
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 13 February 2017
Sec. Movement Disorders
Volume 8 - 2017 | https://doi.org/10.3389/fneur.2017.00037
 Carmen D. Rietdijk1
Carmen D. Rietdijk1 Paula Perez-Pardo1
Paula Perez-Pardo1 Johan Garssen1,2
Johan Garssen1,2 Richard J. A. van Wezel3,4
Richard J. A. van Wezel3,4 Aletta D. Kraneveld1*
Aletta D. Kraneveld1*
Parkinson’s disease (PD) is a neurodegenerative disorder for which there is no cure. Most patients suffer from sporadic PD, which is likely caused by a combination of genetic and environmental factors. Braak’s hypothesis states that sporadic PD is caused by a pathogen that enters the body via the nasal cavity, and subsequently is swallowed and reaches the gut, initiating Lewy pathology (LP) in the nose and the digestive tract. A staging system describing the spread of LP from the peripheral to the central nervous system was also postulated by the same research group. There has been criticism to Braak’s hypothesis, in part because not all patients follow the proposed staging system. Here, we review literature that either supports or criticizes Braak’s hypothesis, focused on the enteric route, digestive problems in patients, the spread of LP on a tissue and a cellular level, and the toxicity of the protein αSynuclein (αSyn), which is the major constituent of LP. We conclude that Braak’s hypothesis is supported by in vitro, in vivo, and clinical evidence. However, we also conclude that the staging system of Braak only describes a specific subset of patients with young onset and long duration of the disease.
Parkinson’s disease (PD) is an incurable neurodegenerative disease hallmarked by damage to the dopaminergic neurons of the substantia nigra (SN), and αSynuclein (αSyn) containing inclusion bodies (Lewy pathology; LP) in the surviving neurons, resulting in characteristic motor impairment. The prevalence of PD in Europe ranges between 65.6 and 12,500 per 100,000, and the annual incidence rate ranges between 5 and 346 per 100,000 (1). The variation in these prevalence and incidence rates could be due to genetic or environmental factors, differences in case ascertainment or diagnostic criteria, or different age distributions in the populations (countries) studied (1). In the US population of 65 years and older, PD is more common in Caucasians and Hispanics, than Afro-Americans and Asians (2, 3), indicating a genetic factor may be (partially) responsible for the differences found in the European study. Current treatments for PD include medicinal treatment using levodopa (4, 5), and surgical treatment using deep brain stimulation (6). Although these treatments offer relief of symptoms, they do not cure the disease. All in all, it is clear that PD is an important neurodegenerative disorder to study, even with the more conservative estimations of prevalence and incidence, since currently no cure or preventative treatment exists.
There are two forms of PD: familial and sporadic. The familial form is caused by genetic aberrations, among others in the gene for αSyn [point mutations A30P (7), A53T (8), E46K (9), H50Q (10, 11), and G51D (12), or locus duplication (13, 14) or triplication (15, 16)]. The cause for sporadic PD is not known, but some progress has been made in the search for potential causes, implicating both genetic and environmental factors. The pesticides rotenone and paraquat (17), and the toxin MPTP (18) (1-methyl-4-fenyl-1,2,3,6-tetrahydropyridine; a toxic byproduct of the opioid analgesic desmethylprodine, MPPP, a synthetic heroin), are known to cause PD in humans, explaining some cases of sporadic PD. Additionally, two twin studies have found that sporadic PD has a significant genetic component (19, 20). As mentioned above, in the US, a difference was found in the incidence and prevalence of PD between the Caucasian and Hispanic versus Afro-American and Asian population, also showing a genetic influence (2). On the other hand, a recent review by Pan-Montojo and Reichmann suggests an important role of toxic environmental substances in the etiology of sporadic PD (21). Although the exact influence of genetic and environmental factors in sporadic PD is not known, some elements of disease development have been identified, most importantly neuroinflammation, oxidative stress, and αSyn misfolding and aggregation (22–29). Misfolding and aggregation of αSyn is suspected to lead to LP in surviving neurons, and thus combatting αSyn aggregation has been suggested to be of potential therapeutic value (30). It seems likely that both environmental and genetic factors interact to cause sporadic PD. As a result, the search for potential environmental factors has been ongoing in PD research.
In 2003, Braak et al. postulated the hypothesis that an unknown pathogen (virus or bacterium) in the gut could be responsible for the initiation of sporadic PD (31), and they presented an associated staging system for PD based on a specific pattern of αSyn spreading (32). These publications were followed by the more encompassing dual-hit hypothesis, stating that sporadic PD starts in two places: the neurons of the nasal cavity and the neurons in the gut (33, 34). This is now known as Braak’s hypothesis. From these places, the pathology is hypothesized to spread according to a specific pattern, via the olfactory tract and the vagal nerve, respectively, toward and within the central nervous system (CNS). This process has been visualized in Figure 1. Interestingly, the hypothesized spread of disease to the spinal cord only takes place after the CNS has already become involved, and so the spinal cord is not considered to be a potential route for the spread of the disease from the periphery to the brain (33, 35).
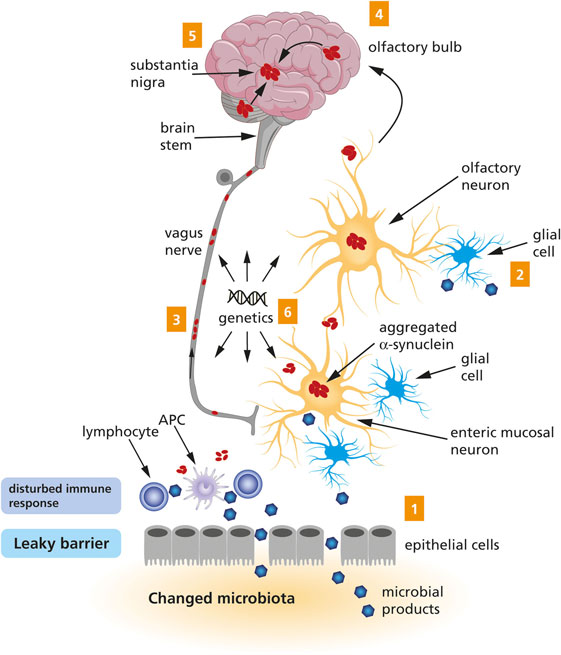
Figure 1. A schematic representation of the Braak’s hypothesis of Parkinson’s disease (PD). Microbial products come into contact with olfactory and/or enteric neurons, which trigger the aggregation of α-Synuclein (1 and 2). The aggregated α-Synuclein spreads toward the central nervous system via the olfactory bulb and the vagus nerve (3 and 4). Eventually, the aggregated α-Synuclein arrives at the substantia nigra (5). Genetic factors are likely to contribute to PD, but the exact mechanism remains to be elucidated (6).
There is experimental and clinical evidence supporting Braak’s hypothesis. Gastrointestinal problems like dysphagia, nausea, constipation and defecatory difficulty (36, 37), and the olfactory problem of the loss of smell (38) have been reported in PD. Additionally, the presence of LP in the neurons of the olfactory tract (39, 40) and the enteric nervous system (ENS) (41–43) has been confirmed. Severe LP in the ENS is positively correlated with constipation and motor problems in PD patients (44). There is also clinical evidence that LP in the nasal and gastrointestinal regions potentially precedes the diagnosis of the disease (32, 43, 45), leading to complaints of the digestive tract (46, 47) and problems with olfaction (48, 49) during the earlier stages of PD, before the onset of motor symptoms [this stage is also known as incidental Lewy body (LB) disease (50)].
In animal models, similar results have been found. Gastrointestinal problems have been described in models of advanced PD suffering from motor impairment (51–58), and in both genetic and toxin-induced models for earlier stages of PD without motor problems (59–61). Additionally, αSyn aggregations were found in the gastrointestinal tract of animal models of early (59, 60, 62) and advanced (51, 55) PD.
From here on, this review will focus on the enteric route of Braak’s hypothesis. The importance of the ENS for PD is emphasized by circumstantial clinical evidence. The microbiome of control subjects contains a higher relative abundance of Prevotellaceae bacteria compared to PD patients, and within PD patients, a higher relative abundance of Enterobacteriaceae is associated with more postural and gait symptoms and less tremors (63). PD patients also suffer from increased inflammation in the colon, although colonic inflammation does not seem to be related to severity of gastrointestinal or motor problems (64). However, in PD patients, another sign of intestinal inflammation, an increased permeability of the intestinal barrier, seems to be related to increased staining in the intestinal mucosa for bacteria, oxidative stress, and αSyn (65). If changes in the microbiome predispose the (future) PD patient to a more pro-inflammatory environment in the intestines and increased barrier permeability, this could potentially lead to oxidative stress in the ENS. This oxidative stress could then trigger αSyn misfolding and aggregation, which could potentially spread from the ENS to the CNS, and eventually cause the hallmark motor problems. Therefore, changes in the microbiome and increased inflammation could directly negatively affect neurons of the ENS and be related to PD development, which is in accordance with Braak’s hypothesis.
Dietary components and dietary patterns have a considerable effect on the composition of the gut microbiome (66). The commensal gut microbiota thrive on the substrates that escape absorption in the small intestine and are available for colonic bacterial fermentation (67). For example, fiber-rich diets can enhance the growth of colonic bacteria that produce short-chain fatty acids (SCFA). These SCFA have systemic anti-inflammatory effects (68) and could therefore influence PD pathogenesis through this gut-mediated mechanism. Another example is Western diet (high in saturated fat and refined carbohydrates) that might result in dysbiotic microbiota (e.g., lower bifidobacteria, higher firmicutes, and proteobacteria) (69–71) and that could ultimately lead to a pro-inflammatory response and promote αSyn pathology. Therefore, it is essential to continue to research specific foods and dietary patterns that can improve gut health for PD risk reduction.
Another vital part of Braak’s hypothesis is the spread of αSyn pathology from the ENS to the CNS via the vagal nerve and the dorsal motor nucleus of the vagus (DMV) in the medulla oblongata, and the spread of pathology within the CNS from lower brainstem regions, toward the SN, and eventually the neocortex. Although these specific areas of the nervous system are affected by PD, certain neighboring areas seem to be spared, such as the nucleus tractus solitarius that is located next to and connected to the DMV. This indicates a non-uniform and specific pattern of the spreading of disease, which cannot be explained by the nearest neighbor rule (72). This specific pattern of spreading is supported by experimental and clinical evidence, although discussion about the validity of Braak’s hypothesis is still ongoing. In PD patients, LP has been found in the vagal nerve (73, 74) and the DMV (73, 75–78), and cell loss in the DMV of PD patients has also been reported (79). LP has been shown to occur in vagal nerves and DMV before it spreads to other parts of the CNS (32, 45, 76, 80), like the locus coeruleus and the SN, the mesocortex, the neocortex, and the prefrontal cortex (32). Additionally, truncal vagotomy might be associated with a decreased long-term risk of developing PD, which could be related to a hindrance of the spreading of disease via the vagal nerve, although this cannot yet be concluded from this single study (81). The spread of αSyn from the ENS to the CNS has also been studied in animal models. When the protein αSyn was injected in the wall of the stomach and duodenum of rats, it was able to spread through the vagal nerve to the DMV (82). Additionally, intragastric rotenone treatment of mice resulted in αSyn inclusions in the ENS, DMV, and SN, and cell loss in the SN (83). This rotenone-induced αSyn spreading could be stopped by vagotomy (84). These results show that the vagus nerve is involved in and essential for the spread of αSyn pathology from the ENS to the CNS in both rats and mice.
Clinical evidence for the cellular transport of LP within the CNS comes from studies of PD patients whose grafts of fetal dopaminergic neurons showed LP and degeneration, indicating potential spread of pathology from host cells to graft cells (85–90). Host-to-graft transmission of αSyn has also been shown for mouse cortical neuronal stem cells (91) and mouse embryonic dopaminergic neurons (92) implanted in transgenic mice overexpressing human αSyn, and for rat embryonic dopaminergic neurons implanted in human αSyn overexpressing rats with (93) or without (94) striatal dopamine depletion. These results show that healthy neurons in the CNS are vulnerable to spread of disease by taking up LP from surrounding LP-affected neurons, although it does not indicate any specific pattern for this spreading.
The ability of LP to spread through the nervous system raises the question what is the exact mechanism of transport of LP between neurons, and why the spread of LP follows a specific pattern, as suggested by Braak’s hypothesis. Both neuronal cell lines and primary neurons are able to excrete αSyn monomers, oligomers, and fibrils through unconventional calcium-dependent exocytosis from large dense core vesicles or via exosomes (84, 95–97). Once the αSyn is present in their environment, both neuronal cell lines and primary neurons seem to be able to take up free or exosome-bound fibrils and oligomers by endocytosis after which they are degraded in lysosomes (SH-SY5Y cells), while monomers seem to diffuse across the cell membrane and are not degraded (91, 97, 98). In a different study, the uptake was only found in proliferating SH-SY5Y neurons, but not in differentiated SH-SY5Y neurons, which could be due to the type of αSyn that was different from the other studies (radioactively labeled cell produced αSyn, versus different forms of recombinant human or non-human αSyn) (96). The transfer of specific αSyn molecules between cells of neuronal cell lines was proven in a coculture study of SH-SY5Y neurons expressing the same human αSyn labeled either green or red (92). Coculture resulted in double-labeled neurons, showing the process of subsequent excretion and uptake of αSyn by neighboring cells. After uptake, αSyn can be transported anterograde or retrograde through axons and passed on to other neurons (82, 84, 99–101), providing a potential highway for the spread of LP between connected nervous system regions in PD patients. A recent study shows that neuron-to-neuron αSyn transmission could be initiated by binding the transmembrane protein lymphocyte-activation gene 3 (LAG3). The study demonstrated that LAG3 binds αSyn preformed fibrils (PFFs) with high affinity and initiates αSyn PFF endocytosis, transmission, and toxicity in SH-SY5Y cells. Moreover, mice lacking LAG3 showed delayed αSyn PFF-induced pathology and reduced toxicity (102).
It is known that the neurons in the areas affected by LP in PD have specific characteristics that cause a high metabolic burden, which seems to make these neurons especially sensitive to oxidative stress and αSyn misfolding. These neurons have high levels of endogenous αSyn, they use monoamine neurotransmitters, have long and highly branched axons with no or poor myelination, and characteristic continuous activity patterns (72, 103, 104). Together this could explain why PD pathology develops in the specific pattern proposed by Braak, specifically affecting interconnected regions with vulnerable neurons like the DMV, while sparing neighboring areas like the nucleus tractus solitaries (72).
It has been suggested that αSyn acts prion-like in PD. In this theory, pathologic, misfolded αSyn is an infectious protein spreading toxicity by forming a toxic template that seeds misfolding for nearby αSyn protein, turning the previously healthy protein into a toxic protein, causing LP. Excellent reviews on the prion-like theory of αSyn have been previously published (105, 106). The prion-like theory fits into Braak’s hypothesis, since the staging system of Braak is based on the regional presence (or absence) of LP and the spreading of LP, linking LP to severity of disease (32). The toxicity of αSyn in its different form is still undecided and remains the topic of many experiments, with one study reporting a cytoprotective function of αSyn aggregation (107), while others suggest that the oligomeric form of αSyn is the most toxic form of the protein (108–110). Foreign αSyn induces LP-resembling inclusion bodies in recipient neurons (91), caused by fibrils acting as exogenous seeds and recruiting endogenous αSyn into the inclusion body (92, 111), even in cells not overexpressing αSyn (101). Neuronal death resulting from αSyn exposure has also been shown (91), with a higher toxicity for oligomeric compared to monomeric species (96), and a higher toxicity of exosome bound oligomers compared to free oligomers (97). Inclusion bodies are linked to cell death, involving the loss of synaptic proteins and reduction in network connectivity (101).
In animal studies, injection of aggregated αSyn (derived from symptomatic transgenic mice) or synthetic αSyn fibrils into the brain of young, asymptomatic transgenic mice accelerated the formation and spread of αSyn inclusions throughout the brain resulted in early-onset motor symptoms, and reduced the lifespan of these mice (112, 113). Synthetic αSyn fibrils injected in the striatum also induced widespread LP, cell death of dopamine neurons in the SN, and motor deficits in wild-type mice (114). It has even been shown that fibril-seeded αSyn inclusions specifically increase neuronal death in αSyn transgenic mice in an experiment where neurons with or without inclusions were followed in vivo, providing direct evidence that αSyn inclusions were responsible for neuronal death (115). Injection of wild-type mice with patient-derived LB αSyn just above the SN resulted in degeneration of the dopamine fibers and cell bodies in the SN, and concomitant development of inclusion bodies exclusively consisting of endogenous αSyn, and reduced motor coordination and balance (116). Mice treated with non-LB αSyn (monomers) did not develop these lesions. Similar results were found in rhesus monkeys; injection of patient-derived LB αSyn in the striatum or SN resulted in reduced nigrostriatal dopaminergic innervation, increased αSyn immunoreactivity in connected brain regions after striatal injection (but not after SN injection), without LP or motor symptoms (116). Taken together, these results do not definitively confirm or reject the prion-like theory in the context of Braak’s hypothesis. However, a picture emerges where αSyn oligomers are likely toxic to neurons, and inclusion bodies are linked to neuronal death, which might or might not lead to motor symptoms. Although the studies included here were performed in the CNS, the emerging picture of oligomer toxicity and inclusion body-induced neuronal death could also be applicable to the ENS and other parts of the peripheral nervous system.
Despite the in vitro, in vivo, and clinical support for Braak’s hypothesis, there is also doubt whether it accurately describes the development of PD in all patients (117, 118). A large subset of 51–83% of PD patients follow Braak’s staging, while a smaller subset of 7–11% do not have LP in the DMV while higher brain regions are affected (119–124). Additionally, there is no correlation between severity of LP in the DMV and in the limbic system or neocortex (125). Also, LP in the ENS is not correlated to olfactory problems, and 27–33% of PD patients did not show any LP in the ENS, which does not support the dual-hit hypothesis (64, 126), although it is known that LP can be restricted to the olfactory system in the early stage of the disease (124). Additionally, people with incidental LB disease seem to have a similar distribution but milder expression of LP compared to PD patients (50, 127) and can show LP in the SN and other areas of the brain without LP or neuronal loss in the DMV (77, 122, 128, 129) or LP in the vagus nerve (45), favoring multiple origination sites for LP instead of a spread from ENS to CNS via the vagus nerve. Additionally, Braak’s hypothesis does not explain how or why cardiac sympathetic nerves are affected in early PD (129). Therefore, it seems safe to conclude that not all PD patients adhere to the specific pattern of LP spread proposed by Braak.
Other studies have shown that the link between LP and clinical PD symptoms should be questioned. Only 45% of people with widespread LP in the brain are diagnosed with dementia or motor symptoms (121) and only about 10% of people with LP in the SN, DMV, and/or basal forebrain are diagnosed with PD (130). Additionally, neurodegeneration in the SN might precede LP (131). Therefore, the spreading of LP, whether according to Braak’s staging system or not, might not be as tightly bound to clinical symptoms as has been suggested by Braak.
The basic science underlying Braak’s hypothesis has also been questioned (118, 132), because in the initial studies all cases were preselected for LP in the DMV (32, 76), systematically excluding any cases where LP in higher brain regions was found in the absence of LP in the DMV, which seems to have led to a selection bias and the inclusion of non-representative samples in the preclinical PD group in the original research (132). The limited clinical information on the preclinical PD group and the absence of information on neuronal cell loss in the original Braak papers have also been criticized (117, 118, 132). It has been suggested that neuronal loss and activation of glial cells should be part of future pathological analysis of PD to better describe disease progression, since the clinical significance of LP is not yet clear and might be less important than previously thought (121, 130, 131).
Studying neuronal loss together with LP during PD development is important because neuronal loss in the SN shows a linear relationship with motor symptoms (133), while LP in the overall brain only shows a trend for positive correlation with motor symptoms (124). Additionally, LP is not related to dopaminergic cell loss in the striatum (124), and may (124) or may not (134) be related to dopaminergic cell loss in the SN of PD patients. Therefore, it can be concluded that neuronal loss and LP are not interchangeable hallmarks for PD progression or severity of disease, but should rather be seen as complimentary to each other.
Studying the activation of glial cells is important because neuroinflammation is an important factor in PD development, and glial cells are major contributors to neuroinflammation, partially through toll-like receptors (TLRs) (22–27). Especially TLR2 and -4 are important in PD, since their expression is increased in the brain of PD patients, and a polymorphism resulting in lower expression of TLR2 tends to be linked to an increased risk of PD (135–138). Preclinical research has confirmed the importance of TLR2 and -4 for PD and has specifically shown their importance in the context of glial-induced inflammation and αSyn uptake by glial cells (138–149).
Reviewing the current literature it can be concluded that there is much evidence to support Braak’s hypothesis. Enteric and olfactory pathology and dysfunction are well-known characteristics of early and late PD. The vagus nerve and DMV form a likely route for αSyn pathology to spread from the ENS to the CNS, and αSyn is able to spread cellularly within the CNS. Neurons are able to transmit different forms of αSyn protein to each other and to transport αSyn via their axons, which enables the spread of the potentially toxic oligomeric variety of the protein, which could be the basic mechanism underlying the specific pattern of LP spread in PD as proposed by Braak. It then seems possible that a pathogen or environmental toxin might provoke local inflammation and oxidative stress in the gut, thereby initiating αSyn deposition that is subsequently disseminated to the CNS. Hypothetically, the toxic αSyn can lead to neuronal death. (Micro)glial cells and surviving neurons can then be activated through the release of danger associated molecular patterns and subsequent activation of TLRs. This would trigger a vicious circle of neuroinflammation.
However, it can also be concluded that a significant portion of PD patients do not follow Braak’s staging system. It has been discovered that a subgroup of levodopa-responsive PD patients who develop PD at a young age and have a long duration clinical course with predominantly motor symptoms, and dementia only at the later stages, seem to follow Braak’s staging, while other levodopa-responsive PD patients did not (80). In addition to this, a LB staging system has been proposed, which encompasses all patient groups, a system wherein LP staging correlates well with motor symptoms and cognitive decline (124), and allowing for patients who show a spread of LP not accounted for in Braak’s hypothesis. Unfortunately, the staging system is only describing the different observed patterns of LP spread, while not answering the question as to the cause of the non-Braak patterns. What is the reason or explanation for these other types of patterns to occur? This question remains to be answered.
We conclude that Braak’s hypothesis and the Braak staging system are valuable and useful for the future study of PD, and these theories are likely to accurately describe disease initiation and progression in a subgroup of PD patients with young onset and long duration of disease. However, a similar theory describing the initiation and disease progression in other PD patients is still sorely lacking and deserves to be elucidated. To better understand the progression of LP and PD in different patient groups, it is necessary to study people longitudinally during disease development, and especially in the earliest stages of PD. This should lead to a larger theory describing different disease processes, all leading to PD, including Braak’s hypothesis. This theory could offer useful insight into specific targets for disease prevention or disease treatment, dependent on the type of LP disease the patient is likely suffering from. Either more optimal treatment with currently available drugs and technology, or the development of new treatments could be the result.
CR, PP-P, JG, RW, and AK conceived and designed the review. CR wrote the first draft of the review with PP-P’s assistance. JG, RW, and AK reviewed and critiqued the manuscript. All the authors were responsible for the decision to submit the manuscript for publication.
JG is an employee of Nutricia Research, Utrecht, The Netherlands. All other authors report no potential conflicts of interest.
Our research was funded by Utrecht University “Focus en Massa program.”
1. von Campenhausen S, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, et al. Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol (2005) 15:473–90. doi:10.1016/j.euroneuro.2005.04.007
2. Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology (2010) 34:143–51. doi:10.1159/000275491
3. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol (2003) 157:1015–22. doi:10.1093/aje/kwg068
4. Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord (2015) 30:4–18. doi:10.1002/mds.26102
5. Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord (2013) 28:1064–71. doi:10.1002/mds.25364
6. Williams ZM. Good vibrations with deep brain stimulation. Nat Neurosci (2015) 18:618–9. doi:10.1038/nn.4007
7. Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet (1998) 18:106–8. doi:10.1038/ng0298-106
8. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science (1997) 276:2045–7. doi:10.1126/science.276.5321.2045
9. Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol (2004) 55:164–73. doi:10.1002/ana.10795
10. Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord (2013) 28:811–3. doi:10.1002/mds.25421
11. Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, et al. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology (2013) 80:1062–4. doi:10.1212/WNL.0b013e31828727ba
12. Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, et al. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol (2013) 73:459–71. doi:10.1002/ana.23894
13. Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet (2004) 364:1167–9. doi:10.1016/S0140-6736(04)17103-1
14. Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, et al. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol (2006) 59:298–309. doi:10.1002/ana.20753
15. Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science (2003) 302:841. doi:10.1126/science.1090278
16. Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, et al. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol (2004) 55:174–9. doi:10.1002/ana.10846
17. Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect (2011) 119:866–72. doi:10.1289/ehp.1002839
18. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science (1983) 219:979–80. doi:10.1126/science.6823561
19. Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ. The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol (1999) 45:577–82. doi:10.1002/1531-8249(199905)45:5<577::AID-ANA5>3.0.CO;2-O
20. Wirdefeldt K, Gatz M, Reynolds CA, Prescott CA, Pedersen NL. Heritability of Parkinson disease in Swedish twins: a longitudinal study. Neurobiol Aging (2011) 32:1923.e1–8. doi:10.1016/j.neurobiolaging.2011.02.017
21. Pan-Montojo F, Reichmann H. Considerations on the role of environmental toxins in idiopathic Parkinson’s disease pathophysiology. Transl Neurodegener (2014) 3:10. doi:10.1186/2047-9158-3-10
22. Drouin-Ouellet J, Cicchetti F. Inflammation and neurodegeneration: the story “retolled”. Trends Pharmacol Sci (2012) 33:542–51. doi:10.1016/j.tips.2012.07.002
23. Tomé CML, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and α-synuclein’s prion-like behavior in Parkinson’s disease—is there a link? Mol Neurobiol (2013) 47:561–74. doi:10.1007/s12035-012-8267-8
24. Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord (2012) 18:S210–2. doi:10.1016/S1353-8020(11)70065-7
25. Clairembault T, Leclair-Visonneau L, Neunlist M, Derkinderen P. Enteric glial cells: new players in Parkinson’s disease? Mov Disord Off J Mov Disord Soc (2015) 30:494–8. doi:10.1002/mds.25979
26. Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem (2010) 285:9262–72. doi:10.1074/jbc.M109.081125
27. Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A. Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: are microglia pathogenic in either disorder? Int Rev Neurobiol (2007) 82:235–46. doi:10.1016/S0074-7742(07)82012-5
28. Niranjan R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol Neurobiol (2014) 49:28–38. doi:10.1007/s12035-013-8483-x
29. Zaltieri M, Longhena F, Pizzi M, Missale C, Spano P, Bellucci A. Mitochondrial dysfunction and-synuclein synaptic pathology in Parkinson’s disease: who’s on first? Park Dis (2015) 2015:1–10. doi:10.1155/2015/108029
30. Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, et al. Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol (2015) 14:855–66. doi:10.1016/S1474-4422(15)00006-X
31. Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) (2003) 110:517–36. doi:10.1007/s00702-002-0808-2
32. Braak H, Del Tredici K, Rüb U, de Vos RAI, Steur ENHJ, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging (2003) 24:197–211. doi:10.1016/S0197-4580(02)00065-9
33. Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol (2007) 33:599–614. doi:10.1111/j.1365-2990.2007.00874.x
34. Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci (2009) 1170:615–22. doi:10.1111/j.1749-6632.2009.04365.x
35. Del Tredici K, Braak H. Review: sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol (2016) 42:33–50. doi:10.1111/nan.12298
36. Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat Disord (2011) 17:10–5. doi:10.1016/j.parkreldis.2010.08.003
37. Cersosimo MG, Benarroch EE. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis (2012) 46:559–64. doi:10.1016/j.nbd.2011.10.014
38. Doty RL. Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis (2012) 46:527–52. doi:10.1016/j.nbd.2011.10.026
39. Beach TG, White CL III, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol (Berl) (2009) 117:169–74. doi:10.1007/s00401-008-0450-7
40. Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat (2007) 211:117–24. doi:10.1111/j.1469-7580.2007.00748.x
41. Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol (Berl) (1988) 76:217–21. doi:10.1007/BF00687767
42. Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett (2006) 396:67–72. doi:10.1016/j.neulet.2005.11.012
43. Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord (2012) 27:709–15. doi:10.1002/mds.23838
44. Lebouvier T, Neunlist M, des Varannes SB, Coron E, Drouard A, N’Guyen J-M, et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One (2010) 5:e12728. doi:10.1371/journal.pone.0012728
45. Bloch A, Probst A, Bissig H, Adams H, Tolnay M. α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol (2006) 32:284–95. doi:10.1111/j.1365-2990.2006.00727.x
46. Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord (2007) 22:1581–6. doi:10.1002/mds.21560
47. Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutiérrez C, et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol (2013) 260:1332–8. doi:10.1007/s00415-012-6801-2
48. Ross GW, Abbott RD, Petrovitch H, Tanner CM, Davis DG, Nelson J, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord (2006) 21:2062–7. doi:10.1002/mds.21076
49. Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol (2008) 63:167–73. doi:10.1002/ana.21291
50. Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol (Berl) (2008) 115:437–44. doi:10.1007/s00401-008-0345-7
51. Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O. Alpha-synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis (2012) 47:258–67. doi:10.1016/j.nbd.2012.04.009
52. Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet (2010) 19:1633–50. doi:10.1093/hmg/ddq038
53. Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, Srinivasan S, et al. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp Neurol (2007) 207:4–12. doi:10.1016/j.expneurol.2007.05.010
54. Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp Neurol (2009) 218:154–61. doi:10.1016/j.expneurol.2009.04.023
55. Natale G, Kastsiushenka O, Fulceri F, Ruggieri S, Paparelli A, Fornai F. MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res (2010) 1355:195–206. doi:10.1016/j.brainres.2010.07.076
56. Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, et al. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci (2004) 24:9434–40. doi:10.1523/JNEUROSCI.3080-04.2004
57. Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, et al. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A (2004) 101:665–70. doi:10.1073/pnas.0307453101
58. Fleming SM, Zhu C, Fernagut P-O, Mehta A, DiCarlo CD, Seaman RL, et al. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol (2004) 187:418–29. doi:10.1016/j.expneurol.2004.01.023
59. Wang L, Magen I, Yuan P, Subramaniam SR, Richter F, Chesselet M, et al. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil (2012) 24:e425–36. doi:10.1111/j.1365-2982.2012.01974.x
60. Drolet RE, Cannon JR, Montero L, Greenamyre JT. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol Dis (2009) 36:96–102. doi:10.1016/j.nbd.2009.06.017
61. Noorian AR, Rha J, Annerino DM, Bernhard D, Taylor GM, Greene JG. Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol Dis (2012) 48:9–19. doi:10.1016/j.nbd.2012.06.005
62. Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RAE, et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord (2014) 29:999–1009. doi:10.1002/mds.25736
63. Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord Off J Mov Disord Soc (2015) 30:350–8. doi:10.1002/mds.26069
64. Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis (2013) 50:42–8. doi:10.1016/j.nbd.2012.09.007
65. Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One (2011) 6:e28032. doi:10.1371/journal.pone.0028032
66. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med (2009) 1:6ra14. doi:10.1126/scitranslmed.3000322
67. Wong JMW, Esfahani A, Singh N, Villa CR, Mirrahimi A, Jenkins DJA, et al. Gut microbiota, diet, and heart disease. J AOAC Int (2012) 95:24–30. doi:10.5740/jaoacint.SGEWong
68. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity (2014) 40:128–39. doi:10.1016/j.immuni.2013.12.007
69. Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr (2009) 101:1493–502. doi:10.1017/S0007114508094658
70. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A (2010) 107:14691–6. doi:10.1073/pnas.1005963107
71. Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int J Obes 2005 (2013) 37:216–23. doi:10.1038/ijo.2012.33
72. Surmeier DJ, Sulzer D. The pathology roadmap in Parkinson disease. Prion (2013) 7:85–91. doi:10.4161/pri.23582
73. Del Tredici K, Braak H. A not entirely benign procedure: progression of Parkinson’s disease. Acta Neuropathol (2008) 115:379–84. doi:10.1007/s00401-008-0355-5
74. Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol (2010) 119:703–13. doi:10.1007/s00401-010-0665-2
75. Takeda S, Yamazaki K, Miyakawa T, Arai H. Parkinson’s disease with involvement of the parasympathetic ganglia. Acta Neuropathol (1993) 86:397–8. doi:10.1007/BF00369454
76. Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol (2002) 61:413–26. doi:10.1093/jnen/61.5.413
77. Jellinger KA. Lewy body-related α-synucleinopathy in the aged human brain. J Neural Transm (2004) 111:1219–35. doi:10.1007/s00702-004-0138-7
78. Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord (2008) 23:837–44. doi:10.1002/mds.21956
79. Gai WP, Blumbergs PC, Geffen LB, Blessing WW. Age-related loss of dorsal vagal neurons in Parkinson’s disease. Neurology (1992) 42:2106–11. doi:10.1212/WNL.42.11.2106
80. Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol (2008) 115:409–15. doi:10.1007/s00401-008-0344-8
81. Svensson E, Horváth-Puhó E, Thomsen R, Djurhuus J, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol (2015) 78:522–9. doi:10.1002/ana.24448
82. Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol (2014) 128:805–20. doi:10.1007/s00401-014-1343-6
83. Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One (2010) 5:e8762. doi:10.1371/journal.pone.0008762
84. Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A, et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep (2012) 2. doi:10.1038/srep00898
85. Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med (2008) 14:504–6. doi:10.1038/nm1747
86. Li J-Y, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med (2008) 14:501–3. doi:10.1038/nm1746
87. Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord (2008) 23:2303–6. doi:10.1002/mds.22369
88. Li J, Englund E, Widner H, Rehncrona S, Björklund A, Lindvall O, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord (2010) 25:1091–6. doi:10.1002/mds.23012
89. Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann N Y Acad Sci (2010) 1184:55–67. doi:10.1111/j.1749-6632.2009.05229.x
90. Kurowska Z, Englund E, Widner H, Lindvall O, Li J-Y, Brundin P. Signs of degeneration in 12–22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson’s disease. J Parkinsons Dis (2011) 1:83–92. doi:10.3233/JPD-2011-11004
91. Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A (2009) 106:13010–5. doi:10.1073/pnas.0903691106
92. Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest (2011) 121:715–25. doi:10.1172/JCI43366
93. Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, et al. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis (2011) 43:552–7. doi:10.1016/j.nbd.2011.05.001
94. Angot E, Steiner JA, Tomé CML, Ekström P, Mattsson B, Björklund A, et al. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS One (2012) 7:e39465. doi:10.1371/journal.pone.0039465
95. Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci (2005) 25:6016–24. doi:10.1523/JNEUROSCI.0692-05.2005
96. Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci (2010) 30:6838–51. doi:10.1523/JNEUROSCI.5699-09.2010
97. Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener (2012) 7:42. doi:10.1186/1750-1326-7-42
98. Lee H-J, Suk J-E, Bae E-J, Lee J-H, Paik SR, Lee S-J. Assembly-dependent endocytosis and clearance of extracellular a-synuclein. Int J Biochem Cell Biol (2008) 40:1835–49. doi:10.1016/j.biocel.2008.01.017
99. Jensen PH, Li J, DahlstroÈm A, Dotti CG. Axonal transport of synucleins is mediated by all rate components. Eur J Neurosci (1999) 11:3369–76. doi:10.1046/j.1460-9568.1999.00754.x
100. Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann Neurol (2012) 72:517–24. doi:10.1002/ana.23747
101. Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron (2011) 72:57–71. doi:10.1016/j.neuron.2011.08.033
102. Mao X, Ou MT, Karuppagounder SS, Kam T-I, Yin X, Xiong Y, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science (2016) 353(6307). doi:10.1126/science.aah3374
103. Braak H, Del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol Aging (2004) 25:19–23. doi:10.1016/j.neurobiolaging.2003.04.001
104. Lebouvier T, Chaumette T, Paillusson S, Duyckaerts C, Bruley des Varannes S, Neunlist M, et al. The second brain and Parkinson’s disease. Eur J Neurosci (2009) 30:735–41. doi:10.1111/j.1460-9568.2009.06873.x
105. Visanji NP, Brooks PL, Hazrati L-N, Lang AE. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol Commun (2013) 1:12. doi:10.1186/2051-5960-1-2
106. Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature (2013) 501:45–51. doi:10.1038/nature12481
107. Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem (2004) 279:4625–31. doi:10.1074/jbc.M310994200
108. Andreasen M, Lorenzen N, Otzen D. Interactions between misfolded protein oligomers and membranes: a central topic in neurodegenerative diseases? Biochim Biophys Acta (2015) 1848:1897–907. doi:10.1016/j.bbamem.2015.01.018
109. Lindström V, Fagerqvist T, Nordström E, Eriksson F, Lord A, Tucker S, et al. Immunotherapy targeting α-synuclein protofibrils reduced pathology in (Thy-1)-h [A30P] α-synuclein mice. Neurobiol Dis (2014) 69:134–43. doi:10.1016/j.nbd.2014.05.009
110. Roberts HL, Brown DR. Seeking a mechanism for the toxicity of oligomeric α-synuclein. Biomolecules (2015) 5:282–305. doi:10.3390/biom5020282
111. Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A (2009) 106:20051–6. doi:10.1073/pnas.0908005106
112. Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med (2012) 209:975–86. doi:10.1084/jem.20112457
113. Mougenot A-L, Nicot S, Bencsik A, Morignat E, Verchère J, Lakhdar L, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging (2012) 33:2225–8. doi:10.1016/j.neurobiolaging.2011.06.022
114. Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science (2012) 338:949–53. doi:10.1126/science.1227157
115. Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, Unni VK. Progressive aggregation of alpha-synuclein and selective degeneration of Lewy inclusion-bearing neurons in a mouse model of Parkinsonism. Cell Rep (2015) 10:1252–60. doi:10.1016/j.celrep.2015.01.060
116. Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol (2014) 75:351–62. doi:10.1002/ana.24066
117. Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol (2008) 116:1–16. doi:10.1007/s00401-008-0406-y
118. Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB. Evidence against a reliable staging system of α-synuclein pathology in Parkinson’s disease. Neuropathol Appl Neurobiol (2009) 35:125–6. doi:10.1111/j.1365-2990.2008.00998.x
119. Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: a critical analysis of α-synuclein staging. Neuropathol Appl Neurobiol (2008) 34:284–95. doi:10.1111/j.1365-2990.2007.00923.x
120. Attems J, Jellinger KA. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease. Neuropathol Appl Neurobiol (2008) 34:466–7. doi:10.1111/j.1365-2990.2008.00937.x
121. Parkkinen L, Pirttilä T, Alafuzoff I. Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol (2008) 115:399–407. doi:10.1007/s00401-008-0346-6
122. Jellinger KA. α-Synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution – a pilot study. Acta Neuropathol (2003) 106:191–202. doi:10.1007/s00401-003-0725-y
123. Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG. MRC Cognitive Function ANS. Patterns and stages of alpha-synucleinopathy: relevance in a population-based cohort. Neurology (2008) 70:1042–8. doi:10.1212/01.wnl.0000306697.48738.b6
124. Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol (2009) 117:613–34. doi:10.1007/s00401-009-0538-8
125. Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, Ayling H, Kallis C, Sterlacci W, et al. Brain stem pathology in Parkinson’s disease: an evaluation of the Braak staging model. Mov Disord (2010) 25:2508–15. doi:10.1002/mds.23305
126. Lebouvier T, Pouclet H, Coron E, Drouard A, N’Guyen J-M, Roy M, et al. Colonic neuropathology is independent of olfactory dysfunction in Parkinson’s disease. J Park Dis (2011) 1:389–94. doi:10.3233/JPD-2011-11061
127. Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL III, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol (2010) 119:689–702. doi:10.1007/s00401-010-0664-3
128. Parkkinen L, Soininen H, Alafuzoff I. Regional distribution of α-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol (2003) 62:363–7. doi:10.1093/jnen/62.4.363
129. Orimo S, Takahashi A, Uchihara T, Mori F, Kakita A, Wakabayashi K, et al. Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol (2007) 17:24–30. doi:10.1111/j.1750-3639.2006.00032.x
130. Parkkinen L, Kauppinen T, Pirttilä T, Autere JM, Alafuzoff I. α-Synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol (2005) 57:82–91. doi:10.1002/ana.20321
131. Milber JM, Noorigian JV, Morley JF, Petrovitch H, White L, Ross GW, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology (2012) 79:2307–14. doi:10.1212/WNL.0b013e318278fe32
132. Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB. Controversies over the staging of α-synuclein pathology in Parkinson’s disease. Acta Neuropathol (2008) 116:125–8. doi:10.1007/s00401-008-0381-3
133. Greffard S, Verny M, Bonnet A-M, Beinis J-Y, Gallinari C, Meaume S, et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol (2006) 63:584–8. doi:10.1001/archneur.63.4.584
134. Parkkinen L, O’Sullivan SS, Collins C, Petrie A, Holton JL, Revesz T, et al. Disentangling the relationship between Lewy bodies and nigral neuronal loss in Parkinson’s disease. J Park Dis (2011) 1:277–86. doi:10.3233/JPD-2011-11046
135. Doorn KJ, Moors T, Drukarch B, van de Berg WD, Lucassen PJ, van Dam AM. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun (2014) 2:90–1. doi:10.1186/s40478-014-0090-1
136. Kalinderi K, Bostantjopoulou S, Katsarou Z, Fidani L. TLR9-1237 T/C and TLR2-194 to -174 del polymorphisms and the risk of Parkinson’s disease in the Greek population: a pilot study. Neurol Sci (2013) 34:679–82. doi:10.1007/s10072-012-1106-x
137. Noguchi E, Nishimura F, Fukai H, Kim J, Ichikawa K, Shibasaki M, et al. An association study of asthma and total serum immunoglobin E levels for toll-like receptor polymorphisms in a Japanese population. Clin Exp Allergy (2004) 34:177–83. doi:10.1111/j.1365-2222.2004.01839.x
138. Drouin-Ouellet J, St-Amour I, Saint-Pierre M, Lamontagne-Proulx J, Kriz J, Barker RA, et al. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int J Neuropsychopharmacol (2014) 18:yu103. doi:10.1093/ijnp/pyu103
139. Kim C, Cho E, Kim H, You S, Lee H, Hwang D, et al. β1-Integrin-dependent migration of microglia in response to neuron-released α-synuclein. Exp Mol Med (2014) 46:e91. doi:10.1038/emm.2014.6
140. Qiao H, Zhang Q, Yuan H, Li Y, Wang D, Wang R, et al. Elevated neuronal α-synuclein promotes microglia activation after spinal cord ischemic/reperfused injury. Neuroreport (2015) 26:656–61. doi:10.1097/WNR.0000000000000406
141. Daniele S, Béraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss K. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci Signal (2015) 8:ra45. doi:10.1126/scisignal.2005965
142. Beraud D, Twomey M, Bloom B, Mittereder A, Ton V, Neitzke K, et al. alpha-Synuclein alters toll-like receptor expression. Front Neurosci (2011) 5:80. doi:10.3389/fnins.2011.00080
143. Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Lachaud CC, Guilliams T, Fernandez-Montesinos R, et al. Preconditioning of microglia by α-synuclein strongly affects the response induced by toll-like receptor (TLR) stimulation. PLoS One (2013) 8:e79160. doi:10.1371/journal.pone.0079160
144. Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A (2003) 100:8514–9. doi:10.1073/pnas.1432609100
145. Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol (2011) 179:954–63. doi:10.1016/j.ajpath.2011.04.013
146. Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, et al. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia (2013) 61:349–60. doi:10.1002/glia.22437
147. Rannikko EH, Weber SS, Kahle PJ. Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci (2015) 16:57. doi:10.1186/s12868-015-0192-0
148. Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep (2013) 3:1393. doi:10.1038/srep01393
Keywords: Parkinson’s disease, Braak’s hypothesis, Lewy pathology, αSynuclein, enteric nervous system
Citation: Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJA and Kraneveld AD (2017) Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 8:37. doi: 10.3389/fneur.2017.00037
Received: 23 September 2016; Accepted: 26 January 2017;
Published: 13 February 2017
Edited by:
Oscar Arias-Carrión, Hospital General Dr. Manuel Gea González, MexicoReviewed by:
Graziella Madeo, National Institutes of Health (NIH), USACopyright: © 2017 Rietdijk, Perez-Pardo, Garssen, van Wezel and Kraneveld. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aletta D. Kraneveld, YS5kLmtyYW5ldmVsZEB1dS5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.