- 1Department of Diagnostic Imaging, The Hospital for Sick Children, Toronto, ON, Canada
- 2Neurosciences & Mental Health Program, Sick Kids Research Institute, Toronto, ON, Canada
- 3Department of Medical Imaging, University of Toronto, Toronto, ON, Canada
- 4Holland-Bloorview Kids Rehabilitation Hospital, Toronto, ON, Canada
- 5Division of Neurosurgery, Sunnybrook Hospital, Toronto, ON, Canada
- 6Division of Neurology, The Hospital for Sick Children, Toronto, ON, Canada
- 7Department of Psychology, University of Toronto, Toronto, ON, Canada
Concussion is a common form of mild traumatic brain injury. Despite the descriptor “mild,” a single injury can leave long-lasting and sustained alterations to brain function, including changes to localized activity and large-scale interregional communication. Cognitive complaints are thought to arise from such functional deficits. We investigated the impact of injury on neurophysiological and functionally specialized resting networks, known as intrinsic connectivity networks (ICNs), using magnetoencephalography. We assessed neurophysiological connectivity in 40 males, 20 with concussion and 20 without. Regions-of-interest that comprise nodes of ICNs were defined, and their time courses derived using a beamformer approach. Pairwise fluctuations and covariations in band-limited amplitude envelopes were computed reflecting measures of functional connectivity. Intra-network connectivity was compared between groups using permutation testing and correlated with symptoms. We observed increased resting spectral connectivity in the default mode network (DMN) and motor networks (MOTs) in our concussion group when compared with controls, across alpha through gamma ranges. Moreover, these differences were not explained by power spectrum density within the ICNs. Furthermore, this increased coupling was significantly associated with symptoms in the DMN and MOTs—but once accounting for comorbidities (including, depression, anxiety, and ADHD) only the DMN continued to be associated with symptoms. The DMN plays a critical role in shifting between cognitive tasks. These data suggest even a single concussion can perturb the intrinsic coupling of this functionally specialized network in the brain, and may explain persistent and wide-ranging symptomatology.
Introduction
Concussion is a common form of mild traumatic brain injury (mTBI), and while symptoms are often acute and the majority of patients make a full recovery, a single injury can leave long-lasting and sustained alterations to brain structure and function, even in the absence of symptomatology. A single concussive event has been shown to perturb neurophysiological function, including localized activity (1) and large-scale interregional interactions (2, 3), with cognitive sequelae manifesting from these phenomenon. Resting functional connectivity is known to be affected, with observed changes in inter-areal spectral correlations, a mechanism that subserves dynamic brain networks for cognition (4), across multiple frequency scales replicated in several magnetoencephalography (MEG) studies (5–7). However, the impact of injury on the coordination of neural activity by oscillations in spontaneous and defined intrinsic connectivity networks’ (ICNs), such as the default mode network (DMN), vision, attention, and motor networks (MOTs), has received little consideration. These networks are critical in coordinating and routing information in the brain, and serve functionally defined and specialized purposes, such as visual perception (8, 9), directed attention (10), and self-referential processes in the DMN (11), among others.
Characterizing changes in spectral ICNs after concussion could help our understanding of the functional phenotypes of injury and improve diagnostics. Early imaging studies examined alterations to functionally segregated brain regions and the contribution of these areas to symptoms and specific cognitive deficits (12). However, concussion is beginning to be considered an example of disturbed neuronal network communication (13). Specifically, there has been a gradual paradigm shift toward thinking of the injury as one affecting interregional integration and segregation—in other words, the coordinated action of areas critical in cognition. The compromised communication within and between networks could lead to a better understanding of the sequelae of concussion. Recent research supports this view and connectivity studies have shown that atypical synchronous network interactions can help describe the disorder, in both task-free resting-state and task dependent, cognitive-behavioral paradigms (3, 6, 7, 14). The majority of disturbances to ICN connectivity have been found in concussion using fMRI (15), however, this technique is limited to measuring ultra-slow fluctuations in blood flow and their regional dynamics. In contrast to fMRI, electrophysiological techniques, such as electroencephalographic (EEG) and MEG, have the capability to measure higher frequency neurophysiological oscillations and the synchrony of these networks which operate at behaviorally relevant time-scales required for goal-directed cognition and action (16). Specifically, EEG and MEG are sensitive to the electrophysiological interactions of primary and secondary currents in the brain and can elucidate their frequency composition and dynamics (4, 17, 18) as EEG and MEG captures neuronal activity at the millisecond temporal resolution. Rather than simple epiphenomenology, neuronal oscillations are thought to gate information and coordinate the functional coupling of brain areas (19–25). Importantly, perturbations to cortical oscillations and synchronization are abnormal and atypical in a variety of neuropsychiatric disorders, and characterizing them provides new understanding of neuropsychopathology. In terms of the rationale for the choice of technique here, when compared with EEG, MEG benefits from improved source localization and its ability to disentangle high-frequency physiological artifacts (such as intermingled ocular or muscular contributions to source signals) from veridical, brain-based, physiological measures (26).
In concussion studies, MEG has revealed abnormal local changes in neuronal function, including source amplitude (27) and atypical interactions among brain areas, while other experiments have shown that pattern classification can differentiate those with the disorder from control groups (3, 28). A recent study of persistent concussion symptom patients showed decreased functional connectivity, mediated via phase locking and synchrony, across multiple frequency bands in the DMN (29). Moreover, converging evidence from longitudinal EEG has revealed progressive restoration of EEG functional connectivity within the DMN, which was also paralleled by significant functional recovery (30). Together, this evidence suggests brain oscillations contribute to symptoms and can track recovery in the disorder, and large-scale neuronal oscillatory connectivity may explain emergent cognitive sequelae and comorbidities. In a previous study, we took an atlas-guided region-of-interest approach to characterize these metrics and examined how individual regions and connections were atypical in their graph properties; in this paper, we investigated how atypicalities in specialized ICNs and their connectivity are expressed following injury and related to the presence of symptoms.
Our aim was to determine changes in resting brain connectivity mediated by oscillations in established intrinsic brain networks after a single concussion—we predicted that connectivity would be reduced in concussion across multiple frequency scales, and particularly that of the DMN given recent findings (29). We explored whether these interactions were associated with primary symptoms and secondary conditions (attention, anxiety, and depression). We predicted that those with a concussion would express atypical and reduced ICN oscillatory connectivity and that neurophysiological network interactions would be negatively associated with the presence of primary and secondary symptoms.
Materials and Methods
Participants
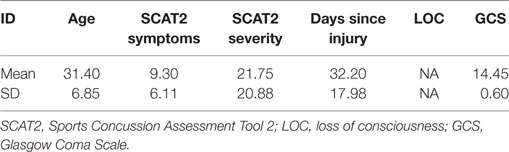
Resting-state MEG data were recorded from 26 male participants with a single concussive episode (scanned <3 months post-injury, mean days since injury = 32.20, SD = 17.98, mean age at injury = 31.4 years, SD = 6.87). Six datasets were excluded from the final analysis due to artifactual data (two due to dental work and four due to excessive head motion during the scan), for a total of 20 participants. The control group consisted of 24 age- and sex-matched participants (mean age = 27.0 years, SD = 5) without any self-reported history of head injury; for final analysis a subset of 20 participants who were most closely matched to the concussion group on age and IQ were used. All participants signed an informed written consent form, after receiving a description of the study. The Research Ethics Board of the Hospital for Sick Children, Toronto, ON, Canada, gave institutional approval for the study.
Participants with concussion were identified at the emergency department of Sunnybrook Health Science Centre (Toronto, ON, Canada). Inclusion criteria were between 20 and 40 years of age; concussion symptoms present during visit to emergency; the MEG scan within 3 months of injury; if loss of consciousness occurred, then less than 30 min; if post-traumatic amnesia occurred, then less than 24 h; causes of head injury were clear (e.g., sustaining a force to the head); Glasgow Coma Scale (GCS) ≥13 (within 24 h of injury); no skull fracture; unremarkable CT scan; and no previous incidence of concussion. Every participant was able to tolerate enclosed space for MR brain imaging; be English speaking; be able to comply with instructions to complete tasks during MEG and MR scans; be able to give informed consent. The control group had no history of TBI (mild, moderate, or severe) or neurological disorders. Exclusion criteria for both groups included ferrous metal inside the body that might be classified as MRI contraindications, or items that might interfere with MEG data acquisition; presence of implanted medical devices; seizures or other neurological disorders, or active substance abuse; certain ongoing medications (anticonvulsants, benzodiazepines, and/or GABA antagonists) known to directly or significantly influence EEG findings.
All participants underwent brief cognitive-behavioral testing in addition to the MEG resting-state scan. These assessments included estimates of IQ from the Wechsler Abbreviated Scale of Intelligence [WASI (31)]; Conner’s Attention-Deficit Hyperactivity Disorder Test (32); the Generalized Anxiety Disorder 7 test [GAD7 (33)]; Patient Health Questionnaire [PHQ9 (34)]; and the Sports Concussion Assessment Tool 2 [SCAT2 (35)].
Procedure and MEG Data Acquisition
Resting-state MEG data were collected while participants were lying supine, and instructed to rest with eyes open and maintain visual fixation on an X within a circle on a screen 60 cm from the eyes. Eyes open resting-state was chosen over eyes closed for two reasons: (1) eye movements are more readily suppressed with a visible target (in that movements are generated more often when eyes are closed, leading to ocular artifacts in the signal) and (2) large amplitude alpha rhythms are relatively suppressed with eyes open, which otherwise dominate the MEG signal. Data were collected inside a magnetically shielded room on a CTF Omega 151 channel system (CTF Systems, Inc., Coquitlam, BC, Canada) at The Hospital for Sick Children, with a sample rate of 600 Hz for 300 s. Throughout the scan, head position was continuously recorded by three fiducial coils placed on the nasion, and left and right pre-auricular points. Head motion exceeding 5 mm on any single epoch was omitted from further analysis (epoching described below).
Following the MEG session, a 3T anatomical MRI image was acquired using a Magnetom Tim Trio (Siemens AG, Erlangen, Germany). T1-weighted magnetic resonance images were acquired using 3D MPRAGE sequences (repetition time = 2,300 ms; echo time = 2.9 ms; flip angle = 9°; field-of-view = 28.8 cm × 19.2 cm; 256 × 240 matrix; 192 slices; 1 mm isovoxel) with a 12-channel head coil. MEG data were coregistered to the MRI structural images using the reference fiducial coil placements. A single-shell head model was constructed for each individual and brain space was normalized to a standard Montreal Neurological Institute brain using SPM2.
MEG Data Processing
Seed Definition and Virtual Electrode Output
Magnetoencephalography data were processed using a mixture of the FieldTrip toolbox (36) and in-house analysis scripts. Time-series were band-pass filtered offline at 1–150 Hz, a Discrete Fourier Transform notch filter applied at the 60 Hz powerline frequency and second harmonic, and a third-order spatial gradient environmental noise-cancellation was applied to the recording. A priori sources (seeds) of interest in cortical and sub-cortical regions were identified using coordinates from de Pasquale et al. (4) [ventral attention network (VAN), dorsal attention network, DMN, MOT, and visual network]. Figure 1A shows the node locations, Figure 1B shows the analysis pipeline.
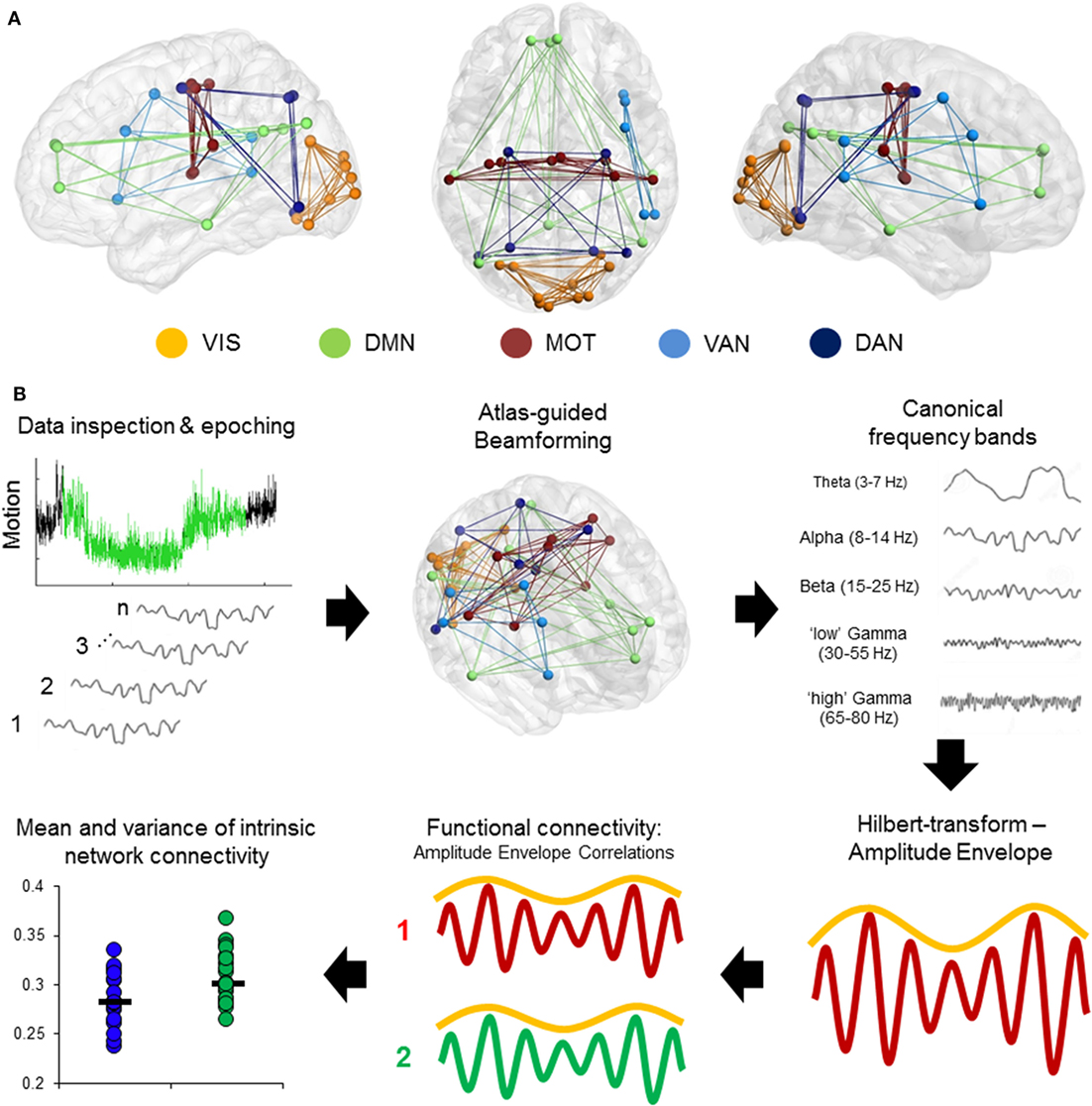
Figure 1. Computing intrinsic connectivity network (ICN) resting-state coupling. (A) Nodes and internal connections of the pre-defined ICNs seed regions based on Montreal Neurological Institute coordinates reported in the literature. (B) Analysis steps in the pipeline. Data were searched for 2 min of minimal head motion from the 5-min recording, and subsequently epoched into 12 s × 10 s segments. A vector beamformer was used to derive “virtual-sensor” time-series from all seeds and filtered into canonical frequency bands. A Hilbert transform was applied to derive estimates of the instantaneous amplitude envelope. Each pairwise combination of time-series amplitude envelopes inside a network were then correlated to define the degree of connectivity between nodes and averaged per epoch to derive a measure of internal connectivity. These were then both averaged over epochs and the SD calculated.
Time-series data were reconstructed from these seed locations using a linearly constrained minimum variance (vector) beamformer (37) for each subject and filtered into five canonical bandwidths for further analysis: theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz), gamma (30–55 Hz), and high-gamma (65–80 Hz). A beamformer is a spatial filter used to suppress signals from extrinsic neural and noise sources, while maintaining unit gain for activity in a target brain location. Individual weight vectors are applied to each sensor signal and summated to give estimated source activity at target seed locations effectively suppressing physiological artifacts not of interest (38, 39).
Statistical Analysis
Each of the extracted time courses for each frequency band from each subject had amplitude envelope correlations (AEC) calculated across each of the 10 s epochs—the envelope was based on the instantaneous amplitude estimate for each sample from the filtered time-series using the Hilbert transform. The magnitude of AEC between all pairwise combinations of the seeds varied between 1 (perfect correlation) and −1 (perfect anti-correlation). These values quantify the time-varying correlation in the envelope between any two sources, referred to henceforth as functional connectivity.
Connectivity matrices with AEC values acting as edge weights for all sources pairs were constructed, and the matrix of weighted undirected graphs in each analyzed frequency band for each participant were averaged to characterize the magnitude of spontaneous intra-network coupling. These were then either averaged over the 12 epochs (2-min run) to derive time-averaged connectivity, or the SD was calculated to define temporal dynamism (39, 40), albeit in network connectivity, rather than local source oscillatory amplitude. Connectivity matrices were then divided into the respective groups and inferential statistics investigating group differences for mean edge weight were implemented using non-parametric permutation testing (20,000 iterations), which do not require the data distributions to be normal. False positives due to multiple comparisons were controlled using Bonferroni-correction across frequency bands. Cognitive-behavioral correlation analyses were conducted using the MATLAB Statistics Toolbox (The Mathworks, Inc.). Networks were visualized and figures produced using BrainNet Viewer (41).
Results
Comorbid Symptoms in Concussion
All clinical outcome measures were greater in the concussion group than the control group (Conners, t = 2.52, p = 0.02; GAD7, t = 2.44, p = 0.02; PHQ9, t = 3.36, p = 0.002; SCAT2 symptoms, t = 4.53, p = < 0.001; SCAT2 severity, t = 3.09, p = 0.004), while being matched on the WASI (t = −1.79, p = 0.08). Table 1 shows concussion group demographic and injury information, including symptom number and severity, days since injury, whether loss of consciousness occurred and for how long, GCS score, presence of post-traumatic amnesia, and mechanism of injury. Symptom severity was most associated with and explained the greatest degree of variance in anxiety (R2 = 0.60, p < 0.001), followed by depression (R2 = 0.28, p < 0.001), and finally attention symptoms (R2 = 0.27, p < 0.001).
Increased DMN and MOT Resting Connectivity Following Concussion
Significantly increased DMN connectivity was observed in the alpha and beta ranges for the concussion group compared to controls (Bonferroni-corrected within frequency bands at p < 0.05; null distributions generated using 20,000 permutations, Figure 2). Connections between the ventro-medial prefrontal cortex (vmPFC), and the dorso-medial prefrontal cortex (dmPFC) and right medial prefrontal cortex (rmPFC) appeared to drive this difference, exhibiting the greatest degree of hypercoupling in the concussion group compared to controls. Elevated coupling in concussion was also found in the MOT across the alpha, beta, and gamma ranges (pcorrected < 0.05). No significant differences were observed in the SD of internal coupling across epochs (all p’s > 0.05).
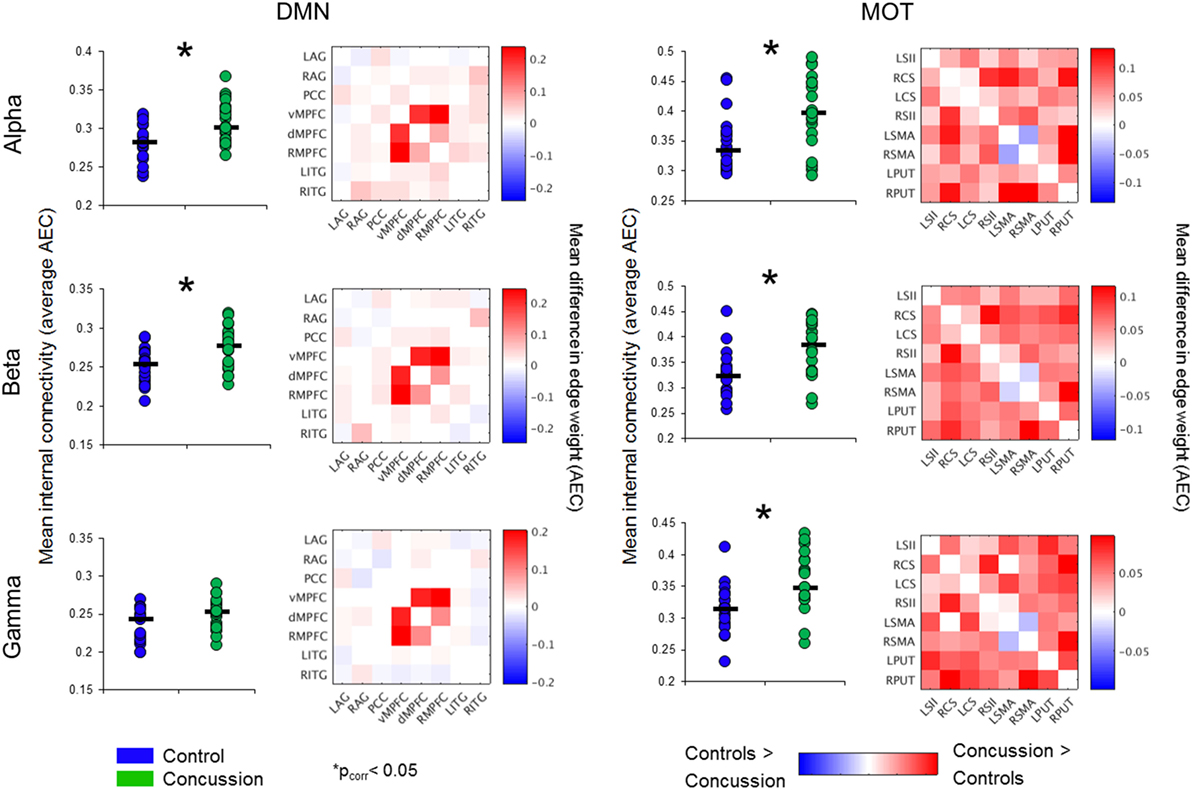
Figure 2. Intrinsic connectivity network spectral power content, averaged over node regions that comprise a network. Mean whole-network spectral power content with ±1 SE bars for the concussion (green) and control groups (blue). Of note is the 10 Hz alpha peak, prominent in the VIS. Abbreviations: DAN, dorsal attention network; VAN, ventral attention network; MOT, motor network; DMN, default mode network, VIS, visual network.
Resting-State Spectral Power Does Not Explain Differences in ICN Coupling
To determine the extent to which changes in intra-network coupling were dependent on oscillatory power, the mean internal power spectrum for each of the ICNs was calculated and divided into canonical frequency ranges—qualitative assessment of the spectrum show an apparent increase in low-frequency power/an alpha peak shift toward low-frequencies, particularly within the DMN (Figure 3). Mixed ANOVAs on each of the ICNs independently revealed a main effect of band (p < 0.001), as expected, but not of group or any interaction (p > 0.05). Post hoc contrasts between groups within bands revealed no significant differences (all p’s > 0.05). This suggests connectivity in the bands exhibiting between group differences is not explained by raw spectral power.
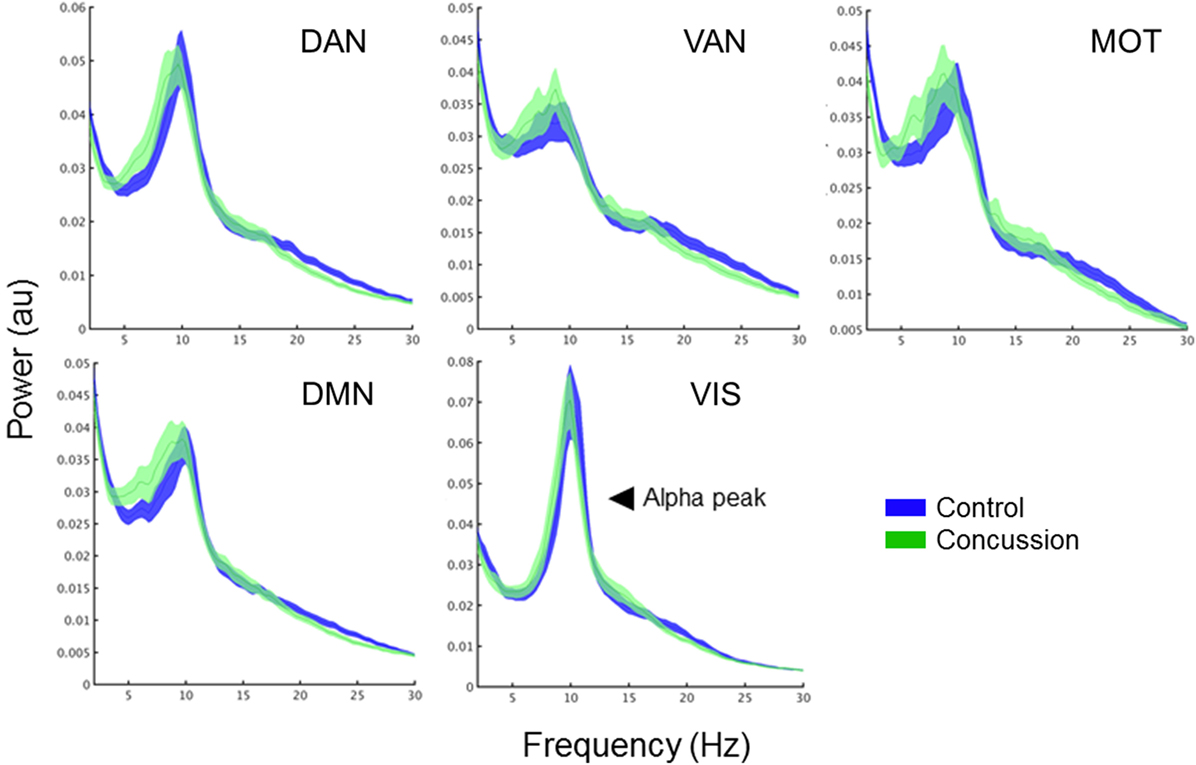
Figure 3. Band-specific ICN connectivity mediated by AEC. Scatter plot dots show intra-subject mean across epochs, black line represents median over participants for each group in the DMN (left column) and MOTs (right), in the alpha (top row), beta (middle row), and gamma ranges (bottom row). *pcorrected < 0.05. Connectivity matrices show edge weight differences (concussion minus controls) in respective ICNs—contrasts revealed the DMN increases in the concussion group were driven by ventro-medial prefrontal cortex (vmPFC) to right medial prefrontal cortex (rmPFC) and dorsal medial prefrontal cortex (dMPFC) connections. Abbreviations: AEC, amplitude envelope correlations; ICN, intrinsic connectivity network; DMN, default mode network; MOTs, motor networks.
DMN Connectivity Correlates With Symptoms
In addition to the main MEG findings, we also conducted follow-up analyses of the relation between network connectivity and concussion symptoms (Figure 4 shows scatter plots of connectivity versus symptom presence, and test statistics for full and partial correlations are detailed in Table 2). Specifically, we examined brain-behavior relations in the DMN and MOTs across frequency ranges where differences were observed in between groups contrasts; the alpha, beta, and gamma bands (Bonferroni-corrected across frequencies). Significant correlations (non-parametric Spearman’s Rho) with symptoms were observed in the DMN and MOT across alpha, beta, and low gamma ranges; however, partial correlations with comorbidity symptom scores (Conner’s, GAD7, and PHQ9) entered as covariates revealed that the variance in MOT connectivity was not solely driven by concussion symptoms. Factoring in these covariates reduced the full correlation coefficients, such that they were found to no longer be significant—critically, the DMN correlations remainedsignificant at the alpha and beta frequencies (Figure 4 shows original scatter plots linear least squares regression line).
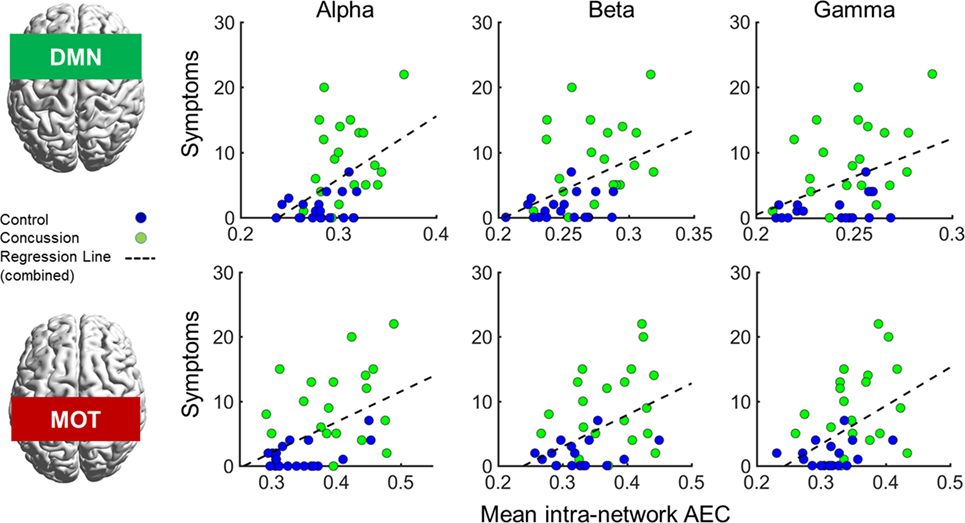
Figure 4. Correlations of internal default mode network (DMN; top row) and motor network (MOT; bottom row) connectivity versus concussion symptoms in the alpha, beta, and gamma ranges. Scatterplots show original (full) correlations and least squares fit line; test statistics for full and partial correlations are given in Table 2. Abbreviation: AEC, amplitude envelope correlations.
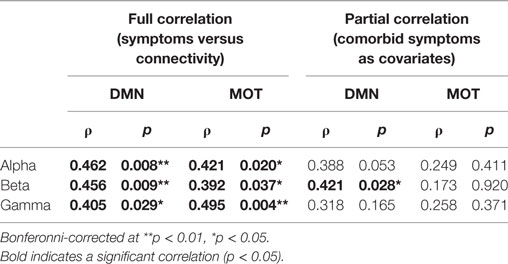
Table 2. Correlations of band-limited connectivity in the default mode network (DMN) and motor network (MOT) against symptoms.
Discussion
Summary
In this study, we used MEG to investigate frequency-specific interactions within functional ICNs in adults with a single concussion compared to a matched control group. We found that a single concussion was associated with increased functional connectivity mediated via band-limited AEC—specifically, elevated coupling within DMN and MOTs, and importantly, in the absence of canonical band power spectrum differences. Intra-DMN connectivity was positively associated with concussion symptoms, even when controlling for secondary/comorbid outcomes (depression, anxiety, and attentional problems), which suggests that the internal coupling of this task-negative (42) and dynamic “cortical-core” network (4) is particularly prone to the effects of even relatively mild traumatic brain injuries. In addition, elevated MOT connectivity was also associated with symptoms and secondary sequelae.
Elevated Default Mode Coupling in Concussion
We found altered oscillatory-mediated connectivity in the DMN in concussion patients. These results appear in opposition to previous fMRI literature examining BOLD fluctuations (13, 15, 43, 44). The DMN, known as the primary resting-state network, involves coordinated action between the posterior cingulate cortex (PCC), bilateral angular gyri, ventro-medial prefrontal cortex (vmPFC), dorso-medial prefrontal cortex (dmPFC), and inferior temporal gyri. Studies evaluating the slow frequency hemodynamic components have shown overall reductions in connectivity of the DMN in mTBI, especially in posterior regions such as the PCC, inferior parietal, and precuneus (13, 45). By contrast, we found overall increased mean coupling within the default mode. Further evaluation of internal edge weights found that these changes were mainly driven by hypercoupling between the vmPFC, dmPFC, and rmPFC frontal regions, in contrast to the posterior bias reported in fMRI work.
This study shows the value of using MEG to determine which neurophysiological mechanisms underlying resting-state networks are impacted by concussion. While it has been shown that the ultra-slow BOLD is recapitulated and positively correlated with AEC in alpha and beta bands in MEG (18, 46, 47), this is possibly not true in brain injury where the mechanisms of interregional coupling (that is phase based or envelope coupled) do not function as expected. Apparently conflicting observations—that DMN connectivity in fMRI and MEG positively correlate in healthy participants, but in concussion, patients show differential connectivity across modalities, decreased in fMRI and increased in MEG—could be due to the impact of injury on distinct mechanisms, such as hemodynamics and neurovascular coupling, changes in regional or global cerebral perfusion, disruption to the neurometabolic balance, and oxidative stress (i.e., endogenous changes in neuronal excitability). Other recent electrophysiological studies have found reduced functional connectivity based on synchronization in the DMN, in a case study of severe TBI using EEG (30), and in a study of chronic mTBI patients using MEG. Together, these further suggest that brain injury, across the severity spectrum, affects neurophysiological fingerprints. These varying results should be considered in light of the fact that all of these measures of connectivity differ (amplitude envelope coupling versus phase synchrony and BOLD correlations), the severity of injury [severe TBI in the case of Ref. (30)] and capture distinct neural mechanisms in brain communication that are either more closely associated with inner mental state and cognition (phase ICNs) or the underlying structural connectivity (21, 48). Moreover, the time since injury to scan was different in all studies, capturing either acute/subacute (as here), or chronic stages (29). These different results could perhaps be explained by compensation mechanisms—that during the acute/subacute recovery, the hyperconnectivity is a reflection of an attempt to reorganize networks. Clearly, these results suggest a multimodal imaging and a synergistic approach to connectivity is required to explain the complexity of pathology in concussion.
The DMN is a task-negative network involved in self-referential processes, such as introspection and experiential memory (11, 49–52), that contains “hubs” that also facilitate task-switching and engagement of other functional networks (4), such as those linked to memory, attention, and executive function (53, 54). These are cognitive domains often reported to be dysfunctional in concussion (55–57), and in light of the DMN’s role in cognition and network toggling, the hyperconnectivity we report might reflect an inability to disengage the DMN in response to shifting task demands, and explain some of the emergent cognitive deficits seen here. The DMN’s critical role in neuropsychopathology and cognitive deficits is further supported by studies of other disorders, including ADHD (58), Alzheimer’s disease (59, 60), PTSD (28, 61), ASD (62, 63), depression (64), and schizophrenia (65).
Oscillations underlie communication processes between brain regions (i.e., within or between brain network connectivity) (21, 23, 66). The complex interplay among regions that comprise the DMN, and ICNs more generally, and how they are mediated by frequency-specific connectivity mechanisms, further highlights the important role of oscillations and their contribution to network architecture and interregional communication—perhaps most importantly, these results show that they are susceptible to perturbations through injury. These neurophysiological processes are intrinsically linked to the topological patterns of communication that constitute functional resting networks (18, 67). Any disruption in the internal balance of these networks may impact the ability for them to switch, engage, or separate—altering cognitive efficiency or recruitment processes. For example, the task-positive executive network is active during externally directed behavior and requires communication between bilateral DLPFC and PCC. However, in the concussion cohort, the connectivity patterns of these brain regions were altered and thus we speculate that task related activity would also be effected. We believe these observations show dysfunctional integration within networks, and an inability to efficiently segregate/decouple areas (6). Thus, a disruption in the internal balance could lead to altered cognition, consistently shown to occur following concussion (56, 68, 69).
Increased MOT Connectivity
In addition to our DMN observations, we also found that the MOT showed multiscale network dysfunction, including the beta band. Beta oscillations serve a critical role in long-range synchrony (70), with the connectivity of these ICNs related to structural connections linking distinct anatomical areas (71); these connections rely on white matter tracts to facilitate signal conduction and increase velocity (72). In patients with Parkinson’s disease, increases in resting-state MOT beta connectivity has been related to slower reaction times during visual-motor-related tasks (73) and similar changes to reaction time are evident in concussion (74). However, the increased MOT connectivity could be a general indicator of functional changes following concussion (e.g., fatigue) and not specific to the symptom scale we used in this study.
Increased beta in the MOT may be due to a variety of neural circuitry changes, possibly related to GABAergic action and concentration (75, 76). Changes to spectral connectivity could cause myriad neurophysiological changes, including altered inhibitory input and changes to excitatory threshold which could alter the connectivity and oscillatory patterns among the brain regions within the MOT. In the motor cortex, beta oscillatory power is high during rest and decreases during movement, known as the event-related-beta desynchronization, related to local processing during movement (73).
As neural oscillations are thought to be a managed through a relation between excitatory and inhibitory neurotransmission and related to resting GABA concentration (76), the increased beta amplitude coupling within the MOT could reflect an increase in cross-regional inhibitory processes, that could be the cause of increased motor threshold (77), and inhibition and excitability in motor cortex, as measured by transcranial magnetic stimulation in concussion (78). Furthermore, increased GABA concentration, an inhibitory neurotransmitter in the motor cortex has been reported in animal and human studies of concussion (79, 80). Although these changes in circuitry are evident at the neuronal level, they will also impact short and long-range connectivity patterns to which MEG is sensitive.
Local alpha oscillations and their regional coupling are thought to facilitate information integration and segregation within and across neural populations via modulation and gating of local inhibition and excitation (81, 82). These multi-frequency abnormalities suggest disruption to communication channels and align with mechanisms of injury where high tensile and sheering forces have been shown to alter white matter connections and therefore local neural activity and large-scale synchrony. Thus, the functional deficits from injury may alter the coordination of information transfer and integration across a variety of complex processes, including oscillatory coupling (investigated here), and phase-amplitude coupling and cross-frequency interactions to which MEG is sensitive (26, 83) and have shown to be affected by concussion (84). As such, the heterogeneous nature of concussion can lead to various changes in brain function on local and global scales, which aligns with the results we found of spectral connectivity changes.
Relations Between Functional Connectivity and Structural Architecture
Cartography of the human “connectome” has seen a surge in recent years, but progress has been slow in elucidating the association between electrophysiological connectivity and structural connectivity. Preclinical work using optogenetics to stimulate neuronal (electrical) activity has shown that this promotes adaptive myelination (85), and a recent human study has proposed that there is a strong link between neural connectonomics, their dynamics, and cortical white matter structure [or “myeloarchitecture” (67)]. It seems reasonable to posit, given these findings, that our observations in altered connectivity of cortical ICNs could be related to neuromorphological changes (86), and more specially microscopic white matter pathology following concussion, including demyelination and/or differentiation (87, 88), reductions in cortical thickness (89–91), and Wallerian degeneration (92, 93).
Several hypotheses have been proposed to better understand the underlying neurophysiological causes of increases in resting-state connectivity. One frame of thought suggests that connectivity increases could be a result of compensation for injury. Specifically, hyperconnectivity could be representative of recruitment of additional resources to maintain cognitive function—the elevated connectivity in frontal regions of the DMN in the concussion group aligns to the general findings of frontal susceptibility to injury [for a review, see Ref. (94)]. The disruption to white matter tracts could cause changes in the long-range connections (such as between PCC and frontal regions) and result in increases in small-world connectivity. This can be seen in our results across the DMN, particularly that increases in spectral connectivity can be attributed to the hypercoupling in frontal regions.
Conclusion
A particular focus in concussion research is to better understand the link between symptom severity/presence and altered brain function, both in the short and long term. We found a significant relation between symptoms and increases in spectrum connectivity in the DMN and MOT. The association between DMN and symptoms was maintained even while controlling for secondary factors as anxiety and depression. Given the critical role of the DMN in cognitive functions, these abnormal patterns may well underlie the cognitive difficulties so often reported following concussion. These results, when combined with observations from other studies, suggest that MEG may be able to identify electrophysiological changes along the temporal continuum of recovery, from the short term, acute stages of injury, through to later, chronic phases. In conclusion, longitudinal MEG studies of neurophysiological function may be able to link symptoms with intrinsic function and predict the course of recovery in individuals.
Ethics Statement
All participants signed an informed written consent form, after receiving a description of the study. The Research Ethics Board of the Hospital for Sick Children gave institutional approval for the study.
Author Contributions
BD, LC, EP, and MT conceived and designed the study, and collected the data. BD, KU, and SW analyzed the data. BD, KU, LC, EP, and MT interpreted the data and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks are given to Amanda Robertson, Allison Bethune, and Marc Lalancette for help in the data collection, and Simeon Wong for help with the data pre-processing. This work was supported by funding from Defence Research and Development Canada (DRDC) (Contract #: W7719-135182/001/TOR) and the Canadian Forces Health Services to MT and EP.
References
1. Shah-Basak P, Urbain C, Wong S, Da Costa L, Pang EW, Dunkley B, et al. Concussion alters the functional brain processes of visual attention and working memory. J Neurotrauma (2017) 35(2):267–77. doi:10.1089/neu.2017.5117
2. Dunkley BT, Pang EW, Sedge PA, Jetly R, Doesburg SM, Taylor MJ. Corrigendum to “Threatening faces induce fear circuitry hypersynchrony in soldiers with post-traumatic stress disorder”. Heliyon (2016) 2(12):e00145. doi:10.1016/j.heliyon.2016.e00145
3. Vakorin VA, Doesburg SM, da Costa L, Jetly R, Pang EW, Taylor MJ. Detecting mild traumatic brain injury using resting state magnetoencephalographic connectivity. PLoS Comput Biol (2016) 12(12):e1004914. doi:10.1371/journal.pcbi.1004914
4. de Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, et al. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron (2012) 74(4):753–64. doi:10.1016/j.neuron.2012.03.031
5. Antonakakis M, Dimitriadis SI, Zervakis M, Papanicolaou AC, Zouridakis G. Reconfiguration of dominant coupling modes in mild traumatic brain injury mediated by δ-band activity: a resting state MEG study. Neuroscience (2017) 356:275–86. doi:10.1016/j.neuroscience.2017.05.032
6. Dunkley BT, Da Costa L, Bethune A, Jetly R, Pang EW, Taylor MJ, et al. Low-frequency connectivity is associated with mild traumatic brain injury. Neuroimage Clin (2015) 7:611–21. doi:10.1016/j.nicl.2015.02.020
7. Huang M-X, Harrington DL, Robb Swan A, Angeles Quinto A, Nichols S, Drake A, et al. Resting-state magnetoencephalography reveals different patterns of aberrant functional connectivity in combat-related mild traumatic brain injury. J Neurotrauma (2016) 34(7):1412–26. doi:10.1089/neu.2016.4581
8. Doesburg SM, Ribary U, Herdman AT, Miller SP, Poskitt KJ, Moiseev A, et al. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage (2011) 54(3):2330–9. doi:10.1016/j.neuroimage.2010.10.044
9. Doesburg SM, Ribary U, Herdman AT, Moiseev A, Cheung T, Miller SP, et al. Magnetoencephalography reveals slowing of resting peak oscillatory frequency in children born very preterm. Pediatr Res (2011) 70(2):171–5. doi:10.1203/PDR.0b013e3182225a9e
10. Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci (2007) 30(7):317–24. doi:10.1016/j.tins.2007.05.001
11. Fingelkurts AA, Fingelkurts AA. Persistent operational synchrony within brain default-mode network and self-processing operations in healthy subjects. Brain Cogn (2011) 75(2):79–90. doi:10.1016/j.bandc.2010.11.015
12. Chen J-K, Johnston KM, Collie A, McCrory P, Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry (2007) 78(11):1231–8. doi:10.1136/jnnp.2006.110395
13. Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Map (2011) 32(11):1825–35. doi:10.1002/hbm.21151
14. Tarapore PE, Findlay AM, Lahue SC, Lee H, Honma SM, Mizuiri D, et al. Resting state magnetoencephalography functional connectivity in traumatic brain injury. J Neurosurg (2013) 118(6):1306–16. doi:10.3171/2013.3.JNS12398
15. Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, et al. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage (2012) 59(1):511–8. doi:10.1016/j.neuroimage.2011.07.081
16. Hari R, Salmelin R. Magnetoencephalography: from SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. Neuroimage (2012) 61(2):386–96. doi:10.1016/j.neuroimage.2011.11.074
17. Baker AP, Brookes MJ, Rezek IA, Smith SM, Behrens T, Smith PJP, et al. Fast transient networks in spontaneous human brain activity. Elife (2014) 3:1–18. doi:10.7554/eLife.01867
18. Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale, Stephenson M, et al. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci U S A (2011) 108(40):16783–8. doi:10.1073/pnas.1112685108
19. Başar E, Başar-Eroglu C, Karakaş S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett (1999) 259(3):165–8. doi:10.1016/S0304-3940(98)00934-3
20. Başar E, Özgören M, Karakaş S, Başar-Eroğlu C. Super-synergy in the brain: the grandmother percept is manifested by multiple oscillations. Int J Bifurcation Chaos (2004) 14(2):453–91. doi:10.1142/S0218127404009272
21. Engel AK, Gerloff C, Hilgetag CC, Nolte G. Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron (2013) 80(4):867–86. doi:10.1016/j.neuron.2013.09.038
22. Fingelkurts AA, Fingelkurts AA. EEG oscillatory states: universality, uniqueness and specificity across healthy-normal, altered and pathological brain conditions. PLoS One (2014) 9(2):e87507. doi:10.1371/journal.pone.0087507
23. Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci (2005) 9(10):474–80. doi:10.1016/j.tics.2005.08.011
24. Stitt I, Galindo-leon E, Pieper F, Engler G, Fiedler E, Stieglitz T, et al. Intrinsic coupling modes reveal the functional architecture of cortico-tectal networks. Sci Adv (2015) 1(7):e1500229. doi:10.1126/sciadv.1500229
25. Zumer JM, Scheeringa R, Schoffelen JM, Norris DG, Jensen O. Occipital alpha activity during stimulus processing gates the information flow to object-selective cortex. PLoS Biol (2014) 12(10):e1001965. doi:10.1371/journal.pbio.1001965
26. Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat Neurosci (2017) 20(3):327–39. doi:10.1038/nn.4504
27. da Costa L, Robertson A, Bethune A, MacDonald MJ, Shek PN, Taylor MJ, et al. Delayed and disorganised brain activation detected with magnetoencephalography after mild traumatic brain injury. J Neurol Neurosurg Psychiatry (2014) 86:1008–15. doi:10.1136/jnnp-2014-308571
28. Huang M-X, Yurgil KA, Robb A, Angeles A, Diwakar M, Risbrough VB, et al. Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. Neuroimage Clin (2014) 5:408–19. doi:10.1016/j.nicl.2014.08.004
29. Alhourani A, Wozny TA, Krishnaswamy D, Pathak S, Walls SA, Ghuman AS, et al. Magnetoencephalography-based identification of functional connectivity network disruption following mild traumatic brain injury. J Neurophysiol (2016) 116(4):1840–7. doi:10.1152/jn.00513.2016
30. Fingelkurts AA, Fingelkurts AA. Longitudinal dynamics of 3-dimensional components of selfhood after severe traumatic brain injury: a qEEG case study. Clin EEG Neurosci (2017) 48(5):327–37. doi:10.1177/1550059417696180
31. Wechsler D. The Wechsler Abbreviated Scale of Intelligence (WASI). New York, NY: The Psychological Corporation, Harcourt Brace & Company (1999).
32. Conners CK, Pitkanen J, Rzepa SR. Conners 3rd edition (Conners 3; Conners 2008). In: Kreutzer JS, DeLuca J, Caplan B editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer (2011). p. 675–678.
33. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med (2006) 166(10):1092–97. doi:10.1001/archinte.166.10.1092
34. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med (2001) 16(9):606–13. doi:10.1046/j.1525-1497.2001.016009606.x
35. McCrory P. Sport concussion assessment tool 2. Scand J Med Sci Sports (2009) 19:452. doi:10.1111/j.1600-0838.2009.00978.x
36. Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci (2011) 2011:156869. doi:10.1155/2011/156869
37. Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Bio Med Eng (1997) 44(9):867–80. doi:10.1109/10.623056
38. Cheyne D, Bostan AC, Gaetz W, Pang EW. Event-related beamforming: a robust method for presurgical functional mapping using MEG. Clin Neurophysiol (2007) 118(8):1691–704. doi:10.1016/j.clinph.2007.05.064
39. Muthukumaraswamy SD. High-frequency brain activity and muscle artifacts in MEG/EEG: a review and recommendations. Front Hum Neurosci (2013) 7(April):138. doi:10.3389/fnhum.2013.00138
40. Koelewijn L, Hamandi K, Brindley LM, Brookes MJ, Routley BC, Muthukumaraswamy SD, et al. Resting-state oscillatory dynamics in sensorimotor cortex in benign epilepsy with centro-temporal spikes and typical brain development. Hum Brain Mapp (2015) 3949:3935–49. doi:10.1002/hbm.22888
41. Xia M, Wang J, He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One (2013) 8(7):e68910. doi:10.1371/journal.pone.0068910
42. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A (2001) 98(2):676–82. doi:10.1073/pnas.98.2.676
43. McDonald BC, Saykin AJ, McAllister TW. Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav (2012) 6(2):193–207. doi:10.1007/s11682-012-9173-4
44. Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, et al. Default-mode network disruption in mild traumatic brain injury. Radiology (2012) 265(3):882–92. doi:10.1148/radiol.12120748
45. Kou Z, Iraji A. Imaging brain plasticity after trauma. Neural Regen Res (2014) 9(7):693–700. doi:10.4103/1673-5374.131568
46. Brookes MJ, O’Neill GC, Hall EL, Woolrich MW, Baker A, Palazzo Corner S, et al. Measuring temporal, spectral and spatial changes in electrophysiological brain network connectivity. Neuroimage (2014) 91(2014):282–99. doi:10.1016/j.neuroimage.2013.12.066
47. Brookes MJ, Hale JR, Zumer JM, Stevenson CM, Francis ST, Barnes GR, et al. Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage (2011) 56(3):1082–104. doi:10.1016/j.neuroimage.2011.02.054
48. Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci (2012) 13:20–5. doi:10.1038/nrn3137
49. Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci (2007) 11(2):49–57. doi:10.1016/j.tics.2006.11.004
50. Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage (2011) 57(3):1221–33. doi:10.1016/j.neuroimage.2011.05.028
51. Raichle ME. The brain’s default mode network. Annu Rev Neurosci (2015) 38(1):433–47. doi:10.1146/annurev-neuro-071013-014030
52. Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn (2008) 17(2):457–67. doi:10.1016/j.concog.2008.03.013
53. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci (2008) 1124:1–38. doi:10.1196/annals.1440.011
54. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus independant thought. Science (2007) 315(5810):393–5. doi:10.1126/science.1131295
55. Baillargeon A, Lassonde M, Leclerc S, Ellemberg D. Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Inj (2012) 26(3):211–20. doi:10.3109/02699052.2012.654590
56. Tapper A, Gonzalez D, Roy E, Niechwiej-Szwedo E. Executive function deficits in team sport athletes with a history of concussion revealed by a visual-auditory dual task paradigm. J Sports Sci (2016) 414:1–10. doi:10.1080/02640414.2016.1161214
57. van der Naalt J, van Zomeren AH, Sluiter WJ, Minderhoud JM. One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complaints and return to work. J Neurol Neurosurg Psychiatry (1999) 66(2):207–13. doi:10.1136/jnnp.66.2.207
58. Lansbergen MM, Arns M, van Dongen-Boomsma Martine M, Spronk D, Buitelaar JK. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog Neuropsychopharmacol Biol Psychiatry (2011) 35(1):47–52. doi:10.1016/j.pnpbp.2010.08.004
59. Stam CJ, Jones BF, Manshanden I, van Cappellen, van Walsum AM, Montez T, et al. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuroimage (2006) 32(3):1335–44. doi:10.1016/j.neuroimage.2006.05.033
60. Stam CJ, De Haan W, Daffertshofer A, Jones BF, Manshanden I, Van Cappellen Van Walsum AM, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain (2009) 132(1):213–24. doi:10.1093/brain/awn262
61. Dunkley BT, Doesburg SM, Sedge PA, Grodecki RJ, Shek PN, Pang EW, et al. Resting-state hippocampal connectivity correlates with symptom severity in post-traumatic stress disorder. Neuroimage Clin (2014) 5:377–84. doi:10.1016/j.nicl.2014.07.017
62. Ye AX, Leung RC, Schaefer CB, Taylor MJ, Doesburg SM, Schäfer CB, et al. Atypical resting synchrony in autism spectrum disorder. Hum Brain Mapp (2014) 35(12):6049–66. doi:10.1002/hbm.22604
63. Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, et al. Default mode network segregation and social deficits in autism spectrum disorder: evidence from non-medicated children DMN in children with ASD. Neuroimage Clin (2015) 9:223–32. doi:10.1016/j.nicl.2015.07.018
64. Nugent AC, Robinson SE, Coppola R, Furey ML, Zarate CA. Group differences in MEG-ICA derived resting state networks: application to major depressive disorder. Neuroimage (2015) 118:1–12. doi:10.1016/j.neuroimage.2015.05.051
65. Camchong J, MacDonald AW, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull (2011) 37(3):640–50. doi:10.1093/schbul/sbp131
66. Fries P. Rhythms for cognition: communication through coherence. Neuron (2015) 88(1):220–35. doi:10.1016/j.neuron.2015.09.034
67. Hunt BAE, Tewarie PK, Mougin OE, Geades N, Jones DK, Singh KD, et al. Relationships between cortical myeloarchitecture and electrophysiological networks. Proc Natl Acad Sci U S A (2016) 113(47):13510–5. doi:10.1073/pnas.1608587113
68. Banks SD, Coronado RA, Clemons LR, Abraham CM, Pruthi S, Conrad BN, et al. Thalamic functional connectivity in mild traumatic brain injury: longitudinal associations with patient-reported outcomes and neuropsychological tests. Arch Phys Med Rehabil (2016) 97(8):1254–61. doi:10.1016/j.apmr.2016.03.013
69. Brown JA, Dalecki M, Hughes C, Macpherson AK, Sergio LE. Cognitive-motor integration deficits in young adult athletes following concussion. BMC Sports Sci Med Rehabil (2015) 7(25):25. doi:10.1186/s13102-015-0019-4
70. Engel AK, Fries P. Beta-band oscillations-signalling the status quo? Curr Opin Neurobiol (2010) 20(2):156–65. doi:10.1016/j.conb.2010.02.015
71. Park H-J, Friston K. Structural and functional brain networks: from connections to cognition. Science (2013) 342(6158):1238411. doi:10.1126/science.1238411
72. Voets NL, Beckmann CF, Cole DM, Hong S, Bernasconi A, Bernasconi N. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain (2012) 135(8):2350–7. doi:10.1093/brain/aws137
73. Pfurtscheller G, Zalaudek K, Neuper C. Event-related beta synchronization after wrist, finger and thumb movement. Electroencephalogr Clin Neurophysiol (1998) 109(2):154–60. doi:10.1016/S0924-980X(97)00070-2
74. Warden DL, Bleiberg J, Cameron KL, Ecklund J, Walter J, Sparling MB, et al. Persistent prolongation of simple reaction time in sports concussion. Neurology (2001) 57(3):524–6. doi:10.1212/WNL.57.3.524
75. Fingelkurts AA, Fingelkurts AA, Kivisaari R, Pekkonen E, Ilmoniemi RJ, Kähkönen S. Enhancement of GABA-related signalling is associated with increase of functional connectivity in human cortex. Hum Brain Map (2004) 22(1):27–39. doi:10.1002/hbm.20014
76. Gaetz W, Edgar JC, Wang DJ, Roberts TPL. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage (2011) 55(2):616–21. doi:10.1016/j.neuroimage.2010.12.077
77. Tallus J, Lioumis P, Hämäläinen H, Kähkönen S, Tenovuo O. Long-lasting TMS motor threshold elevation in mild traumatic brain injury. Acta Neurol Scand (2012) 126(3):178–82. doi:10.1111/j.1600-0404.2011.01623.x
78. Pearce AJ, Hoy K, Rogers MA, Corp DT, Maller JJ, Drury HGK, et al. The long-term effects of sports concussion on retired Australian football players: a study using transcranial magnetic stimulation. J Neurotrauma (2014) 31:1139–45. doi:10.1089/neu.2013.3219
79. Guerriero R, Jean M, Giza CC, Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep (2015) 15(5):1–11. doi:10.1007/s11910-015-0545-1
80. Tremblay S, Beaulé V, Proulx S, Tremblay S, Marjańska M, Doyon J, et al. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Neurophysiol Clin (2014) 125(7):1371–9. doi:10.1016/j.clinph.2013.11.040
81. Doesburg SM, Green JJ, McDonald JJ, Ward LM. From local inhibition to long-range integration: a functional dissociation of alpha-band synchronization across cortical scales in visuospatial attention. Brain Res (2009) 1303:97–110. doi:10.1016/j.brainres.2009.09.069
82. Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci (2010) 4:186. doi:10.3389/fnhum.2010.00186
83. Florin E, Baillet S. The brain’s resting-state activity is shaped by synchronized cross-frequency coupling of oscillatory neural activity. Neuroimage (2015) 111:26–35. doi:10.1016/j.neuroimage.2015.01.054
84. Antonakakis M, Dimitriadis SI, Zervakis M, Micheloyannis S, Rezaie R, Babajani-Feremi A, et al. Altered cross-frequency coupling in resting-state MEG after mild traumatic brain injury. Int J Psychophysiol (2016) 102:1–11. doi:10.1016/j.ijpsycho.2016.02.002
85. Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science (2014) 344(6183):1252304. doi:10.1126/science.1252304
86. Sussman D, da Costa L, Chakravarty M, Pang EW, Taylor MJ, Dunkley BT. Concussion induces focal and widespread neuromorphological changes. Neurosci Lett (2017) 650:52–9. doi:10.1016/j.neulet.2017.04.026
87. Huang M-X, Nichols S, Robb A, Angeles A, Drake A, Holland M, et al. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage (2012) 61(4):1067–82. doi:10.1016/j.neuroimage.2012.04.029
88. McAllister TW, Ford JC, Ji S, Beckwith JG, Flashman LA, Paulsen K, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng (2012) 40(1):127–40. doi:10.1007/s10439-011-0402-6
89. Hamberger A, Viano DC, Säljö A, Bolouri H. Concussion in professional football: morphology of brain injuries in the NFL concussion model – part 16. Neurosurgery (2009) 64(6):1174–82. doi:10.1227/01.NEU.0000316855.40986.2A
90. Lewén A, Fredriksson A, Li GL, Olsson Y, Hillered L, Lobato RD, et al. Behavioural and morphological outcome of mild cortical contusion trauma of the rat brain: influence of NMDA-Receptor blockade. Acta Neurochir (1999) 141(2):193–202. doi:10.1007/s007010050286
91. Urban KJ, Riggs L, Wells GD, Keightley M, Chen J-K, Ptito A, et al. Cortical thickness changes and their relationship to dual-task performance following mild traumatic brain injury in youth. J Neurotrauma (2016) 34(4):816–23. doi:10.1089/neu.2016.4502
92. Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol (2015) 127:45–66. doi:10.1016/B978-0-444-52892-6.00004-0
93. Smith DH, Hicks R, Povlishock JT. Therapy development for diffuse axonal injury. J Neurotrauma (2013) 30(5):307–23. doi:10.1089/neu.2012.2825
Keywords: concussion, mild traumatic brain injury, magnetoencephalography, resting-state, functional connectivity, neural oscillations, synchrony
Citation: Dunkley BT, Urban K, Da Costa L, Wong SM, Pang EW and Taylor MJ (2018) Default Mode Network Oscillatory Coupling Is Increased Following Concussion. Front. Neurol. 9:280. doi: 10.3389/fneur.2018.00280
Received: 19 December 2017; Accepted: 09 April 2018;
Published: 26 April 2018
Edited by:
Marco Sarà, San Raffaele Cassino, ItalyReviewed by:
Sergio Bagnato, Fondazione Istituto G. Giglio di Cefalù, ItalyAlexander Fingelkurts, BM-Science, Finland
Copyright: © 2018 Dunkley, Urban, Da Costa, Wong, Pang and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin T. Dunkley, YmVuLmR1bmtsZXlAc2lja2tpZHMuY2E=
 Benjamin T. Dunkley
Benjamin T. Dunkley Karolina Urban
Karolina Urban Leodante Da Costa
Leodante Da Costa Simeon M Wong
Simeon M Wong Elizabeth W. Pang
Elizabeth W. Pang Margot J. Taylor
Margot J. Taylor