
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 26 March 2018
Sec. Epilepsy
Volume 9 - 2018 | https://doi.org/10.3389/fneur.2018.00195
 Iris Unterberger1
Iris Unterberger1 Laura Zamarian1*
Laura Zamarian1* Manuela Prieschl1
Manuela Prieschl1 Melanie Bergmann1
Melanie Bergmann1 Gerald Walser1
Gerald Walser1 Gerhard Luef1
Gerhard Luef1 Andrija Javor2
Andrija Javor2 Gerhard Ransmayr2
Gerhard Ransmayr2 Margarete Delazer1
Margarete Delazer1
It is not known whether patients with juvenile myoclonic epilepsy (JME) differ from healthy people in decision making under risk, i.e., when the decision-making context offers explicit information about options, probabilities, and consequences already from the beginning. In this study, we adopted the Game of Dice Task-Double to investigate decision making under risk in a group of 36 patients with JME (mean age 25.25/SD 5.29 years) and a group of 38 healthy controls (mean age 26.03/SD 4.84 years). Participants also underwent a comprehensive neuropsychological assessment focused on frontal executive functions. Significant group differences were found in tests of psychomotor speed and divided attention, with the patients scoring lower than the controls. Importantly, patients made risky decisions more frequently than controls. In the patient group, poor decision making was associated with poor executive control, poor response inhibition, and a short interval since the last seizure episode. Executive control and response inhibition could predict 42% of variance in the frequency of risky decisions. This study indicates that patients with JME with poorer executive functions are more likely to make risky decisions than healthy controls. Decision making under risk is of major importance in every-day life, especially with regard to treatment decisions and adherence to long-term medical therapy. Since even a single disadvantageous decision may have long-lasting consequences, this finding is of high relevance.
Juvenile myoclonic epilepsy (JME) is the most common idiopathic generalized epilepsy syndrome, accounting for up to 12% of all epilepsies (1), with seizures typically manifesting around adolescence (2–4). Behavioral disturbances and psychiatric disorders have been reported to occur in approximately one third of patients with JME (5, 6), despite normal intelligence and unremarkable routine brain imaging (7). Functional and microstructural changes in the medial and dorsolateral prefrontal cortex and related subcortical structures have been recently reported for patients with JME by adopting advanced brain imaging methods (5–10). Correspondingly, several studies have suggested a distinct cognitive profile in JME, resembling predominantly frontal lobe dysfunction with disturbances in attention and executive functions (11–14). Recent studies have also shown that patients with JME perform lower than healthy controls when the decision-making context is (at least initially) ambiguous and learning from feedback is required (10, 14). So far, it is not known whether patients with JME would differ from healthy people in decision making under risk, i.e., when the decision-making context offers explicit information about options, probabilities, and consequences from the beginning, and optimal decisions can be estimated or also computed. This type of decision is of major importance in every-day life, especially with regard to treatment decisions and adherence to long-term medical therapy. The Game of Dice Task-Double (GDT-D) (15), a modified version of the GDT (16), is a computerized gambling task assessing decision making under risk. As executive functions play a major role in decision making under risk (17, 18), it could be hypothesized that patients with JME and lower executive functions tend to make more risky decisions compared to healthy controls. Difficulties in risk-related decision making are associated with lower quality of life and may even lead to long-lasting, negative consequences (19). An exact characterization of the problems encountered by this younger patient group is therefore highly relevant.
In this study, we aimed to investigate decision making under risk, when full information about options and consequences is provided, in both patients with JME and healthy controls which to our knowledge has not been done before. Furthermore, we wanted to examine the relationship between decision making under risk, neuropsychological performance, and clinical characteristics of patients with JME.
A total of 36 consecutive patients with classical JME were recruited at the outpatient epilepsy clinic of Medical University of Innsbruck between July 2013 and July 2016. The diagnosis of JME was based on the criteria of the International League against Epilepsy (2). Patients with epilepsy were compared to 38 gender-, education-, and age-matched (±2.5 years) healthy controls. These controls were recruited either from non-related acquaintances of patients or from hospital staff. All participants had normal intelligence (see Table 1) and had no history of neurological, psychiatric, and physical illnesses, except for epilepsy in the patient group. Moreover, participants did not take any other medication than antiepileptic drugs (AEDs) in the patient group. Patients did not have any types of seizure 1 week prior to study inclusion. A routine electroencephalography was performed in all patients at the day of study inclusion to exclude ictal discharges.
All participants performed tests of psychomotor speed [Trail Making Test-A (TMT-A)] (20), cognitive flexibility (TMT-B) (20), categorical verbal fluency [animals/min; Regensburger Wortflüssigkeits-Test (RWT)] (21), phonological verbal fluency (s-words/min; RWT) (21), divided attention [subtest of Tests of Attentional Performance (TAP)] (22), response inhibition (TAP) (22), executive control (TAP) (22), and set-shifting (TAP) (22). They also responded to an inventory of sensation seeking (Arnett Inventory of Sensation Seeking) (23), to a multiple-choice test of estimated verbal intelligence (Mehrfachwahl Wortschatz Intelligenztest) (24), and to a self-rated questionnaire on anxiety and depression symptoms (Hospital Anxiety and Depression Scale—German version) (25).
We tested decision making under risk by means of the GDT-D (15), a modified version of the GDT (16). The GDT is a computerized gambling task, where participants have to maximize their fictitious starting capital of €1000 within 18 throws of a single virtual die. Participants are asked to guess which number will be thrown next by selecting one of the presented answer alternatives: single numbers (e.g., 3) or combinations of two (e.g., 1 2), three (e.g., 1 2 3), or four numbers (e.g., 1 2 3 4). Each alternative is associated with a specific winning probability and a specific amount of gain/loss. For example, the single-number alternative is associated with a winning probability of 1/6 and a gain/loss of €1000. By choosing the single-number alternative, the participant can win €1000 if the selected number is thrown; otherwise, s/he loses €1000. Alternatives and associated gains/losses remain visible on the computer screen for the whole task duration. Before beginning the task, participants are explicitly instructed about the rules and the amount of gains/losses associated with each alternative but not about which alternative is the most advantageous. After a choice is made, the computer indicates which number is thrown, whether the participant has won/lost, the residual capital, and the number of remaining throws (16). The GDT-D differs from the original task (16) in that participants are asked, after they make a choice but before they receive a feedback, whether they want to double their potential gain/loss. In the GDT-D, participants decide optimally when they choose to double their potential gain/loss after selecting the four-numbers alternative. Conversely, doubling the potential gain/loss after selecting the single-number alternative is the most risky choice. The GDT-D allows for the distinguishment between optimal choices and conservative choices as well as between risky decisions and non-risky decisions.
We computed the net score by subtracting the number of high-risk choices (selection of single number and two-numbers combinations) from the number of low-risk choices (selection of three-numbers combinations and four-numbers combinations). A positive net score indicates a low-risk performance (advantageous behavior), whereas a negative net score indicates a high-risk performance (disadvantageous behavior). Furthermore, we analyzed how often participants selected each alternative and how often they decided to double the potential gain/loss (overall as well as after selection of the single-number alternative and the four-numbers alternative). Doubling the single-number alternatives is of special importance as it is extremely disadvantageous. Conversely, doubling the four-numbers alternatives can be considered an optimal decision. We also compared groups with respect to the mean expected value (MEV). The expected value for each single decision was calculated as follows: [(gain × winning probability) − (loss × losing probability)].
Statistical analysis was performed using SPSS 24.0 for Windows. The gender distribution was examined by χ2-test. Group comparisons in age, education, and estimated verbal intelligence were assessed using Student’s t-tests. Group differences in neuropsychological tests and in the GDT-D were investigated using multivariate analyses of variance (MANOVAs). With regard to latency variables, we submitted log-transformed reaction times to analysis. Tables report untransformed data. A Pearson correlation analysis was performed for each group separately between measures of decision making (no. of single-number selections, no. of four-numbers selections, net score, MEV, no. of optimal decisions, and total no. of doubling choices) and neuropsychological variables (categorical verbal fluency, phonological verbal fluency, psychomotor speed, cognitive flexibility, divided attention errors and omissions, response inhibition errors and omissions, executive control errors and omissions, set-shifting errors, intensity seeking, novelty seeking, anxiety, and depression). A correlation analysis was also performed for the patient group between decision making, demographical variables (age and education), and clinical variables (epilepsy duration, age at epilepsy onset, seizure frequency, and time since last seizure in months). For the patient group, we also performed a stepwise regression analysis to investigate which of the neuropsychological variables showing a significant correlation with the GDT-D could predict the patients’ decision-making performance. Significance was set at α = 0.05.
A total of 36 epilepsy patients with a mean age of 25.25 years (SD 2.7) and a mean age at epilepsy onset of 14.28 years (SD 3.4) were included in this study. Patients were compared to 38 gender-, education-, and age-matched controls. Table 1 summarizes the demographic characteristics of the study population. Groups did not differ from each other in terms of age, years of formal education, and gender distribution. They were also comparable in terms of mean estimated verbal intelligence.
All but four patients (88.9%) were under current AED therapy (84.4% monotherapy, 15.6% polytherapy). The three most commonly used drugs were levetiracetam (20 patients), valproic acid (11 patients), and lamotrigine (4 patients). Epilepsy was well controlled in the majority of patients. Overall, during the last year, 21 out of 36 patients (58.4%) were completely seizure free. A more detailed patient description can be found in the Table S1 in Supplementary Material. We did not find any significant differences between seizure-free patients and non-seizure-free patients with regard to demographical, neuropsychological, and decision-making variables (see Table S1 in Supplementary Material).
Descriptive statistics and results of the MANOVA are presented in Table 2. In all tests, group scores were in the average range (16–84 percentiles) of standardized published norms (20–25). Group differences were significant in tests of psychomotor speed and divided attention, with the patients scoring lower than the controls. Group differences in other tests were not significant.
Descriptive statistics and results of the MANOVA are presented in Table 3. Since only few participants decided to double their win/loss with the single-number alternative (extremely risky decisions; JME: 5/36, 13.89%; HC: 5/38, 13.16%), we did not include this variable into the analysis. Results of the MANOVA indicated a significant difference in how frequent groups selected the single-number alternative, with the patients making risky choices more often than controls. Group differences were not significant in other measures of decision making.
For the patient group, we found that poor decision-making performance was associated with poor executive control, poor response inhibition, and a short interval since the last seizure episode (see Table 4). Similarly, for the control group, we found that poor decision-making performance was related to poor executive control. Additionally, high frequency of doubling choices with all types of alternatives was associated with poor set-shifting.
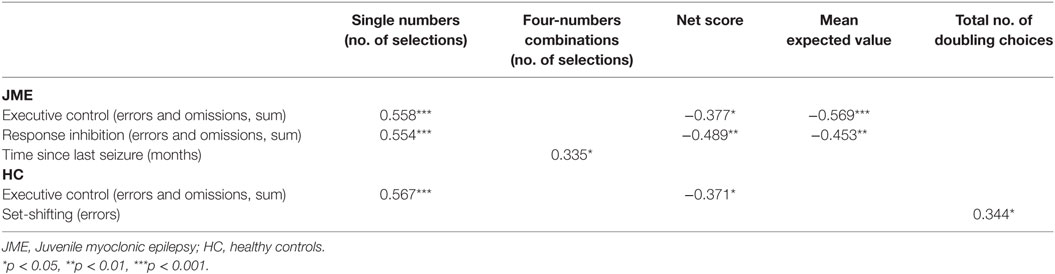
Table 4. Pearson correlation analysis between game of dice task-double measures and neuropsychological/clinical variables.
A stepwise regression analysis was conducted to evaluate whether executive control and response inhibition would predict the patients’ performance on the GDT-D. As dependent variable we used the number of single-number selections since patients significantly differed from controls in this measure. At step 1 of the analysis, “Executive Control” entered into the regression equation and was significantly correlated with the dependent variable [F(1, 34) = 15.40, p < 0.001]. The multiple correlation coefficient was 0.31, indicating that approximately 29.1% of variance in the GDT-D could be accounted for by performance on the executive control test. At step 2 of the analysis, “Response Inhibition” was added into the equation and was significantly correlated with the dependent variable [F(2, 33) = 13.48, p < 0.001]. “Response Inhibition” explained significantly more variance [R2 change = 0.14; F(1, 33) = 8.27, p < 0.01]. The final multiple correlation coefficient was 0.45, indicating that approximately 41.6% of variance in the GDT-D (single-number choices) could be accounted for by both executive control and response inhibition. Table 5 gives additional information about the predictor variables included in the final statistical model.

Table 5. Unstandardized and standardized regression coefficients for the variables included in the model.
In this study, we compared performance of patients with JME to that of healthy controls on the GDT-D, a task of decision making under explicit risk conditions (information about options, probabilities, and consequences is explicitly given from the beginning) and on a comprehensive neuropsychological test battery.
Patients with JME scored significantly lower than healthy controls in tests of psychomotor speed and divided attention. This finding is in line with previous studies reporting predominantly frontal lobe dysfunction in patients with JME (11–14). In the GDT-D, patients scored at the same level as controls in several outcome measures (net score, MEV, and number of optimal decisions). However, patients made significantly more risky decisions than controls. Results of a correlation analysis for the patient group indicated that poor decision making was associated with poor executive control and response inhibition. To evaluate whether both executive control and response inhibition would predict the patients’ performance on the GDT-D, we also performed a stepwise regression analysis. Results indicated that 42% of variance in the number of risky selections could be predicted by these two measures of executive functions. The importance of executive functions in decision making under risk has been shown in previous studies, both in healthy participants and in patients with various neurological disorders (15, 26–29). As outlined by Schiebener et al. (30), not all executive functions contribute to advantageous decision making to the same extent. In a large study with healthy participants, three components were assessed, i.e., general cognitive self-control, concept formation, and monitoring. General cognitive self-control was identified to be the strongest predictor of decision making under risk and to mediate the effect of concept formation and monitoring. General cognitive self-control implies the ability to inhibit automatic responses which are not in accordance with the task’s rules and goals (30). With regard to performance on the decision-making task, cognitive self-control may be responsible for inhibiting the activation of a schema linked to unplanned choices or for inhibiting the impulse of choosing the option associated with the highest possible monetary gain. Cognitive self-control also enables individuals to apply a new strategy when the current strategy does not seem appropriate. Results of our study are in line with Schiebener et al. (30) as they point to the importance of response inhibition and executive control in avoiding disadvantageous, risky choices in the JME group. It should be noted, however, that executive functions account only for part of the variance found in decision making. Other cognitive abilities, including numerical abilities and ratio processing, either contribute directly to advantageous decisions or are mediated via executive functions (15, 31).
Our findings add to previous studies on decision making in patients with JME. While this study investigated decision making under risk with explicit information on task contingencies, previous studies on JME have focused on decision making under initial ambiguity adopting the Iowa Gambling Task (IGT) (10, 14). When the decision situation is ambiguous, probabilities and outcomes are, at least at the beginning of a task, unknown. However, feedback on previous decisions can be used to choose the most advantageous options. Conversely, in decisions under risk, the situation is exactly defined from the beginning. Also, options, probabilities, and consequences are explicitly given or can be estimated (15). Recent research has provided evidence that the neural signals vary between decision situations reflecting different degrees of uncertainty and that different brain circuits are involved in decisions under risk and in decisions under ambiguity (32, 33). In both studies adopting the IGT (10, 14), patients with JME showed difficulties in learning to choose advantageous options. Both studies also reported a correlation between executive functions and decision making. Poor performance on the IGT was associated with an increased activation in the dorsolateral prefrontal cortex during a functional magnetic resonance working memory task, emphasizing frontal lobe dysfunction in patients with JME (10). Our study adds to these findings suggesting that low executive control and response inhibition also predict disadvantageous decisions under exactly defined conditions.
In this study, the direct comparison of seizure-free patients and non-seizure-free patients did not yield any significant results. However, we found that poor decision making was associated with a short interval since the last seizure episode. These results add to previous investigations suggesting that problems in decision making are more pronounced in patients with ongoing seizures (14). The fact that we did not find any significant differences between seizure-free patients and non-seizure-free patients when directly compared to each other might be due to the small sizes of the two subgroups. Future studies should investigate the impact of disease-related variables in larger groups of patients with JME.
Another limitation of our study regards the lack of an additional group of patients taking the same medications but having a different type of epilepsy. Although cognitive side effects of AEDs are very often subtle (34), they may have an impact on complex cognitive mechanisms such as decision making. Indeed, previous investigations have shown that AEDs compared to nondrug conditions may impair performance in executive functions tasks (35). In this study, all but four patients (88.9%) were under AED therapy, and we cannot disentangle the possible influence of AED treatment from other disease-related conditions. As in other investigations (10, 14, 36–38), decision-making deficits were observed under current antiepileptic medication. However, a recent study on TLE and decision-making (39) indicates that AED are unlikely to be the major cause of risky decision making. In the study by Delazer et al. (39), two groups of patients (one with structural abnormalities in the mesial temporal lobe, the other with abnormalities in the lateral, basal, or polar parts of the temporal lobe) received standard AED therapy, but only the group with mesial temporal lobe epilepsy showed decision-making deficits. It should be noted, however, that Delazer et al. (39) used a different decision-making task (IGT) as the one adopted here (GDT-D). In our study, specific AED effects on cognition could not be explored due to the small sample sizes of different medications. Future studies might investigate the impact of AED therapy on decision making and risky behavior in more detail.
In conclusion, our findings show that the number of optimal decisions did not differ between patients and healthy controls. However, patients with JME made more risky decisions. High levels of executive control and response inhibition seem to be essential for making less risky decisions. Several cognitive models have been proposed where reflective processing, including executive control, guides decision making. Apart from reflective processing, heuristics, intuition, and emotions are crucial in the process of decision making. Recent studies propose that reflective processing and intuitive processing interact and inform each other and that advantageous decision-making relies on both, intuitions and reflections (18, 40, 41). While healthy people switch between resources without any effort and may safely follow their intuitions without hesitation, patients with low cognitive control may be attracted by risky, disadvantageous alternatives. Providing full and exact information (e.g., about treatment options and consequences) and encouraging deliberate and slow reasoning may lead to safe and advantageous decisions also in patients with JME. This may be of particular importance when discussing possible treatment options with patients.
This study was carried out in accordance with the recommendations of the Declaration of Helsinki with written informed consent from all subjects. The protocol was approved by the research ethics committee at Innsbruck Medical University.
IU, LZ, AJ, GR, and MD generated the research idea and concept. IU, LZ, and MD were responsible for data acquisition and interpretation, literature review, and manuscript preparation. IU, LZ, MP, MB, GW, GL, AJ, GR, and MD made critical revisions for important intellectual content and approved the final manuscript.
AJ has moved to Biogen International GmbH, Zug, Switzerland since completion of the work. None of the other authors has any conflict of interest to disclose.
The authors thank their patients and healthy controls for participation. We are particularly grateful to Nicole Mayr for her dedicated work in coordinating the survey schedule and for her optimal participant support and to Laura Lettenbichler for her help in data collection.
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fneur.2018.00195/full#supplementary-material.
1. Genton P, Gelisse P, Thomas P. Juvenile myoclonic epilepsy today: current definitions and limits. Juvenile Myoclonic Epilepsy The Janz Syndrome. Petersfield: Wrightson Biomedial Publishing (2000). p. 11–32.
2. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia (1989) 30(4):389–99. doi:10.1111/j.1528-1157.1989.tb05316.x
3. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia (2017) 58(4):512–21. doi:10.1111/epi.13709
5. de Araujo Filho GM, Yacubian EMT. Juvenile myoclonic epilepsy: psychiatric comorbidity and impact on outcome. Epilepsy Behav (2013) 28(Suppl 1):S74–80. doi:10.1016/j.yebeh.2013.03.026
6. Gelisse P, Genton P, Thomas P, Rey M, Samuelian JC, Dravet C. Clinical factors of drug resistance in juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry (2001) 70(2):240–3. doi:10.1136/jnnp.70.2.240
7. Schmitz B, Yacubian EM, Feucht M, Hermann B, Trimble M. Neuropsychology and behavior in juvenile myoclonic epilepsy. Epilepsy Behav (2013) 28(Suppl 1):S72–3. doi:10.1016/j.yebeh.2013.02.019
8. Vollmar C, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, et al. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain J Neurol (2011) 134(Pt 6):1710–9. doi:10.1093/brain/awr098
9. O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, Thompson P, et al. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology (2011) 76(1):34–40. doi:10.1212/WNL.0b013e318203e93d
10. Wandschneider B, Centeno M, Vollmar C, Stretton J, O’Muircheartaigh J, Thompson PJ, et al. Risk-taking behavior in juvenile myoclonic epilepsy. Epilepsia (2013) 54(12):2158–65. doi:10.1111/epi.12413
11. Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, Lin K, Caboclo LOSF, Vidal-Dourado M, et al. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav (2007) 10(2):263–7. doi:10.1016/j.yebeh.2006.11.012
12. Piazzini A, Turner K, Vignoli A, Canger R, Canevini MP. Frontal cognitive dysfunction in juvenile myoclonic epilepsy. Epilepsia (2008) 49(4):657–62. doi:10.1111/j.1528-1167.2007.01482.x
13. Wandschneider B, Thompson PJ, Vollmar C, Koepp MJ. Frontal lobe function and structure in juvenile myoclonic epilepsy: a comprehensive review of neuropsychological and imaging data. Epilepsia (2012) 53(12):2091–8. doi:10.1111/epi.12003
14. Zamarian L, Höfler J, Kuchukhidze G, Delazer M, Bonatti E, Kemmler G, et al. Decision making in juvenile myoclonic epilepsy. J Neurol (2013) 260(3):839–46. doi:10.1007/s00415-012-6715-z
15. Brand M, Schiebener J, Pertl M-T, Delazer M. Know the risk, take the win: how executive functions and probability processing influence advantageous decision making under risk conditions. J Clin Exp Neuropsychol (2014) 36(9):914–29. doi:10.1080/13803395.2014.955783
16. Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. Decision-making deficits of korsakoff patients in a new gambling task with explicit rules: associations with executive functions. Neuropsychology (2005) 19(3):267–77. doi:10.1037/0894-4105.19.3.267
17. Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw (2006) 19(8):1266–76. doi:10.1016/j.neunet.2006.03.001
18. Schiebener J, Brand M. Decision making under objective risk conditions-a review of cognitive and emotional correlates, strategies, feedback processing, and external influences. Neuropsychol Rev (2015) 25(2):171–98. doi:10.1007/s11065-015-9285-x
19. Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain J Neurol (2003) 126(Pt 8):1790–800. doi:10.1093/brain/awg177
21. Aschenbrennen S, Tucha O, Lange KW. Regensburger Wortflüssigkeits-Test. Göttingen: Hogrefe (2001).
22. Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (TAP) [Tests of Attentional Performance (TAP)]. Würselen: Vera Fimm/Psychologische Testsysteme (2000).
23. Roth M. Die deutsche Version des Arnett Inventory of Sensation Seeking (AISS): Psychometrische Kennwerte und Befunde zur Reliabilität und Validität. Z Für Differ Diagn Psychol (2003) 24:65–76.
24. Lehrl S. Mehrfach-Wortschatz-Intelligenztest [Multiple Choice Vocabulary Intelligence Test]. Balingen: Spitta Verlag (1999).
25. Hermann C, Buss U, Snaith RP. Hospital Anxiety and Depression Scale – German (HADS-D). Bern: Hans Huber (1995).
26. Zamarian L, Sinz H, Bonatti E, Gamboz N, Delazer M. Normal aging affects decisions under ambiguity, but not decisions under risk. Neuropsychology (2008) 22(5):645–57. doi:10.1037/0894-4105.22.5.645
27. Brand M, Labudda K, Kalbe E, Hilker R, Emmans D, Fuchs G, et al. Decision-making impairments in patients with Parkinson’s disease. Behav Neurol (2004) 15(3–4):77–85. doi:10.1155/2004/578354
28. Delazer M, Sinz H, Zamarian L, Stockner H, Seppi K, Wenning GK, et al. Decision making under risk and under ambiguity in Parkinson’s disease. Neuropsychologia (2009) 47(8–9):1901–8. doi:10.1016/j.neuropsychologia.2009.02.034
29. Schiebener J, Zamarian L, Delazer M, Brand M. Executive functions, categorization of probabilities, and learning from feedback: what does really matter for decision making under explicit risk conditions? J Clin Exp Neuropsychol (2011) 33(9):1025–39. doi:10.1080/13803395.2011.595702
30. Schiebener J, Wegmann E, Gathmann B, Laier C, Pawlikowski M, Brand M. Among three different executive functions, general executive control ability is a key predictor of decision making under objective risk. Front Psychol (2014) 5:1386. doi:10.3389/fpsyg.2014.01386
31. Pertl M-T, Zamarian L, Delazer M. Reasoning and mathematical skills contribute to normatively superior decision making under risk: evidence from the game of dice task. Cogn Process (2017) 18(3):249–60. doi:10.1007/s10339-017-0813-x
32. Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science (2005) 310(5754):1680–3. doi:10.1126/science.1115327
33. Schultz W, Preuschoff K, Camerer C, Hsu M, Fiorillo CD, Tobler PN, et al. Explicit neural signals reflecting reward uncertainty. Philos Trans R Soc Lond B Biol Sci (2008) 363(1511):3801–11. doi:10.1098/rstb.2008.0152
34. Hermann B, Meador KJ, Gaillard WD, Cramer JA. Cognition across the lifespan: antiepileptic drugs, epilepsy, or both? Epilepsy Behav (2010) 17(1):1–5. doi:10.1016/j.yebeh.2009.10.019
35. Hessen E, Lossius MI, Gjerstad L. Antiepileptic monotherapy significantly impairs normative scores on common tests of executive functions. Acta Neurol Scand (2009) 119(3):194–8. doi:10.1111/j.1600-0404.2008.01109.x
36. Delazer M, Zamarian L, Bonatti E, Kuchukhidze G, Koppelstätter F, Bodner T, et al. Decision making under ambiguity and under risk in mesial temporal lobe epilepsy. Neuropsychologia (2010) 48(1):194–200. doi:10.1016/j.neuropsychologia.2009.08.025
37. Labudda K, Frigge K, Horstmann S, Aengenendt J, Woermann FG, Ebner A, et al. Decision making in patients with temporal lobe epilepsy. Neuropsychologia (2009) 47(1):50–8. doi:10.1016/j.neuropsychologia.2008.08.014
38. Yamano M, Akamatsu N, Tsuji S, Kobayakawa M, Kawamura M. Decision-making in temporal lobe epilepsy examined with the Iowa gambling task. Epilepsy Res (2011) 93(1):33–8. doi:10.1016/j.eplepsyres.2010.10.009
39. Delazer M, Zamarian L, Bonatti E, Walser N, Kuchukhidze G, Bodner T, et al. Decision making under ambiguity in temporal lobe epilepsy: does the location of the underlying structural abnormality matter? Epilepsy Behav (2011) 20(1):34–7. doi:10.1016/j.yebeh.2010.11.006
40. Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science (1997) 275(5304):1293–5. doi:10.1126/science.275.5304.1293
Keywords: juvenile myoclonic epilepsy, gambling, risk-taking, neuropsychology, cognition, executive functions
Citation: Unterberger I, Zamarian L, Prieschl M, Bergmann M, Walser G, Luef G, Javor A, Ransmayr G and Delazer M (2018) Risky Decision Making in Juvenile Myoclonic Epilepsy. Front. Neurol. 9:195. doi: 10.3389/fneur.2018.00195
Received: 17 January 2018; Accepted: 13 March 2018;
Published: 26 March 2018
Edited by:
Udaya Seneviratne, Monash Medical Centre, AustraliaReviewed by:
Marino M. Bianchin, Federal University of Rio Grande do Sul (UFRGS), BrazilCopyright: © 2018 Unterberger, Zamarian, Prieschl, Bergmann, Walser, Luef, Javor, Ransmayr and Delazer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Zamarian, bGF1cmEuemFtYXJpYW5AaS1tZWQuYWMuYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.