- 1Graduate Program in Medicine: Medical Sciences, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
- 2Basic Research and Advanced Investigations in Neurology (BRAIN), Experimental Research Centre, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- 3Neurology Division, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- 4Centro de Tratamento de Epilepsia Refratária (CETER), Department of Neurology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
Introduction: Metabolic syndrome (MetS) is an emergent problem among patients with epilepsy. Here, we evaluate and compare the diagnostic yield and accuracy of different MetS criteria among adult patients with epilepsy to further explore the best strategy for diagnosis of MetS among patients with epilepsy.
Materials and methods: Ninety-five epileptic adults from a tertiary epilepsy reference center were prospectively recruited over 22 weeks in a cross-sectional study. MetS was defined according to five international criteria used for the diagnosis of the condition [ATP3, American Association of Clinical Endocrinologists (AACE), International Diabetes Federation (IDF), AHA/NHLBI, and harmonized criteria]. Sensitivity, specificity, positive and negative predictive values (NPVs), and area under the receiver operating characteristic curve (ROC) curve were estimated for each criterion.
Results: In our sample, adult patients with epilepsy showed a high prevalence of obesity, hypertension, and diabetes. However, the prevalence of MetS was significantly different according to each criterion used, ranging from 33.7%, as defined by AACE, to 49.4%, as defined by the harmonized criteria (p < 0.005). IDF criteria showed the highest sensitivity [S = 95.5% (95% CI 84.5–99.4), p < 0.05] and AACE criteria showed the lowest sensitivity and NPV [S = 68.2% (95% CI 52.4–81.4), p < 0.05; NPV = 75.8% (95% CI 62.3–86.1), p < 0.05]. ROC curve for all criteria studied showed that area under curve (AUC) for IDF criterion was 0.966, and it was not different from AUC of harmonized criterion (p = 0.092) that was used as reference. On the other hand, the use of the other three criteria for MetS resulted in significantly lower performance, with AUC for AHA/NHLBI = 0.920 (p = 0.0147), NCEP/ATP3 = 0.898 (p = 0.0067), AACE = 0.830 (p = 0.00059).
Conclusion: Our findings suggest that MetS might be highly prevalent among adult patients with epilepsy. Despite significant variations in the yield of different criteria, the harmonized definition produced the highest prevalence rates and perhaps should be preferred. Correct evaluation of these patients might improve the rates of detection of MetS and foster primary prevention of cardiovascular events in this population.
Introduction
Epilepsy is a common serious chronic neurologic disorder, affecting about 50 million people worldwide (1). Data from 2000 estimated the world’s epilepsy-related burden of disease as 6,223,000 disability-adjusted life years (2), and ILAE/IBE/WHO Global Campaign against Epilepsy reaffirmed the prediction that the global burden of this disease will rise 14.7% in the next decade (3). Although epidemiological studies have pointed out that treatment success rates, public health policies, education, and psychosocial issues are key factors in Health-Related Quality of Life of patients with epilepsy, they have hardly addressed the impact of some common general medical conditions in patients with epilepsy (4). There is, indeed, growing concern regarding comorbidity management in epilepsy and the overall impact that they play in the global quality of life of patients with epilepsy.
Cardiovascular disease (CVD) has become the leading cause of death and has lifetime prevalence greater than 70% in western civilizations (5). In a cohort of 9,061 adult patients hospitalized due to epilepsy, estimated coronary heart disease mortality was 2.5 times the predicted rate; even greater rates were observed regarding stroke (6). A cross-sectional population-based study showed 34% increase in risk of coronary heart disease and 68% increase in risk of fatal CVD among patients with epilepsy (7). Also, a Swedish case–control study linked epilepsy to a significantly higher incidence of myocardial infarction and worse cardiovascular outcomes (8).
Among the clinical tools for prediction of future CVD, the concept of metabolic syndrome (MetS) is well accepted. MetS is defined as a cluster of metabolic risk factors that include central obesity, dyslipidemia, insulin resistance, and/or glucose intolerance, and abnormally high blood pressure, in variable associations that increases the risk to develop CVD and diabetes (9). The occurrence and relevance of MetS in patients with epilepsy has been gaining growing emphasis in the neurological literature (10–15). These studies are resumed in Table 1, and they focused mainly on prevalence and metabolic aspects of MetS, but the definitions used were heterogeneous and data not readily comparable. In fact, various medical societies had published their own criteria for the MetS diagnosis, but how these criteria correlate, and more importantly, which is the one that best fits the epileptic population, is currently unknown. The objective of our study is to report the prevalence of MetS in patients with epilepsy in a cohort of an outpatient clinic of a tertiary hospital and evaluate diagnostic yield and accuracy of five different internationally accepted MetS criteria in these patients. We hope our work can drive attention to an underestimated health problem in patients with epilepsy, perhaps helping to improve care of these patients in near future.
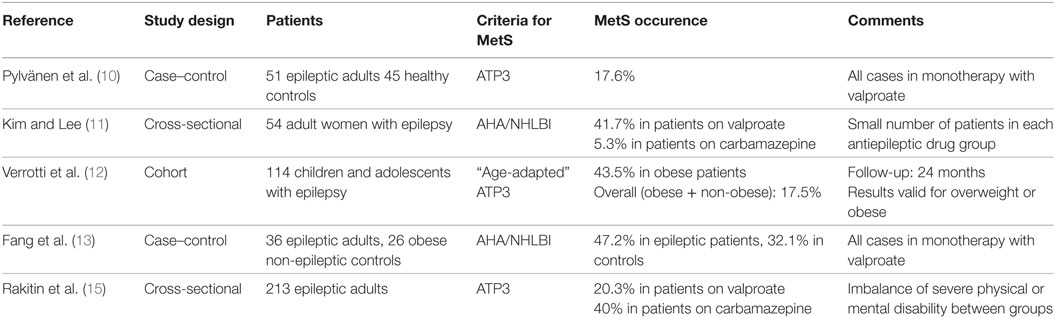
Table 1. Summary of published studies evaluating metabolic syndrome (MetS) occurrence in epilepsy patients.
Materials and Methods
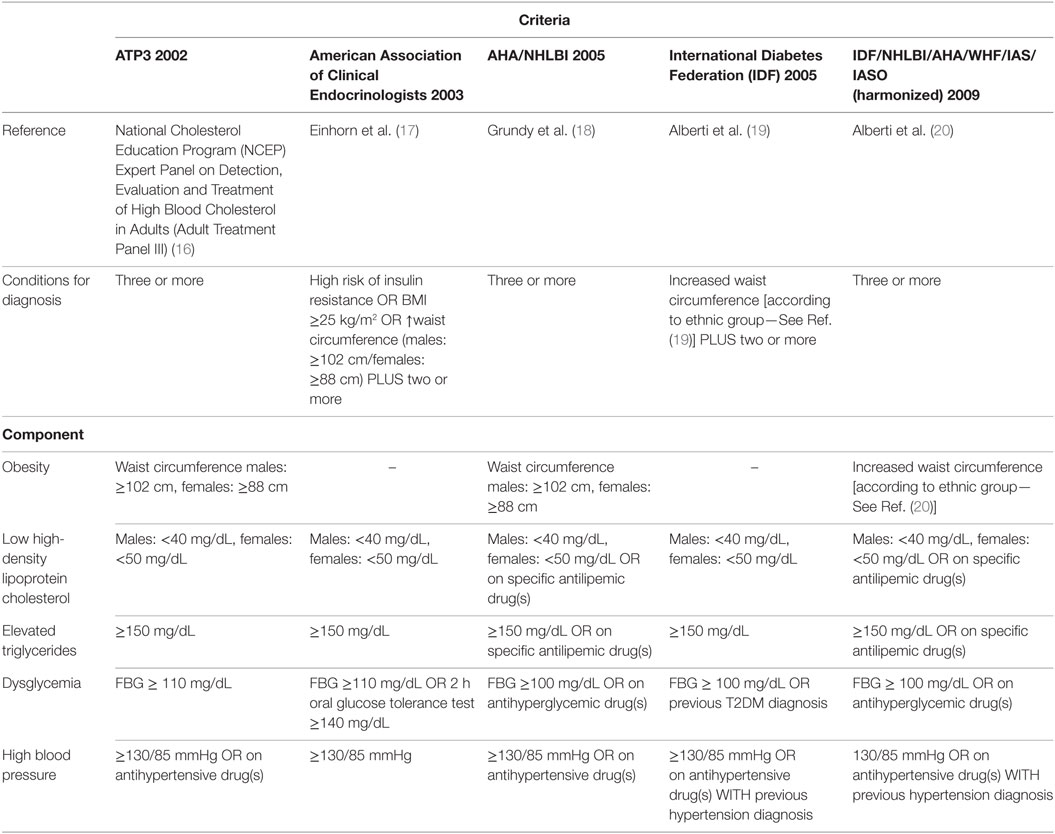
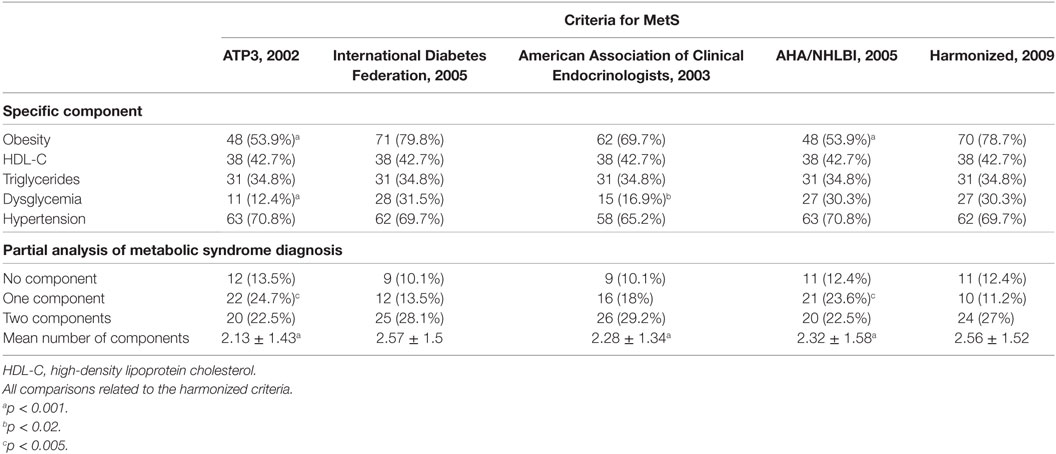
This study aimed to determine the prevalence of MetS in a cohort of patients with epilepsy in an outpatient clinic of a tertiary hospital and to determine the general and specific performance of five international criteria used for the diagnosis of MetS. For this, we investigated National Cholesterol Education Program’s Adult Treatment Panel III (NCEP ATP3) (16), American Association of Clinical Endocrinologists (AACE) (17), American Heart Association/National Heart, Lung and Blood Institute (AHA/NHLBI) (18), International Diabetes Federation (IDF) (19), and the harmonized criteria (IDF/NHLBI/AHA/WHF/IAS/IASO) (20). Harmonized criterion was used as gold standard to compare other criteria. Each criterion is composed of five specific subsets of criteria [obesity, high-density lipoprotein (HDL) cholesterol, triglycerides, dysglycemia, hypertension], each one with variable cutoffs. Table 2 presents a comparative view of components of five internationally accepted criteria for MetS diagnosis used in this study. Table 3 is revising cutoff values for waist circumference, by ethnic group, for the definition of central obesity in the IDF criteria. Table 4 is revising the cutoff values for waist circumference, by ethnic group, for the definition of central obesity in the harmonized criteria for comparison (IDF/NHLBI/AHA/WHF/IAS/IASO, 2009).
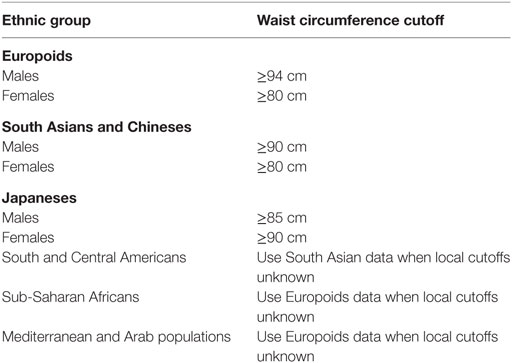
Table 3. Cutoff values for waist circumference, by ethnic group, for the definition of central obesity in the International Diabetes Federation criteria (2005) (19).
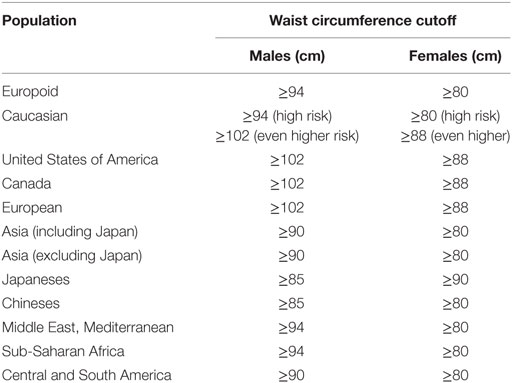
Table 4. Cutoff values for waist circumference, by ethnic group, for the definition of central obesity in the harmonized criteria (International Diabetes Federation/NHLBI/AHA/WHF/IAS/IASO, 2009) (20).
Study Design and Patient Population
A cross-sectional, consecutive, single-center study was carried out at the Epilepsy Outpatient Clinic, Hospital de Clínicas de Porto Alegre. This is a tertiary hospital located in the Southern region of Brazil. Porto Alegre is the capital of Rio Grande do Sul State, with a population of 1,416,735 individuals distributed in an area of 496.8 km2. A large fraction of the State population consists of Caucasian European immigrants, e.g., German, Italian, and Portuguese ones.
Patients were eligible if older than 18 years of age, received a definite diagnosis of epilepsy, attended to the center for 6 months or more and had used antiepileptic drugs (AEDs) for at least 1 year. We excluded patients with major adverse cardiovascular events (myocardial infarction, ischemic stroke, revascularization procedures) since, by definition, these patients had high cardiovascular risk (21). Patients with cerebrovascular disorders presumed to be of atherosclerotic origin (i.e., asymptomatic carotid stenosis) were also excluded. Other kinds of cerebrovascular disorders (e.g., arteriovenous malformations, aneurysms) were included. The inability to obtain accurate biometrical data was also an exclusion criterion. Personal demographic data (age, sex, ethnicity, disability) were derived from medical records, and when unavailable, from clinical interview with patients or their proxies. Electronic medical records were reviewed on a weekly basis. Subjects were recruited by phone or personal contact before routine medical visits. The study was approved by the Ethics Committee of our Institutional Review Board (GPPG-HCPA; Approval Protocol Number: 110311) and is fully compliant with the Declaration of Helsinki. All individuals enrolled in the study, or their legal proxies, gave written informed consent prior to their inclusion, and were free to withdraw such consent at any given time.
Variables and Clinical Assessments
All clinical assessments were performed as recommended elsewhere (20, 22, 23). After data collection, the investigators were blinded to the results and then received anonymous data to classify the patients according to the five MetS criteria. Diagnostic yield and accuracy parameters were estimated for each criterion. Epilepsy syndromes were classified according to ILAE recommendations (24, 25). Epilepsy cause, treatment, control, and duration were also investigated. Electronic medical records were reviewed for EEGs and neuroimaging studies. Seizures were deemed controlled if the current interictal period was greater than 1 year. Population-specific thresholds for increased waist circumference were set at 80 cm for females and 90 cm for males (19). Anthropometric measuring devices and sphygmomanometers were checked biweekly and calibrated as needed. Regular physical exercise was defined as at least 12 MET-hours per week (26). All blood samples were obtained at 8:00 a.m. after an overnight fasting, and handled independently by the central laboratory. Missing data were handled by deleting the given case from final analysis.
Sample Size Determination and Statistical Analysis
We used as gold standard the harmonized criteria to perform diagnostic of MetS in patients with epilepsy (IDF/NHLBI/AHA/WHF/IAS/IASO) (20). We hypothesized that at least one criteria would show significantly different sensitivity, specificity, or predictive values from harmonized criteria. Based on data from previous reports, we estimated a sample size of at least 88 participants to reject the null hypothesis with 0.8 probability (27). Categorical data were expressed as counts (%), and continuous data as mean (±SD). Since MetS is a clinical diagnosis, the harmonized criterion was elected as the reference standard, in accordance to previous recommendations (28); unadjusted and adjusted sensitivities, specificities, and predictive values were plotted using the random effects model. Specific reporting are in agreement with Standards for Reporting Diagnostic Accuracy (STARD) statement when applicable (29). The area under the receiver operating characteristic curve (ROC curve) was estimated to further compare MetS criteria other than harmonized criteria (30). Cohen’s kappa or McNemar test was used to evaluate concordance of different MetS criteria with the harmonized reference, when appropriate. Other categorical variables were compared using the chi-square or Fisher’s exact test, and continuous data were compared using one-way ANOVA or the Kruskal–Wallis test (with subsequent post hoc tests). All tests were two-sided and all statistics were performed using SPSS Statistics 19.0 and MedCalc 16.4. A p-value less than 0.05 was considered significant.
Results
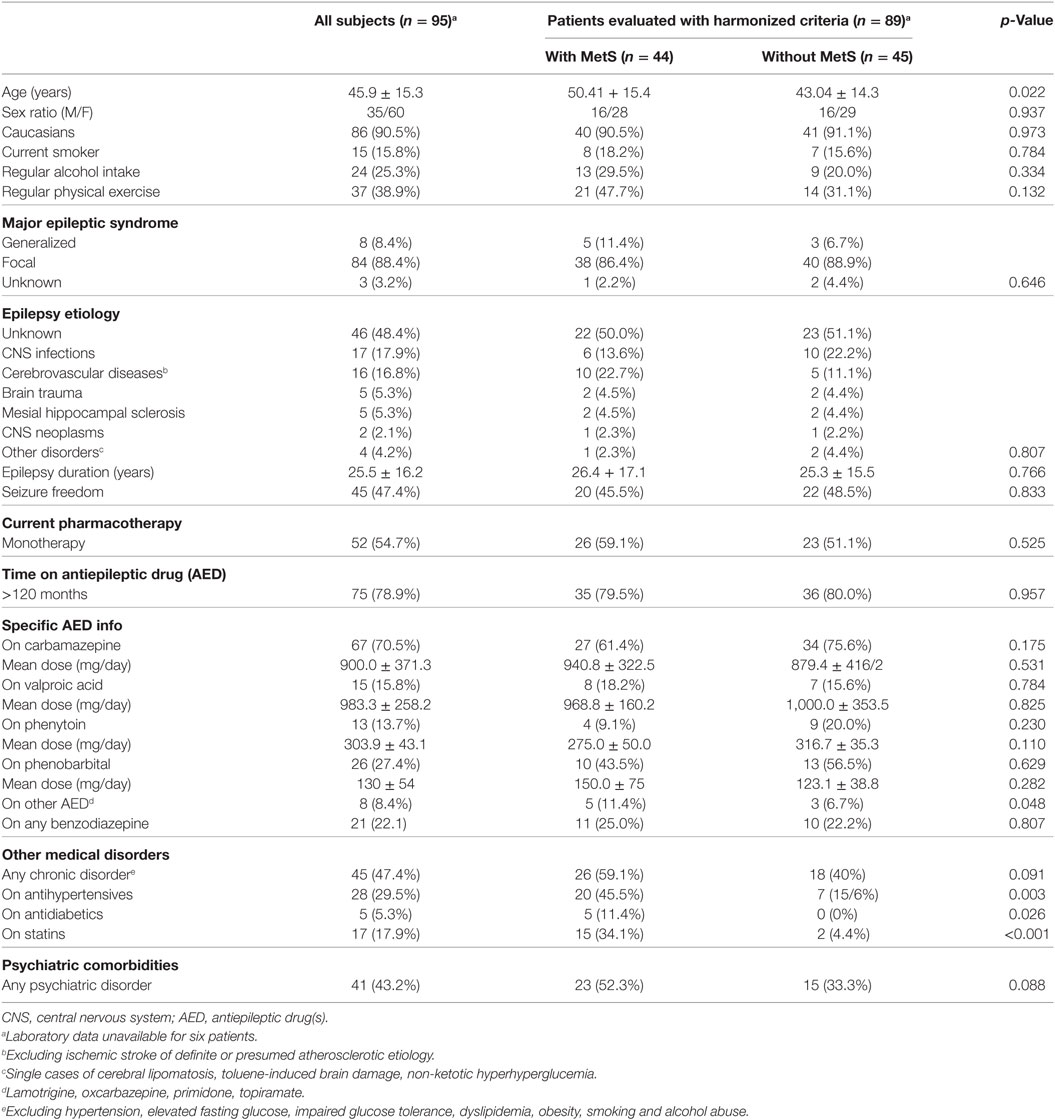
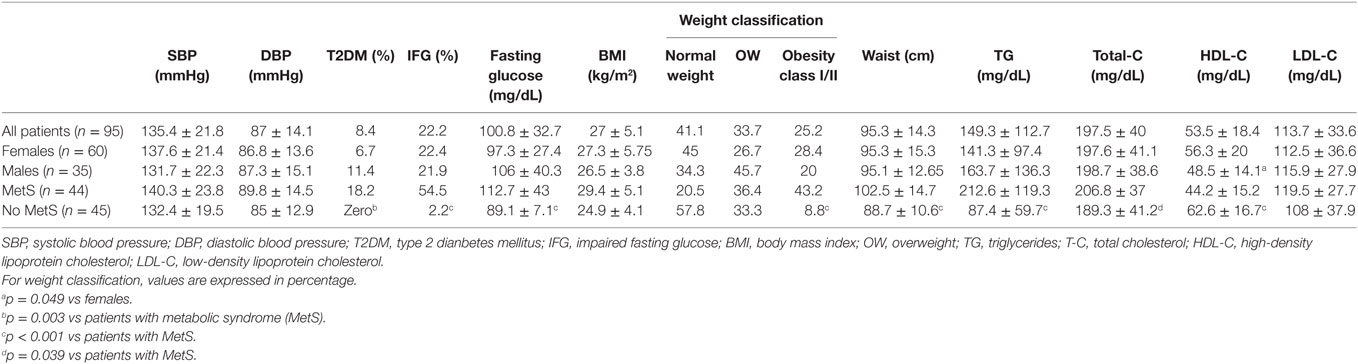
A total of 752 patients with epilepsy attended the outpatient clinic over a period of 22 weeks (from February to July 2011). Ninety-five patients fulfilled the inclusion criteria and agreed to participate in the study. In five cases, the laboratory samples were not handled properly, so part of their data were unavailable for analysis. One patient withdrew consent to the use of his biochemical data, totaling 89 patients available for MetS evaluation (Figure 1). No adverse effects were noted during data collection. Clinical and demographic characteristics of the patients are presented in Table 5.
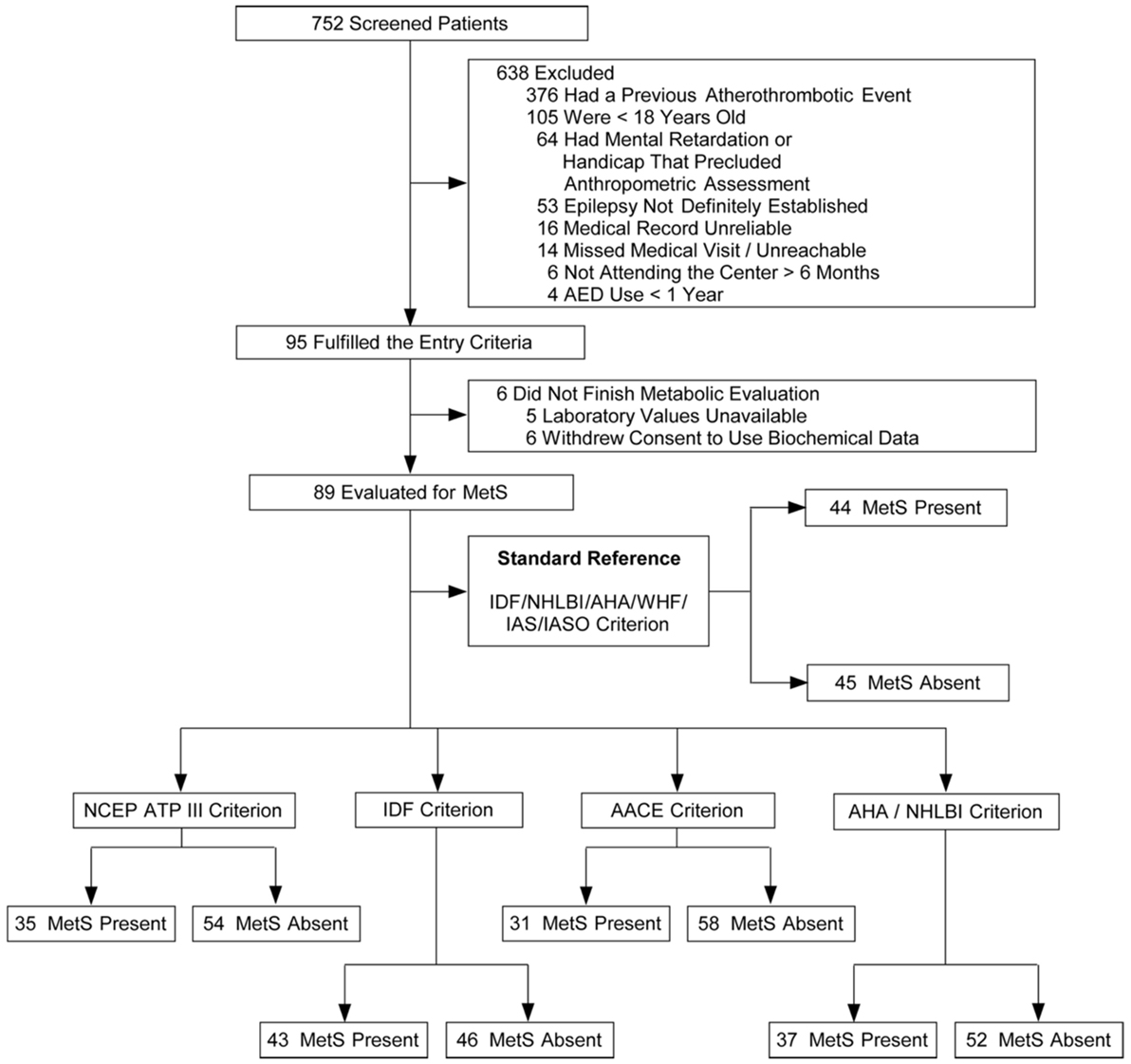
Figure 1. Flowcharts of the selection of patients and classification according with different metabolic syndrome (MetS) criteria.
Epilepsy-Related Features
In our sample, 88.4% of patients presented focal epilepsy, with a mean duration of approximately 25 years. A composite of unknown causes (48.4%), infections (17.9%), and cerebrovascular disorders (16.8%) accounted for most causes of epilepsy. Neurocysticercosis (n = 12) and pneumococcal meningitis (n = 2) were the most common infectious causes. In line with the predominance of focal epilepsy, more individuals were on carbamazepine than on valproic acid (70.1 vs 15.8%, p = 0.02). About half of the patients were seizure-free at the study time. Patients on monotherapy showed statistical trend for seizure control when compared with patients on polytherapy (55.8 vs 37.2%, p = 0.055).
General Medical Conditions
The average occurrence of general medical and psychiatric comorbidities was 50% each. Hypertension (40%) was significantly more prevalent than any other comorbidity. Diabetes was detected in 8 (8.4%) patients, and all of them were in the MetS group, as expected. The use of antihypertensive (45.5 vs 15.6%, p = 0.003), antidiabetics (11.4 vs 0%, p = 0.026), and statins (34.1 vs 4.4%, p < 0.001) were more common in the MetS group. Overall, psychiatric comorbidities were observed in 41 (43.2%) of patients, with no differences between patients with MetS and without MetS (52.3 vs 33.3%, p = 0.088).
Diagnostic Yield and Accuracy
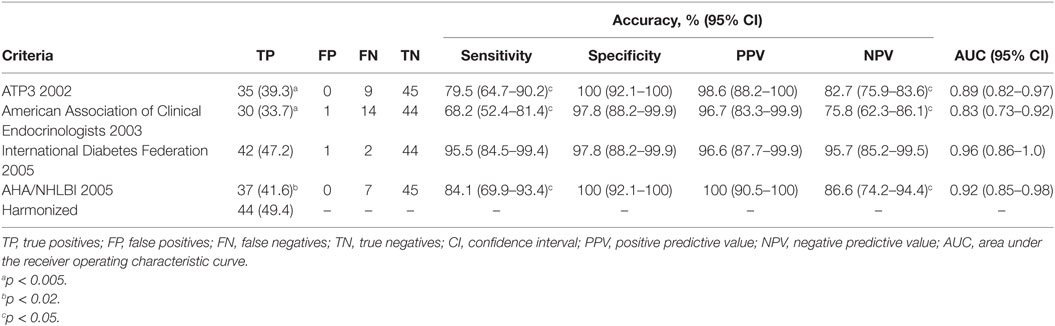
The reference criterion, the harmonized criterion, identified 44 individuals with MetS. MetS prevalence ranged from 33.7% (AACE) to 49.4% (harmonized criterion), a statistically significant difference (p < 0.005) (Table 6). It is of note that even ATP3 or AHA/NHLBI criteria also were unable to identify 9 and 7 MetS cases each, and so disclosed significantly lower prevalence of MetS than the reference harmonized criterion (39.3 vs 49.4%, p < 0.005 and 41.6 vs 49.4%, p < 0.02, respectively).
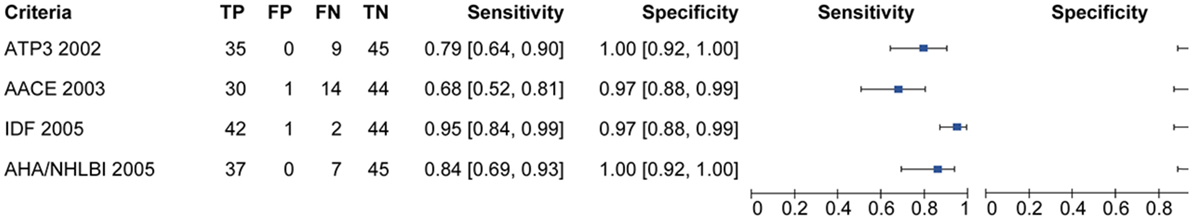
Figure 2 is showing tabulation and graphical plots of sensitivities and specificities for different criteria analyzed. Regarding sensitivity, the IDF criterion showed the highest value [S = 95.5% (95% CI) 84.5–99.4%], and all criteria showed significantly lower sensitivities when compared to the harmonized one. On further analysis, the IDF criterion also showed a significantly higher sensitivity than the AACE criterion (95.5 vs 68.2%, p < 0.05). Unadjusted analysis showed that both the ATP3 (79.5%) and AHA/NHLBI (84.1%) criteria had significantly higher sensitivities than the AACE criterion (68.2%), but this significance was lost after adjustment. All criteria showed similarly high specificities and positive predictive values (p > 0.5).
The negative predictive value (NPV) of IDF (94.7%) outperformed all other definitions (p < 0.05). IDF and AHA/NHLBI definition also showed higher NPV than AACE (95.7 and 85.8 vs 75.8%, p < 0.05), but again, significance was lost after adjustment. In terms of overall performance of the definitions, the area under the ROC curve (AUC) varied from 0.83 (0.73–0.92) in the AACE definition to 0.96 (0.86–1.0) in the IDF definition. Further exploratory analysis showed that when diabetic patients were not excluded in the AACE definition (as default), the NPV overlapped with all others (p = 0.65). The inter-definition agreement was more robust for the IDF criterion, but remained significant for all definitions, as can be seen on Table 7. Figure 3 is showing ROC curve for all criteria studied, and we performed a statistical analysis comparing all AUC with the AUC for harmonized criterion used as reference. This analysis showed that AUC for IDF criterion was 0.966, and it was not different from AUC of harmonized criterion (p = 0.092). On the other hand, the use of the other three criteria for MetS resulted in significantly lower performance, with AUC for AHA/NHLBI = 0.920 (p = 0.0147), NCEP/ATP3 = 0.898 (p = 0.0067), AACE = 0.830 (p = 0.00059) when compared with harmonized criterion.
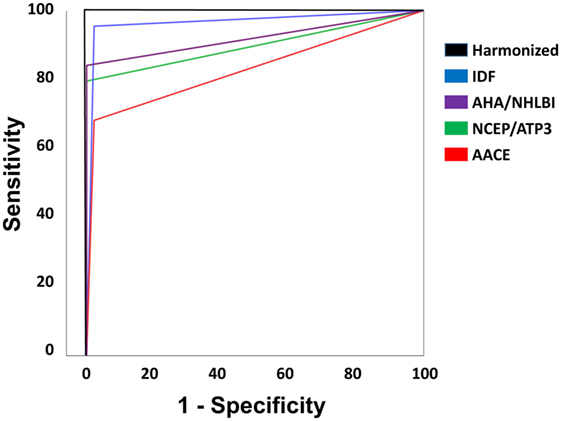
Figure 3. Receiver operating characteristic curve for all criteria studied. Statistical analysis comparing all area under curve (AUC) with the AUC for harmonized criterion, used as reference. AUC for International Diabetes Federation (IDF) criterion was 0.966, and it was not different from AUC of harmonized criterion (p = 0.092). On the other hand, the use of the other three criteria for MetS resulted in significantly lower performance, with AUC for AHA/NHLBI = 0.920 (p = 0.0147), NCEP/ATP3 = 0.898 (p = 0.0067), American Association of Clinical Endocrinologists (AACE) = 0.830 (p = 0.00059) when compared with harmonized criterion.
MetS Individual Components Analysis
In our patients, the prevalence of obesity ranged from 53.9% (according to ATP3 and AHA/NHLBI criterion) to 79.8% (according to the IDF criterion) (p < 0.001). Fewer individuals fulfilled the ATP3 dysglycemia criterion (12.4%, p < 0.001) and AACE (16.9%, p < 0.02) when compared with the harmonized definition (30.3%) (Table 8). The oral glucose tolerance test (OGTT) was necessary to correctly classify six patients, but it changed AACE classification in only three patients. Additional analyses of selected anthropometric and biochemical are shown in Table 9.
Discussion
In this study, we estimated the prevalence of MetS according to five internationally accepted criteria and their diagnostic performance in a cohort of adult patients with epilepsy without previous major cardiovascular events. In these patients, we observed high rates of MetS, obesity, hypertension, and diabetes. Also, the AUC using IDF criterion was not different from AUC of harmonized criterion. On the other hand, the use of the other evaluated criteria for MetS resulted in significantly lower diagnostic performance. Thus, our findings suggest that the use of the harmonized or IDF criteria might result in higher detection rates of MetS in adult patients with epilepsy.
The present data showed that MetS prevalence varied between 33.7 and 49.4%, and it is variable according to the used criteria. Unadjusted prevalence in unselected adults vary between 34.8 and 45.9% (19). Neurologic publications found rates like 11.1% in a selected population of Korean women (11), 29.5% in an Indian population with higher valproate exposure and adapted ATP-3 criteria (14), and up to 43.5% in a highly selected cohort of overweight youngster using valproate evaluated by another adaptation of ATP3 criteria (12). As one struggles to draw valid conclusions when summarizing these studies, the strengths of our work start to become clear. First, it provides a common framework for comparison of different findings by diverse criteria and their inherent relationship. In fact, as far as we can track, the IDF and AACE definitions had not been formally applied in medical studies in patients with epilepsy, yet, and perhaps our data are able support the use of IDF definition better than AACE definition. Second, most patients included in our study were adults with focal epilepsies and most patients were using sodium channel inhibitors. Accordingly, TIGER team reports in the VA study showed that up to 80% of those who had epilepsy at age 65 or greater were on a similar therapeutic regimen, and that remained stable (31), an observation in line with our findings. Third, the underrepresentation of valproic acid in the sample helps to minimize potential bias for drug-induced metabolic changes. Fourth, we purposefully excluded patients with defined major adverse cardiovascular events or known high cardiovascular risk. Therefore, our findings might be more representative of the general epileptic population that would benefit from screening regarding MetS.
Our diagnostic accuracy analysis showed better performance of harmonized or IDF criteria for patients with epilepsy. In this venue, some observations are possible and need to be pointed out. The sensitivities observed varied from 68 to 95%; this implies that for each four given patients with MetS screened with the harmonized or IDF definitions, one would be missed by the AACE criteria. Besides that, in our study, IDF criteria showed the highest sensitivity and inter-definition agreement with the harmonized criteria. This is possibly related to the tighter cut offs for waist circumference (32), and a closer look at our population showed higher than expected values, especially in females. Lofgren and coworkers also found that epileptic women had higher risk of obesity, and that sedentarism and long-term use of AED were linked to higher BMI (33). Furthermore, the AACE criterion showed a low NPV (about 75%) in our population, which may hinder its clinical applicability. One possible explanation is the fact the AACE does not accept the coexistence of MetS and diabetes (17); the exploratory analysis showed above corroborates this proposition, and points out that OGTT adds little to the diagnosis. Taken together, these findings suggest that in patients with epilepsy, the AACE criterion should be used with more caution, especially in females, while the harmonized (and secondarily IDF) might be more suitable for the diagnosis of MetS.
The prevalence of MetS in Brazilian population is variable, and it has been underreported. In a recent systematic review, de Carvalho Vidigal et al. revised 10 cross sectional studies that reported a prevalence of MetS of 29.6%, ranging from 14.9 to 65.3% (34). In this study, the highest prevalence of MetS (65.3%) was found in an indigenous population, whereas the lowest prevalence of MS (14.9%) was reported in a rural area. The most frequent MetS components were low HDL-cholesterol (59.3%) and hypertension (52.5%). The two studies that evaluated urban population closer to our sample showed a prevalence of MetS that was variable from 35.9 to 43.2%. Silva et al. evaluated the prevalence of MetS in 287 adults from the urban region of the city of São Paulo using IDF criterion and observed an overall prevalence of 36.6% of MetS (35). In its turn, Gronner et al. evaluated 1,116 adults from São Carlos, a medium-size city in the State of Sao Paulo, using NCEP-ATPIII and IDF and observed an overall prevalence of 35.9 and 43.2%, respectively (36). These results overlap with our observations in patients with epilepsy and suggest that MetS is highly prevalent in Brazilian population and that might be also true for adult patients with epilepsy in Brazil. At this point, we cannot conclude that MetS is more prevalent in patients with epilepsy, when compared with controls without epilepsy. However, our results might suggest that MetS can be highly prevalent in adult patients with epilepsy, especially those living in developing regions of the world, and it might have impact in the health conditions of these patients. Further studies are clearly needed to evaluate the prevalence of MetS in patients with epilepsy, its variability according to world region, and its impact in health quality of these individuals.
Our study had methodological limitations that should be addressed. Besides those inherent to cross-sectional studies, the population characteristics hinder the applicability of our conclusions to youngsters and to those who already had their first atherothrombotic event. The absence of a control group also limits generalization of our findings and forbid any speculation how MetS might differ between epileptic and healthy subjects. We cannot firmly exclude that differences in etiologic evaluation could have led to some misclassification. Newer AEDs were underrepresented in out sample. Insulin kinetics parameters, as basal insulin levels and HOMA-IR, were not assessed. Diabetes was not exhaustively screened in all patients, a situation that may have changed since the recommendations for using HBA1C in routine practice (23). Finally, reference center selection bias and the lack of a control group preclude a definitive conclusion that all adult patients with epilepsy are at increased risk for MetS. Said that, it is worthwhile to mention that our work aimed primarily at providing answers for implementing future actions in clinical grounds. In that matter, it was not only successful but also the first study to provide a structured comparison of MetS criteria in the very special population of patients with epilepsy.
Conclusion
Our study was not adequate to access the real prevalence of MetS in all adult patients with epilepsy. As consequence, our data do not provide sufficient evidence to support the general incorporation of protocols for evaluating MetS in patients with epilepsy. However, we observed a higher prevalence of MetS and cardiovascular risk, irrespective of VPA use, in our cohort. Moreover, our study might suggest that harmonized or IDF criteria could present better sensitivity/specificity for evaluating these patients. However, further studies with large group of patients and adequate controls are necessary to evaluate the real prevalence of MetS in adult patients with epilepsy. For now, we believe that it is reasonable to alert physicians about the possibility of occurrence of MetS in patients with epilepsy and suggest that these patients should receive adequate evaluations, recommendations, and treatments. Additional prospective studies are necessary to confirm our preliminary observations as well as to broadly assess the clinical implications of our findings.
Ethics Statement
The study was approved by the Ethics Committee of our Institutional Review Board (GPPG-HCPA; Approval Protocol Number: 110311) and is fully compliant with the Declaration of Helsinki. All individuals enrolled in the study, or their legal proxies, gave written informed consent prior to their inclusion, and were free to withdraw such consent at any given time.
Author Contributions
Conception and design of the work: LC, PC, MO, LB, CT, and MB. Acquisition, analysis, and interpretation of data for the work: LC, PC, MO, LB, CT, and MB. Drafting the work and revising the manuscript: LC, PC, MO, LB, CT, and MB. Final approval of the version to be published: LC, PC, MO, LB, CT, and MB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was fully supported by the Brazilian Government research grant agencies CNPq, and FAPERGS. MB is further supported by CNPq (#485423/2012-0, #307084/2014-0) and PRONEM-FAPERGS/CNPq (#11/2043.0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. WHO. WHO Fact Sheet Epilepsy. WHO Media Centre (2009). Available from: http://www.who.int/mediacentre/factsheets/fs999/en
2. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet (2006) 367:1754–7. doi:10.1016/S0140-6736(06)68770-9
3. Reynolds EH. The ILAE/IBE/WHO epilepsy global campaign history. International League Against Epilepsy. International Bureau for Epilepsy. Epilepsia (2002) 43(Suppl 6):9–11. doi:10.1046/j.1528-1157.43.s.6.5.x
4. Stavem K, Bjornaes H, Langmoen IA. Long-term seizures and quality of life after epilepsy surgery compared with matched controls. Neurosurgery (2008) 62:326–34. doi:10.1227/01.neu.0000315999.58022.1c
5. Mcneill AM, Rosamond WD, Girman CJ, Heiss G, Golden SH, Duncan BB, et al. Prevalence of coronary heart disease and carotid arterial thickening in patients with the metabolic syndrome (The ARIC Study). Am J Cardiol (2004) 94:1249–54. doi:10.1016/j.amjcard.2004.07.107
6. Nilsson L, Tomsom T, Farahmand BY, Diwan V, Persson PG. Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia (1997) 38:1062–8. doi:10.1111/j.1528-1157.1997.tb01194.x
7. Gaitatzis A, Carrol K, Majeed A, W Sander J. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia (2004) 45:1613–22. doi:10.1111/j.0013-9580.2004.17504.x
8. Jansky I, Hallqvist J, Tomson T, Ahlbom A, Mukamal KJ, Ahvne S. Increased risk and worse prognosis of myocardial infarction in patients with prior hospitalization for epilepsy – The Stockholm Heart Epidemiology Program. Brain (2009) 132:2798–804. doi:10.1093/brain/awp216
9. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA (2002) 288:2709–16. doi:10.1001/jama.288.21.2709
10. Pylvänen V, Pakarinen A, Knip M, Isojarvi J. Insulin-related metabolic changes during treatment with valproate in patients with epilepsy. Epilepsy Behav (2006) 8:643–8. doi:10.1016/j.yebeh.2006.02.008
11. Kim JY, Lee HW. Metabolic and hormonal disturbances in women with epilepsy on antiepileptic drug monotherapy. Epilepsia (2007) 48:1366–70. doi:10.1111/j.1528-1167.2007.01052.x
12. Verrotti A, Manco R, Agostinelli S, Coppola G, Chiarelli F. The metabolic syndrome in overweight epileptic patients treated with valproic acid. Epilepsia (2010) 51:268–73. doi:10.1111/j.1528-1167.2009.02206.x
13. Fang J, Chen S, Tong N, Chen L, An D, Mu J, et al. Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure (2012) 21:578–82. doi:10.1016/j.seizure.2012.06.001
14. Nair SS, Harikrishnan S, Sankara Sarma P, Thomas SV. Metabolic syndrome in young adults with epilepsy. Seizure (2016) 37:61–4. doi:10.1016/j.seizure.2016.03.002
15. Rakitin A, Kõks S, Haldre S. Metabolic syndrome and anticonvulsants: a comparative study of valproic acid and carbamazepine. Seizure (2016) 38:11–6. doi:10.1016/j.seizure.2016.03.008
16. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III): final report. Circulation (2002) 106:3143–421.
17. Einhorn D, Reaven GM, Cobin RH, Ford E, Ganada OP, Handelsman YM, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract (2003) 9:237–52. doi:10.4158/EP.9.S2.5
18. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation (2005) 112:2735–52. doi:10.1161/CIRCULATIONAHA.105.169404
19. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet (2005) 366:1059–62. doi:10.1016/S0140-6736(05)67402-8
20. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120:1640–5. doi:10.1161/CIRCULATIONAHA.109.192644
21. Law MR, Watt HC, Wald NJ. The underlying risk of death after myocardial infarction in the absence of treatment. Arch Intern Med (2002) 162:2405–10. doi:10.1001/archinte.162.21.2405
22. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA (2014) 311:507–20. doi:10.1001/jama.2013.284427
23. American Diabetes Association. Standards of medical care in diabetes – 2011. Diabetes Care (2011) 34(Suppl 1):S11–61. doi:10.2337/dc11-S011
24. Scheffer IE, French J, Hirsch E, Jain S, Mathern GW, Moshé SL, et al. Classification of the epilepsies: new concepts for discussion and debate—special report of the ILAE Classification Task Force of the Commission for Classification and Terminology. Epilepsia Open (2016) 1:37–44. doi:10.1002/epi4.5
25. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus – report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia (2015) 56:1515–23. doi:10.1111/epi.13121
26. Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King A, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation (2007) 116:1094–105. doi:10.1161/CIRCULATIONAHA.107.185650
27. Dupont WD, Plummer WD Jr. Power and sample size calculations for studies involving linear regression. Control Clin Trials (1998) 19:589–601. doi:10.1016/S0197-2456(98)00037-3
28. Rutjes AW, Reitsma JB, Coormarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess (2007) 11:iii–ix,51. doi:10.3310/hta11500
29. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ (2015) 351:h5527. doi:10.1136/bmj.h5527
30. Stevens J. Applied Multivariate Statistics for the Social Sciences. 4th ed. Mahwah, NJ: Lawrence Erlbaum Associates (2002).
31. Pugh MJ, Van Cott AC, Cramer KA, Knoefel JE, Amuan ME, Tabares J, et al. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000-2004. Neurology (2008) 70:2171–8. doi:10.1212/01.wnl.0000313157.15089.e6
32. Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care (2004) 27:1182–6. doi:10.2337/diacare.27.5.1182
33. Lofgren E, Mikkonen K, Tolonen U, Pakarinen A, Koivunen R, Myllyla VV, et al. Reproductive endocrine function in women with epilepsy: the role of epilepsy type and medication. Epilepsy Behav (2007) 10:77–83. doi:10.1016/j.yebeh.2006.09.011
34. de Carvalho Vidigal F, Bressan J, Babio N, Salas-Salvadó J. Prevalence of metabolic syndrome in Brazilian adults: a systematic review. BMC Public Health (2013) 13:1198. doi:10.1186/1471-2458-13-1198
35. Silva EC, Martins IS, de Araújo EA. Metabolic syndrome and short stature in adults from the metropolitan area of São Paulo city (SP, Brazil). Cien Saude Colet (2011) 16:663–8. doi:10.1590/S1413-81232011000200030
Keywords: metabolic syndrome, comorbidities in epilepsy, general medical conditions, risk factors, cardiovascular risk
Citation: Cabral LS, Cherubini PA, de Oliveira MA, Bianchini L, Torres CM and Bianchin MM (2017) Diagnostic Yield and Accuracy of Different Metabolic Syndrome Criteria in Adult Patients with Epilepsy. Front. Neurol. 8:460. doi: 10.3389/fneur.2017.00460
Received: 17 March 2017; Accepted: 18 August 2017;
Published: 01 September 2017
Edited by:
Fernando Cendes, Universidade Estadual de Campinas, BrazilReviewed by:
Luiz Eduardo Betting, Universidade Estadual Paulista Júlio Mesquita Filho, BrazilMarco Mula, St George’s, University of London, United Kingdom
Copyright: © 2017 Cabral, Cherubini, de Oliveira, Bianchini, Torres and Bianchin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marino Muxfeldt Bianchin, bWJpYW5jaGluQGhjcGEuZWR1LmJy
 Lucas Scotta Cabral1,2,3
Lucas Scotta Cabral1,2,3 Marino Muxfeldt Bianchin
Marino Muxfeldt Bianchin