- Department of Medical Imaging, Guangdong Second Provincial General Hospital, Guangzhou, China
Objective: Daytime cognitive impairment is an essential symptom of primary insomnia (PI). However, the underlying neural substrate remains largely unknown. Many studies have shown that the right anterior insula (rAI) as a key node of salience network (SN) plays a critical role in switching between the executive control network (ECN) and the default mode network (DMN) for better performance of cognitively demanding tasks. Aberrant effective connectivity (directional functional connectivity) of rAI with ECN or DMN may be one reason for daytime cognitive impairment in PI patients. Up to now, no effective connectivity study has been conducted on patients with PI during resting state. Our aim is to investigate the effective connectivity between the rAI and the other voxels in the whole brain in PI.
Materials and methods: Fifty drug-naive patients with PI and forty age- and sex-matched healthy controls were scanned using resting-state functional MRI. Seed-based Granger causality analysis was used to examine effective connectivity between the rAI, including ventral and dorsal part, and the whole brain. The effective connectivity was compared between the two groups and was correlated with clinical characteristics.
Results: Compared with controls, patients showed decreased effective connectivity from the rAI to the bilateral precuneus, the left postcentral gyrus (extending to bilateral precuneus) and the bilateral cerebellum posterior lobe, and decreased effective connectivity from the bilateral orbitofrontal cortex (OFC) to the rAI (single voxel P < 0.001, AlphaSim corrected with P < 0.01). In addition, effective connectivity from the ventral rAI to the left postcentral gyrus and from the left OFC to the ventral rAI were significantly negatively correlated with Insomnia Severity Index scores (r = −0.28/P = 0.046 and r = −0.29/P = 0.038, respectively).
Conclusion: The present study is the first to reveal aberrant effective connectivity between the SN hub (rAI) and the posterior DMN hub (precuneus) as well as decision-making region (OFC) and sensori-motor region in PI. These findings suggest an aberrant salience processing system of the rAI in PI patients.
Introduction
Primary insomnia (PI) is one of the most common health problems. It is characterized by difficulties in falling asleep, maintaining sleep, or early awakening for at least 1 month (1). The worldwide prevalence of insomnia symptoms is approximately 30–35% and approximately 10% of people are diagnosed with PI (2, 3). Insomnia is associated with cognitive impairment, daytime fatigue, and mood disruption (4, 5). Among a series of adverse consequences caused by insomnia, daytime cognitive impairment is an essential symptom with regard to working memory, episodic memory, and some aspects of executive functioning (4, 6). However, the underlying neural substrate is incompletely understood.
Neuroimaging techniques provided a new avenue to study the pathophysiological mechanisms underlying many psychiatric disorders (7–12). With regard to PI, PET, functional, and structural MR imaging have shown abnormal glucose metabolism (13), activation (14, 15), spontaneous activity (16–18), functional or structural connectivity (19–25), or atrophic structure (26–28) related to the cognitive system, especially the salience network (SN) (11, 21), executive control network (ECN), and default mode network (DMN) (22, 29).
It is worth noting that failed reducing activities of DMN is an important feature of PI during cognitively demanding tasks, such as during working memory task (15). During working memory task, with increasing task difficulty PI patients not only showed reduced activation in task-positive regions but also showed reduced deactivation in task-negative regions (DMN) (15). This study suggested that it was not simply the failure to recruit ECN that was associated with the reduced cognitively demanding task performance, but there was a conjoint failure to deactivate the DMN. However, very little is known about the neural mechanism behind this phenomenon.
The right anterior insula (rAI) as a key node of SN can modulate activity in the ECN and the DMN in healthy individuals for better performance of cognitively demanding tasks (30, 31). It is worth noting that the modulating function of anterior insula is right lateralized. So, we only choose the right side as the seed region but not left insula. Present theory holds that one fundamental mechanism underlying cognitive control is a transient signal from the right fronto-insular cortex, which engages the brain’s attention, working memory, and higher-order control processes while disengaging other systems (such as DMN) that are not immediately task relevant (30, 32). Therefore, aberrant directed functional connectivity (FC) (effective connectivity) of rAI over ECN or DMN may be one reason of daytime cognitive impairment in PI patients. Previous studies have found altered FC in the right insula in PI patients (20–22, 33–35). However, there is no research to study the effective connectivity of the rAI.
In contrast to FC, which is zero time-lagged correlation between time series at spatially distinct regions of brain, the effective connectivity is the time-lagged correlation between time series. Effective connectivity from a region X to another region Y implies that the neuronal activity in region X precedes and predicts the neuronal activity that occurs in region Y. As mentioned above, some FC studies have already been performed to study the right insula (including rAI). However, there is still no research to study the directional FC (effective connectivity) in PI. Investigation of effective connectivity in PI patients may deepen our understanding of neurologic mechanism of PI.
We employed Granger causality analysis (36–38) in resting-state functional MR imaging to investigate the rAI-centered effective connectivity. Granger causal influence from a region X to another region Y implies that the neuronal activity in region X precedes and predicts the neuronal activity that occurs in region Y (38). Thus, the whole-brain Granger causal analysis is a useful approach to study the effective connectivity that may exist across networks. In contrast to undirected FC which does not support inferences about directed (causal) brain connections, effective connectivity refers to the influence that one neural system exerts over another and quantifies the directed coupling among brain regions. In addition to Granger causal analysis, there are also several ways to capture the directional brain dynamics, such as dynamic causal modeling and structural equation modeling. Granger causal analysis has been widely used to study effective connectivity in normal brains (30), schizophrenia (38), and major depressive disorders (37). To date, to the best of our knowledge, no studies have been published reporting effective connectivity in PI patients. The purpose of this study was to analyze the effective connectivity between the rAI and the whole brain in PI patients using first-order Granger causality analysis and its association with sleep and emotion scales of PI. We hypothesized that the effective connectivity between rAI and ECN or DMN was disrupted.
Materials and Methods
Participants
This prospective study was approved by the ethics committee of the Guangdong Second Provincial General Hospital. All PI patients were recruited from the Department of Neurology at Guangdong Second Provincial General Hospital, Guangzhou, China from April 2010 to May 2016. Written informed consent was obtained from all patients. The inclusion criteria for PI patients were (a) all patients must meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) for diagnosis of PI; (b) patients had been complaining of difficulty falling asleep, maintaining sleep, or early awakening for at least 1 month; (c) patients had no other sleep disorders such as hypersomnia, parasomnia, sleep-related movement disorder, or other psychiatric disorders; (d) patients were younger than 60 years old; (e) free of any psychoactive medication at least 2 weeks prior to and during the study; and (f) patients were right-hand dominant as assessed with the Edinburgh Handedness Inventory. Exclusion criteria were as follows: (a) patients had an abnormal signal in any region of the brain verified by conventional T1-weighted or T2-fluid-attenuated inversion recovery MR imaging; (b) the insomnia disorder was caused by organic disease or severe mental disease such as secondary to depression or generalized anxiety; (c) other sleep disorder; (d) women who were pregnant, nursing, or menstruating; and (e) head motion more than or equal to 1.5 mm or 1.5° during MR imaging. Then three patients were discarded. Finally, 50 PI patients who met the requirements were included in the study.
A total of 40 age-, gender-, and education-matched healthy control (HC) subjects were recruited (17 men, 23 women; mean age, 39.38 ± 9.26 years) from the local community by using advertisements. Each HC subject gave written informed consent. HCs must met the following criterion: (a) Insomnia Severity Index (ISI) score was less than 7; (b) no history of swing shifts, shift work, or sleep complaints; (c) no medication or substance abuse such as caffeine, nicotine, or alcohol; (d) no brain lesions or prior substantial head trauma, which was verified by conventional T1-weighted or T2-fluid-attenuated inversion recovery MR imaging; (e) no history of psychiatric or neurological diseases; (f) head motion less than 1.5 mm or 1.5° during MR scan; and (g) right-hand dominant. Three controls were discarded due to head motion.
Sleep and Emotion Scales
Several questionnaires were filled out by study participants. These questionnaires included the ISI (39), the Pittsburgh Sleep Quality Index (PSQI) (40), the Self-rating Anxiety Scale (SAS) (41), and the Self-rating Depression Scale (SDS) (42).
MR Imaging
Resting-state functional MR imaging data were acquired using a 1.5 T MR scanner (Achieva Nova-Dual; Philips, Best, the Netherlands) in the Department of Medical Imaging, Guangdong Second Provincial General Hospital. To minimize head movements, a belt and foam pads were used. During the scanning, subjects were instructed to rest with their eyes closed and remain still but emphatically without falling asleep. The functional MR images were acquired in about 10 min using a gradient-echo planar imaging sequence as follows: interleaved scanning, repetition time = 2,500 ms, echo time = 50 ms, matrix = 64 × 64, field of view = 224 mm × 224 mm, flip angle = 90°, section thickness = 4 mm, gap = 0.8 mm, 27 axial slices, and 240 volumes.
Data Preprocessing
The Data Processing Assistant for Resting-State Functional MR Imaging toolbox1 (version 2.3) was used to process the resting-state functional MR imaging data. Volumes at the first 10 time points were discarded so that magnetization reached a steady state and subjects had adapted to the MR scanning noise. The slice timing and realignment for head motion correction were conducted on the remaining images. Then, the realigned images were spatially normalized to the Montreal Neurological Institute template by applying the EPI template, and each voxel was resampled to 3 mm × 3 mm × 3 mm. We spatially smoothed the spatially normalized images with a 6-mm full-width half-maximum isotropic Gaussian kernel. In order to reduce effects of low-frequency drift and high-frequency noise, we processed the data to remove linear trends and filtered temporally (band-pass, 0.01–0.08 Hz). Nine nuisance covariates, including cerebrospinal fluid signals, white matter signals, global brain signal, and six head motion parameters were regressed from the imaging data. The residuals of these regressions were used for the following analysis.
Granger Causality Analysis
We calculated the effective connectivity of the time series of the dorsal and ventral rAI on every voxel in the whole brain (X to Y) and the effective connectivity of the time series of every voxel in the whole brain on the dorsal and ventral rAI (Y to X). The mean temporal-domain bold signals for the dorsal and ventral rAI are displayed in Figure S10 in Supplementary Material. Regions of interest (ROI) in the dorsal and ventral rAI were selected based on the brain atlas based on connectional architecture (43).2 Bivariate first-order coefficient-based voxel-wise Granger causality analysis was performed using REST-GCA (44). We followed Chen’s (37) extended model as following:
where Yt is the BOLD time series of one voxel in the brain at time t; X is the BOLD time series of seed region; Zt is a q × 1 vector containing exogenous variables (covariates or confounds) at time t; εt is the error term; p and q are the number of lags and confounds, respectively; Ai is the signed path coefficient at time lag i (i = 1, …, p); Bi is the autoregression coefficient. In the present study, the number of lags p = 1 (1 TR = 2.5 s).
Explanation of the coefficient was the same as the previous study (38). The positive coefficient is referred as excitatory influence and vice visa.
Statistical Analysis
Differences in age, education level, ISI, PSQI, SAS, and SDS scores between PI patients and HCs were compared by using two-sample t tests. Differences associated with gender between the two groups were assessed by using chi-squared tests.
First, the effective connectivity maps were analyzed using one-sample t-test for the entire sample (both PI patients and HCs) with an uncorrected P < 0.001, cluster size = 50. Then, between-group differences in effective connectivity were compared by using two-sample t tests in a voxel-by-voxel fashion with age, sex, and education level imported as covariates. Multiple comparisons were corrected by an AlphaSim method implemented in the DPABI software [DPABI version 2.3, Data Processing & Analysis for (Resting-State) Brain Imaging] (45) and using significant corrected thresholds of P < 0.01 with combined with single voxel P < 0.001. The estimated FWHM (x–y–z) for the 4T maps (from ventral rAI, from dorsal rAI, to ventral rAI, and to dorsal rAI) were 6.9857–7.1202–7.9652, 5.8353–5.9840–6.7710, 9.1891–9.4015–9.4269, and 5.0546–5.1626–5.8403. The cluster size thresholds for the 4T maps were 22, 16, 48, and 26.
Besides, we used permutation threshold-free cluster enhancement (TFCE) correction method to perform statistical analysis (46, 47). The permutation TFCE correction method implements correction through a permutation testing approach which controls family-wise error rate by comparing voxel-wise statistics (TFCE) to the maximal statistics obtained from repeating the analysis with randomized data. The Matlab scripts for the permutation TFCE correction have been made available online: https://github.com/markallenthornton. ROI were defined as 6-mm-diameter spheres centered on voxels that exhibited the largest absolute t value in each of the significant clusters in the t map of between-group differences in effective connectivity. Then, effective connectivity was calculated for each subject by averaging the values of effective connectivity across all voxels within each of the ROI and correlated with the sleep and emotion scales using Pearson’s correlation analysis.
Results
Sleep and Emotion Scales
As Table 1 shown, the PI patients and the controls showed no significant differences in age (P = 0.37), sex (P = 0.81), and education level (P = 0.28). PI patients had higher ISI, PSQI, SAS, and SDS scores than those of HCs (all P < 0.001).
Effective Connectivity
One-sample t-test showed that the rAI exerted excitatory influence on the bilateral dorsolateral prefrontal cortex (DLPFC), the inferior parietal regions, the cingulate gyrus, and the left cerebellar crus. Inhibitory influence of the rAI was noted on the left precentral gyrus, the postcentral gyrus, and the bilateral occipital lobe. Furthermore, the bilateral DLPFC, the inferior parietal regions, and the cingulate gyrus, in turn, had inhibitory influence on the rAI, and in the same way, the bilateral occipital lobe had excitatory influence on the rAI. It is worth noting that the results of one-sample t-test were very similar to those of previous study (38). The results of the one-sample t tests are presented in Figures S4–S7 in Supplementary Material.
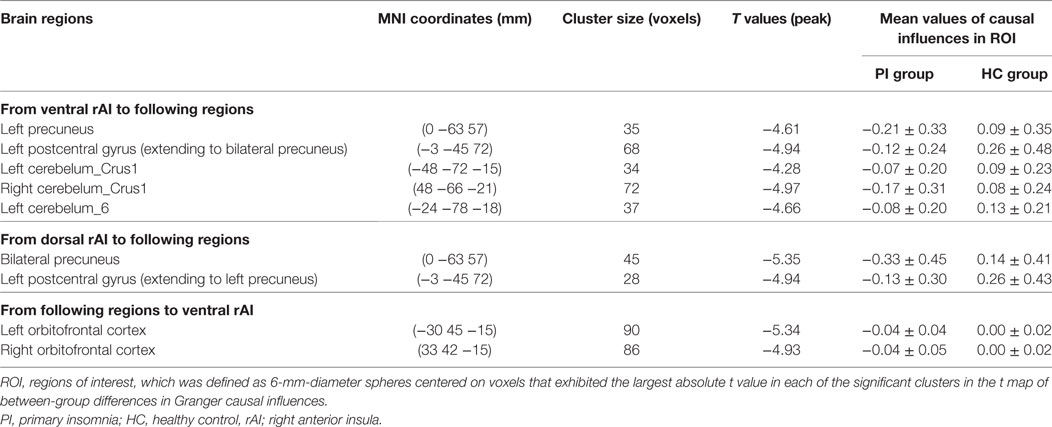
Compared with HCs, patients with PI showed negative effective connectivity (inhibitory influences) from the ventral rAI to the left precuneus, the left postcentral gyrus extending to the bilateral precuneus, and bilateral cerebellum posterior lobe including the bilateral cerebelum_crus1 and left cerebelum_6 (Figure 1A), and negative effective connectivity from the dorsal rAI to the bilateral precuneus and left postcentral gyrus extending to the left precuneus (Figure 1B). Also, patients with PI showed negative effective connectivity from bilateral orbitofrontal cortex (OFC) to ventral rAI (Figure 1C) (single voxel P < 0.001, corrected by AlphaSim correction with cluster P < 0.01). All above results of between-group differences in effective connectivity are shown in Table 2. Figures S1–S3 in Supplementary Material showed the bar graphs demonstrating the mean effective connectivity values in the ROI defined as 6-mm-diameter spheres centered on voxels that exhibited the largest absolute t value in each of the significant clusters in the t map.
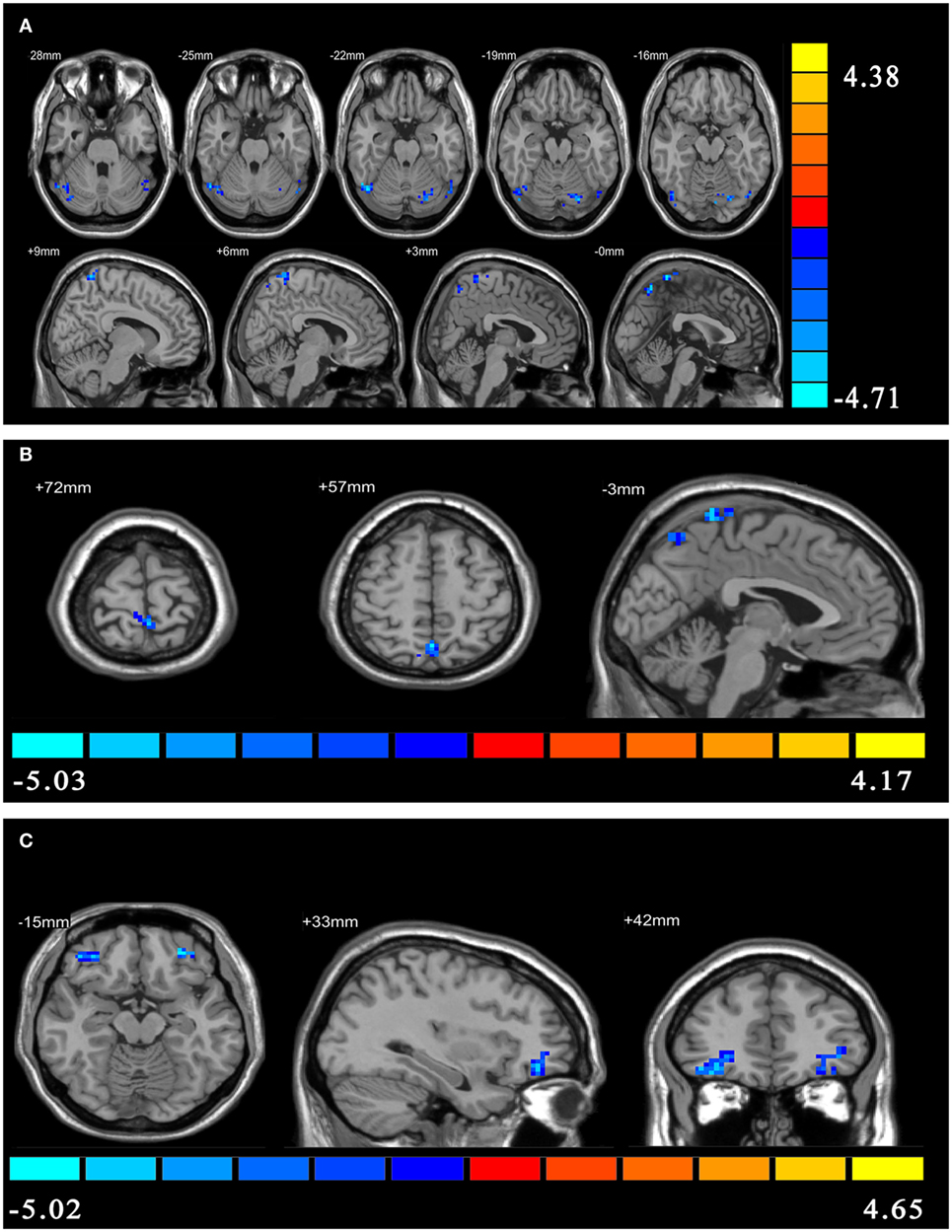
Figure 1. The between-group differences in effective connectivity. (A) The between-group differences in effective connectivity from ventral right anterior insula (rAI) to the whole brain. (B) The between-group differences in effective connectivity from dorsal rAI to the whole brain. (C) The between-group differences in effective connectivity from the whole brain to ventral rAI.
Results from permutation TFCE correction (5,000 times permutation, default parameters, FWE corrected, P < 0.05) were very similar to those derived from our parameter statistical method (two-sample t test with AlphaSim correction). Therefore, we only discussed these results. Figure S8 in Supplementary Material showed the P map of the permutation TFCE correction.
Relationships Between Effective Connectivity and Sleep and Emotion Scales
As Figure 2 shown, effective connectivity from the ventral rAI to the left postcentral gyrus extending to the bilateral precuneus and from the left OFC to the ventral rAI were significantly negatively correlated with ISI scores in PI group (r = −0.28/P = 0.046 and r = −0.29/P = 0.038, respectively).
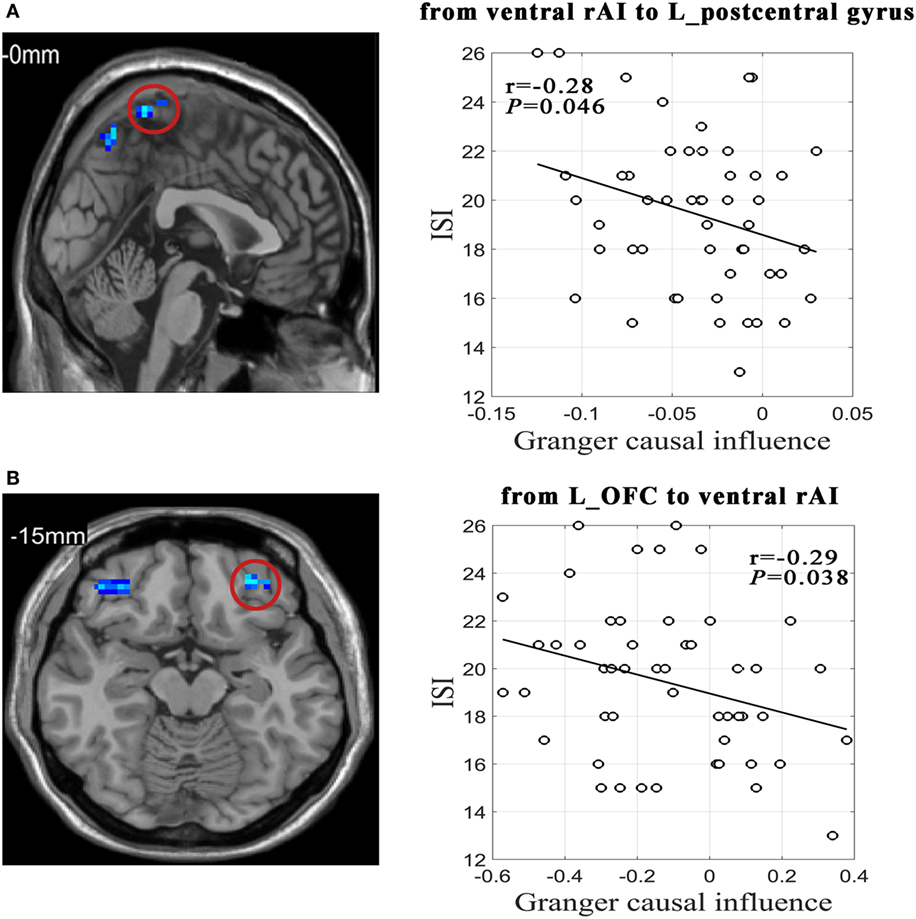
Figure 2. Relationships between effective connectivity and sleep and emotion scales in primary insomnia group. (A) Scatter plot shows correlation between effective connectivity value from ventral right anterior insula (rAI) to left postcentral gyrus extending to bilateral precuneus and Insomnia Severity Index (ISI) scores. (B) Scatter plot shows correlation between effective connectivity value from left orbitofrontal cortex (L_OFC) to ventral rAI and ISI.
Effective connectivity from the ventral rAI to the left precuneus was significantly negatively correlated with PSQI and ISI scores in HC group (r = −0.31/P = 0.047 and r = −0.32/P = 0.045, respectively). Figure S9 in Supplementary Material shows the results of the correlation analysis of the HC group.
Discussion
The present study investigated the effective connectivity between the rAI and the whole brain in PI patients. Our findings showed aberrant effective connectivity of rAI (a key node of SN) with the posterior DMN hub (precuneus) as well as regions involved in decision-making (OFC) and regions involved in sensori-motor function in PI. In addition, effective connectivity from the ventral rAI to the left postcentral gyrus extending to the bilateral precuneus and from the left OFC to the ventral rAI were significantly negatively correlated with ISI scores in PI group.
In contrast to FC which does not support inferences about directional brain connections, effective connectivity refers to the influence that one neural system exerts over another and quantifies the directional connectivity among brain regions (38). Consequently, effective connectivity may provide new insight into the neurological mechanism of insomnia.
The important findings in the current study were aberrant effective connectivity from the ventral rAI to the left precuneus and from the dorsal rAI to the bilateral precuneus at resting state. The rAI was a hub node of SN which is involved in detecting and orienting to both external and internal salient stimuli and events (31, 32). The precuneus was a hub node of DMN which is involved in self-referential/internally oriented processes (48). Previous study using chronometry and Granger causality analysis confirmed that rAI plays a critical and causal role in switching between the ECN and the DMN during visual attention tasks, oddball tasks, and even resting state (30). Furthermore, the present theory holds that one fundamental mechanism underlying cognitive control is a transient signal from the right fronto-insular cortex, which engages the brain’s attentional, working memory, and higher-order control processes while disengaging other systems (such as DMN) that are not immediately task relevant (30, 32). Interestingly, a recent study found that PI patients showed both reduced activation in task-related working memory regions and reduced deactivation in regions of the DMN with increasing task difficulty (15). This finding demonstrated a failed disengagement from DMN during working memory tasks in PI patients. It is a complement to previous studies that only found decreased metabolism or decreased activation in cognitive or task-related regions (13, 14). In our study, although we did not found any altered effective connectivity from rAI to ECN, we found aberrant effective connectivity from the rAI to regions of DMN at resting state or baseline condition. Our findings offer a parsimonious explanation for failed disengagement from DMN during cognitively demanding tasks (especially the working memory task) in PI patients.
Another finding was that PI patients showed aberrant effective connectivity from the bilateral OFC to the ventral rAI. Besides, the effective connectivity from left OFC to ventral rAI was significantly negatively correlated with ISI scores in PI group. The OFC is an important brain area responsible for emotion and decision-making (49). Previous study showed that PI patients’ speed are slower than controls on a vigilance task which only need decision-making (50). Impaired decision-making may also lead to a lack of ability to solve problems in the insomnia patients (51). Indeed, PI patients showed reduced orbitofrontal gray matter volume or density (27, 28). Recent studies found that rAI also acted as a main outflow hub within SN for easier decision-making task (52). Together with previous studies, our findings suggest an abnormal OFC-rAI circuit in PI patients, which might be one of underlying substrate of impaired decision-making observed in PI patients.
We also found that PI patients showed aberrant effective connectivity from the rAI to the left postcentral gyrus (extending to bilateral precuneus) and the bilateral cerebellum posterior lobe. In addition, effective connectivity from ventral rAI to the left postcentral gyrus was significantly negatively correlated with ISI scores in the PI group. The postcentral gyrus and the cerebellum are locations of primary somatosensory cortex and motor control area, respectively. In recent years, several studies have also frequently reported abnormal spontaneous brain activity in the cerebellum posterior lobe as well as the postcentral gyrus in PI (16, 17, 53). On the other hand, existing evidence suggests the anterior insula and the anterior cingulate cortex serve as complementary limbic sensory and motor regions. They work together, similar to the somatosensory and motor cortices (54, 55). Relative to rAI, which is high on the level of the hierarchy due to its function of switching between other large-scale networks, the postcentral gyrus and the cerebellum is lower in the hierarchy (32, 56). Therefore, our findings that aberrant effective connectivity from the rAI to the left postcentral gyrus and the bilateral cerebellum posterior lobe may reflect aberrant top-down sensory and motor control of rAI in PI patients.
Our study had several limitations. First, it was a cross-sectional study, and we cannot directly identify the causal relation between PI and the abnormal effective connectivity. Longitudinal studies may help address this question. Second, we did not directly investigate the inter-network effective connectivity among SN, ECN, and DMN using independent component analysis, even though selected seed regions of rAI for Granger causality analysis was the widely recognized hub node of SN. Future researchers can use independent component analysis to study inter-network effective connectivity in PI. Third, analyses of combination of mental chronometry and Granger causality analysis will increase our understanding of PI. However, we did not do these analyses for technical reasons. Future study is suggested to do these analyses. Finally, the activity of the brain at resting state is not static but is a highly dynamic system. Therefore, static effective connectivity may not be enough to fully characterize the human brain. Future study is suggested to use dynamic FC to investigate the brain in PI.
In summary, we for the first time found aberrant effective connectivity of rAI (a key node of SN) with the posterior DMN hub (precuneus) as well as regions involved in decision-making (OFC) and regions involved in sensori-motor function in PI. These findings suggest an aberrant salience processing system of the rAI, which may be a candidate substrate for cognitive impairment, especially the impairment of working memory and decision-making in PI patients.
Ethics Statement
This study was carried out in accordance with the recommendations of “ethics committee of the Guangdong Second Provincial General Hospital” with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the “ethics committee of the Guangdong Second Provincial General Hospital.”
Author Contributions
Study concepts/study design or data acquisition, manuscript drafting for important intellectual content, and approval of final version of submitted manuscript: all authors; literature research: CL, YY, KH, SF, and GJ; clinical studies: CL, MD, and GJ; experimental studies: CL and GJ; statistical analysis: CL and SF; and manuscript editing: CL, MD, and GJ.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study has received funding by the National Natural Science Foundation of China (grant no.: 81471639); the National Natural Science Foundation of China (grant no.: 81771807); the Natural Science Foundation of Guangdong Provincial (grant no.: 2015A030313723); the Science and Technology Foundation of Guangdong Province (grant no.: 2016A020215125; 2017A020215077); and the Science and Technology Foundation of Guangzhou City (grant no.: 201607010056).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fneur.2018.00317/full#supplementary-material.
Figure S1. The bar graphs demonstrating the mean effective connectivity values in the regions of interest defined as 6-mm-diameter spheres centered on voxels that exhibited the largest absolute t value in each of the significant clusters in the t map. Error bars indicate SD. Abbreviations: L_CER_crus1, left cerebelum_crus1; R_CER_crus1, right cerebelum_crus1; L_CER_6, left cerebelum_6; L_precuneus, left precuneus; L_postcentral gyrus, left postcentral gyrus (extending to bilateral precuneus).
Figure S2. The bar graphs demonstrating the mean effective connectivity values in the regions of interest defined as 6-mm-diameter spheres centered on voxels that exhibited the largest absolute t value in each of the significant clusters in the t map. Error bars indicate SD. Abbreviations: Bi_precuneus, bilateral precuneus; L_postcentral gyrus, left postcentral gyrus (extending to left precuneus).
Figure S3. The bar graphs demonstrating the mean effective connectivity values in the regions of interest defined as 6-mm-diameter spheres centered on voxels that exhibited the largest absolute t value in each of the significant clusters in the t map. Error bars indicate SD. Abbreviations: R_OFC, right orbitofrontal cortex; L_OFC, left orbitofrontal cortex.
Figure S4. The effective connectivity from ventral right anterior insula to the whole brain.
Figure S5. The effective connectivity from dorsal right anterior insula to the whole brain.
Figure S6. The effective connectivity from the whole brain to ventral right anterior insula.
Figure S7. The effective connectivity from the whole brain to dorsal right anterior insula.
Figure S8. The statistical results of the permutation threshold-free cluster enhancement correction (5,000 times permutation, default parameters, FWE corrected, P < 0.05). (A) Between-group differences in effective connectivity from ventral right anterior insula (rAI) to the whole brain. (B) Between-group differences in effective connectivity from dorsal rAI to the whole brain. (C) Between-group differences in effective connectivity from the whole brain to ventral right anterior insula.
Figure S9. Relationships between effective connectivity and sleep and emotion scales in healthy control group.
Figure S10. Temporal-domain bold signals for seed regions.
Footnotes
- ^http://www.restfMRI.net/forum/DPARSF (Accessed: July, 2017).
- ^http://atlas.brainnetome.org/ (Accessed: July, 2017).
References
1. Gmitrowicz A, Kucharska A. [Developmental disorders in the fourth edition of the American classification: diagnostic and statistical manual of mental disorders (DSM IV – optional book)]. Psychiatr Pol (1994) 28(5):509–21.
2. Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, et al. Insomnia disorder. Nat Rev Dis Primers (2015) 1:15026. doi:10.1038/nrdp.2015.26
3. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev (2002) 6(2):97–111. doi:10.1053/smrv.2002.0186
4. Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev (2012) 16(1):83–94. doi:10.1016/j.smrv.2011.03.008
5. Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone (2003) 5(3):5–15. doi:10.1016/S1098-3597(03)90031-7
6. Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev (2001) 5(6):423–45. doi:10.1053/smrv.2001.0157
7. Liu F, Guo W, Fouche JP, Wang Y, Wang W, Ding J, et al. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct Funct (2015) 220(1):101–15. doi:10.1007/s00429-013-0641-4
8. Liu F, Guo W, Liu L, Long Z, Ma C, Xue Z, et al. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord (2013) 146(3):401–6. doi:10.1016/j.jad.2012.10.001
9. Sommer IEC, Ramsey NF, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res (2001) 52(1):57–67. doi:10.1016/S0920-9964(00)00180-8
10. Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord (2011) 13(1):1–15. doi:10.1111/j.1399-5618.2011.00893.x
11. Khazaie H, Veronese M, Noori K, Emamian F, Zarei M, Ashkan K, et al. Functional reorganization in obstructive sleep apnoea and insomnia: a systematic review of the resting-state fMRI. Neurosci Biobehav Rev (2017) 77:219–31. doi:10.1016/j.neubiorev.2017.03.013
12. Sheng J, Shen Y, Qin Y, Zhang L, Jiang B, Li Y, et al. Spatiotemporal, metabolic, and therapeutic characterization of altered functional connectivity in major depressive disorder. Hum Brain Mapp (2018) 39(5):1957–71. doi:10.1002/hbm.23976
13. Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry (2004) 161(11):2126. doi:10.1176/appi.ajp.161.11.2126
14. Altena E, Yd VDW, Sanzarigita EJ, Voorn TA, Rombouts SA, Kuijer JP, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep (2008) 31(9):1271. doi:10.5665/sleep/31.9.1271
15. Drummond SP, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural correlates of working memory performance in primary insomnia. Sleep (2013) 36(9):1307. doi:10.5665/sleep.2952
16. Dai XJ, Nie X, Liu X, Pei L, Jiang J, Peng DC, et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J Clin Sleep Med (2015) 12(3):363. doi:10.5664/jcsm.5586
17. Li C, Ma X, Dong M, Yin Y, Hua K, Li M, et al. Abnormal spontaneous regional brain activity in primary insomnia: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat (2016) 12:1371–8. doi:10.2147/NDT.S109633
18. Dai XJ, Peng DC, Gong HH, Wan AL, Nie X, Li HJ, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat (2014) 10:2163–75. doi:10.2147/NDT.S69681
19. Wang T, Yan J, Li S, Zhan W, Ma X, Xia L, et al. Increased insular connectivity with emotional regions in primary insomnia patients: a resting-state fMRI study. Eur Radiol (2017) 27(9):3703–9. doi:10.1007/s00330-016-4680-0
20. Li C, Dong M, Yin Y, Hua K, Fu S, Jiang G. Abnormal whole-brain functional connectivity in patients with primary insomnia. Neuropsychiatr Dis Treat (2017) 13:427–35. doi:10.2147/NDT.S128811
21. Lu FM, Liu CH, Lu SL, Tang LR, Tie CL, Zhang J, et al. Disrupted topology of frontostriatal circuits is linked to the severity of insomnia. Front Neurosci (2017) 11:214. doi:10.3389/fnins.2017.00214
22. Pang R, Zhan Y, Zhang Y, Guo R, Wang J, Guo X, et al. Aberrant functional connectivity architecture in participants with chronic insomnia disorder accompanying cognitive dysfunction: a whole-brain, data-driven analysis. Front Neurosci (2017) 11:259. doi:10.3389/fnins.2017.00259
23. Huang Z, Liang P, Jia X, Zhan S, Li N, Ding Y, et al. Abnormal amygdala connectivity in patients with primary insomnia: evidence from resting state fMRI. Eur J Radiol (2012) 81(6):1288–95. doi:10.1016/j.ejrad.2011.03.029
24. Lu FM, Dai J, Couto TA, Liu CH, Chen H, Lu SL, et al. Diffusion tensor imaging tractography reveals disrupted white matter structural connectivity network in healthy adults with insomnia symptoms. Front Hum Neurosci (2017) 11:583. doi:10.3389/fnhum.2017.00583
25. Lu Z, Wang E, Zhang X, Sherif K, Budhachandra K, Zhang H, et al. Cortical structural connectivity alterations in primary insomnia: insights from MRI-based morphometric correlation analysis. Biomed Res Int (2015) 2015(3):1–23. doi:10.1155/2015/817595
26. Suh S, Kim H, Dang-Vu TT, Joo E, Shin C. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep (2016) 39(1):161–71. doi:10.5665/sleep.5340
27. Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry (2010) 67(2):182–5. doi:10.1016/j.biopsych.2009.08.003
28. Stoffers D, Moens S, Benjamins J, van Tol MJ, Penninx BW, Veltman DJ, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol (2012) 3:105. doi:10.3389/fneur.2012.00105
29. Nie X, Shao Y, Liu SY, Li HJ, Wan AL, Nie S, et al. Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr Dis Treat (2015) 11:3085–93. doi:10.2147/NDT.S95224
30. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A (2008) 105(34):12569–74. doi:10.1073/pnas.0800005105
31. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci (2007) 27(9):2349–56. doi:10.1523/JNEUROSCI.5587-06.2007
32. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct (2010) 214(5–6):655–67. doi:10.1007/s00429-010-0262-0
33. Chen MC, Chang C, Glover GH, Gotlib IH. Increased insula coactivation with salience networks in insomnia. Biol Psychol (2014) 97:1–8. doi:10.1016/j.biopsycho.2013.12.016
34. Li X, Guo S, Wang C, Wang B, Sun H, Zhang X. Increased interhemispheric resting-state functional connectivity in healthy participants with insomnia symptoms: a randomized clinical consort study. Medicine (Baltimore) (2017) 96(27):e7037. doi:10.1097/MD.0000000000007037
35. Liu X, Zheng J, Liu BX, Dai XJ. Altered connection properties of important network hubs may be neural risk factors for individuals with primary insomnia. Sci Rep (2018) 8(1):5891. doi:10.1038/s41598-018-23699-3
36. Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage (2005) 25(1):230–42. doi:10.1016/j.neuroimage.2004.11.017
37. Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in major depressive disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry (2011) 16(7):763–72. doi:10.1038/mp.2010.46
38. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron (2013) 79(4):814–28. doi:10.1016/j.neuron.2013.06.027
39. Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med (2001) 2(4):297–307. doi:10.1016/S1389-9457(00)00065-4
40. Buysse DJ, Reynolds CR, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res (1989) 28(2):193–213. doi:10.1016/0165-1781(89)90047-4
41. Zung WW. A rating instrument for anxiety disorders. Psychosomatics (1971) 12(6):371–9. doi:10.1016/S0033-3182(71)71479-0
42. Zung WW. A self-rating depression scale. Arch Gen Psychiatry (1965) 12:63–70. doi:10.1001/archpsyc.1965.01720310065008
43. Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex (2016) 26(8):3508–26. doi:10.1093/cercor/bhw157
44. Zang ZX, Yan CG, Dong ZY, Huang J, Zang YF. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods (2012) 203(2):418–26. doi:10.1016/j.jneumeth.2011.10.006
45. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics (2016) 14(3):339–51. doi:10.1007/s12021-016-9299-4
46. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage (2009) 44(1):83–98. doi:10.1016/j.neuroimage.2008.03.061
47. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp (2002) 15(1):1–25. doi:10.1002/hbm.1058
48. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A (2003) 100(1):253–8. doi:10.1073/pnas.0135058100
49. Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex (2000) 10(3):295–307. doi:10.1093/cercor/10.3.295
50. Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res (2008) 17(3):335–43. doi:10.1111/j.1365-2869.2008.00671.x
51. Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther (2000) 38(7):679–93. doi:10.1016/S0005-7967(99)00136-9
52. Chand GB, Dhamala M. The salience network dynamics in perceptual decision-making. Neuroimage (2016) 134:85–93. doi:10.1016/j.neuroimage.2016.04.018
53. Zhou F, Huang S, Zhuang Y, Gao L, Gong H. Frequency-dependent changes in local intrinsic oscillations in chronic primary insomnia: a study of the amplitude of low-frequency fluctuations in the resting state. Neuroimage Clin (2017) 15:458–65. doi:10.1016/j.nicl.2016.05.011
54. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev (1996) 22(3):229–44. doi:10.1016/S0165-0173(96)00011-2
55. Craig AD. How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci (2009) 10(1):59–70. doi:10.1038/nrn2555
Keywords: primary insomnia, functional magnetic resonance imaging, effective connectivity, insular cortex, executive function, cognitive impairment
Citation: Li C, Dong M, Yin Y, Hua K, Fu S and Jiang G (2018) Aberrant Effective Connectivity of the Right Anterior Insula in Primary Insomnia. Front. Neurol. 9:317. doi: 10.3389/fneur.2018.00317
Received: 28 January 2018; Accepted: 23 April 2018;
Published: 08 May 2018
Edited by:
Xi-jian Dai, Medical School of Nanjing University, ChinaReviewed by:
Zhen Yuan, University of Macau, MacauAxel Steiger, Max-Planck-Institut für Psychiatrie, Germany
Copyright: © 2018 Li, Dong, Yin, Hua, Fu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guihua Jiang, amlhbmdndWlodWExOTcwQDE2My5jb20=
 Chao Li
Chao Li Mengshi Dong
Mengshi Dong