- 1Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
- 2Mayo Clinic, Rochester, MN, United States
Cerebral ischemia and stroke are increasing in prevalence and are among the leading causes of morbidity and mortality in both developed and developing countries. Despite the progress in endovascular treatment, ischemia/reperfusion (IR) injury is an important contributor to post-surgical mortality and morbidity affecting a wide range of neurointerventional procedures. However, pharmacological recruitment of effective cerebral protective signaling has been largely disappointing to date. In remote ischemic conditioning (RIC), repetitive transient mechanical obstruction of vessels at a limb remote from the IR injury site protects vital organs from IR injury and confers infarction size reduction following prolonged arterial occlusion. Results of pharmacologic agents appear to be species specific, while RIC is based on the neuroprotective influences of phosphorylated protein kinase B, signaling proteins, nitric oxide, and transcriptional activators, the benefits of which have been confirmed in many species. Inducing RIC protection in patients undergoing cerebral vascular surgery or those who are at high risk of brain injury has been the subject of research and has been enacted in clinical settings. Its simplicity and non-invasive nature, as well as the flexibility of the timing of RIC stimulus, also makes it feasible to apply alongside neurointerventional procedures. Furthermore, despite nonuniform RIC protocols, emerging literature demonstrates improved clinical outcomes. The aims of this article are to summarize the potential mechanisms underlying different forms of conditioning, to explore the current translation of this paradigm from laboratory to neurovascular diseases, and to outline applications for patient care.
Introduction
Recent studies show that ischemia/reperfusion (IR) injury is an important contributor to post-surgical mortality and morbidity affecting those undergoing a wide range of neurointerventional procedures (1, 2). Effective protection attenuating IR injury is therefore an important factor in improving patient prognosis. However, pharmacological strategy to protect the brain against IR injury has been largely disappointing to date.
Ischemic conditioning, a powerful non-pharmacological strategy for reducing IR injury, was recognized in animal models in 1986 (3), though this innate cytoprotective mechanism in the brain was noted as early as the 1940s (4). By 1996, its use extended to organs remote from the heart in the form of remote ischemic conditioning (RIC) (5). Today, RIC is a remarkably simple and low-cost intervention that employs repetitive inflation and deflation of a standard arm or leg blood pressure cuff and constitutes a highly effective therapy for protecting vital organs from IR injury. Base on its simplicity, accessibility, and non-invasive nature, RIC has the potential for treatment in a wide variety of conditions including acute, subacute, and chronic neurological diseases with an ischemic basis, such as acute ischemic stroke (AIS) (6).
The aims of this article are to summarize the potential mechanisms underlying different forms of conditioning, to explore the current translation of this paradigm from laboratory to neurovascular diseases, and to outline applications for patient care.
RIC Protocol
The most effective RIC protocol has yet to be fully defined. Currently, the most commonly employed technique across clinical settings is three to four repetitions of 5-min inflation/deflation using a standard blood pressure cuff. Tourniquet pressure should be above the systolic pressure to ensure arterial occlusion. Its localization (arm versus thigh) does not affect cytoprotection (7). However, more than eight ischemic cycles or cycles >10 min did not lead to better results and possibly even increased injury in mice (8). If RIC were considered in the manner one would analyze a therapeutic drug, its exact dosage, pharmacokinetics, and pharmacodynamics would remain largely unclear.
Experimental and clinical evidence suggests that RIC, as well as other preconditioning stimuli, activates at least two distinct time frames of protection against IR injury of brain and heart. The time window of brain protection by preconditioning has also been demonstrated in vitro model (9). The initial time window of brain protection is short lasting as a result of changes in ion channel permeabilities, protein phosphorylation, and release of several mediators [including adenosine and bradykinin (BK)]. It occurs immediately after the RIC stimulus and lasts 2 h (10). The delayed form of protection, referred to as the second window of protection (SWOP), follows 12–24 h later, and lasts 48–72 h (as shown across multiple species) (11). SWOP may be triggered by the reactive oxygen species (ROS) and mediated by modulated inflammatory response, improved endothelial function, and activation of gene expression (such as HIF, toll-like receptor caspases, and heat shock proteins) (Figure 1) (12, 13). Various clinical studies have demonstrated the SWOP in RIC, although all the studies are in cardiac surgery settings (14).
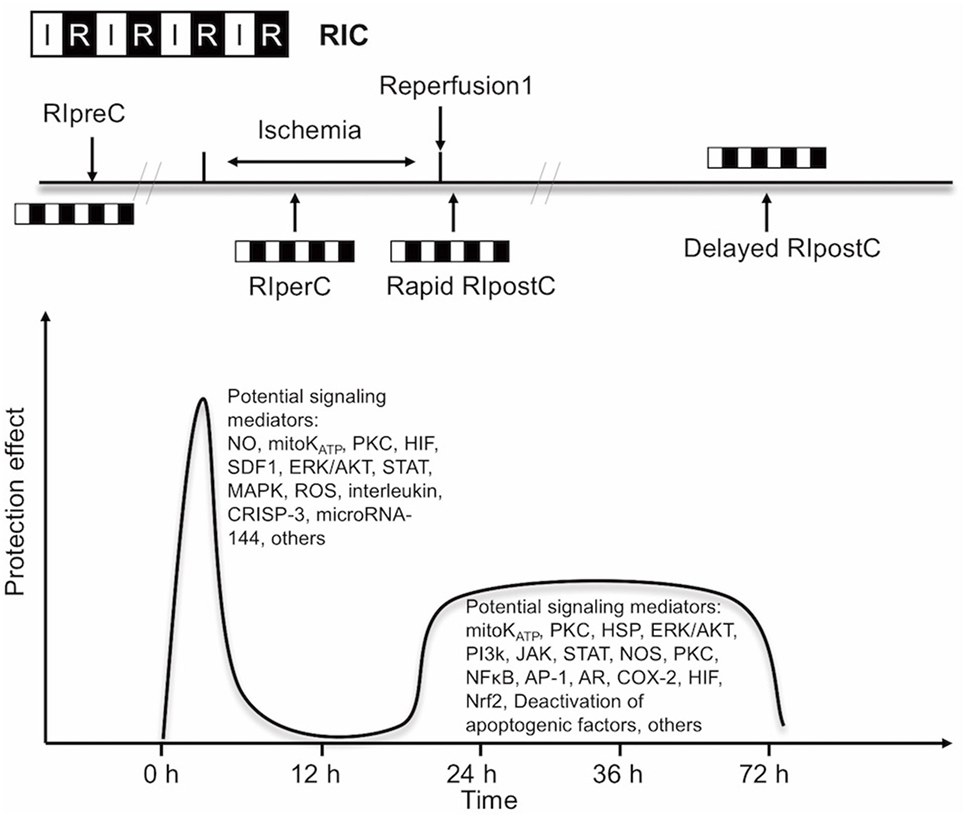
Figure 1. Simplified scheme and possible mechanisms of the temporal nature of the two windows of remote ischemic conditioning (RIC). Abbreviations: AR, aldose reductase; AP-1, activator protein 1; COX-2, cyclooxygenase-2; CRISP-3, cysteine-rich secretory protein 3; NOS, nitric oxide synthase; ERK/AKT, extracellular signal regulated kinase/protein kinase B; HIF, hypoxia-inducible factor; HSP, heat shock protein; JAK, Janus kinase; KATP, ATP-sensitive potassium channel; MAPK, mitogen-activated protein kinase; Mito, mitochondria; NFκB, nuclear factor κB; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor; PI3k, phosphoinositide-3 kinase; PKC, protein kinase C; ROS, reactive oxygen species; SDF1, stromal cell-derived factor 1; STAT, signal transducer and activator of transcription.
The concept of RIC has now expanded into three temporal variants after its initial application: remote ischemic preconditioning (RIPreC), perconditioning (RIPerC), and postconditioning (RIPostC) (15–17). Brain mechanisms are independent of the timing of conditioning strategies (pre-, per-, postconditioning), and their effects have a great deal of overlap.
RIC Mechanisms
The mechanisms underlying RIC include neurovascular protection, anti-inflammatory action, reduced excitotoxicity, and metabolic protection, which are associated with influences on mitochondria, circulating inflammatory cells, or transcriptional upregulation of protective pathways (Figure 2) (18, 19). There is a consensus that the infarct-sparing effect of all forms of ischemic conditioning involves the upregulation of several signal transduction cascades, which serve to stabilize the mitochondria (20).
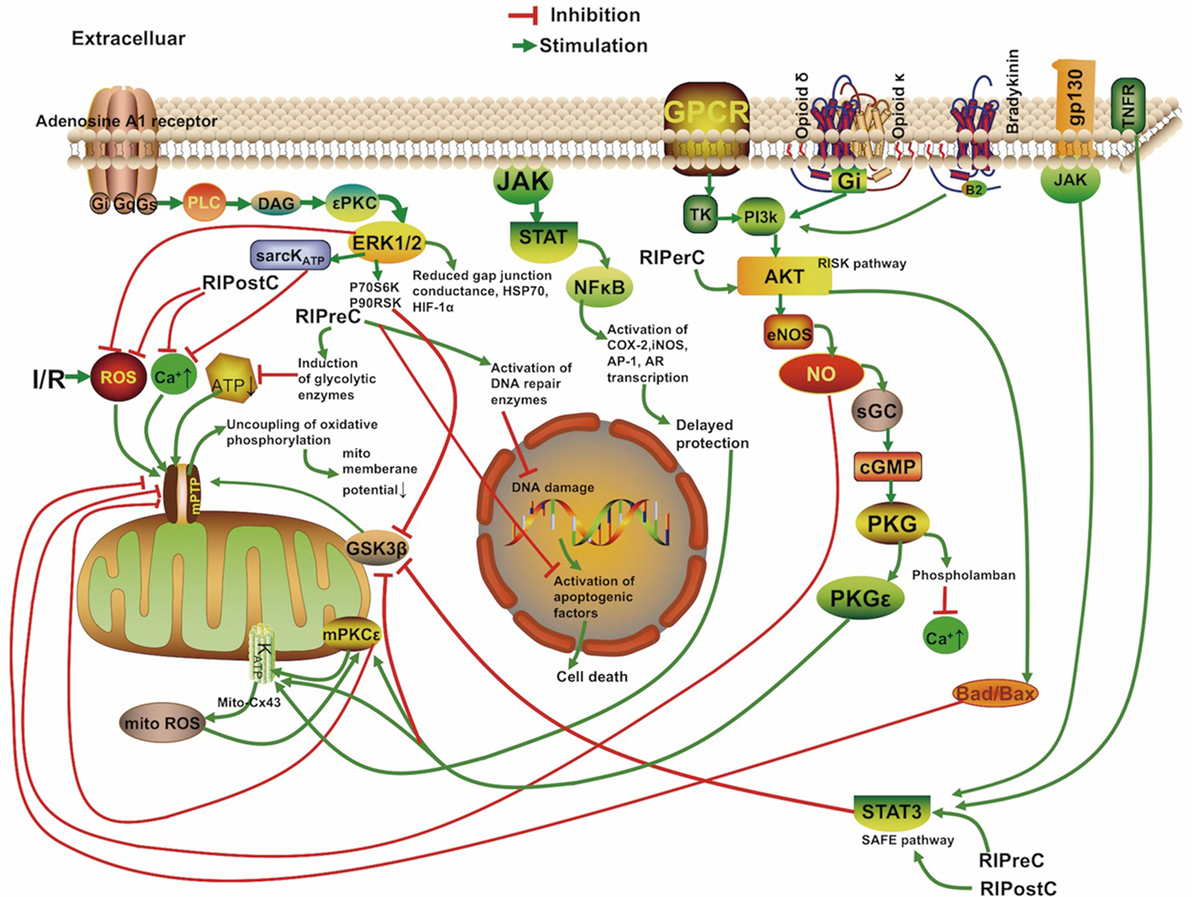
Figure 2. Overview of the proposed signaling cascades recruited in the setting of remote ischemic conditioning based on available data. Abbreviations: Akt, protein kinase B; AR, aldose reductase; AP-1, activator protein 1; cGMP, cyclic guanosine monophosphate; COX-2, cyclooxygenase-2; Cx 43, connexin 43; DAG, diacylglycerol; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal regulated kinase; Gs/Gi/q, stimulatory/inhibitory G protein; GPCR, G protein-coupled receptor; gp130, glycoprotein 130; GSK3β, glycogen synthase kinase 3 β; HIF-1α, hypoxia-inducible factor 1α; HSP, heat shock protein; IR, ischemia/reperfusion; iNOS, inducible nitric oxide synthase; JAK, Janus kinase; KATP, ATP-sensitive potassium channel; mPTP, mitochondrial permeability transition pore; Mito, mitochondria; NFκB, nuclear factor κB; NO, nitric oxide; P70S6K, p70 ribosomal S6 protein kinase; P90RSK, 90 ribosomal S6 kinase; PI3k, phosphoinositide-3 kinase; PKC, protein kinase C; PKG, protein kinase G; PLC, phospholipase C; RISK, reperfusion injury salvage kinase pathway; ROS, reactive oxygen species; sarcKATP, sarcolemmal potassium channels; sGC, soluble guanylate cyclase; SAFE, survivor activating factor enhancement; STAT, signal transducer and activator of transcription; TK, tyrosine kinase; TNFR, tumor necrosis factor receptor.
Although neurons are assumed to be the cellular target of cerebral conditioning, ischemic tolerance occurring at the level of endothelial and smooth muscle cells contributes to neuronal protection (21). RIPreC was first shown to protect against endothelial injury during IR in humans in 2002 (22), and vasodilation was shown to be better preserved in a preconditioned brain (23). Trans-cranial Doppler measurements of patients undergoing RIC indicated transient cerebral vasodilation over the duration of conditioning (24). All temporal variants of RIC have been proven to prolong protein kinase B (Akt) activity in the endothelium, which increases nitric oxide (NO) production through improved endothelial nitric oxide synthase (eNOS) activity and helps to maintain vascular homeostasis (25–27).
Cell-Level Mechanisms Underlying RIPreC
The mechanism of brain preconditioning involves a shift in the neuronal excitotoxic/inhibitory balance and a reduction in inflammatory sequelae. Several intracellular signaling pathways and various intercellular mediators and kinases have been identified in tissue protection by RIC. The protective reperfusion injury salvage kinase pathway (RISK) including the phosphoinositide-3 kinase/Akt signaling cascade and the pro-survival survivor activating factor enhancement (SAFE) pathway including the Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT)3 signaling cascade are the most important pathways involved in ischemia cytoprotection and eNOS activation (28, 29). And the SAFE pathway was shown to lead to tissue protection independently of the RISK pathway (28). Phosphorylation of JAK2, STAT3, STAT5, Akt, and other signaling complexes may ultimately reduce apoptosis, ROS production, and inflammation (30, 31). In addition, STAT3 located in the matrix of subsarcolemmal and interfibrillar mitochondria also serves to improve mitochondrial respiration and attenuate mPTP opening, and ROS formation (32, 33). And Akt activation, in interaction with STAT3 activation, was mandatory for ischemic preconditioning (34). The activation of the STATs also results in transcriptional upregulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2, known distal mediators/effectors of protection (35, 36). There are direct evidences for STATs involvement in patients with RIC (37, 38). A recent study demonstrated that RIPreC could enhance the phosphorylated Akt, STAT3, STAT5, and eNOS expression levels and activating the pro-survival signaling pathway in humans (39). In addition, previous reports showed that NO, hypoxia-inducible factor (HIFs), erythropoietin, free radicals, BK, adenosine, opioids, activation of the ATP-sensitive potassium (KATP) channel, and norepinephrine all have roles in RIPreC (40–42). One of the key regulators of the genomic response after RIPreC is the transcriptional activator HIF. HIF-1 activation is neuroprotective, and a neuron-specific HIF-1α deletion demonstrated exacerbation of brain injury in an experimental model of stroke (43). The growth of new vessels stimulated by the VEGF and erythropoietin cytokines are also regulated by HIF-1 (43). Some researchers believe that expression of HIF-1α—but not phosphorylation of extracellular signal regulated kinase 1/2 (ERK1/2), Akt, or STAT5—is required for RIPreC (44). Inflammatory mediators, such as interleukin-6, tumor necrosis factor (TNF), intracellular adhesion molecule, matrix metalloproteinase 9, and C-reactive protein are downregulated through RIPreC (45).
Microarrays indicate that preconditioning stimulates a genomic reprogramming of cells that confers cytoprotection, recovery, neurogenesis, and angiogenesis (46). In particular, genes regulating cell metabolism, signal transport, growth factors, ion channels, metallothionins, or cell cycle/apoptosis are selectively upregulated (46, 47). The microRNA for glutamate receptor, ionotropic delta 2, was reported to be downregulated in the mouse brain after RIPreC (46).
Using a global model of ischemia preconditioning in gerbils, short stimuli were shown to induce an increase in dendritic spine density of vulnerable hippocampal CA1 neurons 3 days after reperfusion, comparable to the SWOP of the neuroprotective effect induced by preconditioning (48). Preconditioning in immature brains also increases the concentration of astrocytic glycogen, which is neuroprotective, and delays energy depletion caused by ischemia (49). Moncada found that preconditioning increases expression of cyclooxygenase 1 and prostacyclin synthase; these enzymes act successively to produce prostacyclin, which inhibits platelet aggregation and vasoconstriction (50). Røpcke et al. also demonstrated that RIPreC reduces arterial thrombus formation and embolization in rats (51). Several clinical trials are underway to test the safety and efficacy of RIPreC for protecting the brain against anticipated damage (52, 53), and its procedural simplicity makes it an excellent candidate for study in future clinical trials.
Corroborating Evidence Based on Transient Ischemic Attack (TIA) Neuroprotection
Patients who suffer a TIA show better clinical outcomes in subsequent strokes compared to those who suffer similar strokes without first having suffered a TIA, which may be due to activation of the same neuroprotective pathways as RIPreC (54). Schaller found that stroke patients showed more favorable neurological outcomes when the preceding TIAs occurred 1–7 days prior to stroke (55). Similarly, in a German study comprised of 7,611 patients, TIA was associated with reduced stroke severity (56). Recent data also suggests that peripheral vascular disease with chronic limb hypoperfusion was associated with less disability and lower mortality in AIS (57). In contrast to the findings, Kim et al. reported that a low ankle-brachial blood pressure index (ABI) (<0.9) was associated with an increased risk of poor functional outcome in patients with acute cerebral infarction (odds ratio 3.452, P < 0.001) than patients without low ABI (58). However, in this study, the patients with a low ABI were more likely to have a high NIHSS score at baseline. Besides, the patients with a low ABI more often had diabetes mellitus (44.9 versus 29.5%, P = 0.007). Diabetes mellitus itself may attenuate the effectiveness of RIC (59). In future trials, subgroup analysis of patients with comorbidities such as diabetes is needed.
Alternative Method: RIPerC
Remote ischemic preconditioning may be not practical in acute clinical settings because it must be initiated before the ischemic event. The neuroprotective efficacy of RIPerC has been proven in a number of animal models (10, 14, 25, 60). Furthermore, mild to moderate hemorrhage after tissue plasminogen activator (tPA) was attenuated when RIPerC therapy was performed 2 h before tPA infusion, making it an excellent candidate for combination therapy with tPA (61). Clinical MRI evidence suggests RIPerC treatment induces an immediate neuroprotective effect by reducing cytotoxic cerebral edema when perfusion is restored (62). RIPerC also upregulates mRNA expression of eNOS about 10-fold in the blood vessels, from the site of conditioning, and increases the concentration of NO in plasma (63).
The Reasoning Behind RIPostC
Remote ischemic postconditioning can be used in both elective and acute settings. Evidence from experimental and trial studies supports an additive protective effect of combined RIPreC and postconditioning, as reperfusion itself is associated with cell injury and cell death in its very early moments (64–66). Postconditioning likely mitigates damage from sudden reperfusion, plausibly blocking production of ROS and reactive nitrogen species and thus attenuating reperfusion-induced brain injury (67), or possibly by attenuating endoplasmic reticulum stress response-induced apoptosis (68). The pro-survival protein kinases extracellular signal-regulated kinases (ERK), p38 mitogen-activated protein kinase (MAPK), and Akt showed prolonged phosphorylation in the cortex of postconditioned rats (69). Protection from RIPostC is blocked in animal models by removing the influence of STAT3 and mitochondrial KATP channels, as well as TNF α (33, 70).
Mitochondria and RIC
Mitochondria play critical roles in all pathways triggered by RIC. RIC causes recruitment of ligands such as adenosine and opioids to Gprotein-coupled receptors. This action leads to the activation of signaling protein kinases and the opening of mitochondrial KATP channels, which subsequently prevents the opening of the mitochondrial permeability transition pore (mPTP) after the first minutes of reperfusion whereby tissue protection is activated (71–73).
The role of signal transduction pathways during RIC has predominately been demonstrated in the heart. However, the presence of STATs in the mitochondria was confirmed in a number of organs including heart, kidney, and brain (74). A few reports in the literature have suggested the involvement of MAPKs, Akt, HIF-1α, and STATs in mitochondrial neuroprotection following preconditioning (30, 75–77). STATs have been shown to regulate mitochondrial function by preserving efficiency of electron transport chain complexes (35, 78).
Transfer of the Cerebral Protective Stimulus
In RIC, transient, reversible episodes of ischemia with reperfusion in the stimulus location render remote tissues and target organs resistant to IR injury. At present, transfer of the cerebral protective stimulus is not well understood, though studies have shown it to act through multiple pathways (15).
Humoral Pathways
The humoral pathway has been most extensively studied. Some studies have identified specific factors, such as stromal cell-derived factor-1 α, interleukin, nitrite, cysteine-rich secretory protein 3, and microRNA-144 as possible candidate transfer factors (51, 79, 80). Ueno et al. suggest that RIPreC transiently increases plasma VEGF levels by downregulating miR-762 and miR-3072-5p in CD34-positive bone marrow cells, leading to protection against organ ischemia (81). In a recent human study, only STAT5 signaling was identified to be associated with RIPreC humoral transfer (38). Endothelial cells were suggested as the target for RIPreC-released mediators (82). Finally, Dong et al. suggest that humoral factors, rather than the neural pathway, play an important role in the formation of the tolerance against spinal cord ischemia by limb RIPreC (83).
Nerve Pathway
Occlusion with a tourniquet on the arm can stimulate the release of autacoids that activate an afferent neural pathway and/or cause the release of NO from blood vessels (80, 84, 85). Transection of the femoral nerve or spinal cord can abrogate the effect of RIC in rabbits (86). The dependence of remote conditioning on intact neural pathways also may explain why its effects seem to be attenuated in patients with neuropathy (87).
Mastitskaya et al.’s study used viral gene transfer and optogenetics to show that the dorsal motor neurons of the vagus in the brainstem were required for RIPreC to have a cardioprotective effect, and that stimulation of these neurons mimicked the effect of RIPreC (88). Interestingly, femoral nerve or sciatic nerve resection alone only partially abolished the infarct-limiting effect of RIPreC in mice, suggesting the influence of both neural and humoral pathways (89).
Inflammatory Pathway
Remote ischemic preconditioning has been shown to have a systemic anti-inflammatory influence through upregulation of cytoprotective genes and suppression of proinflammatory genes in immune cells (90). Circulating monocytes and neutrophil infiltration play a key role in IR injury. RIPreC downregulated the expression of a broad spectrum of proinflammatory genes in circulating monocytes. For circulating neutrophil, RIPreC activated signal pathways in neutrophils modulating the release of proinflammatory cytokines and the expression of adhesion markers. Consequently, RIPreC negatively affected their function (18). Microarray analysis showed that reduction of inflammatory gene expression takes place within 15 min of RIC and at 24 h after conditioning in humans (18). Humoral, neural, and anti-inflammatory pathways probably interact with each other and are not necessarily mutually exclusive (91).
Clinical Applications
Larger trials of RIC, especially for cardioprotection but also for kidney and neuroprotection, have largely supported the consensus of RIC’s lack of harmful influence and reduction of IR injury when established protocols are used and in the absence of propofol (6, 92). Several clinical studies are also underway to expand the literature on neuroprotection specifically (52, 53).
RIC in AIS
Over 10 million people worldwide suffer an AIS each year (93), yet few neuroprotective treatments against IR injury have been proven effective: clinical trials of more than 50 compounds for treatment of IR injury secondary to AIS all showed negative results. Mechanical thrombectomy has been widely accepted as an effective treatment for AIS. Despite the sharp increase in recanalization rate with current thrombectomy devices compared with tPA, cerebral reperfusion after endovascular embolectomy and/or tPA may cause deterioration of penumbra, disruption of the blood–brain barrier, cerebral edema, and intracerebral hemorrhage (94). Thus, there is an urgent need for effective forms of secondary prevention after the acute phase of AIS intervention, for which RIC is an excellent candidate.
In a model of autologous thromboembolic clots, RIPerC has been effective in mice models when applied 2 h after stroke onset with or without late (4 h after stroke onset) intravenous (IV) tPA (25). Hahn et al. show that infarct size in a rat AIS model was reduced by RIPreC but even further by RIPerC (17). In an analogous study, RIPerC therapy also improved the cerebral blood flow (CBF) and the hemorrhage, edema, and neurobehavioral outcomes significantly on top of the reduction in infarction size compared to IV-tPA alone at 4 h post-stroke (95). Hess et al. show optimal results occurred when RIPerC was started as soon as possible after stroke onset and RIPostC was administered two to three times during first day and repeated daily during the following week (96).
Trials in AIS
Several trials studying the effect of RIC on AIS patient outcomes have shown benefits when RIC is administered during ischemia. Hougaard et al. (62) found an overall reduction in the risk of infarction for tissue subjected to pre-hospital RIPerC at 1 month but the study was not powered to show effect in clinical outcome at 3 months. The Remote Ischemic Conditioning After Stroke Trial study (64), a blinded placebo-controlled trial of RIC in AIS patients, showed improved clinical outcome in the RIC group. Compared with sham, 90-day NIHSS score was significantly lower in the RIC group (1 versus 3, P = 0.04). RIC also increased plasma heat shock protein 27 (HSP27, P < 0.05) level in the study, compared with control. The investigators suggested that the neuroprotective effects may be mediated through phosphorylated HSP27. A research group in Denmark administered RIPerC during transportation in the ambulance as a pretreatment to IV alteplase. Overall, the study showed RIPerC to be safe and feasible in the setting of AIS, with the likely benefit of greater tissue survival in the penumbra than the control (62). Another randomized trial also found that high prestroke physical activity is associated with reduced infarct size after IV tPA treatment only in patients receiving adjuvant RIPerC (97). While a French multicentric trial of RIC for ischemic stroke within 6 h of symptom onset is currently underway. Results of this trial have not yet been reported (98).
Other Clinical Applications for RIC
Intracranial Atherosclerotic Stenosis
Endovascular treatment of ICAS carries a risk of intraoperative and postoperative ischemic events, allowing for non-urgent consideration of protection against IR injury. RIPreC alone was recently found to significantly decrease the incidence of stroke in patients with ICAS (26.7 versus 7.9%), increase CBF, and protect against ischemia-related neurological morbidity (99). Meng et al. (99) found that RIC could improve the cerebral circulation in patients with intracranial arterial stenosis. While RIC was also reported to be effective in cerebral small vessel disease (SVD) related cognitive impairment. Wang et al. (100) randomly assigned 30 patients with mild cognitive impairment caused by cerebral SVD to receive RIC (by the method used by Meng et al. twice daily for 12 months) or to receive a sham intervention; the patients who received RIC had a higher reduction of white matter hyperintensities volume (−2.632 versus −0.935 cm3, P = 0.049), with a better visuospatial and executive ability at 1 year (0.639 versus 0.191, P = 0.048). Meanwhile, in a bilateral carotid artery stenosis mouse model with vascular cognitive impairment, RIC was effective in improving cognition and CBF, attenuating tissue damage (101).
Carotid Artery Stenting (CAS)
Carotid artery stenting is a selective procedure used to tread carotid artery stenosis, RIC has been evaluated in surgical brain injury paradigms such as hypothermic circulatory arrest and following carotid endarterectomy. Though a pilot study of 70 patients who received RIC showed no statistically significant improvement in neurological outcome (53), the first proof-of-concept trial of RIC before CAS found that RIC can ameliorate the complications of distal thromboembolization (102). This is the first study to show effect of RIC given before CAS on ischemic lesions size and number assessed by MRI. The authors reported that the incidence of new ischemic lesions were lower in patients who received RIC than in patients who did not (15.87 versus 36.51%, P < 0.01), with smaller infarct volume (0.06 versus 0.17 ml).
Subarachnoid Hemorrhage (SAH) From Intracranial Aneurysm
The leading cause of SAH is rupture of an intracranial aneurysm, accounting for roughly 80% of cases. Even if embolization of the ruptured intracranial aneurysm is successful, delayed cerebral ischemia may occur (103). Preconditioning before the induction of SAH in rats was shown to improve vasospasm, reduce cerebral inflammatory cytokines, attenuate tissue hypoxia, and prevent neurological deterioration (51). Some authors believe that SAH is a particularly feasible clinical setting to evaluate human response because RIPreC activates multiple pathways that have been invoked in SAH (104).
Laiwalla et al. reported a matched cohort analysis of RIPostC for patients with aSAH.
Remote ischemic conditioning was independently associated with good outcomes and lower incidence of delayed cerebral ischemia (105). A longitudinal human pilot study in aSAH patients undergoing RIC found coordinated expression and methylation of a small set of key genes in mitotic cell cycle, defense, and inflammatory responses after RIC (106). Other human studies have confirmed the safety and feasibility of lower limb RIC in individuals with aSAH in which no patient experienced delayed cerebral ischemia (51).
Limitations of RIC
Remote ischemic conditioning can be initiated during pre-hospital transport, through which the patient would receive benefit during triage, imaging, and reperfusion therapy by IV or endovascular methods with low known risk of adverse effects. In the study by Botker et al. (107), the RIC stimulus was initiated in ambulance during transfer for angioplasty, resulting in increased myocardial salvage (36%). RIC intervention can also be delivered on immediate arrival at interventional center when ambulance transit times are short, and even at the onset of reperfusion (108). However, most of the current trials are studies mainly focusing on cardioprotective effects. These studies provided further opportunities to investigate the neuroprotective effect of limb RIC applied in an ambulance, helicopter, or emergency departments, in advance of interventional reperfusion. Moreover, preclinical trial in murine thromboembolic stroke model and pilot trials suggest that RIC can be combined with recombinant tissue plasminogen activator in the pre-hospital setting to increase the protective effect. In the Denmark trial, patients were randomly assigned to receive or not receive RIPerC treatment, and RIPerC was completed during transportation in the ambulance before a final diagnosis of ischemic stroke (62, 109). However, it has been reported that about 3% patients will not able to tolerate tourniquet inflation on their arm (94). Furthermore, RIC would also predetermine the arm to be used for arterial and venous access. Other considerations include the influence on obtaining endovascular access during vascular intervention (110). Finally, the time window and the primary RIC protocol in neuroprotection are still not fully determined.
In two large trials, the benefits from RIC were not confirmed in patients undergoing cardiovascular surgery. However, a point of critique in their studies is that the use of propofol anesthesia in most (111) or all patients (112). The second problem is the inclusion of many patients who also underwent valve surgery. RIC protects only from IR injury and not from traumatic injury at the target organ. Propofol is known to disrupt RIC (113–115). Neither RIC cardioprotection nor STAT5 activation were observed under propofol anesthesia (115). In clinical studies reporting protective effects of RIC, the RIC procedure was either completed without anesthetic intervention or completed during anesthesia induction with anesthetics other than propofol (116). The use of propofol has been suggested to be avoided in future studies on RIC (117). And the efficacy of RIC could also be influenced by many other variables including conditioning protocol, concomitant medications, and coexisting conditions (118–121).
Most animal studies have been performed in reductionist approaches which lack risk factors and comorbidities (122). Additional sources of variation should be considered in future studies, including the choice of anesthesia, patient’s comorbidities and comedications, and the temporal aspects of the remote conditioning algorithm (122). Caution should be exercised when assessing outcomes because patient selection and trial design may affect outcomes.
Conclusion
Remote ischemic conditioning is protective against reperfusion injury, and further research will expand our knowledge in the field of cerebral vascular diseases. Its simplicity and non-invasive nature, as well as the flexibility of the timing of RIC stimulus, make it feasible to apply alongside neurointerventional procedures. Precise knowledge of its optimal dosage and timing of administration is yet to be found. RIC has promising but understudied potential neuroprotective influences on patients undergoing endovascular treatments who have risks of IR injury. Further validation using well-designed randomized controlled trials is necessary to document the efficacy of differing RIC protocols across a range of cerebrovascular diseases.
Author Contributions
DK: concept, design, and development of the study; MHL: development of the study; GZ: acquisition and analysis of the data, writing of the article; GT: article writing; HTL: development of the study; RK: critical review of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by grants from National Natural Science Foundation of China, grant no. 81370041, 81471760, 81671655 and National Institutes of Health, grant no. NS076491.
References
1. Anzell AR, Maizy R, Przyklenk K, Sanderson TH. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol (2018) 55:2547–64. doi:10.1007/s12035-017-0503-9
2. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med (2007) 357:1121–35. doi:10.1056/NEJMra071667
3. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation (1986) 74:1124–36. doi:10.1161/01.CIR.74.5.1124
4. Noble R. The development of resistance by rats and guinea pigs to amount of trauma usually fatal. Am J Physiol (1943) 138:346–51.
5. Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation (1996) 94:2193–200. doi:10.1161/01.CIR.94.9.2193
6. Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, et al. Remote ischaemic conditioning – a new paradigm of self-protection in the brain. Nat Rev Neurol (2015) 11:698–710. doi:10.1038/nrneurol.2015.223
7. Dezfulian C, Taft M, Corey C, Hill G, Krehel N, Rittenberger JC, et al. Biochemical signaling by remote ischemic conditioning of the arm versus thigh: is one raise of the cuff enough? Redox Biol (2017) 12:491–8. doi:10.1016/j.redox.2017.03.010
8. Johnsen J, Pryds K, Salman R, Løfgren B, Kristiansen SB, Bøtker HE. The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol (2016) 111:10. doi:10.1007/s00395-016-0529-6
9. McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, et al. Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci U S A (2003) 100:715–20. doi:10.1073/pnas.0232966100
10. Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience (2008) 151:1099–103. doi:10.1016/j.neuroscience.2007.11.056
11. Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res (1993) 72:1293–9. doi:10.1161/01.RES.72.6.1293
12. Yellon DM, Baxter GF. A “second window of protection” or delayed preconditioning phenomenon: future horizons for myocardial protection? J Mol Cell Cardiol (1995) 27:1023–34. doi:10.1016/0022-2828(95)90071-3
13. Kis A, Yellon DM, Baxter GF. Second window of protection following myocardial preconditioning: an essential role for PI3 kinase and p70S6 kinase. J Mol Cell Cardiol (2003) 35:1063–71. doi:10.1016/S0022-2828(03)00208-6
14. Hausenloy DJ, Yellon DM. The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther (2010) 24:235–54. doi:10.1007/s10557-010-6237-9
15. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol (2015) 65:177–95. doi:10.1016/j.jacc.2014.10.031
16. Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res (2009) 1288:88–94. doi:10.1016/j.brainres.2009.07.029
17. Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke (2011) 42:2960–2. doi:10.1161/STROKEAHA.111.622340
18. Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics (2004) 19:143–50. doi:10.1152/physiolgenomics.00046.2004
19. Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci (2006) 7:437–48. doi:10.1038/nrn1927
20. Przyklenk K. Reduction of myocardial infarct size with ischemic “conditioning”: physiologic and technical considerations. Anesth Analg (2013) 117:891–901. doi:10.1213/ANE.0b013e318294fc63
21. Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, et al. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke (2002) 33:1677–84. doi:10.1161/01.STR.0000016332.37292.59
22. Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation (2002) 106:2881–3. doi:10.1161/01.CIR.0000043806.51912.9B
23. Bastide M, Gelé P, Pétrault O, Pu Q, Caliez A, Robin E, et al. Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J Cereb Blood Flow Metab (2003) 23:399–405. doi:10.1097/01.WCB.0000050064.57184.F2
24. Gonzalez NR, Hamilton R, Bilgin-Freiert A, Dusick J, Vespa P, Hu X, et al. Cerebral hemodynamic and metabolic effects of remote ischemic preconditioning in patients with subarachnoid hemorrhage. Acta Neurochir Suppl (2013) 115:193–8. doi:10.1007/978-3-7091-1192-5_36
25. Hoda MN, Siddiqui S, Herberg S, Periyasamy-Thandavan S, Bhatia K, Hafez SS, et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke (2012) 43:2794–9. doi:10.1161/STROKEAHA.112.660373
26. Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation (2007) 116:1386–95. doi:10.1161/CIRCULATIONAHA.106.653782
27. Gustavsson M, Anderson MF, Mallard C, Hagberg H. Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr Res (2005) 57:305–9. doi:10.1203/01.PDR.0000151122.58665.70
28. Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol (2009) 47:32–40. doi:10.1016/j.yjmcc.2009.03.019
29. Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, et al. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther (2012) 26:87–93. doi:10.1007/s10557-011-6364-y
30. Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci (2004) 25:577–83. doi:10.1016/j.tips.2004.09.006
31. Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci (2006) 26:1269–74. doi:10.1523/JNEUROSCI.4480-05.2006
32. Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res (2011) 109:1302–8. doi:10.1161/CIRCRESAHA.111.255604
33. Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science (2009) 323:793–7. doi:10.1126/science.1164551
34. Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res (2008) 79:127–33. doi:10.1093/cvr/cvn067
35. Kim EJ, Raval AP, Perez-Pinzon MA. Preconditioning mediated by sublethal oxygen-glucose deprivation-induced cyclooxygenase-2 expression via the signal transducers and activators of transcription 3 phosphorylation. J Cereb Blood Flow Metab (2008) 28:1329–40. doi:10.1038/jcbfm.2008.26
36. Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, et al. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol (2003) 35:525–37. doi:10.1016/S0022-2828(03)00076-2
37. Liang Y, Li YP, He F, Liu XQ, Zhang JY. Long-term, regular remote ischemic pre-conditioning improves endothelial function in patients with coronary heart disease. Braz J Med Biol Res (2015) 48:568–76. doi:10.1590/1414-431X20144452
38. Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ Res (2012) 110:111–5. doi:10.1161/CIRCRESAHA.111.259556
39. Wu Q, Wang T, Chen S, Zhou Q, Li H, Hu N, et al. Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of fallot repair surgery: a randomized controlled trial. Eur Heart J (2018) 39:1028–37. doi:10.1093/eurheartj/ehx030
40. Diwan V, Kant R, Jaggi AS, Singh N, Singh D. Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem (2008) 315:195–201. doi:10.1007/s11010-008-9808-3
41. Weinbrenner C, Schulze F, Sarvary L, Strasser RH. Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res (2004) 61:591–9. doi:10.1016/j.cardiores.2003.10.008
42. Zhao HG, Sun XC, Xian XH, Li WB, Zhang M, Li QJ. The role of nitric oxide in the neuroprotection of limb ischemic preconditioning in rats. Neurochem Res (2007) 32:1919–26. doi:10.1007/s11064-007-9381-2
43. Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci (2007) 27:6320–32. doi:10.1523/JNEUROSCI.0449-07.2007
44. Albrecht M, Zitta K, Bein B, Wennemuth G, Broch O, Renner J, et al. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol (2013) 108:314. doi:10.1007/s00395-012-0314-0
45. Szabó A, Varga R, Keresztes M, Vízler C, Németh I, Rázga Z, et al. Ischemic limb preconditioning downregulates systemic inflammatory activation. J Orthop Res (2009) 27:897–902. doi:10.1002/jor.20829
46. Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet (2003) 362:1028–37. doi:10.1016/S0140-6736(03)14412-1
47. Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem (2004) 89:73–89. doi:10.1111/j.1471-4159.2004.02316.x
48. Corbett D, Giles T, Evans S, McLean J, Biernaskie J. Dynamic changes in CA1 dendritic spines associated with ischemic tolerance. Exp Neurol (2006) 202:133–8. doi:10.1016/j.expneurol.2006.05.020
49. Brucklacher RM, Vannucci RC, Vannucci SJ. Hypoxic preconditioning increases brain glycogen and delays energy depletion from hypoxia-ischemia in the immature rat. Dev Neurosci (2002) 24:411–7. doi:10.1159/000069051
50. Moncada S. Biology and therapeutic potential of prostacyclin. Stroke (1983) 14:157–68. doi:10.1161/01.STR.14.2.157
51. Røpcke DM, Hjortdal VE, Toft GE, Jensen MO, Kristensen SD. Remote ischemic preconditioning reduces thrombus formation in the rat. J Thromb Haemost (2012) 10:2405–6. doi:10.1111/j.1538-7836.2012.04914.x
52. England TJ, Hedstrom A, O’Sullivan S, Donnelly R, Barrett DA, Sarmad S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke (2017) 48:1412–5. doi:10.1161/STROKEAHA.116.016429
53. Walsh SR, Nouraei SA, Tang TY, Sadat U, Carpenter RH, Gaunt ME. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovascular Surg (2010) 44:434–9. doi:10.1177/1538574410369709
54. Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, et al. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke (1999) 30:1851–4. doi:10.1161/01.STR.30.9.1851
55. Schaller B. Ischemic preconditioning as induction of ischemic tolerance after transient ischemic attacks in human brain: its clinical relevance. Neurosci Lett (2005) 377:206–11. doi:10.1016/j.neulet.2004.12.004
56. Weber R, Diener HC, Weimar C; German Stroke Study Collaboration. Why do acute ischemic stroke patients with a preceding transient ischemic attack present with less severe strokes? Insights from the German Stroke Study. Eur Neurol (2011) 66:265–70. doi:10.1159/000331593
57. Connolly M, Bilgin-Freiert A, Ellingson B, Dusick JR, Liebeskind D, Saver J, et al. Peripheral vascular disease as remote ischemic preconditioning, for acute stroke. Clin Neurol Neurosurg (2013) 115:2124–9. doi:10.1016/j.clineuro.2013.07.038
58. Kim J, Lee DH, Cha MJ, Song TJ, Park JH, Lee HS, et al. Low ankle-brachial index is an independent predictor of poor functional outcome in acute cerebral infarction. Atherosclerosis (2012) 224:113–7. doi:10.1016/j.atherosclerosis.2012.06.058
59. Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, et al. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv (2013) 6:246–51. doi:10.1161/CIRCINTERVENTIONS.112.000184
60. Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg (2010) 25:127–34. doi:10.1111/j.1540-8191.2009.00820.x
61. Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab (2000) 20:452–7. doi:10.1097/00004647-200003000-00002
62. Hougaard KD, Hjort N, Zeidler D, Sørensen L, Nørgaard A, Hansen TM, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke (2014) 45:159–67. doi:10.1161/STROKEAHA.113.001346
63. Hoda MN, Hess DC, Ergul A, Fagan SC. Response to letter regarding article, “Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type”. Stroke (2013) 44:e37. doi:10.1161/STROKEAHA.111.000541
64. Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin H, Halkos ME, Kerendi F. Postconditioning – a new link in nature’s armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol (2005) 100:295–310. doi:10.1007/s00395-005-0523-x
65. Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croué A, Henrion D, et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol (2011) 106:1329–39. doi:10.1007/s00395-011-0210-z
66. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol (2012) 298:229–317. doi:10.1016/B978-0-12-394309-5.00006-7
67. Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab (2009) 29:873–85. doi:10.1038/jcbfm.2009.13
68. Liu X, Zhao S, Liu F, Kang J, Xiao A, Li F, et al. Remote ischemic postconditioning alleviates cerebral ischemic injury by attenuating endoplasmic reticulum stress-mediated apoptosis. Transl Stroke Res (2014) 5:692–700. doi:10.1007/s12975-014-0359-5
69. Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab (2008) 28:232–41. doi:10.1038/sj.jcbfm.9600579
70. Sun J, Tong L, Luan Q, Deng J, Li Y, Li Z, et al. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J Cereb Blood Flow Metab (2012) 32:851–9. doi:10.1038/jcbfm.2011.199
71. Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol (2009) 46:858–66. doi:10.1016/j.yjmcc.2008.11.019
72. Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol (2010) 105:151–4. doi:10.1007/s00395-009-0080-9
73. de Lima Portella R, Lynn Bickta J, Shiva S. Nitrite confers preconditioning and cytoprotection after ischemia/reperfusion injury through the modulation of mitochondrial function. Antioxid Redox Signal (2015) 23:307–27. doi:10.1089/ars.2015.6260
74. Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science (2009) 324:1713–6. doi:10.1126/science.1171721
75. Lin HW, Thompson JW, Morris KC, Perez-Pinzon MA. Signal transducers and activators of transcription: STATs-mediated mitochondrial neuroprotection. Antioxid Redox Signal (2011) 14:1853–61. doi:10.1089/ars.2010.3467
76. Perez-Pinzon MA, Stetler RA, Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab (2012) 32:1362–76. doi:10.1038/jcbfm.2012.32
77. Nakajima T, Iwabuchi S, Miyazaki H, Okuma Y, Kuwabara M, Nomura Y, et al. Preconditioning prevents ischemia-induced neuronal death through persistent Akt activation in the penumbra region of the rat brain. J Vet Med Sci (2004) 66:521–7. doi:10.1292/jvms.66.521
78. Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, et al. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One (2010) 5:e9532. doi:10.1371/journal.pone.0009532
79. Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol (2014) 109:423. doi:10.1007/s00395-014-0423-z
80. Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res (2014) 114:1601–10. doi:10.1161/CIRCRESAHA.114.303822
81. Ueno K, Samura M, Nakamura T, Tanaka Y, Takeuchi Y, Kawamura D, et al. Increased plasma VEGF levels following ischemic preconditioning are associated with down regulation of miRNA-762 and miR-3072-5p. Sci Rep (2016) 6:36758. doi:10.1038/srep36758
82. Weber NC, Riedemann I, Smit KF, Zitta K, van de Vondervoort D, Zuurbier CJ, et al. Plasma from human volunteers subjected to remote ischemic preconditioning protects human endothelial cells from hypoxia-induced cell damage. Basic Res Cardiol (2015) 110:17. doi:10.1007/s00395-015-0474-9
83. Dong HL, Zhang Y, Su BX, Zhu ZH, Gu QH, Sang HF, et al. Limb remote ischemic preconditioning protects the spinal cord from ischemia-reperfusion injury: a newly identified nonneuronal but reactive oxygen species-dependent pathway. Anesthesiology (2010) 112:881–91. doi:10.1097/ALN.0b013e3181d0486d
84. Kolh P. Remote ischaemic pre-conditioning in cardiac surgery: benefit or not? Eur Heart J (2014) 35:141–3. doi:10.1093/eurheartj/eht517
85. Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, et al. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol (2010) 299:H1598–603. doi:10.1152/ajpheart.00396.2010
86. Donato M, Buchholz B, Rodríguez M, Pérez V, Inserte J, García-Dorado D, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol (2013) 98:425–34. doi:10.1113/expphysiol.2012.066217
87. Oosterlinck W, Dresselaers T, Geldhof V, Nevelsteen I, Janssens S, Himmelreich U, et al. Diabetes mellitus and the metabolic syndrome do not abolish, but might reduce, the cardioprotective effect of ischemic postconditioning. J Thorac Cardiovasc Surg (2013) 145:1595–602. doi:10.1016/j.jtcvs.2013.02.016
88. Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res (2012) 95:487–94. doi:10.1093/cvr/cvs212
89. Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol (2010) 105:651–5. doi:10.1007/s00395-010-0099-y
90. Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet (2009) 374:1557–65. doi:10.1016/S0140-6736(09)61421-5
91. Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis (2009) 204:334–41. doi:10.1016/j.atherosclerosis.2008.10.029
92. Zarbock A, Kellum JA, Van Aken H, Schmidt C, Küllmar M, Rosenberger P, et al. Long-term effects of remote ischemic preconditioning on kidney function in high-risk cardiac surgery patients: follow-up results from the RenalRIP trial. Anesthesiology (2017) 126:787–98. doi:10.1097/ALN.0000000000001598
93. Feigin VL, Krishnamurthi R, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology (2015) 45:161–76. doi:10.1159/000441085
94. Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke (2015) 10:143–52. doi:10.1111/ijs.12434
95. Zhao L, Nowak TS Jr. CBF changes associated with focal ischemic preconditioning in the spontaneously hypertensive rat. J Cereb Blood Flow Metab (2006) 26:1128–40. doi:10.1038/sj.jcbfm.9600269
96. Hess DC, Hoda MN, Bhatia K. Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke (2013) 44:1191–7. doi:10.1161/STROKEAHA.112.678482
97. Blauenfeldt RA, Hougaard KD, Mouridsen K, Andersen G. High prestroke physical activity is associated with reduced infarct growth in acute ischemic stroke patients treated with intravenous tPA and randomized to remote ischemic perconditioning. Cerebrovasc Dis (2017) 44:88–95. doi:10.1159/000477359
98. Pico F, Rosso C, Meseguer E, Chadenat ML, Cattenoy A, Aegerter P, et al. A multicenter, randomized trial on neuroprotection with remote ischemic per-conditioning during acute ischemic stroke: the REmote iSchemic Conditioning in acUtE BRAin INfarction study protocol. Int J Stroke (2016) 11:938–43. doi:10.1177/1747493016660098
99. Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology (2012) 79:1853–61. doi:10.1212/WNL.0b013e318271f76a
100. Wang Y, Meng R, Song H, Liu G, Hua Y, Cui D, et al. Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke (2017) 48:3064–72. doi:10.1161/STROKEAHA.117.017691
101. Khan MB, Hoda MN, Vaibhav K, Giri S, Wang P, Waller JL, et al. Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl Stroke Res (2015) 6:69–77. doi:10.1007/s12975-014-0374-6
102. Zhao W, Meng R, Ma C, Hou B, Jiao L, Zhu F, et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, randomized controlled trial. Circulation (2017) 135:1325–35. doi:10.1161/CIRCULATIONAHA.116.024807
103. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol (2014) 10:44–58. doi:10.1038/nrneurol.2013.246
104. Koch S, Gonzalez N. Preconditioning the human brain: proving the principle in subarachnoid hemorrhage. Stroke (2013) 44:1748–53. doi:10.1161/STROKEAHA.111.000773
105. Laiwalla AN, Ooi YC, Liou R, Gonzalez NR. Matched cohort analysis of the effects of limb remote ischemic conditioning in patients with aneurysmal subarachnoid hemorrhage. Transl Stroke Res (2016) 7:42–8. doi:10.1007/s12975-015-0437-3
106. Nikkola E, Laiwalla A, Ko A, Alvarez M, Connolly M, Ooi YC, et al. Remote ischemic conditioning alters methylation and expression of cell cycle genes in aneurysmal subarachnoid hemorrhage. Stroke (2015) 46:2445–51. doi:10.1161/STROKEAHA.115.009618
107. Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet (2010) 375:727–34. doi:10.1016/S0140-6736(09)62001-8
108. Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv (2013) 6:1055–63. doi:10.1016/j.jcin.2013.05.011
109. Hoda MN, Fagan SC, Khan MB, Vaibhav K, Chaudhary A, Wang P, et al. A 2 × 2 factorial design for the combination therapy of minocycline and remote ischemic perconditioning: efficacy in a preclinical trial in murine thromboembolic stroke model. Exp Transl Stroke Med (2014) 6:10. doi:10.1186/2040-7378-6-10
110. Mouton R, Soar J. Remote ischaemic preconditioning: an intervention for anaesthetists? Br J Anaesth (2017) 118:288–91. doi:10.1093/bja/aew409
111. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med (2015) 373:1408–17. doi:10.1056/NEJMoa1413534
112. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med (2015) 373:1397–407. doi:10.1056/NEJMoa1413579
113. Landoni G, Greco T, Biondi-Zoccai G, Nigro Neto C, Febres D, Pintaudi M, et al. Anaesthetic drugs and survival: a Bayesian network meta-analysis of randomized trials in cardiac surgery. Br J Anaesth (2013) 1116:886–96. doi:10.1093/bja/aet231
114. Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand (2012) 56:30–8. doi:10.1111/j.1399-6576.2011.02585.x
115. Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg (2014) 147:376–82. doi:10.1016/j.jtcvs.2013.01.005
116. Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet (2013) 382:597–604. doi:10.1016/S0140-6736(13)61450-6
117. Heusch G. Critical issues for the translation of cardioprotection. Circ Res (2017) 120:1477–86. doi:10.1161/CIRCRESAHA.117.310820
118. Zhou C, Liu Y, Yao Y, Zhou S, Fang N, Wang W, et al. β-Blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: a meta-analysis of 15 randomized trials. J Cardiothorac Vasc Anesth (2013) 27:305–11. doi:10.1053/j.jvca.2012.09.028
119. van den Munckhof I, Riksen N, Seeger JP, Schreuder TH, Borm GF, Eijsvogels TM, et al. Aging attenuates the protective effect of ischemic preconditioning against endothelial ischemia-reperfusion injury in humans. Am J Physiol Heart Circ Physiol (2013) 304:H1727–32. doi:10.1152/ajpheart.00054.2013
120. Przyklenk K. Efficacy of cardioprotective ‘conditioning’ strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging (2011) 28:331–43. doi:10.2165/11587190-000000000-00000
121. Bickler PE, Fahlman CS, Gray JJ. Hypoxic preconditioning failure in aging hippocampal neurons: impaired gene expression and rescue with intracellular calcium chelation. J Neurosci Res (2010) 88:3520–9. doi:10.1002/jnr.22508
122. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev (2014) 66:1142–74. doi:10.1124/pr.113.008300
Keywords: remote ischemic conditioning, acute ischemic stroke, ischemia/reperfusion injury, neuroprotection, neurointerventional procedures
Citation: Zhou G, Li MH, Tudor G, Lu HT, Kadirvel R and Kallmes D (2018) Remote Ischemic Conditioning in Cerebral Diseases and Neurointerventional Procedures: Recent Research Progress. Front. Neurol. 9:339. doi: 10.3389/fneur.2018.00339
Received: 12 February 2018; Accepted: 30 April 2018;
Published: 16 May 2018
Edited by:
Bruce Campbell, University of Melbourne, AustraliaReviewed by:
David Charles Hess, Augusta University, United StatesNeil James Spratt, Hunter Medical Research Institute, University of Newcastle and Hunter New England Local Health District, Australia
Copyright: © 2018 Zhou, Li, Tudor, Lu, Kadirvel and Kallmes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramanathan Kadirvel, a2FkaXJAbWF5by5lZHU=
 Geng Zhou
Geng Zhou Ming Hua Li1
Ming Hua Li1