
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neurol. , 23 December 2016
Sec. Sleep Disorders
Volume 7 - 2016 | https://doi.org/10.3389/fneur.2016.00236
Sudden infant death syndrome (SIDS) is the unexplained death, usually during sleep, of a baby younger than 1-year-old. Even though researchers have discovered some factors that may put babies at extra risk, SIDS remains unpredictable up until now. One hypothesis is that impaired cardiovascular control may play a role in the underlying mechanism of SIDS. A reduction of heart rate variability (HRV) and progressive decrease in heart rate (HR) have been observed in infants who have later succumbed to SIDS. Many clues indicated the heart could be the final weakness in SIDS. Therefore, continuous monitoring of the dynamic changes within the heart may provide a possible preventive strategy of SIDS. Camera-based photoplethysmography was recently demonstrated as a contactless method to determine HR and HRV. This perspective presents a hypothesis that a camera-based, non-contact, vital-sign monitoring technology, which can indicate abnormal changes or a sudden loss of vital signs in a timely manner, may enable a crucial and low-cost means for the early prevention of SIDS in newborn infants.
Sudden infant death syndrome, which usually occurs during sleep with a peak incidence at 2–3 months of age, remains as one of the current leading causes of post-neonatal infant death. The pathophysiological mechanism underlying the death is still unclear. This old problem will continue to plague us until the answers eventually fall into place. The majority of findings suggests that impaired cardiovascular control could lead to SIDS. Infants who have succumbed to SIDS have a higher basal heart rate (HR) (1), reduced heart rate variability (HRV) (2, 3), familial long QT syndrome (4), Brugada syndrome (5), lower parasympathetic activity, and/or higher sympathovagal balance (6).
There are many clinical studies that have linked a progressive decrease in HR with SIDS cases (7–13). These studies are based on the objective data that immediately precede SIDS obtained from physiological memory-monitor recordings. The primary event in every case was a progressive decrease in HR that developed over minutes or hours before SIDS (14). The exact causes of the bradycardia could not be determined from the recordings, but there is an assumption that the progressive decrease in HR is probably due to hypoxic cardiac depression, which was deduced from the evidence obtained from similar recordings during apparent life-threatening events (ALTEs).
Subsequently, some risk factors related to autonomic dysfunction have been implicated in SIDS. Reduction in cardiac autonomic function induced by these risk factors has been demonstrated by the analysis of the spontaneous beat-to-beat changes in HR, known as HRV. Prone sleeping is well established as a major risk factor for SIDS (15), which is associated with a reduction in cardiovascular and autonomic control (16–18). HRV parameters were found to be reduced in prone position compared to supine position, suggesting diminished sympathetic activity in prone position (17–20). Maternal cigarette smoking is another modifiable risk factor for SIDS. The increased risk for SIDS associated with maternal smoking has been attributed to various mechanisms, including impairment of autonomic functions (21, 22). Infants from smoking mothers have reduced HRV and lower frequency power normalized to the total spectral power (LF/TP) (23). Larger infant HR decreases during hyperoxia and smaller HR rises during hypoxia are correlated with the increasing number of cigarettes smoked by the mother (24). Thermal stress could also increase an infant’s vulnerability to SIDS by disrupting autonomic functions (25). The elevated ambient temperature is associated with a higher basal HR and lower short- and long-term HRV during all sleep stages (26), and a cool environment is linked to greater autonomic dysfunction (27). It has been suggested that immature autonomic cardiovascular control contributes to the increase risk for SIDS in preterm infants. The delayed maturation of autonomic cardiovascular control in preterm infants, reflected in a shorter inter-beat (RR)-interval (28) and a lower power of HRV (29, 30).
The high incidence, unknown pathophysiological mechanisms, and the catastrophic impact on affected families are what make SIDS so frightening. Given the strong and mounting evidence implicating reduced cardiovascular control in SIDS cases, questions regarding home monitoring in order to provide the warning needed in time for intervention arise in families.
Current HR monitoring techniques usually require the use of adhesive electrodes or sensors. Long-term attachment to the sensors can cause skin irritation, stress, pain, and even damage to the fragile skin of infants. In particular, epidermal stripping from removal of adhesives may occur, particularly in preterm infants less than 27 weeks of gestational age due to incompletely developed stratum corneum and the decreased number of fibrils that connect the epidermis to the dermis (31, 32). Moreover, the obtrusiveness of the wires has a negative impact on parent-child bonding, especially during the care by a kangaroo mother. The stress caused by the repetitive application and removal of patches can interfere with the infant’s normal growth and hamper the infant’s cognitive development (33, 34).
An alternative solution might be technologies for non-contact monitoring like capacitive ECG (35), ballistocardiograph (36), Laser Doppler (37), Microwave Doppler Radar (38), Ultra-Wideband Radar (39, 40), or thermography (41). In spite of varying success that these technologies have obtained, they share some common problems in that they all require expensive and specialized hardware. One of the most recent and promising non-contact techniques is the camera-based photoplethysmography (PPG) of sensing the blood volume pulse (BVP) through variations in reflected light (42). Its ease of use, low cost, and convenience make it a tantalizing prospect that would enhance the delivery of primary health care for infants.
There have been many attempts made to develop an ambient light-based approach, utilizing normal ambient light as the source of light (43–51). The ability to accurately measure cardiovascular parameters – namely, HR and HRV—via a simple consumer-level digital camera with normal ambient light as the light source has been demonstrated on healthy adult humans in controlled environments for short periods of time. Many ambient light-based approaches are based on the fact that the red, green, and blue (RGB) channels that are produced by ambient light register the BVP with different relative strengths (46–51). The underlying BVP can be the recovery from a linear combination of RGB channels. Poh et al. exploited this difference to achieve motion robustness by recovering independent source signals from a linear combination of RGB channels using ICA (46, 47). The linear combination coefficients could be estimated by maximizing the non-Gaussianity within ICA output components. It has been demonstrated that utilizing ICA could further attenuate the motion artifact and improve the estimation accuracy of BVP signals, when compared with using the raw green channel without ICA. Lewandowska and Nowak later utilized PCA instead of ICA to discover the linear combinations of RGB channels (48). De Haan and Jeanne proposed a chrominance-based approach that constructs two orthogonal chrominance signals from the linear combination of RGB color channels (49). It is superior in the SNR in the presence of periodic motion artifacts. More recently, McDuff et al. moved a step forward through employing a novel five-band camera with red, green, blue, cyan, and orange color channels (51), which provides higher flexibility in the number of source signals for blind source separation methods, like PCA and ICA. The authors showed that the orange channel helps boost the measurement performance and the GCO channel combination outperforms the RGB channels showing the best performance in HR and HRV measurement. Regarding distance, a measureable distance up to 3 m was observed with GCO channel combination.
There are also some papers in the literature that reported a camera-based estimation of HR in the neonatal intensive care unit (NICU) with the normal NICU lighting as the illumination source (52–55). Scalise et al. demonstrated for the first time the feasibility of camera-based PPG for the NICU on seven infants (52). An algorithm based on ICA was used in this study to measure HR on a 30-s video with a webcam 20 cm away from the face. Aarts et al. studied 19 infants in the NICU in both the USA and the Netherlands (53). Recordings were up to 5 min with the camera at approximately 1 m from the infants. A good result, which is defined as a HR obtained from fast Fourier transform (FFT) of the green channel signal matching control in >90% of the time, has derived in 13 out of 19 infants. Villarroel et al. continuously recorded two preterm infants using a digital video camera for nearly a total of 40 h in the high-dependency care area of the NICU in Oxford (54). The authors have shown that continuous estimating of HR, RR, and changes in SpO2 with an accuracy that is clinically useful can be achieved with their chrominance-based algorithms (50). Episodes of bradycardia (decrease of HR) accompanied by a major desaturation, as a result of the immaturity of the cardiorespiratory system, can be identified. The Xerox Innovation Group – PARC monitored eight neonates with gestational ages of 37–40 weeks in the NICU (55). Video data from the infants’ chests and faces were recorded for 30 min each, with a camera positioned at a distance of ~3 feet. Mean bias of 2.52 bpm and 95% limits of agreement of ±5.48 bpm, which are close to medical standards, were obtained.
Regardless of how successful these ambient-light-based methods have been in acquiring physiological parameters, a key limitation regarding the utilization of ambient light as the illumination source is that they cannot work when not enough ambient light is available, such as during nighttime. However, the ability to function in low light or without visible light is particularly intriguing for the study of SIDS since the SIDS events happen exclusively during sleep and often in dimly lit rooms. To conquer this limitation, we have developed a single-channel based algorithm (56) that can isolate multiple underlying components using only the temporal information inherent in the single-channel recordings. Therefore, our method lends itself well to applications where multichannel images are not available or are undesirable, such as during nighttime. Figure 1 provides an overview of our approach. At the heart of our method is to perform ICA on the embedding matrix that is constructed out of a series of delay vectors from the single-channel recordings. In our initial study, we demonstrated non-contact measurements of HR for both day and night conditions on 15 adults and a 1-month-old infant while the baby is asleep (56). We mimicked the night in a dark room with a near-infrared LED serving as a source of illumination. We have now begun a clinical study with infants at Shanghai Children’s Medical Center in China. Our single-channel method that has the potential to automatically detect the onset of devastating events, such as SIDS, at night and promptly alert parents, family members, or medical staff will be of significant value to patients and family. It can also improve efficiency and reduce medical and legal costs for hospitals and society.
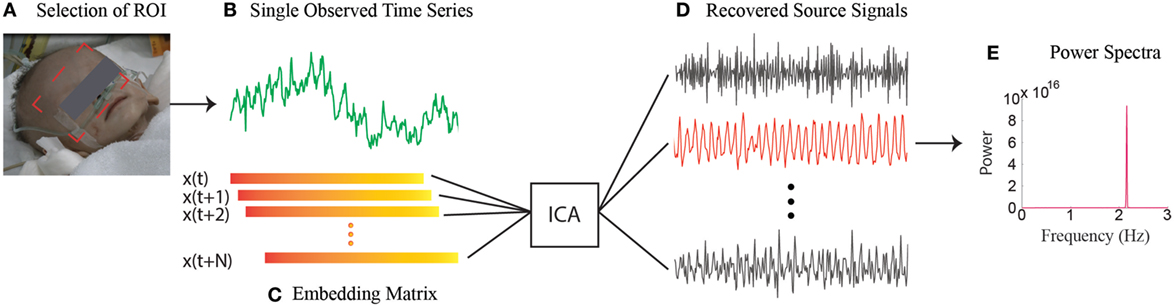
Figure 1. Overview of the single-channel based approach. (A) Selection of the ROI. (B) Spatial average of pixels in ROI over time yields a raw single-channel signal. (C) Construction of the embedding matrix by a number of consecutive delay vectors from the raw single-channel signal. (D) Recovery of source signals by implementing ICA on embedding matrix. (E) The power spectra of the selected source signal.
There is a lack of study that could provide evidence as to whether or not home monitoring could provide warning in time for intervention—or even if intervention would prevent the unexpected death. One of the unanswered questions regarding home monitoring is how to identify the infants who may be at risk for SIDS to be monitored. Evidence that siblings of SIDS victims and infants who have had episodes of extreme apnea, bradycardia, or an ALTE are at any increased risk for SIDS is inconclusive and inadequate. Furthermore, false alarms can occur when the sensors inadvertently are shifted out of position due to infant’s moving in the bed. Frequent false alarms may increase parents’ depression and hostility. Moreover, widespread adoption of home monitoring has been limited by the cost associated with purchasing the device and the low utilization coefficient by the parents (who may unwilling to attach the special designed sensors on their baby). With the emergence of camera-based, non-contact technologies, the camera integrated in mobile phones, tablets, or notebooks could easily double as a heart-rate monitor. In particular, the HR measurement function could also be readily integrated in infant or home-security monitoring systems. The non-contact nature makes the camera-based technology an ideal mean for HR and HRV monitoring for infants. Given the evidence that a reduction of HRV and progressive decrease in HR has been observed in infants who have later succumbed to SIDS, a camera-based, non-contact vital-sign monitoring technology, which can indicate abnormal changes or a sudden loss of vital signs in a timely manner, may enable a crucial and low-cost means for the early prevention of SIDS in newborn infants.
FZ, ZJ, and JZT conceived the idea. FZ, ML, and ZL wrote the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We greatly appreciate the help of Sandra Jackson for proofreading and editing.
This work was supported by funds from the International S&T Cooperation Program of China (ISTCP) (No. S2015ZR1146).
1. Kelly DH, Golub H, Carley D, Shannon DC. Pneumograms in infants who subsequently died of the sudden infant death syndrome. J Pediatr (1986) 109:249–54. doi:10.1016/S0022-3476(86)80380-8
2. Franco P, Szliwowski H, Dramaix M, Kahn A. Polysomnographic study of the autonomic nervous system in potential victims of sudden infant death syndrome. Clin Auton Res (1998) 8:243–9. doi:10.1007/BF02277969
3. Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP, et al. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res (1992) 31:606–12. doi:10.1203/00006450-199206000-00014
4. Schwartz PJ, Stramba-Badiale M, Segantini A, Austoni P, Bosi G, Giorgetti R, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med (1998) 338:1709–14. doi:10.1056/NEJM199806113382401
5. Franco E, Dias A, Teresa D, Hebert K. EKG pattern of brugada syndrome and sudden infant death syndrome – is it time to review the diagnostic criteria? Case report and review of literature. Ann Noninvasive Electrocardiol (2014) 19:198–202. doi:10.1111/anec.12086
6. Franco P, Verheulpen D, Valente F, Kelmanson I, de Broca A, Scaillet S, et al. Autonomic responses to sighs in healthy infants and in victims of sudden infant death. Sleep Med (2003) 4:569–77. doi:10.1016/S1389-9457(03)00107-2
7. Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res (1999) 45:350–4. doi:10.1203/00006450-199903000-00010
8. Kelly DH, Pathak A, Meny R. Sudden severe bradycardia in infancy. Pediatr Pulmonol (1991) 10:199–204. doi:10.1002/ppul.1950100312
9. Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics (1994) 93:44–9.
10. Poets CF, Southall DP. Prone sleeping position and sudden infant death. N Engl J Med (1993) 329:425–6. doi:10.1056/NEJM199308053290610
11. Haas JE, Taylor JA, Bergman AB, van Belle G, Felgenhauer JL, Siebert JR, et al. Relationship between epidemiologic risk factors and clinicopathologic findings in SIDS. Pediatrics (1993) 91:106–12.
12. Hilton JMN. The pathology of sudden infant death syndrome. In: Mason JK, editor. Paediatric Forensic Medicine and Pathology. London: Chapman (1998). p. 156–64.
13. Byard RW, Krous HF. Specific pathologic problems and possible solutions. In: Byard RW, editor. Sudden Infant Death Syndrome: Problems, Progress and Possibilities. London: Hodder Arnold (2001). p. 230–1.
14. Poets CF. Status thymico-lymphaticus, apnea, and sudden infant death-lessons learned from the past? Eur J Pediatr (1996) 155:165–7. doi:10.1007/BF01953930
15. Colvin JD, Collie-Akers V, Schunn C, Moon RY. Sleep environment risks for younger and older infants. Pediatrics (2014) 134:e406–12. doi:10.1542/peds.2014-0401
16. Yiallourou SR, Sands SA, Walker AM, Horne RS. Baroreflex sensitivity during sleep in infants: impact of sleeping position and sleep state. Sleep (2011) 34:725–32. doi:10.5665/SLEEP.1036
17. Jean-Louis M, Anwar M, Rosen H, Craelius W, Hiatt M, Hegyi T. Power spectral analysis of heart rate in relation to sleep position. Biol Neonate (2004) 86:81–4. doi:10.1159/000077782
18. Ariagno RL, Mirmiran M, Adams MM, Saporito AG, Dubin AM, Baldwin RB. Effect of position on sleep, heart rate variability, and QT interval in preterm infants at 1 and 3 months’ corrected age. Pediatrics (2003) 111:622–5. doi:10.1542/peds.111.3.622
19. Galland BC, Taylor BJ, Bolton DP, Sayers RM. Heart rate variability and cardiac reflexes in small for gestational age infants. J Appl Physiol (2006) 100:933–9. doi:10.1152/japplphysiol.01275.2005
20. Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. The effect of gestational age at birth on post-term mMaturation of heart rate variability. Sleep (2015) 38:1635–44. doi:10.5665/sleep.5064
21. Browne CA, Colditz PB, Dunster KR. Infant autonomic function is altered by maternal smoking during pregnancy. Early Hum Dev (2000) 59:209–18. doi:10.1016/S0378-3782(00)00098-0
22. Franco P, Chabanski S, Szliwowski H, Dramaix M, Kahn A. Influence of maternal smoking on autonomic nervous system in healthy infants. Pediatr Res (2000) 47:215–20. doi:10.1203/00006450-200002000-00011
23. Thiriez G, Bouhaddi M, Mourot L, Nobili F, Fortrat JO, Menget A, et al. Heart rate variability in preterm infants and maternal smoking during pregnancy. Clin Auton Res (2009) 19:149–56. doi:10.1007/s10286-009-0003-8
24. Sovik S, Lossius K, Walloe L. Heart rate response to transient chemoreceptor stimulation in term infants is modified by exposure to maternal smoking. Pediatr Res (2001) 49:558–65. doi:10.1203/00006450-200104000-00019
25. Stanton AN, Scott DJ, Downham MA. Is overheating a factor in some unexpected infant deaths? Lancet (1980) 1:1054–7. doi:10.1016/S0140-6736(80)91499-3
26. Stéphan-Blanchard E, Chardon K, Léké A, Delanaud S, Bach V, Telliez F. Heart rate variability in sleeping preterm neonates exposed to cool and warm thermal conditions. PLoS One (2013) 8:e68211. doi:10.1371/journal.pone.0068211
27. Fox GP, Matthews TG. Autonomic dysfunction at different ambient temperatures in infants at risk of sudden infant death syndrome. Lancet (1989) 2:1065–7. doi:10.1016/S0140-6736(89)91080-5
28. Patural H, Barthelemy JC, Pichot V, Mazzocchi C, Teyssier G, Damon G, et al. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin Auton Res (2004) 14:391–5. doi:10.1007/s10286-004-0216-9
29. Longin E, Gerstner T, Schaible T, Lenz T, König S. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J Perinat Med (2006) 34:303–8. doi:10.1515/JPM.2006.058
30. De Rogalski Landrot I, Roche F, Pichot V, Teyssier G, Gaspoz JM, Barthelemy JC, et al. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Auton Neurosci (2007) 136:105–9. doi:10.1016/j.autneu.2007.04.008
31. McManus KJ. Skin breakdown: risk factors, prevention, and treatment. Newborn Infant Nurs Rev (2001) 1:35–42. doi:10.1053/nbin.2001.22874
32. Afsar FS. Skin care for pretermand termneonates. Clin Exp Dermatol (2009) 34:855–8. doi:10.1111/j.1365-2230.2009.03424.x
33. Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate (2000) 77:69–82. doi:10.1159/000014197
34. Chen W, Bouwstra S, Bambang OS, Feijs L. Intelligent design for neonatal monitoring with wearable sensors. In: Somerset VS, editor. Intelligent and Biosensors. Croatie: InTech (2010). p. 386–410.
35. Lee JS, Heo J, Lee WK, Lim YG, Kim YH, Park KS. Flexible capacitive electrodes for minimizing motion artifacts in ambulatory electrocardiograms. Sensors (Basel) (2014) 14:14732–43. doi:10.3390/s140814732
36. Giovangrandi L, Inan OT, Wiard RM, Etemadi M, Kovacs GT. Ballistocardiography – a method worth revisiting. Conf Proc IEEE Eng Med Biol Soc (2011) 2011:4279–82. doi:10.1109/IEMBS.2011.6091062
37. Humeau A, Buard B, Mahé G, Chapeau-Blondeau F, Rousseau D, Abraham P. Multifractal analysis of heart rate variability and laser Doppler flowmetry fluctuations: comparison of results from different numerical methods. Phys Med Biol (2010) 55:6279–97. doi:10.1088/0031-9155/55/20/015
38. Qiao D, He T, Hu B, Li Y. Non-contact physiological signal detection using continuous wave Doppler radar. Biomed Mater Eng (2014) 24:993–1000. doi:10.3233/BME-130896
39. Higashikaturagi K, Nakahata I, Matsunami I, Kajiwara A. Non-invasive respiration monitoring sensor using UWB-IR. Ultra-Wideband IEEE Int Conf (2008) 1:101–4. doi:10.1109/ICUWB.2008.4653294
40. Lazaro A, Girbau D, Villarino R. Analysis of vital signs monitoring using and IR-UWB radar. Prog Electromagn Res (2010) 100:265–84. doi:10.2528/PIER09120302
41. Garbey M, Sun N, Merla A, Pavlidis I. Contact-free measurement of cardiac pulse based on the analysis of thermal imagery. IEEE Trans Biomed Eng (2007) 54:1418–26. doi:10.1109/TBME.2007.891930
42. Zhao F, Li M, Tsien JZ. Technology platforms for remote monitoring of vital signs in the new era of telemedicine. Expert Rev Med Devices (2015) 12:411–29. doi:10.1586/17434440.2015.1050957
43. Takano C, Ohta Y. Heart rate measurement based on a time-lapse image. Med Eng Phys (2007) 29:853–7. doi:10.1016/j.medengphy.2006.09.006
44. Verkruysse W, Svaasand LO, Nelson JS. Remote plethysmographic imaging using ambient light. Opt Express (2008) 16:21434–45. doi:10.1111/j.1365-2230.2009.03424.x
45. Wu HY, Rubinstein M, Shih E, Guttag J, Durand F, Freeman WT. Eulerian video magnification for revealing subtle changes in the world. ACM Trans Graph (2012) 31:1–8. doi:10.1145/2185520.2185561
46. Poh MZ, McDuff DJ, Picard RW. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt Express (2010) 18:10762–74. doi:10.1364/OE.18.010762
47. Poh MZ, McDuff DJ, Picard RW. Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans Biomed Eng (2011) 58:7–11. doi:10.1109/TBME.2010.2086456
48. Lewandowska M, Nowak J. Measuring pluse rate with a webcam – a non-contact method for evaluation cardiac activity. J Med Image Health Informat (2012) 2:87–92. doi:10.1166/jmihi.2012.1064
49. De Haan G, Jeanne V. Robust pulse rate from chrominance-based rPPG. IEEE Trans Biomed Eng (2013) 60:2878–86. doi:10.1109/TBME.2013.2266196
50. Tarassenko L, Villarroel M, Guazzi A, Jorge J, Clifton DA, Pugh C. Non-contact video-based vital sign monitoring using ambient light and auto-regressive models. Physiol Meas (2014) 35:807–31. doi:10.1088/0967-3334/35/5/807
51. McDuff DJ, Gontarek S, Picard RW. Improvements in remote cardiopulmonary measurement using a five band digital camera. IEEE Trans Biomed Eng (2014) 61:2593–601. doi:10.1109/TBME.2014.2323695
52. Scalise L, Bernacchia N, Ercoli I, Marchionni P. Heart rate measurement in neonatal patients using a webcamera. IEEE Int Symp on Medical Measurements and Applications Proc. (2012). 2012:1–4.
53. Aarts LA, Jeanne V, Cleary JP, Lieber CA, Nelson JS, Bambang-Oetomo S, et al. Non-contact heart rate monitoring utilizing camera photoplethysmography in the neonatal intensive care unit – a pilot study. Early Hum Dev (2013) 89:943–8. doi:10.1016/j.earlhumdev.2013.09.016
54. Villarroel M, Guazzi A, Jorge J, Davis S, Watkinson P, Green G, et al. Continuous non-contact vital sign monitoring in neonatal intensive care unit. Healthc Technol Lett (2014) 1:87–91. doi:10.1049/htl.2014.0077
55. Mestha LK, Kyal S, Xu B, Lewis LE, Kumar V. Towards continuous monitoring of pulse rate in neonatal intensive care unit with a webcam. Conf Proc IEEE Eng Med Biol Soc (2014) 2014:3817–20. doi:10.1109/EMBC.2014.6944455
Keywords: SIDS, prevention, non-contact monitoring, vital signs, camera, video
Citation: Zhao F, Li M, Jiang Z, Tsien JZ and Lu Z (2016) Camera-Based, Non-Contact, Vital-Signs Monitoring Technology May Provide a Way for the Early Prevention of SIDS in Infants. Front. Neurol. 7:236. doi: 10.3389/fneur.2016.00236
Received: 05 June 2016; Accepted: 09 December 2016;
Published: 23 December 2016
Edited by:
David Gozal, University of Chicago, USAReviewed by:
Karl Æ. Karlsson, Reykjavík University, IcelandCopyright: © 2016 Zhao, Li, Jiang, Tsien and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zhao, ZnpoYW9AYXVndXN0YS5lZHU=;
Zhaohui Lu, bHV6aGFvaHVpQHNjbWMuY29tLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.