- 1Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
- 2Bellaria Hospital, IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy
Sleep disorders (SDs) are one of the most frequent non-motor symptoms of Parkinson’s disease (PD), usually increasing in frequency over the course of the disease and disability progression. SDs include nocturnal and diurnal manifestations such as insomnia, REM sleep behavior disorder, and excessive daytime sleepiness. The causes of SDs in PD are numerous, including the neurodegeneration process itself, which can disrupt the networks regulating the sleep–wake cycle and deplete a large number of cerebral amines possibly playing a role in the initiation and maintenance of sleep. Despite the significant prevalence of SDs in PD patients, few clinical trials on SDs treatment have been conducted. Our aim is to critically review the principal therapeutic options for the most common SDs in PD. The appropriate diagnosis and treatment of SDs in PD can lead to the consolidation of nocturnal sleep, the enhancement of daytime alertness, and the amelioration of the quality of life of the patients.
Introduction
Non-motor symptoms (NMSs) are present in almost all patients with Parkinson’s disease (PD) frequently preceding the onset of motor symptoms (1). NMSs usually impact on the patients’ quality of life, representing a possible cause of institutionalization (2, 3). Among NMSs, after psychiatric symptoms, sleep disorders (SDs) constitute the second most frequent complaint, affecting 64% of PD patients, ranging from 41.1% in naïve patients to 78.3% in complicated patients (1). SDs in PD are multifactorial and include nocturnal and diurnal manifestations. Reduced sleep efficiency and an increased number of awakenings characterize sleep in PD (4). These disturbances are linked, on one side, to PD motor (akinesia, rigidity, and dystonia) and autonomic symptoms (nocturia) and, on the other side, to the presence of concomitant SDs such as REM sleep behavior disorder (RBD), restless legs syndrome (RLS), or breathing disorders such as obstructive sleep apnea (OSA) (5). Diurnal manifestations include excessive daytime sleepiness (EDS) and sudden sleep attacks, which could be a consequence of nocturnal sleep impairment or dopaminergic treatment or, more interestingly, to the neurodegenerative process of PD itself dysregulating the circadian sleep–wake rhythm (4–8). Apart from instrumental tools, specific and validated scales have been designed to evaluate SDs in PD [Parkinson’s Disease Sleep Scale (PDSS) and its modified version (PDSS-2), the Scales for Outcomes in PD-Sleep (SCOPA-S)] (9, 10). Despite the importance of identifying and treating SDs in PD patients, large-scale, randomized controlled trials for treatment options in this population are lacking. Our narrative review will discuss the available optimal therapeutic strategies, based on the literature data, to use in clinical practice.
Nocturnal SDs
Insomnia
According to the International Classification of SDs, chronic insomnia is characterized by difficulties initiating sleep, maintaining sleep or waking up earlier than desired in the morning (Table 1) (11). Insomnia is the most frequent SD in PD patients (36.9%) with a prevalence ranging from 27 to 80%. The most frequent complaint of PD patients is maintenance insomnia with frequent awakenings and sleep fragmentation (81%), but initial (18%) and terminal insomnia (40%) are also possible (12). Video-polysomnographic studies showed an increased sleep latency and frequent and sometimes prolonged intra-sleep awakenings. The representation of the different sleep stages is physiological (4, 13). In addition to the neurodegeneration process of PD, the most important factors for developing insomnia are female gender, PD duration, and the presence of depression and anxiety. Other factors, such as cough, cold sensations, heat sensations, and pain, which are more common in PD compared to controls, are suggested as contributing factors for sleep fragmentation. Moreover, some drugs such as dopamine agonists and their withdrawal, entacapone, selegiline, and rasagiline (although literature data are conflicting) could increase the risk of insomnia if compared to placebo (14–19).

Table 1. Diagnostic criteria for chronic insomnia (International Classification of Sleep Disorders).
Instead, akinesia, bed-turning difficulties, cramps, nocturia, difficult breathing, and nightmares seem not to be direct causes of insomnia in PD (5, 13, 20).
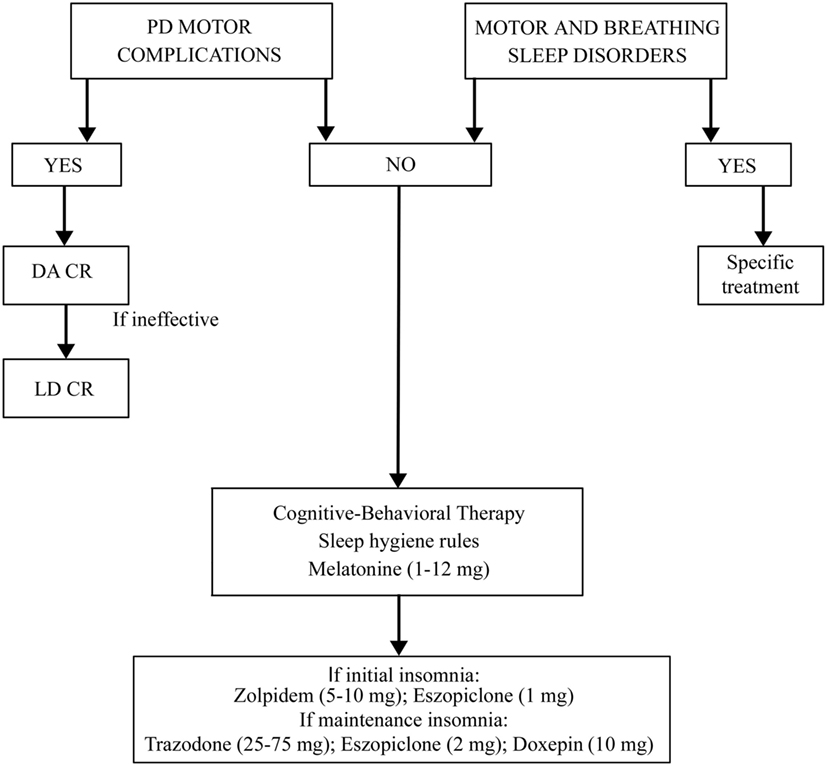
The treatment for insomnia (pharmacological or behavioral) must be preceded by a correct identification of the type of insomnia (initial, of maintenance, or terminal) and of possible disorders and factors causing it (Figure 1). It is necessary to rule out and treat motor or sleep breathing disorders, if present. If insomnia is iatrogenic or due to motor complications of PD, it is useful to modify the therapy. Levodopa–carbidopa controlled-release (LD-CR) improves sleep-associated motor symptoms that may contribute to insomnia, although data documenting an objective improvement in sleep parameters or in sleep satisfaction are insufficient. A double-blind crossover study, comparing the effect of a single dose of 100/25 mg LD-CR with placebo, in 40 fluctuating PD patients, showed an improvement in nocturnal akinesia and only a not-significant trend of increase in total sleep time without any improvement in sleep latency and sleep fragmentation (21). In another randomized study on 32 akinetic rigid PD patients, LD-CR 200 mg before sleeping reduced nocturnal akinesia but did not significantly improve any objective sleep parameter, such as sleep latency, total sleep time, and number of arousals, compared to placebo (22).
The effect of dopamine agonists (DAs) on SDs in PD has been evaluated usually as secondary endpoint in many randomized controlled trials showing an improvement in sleep parameters. Ropinirole 24-h prolonged release (2–24 mg/day) as adjunctive therapy to levodopa, significantly improved PDSS scores in 93/198 patients with motor fluctuations enrolled in a randomized, placebo-controlled study (23). A double-blind, double-dummy, randomized, placebo-controlled trial, comparing the efficacy of pramipexole (up to 4.5 mg/day) and transdermal rotigotine patch (up to 16 mg/day) on advanced-stage PD patients treated with LD showed for both drugs small, but significant, improvement in sleep parameters assessed by PDSS (24). Rotigotine transdermal patch (2–16 mg/day) improved early morning motor function and PDSS-2 scores in a multinational, randomized double-blind placebo-controlled study on 287 PD patients with unsatisfactory early morning motor symptom control and motor symptoms during the night (25). These data have been confirmed by two successive actigraphic and polysomnographic studies specifically designed to objectively evaluate the effect of rotigotine on sleep. These studies demonstrated that the rotigotine patch reduces nocturnal motor activity, duration of wake after sleep onset (WASO), nocturia, pain, and coexisting SDs such as RLS in PD patients. The rotigotine patch also reduces the number and the duration of daytime sleep episodes and improves quality of life (Figure 2) (26, 27). Finally, rasagiline, a monoamine oxidase B inhibitor, can improve insomnia probably increasing endogenous melatonin levels. A single-center, prospective, observational study compared 19 PD patients treated with LD 200–300 mg/day associated with rasagiline 1 mg/day with 19 PD patients treated only with LD 200–300 mg/day. After 12 weeks of treatment, the group treated with LD and rasagiline showed a significant reduction in mean sleep latency and an increase in mean total sleep time (28).
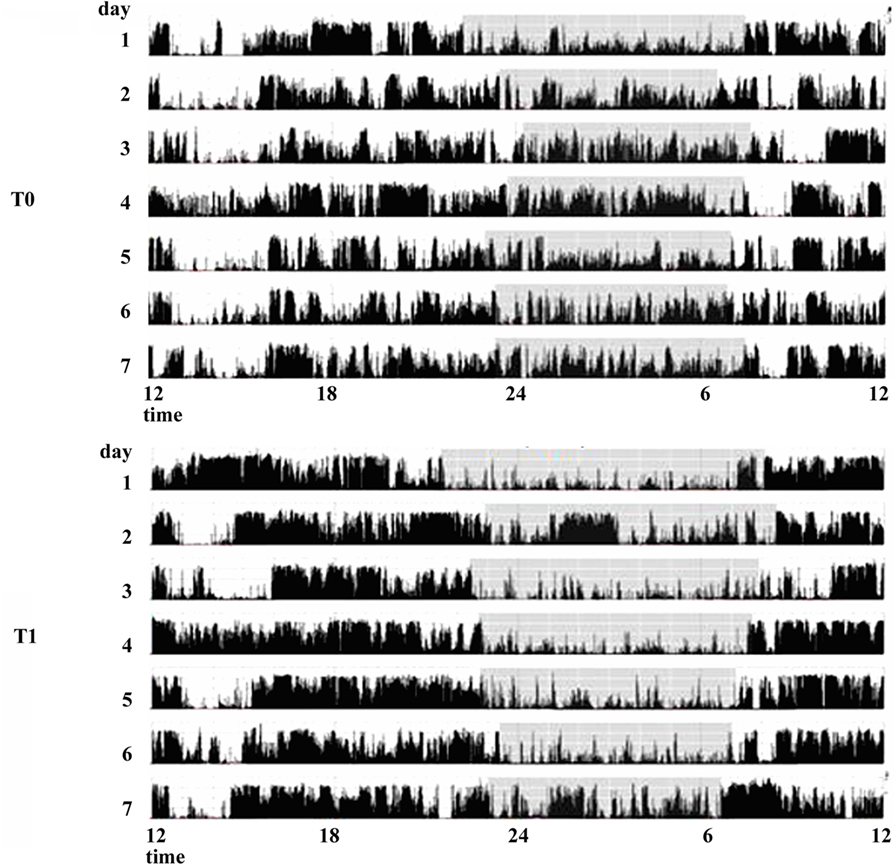
Figure 2. Actigraphic recordings of a patient with Parkinson’s disease with sleep complaints in basal condition (T0) and during therapy with rotigotine 24-h transdermal patch (T1). During rotigotine therapy (T1), the actigraphic recording demonstrates a marked reduction in nighttime motor activity (gray stripe) compared to the basal period. A daytime reduction in diurnal naps is also noticeable (see period between 9 and 11 a.m.).
If insomnia is not iatrogenic and not due to PD motor complications, the principal therapy remains the cognitive behavioral therapy (29, 30). This treatment comprises advice on sleep–wake behavior hygiene, stimulus control, sleep restriction, relaxation, and cognitive techniques (20).
In the cases in which pharmacological therapy is necessary, eszopiclone, doxepin, zolpidem, trazodone, ramelteon, and melatonin could be useful although these drugs are investigational for the treatment of insomnia in PD because there is insufficient evidence regarding their efficacy. To avoid tolerance, hypnotic drugs should ideally be used for no longer than 4–5 weeks (30). Eszopiclone (2–3 mg/day) was tested in 30 PD patients with insomnia in a placebo-controlled trial. This drug does not increase total sleep time but reduces awakenings during the night and improves sleep quality. Thirteen percent of patients reported mild adverse events such as dizziness and sedation (31). In a three-arm 6-week placebo randomized pilot study in 18 PD patients, treated respectively with doxepin (10 mg daily) and cognitive behavioral therapy, doxepin improved the Pittsburgh Sleep Quality Index-Sleep Disturbances Subscale, the SCOPA-night score, the Insomnia Severity Index, the Fatigue Severity Scale, and the Montreal Cognitive Assessment. Adverse events, such as mild fatigue, transient mild nausea and unsteadiness were reported in only three cases (32). The use of zolpidem for insomnia in PD patients is still debated because randomized controlled trials in this population are lacking. Drowsiness and risk of falls are potential relevant side effects in PD (33, 34). Also, trazodone, one of the most used drugs for insomnia in elderly people with a safety profile could present possible side effects such as increased rates of falls, dizziness, impairments in short-term memory, and worsening in verbal memory (35–39). Ramelteon has been studied in PD patients with RBD improving sleep latency (40). Melatonin is established as effective in improving patients’ perception of sleep quality, but data are conflicting regarding objective improvement in sleep polysomnographic parameters (41, 42). A multi-site double-blind placebo-controlled crossover trial in 40 PD patients showed that melatonin 50 mg compared to placebo improved total sleep time, while melatonin 5 mg improved the subjective sleep disturbances perception and reduced daytime sleepiness (43). Another trial in 16 PD patients showed that melatonin 3 mg improved the subjective quality of sleep (44). Globally considering the circadian sleep–wake cycle dysregulation affecting PD patients, melatonin is effective with a good safety profile (8, 45).
Summing up, the treatment of insomnia in PD needs to rule out and eventually to treat other sleep-related motor and breathing disorders. The second step is to treat PD motor complications, if present, using prolonged release dopaminergic drugs or LD. In all the cases, it is mandatory to recommend sleep hygiene rules and cognitive-behavioral therapy, alone or in association with melatonine also at high dosages. If these measures are ineffective, a pharmacological therapy with Zolpidem, Eszopiclone, Trazodone, or Doxepine could be added (Figure 1).
Nocturia
Nocturia affects 35% of PD patients (1). The mechanism underlying nocturia remains unclear, but it has been suggested that it may be related to autonomic modifications and to the loss of the normal D1-mediated inhibition of micturition associated with detrusor hyperreflexia (46–50).
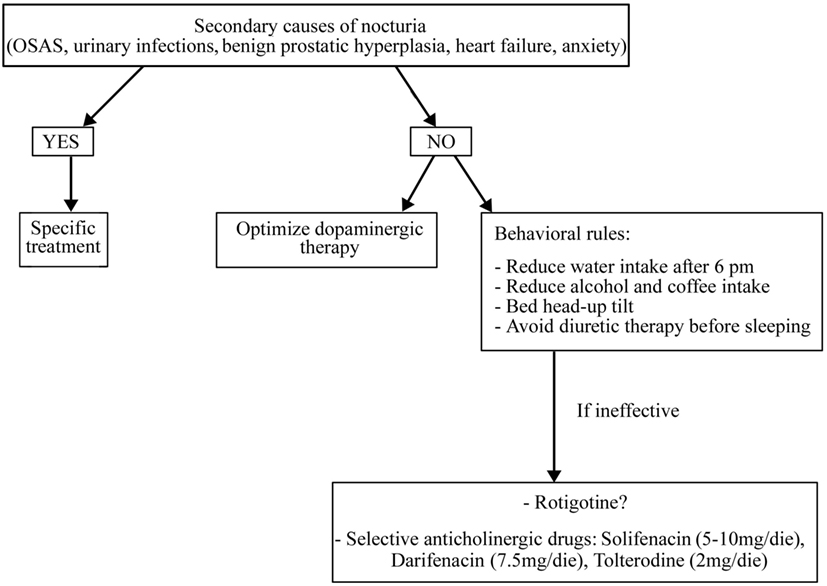
Before starting treatment, it is necessary to rule out and treat all the secondary causes of nocturia, if present (such as OSAS, urinary infections, benign prostatic hypertrophy, heart failure, and anxiety) and optimize dopaminergic therapy (51) (Figure 3).
In addition, it is necessary to apply some behavioral rules such as reducing water intake late in the afternoon and avoid diuretic therapy before sleeping (for more details, see Figure 3). If these measures are ineffective, a pharmacological treatment could be started also if randomized controlled trials on the drug treatment of nocturia in PD patients are lacking. It is uncertain whether LD can improve micturition disorders. In a study on 26 LD-naïve PD patients, an acute challenge of LD worsened the detrusor’s overactivity while, after 2 months of chronic LD therapy, an acute challenge showed contrary results (52). Some reports have shown storage-facilitating effects of dopaminergic drugs, but there are no conclusive data regarding these drugs (50). Subcutaneous apomorphine (3–8 mg) reduced detrusor hyperreflexia in 12 PD patients without dopaminergic therapy (53). Rasagiline 1 mg incremented bladder capacity and significantly decreased residual volume in 20 mild PD patients treated with DAs (54). The efficacy of intranasal desmopressin and botulinum toxin injections into the detrusor muscle are not sufficiently proven as well as the anthicholinergic drugs, which are the most used drugs for overactive bladder in elderly people. These drugs need caution in PD patients for their side effects such as sleepiness, confusion, and cognitive impairment. Among anthicholinergic drugs, M3 receptor selective agents such as solifenacin (5–10 mg/day), darifenacin (7.5 mg/day), and tolterodine (2 mg/day) have a better tolerability than non-selective drugs such as oxybutinin (5 mg/bid) and trospium chloride (10–20 mg/bid) (50).
Sleep-Related Movement Disorders
Restless Legs Syndrome
Restless legs syndrome is a sensorimotor disorder occurring in the evening or at night, characterized by an urge to move the legs usually associated with uncomfortable and unpleasant sensations in the legs relieved by movement (Table 2) (11). RLS affects 15% of patients with PD and occurs either before or after the onset of PD (1). In the de novo untreated populations, the prevalence of RLS seems to be similar to that of controls across populations. On the contrary, RLS prevalence increases during the course of PD and treatment duration, independent of the drug dosages (55). PD patients with RLS have higher age at PD onset, worse sleep quality, and more cardiovascular and anxiety disturbances (56, 57). Also, the presence of a secondary condition, such as iron deficiency, could explain the association between RLS and PD. The pathophysiology of RLS in PD patients is still debated but differs from that of idiopathic RLS (12). PD patients with RLS could have more preserved nigrostriatal dopaminergic pathways than those without RLS suggesting a non-linear relationship between dopaminergic dysfunction and RLS (57–60).

Table 2. Diagnostic criteria for restless legs syndrome (RLS) (International Classification of Sleep Disorders).
The RLS diagnosis is clinically based on the presence of the five international diagnostic criteria (61, 62). The Hening Telephone Diagnostic Interview (HTDI), the Cambridge–Hopkins diagnostic questionnaire for RLS (CH-RLSq), and the RLS diagnostic index (RLS-DI) are useful diagnostic instruments, although not specific for PD patients (61).
If RLS is mild, it can be managed by only lifestyle changes. Therefore, before initiating any pharmacological treatment, it is necessary to evaluate the frequency and duration of symptoms and their impact on the patient’s quality of life. Chronic renal failure, iron, vitamin B12 and folic acid deficiency, serum glucose, and HbA1C need to be investigated in order to exclude secondary forms. The serum ferritin level should be measured, and, if the concentration is <50–75 μg/mL, or if transferrin saturation is less than 20%, supplementation with oral iron is recommended. If oral iron is poorly tolerated or contraindicated, the intravenous administration can also be considered (63). The withdrawal of drugs that potentially exacerbate RLS such as antidopaminergic drugs, antihistamines, and antidepressants (except for bupropion) is also recommended (63).
Treatment of RLS in PD patients has not been evaluated in controlled studies. DAs have proven effective for RLS. The lowest possible cumulative daily dose is recommended to prevent augmentation, which is a side effect characterized by an overall increase in RLS symptoms severity during therapy (63, 64). To prevent such augmentation, long-acting DAs should be preferred to short acting ones. Alternatively, α2δ ligands (pregabalin 150–450 mg/day; gabapentin 900–2.400 mg/day; enacarbil 600–1.800 mg/day) are useful. Dizziness, somnolence, and fatigue are common α2δ ligands side effects. In resistant cases, low doses of opioids such as long-acting oxycodone or methadone should be considered, except for patients with high risk of addiction or with preexisting severe constipation, sleep apnea syndrome, or prolonged QTc (63). Finally, patients may obtain temporally relief by rubbing or massaging the affected limbs, bathing in hot or cold water, physical activity, or distracting themselves with mental exercises (for example, reading an interesting book at the onset of the symptoms) (65).
Periodic Limb Movements (PLMs)
Periodic limb movements are stereotyped and repetitive movements affecting the limbs, in particular, the legs, which can cause non-restorative sleep (11). More than 15 movements per hour of sleep are considered pathological, although the prevalence of PLMs increases with age also in the normal population. In elderly, the prevalence of PLMs is estimated between 25 and 58% (11, 66).
Subjects with PLMs may or may not be aware of the movements that are instead reported by the bed partner. Some authors reported a possible link between the presence of PLMs and frequent awakenings, daytime sleepiness, fatigue or, more rarely, EDS (67–69). PLMs are often associated with RLS, narcolepsy, or sleep apnea (70). Eighty percent or more of all RLS patients have PLMs (71–73).
It is not clear, if prevalence of PLMs in PD is higher than in the general population (74–77). Age, secondary causes such as hyposideremia and the loss of dopaminergic cells may explain the link existing between PLMs and PD (78, 79). Antidepressants, neuroleptics, and lithium could be other potential inducing factors (11).
Before starting a specific therapy for PLMs, it is important to evaluate their clinical impact because PLMs could be only an incidental videopolysomnography (VPSG) finding that does not disturb sleep. There are few studies directly assessing the therapy for PLMs in PD and the principal information regarding PLMs treatment comes from RLS patients (63) In RLS patients, randomized placebo-controlled studies have established the efficacy of DAs for both improvement of RLS symptoms and reduction in PLMs. In a cross-sectional study on 19 untreated PD patients, the introduction of DAs therapy almost totally suppressed PLMs (80).
Parasomnias
NREM Parasomnias
NREM parasomnias are undesired events characterized by an incomplete arousal from NREM sleep and include confusional arousals, sleepwalking, and sleep terrors (Table 3) (11). The prevalence of NREM parasomnias in PD is not clear. A questionnaire study on 661 PD patients showed that sleepwalking had a prevalence of 1.8% while night terrors 3.9% (81). In PD patients with RBD, the prevalence of NREM parasomnias (parasomnia overlap disorder) is higher, reaching respectively, 4.3 and 8.7% (81). Somnambulism in PD is associated with higher incidence of depression and advanced PD stage (82). Probably, the neurodegenerative changes of advanced PD influence not only the control of muscle tone but also the mechanisms of state transition (83).

Table 3. Diagnostic criteria for NREM parasomnias (International Classification of Sleep Disorders).
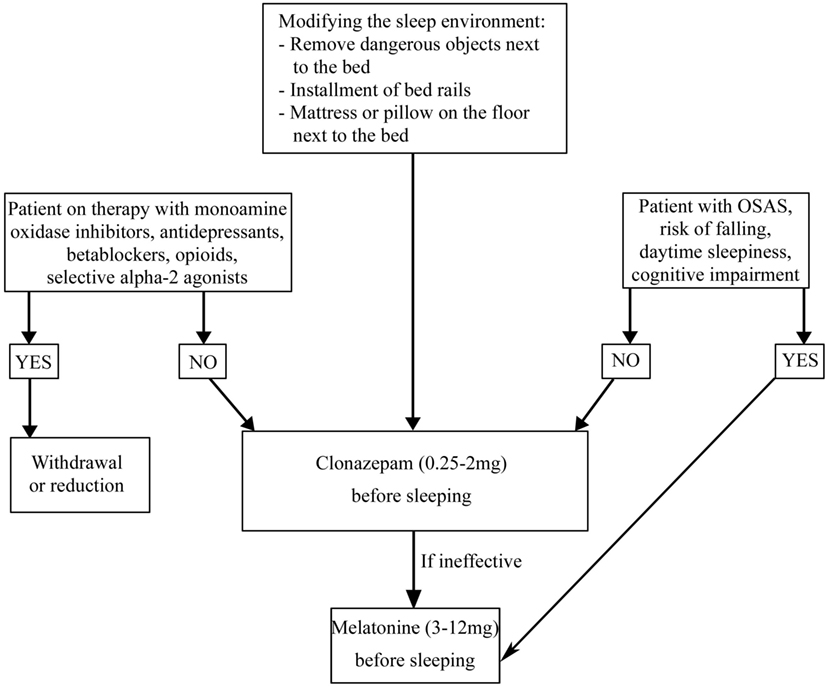
The first approach to a patient with NREM parasomnias, independently of its causes, is to secure the bedroom in which the patient sleeps, closing and locking doors or windows, blocking stairways, and removing all potentially dangerous objects. It is also important to reduce all the possible precipitating and predisposing factors such as alcohol consumption, stress, fever, sleep deprivation, sleeping in unfamiliar or noise-exposed bedrooms. If present, it is necessary to treat OSA and reduce or withdraw psychotropic drugs such as phenothiazines, anticholinergic agents, and sedative/hypnotic agents (11). Clonazepam (CNZ) (0.25–2 mg at bedtime) is the first line pharmacologic treatment for NREM parasomnias in adults, but its efficacy has not been proved in randomized controlled trials (84–87). CNZ was effective in 90% of a series of 20 patients with parasomnia overlap disorder (88), but there are no studies in PD patients. Only four sleepwalkers were identified in a set of 165 PD patients. After a 2-year follow-up, two patients have had a spontaneous remission of sleepwalking, one patient responded to topiramate treatment (100 mg/day) and one patient showed a resolution of the episodes after clozapine treatment (25/mg die) (89).
REM Parasomnias
Nightmares
Nightmares are vivid and unpleasant dreams recurring in REM sleep and causing awakening (Table 4) (11). Their prevalence in PD patients seems to be 17.2%, but these data are only based on questionnaires (81). Dream contents in PD patients seem to be different from controls in particular regarding violence, misfortune, and presence of animals. Some authors explain these contents in relation to the cognitive and frontal impairment of PD patients instead of therapy, mood disorders, hallucinations, and the presence of RBD (90). Traumatic events, stress, use of antidepressants, alpha-agonists, beta-blockers, and cholinergic antagonists are all predisposing and precipitating factors for nightmares (91).
The first-line treatment for nightmares is cognitive behavioral therapy, in particular, the imagery rehearsal therapy (IRT), but these techniques have not been systematically tested in PD patients and need to be confirmed. A placebo-controlled study showed that prazosin (9.5 mg/at bedtime) reduced nightmares in 10 Vietnam veterans. Other studies proved prazosin efficacy, but its real effectiveness needs to be tested in larger placebo-controlled trials (92–95). Side effects such as orthostatic hypotension could discourage its use in PD patients.
REM Sleep Behavior Disorder
REM sleep behavior disorder is a REM parasomnia characterized by complex, sometimes violent, and dangerous motor behaviors during which the patient acts out the content of his/her dream (Table 5) (11). RBD is present in 30% of patients with PD and often precedes the onset of motor symptoms (1). The association between PD and RBD may be explained considering the brainstem abnormalities in regions that control REM sleep such as the peduncle pontine nucleus and the laterodorsal tegmental nuclei, which are affected during Braak’s stages 1 and 2 (96). Independent of pharmacological therapy, in order to guarantee the patient and bed partner’s safety, securing the bedroom is necessary (Figure 4) (97). It is also necessary to withdraw or reduce drugs potentially causing RBD, such as monoamine oxidase inhibitors, antidepressants, beta blockers (bisoprolol), opioids (tramadol), and centrally acting alpha-agonist hypotensive agents (clonidine) (98–108). If RBD causes sleep disruption or if it influences the patient and bed partner’s safety, pharmacological treatment is indicated (97). CNZ, 0.25–2 mg 30 min prior to bedtime has been a first-line therapy for RBD (97). Several large case series proved the efficacy of CNZ in 87–90% RBD patients with or without PD, also if no randomized placebo-controlled studies have been performed (85, 109–111). Sedation and increased risk of fallings are CNZ possible side effects. CNZ is contraindicated in moderate and severe OSAS (85, 110, 112). The mechanism of action of CNZ is unknown. Considering that it does not suppress REM sleep and it does not influence REM muscle tone, probably it could modify dream contents or inhibit brainstem locomotor pattern generators (113, 114). Recent studies proved that CNZ moderately increases total sleep time, sleep efficiency, NREM sleep stages (except stage 1), and decreases wake after sleep onset (WASO) (115). In PD patients in which CNZ is contraindicated (for example, patient with OSAS, cognitive impairment, and a high baseline risk of falling), melatonin (3–12 mg, before sleeping) could be the treatment of choice. CNZ and melatonin appear comparably effective for RBD symptoms and injury prevention. Melatonin’s favorable safety and tolerability profile is very useful for patients receiving polytherapy and for neurologically impaired RBD patients who are more sensitive to adverse drugs effects (116, 117). A recent meta-analysis of randomized clinical trials suggested that exogenous melatonin, improving sleep quality in patients with neurodegenerative disorders can be considered as a possible mono or add-on therapy in patients with RBD (118).

Table 5. Diagnostic criteria for REM sleep behavior disorder (International Classification of Sleep Disorders).
Melatonin mechanisms of action are still not completely known, but its use seems to reduce RBD and decrease muscle tone during REM sleep. Side effects (morning headache, morning sleepiness, and delusions/hallucinations) are usually related to high doses (117, 119, 120). Pramipexole (0.2–1 mg/night), paroxetine (10–40 mg), donepezil (10–15 mg), and rivastigmine (4.5–6 mg) may be effective in some refractory cases, but the evidence of their efficacy is inconclusive due to the lack of randomized controlled trials (97, 121–123). Two studies have been carried out on rivastigmine. Rivastigmine compared to placebo induced a reduction in the frequency of RBD episodes in a pilot placebo-controlled crossover trial on 25 patients with mild cognitive impairment (but without PD). Similar results have been found in a double-blind, crossover trial in 12 patients with PD and RBD resistant to CNZ and melatonin (124, 125). Very limited evidence has been reported for zopiclone, benzodiazepines other than CNZ, yi-gan san, desipramine, clozapine, carbamazepine, and sodium oxybate (97). Ramelteon was effective in RBD in PD patients in two recent open trials, conducted, respectively, in 24 and 12 PD patients, but these promising results could be confirmed in larger population and for longer follow-up. Daytime sleepiness, nausea, delirium, giddiness, and worsening of constipation are possible ramelteon side effects (40, 126).
Sleep-Related Breathing Disorders
Obstructive Sleep Apnea
Obstructive sleep apnea is characterized by snoring and repetitive episodes of complete (apnea) or partial (hypopnea) upper airway obstruction occurring during sleep. The patient typically complains of lack of breath, gasping or choking, insomnia, non-restorative sleep, and EDS (11). The prevalence of OSA in PD is highly controversial, widely ranging from 20 to 60% (5, 7, 127–129). Although middle-elderly age, fluctuating respiratory muscle coordination, autonomic dysfunction, and reduced respiratory drive may justify a higher prevalence of OSA in PD patients, recent studies demonstrated that OSA’s prevalence in PD is the same of the general population. At the same time, sleep fragmentation and intermittent hypoxemia due to OSA may contribute to and worsen PD (4, 130–136).
The gold standard therapy for OSA is the continuous positive airway pressure (CPAP), which normalizes nocturnal respiration improving nighttime oxygenation, sleep architecture (particularly deepening sleep), and daytime sleepiness (136). The control of body weight and sleeping on one’s side during the night could be useful. In this case, to relieve the difficulties of turning around, adapted physiotherapy and increased dopaminergic treatment at night by extra nocturnal doses or sustained release drugs may improve OSA (137). Recently, mandibular advancement devices have been shown to be similarly beneficial to CPAP in improving OSA (138). There are contradictory reports on the modulation of respiratory function by antiparkinsonian treatment and the real effect of DAs remains unknown (139, 140). DAs seem to enhance the risk of central SDs of breathing (141). On the contrary, bedtime long-acting LD seems to improve OSA in PD patients as shown in a polygraphic study in 57 patients, even if the real mechanism of action of LD has not been proven (142).
Diurnal SDs
Excessive Daytime Sleepiness
Excessive daytime sleepiness, first described as “sleep attack” characterized by sudden and irresistible overwhelming sleepiness without awareness of falling asleep (143), is more often reported as sleepiness with prodromes such as tearing or yawning (144, 145) (Table 6). EDS is present in 21% of patients with PD and increases with disease progression (1). Age, sleep–wake cycle alteration related to PD, PLM, daytime immobility, and dopaminergic medications may contribute to EDS (4, 8, 146–148). Moreover, a study on 10,053 drug-naive patients with early PD proved that patients who developed EDS after 5 years of therapy were those with higher sleepiness at the baseline (149). The Multiple Sleep Latency Test (MSLT), the Maintenance of Wakefulness Test (MWT), and the Epworth Sleeping Scale (ESS) may be inadequate to detect sleepiness in PD patients (4, 5, 8, 146, 147, 150). The Inappropriate Sleep Composite Score (ISCS) instead has a higher specificity of predicting PD patients with increased risk of accidents with motor vehicle due to fall asleep while driving (151).

Table 6. Diagnostic criteria for excessive daytime sleepiness (International Classification of Sleep Disorders).
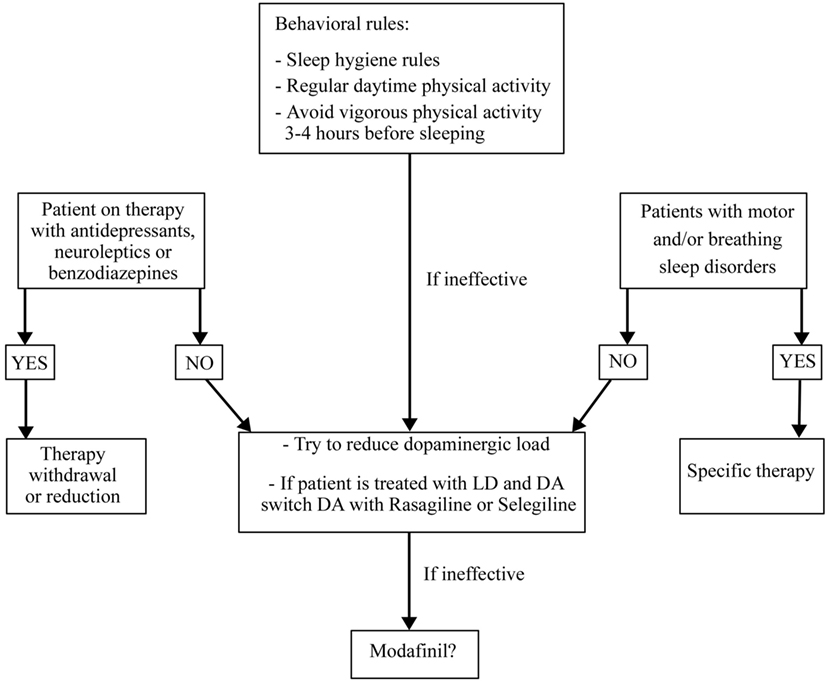
Treatment of EDS in PD is a challenge. First of all, it is necessary to identify and treat any possible SDs that could disrupt nocturnal sleep and to withdraw or reduce any possible drugs causing hypersomnia such as antidepressants, antipsychotics, or sedatives. At the same time, it is important to educate patients to apply the sleep hygiene rules (Figure 5). All DAs cause more EDS than LD without differences between drugs but with a direct relationship with drug dose (152, 153). Combination therapy with LD and DAs shows the highest risk of EDS (154). Instead, selegeline, amantadine, and entacapone had no influence on EDS (155, 156). The association between orally dispersible selegiline and DAs may reduce or resolve EDS (150, 153, 154, 156). If the above strategies do not improve EDS, the use of stimulating drugs such as modafinil (100–400 mg/day) should be a solution. It seems to improve patients’ perception of wakefulness without any objective confirmation (41). Two randomized, double-blind, crossover, placebo-controlled trials on small groups of PD patients with EDS showed that modafinil 100–200 mg/day after 2 weeks of treatment, improved the ESS scores but not sleep latency on the MWT (157). On the other hand, a double-blind, placebo-controlled parallel design trial on 40 PD patients treated with modafinil 200–400 mg/day for 4 weeks showed no improvement of ESS scores (158). A recent review and meta-analysis of pharmacological interventions for EDS in Parkinson’s disease concluded that modafinil improves daytime sleepiness of PD patients also if the significance of this improvement was not robust to all sensitivity analyses (42). Possible adverse events related to modafinil therapy are headache, dry mouth, dizziness, nausea, nervousness, insomnia, and generalized itching (42, 159, 160). These side effects seem to be mild and decrease with dose reduction (161). Nocturnally administered sodium oxybate 3–9 g/night in two split doses (at bedtime and 4 h later) may improve EDS and fatigue in PD, but further and larger studies are needed to prove its efficacy and safety considering also its depressant function on the respiratory centers and its abuse potential. Only one multicenter, open-label, polysomnographic study tested the efficacy of sodium oxybate after 12 weeks of therapy on 30 PD patients demonstrating an improvement of ESS, Pittsburgh Sleep Quality Inventory score, Fatigue Severity Scale score, and an increase in slow-wave sleep (162). Methylphenidate (1 mg/kg three times daily, for 3 months) was effective after a night of LD withdrawal and after an acute administration of LD, in reducing ESS in 17 patients with advanced PD (163). Unlike modafinil and sodium oxybate, which did not influence motor PD symptoms, methylphenidate improved them and, in particular, gait (163). Finally, caffeine (200 mg/bid) seems to improve motor symptoms after 3 weeks of treatment, in the absence of any real influence on EDS in a randomized, double-blind, crossover, placebo-controlled, multicenter trial on 61 patients (164).
Deep Brain Stimulation (DBS)
Deep brain stimulation is an established therapy for PD. The effects of DBS on sleep have been proved in many studies, but it is not clear if DBS modifies sleep directly or indirectly improving PD symptoms. Subthalamic nucleus (STN)-DBS improves PSQI and PDSS scores with contrasting data on objective sleep parameters evaluated by polysomnography in small cohorts of patients (165–174). Fifteen PD patients who underwent STN-DBS showed an improvement in total sleep time and sleep efficiency with a decrease in WASO and arousal index assessed by VPSG. This study found no link between sleep modifications and motor improvement (170). Two other studies, respectively, in 10 and 11 patients, did not find any significant changes in sleep efficiency after STN-DBS, although an increase in uninterrupted sleep and REM sleep and a reduction in arousal index has been found (168, 171). Another study conducted in 10 patients showed also an increase in slow-wave sleep after STN-DBS, without any changes in sleep latency and number of awakenings (175, 176). DBS seems effective in reducing sleep onset insomnia as documented in two studies that evaluated initial insomnia with PDSS in 5 and 10 PD patients (167, 173). One of these studies, in particular, using stimulation of pedunculopontine nucleus demonstrated also an improvement in maintenance insomnia and daytime sleepiness (173). Another study showed an improvement in RLS symptoms in 16 patients but also an improvement in maintenance insomnia and EDS, after STN-DBS (166). On the contrary, other studies did not show any improvement in EDS at the ESS (177–179).
The effects of STN-DBS on RBD have been evaluated in a study on 90 PD patients among which 47 were affected by RBD ascertained by clinical history assessment. After STN-DBS, RBD developed de novo in 16 patients within 1 year and persisted in other patients (180). Other studies did not report any increase nor improvement in RBD symptoms after DBS, but the cohorts examined in these studies were smaller (168, 175, 176). A study on 195 patients who underwent STN-DBS demonstrated the new onset of RLS in 11 patients (181). The same results have been found in 6 out of 31 PD patients after STN-DBS (182). Other studies have instead reported a reduction in RLS symptoms after surgery (166, 183).
Subthalamic nucleus-DBS seems not to influence PLMs (168, 171) nor the occurrence of apnea–hypopnea (168, 175).
Conclusion
Sleep disorders in PD are frequent. Understanding sleep problems in PD and their treatment is challenging, but it will help to improve the management of PD, improving the quality of life in these patients. An accurate clinical and diagnostic assessment is mandatory before starting the treatment. The first objective to achieve is to understand if SDs are a primary or secondary disorder and if behavioral rules or a dose modification of the ongoing therapy could improve SDs. Many different treatments options are now available to treat SDs in PD but further and larger randomized controlled trials are needed to confirm their efficacy and to solve these conflicting data. A better understanding of the relationship between sleep–wake regulation and PD could guide future research and facilitate the management of the disease.
Author Contributions
GL: drafting of manuscript, critical revisions, and final approval. GC-B: critical revisions and final approval. LS: critical revision and final approval. GG: critical revisions and final approval. AC: critical revisions and final approval. PC: critical revisions and final approval. FP: drafting of manuscript, critical revisions, and final approval.
Conflict of Interest Statement
GL received honoraria for participation in clinical trial as sub-investigator from UCB Pharma; PC received honoraria for speaking engagements or consulting activities from Allergan Italia, Lundbeck Italy, UCB Pharma S.p.A, Chiesi Farmaceutici, AbbVie srl, Eli Lilly and Company, Zambon; FP received honoraria for speaking engagements or consulting activities from Sanofi and Bial. The other authors declare no conflict of interest.
Acknowledgments
The authors thank Cecilia Baroncini and Cecilia Sottilotta for the English revision of the text.
References
1. Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord (2009) 24:1641–9. doi:10.1002/mds.22643
2. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc (2000) 48:938–42. doi:10.1111/j.1532-5415.2000.tb06891.x
3. Findley L, Aujla M, Bain PG, Baker M, Beech C, Bowman C, et al. Direct economic impact of Parkinson’s disease: a research survey in the United Kingdom. Mov Disord (2003) 18:1139–45. doi:10.1002/mds.10507
4. Peeraully T, Yong M-H, Chokroverty S, Tan E-K. Sleep and Parkinson’s disease: a review of case-control polysomnography studies: sleep and Parkinson’s disease. Mov Disord (2012) 27:1729–37. doi:10.1002/mds.25197
5. Arnulf I, Konofal E, Merino-Andreu M, Houeto JL, Mesnage V, Welter ML, et al. Parkinson’s disease and sleepiness: an integral part of PD. Neurology (2002) 58:1019–24. doi:10.1212/WNL.58.7.1019
6. Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol (1988) 11:512–9. doi:10.1097/00002826-198812000-00004
7. Zoccolella S, Savarese M, Lamberti P, Manni R, Pacchetti C, Logroscino G. Sleep disorders and the natural history of Parkinson’s disease: the contribution of epidemiological studies. Sleep Med Rev (2011) 15:41–50. doi:10.1016/j.smrv.2010.02.004
8. Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson’s disease. Exp Neurol (2013) 243:45–56. doi:10.1016/j.expneurol.2012.08.018
9. Martinez-Martin P, Visser M, Rodriguez-Blazquez C, Marinus J, Chaudhuri KR, van Hilten JJ, et al. SCOPA-sleep and PDSS: two scales for assessment of sleep disorder in Parkinson’s disease. Mov Disord (2008) 23:1681–8. doi:10.1002/mds.22110
10. Trenkwalder C, Kohnen R, Högl B, Metta V, Sixel-Döring F, Frauscher B, et al. Parkinson’s disease sleep scale-validation of the revised version PDSS-2: Parkinson’s disease sleep scale. Mov Disord (2011) 26:644–52. doi:10.1002/mds.23476
11. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Diagnostic and Coding Manual. III. Darien, IL: American Academy of Sleep Medicine (2014).
12. Ylikoski A, Martikainen K, Partinen M. Parkinson’s disease and restless legs syndrome. Eur Neurol (2015) 73:212–9. doi:10.1159/000375493
13. Ratti PL, Nègre-Pagès L, Pérez-Lloret S, Manni R, Damier P, Tison F, et al. Subjective sleep dysfunction and insomnia symptoms in Parkinson’s disease: insights from a cross-sectional evaluation of the French CoPark cohort. Parkinsonism Relat Disord (2015) 21:1323–9. doi:10.1016/j.parkreldis.2015.09.025
14. Lyons KE, Friedman JH, Hermanowicz N, Isaacson SH, Hauser RA, Hersh BP, et al. Orally disintegrating selegiline in Parkinson patients with dopamine agonist-related adverse effects. Clin Neuropharmacol (2010) 33:5–10. doi:10.1097/WNF.0b013e3181b7926f
15. Parkinson Study Group. Pramipexole in levodopa-treated Parkinson disease patients of African, Asian, and Hispanic heritage. Clin Neuropharmacol (2007) 30:72–85. doi:10.1097/01.wnf.0000240943.59617.4c
16. Pondal M, Marras C, Miyasaki J, Moro E, Armstrong MJ, Strafella AP, et al. Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic. J Neurol Neurosurg Psychiatry (2013) 84:130–5. doi:10.1136/jnnp-2012-302684
17. Solís-García del Pozo J, Mínguez-Mínguez S, de Groot PWJ, Jordán J. Rasagiline meta-analysis: a spotlight on clinical safety and adverse events when treating Parkinson’s disease. Expert Opin Drug Saf (2013) 12:479–86. doi:10.1517/14740338.2013.790956
18. Elmer L, Schwid S, Eberly S, Goetz C, Fahn S, Kieburtz K, et al. Rasagiline-associated motor improvement in PD occurs without worsening of cognitive and behavioral symptoms. J Neurol Sci (2006) 248:78–83. doi:10.1016/j.jns.2006.05.014
19. Stowe R, Ives N, Clarke CE, Handley K, Furmston A, Deane K, et al. Meta-analysis of the comparative efficacy and safety of adjuvant treatment to levodopa in later Parkinson’s disease. Mov Disord (2011) 26:587–98. doi:10.1002/mds.23517
20. Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol (2015) 14:547–58. doi:10.1016/S1474-4422(15)00021-6
21. Stocchi F, Barbato L, Nordera G, Berardelli A, Ruggieri S. Sleep disorders in Parkinson’s disease. J Neurol (1998) 245(Suppl 1):S15–8. doi:10.1007/PL00007731
22. Wailke S, Herzog J, Witt K, Deuschl G, Volkmann J. Effect of controlled-release levodopa on the microstructure of sleep in Parkinson’s disease. Eur J Neurol (2011) 18:590–6. doi:10.1111/j.1468-1331.2010.03213.x
23. Ray Chaudhuri K, Martinez-Martin P, Rolfe KA, Cooper J, Rockett CB, Giorgi L, et al. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson’s disease. Eur J Neurol (2012) 19:105–13. doi:10.1111/j.1468-1331.2011.03442.x
24. Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol (2007) 6:513–20. doi:10.1016/S1474-4422(07)70108-4
25. Trenkwalder C, Kies B, Rudzinska M, Fine J, Nikl J, Honczarenko K, et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord (2011) 26:90–9. doi:10.1002/mds.23441
26. Calandra-Buonaura G, Guaraldi P, Doria A, Zanigni S, Nassetti S, Favoni V, et al. Rotigotine objectively improves sleep in Parkinson’s disease: an open-label pilot study with actigraphic recording. Parkinsons Dis (2016) 2016:3724148. doi:10.1155/2016/3724148
27. Pierantozzi M, Placidi F, Liguori C, Albanese M, Imbriani P, Marciani MG, et al. Rotigotine may improve sleep architecture in Parkinson’s disease: a double-blind, randomized, placebo-controlled polysomnographic study. Sleep Med (2016) 21:140–4. doi:10.1016/j.sleep.2016.01.016
28. Schettino C, Dato C, Capaldo G, Sampaolo S, Di Iorio G, Melone MA. Rasagiline for sleep disorders in patients with Parkinson’s disease: a prospective observational study. Neuropsychiatr Dis Treat (2016) 12:2497–502. doi:10.2147/NDT.S116476
29. Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine Report. Sleep (2006) 29:1415–9.
30. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med (2016) 165:125–33. doi:10.7326/M15-2175
31. Menza M, Dobkin RD, Marin H, Gara M, Bienfait K, Dicke A, et al. Treatment of insomnia in Parkinson’s disease: a controlled trial of eszopiclone and placebo. Mov Disord (2010) 25:1708–14. doi:10.1002/mds.23168
32. Rios Romenets S, Creti L, Fichten C, Bailes S, Libman E, Pelletier A, et al. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson’s disease – a randomized study. Parkinsonism Relat Disord (2013) 19:670–5. doi:10.1016/j.parkreldis.2013.03.003
33. Lavoisy J, Marsac J. Zolpidem in Parkinson’s disease. Lancet (1997) 350:74. doi:10.1016/S0140-6736(05)66285-X
34. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, Barry MJ, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med (2016) 165:125–33. doi:10.7326/M15-2175
35. Werneck AL, Rosso AL, Vincent MB. The use of an antagonist 5-HT2a/c for depression and motor function in Parkinson’ disease. Arq Neuropsiquiatr (2009) 67:407–12. doi:10.1590/S0004-282X2009000300007
36. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ (2011) 343:d4551. doi:10.1136/bmj.d4551
37. Oderda LH, Young JR, Asche CV, Pepper GA. Psychotropic-related hip fractures: meta-analysis of first-generation and second-generation antidepressant and antipsychotic drugs. Ann Pharmacother (2012) 46:917–28. doi:10.1345/aph.1Q589
38. Ip EJ, Bui QV, Barnett MJ, Kazani A, Wright R, Serino MJ, et al. The effect of trazodone on standardized field sobriety tests. Pharmacotherapy (2013) 33:369–74. doi:10.1002/phar.1210
39. Roth AJ, McCall WV, Liguori A. Cognitive, psychomotor and polysomnographic effects of trazodone in primary insomniacs. J Sleep Res (2011) 20:552–8. doi:10.1111/j.1365-2869.2011.00928.x
40. Kashihara K, Nomura T, Maeda T, Tsuboi Y, Mishima T, Takigawa H, et al. Beneficial effects of ramelteon on rapid eye movement sleep behavior disorder associated with Parkinson’s disease – results of a multicenter open trial. Intern Med (2016) 55:231–6. doi:10.2169/internalmedicine.55.5464
41. Zesiewicz TA, Sullivan KL, Arnulf I, Chaudhuri KR, Morgan JC, Gronseth GS, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the quality standards subcommittee of the American Academy of Neurology. Neurology (2010) 74:924–31. doi:10.1212/WNL.0b013e3181d55f24
42. Rodrigues TM, Castro Caldas A, Ferreira JJ. Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: systematic review and meta-analysis. Parkinsonism Relat Disord (2016) 27:25–34. doi:10.1016/j.parkreldis.2016.03.002
43. Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med (2005) 6:459–66. doi:10.1016/j.sleep.2005.04.004
44. Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhães MC, de Lourdes Seabra M, de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J Neurol (2007) 254:459–64. doi:10.1007/s00415-006-0390-x
45. Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol (2014) 71:589–95. doi:10.1001/jamaneurol.2014.65
46. Micieli G, Tosi P, Marcheselli S, Cavallini A. Autonomic dysfunction in Parkinson’s disease. Neurol Sci (2003) 24(Suppl 1):S32–4. doi:10.1007/s100720300035
47. Blackett H, Walker R, Wood B. Urinary dysfunction in Parkinson’s disease: a review. Parkinsonism Relat Disord (2009) 15:81–7. doi:10.1016/j.parkreldis.2007.10.016
48. Aviles-Olmos I, Foltynie T, Panicker J, Cowie D, Limousin P, Hariz M, et al. Urinary incontinence following deep brain stimulation of the pedunculopontine nucleus. Acta Neurochir (Wien) (2011) 153:2357–60. doi:10.1007/s00701-011-1155-6
49. Blanco L, Ros CM, Tarragón E, Fernández-Villalba E, Herrero MT. Functional role of Barrington’s nucleus in the micturition reflex: relevance in the surgical treatment of Parkinson’s disease. Neuroscience (2014) 266:150–61. doi:10.1016/j.neuroscience.2014.02.002
50. Sakakibara R, Panicker J, Finazzi-Agro E, Iacovelli V, Bruschini H. A guideline for the management of bladder dysfunction in Parkinson’s disease and other gait disorders. Neurourol Urodyn (2016) 35:551–63. doi:10.1002/nau.22764
51. Dani H, Esdaille A, Weiss JP. Nocturia: aetiology and treatment in adults. Nat Rev Urol (2016) 13:573–83. doi:10.1038/nrurol.2016.134
52. Brusa L, Petta F, Pisani A, Moschella V, Iani C, Stanzione P, et al. Acute vs chronic effects of l-DOPA on bladder function in patients with mild Parkinson disease. Neurology (2007) 68:1455–9. doi:10.1212/01.wnl.0000260605.12506.86
53. Aranda B, Cramer P. Effects of apomorphine and l-DOPA on the parkinsonian bladder. Neurourol Urodyn (1993) 12:203–9. doi:10.1002/nau.1930120302
54. Brusa L, Musco S, Bernardi G, Iani C, Pierantozzi M, Stanzione P, et al. Rasagiline effect on bladder disturbances in early mild Parkinson’s disease patients. Parkinsonism Relat Disord (2014) 20:931–2. doi:10.1016/j.parkreldis.2014.04.020
55. Trenkwalder C, Allen R, Högl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology (2016) 86(14):1336–43. doi:10.1212/WNL.0000000000002542
56. Fereshtehnejad SM, Shafieesabet M, Shahidi GA, Delbari A, Lökk J. Restless legs syndrome in patients with Parkinson’s disease: a comparative study on prevalence, clinical characteristics, quality of life and nutritional status. Acta Neurol Scand (2015) 131:211–8. doi:10.1111/ane.12307
57. Moccia M, Erro R, Picillo M, Santangelo G, Spina E, Allocca R, et al. A four-year longitudinal study on restless legs syndrome in Parkinson disease. Sleep (2016) 39:405–12. doi:10.5665/sleep.5452
58. Slow EJ, Postuma RB, Lang AE. Implications of nocturnal symptoms towards the early diagnosis of Parkinson’s disease. J Neural Transm (2014) 121(Suppl 1):S49–57. doi:10.1007/s00702-014-1168-4
59. Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain (2009) 132:2403–12. doi:10.1093/brain/awp125
60. Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord (2010) 16:79–84. doi:10.1016/j.parkreldis.2009.08.007
61. Walters AS, Frauscher B, Allen R, Benes H, Chaudhuri KR, Garcia-Borreguero D, et al. Review of diagnostic instruments for the restless legs syndrome/Willis-Ekbom disease (RLS/WED): critique and recommendations. J Clin Sleep Med (2014) 10:1343–9. doi:10.5664/jcsm.4298
62. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – history, rationale, description, and significance. Sleep Med (2014) 15:860–73. doi:10.1016/j.sleep.2014.03.025
63. Garcia-Borreguero D, Silber MH, Winkelman JW, Högl B, Bainbridge J, Buchfuhrer M, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med (2016) 21:1–11. doi:10.1016/j.sleep.2016.01.017
64. Allen RP, Ondo WG, Ball E, Calloway MO, Manjunath R, Higbie RL, et al. Restless legs syndrome (RLS) augmentation associated with dopamine agonist and levodopa usage in a community sample. Sleep Med (2011) 12:431–9. doi:10.1016/j.sleep.2011.03.003
65. Klingelhoefer L, Cova I, Gupta S, Chaudhuri KR. A review of current treatment strategies for restless legs syndrome (Willis-Ekbom disease). Clin Med (Lond) (2014) 14:520–4. doi:10.7861/clinmedicine.14-5-520
66. Claman DM, Ewing SK, Redline S, Ancoli-Israel S, Cauley JA, Stone KL, et al. Periodic leg movements are associated with reduced sleep quality in older men: the MrOS sleep study. J Clin Sleep Med (2013) 9:1109–17. doi:10.5664/jcsm.3146
67. Varadharajulu S, Chandrasekaran B. Periodic limb movements and insomnia, a common but under-recognized association. Indian J Psychiatry (2015) 57:205–7. doi:10.4103/0019-5545.158196
68. Mendelson WB. Are periodic leg movements associated with clinical sleep disturbance? Sleep (1996) 19:219–23.
69. Karadeniz D, Ondze B, Besset A, Billiard M. Are periodic leg movements during sleep (PLMS) responsible for sleep disruption in insomnia patients? Eur J Neurol (2000) 7:331–6. doi:10.1046/j.1468-1331.2000.00070.x
70. Dauvilliers Y, Pennestri M-H, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res (2007) 16:333–9. doi:10.1111/j.1365-2869.2007.00601.x
71. Walters AS, Picchietti D, Hening W, Lazzarini A. Variable expressivity in familial restless legs syndrome. Arch Neurol (1990) 47:1219–20. doi:10.1001/archneur.1990.00530110079020
72. Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev (2006) 10:169–77. doi:10.1016/j.smrv.2005.12.003
73. Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep (2009) 32:589–97.
74. Shpirer I, Miniovitz A, Klein C, Goldstein R, Prokhorov T, Theitler J, et al. Excessive daytime sleepiness in patients with Parkinson’s disease: a polysomnography study. Mov Disord (2006) 21:1432–8. doi:10.1002/mds.21002
75. Yong M-H, Fook-Chong S, Pavanni R, Lim L-L, Tan E-K. Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS One (2011) 6:e22511. doi:10.1371/journal.pone.0022511
76. Wetter TC, Brunner H, Högl B, Yassouridis A, Trenkwalder C, Friess E. Increased alpha activity in REM sleep in de novo patients with Parkinson’s disease. Mov Disord (2001) 16:928–33. doi:10.1002/mds.1163
77. Garcia-Borreguero D, Caminero AB, De La Llave Y, Larrosa O, Barrio S, Granizo JJ, et al. Decreased phasic EMG activity during rapid eye movement sleep in treatment-naïve Parkinson’s disease: effects of treatment with levodopa and progression of illness. Mov Disord (2002) 17:934–41. doi:10.1002/mds.10233
78. Wetter TC, Collado-Seidel V, Pollmächer T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep (2000) 23:361–7.
79. Happe S, Pirker W, Klösch G, Sauter C, Zeitlhofer J. Periodic leg movements in patients with Parkinson’s disease are associated with reduced striatal dopamine transporter binding. J Neurol (2003) 250:83–6. doi:10.1007/s00415-003-0957-8
80. Puligheddu M, Figorilli M, Aricò D, Raggi A, Marrosu F, Ferri R. Time structure of leg movement activity during sleep in untreated Parkinson disease and effects of dopaminergic treatment. Sleep Med (2014) 15:816–24. doi:10.1016/j.sleep.2014.03.011
81. Ylikoski A, Martikainen K, Partinen M. Parasomnias and isolated sleep symptoms in Parkinson’s disease: a questionnaire study on 661 patients. J Neurol Sci (2014) 346:204–8. doi:10.1016/j.jns.2014.08.025
82. Di Fabio N, Poryazova R, Oberholzer M, Baumann CR, Bassetti CL. Sleepwalking, REM sleep behaviour disorder and overlap parasomnia in patients with Parkinson’s disease. Eur Neurol (2013) 70:297–303. doi:10.1159/000353378
83. Poryazova R, Waldvogel D, Bassetti CL. Sleepwalking in patients with Parkinson disease. Arch Neurol (2007) 64:1524–7. doi:10.1001/archneur.64.10.1524
84. Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Am J Psychiatry (1989) 146:1166–73. doi:10.1176/ajp.146.9.1166
85. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med (1996) 100:333–7. doi:10.1016/S0002-9343(97)89493-4
86. Guilleminault C, Kirisoglu C, Bao G, Arias V, Chan A, Li KK. Adult chronic sleepwalking and its treatment based on polysomnography. Brain (2005) 128:1062–9. doi:10.1093/brain/awh481
87. Attarian H, Zhu L. Treatment options for disorders of arousal: a case series. Int J Neurosci (2013) 123:623–5. doi:10.3109/00207454.2013.783579
88. Schenck CH, Boyd JL, Mahowald MW. A parasomnia overlap disorder involving sleepwalking, sleep terrors, and REM sleep behavior disorder in 33 polysomnographically confirmed cases. Sleep (1997) 20:972–81.
89. Dumitrascu O, Schenck CH, Applebee G, Attarian H. Parasomnia overlap disorder: a distinct pathophysiologic entity or a variant of rapid eye movement sleep behavior disorder? A case series. Sleep Med (2013) 14:1217–20. doi:10.1016/j.sleep.2013.06.012
90. Bugalho P, Paiva T. Dream features in the early stages of Parkinson’s disease. J Neural Transm (2011) 118:1613–9. doi:10.1007/s00702-011-0679-5
91. Spoormaker VI, Schredl M, Van den Bout J. Nightmares: from anxiety symptom to sleep disorder. Sleep Med Rev (2006) 10:19–31. doi:10.1016/j.smrv.2005.06.001
92. Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol (2002) 22:82–5. doi:10.1097/00004714-200202000-00013
93. Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry (2000) 61:129–33. doi:10.4088/JCP.v61n0208
94. Raskind MA, Thompson C, Petrie EC, Dobie DJ, Rein RJ, Hoff DJ, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry (2002) 63:565–8. doi:10.4088/JCP.v63n0705
95. Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry (2003) 160:371–3. doi:10.1176/appi.ajp.160.2.371
96. Lima MMS. Sleep disturbances in Parkinson’s disease: the contribution of dopamine in REM sleep regulation. Sleep Med Rev (2013) 17:367–75. doi:10.1016/j.smrv.2012.10.006
97. Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med (2010) 6:85–95.
98. Akindele MO, Evans JI, Oswald I. Mono-amine oxidase inhibitors, sleep and mood. Electroencephalogr Clin Neurophysiol (1970) 29:47–56. doi:10.1016/0013-4694(70)90078-7
99. Passouant P, Cadilhac J, Ribstein M. [Sleep privation with eye movements using antidepressive agents]. Rev Neurol (Paris) (1972) 127:173–92.
100. Guilleminault C, Raynal D, Takahashi S, Carskadon M, Dement W. Evaluation of short-term and long-term treatment of the narcolepsy syndrome with clomipramine hydrochloride. Acta Neurol Scand (1976) 54:71–87. doi:10.1111/j.1600-0404.1976.tb07621.x
102. Bental E, Lavie P, Sharf B. Severe hypermotility during sleep in treatment of cataplexy with clomipramine. Isr J Med Sci (1979) 15:607–9.
103. Schenck CH, Mahowald MW, Kim SW, O’Connor KA, Hurwitz TD. Prominent eye movements during NREM sleep and REM sleep behavior disorder associated with fluoxetine treatment of depression and obsessive-compulsive disorder. Sleep (1992) 15:226–35.
104. Niiyama Y, Shimizu T, Abe M, Hishikawa Y. Cortical reactivity in REM sleep with tonic mentalis EMG activity induced by clomipramine: an evaluation by slow vertex response. Electroencephalogr Clin Neurophysiol (1993) 86:247–51. doi:10.1016/0013-4694(93)90105-5
105. Louden MB, Morehead MA, Schmidt HS. Activation by selegiline (Eldepryle) of REM sleep behavior disorder in parkinsonism. W V Med J (1995) 91:101.
106. Iranzo A, Santamaria J. Bisoprolol-induced rapid eye movement sleep behavior disorder. Am J Med (1999) 107:390–2. doi:10.1016/S0002-9343(99)00245-4
107. Onofrj M, Luciano AL, Thomas A, Iacono D, D’Andreamatteo G. Mirtazapine induces REM sleep behavior disorder (RBD) in parkinsonism. Neurology (2003) 60:113–5. doi:10.1212/01.WNL.0000042084.03066.C0
108. Winkelman JW, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep (2004) 27:317–21.
109. Schenck CH, Hurwitz TD, Mahowald MW. Symposium: normal and abnormal REM sleep regulation: REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res (1993) 2:224–31. doi:10.1111/j.1365-2869.1993.tb00093.x
110. Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain (2000) 123(Pt 2):331–9. doi:10.1093/brain/123.2.331
111. Wing YK, Lam SP, Li SX, Yu MW, Fong SY, Tsoh JM, et al. REM sleep behaviour disorder in Hong Kong Chinese: clinical outcome and gender comparison. J Neurol Neurosurg Psychiatry (2008) 79:1415–6. doi:10.1136/jnnp.2008.155374
112. Ferreira JJ, Katzenschlager R, Bloem BR, Bonuccelli U, Burn D, Deuschl G, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol (2013) 20:5–15. doi:10.1111/j.1468-1331.2012.03866.x
113. Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology (1992) 42:1371–4. doi:10.1212/WNL.42.7.1371
114. Li SX, Lam SP, Zhang J, Yu MW, Chan JW, Liu Y, et al. A prospective, naturalistic follow-up study of treatment outcomes with clonazepam in rapid eye movement sleep behavior disorder. Sleep Med (2016) 21:114–20. doi:10.1016/j.sleep.2015.12.020
115. Ferri R, Zucconi M, Marelli S, Plazzi G, Schenck CH, Ferini-Strambi L. Effects of long-term use of clonazepam on nonrapid eye movement sleep patterns in rapid eye movement sleep behavior disorder. Sleep Med (2013) 14:399–406. doi:10.1016/j.sleep.2013.01.007
116. McCarter SJ, Boswell CL, St Louis EK, Dueffert LG, Slocumb N, Boeve BF, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med (2013) 14:237–42. doi:10.1016/j.sleep.2012.09.018
117. McGrane IR, Leung JG, St. Louis EK, Boeve BF. Melatonin therapy for REM sleep behavior disorder: a critical review of evidence. Sleep Med (2015) 16:19–26. doi:10.1016/j.sleep.2014.09.011
118. Zhang W, Chen X, Su S, Jia Q, Ding T, Zhu Z, et al. Exogenous melatonin for sleep disorders in neurodegenerative diseases: a meta-analysis of randomized clinical trials. Neurol Sci (2016) 37:57–65. doi:10.1007/s10072-015-2357-0
119. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med (2003) 4:281–4. doi:10.1016/S1389-9457(03)00072-8
120. Lin L, Huang Q-X, Yang S-S, Chu J, Wang J-Z, Tian Q. Melatonin in Alzheimer’s disease. Int J Mol Sci (2013) 14:14575–93. doi:10.3390/ijms140714575
121. Sasai T, Inoue Y, Matsuura M. Effectiveness of pramipexole, a dopamine agonist, on rapid eye movement sleep behavior disorder. Tohoku J Exp Med (2012) 226:177–81. doi:10.1620/tjem.226.177
122. Sasai T, Matsuura M, Inoue Y. Factors associated with the effect of pramipexole on symptoms of idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord (2013) 19:153–7. doi:10.1016/j.parkreldis.2012.08.010
123. Tan SM, Wan YM. Pramipexole in the treatment of REM sleep behaviour disorder: a critical review. Psychiatry Res (2016) 243:365–72. doi:10.1016/j.psychres.2016.06.055
124. Di Giacopo R, Fasano A, Quaranta D, Della Marca G, Bove F, Bentivoglio AR. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Mov Disord (2012) 27:559–61. doi:10.1002/mds.24909
125. Brunetti V, Losurdo A, Testani E, Lapenta L, Mariotti P, Marra C, et al. Rivastigmine for refractory REM behavior disorder in mild cognitive impairment. Curr Alzheimer Res (2014) 11:267–73. doi:10.2174/1567205011666140302195648
126. Esaki Y, Kitajima T, Koike S, Fujishiro H, Iwata Y, Tsuchiya A, et al. An open-labeled trial of ramelteon in idiopathic rapid eye movement sleep behavior disorder. J Clin Sleep Med (2016) 12:689–93. doi:10.5664/jcsm.5796
127. Maria B, Sophia S, Michalis M, Charalampos L, Andreas P, John ME, et al. Sleep breathing disorders in patients with idiopathic Parkinson’s disease. Respir Med (2003) 97:1151–7. doi:10.1016/S0954-6111(03)00188-4
128. Norlinah MI, Afidah KN, Noradina AT, Shamsul AS, Hamidon BB, Sahathevan R, et al. Sleep disturbances in Malaysian patients with Parkinson’s disease using polysomnography and PDSS. Parkinsonism Relat Disord (2009) 15:670–4. doi:10.1016/j.parkreldis.2009.02.012
129. Cochen de Cock V, Abouda M, Leu S, Oudiette D, Roze E, Vidailhet M, et al. Is obstructive sleep apnea a problem in Parkinson’s disease? Sleep Med (2010) 11:247–52. doi:10.1016/j.sleep.2009.05.008
130. Coccagna G, Martinelli P, Zucconi M, Cirignotta F, Ambrosetto G. Sleep-related respiratory and haemodynamic changes in Shy-Drager syndrome: a case report. J Neurol (1985) 232:310–3. doi:10.1007/BF00313872
131. Vincken W, Cosio MG. “Saw-tooth” pattern in the flow-volume loop. Chest (1985) 88:480–1. doi:10.1378/chest.88.3.480-a
132. Chester CS, Gottfried SB, Cameron DI, Strohl KP. Pathophysiological findings in a patient with Shy-Drager and alveolar hypoventilation syndromes. Chest (1988) 94:212–4. doi:10.1378/chest.94.1.212
133. Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med (2002) 162:893–900. doi:10.1001/archinte.162.8.893
134. Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging (2003) 24:197–211. doi:10.1016/S0197-4580(02)00065-9
135. Seccombe LM, Giddings HL, Rogers PG, Corbett AJ, Hayes MW, Peters MJ, et al. Abnormal ventilatory control in Parkinson’s disease – further evidence for non-motor dysfunction. Respir Physiol Neurobiol (2011) 179:300–4. doi:10.1016/j.resp.2011.09.012
136. Neikrug AB, Liu L, Avanzino JA, Maglione JE, Natarajan L, Bradley L, et al. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep (2014) 37:177–85. doi:10.5665/sleep.3332
137. Cochen de Cock V, Benard-Serre N, Driss V, Granier M, Charif M, Carlander B, et al. Supine sleep and obstructive sleep apnea syndrome in Parkinson’s disease. Sleep Med (2015) 16:1497–501. doi:10.1016/j.sleep.2014.09.014
138. Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med (2013) 187:879–87. doi:10.1164/rccm.201212-2223OC
139. Herer B, Arnulf I, Housset B. Effects of levodopa on pulmonary function in Parkinson’s disease. Chest (2001) 119:387–93. doi:10.1378/chest.119.2.387
140. De Keyser J, Vincken W. l-DOPA-induced respiratory disturbance in Parkinson’s disease suppressed by tiapride. Neurology (1985) 35:235–7. doi:10.1212/WNL.35.2.235
141. Valko PO, Hauser S, Sommerauer M, Werth E, Baumann CR. Observations on sleep-disordered breathing in idiopathic Parkinson’s disease. PLoS One (2014) 9:e100828. doi:10.1371/journal.pone.0100828
142. Gros P, Mery VP, Lafontaine A-L, Robinson A, Benedetti A, Kimoff RJ, et al. Obstructive sleep apnea in Parkinson’s disease patients: effect of Sinemet CR taken at bedtime. Sleep Breath (2016) 20:205–12. doi:10.1007/s11325-015-1208-9
143. Frucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology (1999) 52:1908–10. doi:10.1212/WNL.52.9.1908
144. Reyner LA, Horne JA. Falling asleep whilst driving: are drivers aware of prior sleepiness? Int J Legal Med (1998) 111:120–3. doi:10.1007/s004140050131
145. Hauser RA, Gauger L, Anderson WM, Zesiewicz TA. Pramipexole-induced somnolence and episodes of daytime sleep. Mov Disord (2000) 15:658–63. doi:10.1002/1531-8257(200007)15:4<658::AID-MDS1009>3.0.CO;2-N
146. Fabbrini G, Barbanti P, Aurilia C, Vanacore N, Pauletti C, Meco G. Excessive daytime sleepiness in de novo and treated Parkinson’s disease. Mov Disord (2002) 17:1026–30. doi:10.1002/mds.10193
147. Gjerstad MD, Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology (2006) 67:853–8. doi:10.1212/01.wnl.0000233980.25978.9d
148. Rye DB. Excessive daytime sleepiness and unintended sleep in Parkinson’s disease. Curr Neurol Neurosci Rep (2006) 6:169–76. doi:10.1007/s11910-996-0041-8
149. Tholfsen LK, Larsen JP, Schulz J, Tysnes O-B, Gjerstad MD. Development of excessive daytime sleepiness in early Parkinson disease. Neurology (2015) 85:162–8. doi:10.1212/WNL.0000000000001737
150. Stevens S, Cormella CL, Stepanski EJ. Daytime sleepiness and alertness in patients with Parkinson disease. Sleep (2004) 27:967–72.
151. Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA (2002) 287:455–63. doi:10.1001/jama.287.4.455
152. Zesiewicz TA, Hauser RA. Sleep attacks and dopamine agonists for Parkinson’s disease: what is currently known? CNS Drugs (2003) 17:593–600. doi:10.2165/00023210-200317080-00004
153. Razmy A, Lang AE, Shapiro CM. Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol (2004) 61:97–102. doi:10.1001/archneur.61.1.97
154. Paus S, Brecht HM, Köster J, Seeger G, Klockgether T, Wüllner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Mov Disord (2003) 18:659–67. doi:10.1002/mds.10417
155. Valko PO, Waldvogel D, Weller M, Bassetti CL, Held U, Baumann CR. Fatigue and excessive daytime sleepiness in idiopathic Parkinson’s disease differently correlate with motor symptoms, depression and dopaminergic treatment. Eur J Neurol (2010) 17:1428–36. doi:10.1111/j.1468-1331.2010.03063.x
156. Diederich NJ, McIntyre DJ. Sleep disorders in Parkinson’s disease: many causes, few therapeutic options. J Neurol Sci (2012) 314:12–9. doi:10.1016/j.jns.2011.10.025
157. Högl B, Saletu M, Brandauer E, Glatzl S, Frauscher B, Seppi K, et al. Modafinil for the treatment of daytime sleepiness in Parkinson’s disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep (2002) 25:905–9.
158. Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson’s disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry (2005) 76:1636–9. doi:10.1136/jnnp.2005.065870
159. Kumar R. Approved and investigational uses of modafinil: an evidence-based review. Drugs (2008) 68:1803–39. doi:10.2165/00003495-200868130-00003
160. Generali JA, Cada DJ. Modafinil: Parkinson disease-related somnolence. Hosp Pharm (2014) 49:612–4. doi:10.1310/hpj4907-612
161. Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, et al. The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord (2011) 26(Suppl 3):S42–80. doi:10.1002/mds.23884
162. Ondo WG, Perkins T, Swick T, Hull KL, Jimenez JE, Garris TS, et al. Sodium oxybate for excessive daytime sleepiness in Parkinson disease: an open-label polysomnographic study. Arch Neurol (2008) 65:1337–40. doi:10.1001/archneur.65.10.1337
163. Devos D, Krystkowiak P, Clement F, Dujardin K, Cottencin O, Waucquier N, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry (2007) 78:470–5. doi:10.1136/jnnp.2006.100016
164. Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology (2012) 79:651–8. doi:10.1212/WNL.0b013e318263570d
165. Breen DP, Low HL, Misbahuddin A. The impact of deep brain stimulation on sleep and olfactory function in Parkinson’s disease. Open Neurol J (2015) 9:70–2. doi:10.2174/1874205X01509010070
166. Chahine LMM, Ahmed A, Sun Z. Effects of STN DBS for Parkinson’s disease on restless legs syndrome and other sleep-related measures. Parkinsonism Relat Disord (2011) 17:208–11. doi:10.1016/j.parkreldis.2010.11.017
167. Hjort N, Østergaard K, Dupont E. Improvement of sleep quality in patients with advanced Parkinson’s disease treated with deep brain stimulation of the subthalamic nucleus. Mov Disord (2004) 19:196–9. doi:10.1002/mds.10639
168. Iranzo A, Valldeoriola F, Santamaría J, Tolosa E, Rumià J. Sleep symptoms and polysomnographic architecture in advanced Parkinson’s disease after chronic bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry (2002) 72:661–4. doi:10.1136/jnnp.72.5.661
169. Jiang L-L, Liu J-L, Fu X-L, Xian W-B, Gu J, Liu Y-M, et al. Long-term efficacy of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a 5-year follow-up study in China. Chin Med J (Engl) (2015) 128:2433–8. doi:10.4103/0366-6999.164925
170. Merlino G, Lettieri C, Mondani M, Belgrado E, Devigili G, Mucchiut M, et al. Microsubthalamotomy improves sleep in patients affected by advanced Parkinson’s disease. Sleep Med (2014) 15:637–41. doi:10.1016/j.sleep.2013.12.016
171. Nishida N, Murakami T, Kadoh K, Tohge R, Yamanegi M, Saiki H, et al. Subthalamic nucleus deep brain stimulation restores normal rapid eye movement sleep in Parkinson’s disease. Mov Disord (2011) 26:2418–22. doi:10.1002/mds.23862
172. Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol (2013) 12:37–44. doi:10.1016/S1474-4422(12)70264-8
173. Peppe A, Pierantozzi M, Baiamonte V, Moschella V, Caltagirone C, Stanzione P, et al. Deep brain stimulation of pedunculopontine tegmental nucleus: role in sleep modulation in advanced Parkinson disease patients: one-year follow-up. Sleep (2012) 35:1637–42. doi:10.5665/sleep.2234
174. Liu KD, Shan DE, Kuo TB, Yang CC. The effects of bilateral stimulation of the subthalamic nucleus on heart rate variability in patients with Parkinson’s disease. J Neurol (2013) 260:1714–23. doi:10.1007/s00415-013-6849-7
175. Cicolin A, Lopiano L, Zibetti M, Torre E, Tavella A, Guastamacchia G, et al. Effects of deep brain stimulation of the subthalamic nucleus on sleep architecture in parkinsonian patients. Sleep Med (2004) 5:207–10. doi:10.1016/j.sleep.2003.10.010
176. Monaca C, Ozsancak C, Jacquesson JM, Poirot I, Blond S, Destee A, et al. Effects of bilateral subthalamic stimulation on sleep in Parkinson’s disease. J Neurol (2004) 251:214–8. doi:10.1007/s00415-004-0305-7
177. Lyons KE, Pahwa R. Effects of bilateral subthalamic nucleus stimulation on sleep, daytime sleepiness, and early morning dystonia in patients with Parkinson disease. J Neurosurg (2006) 104:502–5. doi:10.3171/jns.2006.104.4.502
178. Chou KL, Persad CC, Patil PG. Change in fatigue after bilateral subthalamic nucleus deep brain stimulation for Parkinson’s disease. Parkinsonism Relat Disord (2012) 18:510–3. doi:10.1016/j.parkreldis.2012.01.018
179. Lilleeng B, Gjerstad M, Baardsen R, Dalen I, Larsen JP. The long-term development of non-motor problems after STN-DBS. Acta Neurol Scand (2015) 132:251–8. doi:10.1111/ane.12391
180. Kim YE, Jeon BS, Paek SH, Yun JY, Yang HJ, Kim HJ, et al. Rapid eye movement sleep behavior disorder after bilateral subthalamic stimulation in Parkinson’s disease. J Clin Neurosci (2015) 22:315–9. doi:10.1016/j.jocn.2014.07.016
181. Kedia S, Moro E, Tagliati M, Lang AE, Kumar R. Emergence of restless legs syndrome during subthalamic stimulation for Parkinson disease. Neurology (2004) 63:2410–2. doi:10.1212/01.WNL.0000147288.26029.B8
182. Marques A, Fantini ML, Morand D, Pereira B, Derost P, Ulla M, et al. Emergence of restless legs syndrome after subthalamic stimulation in Parkinson’s disease: a dopaminergic overstimulation? Sleep Med (2015) 16:583–8. doi:10.1016/j.sleep.2014.11.020
Keywords: insomnia, sleep-related movement disorders, nocturia, parasomnias, excessive daytime sleepiness
Citation: Loddo G, Calandra-Buonaura G, Sambati L, Giannini G, Cecere A, Cortelli P and Provini F (2017) The Treatment of Sleep Disorders in Parkinson’s Disease: From Research to Clinical Practice. Front. Neurol. 8:42. doi: 10.3389/fneur.2017.00042
Received: 20 November 2016; Accepted: 30 January 2017;
Published: 16 February 2017
Edited by:
Ahmed S. BaHammam, King Saud University, Saudi ArabiaReviewed by:
Pablo Torterolo, University of the Republic, UruguayThien Thanh Dang-Vu, Concordia University, Canada
Copyright: © 2017 Loddo, Calandra-Buonaura, Sambati, Giannini, Cecere, Cortelli and Provini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Provini, ZmVkZXJpY2EucHJvdmluaUB1bmliby5pdA==
 Giuseppe Loddo
Giuseppe Loddo Giovanna Calandra-Buonaura
Giovanna Calandra-Buonaura Luisa Sambati
Luisa Sambati Giulia Giannini
Giulia Giannini Annagrazia Cecere
Annagrazia Cecere Pietro Cortelli
Pietro Cortelli Federica Provini
Federica Provini