
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 10 July 2017
Sec. Stroke
Volume 8 - 2017 | https://doi.org/10.3389/fneur.2017.00325
 Laurent Carteron1,2
Laurent Carteron1,2 Camille Patet1,2
Camille Patet1,2 Daria Solari1,2
Daria Solari1,2 Mahmoud Messerer3
Mahmoud Messerer3 Roy T. Daniel3
Roy T. Daniel3 Philippe Eckert1
Philippe Eckert1 Reto Meuli4
Reto Meuli4 Mauro Oddo1,2*
Mauro Oddo1,2*
Background: The pathophysiology of early brain injury following aneurysmal subarachnoid hemorrhage (SAH) is still not completely understood.
Objective: Using brain perfusion CT (PCT) and cerebral microdialysis (CMD), we examined whether non-ischemic cerebral energy dysfunction may be a pathogenic determinant of EBI.
Methods: A total of 21 PCTs were performed (a median of 41 h from ictus onset) among a cohort of 18 comatose mechanically ventilated SAH patients (mean age 58 years, median admission WFNS score 4) who underwent CMD and brain tissue PO2 (PbtO2) monitoring. Cerebral energy dysfunction was defined as CMD episodes with lactate/pyruvate ratio (LPR) >40 and/or lactate >4 mmol/L. PCT-derived global CBF was categorized as oligemic (CBF < 28 mL/100 g/min), normal (CBF 28–65 mL/100 g/min), or hyperemic (CBF 69–85 mL/100 g/min), and was matched to CMD/PbtO2 data.
Results: Global CBF (57 ± 14 mL/100 g/min) and PbtO2 (25 ± 9 mm Hg) were within normal ranges. Episodes with cerebral energy dysfunction (n = 103 h of CMD samples, average duration 7.4 h) were frequent (66% of CMD samples) and were associated with normal or hyperemic CBF. CMD abnormalities were more pronounced in conditions of hyperemic vs. normal CBF (LPR 54 ± 12 vs. 42 ± 7, glycerol 157 ± 76 vs. 95 ± 41 µmol/L; both p < 0.01). Elevated brain LPR correlated with higher CBF (r = 0.47, p < 0.0001).
Conclusion: Cerebral energy dysfunction is frequent at the early phase following poor-grade SAH and is associated with normal or hyperemic brain perfusion. Our data support the notion that mechanisms alternative to ischemia/hypoxia are implicated in the pathogenesis of early brain injury after SAH.
Increasing evidence suggests that early events occurring during the first 72 h following ictus onset, before the occurrence of secondary delayed neurological deterioration, substantially contribute to the pathophysiology and outcome of aneurysmal subarachnoid hemorrhage (SAH). This so-called early brain injury phase includes multiple physiological derangements that exacerbates the primary cerebral insult and may eventually worsen patient prognosis (1–6). While research efforts have predominantly focused on cerebral hemodynamics and perfusion, and the comprehension of mechanisms involved in brain ischemia, it is now increasingly recognized that a number of alternative “non-ischemic” mechanisms are implicated in the pathogenesis of early brain injury (7).
Energy dysfunction has emerged as one potential pathogenic determinant of cerebral damage following acute brain injury. Using a combination of intra-cerebral microdialysis (CMD)—to quantify regional brain metabolism—and neuroimaging techniques, to assess brain perfusion and oxygenation, several studies have reported profound derangements in the regulation of neuroenergetics in the absence of ischemia at the early phase of acute brain injury (8–15). So far, however, cerebral energy dysfunction has mainly been described following neurotrauma (16–19). Whether cerebral energy dysfunction contributes to early brain injury following SAH has not been extensively studied; moreover, its exact nature and potential cerebral blood flow (CBF) correlates have not been precisely characterized.
The aim of this study in comatose SAH patients who underwent advanced multimodal neuromonitoring was to examine the relationship of cerebral energy metabolism (using CMD) with brain oxygenation (using brain tissue PO2, PbtO2) and CBF. CBF was quantified using brain perfusion CT (PCT) performed at the early phase of SAH. We hypothesized that early brain injury following SAH leads to an increase in neurochemical markers of cerebral energy dysfunction [CMD lactate and lactate/pyruvate ratio (LPR)] and brain cell damage (CMD glycerol) despite the absence of cerebral ischemia/hypoxia.
Subjects were part of a retrospective observational cohort of patients with SAH admitted to the Intensive Care Unit who underwent CMD/PbtO2 monitoring and brain PCT as part of standard care. Indication for intracranial monitoring was a post-resuscitation Glasgow Coma Scale <9. Subjects were recruited over a 2-year period (March 2013–April 2015); only patients who had complete CMD/PbtO2 data and in whom brain PCT was performed within 96 h from SAH were included in the present study. A subset of the present cohort was also included in another clinical investigation that evaluated that value of CMD to detect delayed cerebral ischemia, submitted elsewhere. Approval for the study was obtained from our local Ethical Committee, and a waiver of consent was given due to the retrospective nature of the study.
Cerebral microdialysis consisted of a CMA 70 catheter (20 kDa cutoff, CMA Microdialysis AB®, Stockholm, Sweden; flow rate 0.3 µL/min), inserted through a triple-lumen bolt into subcortical white matter generally in the non-dominant frontal lobe in an area situated between the anterior and the middle cerebral artery territories. CMD samples were collected hourly and analyzed immediately at the bedside with the ISCUS flex system (CMA Microdialysis AB®) for brain extracellular concentrations of lactate, pyruvate, glucose, glutamate, and glycerol. First hour of monitored data, artifacts, and values outside obvious physiological ranges were manually excluded. In all patients, PbtO2 (Licox®, Integra Neurosciences, Plainsboro, NJ, USA) and intracranial pressure (ICP; Codman®, Raynham, MA, USA) probes were also inserted, adjacent to the CMD catheter. The correct placement of all probes was verified within 24 h by a follow-up non-contrast head CT scan.
Patients were managed according to a written standardized algorithm, in line with international guidelines (20). After initial stabilization, the ruptured aneurysm was secured either by surgical clipping or by endovascular coiling. All patients were mechanically ventilated, targeting a PaO2 of 90–100 mm Hg and a PaCO2 of 35–40 mm Hg. Cerebral perfusion pressure (CPP) was maintained at least >70 mm Hg, if needed with the use of fluids (crystalloids) and vasopressors (norepinephrine). Blood glucose was maintained at 6–8 mmol/L, and enteral nutrition was started within 48 h.
Neuroimaging was conducted in stable conditions of ICP, and systemic hemodynamics. PCT was performed using a multi-detector row CT Lightspeed (GE medical systems, Milwaukee, WI, USA). Scanning was initiated 5 s after injection of 50 mL of iohexol (300 mg/mL of iodine; GE Healthcare Europe), perfused at a rate of 5 mL/s, with the following parameters: 80 kV, 240 mAs, 0.4 rotations/s, and total duration of 50 s. The series evaluated 16 adjacent 5-mm-thick sections of brain parenchyma. Postprocessing of PCT data was performed by an experienced neuroradiologist, masked to CMD and PbtO2 variables, using a dedicated software (Brilliance Workspace Portal®, Philips Medical Systems, Cleveland, OH, USA), which uses the central volume principle using deconvolution to measure the mean transit time (MTT). Cerebral blood volume (CBV) is calculated from the time-enhancement curves, and CBF is derived from the equation: CBF = CBV/MTT.
We defined four main regions of interest (ROIs): right and left middle cerebral artery, right and left anterior cerebral artery. CBF from posterior and cerebellar arteries territories were not analyzed in this study. Three-dimensional reconstruction was processed with Carestream Vue PACS (Carestream Health, Rochester, NY, USA) using a series of the thin-slice enhanced brain CT. Because intracranial probes were located in the white matter, ROIs were drawn in areas of predominant white matter to allow concordant measurement of global supratentorial perfusion in the same type of tissue. All procedures and the ROIs’ characteristics were selected in line with previous studies (16). Global CBF was defined as the average of 4 ROIs’ CBF. Once the postprocessing completed, each PCT was then categorized as ischemic (global CBF < 28 mL/100 g/min), normal (global CBF 28–66 mL/100 g/min), or hyperemic (global CBF > 66 mL/100 g/min) (21, 22).
Episodes with cerebral energy dysfunction were defined by LPR >40 and/or lactate >4 mmol/L in brain extracellular fluid, according to recent consensus guidelines (23). Simultaneous levels of CMD glutamate, glycerol, as well as PbtO2 and ICP (averaged to the hour previous to CMD sampling), were also collected. For the purpose of the study, we analyzed CMD data closest to the time of PCT data. Identification of episodes of energy dysfunction was conducted by LC and MO, blinded to PCT data.
Variables of interest included cerebral metabolic (CMD derived) data, PbtO2, and PCT findings. Statistical analysis was performed using JMP-10® package software (SAS Institute, Cary, NC, USA). Results were expressed as mean ± SD or median and interquartile range, and comparisons between groups were explored using Student’s t-test or Mann–Whitney test as appropriate. Linear correlations were tested using the non-parametric Spearman’s correlation coefficient. Significance was set at a p < 0.05.
Out of a total of 29 patients who underwent CMD monitoring during the study period, complete dataset was available for 18 non-consecutive comatose subjects who underwent 21 PCTs during the early post-injury phase [a median of 41 (25–73) h from ictus onset]. Patients were predominantly women (16/18), mean age was 58 ± 9 years, and modified Fisher scale was 3 (1 patient) or 4 (17 patients). All patients were sedated and mechanically ventilated at the time of CT perfusion.
Brain PCT data are summarized in Table 1. No significant differences for CBF were found between the different vascular territories. Global CBF averaged 57 ± 14 mL/100 g/min: among the 21 PCTs, 14 showed normal CBF while 7 were classified as hyperemic. Importantly, among the three patients in whom brain PCT was performed twice, CBF findings were concordant over the two repeated measurements (i.e., two patients with normal CBF, and one patient with hyperemic CBF). When examining concomitant cerebral physiologic variables (n = 157 h of intracranial monitoring, Table 2), we found that ICP and PbtO2 were on average within normal ranges. In contrast, brain extracellular concentrations of LPR, glutamate and glycerol were all elevated (Table 2), indicating substantial cerebral metabolic abnormalities in the early brain injury phase following SAH in our patient cohort, despite the absence of cerebral ischemia/hypoxia.
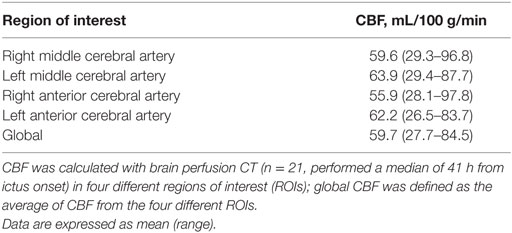
Table 1. Cerebral blood flow (CBF) during the early brain injury phase following aneurysmal subarachnoid hemorrhage.
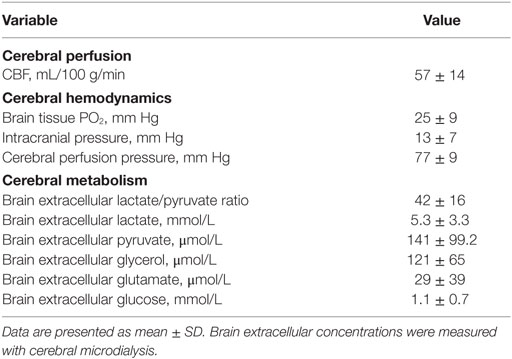
Table 2. Global cerebral blood flow (CBF), main brain physiologic, and cerebral metabolic variables at the time of brain perfusion CT.
The relationship of cerebral energy dysfunction (n = 103 h of CMD samples, average duration 7.4 h) with global CBF was further analyzed, according to CBF classification (normal vs. hyperemic). Figure 1 illustrates two representative patients in whom cerebral energy dysfunction was associated with normal (Figure 1A) or hyperemic (Figure 1B) CBF on PCT, and absence of low PbtO2.
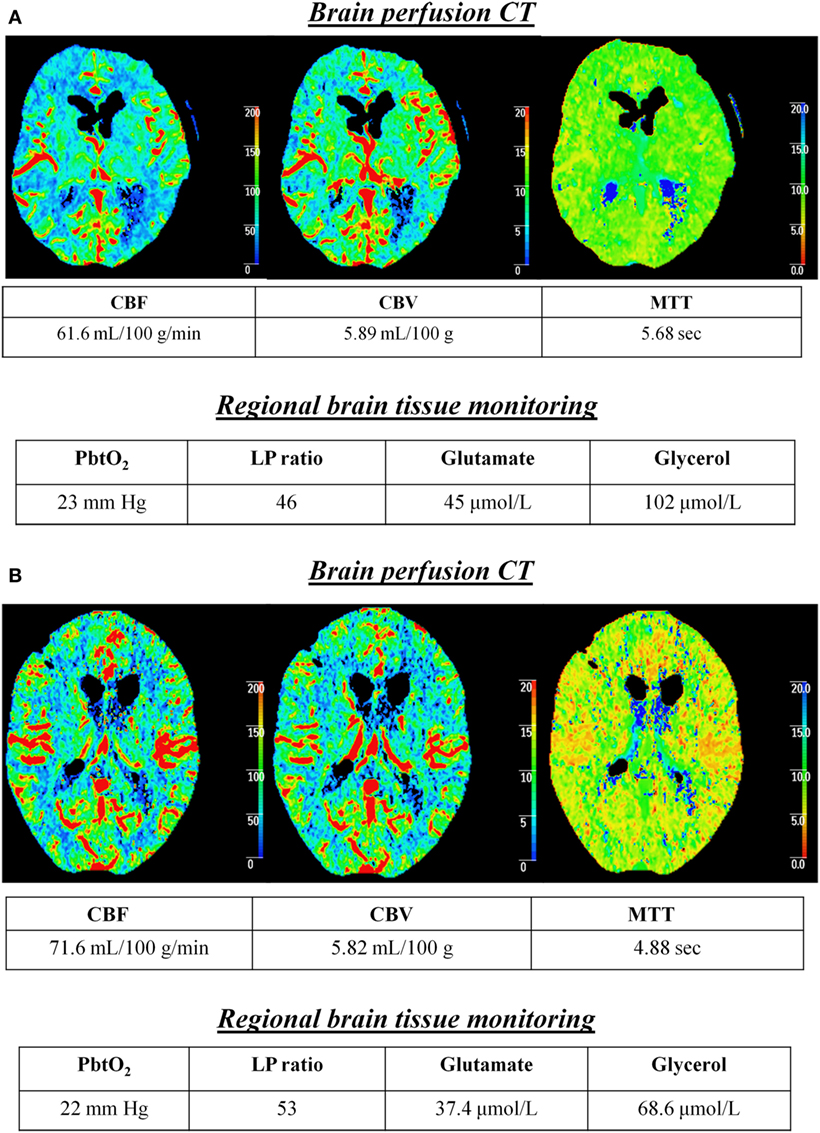
Figure 1. Cerebral energy dysfunction is associated with normal or hyperemic CBF in the early brain injury phase of aneurysmal subarachnoid hemorrhage. Illustrative examples of two patients in whom cerebral energy dysfunction was associated with brain perfusion CT (PCT) showing normal (A) or hyperemic (B) CBF. Time from PCT to subarachnoid hemorrhage was 16 h in patient (A) and 38 h in patient (B). Brain extracellular concentrations of lactate/pyruvate (LP) ratio, glutamate, and glycerol were measured with a 20 kDa cerebral microdialysis catheter that was located in the subcortical white matter, adjacent to a brain tissue PO2 (PbtO2) probe. Abbreviations: CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time.
Complete CMD data were available for 19 PCTs: episodes of cerebral energy dysfunction were observed in 14/19 PCT measurements, corresponding to 66% of total CMD samples examined. Nine PCTs showed normal CBF while five were hyperemic. Cerebral metabolic abnormalities were more pronounced in hyperemic than normal CBF (Table 3): cerebral hyperemia was associated with higher levels of brain extracellular LPR (54.1 ± 11.9 vs. 42.1 ± 6.5 when CBF was normal, p < 0.0001) and glycerol (157.0 ± 76.1 vs. 95.1 ± 41.4 µmol/L, p < 0.0001). Brain CMD glutamate also was higher when CBF was hyperemic (38.0 ± 51.5 vs. 25.9 ± 23.5 µmol/L when CBF was normal), although this did not reach statistical significance (p = 0.18). As shown in Table 3, ICP and PbtO2 did not differ between the two CBF conditions: only a minority of samples (16%) had elevated ICP >20 mm Hg or PbtO2 <15 mm Hg (11%).
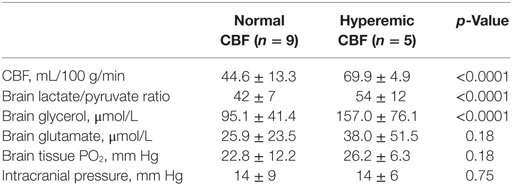
Table 3. Episodes of cerebral energy dysfunction categorized according to cerebral blood flow (CBF), dichotomized as normal vs. hyperemic.
As shown in Figure 2, Spearman’s linear correlation coefficient analysis found a positive linear correlation of higher global CBF with both elevated brain LPR (r = 0.47, p < 0.0001) and brain glycerol (r = 0.58, p < 0.0001) during episodes of cerebral energy dysfunction, thereby suggesting that cerebral hyperemia correlated with more pronounced derangements in brain interstitial tissue biochemistry.
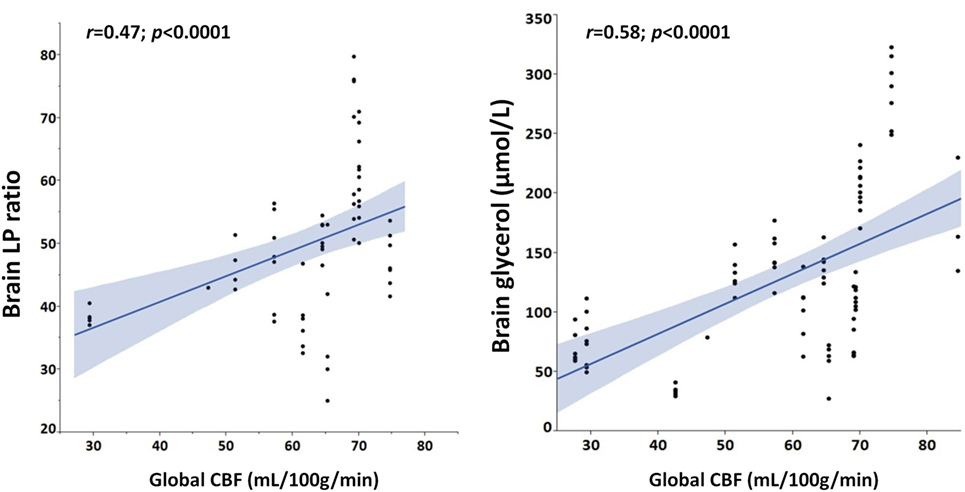
Figure 2. Hyperemic cerebral blood flow (CBF) is positively correlated with cerebral metabolic and cellular distress. Graphs illustrate Spearman’s linear correlations of global CBF, measured with perfusion CT, with brain extracellular concentrations of lactate/pyruvate (LP) ratio and glycerol.
Global CBF was higher during episodes with cerebral energy dysfunction (n = 14; CBF 59.2 ± 15.7 mL/100 g/min) vs. those without (n = 5; CBF 51.2 ± 7.9 mL/100 g/min, p = 0.01).
Our data combining regional brain tissue monitoring with perfusion head CT scan demonstrate that cerebral energy dysfunction may occur despite absence of brain hypoxia/ischemia, since PbtO2 was on average within normal ranges and cerebral perfusion was normal, or even supranormal. This clinical study identifies cerebral energy dysfunction as a novel pathogenic determinant of early brain injury following SAH. Our findings further suggest that, at the early phase of SAH, interventions targeted to cerebral perfusion and oxygenation may not be sufficient to prevent further secondary brain damage. It is plausible to conceive that alternative strategies aimed at improving cerebral energy metabolism may be of value in this setting.
All PCTs were showing normal to supranormal CBF. Our findings (see Table 1) are in line with previous clinical observations showing that CBF measured at the early acute phase following SAH was on average above 54 mL/100 g/min, irrespective of later occurrence of delayed cerebral ischemia (24, 25). Normal CBF was associated with normal PbtO2 and ICP values. Thus, our study combining cerebral perfusion imaging with monitoring of regional brain physiology did not reveal evidence of cerebral ischemia or hypoxia in our patient cohort, at least at the time interval where neuroimaging was performed.
Despite assessment of cerebral perfusion, oxygenation and hemodynamics showed absence of abnormalities, sampling of patient brain extracellular fluid obtained at the time interval of PCT revealed levels of LPR, glutamate, and glycerol that were all pathologically elevated (see Table 2). These neurometabolic abnormalities were all clinically relevant: in particular, brain LPR was on average above 40, a threshold that is associated with worse neurological recovery (17, 23, 26).
When focusing on episodes of cerebral energy dysfunction, we found that these were frequent, corresponding to two thirds of total CMD samples collected during PCTs. We found that cerebral energy dysfunction was not associated with ischemia/oligemia, but rather with normal to supranormal CBF. Previous clinical investigation in patients with traumatic brain injury revealed that energy dysfunction is predominantly non-ischemic (16, 17) and unrelated to reduced cerebral perfusion (27, 28). To the best of our knowledge, this is the first study to show evidence of non-ischemic/hypoxic cerebral energy dysfunction during the early brain injury phase following SAH in humans.
Interestingly, when dichotomizing global CBF data as normal vs. hyperemic (Table 3), hyperemia correlated with a greater impairment of cerebral energy metabolism and higher levels of cerebral glycerol, a marker of brain cell membrane disruption. Brain glutamate, a marker of brain excitotoxicity, was also higher in hyperemic conditions. In line with this, we observed that brain LPR and glycerol were positively correlated with increased CBF (Figure 2). Altogether, these findings establish a link between hyperemia and the severity of neurochemical derangements.
Our data support the notion that mechanisms alternative to ischemia or hypoxia may be implicated in the pathogenesis of cerebral energy dysfunction following SAH. Hyperglycolysis (10, 14, 15, 29, 30), mitochondrial dysfunction (31), electrographic seizures (18), and cortical spreading depression (32) have been associated with cerebral energy dysfunction and metabolic crisis. Cerebral energy dysfunction is now well recognized as an important determinant of secondary brain injury (11) and patient prognosis (19, 33), at least in patients with traumatic brain injury.
The association between hyperemia and cerebral energy dysfunction during the early brain injury phase of SAH is not unprecedented and may help understanding underlying mechanisms. A growing body of evidence suggests that cortical spreading depression might play an important role in the pathophysiology of secondary cerebral damage following acute brain injury in general and are implicated in the early brain injury phase following SAH (34). Sakowitz and colleagues indeed found a relationship between cortical spreading depression and cerebral energy dysfunction in patients with SAH (35). Persistent impairment of cerebral energy metabolism might occur despite apparent hyperemia (36). Further study is needed to examine the exact relationship of causality between spreading depressions and cerebral energy dysfunction following SAH: it is also conceivable that treatment of spreading depressions may improve brain energetics.
Mitochondrial dysfunction is characterized by increased LPR, because of elevated brain lactate but with normal-to-elevated levels of pyruvate and PbtO2 (31). This cerebral metabolic pattern is distinct from that seen in ischemia where LPR elevation is coupled with low pyruvate and PbtO2 (23). Here, episodes of cerebral energy dysfunction were associated with normal PbtO2 and CBF, and also with normal-to-elevated brain extracellular pyruvate (on average 141.0 ± 99.2 µmol/L), thereby suggesting that mitochondrial dysfunction may be potentially implicated. Finally, elevated lactate may be secondary to increased activation of astrocytic glycolysis (29, 30).
Diagnosis of cerebral energy dysfunction requires advanced brain monitoring with the use of the cerebral microdialysis technique, thereby reinforcing the utility of this monitoring modality for the care of patients with poor-grade SAH (37). The fact that cerebral energy dysfunction might occur despite brain physiology (ICP and PbtO2) and CBF are normal (or even supranormal) indicates that therapy targeted solely to improve cerebral oxygenation and perfusion may not be enough to mitigate brain damage during the acute phase following SAH. While ischemia/hypoxia undoubtedly might play a role in the pathogenesis of SAH, the scientific paradigm has increasingly turned its interest toward the search for alternative mechanisms (7), particularly at the early brain injury phase (1, 4). Emerging evidence suggests that therapies aimed at modulating neuroenergetics might be protective after brain injury (38). Recent clinical investigation further demonstrates that resuscitation of the injured human brain with hypertonic lactate solutions exerts a beneficial effect on cerebral energy metabolism (39, 40).
The first limitation is related to the single-center observational cohort and the relatively small sample-size, which limits data generalizability. PCTs, however, were analyzed blinded to intracranial monitoring data; similarly, CMD and other brain physiological data were matched to PCTs in a blinded fashion. Although a correlation between CBF and CMD LPR and glycerol was found during episodes of cerebral energy dysfunction, due to the limited number of patients we could not use multiple regression with adjustment of cofactors, therefore we cannot confirm a causal relationship.
Timing of brain imaging is an important issue. Although brain PCT was performed at a relatively early phase after SAH (median 41 h from ictus onset) the role of initial global ischemia after SAH cannot be extrapolated from our data. Previous studies performed at the very early phase (<12 h) indeed reported acute cerebral hypoperfusion in subjects with SAH undergoing Xenon contrast-enhanced CT scanning (41). Our findings therefore apply more to the post-resuscitation ICU phase (approximately day 1–3).
Based on our local practice in patients with high grade SAH, CPP was kept >70 mm Hg (average CPP was 77 ± 9 mm Hg). As the major finding of this study was that cerebral energy dysfunction was frequent and was associated with normal to hyperemic CBF, it is unclear whether this was due only to the intrinsic SAH pathophysiology or whether this was, at least in part, caused or even exacerbated by elevated CPP. Based on these data, a preventive strategy of CPP augmentation may not seem justified in patients with SAH, since it may potentially aggravate cerebral energy dysfunction. Our data appear in line with previous microdialysis studies in patients with traumatic brain injury, showing that lower CPP (50–60 mm Hg) may be sufficient to preserve normal cerebral energy metabolism (42).
PCTs were performed in stable conditions, after initial resuscitation. This is an advantage in terms of the homogeneity of the data analyzed. However, our findings only apply to early post-SAH phase when ICP and CPP are stable, but we cannot extrapolate our findings to conditions of more disturbed intracranial physiology (e.g., intracranial hypertension). While matching of PCT with CMD and PbtO2 data offers a comprehensive view of the injured brain state, on the other hand it only provides a partial view of the overall picture. This is mainly because PCTs is not available continuously. Our study should therefore be considered as hypothesis generating, and further investigation is needed to confirm our findings. We believe, however, that, notwithstanding these limitations, this clinical study identifies cerebral energy dysfunction as a novel pathogenic determinant of early brain injury following SAH.
Our study in patients with comatose aneurysmal SAH who underwent advanced brain monitoring with cerebral microdialysis and PbtO2, in combination with PCT performed during the early phase following ictus onset, indicates that cerebral energy dysfunction is frequent and is associated with normal to hyperemic CBF. Our data identify an alternative pathogenic mechanism of early brain injury following SAH in humans, which appears unrelated to cerebral ischemia/hypoxia. Management targeted to improve energy substrate delivery—together with adequate cerebral perfusion and oxygenation—may help attenuating secondary brain damage at the early phase of SAH. Additional study is needed to characterize the exact mechanisms of SAH-related cerebral energy dysfunction and to examine whether timely diagnosis and treatment may translate into better patient outcome.
Approval for the study was obtained from the Ethical Committee of the University of Lausanne. A waiver of consent was given due to the retrospective observational cohort study.
LC collected the data, performed data analysis, and drafted the manuscript; CP and DS helped with data collection and revised the manuscript; RD, PE, and RM revised the manuscript and provided important intellectual contribution; MO conceived the study design, supervised data collection and analysis, and revised the manuscript. All the authors have read and approved the content of the present manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supported by grants from the Swiss National Science Foundation (grant nr 32003B_155957), the Novartis Foundation for Biomedical Research (both to MO), the Société Française d’Anesthésie et de Réanimation (SFAR), and the “Fondation des Gueules Cassées” (both to LC).
1. Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry (2014) 85(12):1343–53. doi:10.1136/jnnp-2014-307711
2. Cossu G, Messerer M, Oddo M, Daniel RT. To look beyond vasospasm in aneurysmal subarachnoid haemorrhage. Biomed Res Int (2014) 2014:628597. doi:10.1155/2014/628597
3. Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res (2013) 4(4):432–46. doi:10.1007/s12975-013-0257-2
4. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol (2014) 10(1):44–58. doi:10.1038/nrneurol.2013.246
5. Tso MK, Macdonald RL. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res (2014) 5(2):174–89. doi:10.1007/s12975-014-0323-4
6. Zheng VZ, Wong GK. Neuroinflammation responses after subarachnoid hemorrhage: a review. J Clin Neurosci (2017) 42:7–11. doi:10.1016/j.jocn.2017.02.001
7. Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab (2011) 31(6):1443–51. doi:10.1038/jcbfm.2011.7
8. Bergsneider M, Hovda DA, Lee SM, Kelly DF, McArthur DL, Vespa PM, et al. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma (2000) 17(5):389–401. doi:10.1089/neu.2000.17.389
9. Bergsneider M, Hovda DA, McArthur DL, Etchepare M, Huang SC, Sehati N, et al. Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. J Head Trauma Rehabil (2001) 16(2):135–48. doi:10.1097/00001199-200104000-00004
10. Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg (1997) 86(2):241–51. doi:10.3171/jns.1997.86.2.0241
11. Bouzat P, Sala N, Payen JF, Oddo M. Beyond intracranial pressure: optimization of cerebral blood flow, oxygen, and substrate delivery after traumatic brain injury. Ann Intensive Care (2013) 3(1):23. doi:10.1186/2110-5820-3-23
12. Brooks GA, Martin NA. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front Neurosci (2015) 8:408. doi:10.3389/fnins.2014.00408
13. Carpenter KL, Jalloh I, Hutchinson PJ. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci (2015) 9:112. doi:10.3389/fnins.2015.00112
14. Jalloh I, Carpenter KL, Grice P, Howe DJ, Mason A, Gallagher CN, et al. Glycolysis and the pentose phosphate pathway after human traumatic brain injury: microdialysis studies using 1,2-(13)C2 glucose. J Cereb Blood Flow Metab (2015) 35(1):111–20. doi:10.1038/jcbfm.2014.177
15. Jalloh I, Carpenter KL, Helmy A, Carpenter TA, Menon DK, Hutchinson PJ. Glucose metabolism following human traumatic brain injury: methods of assessment and pathophysiological findings. Metab Brain Dis (2015) 30(3):615–32. doi:10.1007/s11011-014-9628-y
16. Sala N, Suys T, Zerlauth JB, Bouzat P, Messerer M, Bloch J, et al. Cerebral extracellular lactate increase is predominantly nonischemic in patients with severe traumatic brain injury. J Cereb Blood Flow Metab (2013) 33(11):1815–22. doi:10.1038/jcbfm.2013.142
17. Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab (2005) 25(6):763–74. doi:10.1038/sj.jcbfm.9600073
18. Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol (2016) 79(4):579–90. doi:10.1002/ana.24606
19. Xu Y, McArthur DL, Alger JR, Etchepare M, Hovda DA, Glenn TC, et al. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J Cereb Blood Flow Metab (2010) 30(4):883–94. doi:10.1038/jcbfm.2009.263
20. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke (2012) 43(6):1711–37. doi:10.1161/STR.0b013e3182587839
21. Cremers CH, van der Schaaf IC, Wensink E, Greving JP, Rinkel GJ, Velthuis BK, et al. CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab (2014) 34(2):200–7. doi:10.1038/jcbfm.2013.208
22. Huang AP, Tsai JC, Kuo LT, Lee CW, Lai HS, Tsai LK, et al. Clinical application of perfusion computed tomography in neurosurgery. J Neurosurg (2014) 120(2):473–88. doi:10.3171/2013.10.JNS13103
23. Hutchinson PJ, Jalloh I, Helmy A, Carpenter KL, Rostami E, Bellander BM, et al. Consensus statement from the 2014 International Microdialysis Forum. Intensive Care Med (2015) 41(9):1517–28. doi:10.1007/s00134-015-3930-y
24. Lagares A, Cicuendez M, Ramos A, Salvador E, Alén JF, Kaen A, et al. Acute perfusion changes after spontaneous SAH: a perfusion CT study. Acta Neurochir (Wien) (2012) 154(3):405–11; discussion 411–2. doi:10.1007/s00701-011-1267-z
25. van der Schaaf I, Wermer MJ, van der Graaf Y, Hoff RG, Rinkel GJ, Velthuis BK. CT after subarachnoid hemorrhage: relation of cerebral perfusion to delayed cerebral ischemia. Neurology (2006) 66(10):1533–8. doi:10.1212/01.wnl.0000216272.67895.d3
26. Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med (2008) 36(12):3233–8. doi:10.1097/CCM.0b013e31818f4026
27. Asgari S, Vespa P, Hu X. Is there any association between cerebral vasoconstriction/vasodilatation and microdialysis lactate to pyruvate ratio increase? Neurocrit Care (2013) 19(1):56–64. doi:10.1007/s12028-013-9821-6
28. Vespa PM, O’Phelan K, McArthur D, Miller C, Eliseo M, Hirt D, et al. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med (2007) 35(4):1153–60. doi:10.1097/01.CCM.0000259466.66310.4F
29. Oddo M, Levine JM, Frangos S, Maloney-Wilensky E, Carrera E, Daniel RT, et al. Brain lactate metabolism in humans with subarachnoid hemorrhage. Stroke (2012) 43(5):1418–21. doi:10.1161/STROKEAHA.111.648568
30. Samuelsson C, Hillered L, Zetterling M, Enblad P, Hesselager G, Ryttlefors M, et al. Cerebral glutamine and glutamate levels in relation to compromised energy metabolism: a microdialysis study in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab (2007) 27(7):1309–17. doi:10.1038/sj.jcbfm.9600433
31. Jacobsen A, Nielsen TH, Nilsson O, Schalen W, Nordstrom CH. Bedside diagnosis of mitochondrial dysfunction in aneurysmal subarachnoid hemorrhage. Acta Neurol Scand (2014) 130(3):156–63. doi:10.1111/ane.12258
32. Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J Neurotrauma (2016) 33(19):1775–83. doi:10.1089/neu.2015.4226
33. Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab (2003) 23(10):1239–50. doi:10.1097/01.WCB.0000089833.23606.7F
34. Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med (2011) 17(4):439–47. doi:10.1038/nm.2333
35. Sakowitz OW, Santos E, Nagel A, Krajewski KL, Hertle DN, Vajkoczy P, et al. Clusters of spreading depolarizations are associated with disturbed cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke (2013) 44(1):220–3. doi:10.1161/STROKEAHA.112.672352
36. Hashemi P, Bhatia R, Nakamura H, Dreier JP, Graf R, Strong AJ, et al. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leao’s spreading depression. J Cereb Blood Flow Metab (2009) 29(1):166–75. doi:10.1038/jcbfm.2008.108
37. Chen HI, Stiefel MF, Oddo M, Milby AH, Maloney-Wilensky E, Frangos S, et al. Detection of cerebral compromise with multimodality monitoring in patients with subarachnoid hemorrhage. Neurosurgery (2011) 69(1):53–63; discussion 63. doi:10.1227/NEU.0b013e3182191451
38. Bouzat P, Oddo M. Lactate and the injured brain: friend or foe? Curr Opin Crit Care (2014) 20(2):133–40. doi:10.1097/MCC.0000000000000072
39. Bouzat P, Sala N, Suys T, Zerlauth JB, Marques-Vidal P, Feihl F, et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med (2014) 40(3):412–21. doi:10.1007/s00134-013-3203-6
40. Quintard H, Patet C, Zerlauth JB, Suys T, Bouzat P, Pellerin L, et al. Improvement of neuroenergetics by hypertonic lactate therapy in patients with traumatic brain injury is dependent on baseline cerebral lactate/pyruvate ratio. J Neurotrauma (2016) 33(7):681–7. doi:10.1089/neu.2015.4057
41. Schubert GA, Seiz M, Hegewald AA, Manville J, Thome C. Acute hypoperfusion immediately after subarachnoid hemorrhage: a xenon contrast-enhanced CT study. J Neurotrauma (2009) 26(12):2225–31. doi:10.1089/neu.2009.0924
Keywords: cerebral microdialysis, subarachnoid hemorrhage, neuroenergetics, hyperemia, early brain injury
Citation: Carteron L, Patet C, Solari D, Messerer M, Daniel RT, Eckert P, Meuli R and Oddo M (2017) Non-Ischemic Cerebral Energy Dysfunction at the Early Brain Injury Phase following Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 8:325. doi: 10.3389/fneur.2017.00325
Received: 12 April 2017; Accepted: 21 June 2017;
Published: 10 July 2017
Edited by:
Rick Dijkhuizen, University Medical Center Utrecht, NetherlandsReviewed by:
Nikolaus Plesnila, Institute for Stroke and Dementia Research, GermanyCopyright: © 2017 Carteron, Patet, Solari, Messerer, Daniel, Eckert, Meuli and Oddo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mauro Oddo, bWF1cm8ub2Rkb0BjaHV2LmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.