
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 09 June 2017
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 8 - 2017 | https://doi.org/10.3389/fneur.2017.00266
 Jinping Xu1†
Jinping Xu1† Ahmed Elazab1,2†
Ahmed Elazab1,2† Jinhua Liang3
Jinhua Liang3 Fucang Jia1
Fucang Jia1 Huimin Zheng1
Huimin Zheng1 Weimin Wang3
Weimin Wang3 Limin Wang4
Limin Wang4 Qingmao Hu1*
Qingmao Hu1*
Postlesional plasticity has been identified in patients with cerebral gliomas by inducing a large functional reshaping of brain networks. Although numerous non-invasive functional neuroimaging methods have extensively investigated the mechanisms of this functional redistribution in patients with cerebral gliomas, little effort has been made to investigate the structural plasticity of cortical and subcortical structures associated with the glioma volume. In this study, we aimed to investigate whether the contralateral cortical and subcortical structures are able to actively reorganize by themselves in these patients. The compensation mechanism following contralateral cortical and subcortical structural plasticity is considered. We adopted the surface-based morphometry to investigate the difference of cortical and subcortical gray matter (GM) volumes in a cohort of 14 healthy controls and 13 patients with left-hemisphere cerebral gliomas [including 1 patients with World Health Organization (WHO I), 8 WHO II, and 4 WHO III]. The glioma volume ranges from 5.1633 to 208.165 cm2. Compared to healthy controls, we found significantly increased GM volume of the right cuneus and the left thalamus, as well as a trend toward enlargement in the right globus pallidus in patients with cerebral gliomas. Moreover, the GM volumes of these regions were positively correlated with the glioma volumes of the patients. These results provide evidence of cortical and subcortical enlargement, suggesting the usefulness of surface-based morphometry to investigate the structural plasticity. Moreover, the structural plasticity might be acted as the compensation mechanism to better fulfill its functions in patients with cerebral gliomas as the gliomas get larger.
Brain plasticity, defined as the continuous processing allowing short-, middle-, and long-term remodeling of the neurono-synaptic organization (1), has been described as natural plasticity for normal people in learning or memory (2), or postlesional plasticity for patients with traumatic brain injury, stroke, or tumors (3). In particular, more progressive lesions, such as cerebral gliomas, have been identified to induce a large functional reshaping of brain networks around the tumor (4), within the lesioned hemisphere (5) or in parts of the contralateral hemisphere homologous to the structures invaded (6–9), which were thought of as functional compensations (i.e., the absence of deficit). Moreover, these functional reorganizations were utilized to explain why slow infiltrative low-grade gliomas within the so-called “eloquent” areas (such as Broca’s areas, Wernicke’s areas, and the supplementary motor area) usually do not induce detectable neurological deficits (10) or why remove these regions usually does not induce permanent deficits (11–14). For instance, studies have described that totally removing the supplementary motor area can recover in a few weeks (15–18) by recruiting the contralateral homologous regions (19), despite the occurrence of an immediate postsurgical transient supplementary motor area syndrome. For patients with cerebral gliomas, although numerous non-invasive techniques have analyzed almost exclusively mechanisms of functional redistribution, little effort has been made to investigate the structural plasticity of cortical and subcortical structures.
Recently, T1-weighted morphometric analysis has allowed the distinct exploration of cortical morphometry over the entire cortex (20). The precise measurement of gray matter (GM) volume has been adopted in healthy individuals and various neurological diseases (21–24), showing potentials to investigate structural plasticity in patients with cerebral gliomas. The Statistical Parametric Mapping (SPM)1 and FreeSurfer2 are two widely used methods to analyze the structural data. However, using surface geometry to do inter-subject comparisons of cortical brain areas (25), FreeSurfer analyzes GM volumes as structures as a whole without voxel-wise comparisons between individual magnetic resonance (MR) images, which exhibits higher sub-voxel accuracy than SPM based on voxel-based methods and is more robust to missegmentation (26). Therefore, investigating the structural morphometry of the cortical and subcortical structures with FreeSurfer could provide novel insights into the compensation mechanism in the patients with cerebral gliomas.
The goal of this research is to focus on the possible existence of a real cortical and subcortical plastic potential and try to answer whether the subcortical and contralateral cortical structures are able to actively reorganize by themselves in patients with cerebral gliomas. To this end, we adopted the surface-based morphometry to investigate the differences of cortical and subcortical GM volume in a cohort of 13 patients with cerebral gliomas and 14 healthy controls.
In this study, 13 patients with histologically confirmed cerebral gliomas were recruited consecutively from the Guangzhou General Hospital of Guangzhou Military Command. Of the 13 patients, 1 had World Health Organization (WHO) grade I, 8 were WHO II, and 4 were WHO III cerebral glioma (Table 1). Patient data were retrospectively obtained from our institutional database and had been acquired between 2013 and 2016. Patients were filtered to include only those with left frontal and/or temporo-parietal lobe brain gliomas. A small number of patients with right hemispheric gliomas were excluded from further analysis in order to provide a more homogeneous patient population. The glioma volume of each patient was calculated based on the preoperative T2 flair image using the OsiriX Lite (27–30)3 (Table 1). Fourteen healthy controls matched for sex and age were also recruited. All of the healthy controls underwent extensive neurologic, neuropsychologic, and clinical imaging examinations. Participants who had a history of neurologic or psychiatric disease and neurologic sequelae induced by brain trauma were excluded. All the patients and healthy controls are right handed. The study protocol was approved by the Guangzhou General Hospital of Guangzhou Military Command. Written informed consents were obtained from all participants after they received a complete description of the study. All procedures were performed according to the principles expressed in the Declaration of Helsinki.
MRI data were acquired on a 3.0-T MR imaging system (GE Medical Systems) in the Guangzhou General Hospital of Guangzhou Military Command. Whole brain structural images were acquired with a three dimensional (3D) T1-weighted 3D-bravo sequence. Detailed scan parameters were repetition time = 11.952 ms, echo time = 5.036 ms, inversion time = 380 ms, slice thickness = 1 mm, no gaps, flip angle = 15°, acquisition matrix = 256 × 256, and 0.47 mm × 0.47 mm in-plane resolution.
Each scan was processed using the FreeSurfer pipeline, which is a semi-automated approach described in detail in prior publications (31, 32). Briefly, the image processing included skull stripping, automated Talairach transformations, segmentation of the subcortical white matter (WM) and deep GM structures, intensity normalization, tessellation of the boundary between GM and WM, automated topology correction, and surface deformation along intensity gradients for optimal placement of the borders between GM, WM, and cerebrospinal fluid. In case of inaccuracies, manual editing was performed according to the FreeSurfer editing manual,4 either by adding control points to help FreeSurfer identify the WM voxels or removing the skull and dura in case they were considered to be parts of the brain.
The GM volumes of subcortical structures including the bilateral thalamus, hippocampus, amygdala, putamen, globus pallidus, and caudate were calculated using the automated procedure for volumetric measurements of brain structures implemented in the FreeSurfer (33).
The cortical GM volume was analyzed using a surface-based group analysis of FreeSurfer’s Qdec (version 1.5). First, the spatial cortical GM volume of the right hemisphere was smoothed with a circularly symmetric Gaussian kernel of 10 mm full width half maximum to provide normal distribution of the results. Then, we employed a general linear model (GLM) analysis with age and gender as the nuisance factors in the design matrix to directly compare the GM volume in the right hemisphere of the two groups. Finally, the GLM result was corrected for multiple comparisons utilizing a pre-cached cluster-wise Monte Carlo simulation (34) implemented in Qdec (mc-z, threshold: p < 0.001, sign: absolute).
Volumes of the subcortical regions were measured automatically. Comparisons in the volumes of these regions were performed using the GLM analysis with age and gender as covariates using IBM SPSS 19.0 (IBM, Armonk, NY, USA).
Correlations between the glioma volumes of the patients and the GM volumes of the altered regions were analyzed with Pearson’s correlations in SPSS.
There was no significant difference in terms of gender (patients: 10 males and 3 females, healthy controls: 9 males and 5 females, χ2 test, p = 0.678) and age (patients: 38.69 ± 13.68 years, healthy controls: 39.78 ± 15.12 years, two-sample t-test, p = 0.846) between patients with cerebral gliomas and healthy controls in the study.
We found significantly increased GM volume in the right cuneus in patients with cerebral gliomas compared to healthy controls (Figure 1A). We also found significantly increased GM volume in the left thalamus and a trend toward enlargement in the right globus pallidus (Figure 2A; Table 2) in patients with cerebral gliomas.
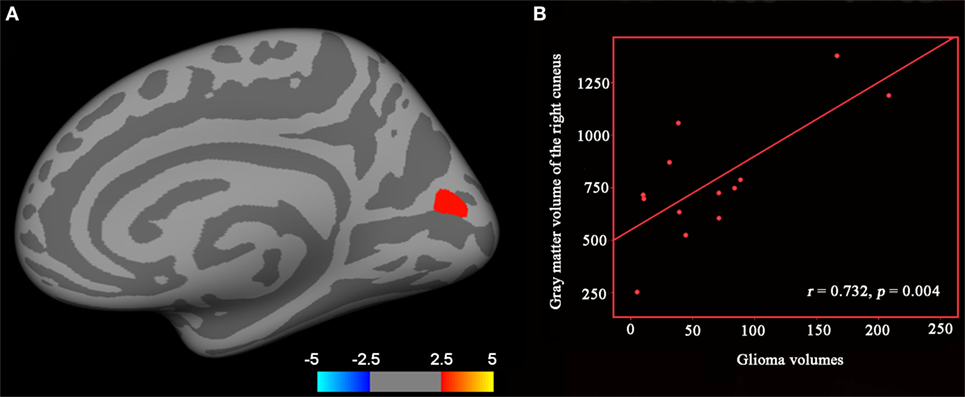
Figure 1. (A) Significantly increased gray matter (GM) volume of the right cuneus in patients with cerebral gliomas compared to healthy controls. Comparison was performed using the GLM analysis and corrected for multiple comparisons utilizing a pre-cached cluster-wise Monte Carlo simulation implemented in Qdec (mc-z, threshold: p < 0.001, sign: absolute). (B) Significantly positive correlation between glioma volumes and the GM volume of the right cuneus was identified in the patients with cerebral gliomas (p < 0.05).
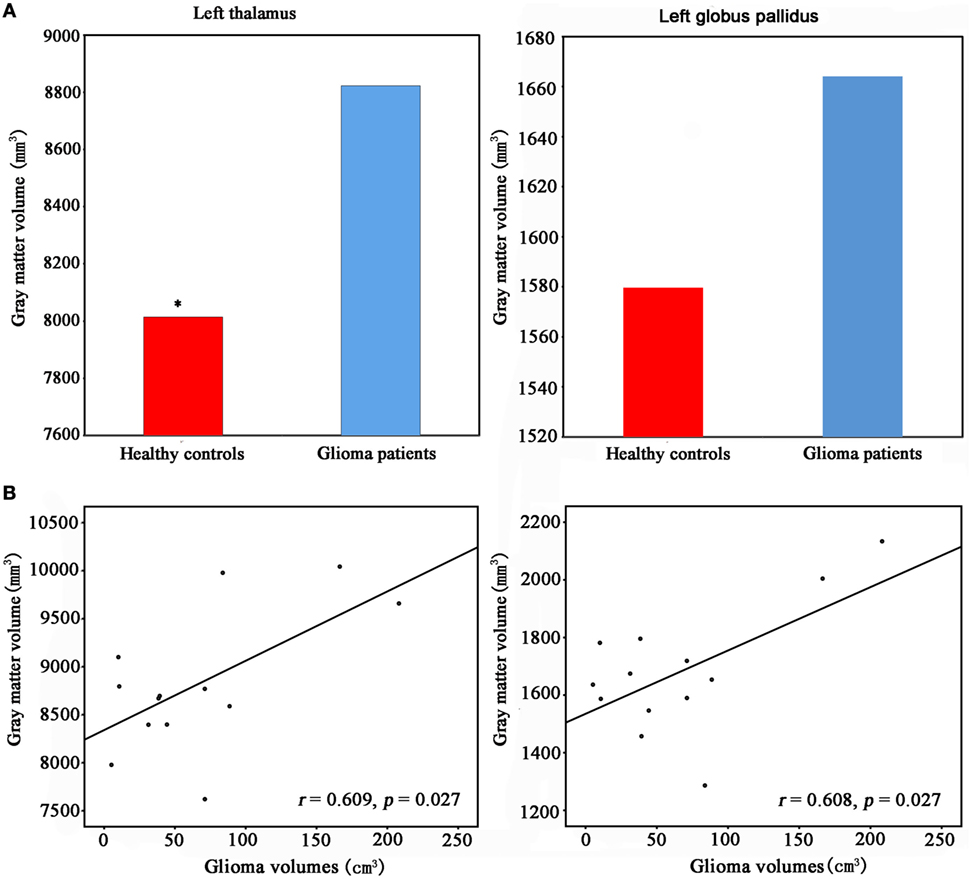
Figure 2. (A) Increased gray matter (GM) volume of the left thalamus and the left palladiums in patients with cerebral gliomas compared to healthy controls. Comparison was performed using the GLM analysis in IBM SPSS 19.0 (IBM, Armonk, NY, USA) with p < 0.05. *represents significant difference between the two groups. (B) Significantly positive correlation between glioma volumes and the GM volume of the left thalamus and palladiums were identified in the patients with cerebral gliomas (p < 0.05).
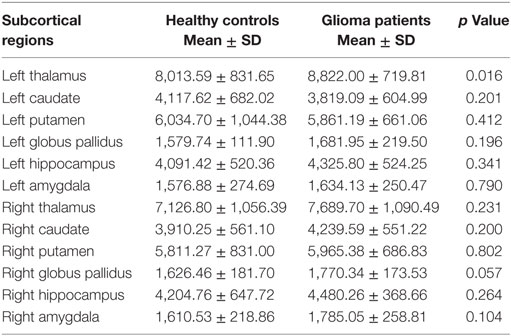
Table 2. The comparisons of gray matter volumes in the subcortical regions between patients with cerebral gliomas and healthy controls.
We found significantly positive correlations between the GM volume in the right cuneus and the glioma volumes in patients with cerebral gliomas (Figure 1B). We also found significantly positive correlations between GM volume in the left thalamus/the left globus pallidus and the glioma volumes in patients with cerebral gliomas (Figure 2B).
Using surface-based morphometric analysis, we found significantly increased GM volume in the right cuneus and the left thalamus, as well as a trend toward enlargement in the right globus pallidus in patients with cerebral gliomas compared to healthy controls. Moreover, the GM volumes of these regions were positively correlated with the glioma volumes of the patients.
Interestingly, we found significantly increased GM volume in the right cuneus and the left thalamus in the patients with cerebral gliomas, both of which were identified as hub regions in brain network. Functionally, the cuneus was identified as a hub region in a functional brain network (35), which connected to a visual network and acted as integrated center of the visual processing. Structurally, the cuneus was also identified as a hub region in the GM structural network and the diffusion-based network (36, 37). As for the thalamus, it has been identified as the a hub region in a brain network analysis and was believed to be a major processor of visual, auditory, and somatosensory information (35). Moreover, the thalamus is believed to act as a relay station by controlling the flow of sensorimotor information to and from the cortex (38, 39), especially in the cortical-striato-thalamic loop. Besides multiple functions, such as coordinating, encoding, retrieval, and planning (40), recent evidence supports a more diverse role of the thalamus in higher order cognitive functions by thalamocortical connections (41, 42). Given all these important parts the cuneus and the thalamus take in the brain functions, it is reasonable to speculate that the enlargement of these regions might be considered as the compensation mechanism to better fulfill its functions in patients with cerebral gliomas as the gliomas get larger. However, a previous review (10) suggested that mechanisms for plasticity are based on a hierarchically organized model involving three levels recruited successively. Our finding of GM volume enlargement in the hub regions might extend their suggestions to involve four levels. Moreover, this structural reorganization demonstrated that the subcortical and contralateral cortical structures are able to actively reorganize by themselves in patients with cerebral gliomas, which might be thought of as a novel explanation to why patients with gliomas usually do not have detectable neurological deficits. However, the mechanisms of brain plasticity for patients with cerebral gliomas were mostly based on functional MRI, which identified a large functional reshaping of brain networks (5–7). To the best of our knowledge, it is the first study that identified cortical and subcortical plasticity in patients with cerebral gliomas using surface-based morphometry. Therefore, our findings of larger GM volume in the cortical and subcortical regions were not hypothesized based on studies published to date and warrant replication.
Whether larger cortical and subcortical GM volume in patients with cerebral gliomas are resulted from the postlesional plasticity, or due to group differences in other confounding factors remain to be determined. Though given the wide range ages in the overall sample as well as a 17-year-old male in the patients group, there is no significant difference of age between the two groups. Moreover, we used GLM analysis with age as the covariate to minimize its impact. Other general confounding factors, such as differences in head motion during scanning would not likely uniquely affect the GM volume of the left cuneus and the left thalamus but would likely affect multiple other regions. Despite of the possibility of various confounding factors, our findings of cortical and subcortical enlargement might be related to the postlesional plasticity in patients with cerebral gliomas.
Some limitations should be stressed in this study. First, due to the loss of cognitive and behavior measurements of the patients, we were unable to address the relationship between these measurements and the GM volumes of the right cuneus/left thalamus, which weaken the interpretation of our finding. Second, our study was limited to patients with left cerebral glioma. Another subgroup of patients with right cerebral glioma is wanted to validate whether there is also a significant increase of GM volumes in the cortical and subcortical regions. Additionally, longitudinal and integrated structural–functional correlations might be necessary to further uncover the compensation mechanism for patients with cerebral gliomas.
To the best of our knowledge, it is the first study that identified cortical and subcortical plasticity in patients with cerebral gliomas, suggesting the usefulness of surface-based morphometry to investigate the structural plasticity. Moreover, the study might imply that the cortical and subcortical structures are able to actively reorganize by themselves and might be acted as the compensation mechanism to better fulfill its functions in patients with cerebral gliomas as the gliomas get larger. This structural reorganization might be thought of as a novel explanation to why patients with cerebral gliomas usually do not have detectable neurological deficits.
The study protocol was approved by the Guangzhou General Hospital of Guangzhou Military Command. Written informed consents were obtained from all participants after they received a complete description of the study. All procedures were performed according to the principles expressed in the Declaration of Helsinki.
QH, FJ, and WW designed the research. LW and JL collected the data. JX and AE analyzed the data. JX wrote the manuscript. QH and FJ discussed the results and offered good suggestions. All the authors reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the reviewers for their constructive comments and suggestions.
This work was supported by the Key Joint Program of National Natural Science Foundation and Guangdong Province (No. U1201257) and the National Natural Science Foundation (No. 61671440).
1. Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity – a review. J Neurooncol (2006) 79:77–115. doi: 10.1007/s11060-005-9109-6
2. Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem (2002) 78:553–64. doi:10.1006/nlme.2002.4091
3. Rijntjes M, Weiller C. Recovery of motor and language abilities after stroke: the contribution of functional imaging. Prog Neurobiol (2002) 66:109–22. doi:10.1016/S0301-0082(01)00027-2
4. Meyer PT, Sturz L, Schreckenberger M, Spetzger U, Meyer GF, Setani KS, et al. Preoperative mapping of cortical language areas in adult brain tumour patients using PET and individual non-normalised SPM analyses. Eur J Nucl Med Mol Imaging (2003) 30:951–60. doi:10.1007/s00259-003-1186-1
5. Meyer PT, Sturz L, Sabri O, Schreckenberger M, Spetzger U, Setani KS, et al. Preoperative motor system brain mapping using positron emission tomography and statistical parametric mapping: hints on cortical reorganisation. J Neurol Neurosurg Psychiatry (2003) 74:471–8. doi:10.1136/jnnp.74.4.471
6. Roux FE, Boulanouar K, Ibarrola D, Tremoulet M, Chollet F, Berry I. Functional MRI and intraoperative brain mapping to evaluate brain plasticity in patients with brain tumours and hemiparesis. J Neurol Neurosurg Psychiatry (2000) 69:453–63. doi:10.1136/jnnp.69.4.453
7. Holodny AI, Schulder M, Ybasco A, Liu WC. Translocation of Broca’s area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J Comput Assist Tomogr (2002) 26:941–3. doi:10.1097/00004728-200211000-00014
8. Baciu M, Le Bas JF, Segebarth C, Benabid AL. Presurgical fMRI evaluation of cerebral reorganization and motor deficit in patients with tumors and vascular malformations. Eur J Radiol (2003) 46:139–46. doi:10.1016/S0720-048X(02)00083-9
9. Taniguchi M, Kato A, Ninomiya H, Hirata M, Cheyne D, Robinson SE, et al. Cerebral motor control in patients with gliomas around the central sulcus studied with spatially filtered magnetoencephalography. J Neurol Neurosurg Psychiatry (2004) 75:466–71. doi:10.1136/jnnp.2002.001834
10. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol (2005) 4:476–86. doi:10.1016/S1474-4422(05)70140-X
11. Duffau H, Bauchet L, Lehericy S, Capelle L. Functional compensation of the left dominant insula for language. Neuroreport (2001) 12:2159–63. doi:10.1097/00001756-200107200-00023
12. Krainik A, Lehericy S, Duffau H, Vlaicu M, Poupon F, Capelle L, et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology (2001) 57:871–8. doi:10.1212/WNL.57.5.871
13. Duffau H, Denvil D, Capelle L. Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy. J Neurol Neurosurg Psychiatry (2002) 72:511–6. doi:10.1136/jnnp.72.4.511
14. Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg (2003) 98:764–78. doi:10.3171/jns.2003.98.4.0764
15. Duffau H, Lopes M, Denvil D, Capelle L. Delayed onset of the supplementary motor area syndrome after surgical resection of the mesial frontal lobe: a time course study using intraoperative mapping in an awake patient. Stereotact Funct Neurosurg (2001) 76:74–82. doi:10.1159/000056496
16. Fukaya C, Katayama Y, Kobayashi K, Kasai M, Oshima H, Yamamoto T. Impairment of motor function after frontal lobe resection with preservation of the primary motor cortex. Acta Neurochir Suppl (2003) 87:71–4. doi:10.1007/978-3-7091-6081-7_15
17. Russell SM, Kelly PJ. Incidence and clinical evolution of postoperative deficits after volumetric stereotactic resection of glial neoplasms involving the supplementary motor area. Neurosurgery (2003) 52:506–16; discussion 515–6. doi:10.1227/01.NEU.0000047670.56996.53
18. Yamane F, Muragaki Y, Maruyama T, Okada Y, Iseki H, Ikeda A, et al. Preoperative mapping for patients with supplementary motor area epilepsy: multimodality brain mapping. Psychiatry Clin Neurosci (2004) 58:S16–21. doi:10.1111/j.1440-1819.2004.01244_5.x
19. Krainik A, Duffau H, Capelle L, Cornu P, Boch AL, Mangin JF, et al. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology (2004) 62:1323–32. doi:10.1212/01.WNL.0000120547.83482.B1
20. Dall’Acqua P, Johannes S, Mica L, Simmen HP, Glaab R, Fandino J, et al. Connectomic and surface-based morphometric correlates of acute mild traumatic brain injury. Front Hum Neurosci (2016) 10:127. doi:10.3389/fnhum.2016.00127
21. Young KD, Bellgowan PS, Bodurka J, Drevets WC. Autobiographical deficits correlate with gray matter volume in depressed and high risk participants. Soc Cogn Affect Neurosci (2015) 10:1588–95. doi:10.1093/scan/nsv047
22. Correa DG, Zimmermann N, Netto TM, Tukamoto G, Ventura N, De Castro Bellini Leite S, et al. Regional cerebral gray matter volume in HIV-positive patients with executive function deficits. J Neuroimaging (2016) 26:450–7. doi:10.1111/jon.12327
23. Drijkoningen D, Chalavi S, Sunaert S, Duysens J, Swinnen SP, Caeyenberghs K. Regional gray matter volume loss is associated with gait impairments in young brain-injured individuals. J Neurotrauma (2016) 34(5):1022–34. doi:10.1089/neu.2016.4500
24. Yang DY, Beam D, Pelphrey KA, Abdullahi S, Jou RJ. Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Mol Autism (2016) 7:11. doi:10.1186/s13229-016-0076-x
25. Eggert LD, Sommer J, Jansen A, Kircher T, Konrad C. Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS One (2012) 7:e45081. doi:10.1371/journal.pone.0045081
26. Clarkson MJ, Cardoso MJ, Ridgway GR, Modat M, Leung KK, Rohrer JD, et al. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage (2011) 57:856–65. doi:10.1016/j.neuroimage.2011.05.053
27. Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging (2004) 17:205–16. doi:10.1007/s10278-004-1014-6
28. Skrap M, Mondani M, Tomasino B, Weis L, Budai R, Pauletto G, et al. Surgery of insular nonenhancing gliomas: volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases. Neurosurgery (2012) 70:1081–93; discussion 1093–84. doi:10.1227/NEU.0b013e31823f5be5
29. Lu J, Wu J, Yao C, Zhuang D, Qiu T, Hu X, et al. Awake language mapping and 3-Tesla intraoperative MRI-guided volumetric resection for gliomas in language areas. J Clin Neurosci (2013) 20:1280–7. doi:10.1016/j.jocn.2012.10.042
30. Patel NV, Jethwa PR, Barrese JC, Hargreaves EL, Danish SF. Volumetric trends associated with MRI-guided laser-induced thermal therapy (LITT) for intracranial tumors. Lasers Surg Med (2013) 45:362–9. doi:10.1002/lsm.22151
32. Boes AD, Caruso P, Duhaime AC, Fischl B. FreeSurfer is useful for early detection of Rasmussen’s encephalitis prior to obvious atrophy. Dev Med Child Neurol (2016) 58:209–10. doi:10.1111/dmcn.12847
33. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron (2002) 33:341–55. doi:10.1016/S0896-6273(02)00569-X
34. Hagler DJ Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage (2006) 33:1093–103. doi:10.1016/j.neuroimage.2006.07.036
35. Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex (2011) 21:2003–13. doi:10.1093/cercor/bhq268
36. Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol (2008) 6:e159. doi:10.1371/journal.pbio.0060159
37. Lim HK, Jung WS, Aizenstein HJ. Aberrant topographical organization in gray matter structural network in late life depression: a graph theoretical analysis. Int Psychogeriatr (2013) 25:1929–40. doi:10.1017/S104161021300149X
38. McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol (1994) 4:550–6. doi:10.1016/0959-4388(94)90056-6
39. Tomasi D, Chang L, Caparelli EC, Ernst T. Sex differences in sensory gating of the thalamus during auditory interference of visual attention tasks. Neuroscience (2008) 151:1006–15. doi:10.1016/j.neuroscience.2007.08.040
40. Sui J, Pearlson GD, Du YH, Yu QB, Jones TR, Chen JY, et al. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol Psychiatry (2015) 78:794–804. doi:10.1016/j.biopsych.2015.02.017
41. Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol (2007) 17:417–22. doi:10.1016/j.conb.2007.07.003
Keywords: structural plasticity, cerebral gliomas, gray matter volume, glioma volumes, surface-based morphometry
Citation: Xu J, Elazab A, Liang J, Jia F, Zheng H, Wang W, Wang L and Hu Q (2017) Cortical and Subcortical Structural Plasticity Associated with the Glioma Volumes in Patients with Cerebral Gliomas Revealed by Surface-Based Morphometry. Front. Neurol. 8:266. doi: 10.3389/fneur.2017.00266
Received: 26 April 2017; Accepted: 26 May 2017;
Published: 09 June 2017
Edited by:
Gordon Li, Stanford University, United StatesReviewed by:
Yuanchao Zhang, University of Science and Technology of China, ChinaCopyright: © 2017 Xu, Elazab, Liang, Jia, Zheng, Wang, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingmao Hu, cW0uaHVAc2lhdC5hYy5jbg==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.