
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 08 June 2018
Sec. Neurotrauma
Volume 9 - 2018 | https://doi.org/10.3389/fneur.2018.00436
This article is part of the Research Topic Phantom Sensation and Pain: Underlying Mechanisms and Innovative Treatments View all 14 articles
 Hannah G. Russell1*
Hannah G. Russell1* Jack W. Tsao1,2,3*
Jack W. Tsao1,2,3*Following the administration of brachial plexus anesthesia for right thumb carpometacarpal arthroplasty with ligament reconstruction, a 54-year-old woman with all limbs intact developed phantom limb sensations, including the misperception of the placement of her right arm and frozen limb sensations in her fingers. Immobility of her fingers in a stacked position was experienced for ~3.5 days after surgery, and she described her phantom sensations as the hand experiencing “tingling” and feeling “heavy.” While the onset of these phantom sensations occurred almost immediately after administration of brachial plexus anesthesia, they lasted for ~69 h after anesthesia wear off, suggesting that cortical effects from denervation resolves much more slowly than initial remapping, giving insight into the mechanisms behind phantom limb sensations that are often experienced by amputees.
Following major limb amputation nearly all amputees will experience phantom limb sensations (PLS), and ~80% will experience phantom limb pain (PLP) (1). PLS have been described as non-painful feelings of a specific shape, movement, position, or temperature of the missing limb, and can include itching and tingling, while PLP is a term used to describe any severely uncomfortable feelings in the phantom limb (2).
Although the etiology behind PLS/PLP remains unknown, one of the leading theories is cortical remapping (3). The cortical remapping theory, otherwise known as the maladaptive plasticity theory, suggests that PLS and PLP arise from the invasion of cortical regions neighboring the zone within the primary sensorimotor cortex previously controlling the amputated limb (3). A direct correlation between the amount of phantom limb pain and the amount of cortical remapping has been found (4). Other theories are a dissociation between vision and proprioception (5) and proprioceptive memories (6). The visual-proprioception dissociation theory suggests that the disconnect between what the amputated limb looked like and how the phantom limb is perceived by the amputee currently is the cause of PLP, and a decrease in the disconnect between the visualization and proprioception of the phantom limb results in a decrease in PLP (5). The theory of proprioceptive memories suggests that memories of the limb's position prior to amputation remaining embedded in the subconscious after amputation contribute to PLP and frozen limb sensations (6).
While studies have been conducted to show the direct relationship between the amount of cortical reorganization and PLS and PLP (4, 7), they have not been able to concretely describe the changes that occur in the peripheral and central nervous systems after amputation. Brachial plexus avulsion injury (BPAI) is a result of the detachment of the nerves of the arm from the nerve roots of the spinal cord, resulting in partial or complete paralysis of the arm (8, 9). BPAI is very common after traffic accidents, and 30–80% of patients with BPAI develop tingling, electric shock, and burning neuropathic pain (10) similar to the PLP and PLS experienced in amputees (2). Over 80% of patients experience this chronic pain following complete BPAI (10, 11). One report described the case of a patient who experienced PLP and PLS after BPAI, even though the patient had an intact limb (12). In another recent case report, a BPAI patient experienced hand-to-face remapping and PLP in his intact, but denervated limb, suggesting that cortical reorganization had occurred following the injury and that the etiology behind the PLP and PLS after BPAI is similar to that experienced in amputees (13). Dorsal root entry zone lesioning has been used to effectively treat pain in both PLP patients and BPAI patients (14), suggesting that the formation of pain after BPAI is similar to that after amputation. Similarly, mirror therapy, which is commonly used to help treat PLP in amputee patients (15–17), has been reported to successfully treat the chronic pain experienced in BPAI patients (13, 18), giving further credence to the suggestion that PLP and PLS after amputation is very similar to the sensations and pain felt after BPAI (13).
The PLP and PLS experienced by both amputee and BPAI patients can be debilitating (19, 20), and a lack of understanding of the mechanisms behind PLP and PLS hinders the ability to develop successful treatments for patients (2, 20). Brachial plexus anesthesia (BPA) is a temporary deafferentation that is often administered for routine upper-extremity surgeries (21) and might serve as a model for the permanent deafferentation experienced in amputees. Therefore, studying patients undergoing BPA has the potential to aid in the understanding of the roles of the peripheral and central nervous systems and in PLP and PLS.
A 54-year-old woman with all limbs intact received BPA in advance of right thumb carpometacarpal arthroplasty with ligament reconstruction. Immediately after BPA onset, she felt her right forearm and hand resting across her chest when it was hanging over the side of the gurney. After surgery, her right hand felt “heavy” with the fingers stacked vertically on top of each other, as shown in Figure 1. She began experiencing right thumb pain 14–16 h after the operation had been completed. However, the sensation of immobility of her 2nd through 5th digits in the stacked position lasted for ~3.5 days after surgery and 69 h after the anesthesia wore off. During this time, although the patient described the phantom sensations as being uncomfortable, she experienced no pain in the fingers. No nerve conduction studies were performed.
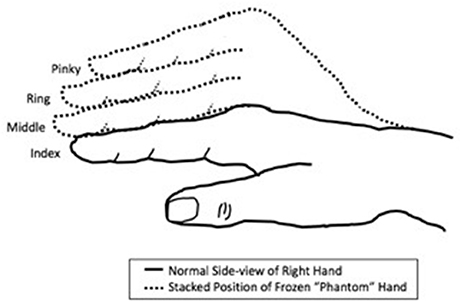
Figure 1. Schematic of frozen limb sensations experienced in the “phantom” hand of the patient. The fingers were experienced as being “stacked” on top of each other in a non-anatomic manner, rather than crossed over on each other.
This study was carried out in accordance with the recommendations of the University of Tennessee Health Science Center. The procedure discussed in this report was not part of a research study but rather routine clinical care. The subject gave written informed consent for publication of her clinical details in accordance with the Declaration of Helsinki.
The etiology of phantom limb phenomena after amputation remains unknown. Phantom sensations and pain have also been described by individuals with intact, but denervated limbs, such as BPAI (12, 13). One study found that administering BPA to amputees with PLP quickly and significantly reduced both the amount of cortical reorganization and the amount of PLP experienced by the amputees, showing a direct relationship between the amount of PLP and cortical remapping (7). While the induction of BPA was found to improve PLP in some, others experienced no improvement in pain levels (7). Additionally, it has been reported that spinal anesthesia induced PLP in an amputee who did not previously experience phantom pain (22). Spinal anesthesia has also been reported to exacerbate the effects of PLP (23). The emergence of PLS under anesthesia in these studies, in addition to what we report here, demonstrates that, although anesthesia has variable effects on reducing PLP, it can rapidly induce phantom limb phenomena in both amputees and persons with intact limbs.
BPA, routinely administered for surgical procedures on the upper limb, is a temporary nerve blockade, and could be considered to be a model for the permanent deafferentation experienced by amputees. Although the patient discussed in this report has all limbs intact, the PLS, similar to those experienced in amputees, emerged within 10 min following onset of the anesthetic effect. The patient's feeling of her arm being in a position in a different area than the actual anatomic position has been reported previously (24). In a study examining phantom sensations after the administration of BPA, it was found that 94% of 77 patients with intact limbs who received an adequate amount of BPA for surgery on the upper limb experienced a feeling of a “phantom” arm resting on his or her chest or abdomen even though it was on the operating table (24).
What is unique about this case is the lingering of apparent frozen limb sensations even after wear-off of the anesthesia. The term “frozen limb” is used to describe the sensations of immobility of a phantom limb in a specific position (25). Although the etiology of frozen limbs is unknown, there have been multiple reports of amputees with phantom limbs “frozen” in the same position the limb was in prior to amputation (26–28). It has been postulated that frozen limbs occur due to proprioceptive memories that store the position of the previously-intact limb prior to amputation (6). However, while our patient experienced similar sensations to those experienced in amputees with frozen phantom limbs, the positioning of her immobile “phantom” fingers after BPA was not the same position of her fingers before the BPA, suggesting that the frozen limb sensations experienced by this patient were of a different proprioceptive memory and indicating that such de novo sensations can arise under BPA and likely represent a different cortical connection pathway than that activated by the last known anatomic position. In addition, although abnormal frozen phantom limb positions have been reported before (29), the stacking position of phantom limbs described by this patient is novel. Of note, although the position in which this patient's fingers were immobilized was abnormal and not anatomical, it was not painful.
While the mechanisms behind phantom limb pain and sensations are unknown, because the patient experienced the sensation that her denervated arm was in a new position soon after the administration of BPA, this suggests that the onset of PLS can occur extremely rapidly after denervation. Rapid remapping has been found to occur within minutes of deafferentation in humans (30), and it has been found that 72% of amputees experience PLP within 8 days after amputation (31). However, since the frozen sensations persisted roughly 69 h after the nerve blockade terminated, it is possible that the return of somatosensory reorganization back to its original state is a much slower process than the initial remapping. The extended persistence of phantom sensations after the wear-off of anesthesia is an important new finding because it suggests that phantom sensations can continue even after nerve functioning is recovered and that the remapping process back to its original state is slower than the initial remapping after denervation. This novel information could give insight into the cortical remapping theory and how it relates to phantom sensations experienced by amputees.
The findings from this patient suggest that PLS experienced by patients with intact limbs receiving routine BPA for upper-extremity surgeries could be studied as a model to help better understand the mechanisms and time course behind how phantom limbs, sensations, and pain arise in both amputee and BPAI patients. Further studies should be conducted to analyze the amount of cortical remapping and PLS that occurs in the setting of BPA. In addition, the positioning of the limb before and after denervation should be studied to better understand frozen limb sensations. This information would improve our understanding of how PLS and PLP arise.
HR collected and analyzed the data and wrote the manuscript. JT supervised conduct of critical input into the drafting and editing of the manuscript and interpretation of the findings.
JT was supported by start-up funds from the University of Tennessee Health Science Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Collins KL, Mckean DL, Huff K, Tommerdahl M, Favorov OV, Waters RS, et al. Hand-to-face remapping but no differences in temporal discrimination observed on the intact hand following unilateral upper limb amputation. Front Neurol. (2017) 8:8. doi: 10.3389/fneur.2017.00008
2. Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. (2002) 1:182–9. doi: 10.1016/s1474-4422(02)00074-1
3. Flor H, Diers M, Andoh J. The neural basis of phantom limb pain. Trends Cogn Sci. (2013) 17:307–8. doi: 10.1016/j.tics.2013.04.007
4. Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumers N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature (1995) 375:482–4. doi: 10.1038/375482a0
5. Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc R Soc B Biol Sci. (1996) 263:377–86. doi: 10.1098/rspb.1996.0058
6. Anderson-Barnes VC, Mcauliffe C, Swanberg KM, Tsao JW. Phantom limb pain – A phenomenon of proprioceptive memory? Med Hypotheses (2009) 73:555–8. doi: 10.1016/j.mehy.2009.05.038
7. Birbaumer N, Lutzenberger W, Montoya P, Larbig W, Unertl K, Töpfner S, et al. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci. (1997) 17:5503–8.
8. Liu Y, Wang L, Meng C, Zhou Y, Lao J, Zhao X. A new model for the study of neuropathic pain after brachial plexus injury. Injury (2017) 48:253–61. doi: 10.1016/j.injury.2016.11.007
9. Wang L, Yuzhou L, Yingjie Z, Jie L, Xin Z. A new rat model of neuropathic pain: complete brachial plexus avulsion. Neurosci Lett. (2015) 589:52–6. doi: 10.1016/j.neulet.2015.01.033
10. Abdel-Aziz S, Ghaleb AH. Cervical spinal cord stimulation for the management of pain from brachial plexus avulsion. Pain Med. (2014) 15:712–4. doi: 10.1111/pme.12313
11. Kretschmer T, Ihle S, Antoniadis G, Seidel JA, Heinen C, Börm W, et al. Patient satisfaction and disability after brachial plexus surgery. Neurosurgery (2009) 65(Suppl. 4):A189–96. doi: 10.1227/01.neu.0000335646.31980.33
12. Shankar H, Hansen J, Thomas K. Phantom pain in a patient with brachial plexus avulsion injury. Pain Med. (2015) 16:777–81. doi: 10.1111/pme.12635
13. Tsao JW, Finn SB, Miller ME. Reversal of phantom pain and hand-to-face remapping after brachial plexus avulsion. Ann Clin Transl Neurol. (2016) 3:463–4. doi: 10.1002/acn3.316
14. Chivukula S, Tempel ZJ, Chen C, Shin SS, Gande AV, Moossy JJ. Spinal and nucleus caudalis dorsal root entry zone lesioning for chronic pain: efficacy and outcomes. World Neurosurg. (2015) 84:494–504. doi: 10.1016/j.wneu.2015.04.025
15. Griffin SC, Curran S, Chan AWY, Finn SB, Baker CI, Pasquina PF, et al. Trajectory of phantom limb pain relief using mirror therapy: retrospective analysis of two studies. Scand J Pain. (2017) 15:98–103. doi: 10.1016/j.sjpain.2017.01.007
16. Ramadugu S, Nagabushnam SC, Katuwal N, Chatterjee K. Intervention for phantom limb pain: a randomized single crossover study of mirror therapy. Indian J Psychiatry (2017) 59:457–64. doi: 10.4103/psychiatry.IndianJPsychiatry_259_16
17. Chan BL, Witt R, Charrow A, Magee A, Howard R, Pasquina PF, et al. Mirror therapy for phantom limb pain. N Engl J Med. (2007) 357:2206–7. doi: 10.1056/NEJMc071927
18. Agon F, Mateo S, Servajean V, Rode G. Regression of supernumerary upper limb phantom and pain after left complete plexus brachial avulsion using mirror therapy: a single case study. Ann Phys Rehabil Med. (2014) 57:e105. doi: 10.1016/j.rehab.2014.03.362
19. Trevelyan EG, Turner WA, Robinson N. Perceptions of phantom limb pain in lower limb amputees and its effect on quality of life: a qualitative study. Br J Pain (2015) 10:70–77. doi: 10.1177/2049463715590884
20. Teixeira MJ, Matheus Gomes Da S, Da P, Bina MT, Santos SN, Raicher I, et al. Neuropathic pain after brachial plexus avulsion - central and peripheral mechanisms. BMC Neurol. (2015) 15:73. doi: 10.1186/s12883-015-0329-x
21. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. (2013) 27:210–21. doi: 10.1002/ca.22254
22. Su C, Liu K, Wang Y. Midazolam as an effective drug for severe phantom limb pain in a patient after undergoing spinal anesthesia for two consecutive surgeries in the contralateral lower limb. Acta Anaesthesiol Taiwan. (2009) 47:32–5. doi: 10.1016/s1875-4597(09)60018-7
23. Shrestha G, Koirala S. Exacerbation of phantom limb pain following spinal anaesthesia: a case report and review of the literatures. Ain Shams J Anaesthesiol. (2016) 9:309. doi: 10.4103/1687-7934.182289
24. Melzack R, Bromage P. Experimental phantom limbs. Exp Neurol. (1973) 39:261–9. doi: 10.1016/0014-4886(73)90228-8
25. Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Arch Neurol. (2000) 57:317. doi: 10.1001/archneur.57.3.317
26. Giummarra MJ, Gibson SJ, Georgiou-Karistianis N, Bradshaw JL. Central mechanisms in phantom limb perception: the past, present and future. Brain Res Rev. (2007) 54:219–32. doi: 10.1016/j.brainresrev.2007.01.009
27. Fraser C. Fact and fiction: a clarification of phantom limb phenomena. Br J Occup Ther. (2002) 65:256–60. doi: 10.1177/030802260206500602
28. Katz J, Melzack R. Pain ‘memories’ in phantom limbs: review and clinical observations. Pain (1990) 43:319–36. doi: 10.1016/0304-3959(90)90029-d
29. Ramachandran V. The perception of phantom limbs. The D. O. Hebb lecture. Brain (1998) 121:1603–30. doi: 10.1093/brain/121.9.1603
30. Moore CI, Stern CE, Dunbar C, Kostyk SK, Gehi A, Corkin S. Referred phantom sensations and cortical reorganization after spinal cord injury in humans. Proc Natl Acad Sci USA. (2000) 97:14703–8. doi: 10.1073/pnas.250348997
Keywords: frozen limb, phantom limb sensation, phantom limb pain, cortical remapping, brachial plexus injury, brachial plexus anesthesia, amputation, cortical reorganization
Citation: Russell HG and Tsao JW (2018) Phantom Sensations Following Brachial Plexus Nerve Block: A Case Report. Front. Neurol. 9:436. doi: 10.3389/fneur.2018.00436
Received: 27 March 2018; Accepted: 24 May 2018;
Published: 08 June 2018.
Edited by:
Peter John Shortland, Western Sydney University, AustraliaReviewed by:
Hariharan Shankar, Medical College of Wisconsin, United StatesCopyright © 2018 Russell and Tsao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah G. Russell, aHJ1c3NlMTJAdXRoc2MuZWR1
Jack W. Tsao, anRzYW9AdXRoc2MuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.