole N. The effects of exercise on memory function among young to middle-aged adults: systematic review and recommendations for future research. Am J Health Promot. (2018) 32:691–704. doi: 10.1177/0890117117737409
24. Song D, Yu DSF, Li PWC, Lei Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int J Nurs Stud. (2018) 79:155–64. doi: 10.1016/j.ijnurstu.2018.01.002
25. Krell-Roesch J, Feder NT, Roberts RO, Mielke MM, Christianson TJ, Knopman DS, et al. Leisure-time physical activity and the risk of incident dementia: the mayo clinic study of aging. J Alzheimers Dis. (2018) 63:149–55. doi: 10.3233/JAD-171141
26. Bray NW, Smart RR, Jakobi JM, Jones GR. Exercise prescription to reverse frailty. Appl Physiol Nutr Metab. (2016) 41:1112–6. doi: 10.1139/apnm-2016-0226
27. Santos-Lozano A, Pareja-Galeano H, Sanchis-Gomar F, Quindós-Rubial M, Fiuza-Luces C, Cristi-Montero C, et al. Physical activity and Alzheimer disease: a protective association. Mayo Clin Proc. (2016) 91:999–1020. doi: 10.1016/j.mayocp.2016.04.024
28. Schuch FB, Vancampfort D, Rosenbaum S, Richards J, Ward PB, Veronese N, et al. Exercise for depression in older adults: a metaanalysis of randomized controlled trials adjusting for publication bias. Rev Bras Psiquiatr. (2016) 38:247–54. doi: 10.1590/1516-4446-2016-1915
29. Law LL, Rol RN, Schultz SA, Dougherty RJ, Edwards DF, Koscik RL, et al. Moderate intensity physical activity associates with CSF biomarkers in a cohort at risk for Alzheimer's disease. Alzheimers Dement. (2018) 10:188–95. doi: 10.1016/j.dadm.2018.01.001
30. Martins RN, Villemagne V, Sohrabi HR, Chatterjee P, Shah TM, Verdile G, et al. Alzheimer's disease: a journey from amyloid peptides and oxidative stress, to biomarker technologies and disease prevention strategies-gains from AIBL and DIAN cohort studies. J Alzheimers Dis. (2018) 62:965–92. doi: 10.3233/JAD-171145
31. Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with metaanalysis. Br J Sports Med. (2018) 52:154–60. doi: 10.1136/bjsports-2016-096587
32. Brasure M, Desai P, Davila H, Nelson VA, Calvert C, Jutkowitz E, et al. Physical activity interventions in preventing cognitive decline and alzheimer-type dementia: a systematic review. Ann Intern Med. (2018) 168:30–8. doi: 10.7326/M17-1528
33. Teri L, Logsdon RG, McCurry SM. Exercise interventions for dementia and cognitive impairment: the Seattle protocols. J Nutr Health Aging (2008) 12:391–4. doi: 10.1007/BF02982672
34. Scherder EJ, Bogen T, Eggermont LH, Hamers JP, Swaab DF. The more physical inactivity, the more agitation in dementia. Int Psychogeriatr. (2010) 22:1203–8. doi: 10.1017/S1041610210001493
35. Thune-Boyle IC, Iliffe S, Cerga-Pashoja A, Lowery D, Warner J. The effect of exercise on behavioural and psychological symptoms of dementia: towards a research agenda. Int Psychogeriatr. (2012) 24:1046–57. doi: 10.1017/S1041610211002365
36. Fleiner T, Dauth H, Gersie M, Zijlstra W, Haussermann P. Structured physical exercise improves neuropsychiatric symptoms in acute dementia care: a hospital-based RCT. Alzheimers Res Ther. (2017) 9:68. doi: 10.1186/s13195-017-0289-z
37. Kwak YS, Um SY, Son TG, Kim DJ. Effect of regular exercise on senile dementia patients. Int J Sports Med. (2008) 29:471–4. doi: 10.1055/s-2007-964853
38. Vidoni ED, Perales J, Alshehri M, Giles AM, Siengsukon CF, Burns JM. Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer disease. J Geriatr Phys Ther. 1. doi: 10.1519/JPT.0000000000000172
39. Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. (2015) 15:CD006489. doi: 10.1002/14651858.CD006489.pub3
40. Norman JE, Rutkowsky J, Bodine S, Rutledge JC. The potential mechanisms of exercise-induced cognitive protection: a literature review. Curr Pharm Des. (2018) 24:1827–31. doi: 10.2174/1381612824666180406105149
41. Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. (2004) 101:3316–21. doi: 10.1073/pnas.0400266101
42. Fleg JL. Aerobic exercise in the elderly: a key to successful aging. Discov Med. (2012) 13: 223–8.
43. Covas MI, Elosua R, Fitó M, Alcántara M, Coca L, Marrugat J. Relationship between physical activity and oxidative stress biomarkers in women. Med Sci Sports Exerc. (2002) 34:814–9. doi: 10.1097/00005768-200205000-00014
44. Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. (2011) 31:137–45. doi: 10.1097/HCR.0b013e3182122827
45. Ghisi GL, Durieux A, Pinho R, Benetti M. Physical exercise and endothelial dysfunction. Arq Bras Cardiol. (2010) 95:30–7. doi: 10.1016/B978-0-12-812348-5.00049-0
46. Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging (2002) 23:941–55. doi: 10.1016/S0197-4580(02)00028-3
47. Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev. (2012) 40:153–8. doi: 10.1097/JES.0b013e3182553430
48. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. (2003) 14:125–30. doi: 10.1111/1467-9280.t01-1-01430
49. Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging (2006) 27:1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016
50. Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. (2006) 1104:64–72. doi: 10.1016/j.brainres.2006.05.066
51. Cotman CW, Berchtold NC. Exercise: a behavioural intervention toenhance brain health and plasticity. Trends Neurosci. (2002) 25:292–8. doi: 10.1016/S0166-2236(02)02143-4
52. Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair (2005) 19:283–95 doi: 10.1177/1545968305280753
53. Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement (2007) 3:S30–7. doi: 10.1016/j.jalz.2007.01.013
54. Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci Biobehav Rev. (2013) 37(9 Pt B):2268–95. doi: 10.1016/j.neubiorev.2013.01.028
55. Hamaide J, De Groof G, Van der Linden A. Neuroplasticity and MRI: a perfect match. Neuroimage (2016) 131:13–28. doi: 10.1016/j.neuroimage.2015.08.005
56. Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, et al. The association between aerobic fitness and executive functions is mediated by prefrontal cortex volume. Brain Behav Immun. (2012) 26:811–9. doi: 10.1016/j.bbi.2011.11.008
57. Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci. (2014) 6:170. doi: 10.3389/fnagi.2014.00170
58. Papenberg G, Ferencz B, Mangialasche F, Mecocci P, Cecchetti R, Kalpouzos G, et al. Physical activity and inflammation: effects on gray-matter volume and cognitive decline in aging. Hum Brain Mapp. (2016) 37:3462–73. doi: 10.1002/hbm.23252
59. Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness and gray matter volume. Neurobiol Aging (2014) 35(Suppl 2): S20–8. doi: 10.1016/j.neurobiolaging.2014.03.034
60. Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. (2003) 258:319–33. doi: 10.1016/S0012-1606(03)00120-9
61. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. (2004) 20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x
62. Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer's disease. Arch Med Res. (2012) 43:615–21. doi: 10.1016/j.arcmed.2012.09.008
63. Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. (2013) 57:47–55. doi: 10.1016/j.nbd.2012.06.011
64. Jensen CS, Portelius E, Høgh P, Wermuth L, Blennow K, Zetterberg H, et al. Effect of physical exercise on markers of neuronal dysfunction in cerebrospinal fluid in patients with Alzheimer's disease. Alzheimers Dement. (2017) 3:284–90. doi: 10.1016/j.trci.2017.03.007
65. Frederiksen KS, Gjerum L, Waldemar G, Hasselbalch SG. Effects of physical exercise on Alzheimer's disease biomarkers: a systematic review of intervention studies. J Alzheimers Dis. (2018) 61:359–72. doi: 10.3233/JAD-170567
66. Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer's disease: a review. Neuropsychol Rehabil. (2004) 14:385–401. doi: 10.1080/09602010443000074
67. D'Onofrio G, Sancarlo D, Seripa D, Ricciardi F, Giuliani F, Panza F, et al. Chapter 18: non-pharmacological approaches in the treatment of dementia. Update on dementia. In: D. Moretti, editor. Update on Dementia. Intech (2016). p. 477–491. Available online at: https://www.intechopen.com/journals/update-on-dementia
68. Wilson BA. Towards a comprehensive model of cognitive rehabilitation. Neuropsychol Rehabil. (2002) 12:97–110. doi: 10.1080/09602010244000020
69. Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. (2012) 15:CD005562. doi: 10.1002/14651858.CD005562.pub2
70. Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. (2010) 30:161–78. doi: 10.1159/000316119
71. Kurz AF, Leucht S, Lautenschlager NT. The clinical significance of cognition-focused interventions for cognitively impaired older adults: a systematic review of randomized controlled trials. Int Psychogeriatr. (2011) 23:1364–75. doi: 10.1017/S1041610211001001
72. Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. (2013) 6:CD003260. doi: 10.1002/14651858.CD003260.pub2
73. Huntley JD, Gould RL, Liu K, Smith M, Howard RJ. Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open (2015) 5:e005247. doi: 10.1136/bmjopen-2014-005247
74. Carrion C, Folkvord F, Anastasiadou D, Aymerich M. Cognitive Therapy for dementia patients: a systematic review. Dement Geriatr Cogn Disord. (2018) 46:1–26. doi: 10.1159/000490851
75. Folstein M, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98.
76. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry (1984) 141:1356–64. doi: 10.1176/ajp.141.11.1356
77. Kallio EL, Öhman H, Kautiainen H, Hietanen M, Pitkälä K. Cognitive training interventions for patients with Alzheimer's disease: a systematic review. J Alzheimers Dis. (2017) 56:1349–72. doi: 10.3233/JAD-160810
78. Spector A, Orrell M, Davies S and Woods B. Can reality orientation be rehabilitated? Development and piloting of an evidence-based programme of cognition-based therapies for people with dementia. Neuropsychol Rehabilitat. (2001) 11:193–6. doi: 10.1080/09602010143000068
79. Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry (2003) 183:248–54. doi: 10.1192/bjp.183.3.248
80. NICE-SCIE(2007). Dementia: Supporting People With Dementia and Their Carers in Health and Social Care. NICE Clinical Guideline 42. London: National Institute for Health and Clinical Excellence (Available online at: www.nice.org.uk)
81. Bacigalupo I, Mayer F, Lacorte E, Di Pucchio A, Marzolini F, Canevelli M, et al. World Alzheimer Report: The Benefits of Early Diagnosis Intervention. Alzheimer's Disease International (ADI) (2011). Available online at: http://www.alz.co.uk/research/WorldAlzheimerReport2011.pdf
82. NHS Institute for Innovation and Improvement. An Economic Evaluation of Alternatives to Antipsychotic Drugs for Individuals Living With Dementia. Coventry: The NHS Institute for Innovation and Improvement (2011).
83. Piras F, Carbone E, Faggian S, Salvalaio E, Gardini S, Borella E. Efficacy of cognitive stimulation therapy for older adults with vascular dementia. Dement Neuropsychol. (2017) 11:434–41. doi: 10.1590/1980-57642016dn11-040014
84. Hall L, Orrell M, Stott J, Spector A. Cognitive Stimulation Therapy (CST): neurpsychological mechanisms of change. Int Psychogeriatr. (2013) 25:479–89. doi: 10.1017/S1041610212001822
85. Orrell M, Yates LA, Burns A, Russell I, Woods RT, Hoare Z, et al. Individual Cognitive Stimulation Therapy for dementia (iCST): study protocol for a randomized controlled trial. Trials (2012) 13:172. doi: 10.1186/1745-6215-13-172
86. Yates LA, Orgeta V, Leung P, Spector A, Orrell M. Field-testing phase of the development of individual cognitive stimulation therapy (iCST) for dementia. BMC Health Services Res. (2016) 16:233. doi: 10.1186/s12913-016-1499-y
87. Orrell M, Yates L, Leung P, Kang S, Hoare Z, Whitaker C, et al. The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: a randomised controlled trial. PLoS Med. (2017) 14:e1002269. doi: 10.1371/journal.pmed.1002269
88. Finkel SI, Costa e Silva J, Cohen G, Miller S, Sartorius N. Behavioural and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr. (1996) 8(Suppl. 3):497–500. doi: 10.1017/S1041610297003943
89. Aalten P, Verhey FR, Boziki M, Brugnolo A, Bullock R, Byrne EJ, et al. Consistency of neuropsychiatric syndromes across dementias: results from the European Alzheimer disease consortium. Part II. Dement Geriatr Cogn Disord. (2008) 28:1–8. doi: 10.1159/000111082
90. Kazui H, Yoshiyama K, Kanemoto H, Suzuki Y, Sato S, Hashimoto M, et al. Differences of behavioural and psychological symptoms of dementia in disease severity in four major dementias. PLoS ONE (2016) 11:e0161092. doi: 10.1371/journal.pone.0161092
91. Gilhooly KJ, Gilhooly ML, Sullivan MP, McIntyre A, Wilson L, Harding E, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. (2016) 16:106–12. doi: 10.1186/s12877-016-0280-8
92. Herrmann N, Lanctôt KL, Sambrook R, Lesnikova N, Hébert R, McCracken P, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry (2006) 21:972–6. doi: 10.1002/gps.1594
93. Azermai M, Petrovic M, Elseviers MM, Bourgeois J, Van Bortel LM, Vander Stichele RH. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Res Rev. (2012) 11:78–86. doi: 10.1016/j.arr.2011.07.002
94. Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, Gurvit H, et al. EFNS-ENS guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. (2012) 19:1159–79. doi: 10.1111/j.1468-1331.2012.03784.x
95. Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioural and psychological symptoms of dementia. Int Psychogeriatr. (2018) 30:295–309. doi: 10.1017/S1041610217002344
96. Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, Robert P, et al. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatr. (2010) 22:346–72. doi: 10.1017/S1041610209991505
97. Seitz DP, Gill SS, Herrmann N, Brisbin S, Rapoport MJ, Rines J, et al. Pharmacological treatments for neuropsychiatric symptoms of dementia in long-term care: a systematic review. Int Psychogeriatr. (2013) 25:185–203. doi: 10.1017/S1041610212001627
98. Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry (2008) 165:844–54. doi: 10.1176/appi.ajp.2008.07111779
99. O'Connor DW, Ames D, Gardner B, King M. Psychosocial treatments of behaviour symptoms in dementia: a systematic review of reports meeting quality standards. Int Psychogeriatr. (2009) 21:225–40. doi: 10.1017/S1041610208007588
100. Gitlin L, Earland TV. Improving Quality of Life in Individuals With Dementia: The Role of Nonpharmacologic Approaches in Rehabilitation. International Encycolpedia of Rehabilitation (2010) 2:22.
101. Norton MJ, Allen RS, Snow AL, Hardin JM, Burgio LD. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. Am J Alzheimer's Dis Other Dem. (1996) 11:10–9.
102. Kovach CR, Noonan PE, Schlidt AM, Wells T. A model of consequences of need-driven, dementia-compromised behavior. J Nurs Scholarsh. (2005) 37:134–40. doi: 10.1111/j.1547-5069.2005.00025_1.x
103. O'Neil ME, Freeman M, Christensen V, Telerant R, Addleman A, Kansagara D. A Systematic Evidence Review of Non-pharmacological Interventions for Behavioural Symptoms of Dementia. Washington DC, Department of Veterans Affairs (2011).
104. Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry (2012) 16:946–53. doi: 10.1176/appi.ajp.2012.11101529
105. McLaren AN, Lamantia MA, Callahan CM. Systematic review of non-pharmacologic interventions to delay functional decline in community-dwelling patients with dementia. Aging Ment Health (2013) 17:655–66. doi: 10.1080/13607863.2013.781121
106. Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. (2014) 18:1–226. doi: 10.3310/hta18610
107. Abraha I, Rimland JM, Trotta FM, Dell'Aquila G, Cruz-Jentoft A, Petrovic M, et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-On Top series. BMJ Open (2017) 7:e012759. doi: 10.1136/bmjopen-2016-012759
108. Cohen-Mansfield J, Thein K, Marx MS. Predictors of the impact of nonpharmacologic interventions for agitation in nursing home residents with advanced dementia. J Clin Psychiatry (2014) 75:666–71. doi: 10.4088/JCP.13m08649
109. Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Thein K. The use and utility of specific nonpharmacological interventions for behavioural symptoms in dementia: an exploratory study. Am J Geriatr Psychiatry (2015) 23:160–70. doi: 10.1016/j.jagp.2014.06.006
110. Scales K, Zimmerman S, Miller SJ. Evidence-based nonpharmacological practices to address behavioural and psychological symptoms of dementia. Gerontologist (2018) 58(suppl_1):S88–102. doi: 10.1093/geront/gnx167
111. Cohen-Mansfield J, Jensen B, Resnick B, Norris M. Assessment and treatment of behaviour problems in dementia in nursing home residents: a comparison of the approaches of physicians, psychologists and nurse practitioners. Int J Geriatr Psychiatry (2012) 27:135–45. doi: 10.1002/gps.2699
112. Cohen-Mansfield J, Dakheel-Ali M, Marx MS, Thein K, Regier NG. Which unmet needs contribute to behaviour problems in persons with advanced dementia? Psychiatry Res. (2015) 30:59–64. doi: 10.1016/j.psychres.2015.03.043
113. Muò R, Schindler A, Vernero I, Schindler O, Ferrario E, Frisoni GB. Alzheimer's disease-associated disability: an ICF approach. Disabil Rehabil. (2005) 27:1405–13. doi: 10.1080/09638280500052542
114. Giebel CM, Sutcliffe C, Stolt M, Karlsson S, Renom-Guiteras A, Soto M, et al. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: a European study. Int Psychogeriatr. (2014) 26:1283–93. doi: 10.1017/S1041610214000775
115. Prizer LP, Zimmerman S. Progressive support for activities of daily living for persons living with dementia. Gerontologist (2018) 58(suppl_1):S74–87. doi: 10.1093/geront/gnx103
116. Sun M, Mainland BJ, Ornstein TJ, Mallya S, Fiocco AJ, Sin GL, et al. The association between cognitive fluctuations and activities of daily living and quality of life among institutionalized patients with dementia. Int J Geriatr Psychiatry (2018) 33:e280–5. doi: 10.1002/gps.4788
117. Egan M, Hobson S, Fearing V. Dementia and occupation: a review of the literature. Can J Occupat Ther. (2006) 73:132–40. doi: 10.2182/cjot.05.0015
118. Georges J, Jansen S, Jackson J, Meyrieux A, Sadowska A, Selmes M. Alzheimer's disease in real life - the dementia carer's survey. Int J Geriatr Psychiatry (2008) 23:546–51. doi: 10.1002/gps.1984
119. Steultjens EM, Dekker J, Bouter LM, Leemrijse CJ, van den Ende CH. Evidence of the efficacy of occupational therapy in different conditions: an overview of systematic reviews. Clin Rehabil. (2005) 19:247–54. doi: 10.1191/0269215505cr870oa
120. Voigt-Radloff S, Graff M, Leonhart R, Schornstein K, Jessen F, Bohlken J, et al. A multicentre RCT on community occupational therapy in Alzheimer's disease: 10 sessions are not better than one consultation. BMJ Open (2011) 9:e000096. doi: 10.1136/bmjopen-2011-000096
121. Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their caregivers: randomised controlled trial. BMJ (2006) 333:1196. doi: 10.1136/bmj.39001.688843.BE
122. Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill-building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH Initiative. Gerontologist (2003) 43:532–46. doi: 10.1093/geront/43.4.532
123. Gitlin LN, Hodgson N, Jutkowitz E, Pizzi L. The cost-effectiveness of a nonpharmacologic intervention for individuals with dementia and family caregivers: the tailored activity program. Am J Geriatr Psychiatry (2010) 18:510–9. doi: 10.1097/JGP.0b013e3181c37d13
124. Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT Jr. A randomized clinical trial of theory-based activities for the behavioural symptoms of dementia in nursing home residents. J Am Geriatr Soc. (2001) 59:1032–41. doi: 10.1111/j.1532-5415.2011.03449.x
125. Rao AK, Chou A, Bursley B, Smulofsky J, Jezequel J. Systematic review of the effects of exercise on activities of daily living in people with Alzheimer's disease. Am J Occup Ther. (2014) 68:50–6. doi: 10.5014/ajot.2014.009035
126. Rao AK. Occupational therapy in chronic progressive disorders: enhancing function and modifying disease. Am J Occup Ther. (2014) 68:251–3. doi: 10.5014/ajot.2014.012120
127. Pimouguet C, Le Goff M, Wittwer J, Dartigues JF, Helmer C. Benefits of occupational therapy in dementia patients: findings from a real-world observational study. J Alzheimers Dis. (2017) 56:509–17. doi: 10.3233/JAD-160820
128. Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatry behavior in persons with dementia and reduce caregiver burden. A randomized pilot study. Am J Geriatr Psychiatry (2008) 16:229–39. doi: 10.1097/01.JGP.0000300629.35408.94
129. Gitlin LN, Arthur P, Piersol C, Hessels V, Wu SS, Dai Y, et al. Targeting behavioral symptoms and functional decline in dementia: a randomized clinical trial. J Am Geriatr Soc. (2018) 66:339–45. doi: 10.1111/jgs.15194
130. Kim SY, Yoo EY, Jung MY, Park SH, Park JH. A systematic review of the effects of occupational therapy for persons with dementia: a meta-analysis of randomized controlled trials. NeuroRehabilitation (2012) 31:107–15.
131. Padilla R. Effectiveness of environment-based interventions for people with Alzheimer's disease and related dementias. Am J Occup Ther. (2011) 65:514–22. doi: 10.5014/ajot.2011.002600
132. Letts L, Edwards M, Berenyi J, Moros K, O'Neill C, O'Toole C, et al. Using occupations to improve quality of life, health and wellness, and client and caregiver satisfaction for people with Alzheimer's disease and related dementias. Am J Occup Ther. (2011) 65:497–504. doi: 10.5014/ajot.2011.002584
133. Gitlin LN, Winter L, Earland TV, Herge EA, Chernett NL, Piersol CV, et al. The Tailored Activity Program (TAP): a nonpharmacologic approach to improving quality of life at home for persons with dementia and their family caregivers. Gerontol Practice Concepts (2009) 49:428–39.
134. Padilla R. Effectiveness of interventions designed to modify the activity demands of the occupations of self-care and leisure for people with Alzheimer's disease and related dementias. Am J Occup Ther. (2011) 65:523–31. doi: 10.5014/ajot.2011.002618
135. National Collaborating Centre for Mental Health (commissioned by the Social Care Institute for Excellence and the National Institute for Health and Clinical Excellence). Dementia. A NICE-SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care. National Clinical Practice Guideline, Number 42. London: The British Psychological Society and Gaskell (2007).
136. German Society for Psychiatry, Psychotherapy, Neurology German Society for Neurology,. Consented Guideline ‘Dementia'. (2009). Available online at: https://www.dgn.org/leitlinien/3176-leitlinie-diagnose-und-therapie-von-demenzen-2016
137. Work Group on Alzheimer's Disease and other dementias. Practice Guideline for the Treatment of Patients With Alzheimer's Disease and Other Dementias (2007). Available online at: http://www.psychiatryonline.com/pracGuide/PracticePDFs/AlzPG101007.pdf
138. Laver K, Cumming R, Dyer S, Agar M, Anstey KJ, Beattie E, et al. Evidence-based occupational therapy for people with dementia and their families: What clinical practice guidelines tell us and implications for practice. Aust Occup Ther J. (2017) 64:3–10. doi: 10.1111/1440-1630.12309
139. Lindbergh CA, Dishman RK, Miller LS. Functional disability in mild cognitive impairment: a systematic review and meta-analysis. Neuropsychol Rev. (2016) 26:129–59. doi: 10.1007/s11065-016-9321-5
140. Kaur N, Belchior P, Gelinas I, Bier N. Critical appraisal of questionnaires to assess functional impairment in individuals with mild cognitive impairment. Int Psychogeriatr. (2016) 28:1425–39. doi: 10.1017/S104161021600017X
141. Reppermund S, Brodaty H, Crawford JD, Kochan NA, Draper B, Slavin MJ, et al. Impairment in instrumental activities of daily living with high cognitive demand is an early marker of mild cognitive impairment: the Sydney memory and ageing study. Psychol Med. (2013) 43:2437–45. doi: 10.1017/S003329171200308X
142. Fieo R, Zahodne L, Tang MX, Manly JJ, Cohen R, Stern Y. The historical progression from ADL scrutiny to IADL to advanced ADL: assessing functional status in the earliest stages of dementia. J Gerontol A Biol Sci Med Sci. (2017) 24:1035–1055. doi: 10.1093/gerona/glx235
143. Aminzadeh F, Byszewski A, Molnar FJ, Eisner M. Emotional impact of dementia diagnosis: exploring persons with dementia and caregivers' perspectives. Aging Ment Health (2007) 11:281–90. doi: 10.1080/13607860600963695
144. Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry (2011) 24:461–72. doi: 10.1097/YCO.0b013e32834bb9d4
145. Weiner MF, Doody RS, Sairam R, Foster B, Liao TY. Prevalence and incidence of major depressive disorder in Alzheimer's disease: findings from two databases. Dement Geriatr Cog Disord. (2002) 13:8–12. doi: 10.1159/000048627
146. Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer's disease. Am J Psychiatry (2005) 162:2086–93. doi: 10.1176/appi.ajp.162.11.2086
147. Hwang TJ, Masterman DL, Ortiz F, Fairbanks LA, Cummings JL. Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Dis Assoc Disord. (2004) 18:17–21.
148. Schultz SK, Hoth A, Buckwalter K. Anxiety and impaired social function in the elderly. Ann Clin Psychiatry (2004) 16:47–51. doi: 10.1080/10401230490281429
149. Gabryelewicz T, Styczynska M, Pfeffer A, Wasiak B, Barczak A, Luczywek E, et al. Prevalence of major and minor depression in elderly persons with mild cognitive impairment–MADRS factor analysis. Int J Geriatr Psychiatry (1998) 19:1168–72. doi: 10.1002/gps.1235
150. Lu PH, Edland SD, Teng E, Tingus K, Petersen RC, Cummings JL, et al. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology (2009) 72:2115–221. doi: 10.1212/WNL.0b013e3181aa52d3
151. Rozzini L, Chilovi BV, Peli M, Conti M, Rozzini R, Trabucchi M, et al. Anxiety symptoms in mild cognitive impairment. Int J Geriatr Psychiatry (2009) 24:300–5. doi: 10.1002/gps.2106
152. Alexopoulos GS, Jeste DV, Chung H, Carpenter D, Ross R, Docherty JP. The expert consensus guideline series. Treatment of dementia and its behavioural disturbances. Introduction: methods, commentary, and summary. Postgrad Med Spec. (2005) 6:22.
153. Salzman C, Jeste DV, Meyer RE, Cohen-Mansfield J, Cummings J, Grossberg GT, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry (2008) 69:889–98. doi: 10.4088/JCP.v69n0602
154. Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry (2005) 76:1070–4. doi: 10.1136/jnnp.2004.052795
155. Spalletta G, Long JD, Robinson RG, Trequattrini A, Pizzoli S, Caltagirone C, et al. Longitudinal neuropsychiatric predictors of death in Alzheimer's disease. J Alzheimers Dis. (2015) 48:627–36. doi: 10.3233/JAD-150391
156. Dauphinot V, Delphin-Combe F, Mouchoux C, Dorey A, Bathsavanis A, Makaroff Z, et al. Risk factors of caregiver burden among patients with Alzheimer's disease or related disorders: a cross-sectional study. J Alzheimers Dis. (2015) 44:907–16. doi: 10.3233/JAD-142337
157. Hongisto K, Hallikainen I, Selander T, Törmälehto S, Väätäinen S, Martikainen J, et al. Quality of Life in relation to neuropsychiatric symptoms in Alzheimer's disease: 5-year prospective ALSOVA cohort study. Int J Geriatr Psychiatry (2018) 33:47–57. doi: 10.1002/gps.4666
158. Hogan DB, Bailey P, Black S, Carswell A, Chertkow H, Clarke B, et al. Diagnosis and treatment of dementia: non pharmacologic and pharmacologic therapy for mild to moderate dementia. CMAJ (2008) 179:1019–26. doi: 10.1503/cmaj.081103
159. Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. Practice parameter: management of dementia (an evidence-based review). Report of the quality standards subcommittee of the American academy of neurology. Neurology (2001) 56:1154–66. doi: 10.1212/WNL.56.9.1154
160. Lyketsos CG, Olin J. Depression in Alzheimer's disease: overview and treatment. Biol Psychiatry (2002) 52:243–52. doi: 10.1016/S0006-3223(02)01348-3
161. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Adv Psychiatr Treat. (2004) 10:171–7. doi: 10.1192/apt.10.3.171
162. World Health Organization. Evidence for Decision Makers. World Health Organization, Regional Office for Europe, Health Evidence Network (2007). Available online at: http://www.euro.who.int/HEN/
163. Orgeta V, Qazi A, Spector AE, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev. (2014) 22:CD009125. doi: 10.1002/14651858.CD009125.pub2
164. Cheston R, Ivanecka A.Individual and group psychotherapy with people diagnosed with dementia: a systematic review of the literature. Int J Geriatr Psychiatry (2017) 32:3–31. doi: 10.1002/gps.4529
165. Goris ED, Ansel KN, Schutte DL. Quantitative systematic review of the effects of non-pharmacological interventions on reducing apathy in persons with dementia. J Adv Nurs. (2016) 72:2612–28. doi: 10.1111/jan.13026
166. Theleritis C, Siarkos K, Politis AA, Katirtzoglou E, Politis A. A systematic review of non-pharmacological treatments for apathy in dementia. Int J Geriatr Psychiatry (2018) 33:e177–92. doi: 10.1002/gps.4783
167. Farina E, Mantovani F, Fioravanti R, Pignatti R, Chiavari L, Imbornone E, et al. Evaluating two group programmes of cognitive training in mild-to-moderate AD: is there any difference between a 'global' stimulation and a 'cognitive-specific' one? Aging Ment Health (2006) 10:211–8. doi: 10.1080/13607860500409492
168. Farina E, Mantovani F, Fioravanti R, Rotella G, Villanelli F, Imbornone E, et al. Efficacy of recreational and occupational activities associated to psychologic support in mild to moderate Alzheimer disease: a multicenter controlled study. Alzheimer Dis Assoc Disord. (2006) 20:275–82. doi: 10.1097/01.wad.0000213846.66742.90
169. Baglio F, Griffanti L, Saibene FL, Ricci C, Alberoni M, Critelli R, et al. Multistimulation group therapy in Alzheimer's disease promotes changes in brain functioning. Neurorehabil Neural Repair (2015) 29:13–24. doi: 10.1177/1545968314532833
170. Dröes RM, Breebaart E, Ettema TP, van Tilburg W, Mellenbergh GJ. The effect of integrated family support versus day care only on behavior and mood of patients with dementia. Int Psychogeriatr. (2000) 12:99–116. doi: 10.1017/S1041610200006232
171. Dröes RM, Breebaart E, Meiland FJM, Van Tilburg W, Mellenbergh GJ. Effect of Meeting Centres Support Programme on feeling of competence of family caregivers and delay of institutionalization of people with dementia. Aging Ment Health (2004) 8:201–11. doi: 10.1080/13607860410001669732
172. Dröes RM, Meiland FJM, Schmitz M, Van Tilburg W. Effect of combined support for people with dementia and carers versus regular day care on behaviour and mood of persons with dementia: results from a multi-centre implementation study. Int J Geriatr Psychiatry (2004) 19:1–12. doi: 10.1002/gps.1142
173. Dröes RM, Meiland FJM, Schmitz M, Van Tilburg W. An evaluation of the Meeting Centres Support Programme among persons with dementia and their carers. Nonpharmacol Therapies Dementia (2011) 2:19–39.
174. Dröes RM, Meiland FJ, Evans S, Brooker D, Farina E, Szcześniak D, et al. Comparison of the adaptive implementation and evaluation of the Meeting Centers Support Program for people with dementia and their family carers in Europe; study protocol of the MEETINGDEM project. BMC Geriatr. (2017) 17:79. doi: 10.1186/s12877-017-0472-x
175. Smits CH, de Lange J, Dröes RM, Meiland F, Vernooij-Dassen M, Pot AM. Effects of combined intervention programmes for people with dementia living at home and their caregivers: a systematic review. Int J Geriatric Psychiatry (2007) 22:1181–93. doi: 10.1002/gps.1805
176. Klapwijk MS, Caljouw MAA, Pieper MJC, Putter H, van der Steen JT, Achterberg WP. Change in quality of life after a multidisciplinary intervention for people with dementia: a cluster randomized controlled trial. Int J Geriatr Psychiatry (2018). 33:1213–19. doi: 10.1002/gps.4912
177. Sinvani L, Warner-Cohen J, Strunk A, Halbert T, Harisingani R, Mulvany C, et al. A multicomponent model to improve hospital care of older adults with cognitive impairment: a propensity score-matched analysis. J Am Geriatr Soc. (2018) 66:1700–7. doi: 10.1111/jgs.15452
178. Baker R, Bell S, Baker E, Gibson S, Holloway J, Pearce R, et al. A randomized controlled trial of the effects of multi-sensory stimulation (MSS) for people with dementia. Br J Clin Psychol. (2001) 40:81–96.
179. Scuteri D, Rombola L, Tridico L, Mizoguchi H, Watanabe C, Sakurada T, et al. Neuropharmacological properties of the essential oil of bergamot for the clinical management of pain-related BPSDs. Curr Med Chem. (2018) 25:1–10. doi: 10.2174/0929867325666180307115546
180. Forrester L, Maayan N, Orrell M, Spector AE, Buchan LD, Soares-Weiser K. Aromatherapy for dementia. Cochr Datab Syst Rev. (2014) 2:CD003150.
181. Brooker DJ, Snape M, Johnson E, Ward D, Payne M. Single case evaluation of the effects of aromatherapy and massage on disturbed behaviour in severe dementia. Br J Clin Psychol. (1997) 36:287–96. doi: 10.1111/j.2044-8260.1997.tb01415.x
182. Ballard CG, O'Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry (2002) 63:553–58. doi: 10.4088/JCP.v63n0703
183. Lin PW, Chan WC, Ng BF, Lam LC. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: a cross-over randomized trial. Int J Geriatr Psychiatry (2007) 22:405–10. doi: 10.1002/gps.1688
184. Nguyen QA, Paton C. The use of aromatherapy to treat behavioural problems in dementia. Int J Geriatr Psychiatry (2008) 23:337–46. doi: 10.1002/gps.1886
185. Yang MH, Lin LC, Wu SC, Chiu JH, Wang PN, Lin JG. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. (2015) 29:93. doi: 10.1186/s12906-015-0612-9
186. VilelaI VC, PachecoI RL, Cruz LatorracaII CO, PachitoIII DV, Riera R. What do Cochrane systematic reviews say about non-pharmacological interventions for treating cognitive decline and dementia? Sao Paulo Med J. (2017) 135:309–20. doi: 10.1590/1516-3180.2017.0092060617
187. Wolfe N, Herzberg J. Can aromatherapy oils promote sleep in severely demented patients? Int J Geriatr Psychiatry (1996) 11:926–7. doi: 10.1002/(SICI)1099-1166(199610)11:10<926::AID-GPS473>3.0.CO;2-1
188. Johannessen B. Nurses experience of aromatherapy use with dementia patients experiencing disturbed sleep patterns. An action research project. Complement Ther Clin Pract. (2013) 19:209–13. doi: 10.1016/j.ctcp.2013.01.003
189. Takeda A, Watanuki E, Koyama S. Effects of inhalation aromatherapy on symptoms of sleep disturbance in the elderly with dementia. Evid Based Complement Alternat Med. (2017) 2017:1902807. doi: 10.1155/2017/1902807
190. MacMahon S, Kermode S. A clinical trial of the effects of aromatherapy on motivational behaviour in a dementia care setting using a single subject design. Aust J Holist Nurs. (1998) 52:47–9.
191. Forrester LT, Maayan N, Orrell M, Spector AE, Buchan LD, Soares-Weiser K. Aromatherapy for dementia. Cochrane Database Syst Rev. (2014) 25:CD003150. doi: 10.1002/14651858.CD003150.pub2
192. van der Steen JT, van Soest-Poortvliet MC, van der Wouden JC, Bruinsma MS, Scholten RJ, Vink AC Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev. (2017) 5:CD003477. doi: 10.1002/14651858.CD003477.pub3
193. Raglio A, Pavlic E, Bellandi D. Music listening for people living with dementia. J Am Med Dir Assoc. (2018) 19:722–3. doi: 10.1016/j.jamda.2018.05.027
194. Sherratt K, Thornton A, Hatton C. Music interventions for people with dementia: a review of the literature. Aging Ment Health (2004) 8:3–12. doi: 10.1080/13607860310001613275
195. Vink AC, Birks JS, Bruinsma MS, Scholten RJ. Music therapy for people with dementia. Cochrane Database Syst Rev. (2004) 3:CD003477. doi: 10.1002/14651858.CD003477.pub2
196. Raglio A, Bellelli G, Mazzola P, Bellandi D, Giovagnoli AR, Farina E, et al. Music, music therapy and dementia: a review of literature and the recommendations of the Italian psychogeriatric association. Maturitas (2012) 72:305–10. doi: 10.1016/j.maturitas.2012.05.016
197. McDermott O, Crellin N, Ridder HM, Orrell M. Music therapy in dementia: a narrative synthesis systematic review. Int J Geriatr Psychiatry (2013) 28:781–94. doi: 10.1002/gps.3895
198. Raglio A, Gianelli MV. Music and music therapy in the management of behavioral disorders in dementia. Neurodegener Dis Manag. (2013) 3:295–8. doi: 10.2217/nmt.13.27
199. Solé C, Mercadal-Brotons M, Galati A, De Castro M. Effects of group music therapy on quality of life, affect, and participation in people with varying levels of dementia. J Music Ther. (2014) 51:103–25. doi: 10.1093/jmt/thu003
200. Zatorre R. Music and the brain. Ann N Y Acad Sci. (2003) 999:4–14. doi: 10.1196/annals.1284.001
201. Zatorre R, McGill J. Music, the food of neuroscience? Nature (2005) 17:312–5. doi: 10.1038/434312a
202. Levitin DJ, Tirovolas AK. Current advances in the cognitive neuroscience of music. Ann N Y Acad Sci. (2009) 1156:211–31. doi: 10.1111/j.1749-6632.2009.04417.x
203. Koelsch S. A neuroscientific perspective on music therapy. Ann N Y Acad Sci. (2009) 1169:374–84. doi: 10.1111/j.1749-6632.2009.04592.x
204. Koelsch S. Towards a neural basis of music-evoked emotions. Trends Cogn Sci. (2010) 14:131–7. doi: 10.1016/j.tics.2010.01.002
205. Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. (2014) 15:170–80. doi: 10.1038/nrn3666
206. Gold C, Solli HP, Kruger V, Lie SA. Dose-response relationship in music therapy for people with serious mental disorders; systematic review and meta-analysis. Clin Psychol Rev. (2009) 29:193–207. doi: 10.1016/j.cpr.2009.01.001
207. Schlaug G. Part VI introduction: listening to and making music facilitates brain recovery processes. Ann N Y Acad Sci. (2009) 1169:372–3. doi: 10.1111/j.1749-6632.2009.04869.x
208. Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Gentile S, et al. Efficacy of music therapy treatment based on cycles of sessions: a randomized controlled trial. Aging Ment Health (2010) 14:900–4. doi: 10.1080/13607861003713158
209. Lin Y, Chu H, Yang CY, Chen CH, Chen SG, Chang HJ, et al. Effectiveness of group music intervention against agitated behavior in elderly persons with dementia. Int J Geriatr Psychiatry (2011) 26:670–8. doi: 10.1002/gps.2580
210. Sung HC, Lee WL, Li TL, Watson R. A group music intervention using percussion instruments with familiar music to reduce anxiety and agitation of institutionalized older adults with dementia. Int J Geriatr Psychiatry (2012) 27:621–7. doi: 10.1002/gps.2761
211. Ridder HM, Stige B, Qvale LG, Gold C. Individual music therapy for agitation in dementia: an exploratory randomized controlled trial. Aging Ment Health (2013) 17:667–78. doi: 10.1080/13607863.2013.790926
212. Ueda T, Suzukamo Y, Sato M, Izumi S. Effects of music therapy on behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. Ageing Res Rev. (2013) 12:628–41. doi: 10.1016/j.arr.2013.02.003
214. Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Villani D, et al. Efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Dis Assoc Disord. (2008) 22:158–62. doi: 10.1097/WAD.0b013e3181630b6f
215. Sakamoto M, Ando H, Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int Psychogeriatr. (2013) 25:775–84. doi: 10.1017/S1041610212002256
216. Jiménez-Palomares M, Rodríguez-Mansilla J, González-López-Arza MV, Rodríguez-Domínguez MT, Prieto-Tato M. Benefits of music therapy as therapy no pharmacology and rehabilitation moderate dementia. Rev Esp Geriatr Gerontol. (2013) 48:238–42. doi: 10.1016/j.regg.2013.01.008
217. Brotons M, Koger SM. The impact of music therapy on language functioning in dementia. J Music Ther. (2000) 37:183–95. doi: 10.1093/jmt/37.3.183
218. Cowles A, Beatty WW, Nixon SJ, Lutz LJ, Paulk J, Paulk K, et al. Musical skill in dementia: a violinist presumed to have Alzheimer's disease learns to play a new song. Neurocase (2003) 9:493–503. doi: 10.1076/neur.9.6.493.29378
219. Van de Winckel A, Feys H, De Weerdt W, Dom R. Cognitive and behavioral effects of music-based exercises in patients with dementia. Clin Rehabil. (2004) 18:253–60. doi: 10.1191/0269215504cr750oa
220. Cuddy LL, Duffin J. Music memory and Alzheimer's disease: is music recognition spared in dementia, and how can it be assessed? Med Hypotheses (2005) 64:229–35. doi: 10.1016/j.mehy.2004.09.005
221. Fornazzari L, Castle T, Nadkarni S, Ambrose M, Miranda D, Apanasiewicz N, et al. Preservation of episodic musical memory in a pianist with Alzheimer disease. Neurology (2006) 66:610–1. doi: 10.1212/01.WNL.0000198242.13411.FB
222. Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, Crociani S, et al. STAM protocol in dementia: a multicenter, single-blind, randomized, and controlled trial. Am J Alzheimers Dis Other Demen. (2012) 27:301–10. doi: 10.1177/1533317512452038
223. Baird A, Samson S. Music and dementia. Prog Brain Res. (2015) 217:207–35. doi: 10.1016/bs.pbr.2014.11.028
224. Samson S, Clément S, Narme P, Schiaratura L, Ehrlé N. Efficacy of musical interventions in dementia: methodological requirements of non-pharmacological trials. Ann NY Acad Sci. (2015) 1337:249–55. doi: 10.1111/nyas.12621
225. Giovagnoli AR, Manfredi V, Schifano L, Paterlini C, Parente A, Tagliavini F. Combining drug and music therapy in patients with moderate Alzheimer's disease: a randomized study. Neurol Sci. (2018) 39:1021–8. doi: 10.1007/s10072-018-3316-3
226. Fusar-Poli L, Bieleninik L, Brondino N, Xi-Jing C, Gold C. The effect of music therapy on cognitive functions in patients with dementia: a systematic review and meta-analysis. Aging Ment Health (2017) 10:1–10. doi: 10.1080/13607863.2017.1348474
227. British Association of Art Therapists website. Available online at: www.baat.org (Accessed on 25 September, 2018).
228. Chancellor B, Duncan A, Chatterjee A. Art therapy for Alzheimer's disease and other dementias. J Alzheimers Dis. (2014) 39:1–11. doi: 10.3233/JAD-131295
229. Silveri MC, Ferrante I, Brita AC, Rossi P, Liperoti R, Mammarella F, et al. The Memory of Beauty survives Alzheimer's disease (but cannot help memory). J Alzheimers Dis. (2015) 45:483–94. doi: 10.3233/JAD-141434
230. Sterritt PF, Pokorny ME. Art activities for patients with Alzheimer's and related disorders. Geriatr Nurs. (1994) 15:155–9. doi: 10.1016/S0197-4572(09)90043-X
231. Rentz CA. Memories in the making: outcome-based evaluation of an art program for individuals with dementing illnesses. Am J Alzheimers Dis Other Demen. (2002) 17:175–81. doi: 10.1177/153331750201700310
232. Bonner C. Reducing Stress-Related Behaviours in People With Dementia-Care-Based Therapy. London: Jessica Kingsley Publishers (2006).
233. Hannemann BT. Creativity with dementia patients. Can creativity and art stimulate dementia patients positively? Gerontology (2006) 52:59–65. doi: 10.1159/000089827
234. Rusted J. A multi-centre randomized control group trial on the use of art therapy for older people with dementia. Group Analysis (2006) 39:517–36. doi: 10.1177/0533316406071447
235. Flood M, Phillips KD. Creativity in older adults: a plethora of possibilities. Issues Ment Health Nurs. (2007) 28:389–411. doi: 10.1080/01612840701252956
236. Schmitt B, Frölich L. Creative therapy options for patients with dementia–a systematic review. Fortschr Neurol Psychiatr. (2007) 75:699–707. doi: 10.1055/s-2006-944298
237. Cummings JL, Miller BL, Christensen DD, Cherry D. Creativity and dementia: emerging diagnostic and treatment methods for Alzheimer's disease. CNS Spectr. (2008) 13(Suppl 2):1–20.
238. Stoukides J. Creative and sensory therapies enhance the lives of people with Alzheimers. Med Health R I. (2008) 91:154.
239. Peisah C, Lawrence G, Reutens S. Creative solutions for severe dementia with BPSD: a case of art therapy used in an inpatient and residential care setting. Int Psychogeriatr. (2001) 23:1011–3. doi: 10.1017/S1041610211000457
240. Safar LT. Use of art making in treating older patients with dementia. Virtual Mentor. (2014) 16:626–30. doi: 10.1001/virtualmentor.2014.16.08.stas1-1408
241. Seifert K, Spottke A, Fliessbach K. Effects of sculpture based art therapy in dementia patients—a pilot study. Helyon (2017) 3:1–13. doi: 10.1016/j.heliyon.2017.e00460
242. Pongan E, Tillmann B, Leveque Y, Trombert B, Getenet JC, Auguste N, et al. Can musical or painting interventions improve chronic pain, mood, quality of life, and cognition in patients with mild Alzheimer's disease? Evidence from a randomized controlled trial. J Alzheimers Dis. (2017) 60:663–77. doi: 10.3233/JAD-170410
243. Hsu TJ, Tsai HT, Hwang AC, Chen LY, Chen LK. Predictors of non-pharmacological intervention effect on cognitive function and behavioral and psychological symptoms of older people with dementia. Geriatr Gerontol Int. (2017) 17:28–35. doi: 10.1111/ggi.13037
244. Rowe M, Alfred D. The effectiveness of slow-stroke massage in diffusing agitated behaviours in individuals with Alzheimer's disease. J Gerontol Nurs. (1999) 25:22–34.
245. Remington R. Calming music and hand massage with agitated elderly. Nurs Res. (2002) 51:317–23. doi: 10.1097/00006199-200209000-00008
247. Moyle W, Johnston A, O'Dwyer S. Exploring the effect of foot massage on agitated behaviours in older people with dementia: a pilot study. Aust J Ageing (2011) 30:159–61. doi: 10.1111/j.1741-6612.2010.00504.x
248. Rodríguez-Mansilla J, González López-Arza MV, Varela-Donoso E, Montanero-Fernández J, González Sánchez B, Garrido-Ardila EM. The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: a randomized controlled trial. Clin Rehabil. (2015) 29:683–93. doi: 10.1177/0269215514554240
249. Moyle W, Cooke ML, Beattie E, Shum DH, O'Dwyer ST, Barrett S, et al. Foot massage and physiological stress in people with dementia: a randomized controlled trial. J Altern Complement Med. (2014) 20:305–11. doi: 10.1089/acm.2013.0177
250. Kapoor Y, Orr R. Effect of therapeutic massage on pain in patients with dementia. Dementia (2015) 1. doi: 10.1177/1471301215583391
251. Wang HL, Keck JF. Foot and hand massage as an intervention for postoperative pain. Pain Manag Nurs. (2004) 5:59–65. doi: 10.1016/j.pmn.2004.01.002
252. Bush E. The use of human touch to improve the well-being of older adults: a holistic nursing intervention. J Holistic Nurs. (2011) 19:256–70. doi: 10.1177/089801010101900306
253. Wu J, Wang Y, Wang Z. The effectiveness of massage and touch on behavioural and psychological symptoms of dementia: a quantitative systematic review and meta-analysis. J Adv Nurs. (2017) 73:2283–95. doi: 10.1111/jan.13311
254. Culler D, Estrin D, Srivastava M. Guest editors' introduction: overview of sensor networks. Computer (2004) 37:41–9. doi: 10.1109/MC.2004.93
255. Cooper RA, Dicianno BE, Brewer B, LoPresti E, Ding D, Simpson R, et al. A perspective on intelligent devices and environments in medical rehabilitation. Med Eng Phys. (2008) 30:1387–98. doi: 10.1016/j.medengphy.2008.09.003
256. Robert PH, Konig A, Andrieu S, Bremond F, Chemin I, Chung PC, et al. Recommendations for ICT use in Alzheimer's disease assessment: Monaco CTAD expert meeting. J Nutr Health Aging (2013) 17:653–60. doi: 10.1007/s12603-013-0046-3
257. Rialle V, Duchene F, Noury N, Bajolle L, Demongeot J. Health “Smart” home: information technology for patients at home. Telemed J E Health (2002) 8:395–409. doi: 10.1089/15305620260507530
258. Powell J, Chiu T, Eysenbach G. A systematic review of networked technologies supporting carers of people with dementia. J Telemed Telecare (2008) 14:154–6. doi: 10.1258/jtt.2008.003018
259. Pilotto A, D'Onofrio G, Benelli E, Zanesco A, Cabello A, Margelí MC, et al. Information and communication technology systems to improve quality of life and safety of Alzheimer's disease patients: a multicenter international survey. J Alzheimers Dis. (2011) 23:131–41. doi: 10.3233/JAD-2010-101164
260. Romdhane R, Mulin E, Derreumeaux A, Zouba N, Piano J, Lee L, et al. Automatic video monitoring system for assessment of Alzheimer's disease symptoms. J Nutr Health Aging (2012) 16:213–8. doi: 10.1007/s12603-012-0039-7
261. Miskelly F. Electronic tracking of patients with dementia and wandering using mobile phone technology. Age Ageing (2005) 34:497–9. doi: 10.1093/ageing/afi145
262. Faucounau V, Riguet M, Orvoen G, Lacombe A, Rialle V, Extra J, et al. Electronic tracking system and wandering in Alzheimer's disease: a case study. Ann Phys Rehabil Med. (2009) 52:579–87. doi: 10.1016/j.rehab.2009.07.034
263. Bantry White E, Montgomery P, McShane R. Electronic tracking for people with dementia who get lost outside the home: a study of the experience of familial carers. Br J Occup Ther. (2010) 73:152–9. doi: 10.4276/030802210X12706313443901
264. Pot AM, Willemse BM, Horjus S. A pilot study on the use of tracking technology: feasibility, acceptability, and benefits for people in early stages of dementia and their informal caregivers. Aging Ment Health (2011) 16:127–34. doi: 10.1080/13607863.2011.596810
265. Teipel S, Babiloni C, Hoey J, Kaye J, Kirste T, Burmeister OK. Information and communication technology solutions for outdoor navigation in dementia. Alzheimer Dement. (2016) 12:695–707. doi: 10.1016/j.jalz.2015.11.003
266. Lauriks S, Reinersmann A, Van der Roest HG, Meiland FJ, Davies RJ, Moelaert F, et al. Review of ICT-based services for identified unmet needs in people with dementia. Ageing Res Rev. (2007) 6:223–46. doi: 10.1016/j.arr.2007.07.002
267. Olsson A, Engström M, Skovdahl K, Lampic C. My, your and our needs for safety and security: relatives' reflections on using information and communication technology in dementia care. Scand J Caring Sci. (2012) 26:104–12. doi: 10.1111/j.1471-6712.2011.00916.x
268. Robinson L, Hutchings D, Corner L, Finch T, Hughes J, Brittain K, et al. Balancing rights and risks: conflicting perspectives in the management of wandering in dementia. Health Risk Soc. (2007) 9:389–406. doi: 10.1080/13698570701612774
269. Landau R, Werner S, Auslander GK, Shoval N, Heinik J. Attitudes of family and professional care-givers towards the use of GPS for tracking patients with dementia: an exploratory study. Br J Soc Work (2009) 39:670–92. doi: 10.1093/bjsw/bcp037
270. Niemeijer AR, Frederiks BJ, Depla MF, Legemaate J, Eefsting JA, Hertogh CM. The ideal application of surveillance technology in residential care for people with dementia. J Med Ethics (2011) 37:303–10. doi: 10.1136/jme.2010.040774
271. Zwijsen SA, Depla MF, Niemeijer AR, Francke AL, Hertogh CM. Surveillance technology: an alternative to physical restraints? A qualitative study among professionals working in nursing homes for people with dementia. Int J Nurs Stud. (2012) 49:212–9. doi: 10.1016/j.ijnurstu.2011.09.002
272. Te Boekhorst S, Depla MF, Francke AL, Twisk JW, Zwijsen SA, Hertogh CM. Quality of life of nursing-home residents with dementia subject to surveillance technology versus physical restraints: an explorative study. Int J Geriatr Psychiatry (2013) 28:356–63. doi: 10.1002/gps.3831
273. Landau R, Werner S. Ethical aspects of using GPS for tracking people with dementia: recommendations for practice. Int Psychogeriatr. (2012) 24:358–66. doi: 10.1017/S1041610211001888
274. Niemeijer AR, Frederiks BJ, Riphagen II, Legemaate J, Eefsting JA, Hertogh CM. Ethical and practical concerns of surveillance technologies in residential care for people with dementia or intellectual disabilities: an overview of the literature. Int Psychogeriatr. (2010) 22:1129–42. doi: 10.1017/S1041610210000037
275. Ienca M, Wangmo T, Jotterand F, Kressig RW, Elger B. Ethical design of intelligent assistive technologies for dementia: a descriptive review. Sci Eng Ethics (2017) 73:1695–1700. doi: 10.1007/s11948-017-9976-1
276. Nelson A, Powell-Cope G, Gavin-Dreschnack D, Quigley P, Bulat T, Baptiste AS, et al. Technology to promote safe mobility in the elderly. Nurs Clin North Am. (2004) 39:649–71. doi: 10.1016/j.cnur.2004.05.001
277. Allum JH, Carpenter MG. A speedy solution for balance and gait analysis: angular velocity measured at the centre of body mass. Curr Opin Neurol. (2005) 18:15–21. doi: 10.1097/00019052-200502000-00005
278. Wu G, Xue S. Portable preimpact fall detector with inertial sensors. IEEE Trans Neural Syst Rehabil Eng. (2008) 16:178–83. doi: 10.1109/TNSRE.2007.916282
279. Schwickert L, Becker C, Lindemann U, Maréchal C, Bourke A, Chiari L, et al. Fall detection with body-worn sensors : a systematic review. Z Gerontol Geriatr. (2013) 46:706–19. doi: 10.1007/s00391-013-0559-8
280. Aud MA, Abbott CC, Tyrer HW, Neelgund RV, Shriniwar UG, Mohammed A, et al. Smart carpet: developing a sensor system to detect falls and summon assistance. J Gerontol Nurs. (2010) 36:8–12. doi: 10.3928/00989134-20100602-02
281. Chaudhuri S, Thompson H, Demiris G. Fall detection devices and their use with older adults: a systematic review. J Geriatr Phys Ther. (2014) 37:178–96. doi: 10.1519/JPT.0b013e3182abe779
282. Danielsen A, Olofsen H, Bremdal BA. Increasing fall risk awareness using wearables: a fall risk awareness protocol. J Biomed Inf. (2016) 63:184–94. doi: 10.1016/j.jbi.2016.08.016
283. Khanuja K, Joki J, Bachmann G, Cuccurullo S. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas (2018) 110:51–6. doi: 10.1016/j.maturitas.2018.01.021
284. König A, Sacco G, Bensadoun G, Bremond F, David R, Verhey F, et al. The role of information and communication technologies in clinical trials with patients with Alzheimer's disease and related disorders. Front Aging Neurosci. (2015) 7:110. doi: 10.3389/fnagi.2015.00110
285. Sacco G, Joumier V, Darmon N, Dechamps A, Derreumaux A, Lee JH, et al. Detection of activities of daily living impairment in Alzheimer's disease and mild cognitive impairment using information and communication technology. Clin Interv Aging (2012) 7:539–49. doi: 10.2147/CIA.S36297
286. Okura T, Langa KM. Caregiver burden and neuropsychiatric symptoms in older adults with cognitive impairments: the aging, demographics, and memory study (ADAMS). Alzheimer Dis Assoc Disord. (2011) 25:116–21. doi: 10.1097/WAD.0b013e318203f208
287. Bankole A, Anderson M, Smith-Jackson T, Knight A, Oh K, Brantley J, et al. Validation of noninvasive body sensor network technology in the detection of agitation in dementia. Am J Alzheimers Dis Other Demen. (2012) 27:346–54. doi: 10.1177/1533317512452036
288. Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. Agitation in cognitive disorders: international psychogeriatric association provisional consensus clinical and research definition. Int Psychogeriatr. (2015) 27:7–17. doi: 10.1017/S1041610214001963
289. Melander C, Martinsson J, Gustafsson S. Measuring electrodermal activity to improve the identification of agitation in individuals with dementia. Dement Geriatr Cogn Disord Extra (2017) 7:430–9. doi: 10.1159/000484890
290. Robinson L, Brittain K, Lindsay S, Jackson D, Olivier P. Keeping In Touch Everyday (KITE) project: developing assistive technologies with people with dementia and their carers to promote independence. Int Psychogeriatr. (2009) 21:494–502. doi: 10.1017/S1041610209008448
291. Kime S, Lamb D, Wilson B. Use of a comprehensive program of external cueing to enhance procedural memory in a patient with dense amnesia. Brain Inj. (1996) 10:17–25. doi: 10.1080/026990596124683
292. Mihailidis A, Fernie GR, Barbenel JC. The use of artificial intelligence in the design of an intelligent cognitive orthosis for people with dementia. Assist Technol. (2001) 13:23–39. doi: 10.1080/10400435.2001.10132031
293. Hartin PJ, Nugent CD, McClean SI, Cleland I, Norton MC, Sanders C, et al. A smartphone application to evaluate technology adoption and usage in persons with dementia. Conf Proc IEEE Eng Med Biol Soc. (2014) 2014:5389–92. doi: 10.1109/EMBC.2014.6944844
294. Lancioni GE, Perilli V, O'Reilly MF, Singh NN, Sigafoos J, Bosco A, et al. Technology-based orientation programs to support indoor travel by persons with moderate Alzheimer's disease: impact assessment and social validation. Res Dev Disabil. (2013) 34:286–93. doi: 10.1016/j.ridd.2012.08.016
295. Stock SE, Davies DK, Secor RR, Wehmeyer ML. Self-directed career preference selection for individuals with intellectual disabilities: using computer technology to enhance self-determination. J Vocat Rehabil. (2003) 19:95–103.
296. Boman IL, Rosenberg L, Lundberg S, Nygard L. The first step in designing a videophone for people with dementia: identification of users' potentials and the requirements of communication technology. Disabil Rehabil Assist Technol. (2012) 7:256–363. doi: 10.3109/17483107.2011.635750
297. Perilli V, Lancioni GE, Singh NN, O'Reilly MF, Sigafoos J, Cassano G, et al. Persons with Alzheimer's disease make phone calls independently using a computer-aided telephone system. Res Dev Disabil. (2012) 33:1014–20. doi: 10.1016/j.ridd.2012.01.007
298. Wimo A, Winblad B, Jönsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimers Dement. (2007) 3:81–91. doi: 10.1016/j.jalz.2007.02.001
299. Wahl HW, Fänge A, Oswald F, Gitlin LN, Iwarsson S. The home environment and disability-related outcomes in aging individuals: what is the empirical evidence? Gerontologist (2009) 3:355–67. doi: 10.1093/geront/gnp056
300. Gitlin LN, Schinfeld S, Winter L, Corcoran M, Boyce AA, Hauck W. Evaluating home environments of persons with dementia: interrater reliability and validity of the Home Environmental Assessment Protocol (HEAP). Disabil Rehabil. (2002) 24:59–71. doi: 10.1080/09638280110066325
301. Marquardt G, Johnston D, Black BS, Morrison A, Rosenblatt A, Lyketsos CG, et al. A descriptive study of home modifications for people with dementia and barriers to implementation. J Hous Elderly (2011) 25:258–73. doi: 10.1080/02763893.2011.595612
302. Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. J Am Geriatr Soc. (2010) 58:1465–74. doi: 10.1111/j.1532-5415.2010.02971.x
303. Allen F, Cain R, Meyer C. How people with dementia and their carers adapt their homes. A qualitative study. Dementia (2017) 1:1471301217712294. doi: 10.1177/1471301217712294
304. Miori V, Russo D, Concordia C. Meeting people's needs in a fully interoperable domotic environment. Sensors (2012) 12:6802–24. doi: 10.3390/s120606802
305. Demiris G, Hensel BK. Technologies for an aging society: a systematic review of “smart home” applications. Yearb Med Inform. (2008) 2008:33–40.
306. Gentry T. Smart homes for people with neurological disability: state of the art. NeuroRehabilitation (2009) 25:209–17. doi: 10.3233/NRE-2009-0517
307. Aiello M, Aloise F, Baldoni R, Cincotti F, Guger C, Lazovik A, et al. Smart homes to improve the quality of life for all. Conf Proc IEEE Eng Med Biol Soc. (2011) 2011:1777–80. doi: 10.1109/IEMBS.2011.6090507
308. Ding D, Cooper RA, Pasquina PF, Fici-Pasquina L. Sensor technology for smart homes. Maturitas (2011) 69:131–6. doi: 10.1016/j.maturitas.2011.03.016
309. Frisardi V, Imbimbo BP. Gerontechnology for demented patients: smart homes for smart aging. J Alzheimers Dis. (2011) 23:143–6. doi: 10.3233/JAD-2010-101599
310. Blasco R, Marco Á, Casas R, Cirujano D, Picking R. A smart kitchen for ambient assisted living. Sensors (2014) 14:1629–53. doi: 10.3390/s140101629
311. Chan M, Campo E, Estève D, Fourniols JY. Smart homes - current features and future perspectives. Maturitas (2009) 64:90–7. doi: 10.1016/j.maturitas.2009.07.014
312. Bossen AL, Kim H, Williams KN, Steinhoff AE, Strieker M. Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technol Telehealth. (2015) 3:49–57. doi: 10.2147/SHTT.S59500
313. Ienca M, Fabrice J, Elger B, Caon M, Pappagallo AS, Kressig RW, et al. Intelligent assistive technology for Alzheimer's disease and other dementias: a systematic review. J Alzheimers Dis. (2017) 56:1301–40. doi: 10.3233/JAD-161037
314. Baus O, Bouchard S. Moving from virtual reality exposure-based therapy to augmented reality exposure-based therapy: a review. Front Hum Neurosci. (2014) 8:112. doi: 10.3389/fnhum.2014.00112
315. Gregg L, Tarrier N. Virtual reality in mental health: a review of the literature. Soc Psychiatry Psychiatr Epidemiol. (2007) 42:343–54. doi: 10.1007/s00127-007-0173-4
316. Cotelli M, Manenti R, Zanetti O, Miniussi C. Non-pharmacological intervention for memory decline. Front Hum Neurosci. (2012) 9:46. doi: 10.3389/fnhum.2012.00046
317. Déjos M, Sauzéon H, N'kaoua B. Virtual reality for clinical assessment of elderly people: early screening for Dementia. Rev Neurol. (2012) 168:404–14. doi: 10.1016/j.neurol.2011.09.005
318. Man DW, Chung JC, Lee GY. Evaluation of a virtual reality-based memory training programme for Hong Kong Chinese older adults with questionable dementia: a pilot study. Int J Geriatr Psychiatry (2012) 27:513–20. doi: 10.1002/gps.2746
319. García-Betances RI, Jiménez-Mixco V, Arredondo MT, Cabrera-Umpiérrez MF. Using virtual reality for cognitive training of the elderly. Am J Alzheimers Dis Other Demen. (2015) 30:49–54. doi: 10.1177/1533317514545866
320. Cogné M, Taillade M, N'Kaoua B, Tarruella A, Klinger E, Larrue F, et al. The contribution of virtual reality to the diagnosis of spatial navigation disorders and to the study of the role of navigational aids: a systematic literature review. Ann Phys Rehab Med. (2017) 60:164–76. doi: 10.1016/j.rehab.2015.12.004
321. Lange BS, Requejo P, Flynn SM, Rizzo AA, Valero-Cuevas FJ, Baker L, et al. The potential of virtual reality and gaming to assist successful aging with disability. Phys Med Rehabil Clin N Am. (2010) 21:339–56. doi: 10.1016/j.pmr.2009.12.007
322. Kalová E, Vlcek K, Jarolímová E, Bures J. Allothetic orientation and sequential ordering of places is impaired in early stages of Alzheimer's disease: corresponding results in real space tests and computer tests. Behav Brain Res. (2005) 159:175–86. doi: 10.1016/j.bbr.2004.10.016
323. Werner P, Rabinowitz S, Klinger E, Korczyn AD, Josman N. Use of the virtual action planning supermarket for the diagnosis of mild cognitive impairment: a preliminary study. Dement Geriatr Cogn Disord. (2009) 27:301–9. doi: 10.1159/000204915
324. Tarnanas I, Tsolaki M, Nef T, M Müri R, Mosimann UP. Can a novel computerized cognitive screening test provide additional information for early detection of Alzheimer's disease? Alzheimers Dement. (2014) 10:790–8. doi: 10.1016/j.jalz.2014.01.002
325. Zygouris S, Giakoumis D, Votis K, Doumpoulakis S, Ntovas K, Segkouli S, et al. Can a virtual reality cognitive training application fulfill a dual role? Using the virtual supermarket cognitive training application as a screening tool for mild cognitive impairment. J Alzheimers Dis. (2015) 44:1333–47. doi: 10.3233/JAD-141260
326. Sauzéon H, N'Kaoua B, Pala PA, Taillade M, Auriacombe S, Guitton P. Everyday-like memory for objects in ageing and Alzheimer's disease assessed in a visually complex environment: The role of executive functioning and episodic memory. J Neuropsychol. (2016) 10:33–58. doi: 10.1111/jnp.12055
327. Burgess N, Trinkler I, King J, Kennedy A, Cipolotti L. Impaired allocentric spatial memory underlying topographical disorientation. Rev Neurosci. (2006) 17:239–51. doi: 10.1515/REVNEURO.2006.17.1-2.239
328. Optale G, Urgesi C, Busato V, Marin S, Piron L, Priftis K, et al. Controlling memory impairment in elderly adults using virtual reality memory training: a randomized controlled pilot study. Neurorehabil Neural Repair (2010) 24:348–57. doi: 10.1177/1545968309353328
329. Weniger G, Ruhleder M, Lange C, Wolf S, Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia (2011) 49:518–27. doi: 10.1016/j.neuropsychologia.2010.12.031
330. Bellassen V, Iglói K, de Souza LC, Dubois B, Rondi-Reig L. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential Alzheimer's disease diagnosis. J Neurosci. (2012) 32:1942–52. doi: 10.1523/JNEUROSCI.4556-11.2012
331. Plancher G, Tirard A, Gyselinck V, Nicolas S, Piolino P. Using virtual reality to characterize episodic memory profiles in amnestic mild cognitive impairment and Alzheimer's disease: influence of active and passive encoding. Neuropsychologia (2012) 50:592–602. doi: 10.1016/j.neuropsychologia.2011.12.013
332. Jebara N, Orriols E, Zaoui M, Berthoz A, Piolino P. Effects of enactment in episodic memory: a pilot virtual reality study with young and elderly adults. Front Aging Neurosci. (2014) 6:338. doi: 10.3389/fnagi.2014.00338
333. Abichou K, La Corte V, Piolino P. Does virtual reality have a future for the study of episodic memory in aging? Geriatr Psychol Neuropsychiatr Vieil. (2017) 15:65–74. doi: 10.1684/pnv.2016.0648
334. Hort J, Laczó J, Vyhnálek M, Bojar M, Bures J, Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci USA. (2007) 104:4042–47. doi: 10.1073/pnas.0611314104
335. Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology (2008) 71:888–95. doi: 10.1212/01.wnl.0000326262.67613.fe
336. Davis R, Ohman JM, Weisbeck C. Salient cues and wayfinding in Alzheimer's disease within a virtual senior residence. Environm Behav. (2017) 49:1038–65. doi: 10.1177/0013916516677341
337. Hofmann M, Rösler A, Schwarz W, Müller-Spahn F, Kräuchi K, Hock C, et al. Interactive computer-training as a therapeutic tool in Alzheimer's disease. Compr Psychiatry (2003) 44:213–9. doi: 10.1016/S0010-440X(03)00006-3
338. Allain P, Foloppe DA, Besnard J, Yamaguchi T, Etcharry-Bouyx F, Le Gall D, et al. Detecting everyday action deficits in Alzheimer's disease using a nonimmersive virtual reality kitchen. J Int Neuropsychol Soc. (2014) 20:468–77. doi: 10.1017/S1355617714000344
339. Fernandez Montenegro JM, Argyriou V. Cognitive evaluation for the diagnosis of Alzheimer's disease based on turing test and virtual environments. Physiol Behav. (2017) 173:42–51. doi: 10.1016/j.physbeh.2017.01.034
340. Zucchella C, Sinforiani E, Tassorelli C, Cavallini E, Tost-Pardell D, Grau S, et al. Serious games for screening pre-dementia conditions: from virtuality to reality? A pilot project. Funct Neurol. (2014) 29:153–8.
341. Manera V, Petit PD, Derreumaux A, Orvieto I, Romagnoli M, Lyttle G, et al. ‘Kitchen and cooking,' a serious game for mild cognitive impairment and Alzheimer's disease: a pilot study. Front Aging Neurosci. (2015) 7:24. doi: 10.3389/fnagi.2015.00024
342. Robert PH, König A, Amieva H, Andrieu S, Bremond F, Bullock R, et al. Recommendations for the use of serious games in people with Alzheimer's Disease, related disorders and frailty. Front Aging Neurosci. (2014) 6:54. doi: 10.3389/fnagi.2014.00054
343. Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, et al. Video game training enhances cognitive control in older adults. Nature (2013) 501:97–101. doi: 10.1038/nature12486
344. Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, et al. Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PLoS ONE (2012) 7:e29676. doi: 10.1371/journal.pone.0029676
345. Oei AC, Patterson MD. Enhancing cognition with video games: a multiple game training study. PLoS ONE (2013) 8:e58546. doi: 10.1371/journal.pone.0058546
346. Powers KL, Brooks PJ, Aldrich NJ, Palladino MA, Alfieri L. Effects of video-game play on information processing: a meta-analytic investigation. Psychon Bull Rev. (2013) 20:1055–79. doi: 10.3758/s13423-013-0418-z
347. Bisoglio J, Michaels TI, Mervis JE, Ashinoff BK. Cognitive enhancement through action video game training: great expectations require greater evidence. Front Psychol. (2014) 5:136. doi: 10.3389/fpsyg.2014.00136
348. Ben-Sadoun G, Sacco G, Manera V, Bourgeois J, König A, Foulon P, et al. Physical and cognitive stimulation using an exergame in subjects with normal aging, mild and moderate cognitive impairment. J Alzheimers Dis. (2016) 53:1299–314. doi: 10.3233/JAD-160268
349. McCallum S, Boletsis C. Dementia Games: a literature review of dementia-related Serious Games. In: Ma M, Oliveira MF, Petersen S, and Hauge JB, editors. Serious Games Development and Applications-Lecture Notes in Computer Science. Berlin, Heidelberg: Springer Publishing, 15–27. doi: 10.1007/978-3-642-40790-1_2
350. Narme P. Benefits of game-based leisure activities in normal aging and dementia. Geriatr Psychol Neuropsychiatr Vieil. (2016) 14:420–8. doi: 10.1684/pnv.2016.0632
351. Ben-Sadoun G, Manera V, Alvarez J, Sacco G, Robert P. Recommendations for the design of serious games in neurodegenerative diseases in neurodegenerative diseases. Front Aging Neurosci. (2018) 10:13. doi: 10.3389/fnagi.2018.00013
352. Lee JH, Kim JH, Jhoo JH, Lee KU, Kim KW, Lee DY, et al. A telemedicine system as a care modality for dementia patients in Korea. Alzheimer Dis Assoc Disord. 14:94–101. doi: 10.1097/00002093-200004000-00007
353. Barton C, Morris R, Rothlind J, Yaffe K. Video-telemedicine in a memory disorders clinic: evaluation and management of rural elders with cognitive impairment. Telemed J E Health (2011) 17:789–93. doi: 10.1089/tmj.2011.0083
354. Brims L, Oliver K. Effectiveness of assistive technology in improving the safety of people with dementia: a systematic review and meta-analysis. Aging Ment Health (2018) 10:1–10. doi: 10.1080/13607863.2018.1455805
355. Barlow J, Singh D, Bayer S, Curry R. A systematic review of the benefits of home telecare for frail elderly people and those with long-term conditions. J Telemed Telecare (2007) 13:172–9. doi: 10.1258/135763307780908058
356. Botsis T, Hartvigsen G. Current status and future perspectives in telecare for elderly people suffering from chronic diseases. J Telemed Telecare (2008) 14:195–203. doi: 10.1258/jtt.2008.070905
357. Cheong CK, Lim KH, Jang JW, Jhoo JH. The effect of telemedicine on the duration of treatment in dementia patients. J Telemed Telecare (2015) 21: 214–8. doi: 10.1177/1357633X14566571
358. Tso JV, Farinpour R, Chui HC, Liu CY. A multidisciplinary model of dementia care in an underserved retirement community, made possible by telemedicine. Front Neurol. (2016) 7:225. doi: 10.3389/fneur.2016.00225
359. Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ (2000) 320:1517–20. doi: 10.1136/bmj.320.7248.1517
360. Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. (2000) 2:CD002098. doi: 10.1002/14651858.CD002098
361. Asghar I, Cang S, Yu H. Usability evaluation of assistive technologies through qualitative research focusing on people with mild dementia. Computers Human Behav. (2018) 79:192–201. doi: 10.1016/j.chb.2017.08.034
362. Brearly TW, Shura RD, Martindale SL, Lazowski RA, Luxton DD, Shenal BV, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev. (2017) 27:174–86. doi: 10.1007/s11065-017-9349-1
363. Czaja SJ, Rubert MP. Telecommunications technology as an aid to family caregivers of persons with dementia. Psychosom Med. (2002) 64: 469–76. doi: 10.1097/00006842-200205000-00011
364. Klimova B. Mobile phone apps in the management and assessment of mild cognitive impairment and/or mild-to-moderate dementia: an opinion article on recent finding. Front Human Neurosci. (2017) 11:461. doi: 10.3389/fnhum.2017.00461
365. Jelcic N, Agostini M, Meneghello F, Bussè C, Parise S, Galano A, et al. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer's disease: a pilot study. Clin Interv Aging (2014) 24:1605–11. doi: 10.2147/CIA.S68145
366. Williams K, Arthur A, Niedens M, Moushey L, Hutfles L. In-home monitoring support for dementia caregivers: a feasibility study. Clin Nurs Res. (2013) 22:139–50. doi: 10.1177/1054773812460545
367. Berwig M, Dichter MN, Albers B, Wermke K, Trutschel D, Seismann-Petersen S, et al. Feasibility and effectiveness of a telephone - based social support intervention for informal caregivers of people with dementia: study protocol of the TALKING TIME project. BMC Health Services Res. (2017) 17:1–11. doi: 10.1186/s12913-017-2231-2
368. Egan KJ, Pinto-Bruno ÁC, Bighelli I, Berg-Weger M, van Straten A, Albanese E, et al. Online training and support programs designed to improve mental health and reduce burden among caregivers of people with dementia: a systematic review. JAMDA (2018) 19:200–6. doi: 10.1016/j.jamda.2017.10.023
369. Godwin KM, Mills WL, Anderson JA, Kunik ME. Technology-driven interventions for caregivers of persons with dementia: a systematic review. Am J Alzheimers Dis Other Demen. (2013) 28:216–22 doi: 10.1177/1533317513481091
370. Len Kelly, Natalie St Pierre-Hansen. So many databases, such little clarity: searching the literature for the topic aboriginal. Can Fam Physician (2008) 54:1572–3.e5.
371. Cogo E, Sampson M, Ajiferuke I, Manheimer E, Campbell K, Daniel R, Moher D. Searching for controlled trials of complementary and alternative medicine: a comparison of 15 databases. Evid Based Complement Alternat Med. (2011) 2011:858246. doi: 10.1093/ecam/nep038
Keywords: Alzheimer's disease, dementia, non-pharmacological intervention, rehabilitation, caregiver, interprofessional team
Citation: Zucchella C, Sinforiani E, Tamburin S, Federico A, Mantovani E, Bernini S, Casale R and Bartolo M (2018) The Multidisciplinary Approach to Alzheimer's Disease and Dementia. A Narrative Review of Non-Pharmacological Treatment. Front. Neurol. 9:1058. doi: 10.3389/fneur.2018.01058
Received: 09 August 2018; Accepted: 21 November 2018;
Published: 13 December 2018.
Edited by:
Stefano F. Cappa, Istituto Universitario di Studi Superiori di Pavia (IUSS), ItalyReviewed by:
Elisabetta Farina, Fondazione Don Carlo Gnocchi Onlus (IRCCS), ItalyFederica Piras, Fondazione Santa Lucia (IRCCS), Italy
Simone Migliore, Casa Sollievo della Sofferenza (IRCCS), Italy
Copyright © 2018 Zucchella, Sinforiani, Tamburin, Federico, Mantovani, Bernini, Casale and Bartolo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Tamburin, c3RlZmFuby50YW1idXJpbkB1bml2ci5pdA==
Michelangelo Bartolo, YmFydG9sb21pY2hlbGFuZ2Vsb0BnbWFpbC5jb20=
 Chiara Zucchella
Chiara Zucchella Elena Sinforiani
Elena Sinforiani Stefano Tamburin
Stefano Tamburin Angela Federico
Angela Federico Elisa Mantovani
Elisa Mantovani Sara Bernini2
Sara Bernini2 Michelangelo Bartolo
Michelangelo Bartolo