
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 15 November 2016
Sec. Neurotrauma
Volume 7 - 2016 | https://doi.org/10.3389/fneur.2016.00199
This article is part of the Research TopicKarolinska Institutet 200-Year Anniversary Symposium on Injuries to the Spinal Cord and Peripheral Nervous System - An Update on Recent Advances in Regenerative NeuroscienceView all 10 articles
NG2 cells, also known as oligodendrocyte progenitor cells, are located throughout the central nervous system and serve as a pool of progenitors to differentiate into oligodendrocytes. In response to spinal cord injury (SCI), NG2 cells increase their proliferation and differentiation into remyelinating oligodendrocytes. While astrocytes are typically associated with being the major cell type in the glial scar, many NG2 cells also accumulate within the glial scar but their function remains poorly understood. Similar to astrocytes, these cells hypertrophy, upregulate expression of chondroitin sulfate proteoglycans, inhibit axon regeneration, contribute to the glial-fibrotic scar border, and some even differentiate into astrocytes. Whether NG2 cells also have a role in other astrocyte functions, such as preventing the spread of infiltrating leukocytes and expression of inflammatory cytokines, is not yet known. Thus, NG2 cells are not only important for remyelination after SCI but are also a major component of the glial scar with functions that overlap with astrocytes in this region. In this review, we describe the signaling pathways important for the proliferation and differentiation of NG2 cells, as well as the role of NG2 cells in scar formation and tissue repair.
Many oligodendrocytes are lost after contusive spinal cord injury (SCI) (1, 2), leaving axons demyelinated and impairing proper conduction of action potentials (3–6). Although new remyelinating oligodendrocytes are formed after SCI (7–11), normal levels of myelination are not achieved (6). Pre-existing oligodendrocytes do not contribute to remyelination (12); however, NG2 cells, also known as oligodendrocyte progenitor cells (OPCs), are ubiquitously distributed throughout the central nervous system (CNS) and are capable of differentiating into oligodendrocytes in the adult CNS (13). Thus, targeting their proliferation and differentiation is an appealing target to promote remyelination after CNS injury. NG2 cells are present in increased numbers surrounding the lesion site (2, 7, 8, 14), and many studies have investigated the mechanisms underlying their differentiation into oligodendrocytes and their contribution to remyelination (15–17).
However, a large number of NG2 cells that do not differentiate into oligodendrocytes are present within the glial scar, which has been traditionally synonymous with reactive astrocytes. Interestingly, similar to astrocytes, these NG2 cells hypertrophy and upregulate expression of chondroitin sulfate proteoglycans (CSPGs) after CNS injury (18). In fact, NG2 is itself a CSPG (gene name is cspg4) and can inhibit axon growth in vitro (19). Interestingly, NG2 cells have the capacity to differentiate into astrocytes at the CNS injury site, as discussed in more detail below. Thus, NG2 cells are potentially a major contributor to the axon regeneration inhibition by the glial scar.
In addition to their role in axon growth inhibition, NG2 cells may share other properties with astrocytes. For example, astrocytes play an important role in preventing the spread of infiltrating leukocytes, and their ablation leads to increased neuron and oligodendrocyte loss (20, 21). Astrocytes also play a major role in the immune response after contusive SCI through secretion of pro-inflammatory cytokines and chemokines (22, 23). In this review article, we will discuss methods of investigating NG2 cells in the context of SCI, the mechanisms underlying the proliferation of NG2 cells after SCI, as well as their contribution to the glial scar including axon regeneration, wound healing and inflammation.
Figure 1 depicts a diagram of the cellular reactions after contusive SCI in mice. Differences between mice, rat, and human SCI will be addressed where appropriate. In the uninjured spinal cord, astrocytes, oligodendrocytes, and NG2 cells are located throughout the parenchyma (Figure 1A). Contusive SCI leads to large scale death of neurons and glia at the site of injury, shearing of ascending and descending axons, and damage to the vasculature. This damage leads to large-scale hemorrhage at the site of the lesion, which leads to the release of factors that contribute to the immune response, and responses from resident glia (24, 25). Microglia reacts within hours after injury by accumulating around the lesion site and secreting pro-inflammatory cytokines and chemokines that which contribute to the immune response (26). While NG2 cells have been shown to proliferate and migrate short distances toward the lesion site after laser induced injury (27), their migration capacity has not been investigated in more clinically relevant traumatic injuries. Astrocytes also proliferate, hypertrophy, and upregulate expression of glial fibrillary acidic protein (GFAP), and secrete cytokines, chemokines, growth factors, and CSPGs (28). Increased inflammation leads to secondary damage to neurons and oligodendrocytes, as well as axonal dieback characterized by dystrophic endings (1, 29) (Figures 1B–D). Myelin debris and CSPGs, both inhibitory to axon regeneration, accumulate in the lesion core and the glial scar. Hematogenous macrophages start to infiltrate the lesion (30, 31) and attract perivascular fibroblasts that separate from blood vessels and form the fibrotic scar (32, 33) peaking in density by 7 days after SCI. By 14 days after SCI, the scar has started to mature and form tight borders between the glial and fibrotic components of the scar (20, 21, 33) (Figure 1E). (At around this time in rats and humans, a fluid-filled cavity starts to form in parts of the fibrotic scar, whereas in mice, the fibrotic scar contracts slightly over time.) The formation of this scar is dependent on the interactions between CNS cells, namely microglia, NG2 cells, and astrocytes, with non-CNS cells, namely hematogenous macrophages and fibroblasts. In human SCI, astrocytes and NG2 cells were readily detected in the glial scar, and macrophages in the lesion core, within days after SCI (34). Understanding their individual contributions to scar formation is essential for designing both regenerative and neuroprotective therapies for SCI. In this review, we will focus primarily on the role of NG2 cells in the context of the glial scar formation after SCI.
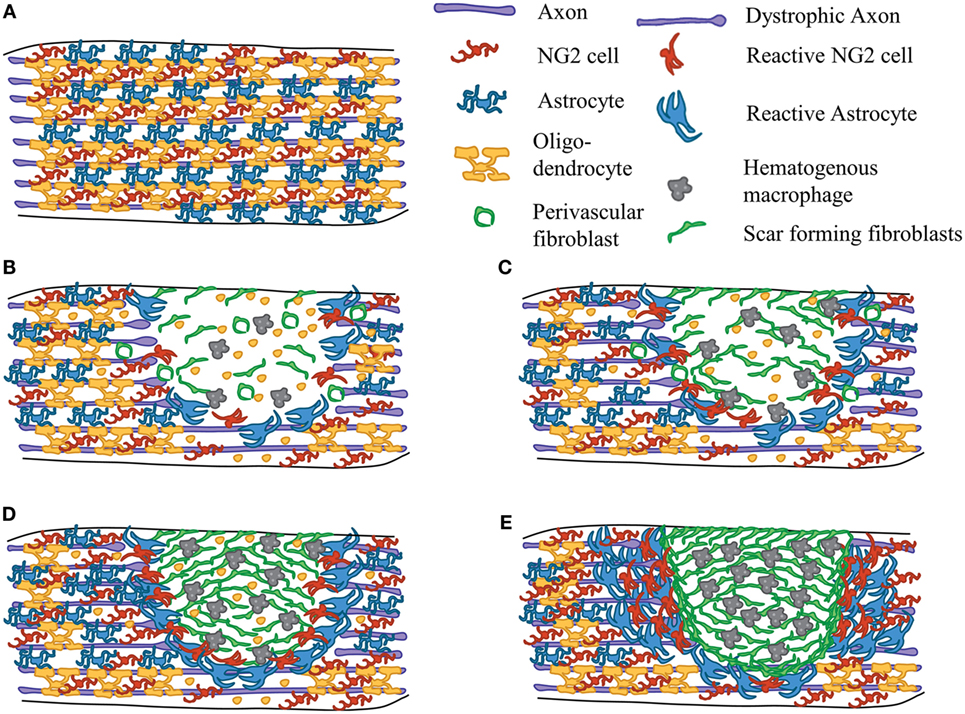
Figure 1. Scar formation after SCI. Diagram depicting the events of scar formation after contusive SCI in mice. Astrocytes (blue), NG2 cells (red), and myelinating oligodendrocytes (yellow) in the uninjured spinal cord white matter (A). Early after SCI, cell death occurs within the lesion site and axons are damaged. Microglia (not shown) and astrocytes respond by secreting cytokines and chemokines. NG2 cells react and proliferate around the lesion site. Macrophages (gray) begin to infiltrate the lesion core and perivascular fibroblasts (green) begin to delaminate from blood vessels (B). Inflammation causes secondary death of oligodendrocytes and neurons leading to accumulation of myelin debris in the injury site (C). Macrophage and fibroblast density peaks at 7 days after SCI (D). By 2 weeks after SCI, the scar has matured. There are tight borders between the fibrotic scar (consisting of fibroblasts and macrophages) and the glial scar (consisting of astrocytes, NG2 cells, and microglia) (E). The relative number of cells may not accurately reflect actual in vivo pathology.
Proper understanding of NG2 cells after SCI requires proper understanding of the tools that have been used to study them, namely antibodies and transgenic mouse lines. In the uninjured brain and spinal cord, antibodies against NG2 and PDGFRα (platelet-derived growth factor receptor alpha) label NG2+ glia, but NG2 antibodies also label pericytes that express this CSPG (35–37) (Table 1). After CNS injury, NG2 expression is upregulated at the injury site (18), but many cells including pericytes, non-myelinating Schwann cells, and macrophages also express NG2 (38, 39) (Table 1), making the use of the NG2 antibody alone insufficient to definitively identify NG2+ glial cells. Similarly, PDGFRα antibodies can label fibroblasts, rather than NG2 glia, at the injury site (39) (Table 1). Since cells that are NG2+ macrophages, NG2+ pericytes, or PDGFRα+ fibroblasts are often counted as NG2 glia, the use of these antibodies as sole markers for NG2 glia has led to the misconception that NG2 glia are located within the injury core (GFAP-negative region) and has mostly likely contributed to the highly variable reports of NG2 cell density across different studies (40). For the remainder of this review, our use of NG2 cells refers to NG2+ glia (and not pericytes), and we use these two terms along with OPC interchangeably. Antibodies against Olig2 has also been used to identify NG2 cells, but they also label mature oligodendrocytes, and several reports have shown that a small population of protoplasmic astrocytes (10, 41) and reactive astrocytes can also express the transcription factor Olig2 after CNS injury (42, 43) (Table 1). Thus, co-labeling with NG2 and Olig2 antibodies may be the best method of histologically detecting NG2 cells after SCI.
Prior to the advent of genetic fate mapping using transgenic mice expressing cell type-specific Cre recombinase, several attempts to understand the fate of NG2 cells after SCI were made. One of the first attempts to study the fate of NG2 cells after SCI utilized a Mahoney retrovirus with reporter expression driven by the NG2 promoter (9). Injection of this virus into the injury site-labeled dividing NG2 cells, however, due to the fact that it was administered after SCI, it also labeled a large number of macrophages (since some of them upregulate NG2 as discussed above) (39, 44). This study also reported that a high percentage of NG2 cells differentiate into GFAP+ astrocytes (35–50%); however, this could have included astrocytes that upregulated NG2 after SCI. Shortly after, Lytle et al. (43) used the CNP-EGFP mice (which labels 2′,3′-cyclic-nucleotide 3′-phosphodiesterase+ NG2 cells and oligodendrocytes) to determine the response of NG2 cells after contusive SCI and reported that a large population of NG2+ cells were EGFP−. This could have been due to CNPase only being expressed in NG2 cells that have already committed to the oligodendrocyte lineage since CNPase is expressed later than PDGFRα and NG2 during development (45, 46). However, it is also possible that scar forming NG2 cells downregulate CNPase expression after injury. Together, these results suggest that NG2 glia comprise both myelinating cells as well as non-myelinating, scar forming cells after contusive SCI.
Transgenic mice expressing tamoxifen-inducible Cre under cell-specific promoters (Cre-ER mice) have been particularly useful for studying fate of NG2 cells after SCI. Although PDGFRα-CreER (13, 47), NG2-CreER (48), and Olig2-CreER (41) mice have been used extensively to either fate map and/or conditionally delete genes in NG2 cells, each mouse line has its advantages and disadvantages. The NG2-CreER mouse line has a recombination efficiency of about 30–40% of NG2 cells (48, 49), while the PDGFRα-CreER has a recombination efficiency of over 90% (47). Low recombination efficiency is often desirable for lineage tracing studies, while high recombination efficiency is often desirable for functional studies. Similar to the limitations of antibodies as discussed above, these transgenic lines label cells other than NG2 glia. The NG2-CreER mice label pericytes (49, 50) whereas the PDGFRα-CreER mice label fibroblasts at the injury site (unpublished observations), and the Olig2-CreER mice label oligodendrocytes as well as a small population of astrocytes (10, 51). Although we did not observe contributions of NG2+ pericytes to scar formation after SCI in mice, it is possible that experimental manipulations (drugs, viruses, or genes deletions) could induce them to contribute to scar formation (49). Thus, these off-target labeling must be carefully considered when interpreting any NG2 cell genetic fate mapping studies involving these mouse lines.
One possible solution to circumvent these technical hurdles is to combine genetic labeling of NG2 cells with antibody co-labeling. Genetically labeled cells in NG2-CreER mice can be co-labeled with Olig2 or PDGFRβ antibody to distinguish NG2 cells from pericytes/fibroblasts respectively. Alternatively, instead of using Rosa26 reporter mice, Olig2 promoter-driven reporter mice can be used in combination with NG2-CreER or PDGFRα-CreER mice, which would label NG2 glia without labeling pericytes. This Olig2 strategy can be used to express not only fluorescent reporters but also proteins such as diphtheria toxin receptor (52) that can be used to probe the function of NG2 cells more specifically. However, such Olig2 reporter mice have not yet been reported in the literature.
NG2 cells have been shown to react to CNS injuries such as traumatic brain injury (18), demyelination (53), and contusive SCI (2). This response is reminiscent of astrocyte reactivity as they surround the lesion site and hypertrophy (18). Whereas NG2 cells are normally evenly dispersed throughout the spinal cord (13) and maintain territories due to the dynamic filopodia being repulsed by neighboring NG2 cells (27), their processes become intertwined as they form the glial scar. Two-photon live imaging has revealed NG2 cells react to laser injury by migrating only short distances toward the lesion (27), suggesting that the large number of NG2 cells at the injury site is most likely due to local proliferation rather than migration. In fact, the percentage of proliferating NG2 cells is increased sixfold (2) and NG2 cells comprise nearly one half of bromodeoxyuridine (BrdU)-labeled cells, 3 days after SCI (7). This is most likely an underestimate since it does not account for NG2 cells that differentiated into oligodendrocytes and/or astrocytes after injury. Overall, these data suggest that NG2 cells have a significant capacity to proliferate after SCI.
As NG2 cells differentiate into oligodendrocytes, they lose expression of the NG2 antigen. NG2 cells are capable of differentiating directly into oligodendrocytes without cell division (27), but they often differentiate after division, where one or both differentiate into oligodendrocytes within 6–8 days. This represents a critical window where their fate after proliferation can be determined by the microenvironment of the injury site (48, 54). For example, myelin damage can accelerate and promote NG2 cell differentiation into oligodendrocytes (54). Sensory deprivation induced by whisker clipping can reduce oligodendrogenesis after NG2 cell division, suggesting that neuronal activity promotes differentiation of NG2 cells into oligodendrocytes (54). Conversely, optogenetic stimulation of neurons can increase the proliferation of NG2 cells and their subsequent differentiation into oligodendrocytes (55). This raises the possibility that the myelin and neuronal damage after SCI may create an environment that significantly influences NG2 cell differentiation.
Several factors important for proliferation and differentiation of NG2 cells are upregulated after SCI. These include fibroblast growth factor 2 (FGF2) (56, 57), glial growth factor 2 (GGF2) (58, 59), and Wnts (60). FGF2 is a potent mitogen for NG2 cells in vitro (56). Deletion of FGFR1 and FGFR2 in NG2 cells reduces oligodendrogenesis and remyelination chronically after cuprizone-induced demyelination (61). FGF2 is increased for at least a month after SCI (57) and intraspinal injection of FGF2 (62) was shown to improve functional recovery after SCI. GGF has been shown to increase the proliferation of NG2 cells while inhibiting their differentiation in vitro (63). Subcutaneous injection of GGF2 increases NG2 cell proliferation, oligodendrogenesis, and functional recovery after SCI (59) as well as increased functional recovery and myelination after experimental autoimmune encephalomyelitis (EAE) (64). Wnts have been shown to play a major role in proliferation of NG2 cells during development (65) and are upregulated after SCI (60). Overexpression of activated β-catenin, a downstream mediator of Wnt signaling, results in developmental hypomyelination and delayed remyelination after demyelination (65). Wnt3A-conditioned media increases proliferation of NG2 cells in vitro and deletion of β-catenin in NG2 cells leads to reduced proliferation of NG2 in the glial scar after contusive SCI (66).
Cytokines such as ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), and tumor necrosis factor (TNF) are also important in the proliferation and differentiation of NG2 cells (67). CNTF and LIF promote oligodendrocyte differentiation in vitro (67), however, CNTF knockout (68) and LIF knockout mice (69) only have a developmental delay in oligodendrogenesis, indicating that these factors may be a mediator of oligodendrogenesis early in development. Daily intraperitoneal administration of CNTF increases numbers of NG2 cells, oligodendrocytes, and neurons and improves outcome after EAE (70). Deletion of the transcription factor signal transducer and activator of transcription 3 (STAT3), which is downstream of CNTF, LIF, and several other cytokines, delays oligodendrogenesis without affecting proliferation after SCI (49). Accordingly, overexpression of a constitutively active STAT3 using an adenovirus leads to increased oligodendrocyte differentiation in vitro (71). Deletion of suppressor of cytokine signaling 3 (SOCS3), a negative regulator of STAT3, leads to enhanced proliferation of NG2 cells in the glial scar but does not affect their differentiation after SCI, suggesting a non-canonical STAT3/SOCS3 signaling mechanism in NG2 cells after SCI (49). Although the proinflammatory cytokine TNFα is typically associated with oligodendrocyte death, it may have important roles in NG2 cell response to injury and subsequent remyelination. TNFα signaling through TNFR2 is important for NG2 cell proliferation and differentiation after cuprizone-induced demyelination (72). Similarly, genetic deletion of TNFR2 using the CNPase-Cre mouse resulted in impaired functional recovery, reduced number of NG2 cells, and impaired oligodendrocyte differentiation and remyelination after EAE (73). While similar genetic studies have not been performed after SCI, pharmacological blockade of TNFR1, but not TNFR2, promotes functional recovery SCI (74).
Both NG2 cells and astrocytes are derived from radial glia during development (75, 76). In addition, NG2 cells isolated in vitro differentiate into astrocytes as well as oligodendrocytes (77). Thus, while NG2 cells differentiate only into oligodendrocytes in the normal CNS, these observations provide a mechanistic basis for the potential of NG2 cells to differentiate into astrocytes in the injured CNS. The advent of Cre-loxP technology has allowed rigorous testing of NG2 cell lineage plasticity in vivo. The NG2-Cre mouse line revealed that, indeed, a population of NG2 cells could differentiate into protoplasmic astrocytes in the ventrolateral forebrain gray matter (78) and spinal cord (79) during development. As mentioned above, Sellers et al. injected Mahoney retrovirus with a reporter driven by the NG2 promoter into the injured spinal cord, and found that 35–54% of reporter-labeled cells were GFAP+ astrocytes (limitations of this technique is discussed above).
Using CreER mice to permanently label a population of NG2 cells prior to injury has led to similar results. The NG2-CreER and PDGFRα-CreER mouse lines both revealed that NG2 cells can only differentiate into oligodendrocytes in the uninjured adult CNS (13, 47, 48). To determine if NG2 cells had lineage plasticity after injury, the Olig2-CreER mice were used in cortical stab injury (41) and dorsal hemisection SCI (10). However, due to the fact that 5% of labeled cells were GFAP+ in the uninjured condition, it was difficult to determine if NG2 cells had astroglial fate. Using the NG2-CreER mice in which astrocytes are not labeled in the uninjured spinal cord, 8% of NG2 cells expressed GFAP at 10 days post cortical stab injury (80). Since the percentage of reporter-labeled cells that co-localized with GFAP decreased to 2% by 30 days after injury and many cells retained NG2 expression, it has been suggested that these NG2 cells transiently express GFAP after injury and that NG2 cell-derived astrocytes are not major contributors to the astroglial scar (80). However, after contusive SCI where inflammation, secondary damage, and astrogliosis is much greater than stab wounds, 25% of reporter-labeled cells in the NG2-CreER mice expressed GFAP at 1 week after SCI and 8% by 4 weeks after injury (49).
Possible mechanisms by which NG2 cells differentiate into astrocytes after SCI could be similar to the mechanisms underlying astrogliogenesis during development. These include the Janus kinase (JAK)/STAT3 (81), bone morphogenetic protein (BMP) (82), and/or Olig2 signaling pathways (83, 84). BMP2 and BMP4 are known to promote astrogliogenesis from NG2 cells in vitro (85). Both BMP2 and BMP4 are upregulated after SCI (86), and intraspinal injection of BMP4 leads to increased differentiation of transplanted NG2 progenitors into GFAP+ astrocytes (9). When NG2 cells are treated with conditioned media from reactive astrocytes isolated from injured spinal cords, it reduces their differentiation into O1+ oligodendrocytes and increases their expression of GFAP (87), suggesting that the injured spinal cord could provide a niche for NG2 cell differentiation into astrocytes. Astrocytes isolated from the injured spinal cord have increased expression of BMP2/BMP4 compared to uninjured spinal cord astrocytes and BMP2 is increased in reactive astrocyte conditioned media, suggesting that astrocytes are a major source of BMPs after SCI (87). NG2 cells increase expression of the BMP downstream effector Smad after exposure to reactive astrocyte-conditioned media with an associated decrease in MBP and increase in GFAP expression, which is reversed upon treatment with the BMP inhibitor noggin (87). Together, these data suggest that BMPs may be derived from reactive astrocytes and promote NG2 cell differentiation into astrocytes after contusive SCI.
In addition to BMPs, the JAK-STAT3 signaling pathway could also be important in astrogliogenesis from NG2 cells after CNS injury. The JAK-STAT3 signaling pathway is important for astrocyte differentiation from nestin+ cortical precursor cells and STAT3 binds to the GFAP promoter (81, 88). In addition, developmental astrogliogenesis is impaired in LIF knockout mice and gp130 knockout mice (89, 90). However, neither deletion of STAT3 nor its negative regulator SOCS3 significantly affects NG2 cell differentiation into astrocytes after SCI (49). Overexpression of the oligodendrocyte transcription factor Olig2 reduced astrocyte differentiation from neural stem cells in vitro (83) while deletion of Olig2 in developing NG2 cells leads to increased astrocyte production at the expense of oligodendrogenesis and myelination (91). However, genetic deletion of Olig2 does not affect astrogliogenesis from NG2 cells after cortical stab injury (80). Together, these data suggest that NG2 cells might differentiate into astrocytes by a mechanism different from developmental processes.
After contusive SCI, there are many Schwann cells at the injury site (92, 93). Since there are no Schwann cells in the normal spinal cord and since the majority of the myelin protein 0 (P0+) myelinating Schwann cells are located in the dorsal column after SCI, it was thought that these Schwann cells had migrated from the dorsal roots. However, genetic lineage tracing revealed that, after focal demyelination in the dorsal column white matter, the majority of Schwann cells were derived from NG2 cells (94). This is also supported by a recent study in which a dorsal rhizotomy did not lead to a significant decrease in Schwann cells at the SCI site, indicating that the peripheral nervous system (PNS) is not a major source of Schwann cells present at the injury site (95). While there is accumulating evidence that NG2 cells can differentiate into Schwann cells after SCI, there are several issues that need to be carefully considered. First, in addition to dorsal roots, the ventral roots as well as nerve fibers on blood vessels may also serve as sources of Schwann cells (96). Second, unlike the ability of NG2 cells to differentiate into astrocytes in vitro, there have been no reports of NG2 cells differentiating into Schwann cells in vitro. Last, whereas NG2 cells and astrocytes are derived from the neural tube, Schwann cells are derived from the neural crest, thereby making the mechanism by which NG2 cells differentiate into Schwann cells ontogenetically more complex than the mechanism of their differentiation into astrocytes.
Increased CSPG expression is widely considered to be a major inhibitory barrier to axon regeneration after CNS injury. Phosphocan, neurocan, versican, and brevican are all upregulated after SCI (97). While reactive astrocytes are considered a major source of CSPGs (98), NG2 cells have also been shown to secrete versican and neurocan in vitro (99–101). Unlike other CSPGs, NG2 is typically expressed on the cell membrane rather than as a secreted factor. However, its extracellular domain can be shed from the cell surface via cleavage by metalloproteinases (MMPs) (102). Increased expression of NG2 in the glial scar and its ability to inhibit neurite outgrowth in vitro indicate that NG2 cells may be major inhibitors of axon regeneration (19). The NG2 proteoglycan leads to inhibition of cerebellar granule neuron neurite outgrowth even after digestion with chrondroitinase ABC (ChABC), indicating that it is not just the glycosaminoglycan (GAG) side chains but also the core proteoglycan that is inhibitory to axon growth (19). Treatment with intraspinal injection of NG2 neutralizing antibody leads to enhanced regeneration of ascending sensory axons after SCI (103), and long-term delivery of NG2 neutralizing antibody through an osmotic pump improves conduction and functional recovery after SCI (104). Together, these studies suggest that NG2 proteoglycan is inhibitory to axon regeneration.
However, the inhibitory properties of NG2 proteoglycan does not necessarily mean that NG2 cells themselves are inhibitory. Despite the increased levels of NG2, several studies have noted that NG2 cells are often associated with regenerating axons, and similar findings have been reported for astrocytes (105–107). Neonatal hippocampal neurites grow better on NG2 cells than on poly-l-lysine and laminin (PLL), even after overexpressing NG2 using an adenovirus (108). In addition, regenerating axons are observed more frequently in areas of the spinal cord that are NG2+ after SCI (39, 109) and may facilitate axon entry into Schwann cell grafts after SCI (110). Also, cspg4 knockout mice display less serotonergic axons that are able to cross into the lesion (111), as well as increased dieback of sensory axons after SCI (112). In addition, regenerating dorsal root ganglion (DRG) axons associate with NG2-expressing cells after dorsal column crush (112). These data suggest that while NG2 proteoglycan may inhibit axon regeneration, NG2 cells themselves may be permissive to axon growth (108, 112). This is similar to the role of reactive astrocytes where even though their expression of CSPG is inhibitory to axon regeneration, reactive astrocytes themselves may be permissive to, and even necessary for, axon regeneration (105–107). Thus, we must be cautious in classifying cells as inhibitory to axon regeneration based solely on their expression of CSPGs.
While axons may be able to use NG2 cells as a growth-permissive substrate, the fact that regenerating axons can form terminal synaptic contacts with NG2 cells implies that the net effect prevents axonal growth (112–114). The ability of NG2 cells to form synapse with axons has been known for quite some time (115), but its significance in the context of axon regeneration is just beginning to be appreciated. NG2 cells form “synaptic-like” structures with DRG axons in vitro (112), and NG2 cells are associated with dystrophic sensory axons (116). Furthermore, there is presynaptic differentiation of injured sensory axons along the CNS/PNS border after dorsal root crush injury (117). Together, these data indicate that NG2 cells may inhibit axon regeneration by both expression of the inhibitory NG2 proteoglycan as well as formation of synaptic contacts.
Astrocytes contribute to the inflammatory response after CNS injury and attenuating their expression of proinflammatory cytokines and chemokines leads to improved functional outcome (118–123). While astrocytes and microglia have been the focus of neuroinflammatory studies, there is accumulating evidence that NG2 cells may also contribute to the inflammatory response. Genetic deletion of Act1, an activator of NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) via interleukin-17 (IL-17) signaling, in NG2 cells, leads to reduced expression of proinflammatory chemokines, reduced leukocyte infiltration, and improved functional outcome after EAE (124). Upon stimulation in vitro, NG2 cells increase expression of multiple proinflammatory chemokines and cytokines as well as MMPs (124, 125). In addition, NG2 cells upregulate IL1β and C-C motif chemokine ligand 2 (CCL2) after cuprizone demyelination (126). Interestingly, deletion of β-catenin in NG2 cells leads to reduced Iba1+ macrophage/microglia density around the lesion after SCI and also reduced astrogliosis, suggesting that NG2 cells may play a role in attracting macrophages after CNS injury (66). Therefore, these studies raise the possibility that NG2 cells may be a major contributor to inflammation after CNS injury, and future studies need to directly address this possibility, especially in the context of SCI.
The glial scar has been synonymous with reactive astrocytes, but there is substantial evidence indicating that NG2 cells are also a major part of the glial scar, both physically and functionally. Similar to astrocytes, NG2 cells react to SCI by proliferating, becoming hypertrophic, and upregulating CSPG expression (Figure 2). However, unlike astrocytes, NG2 cells can differentiate into other cell types, namely oligodendrocytes, astrocytes, and perhaps even Schwann cells. This lineage plasticity of NG2 cells raise the possibility that they can be targeted to promote endogenous repair of the injured spinal cord. Most NG2 cells remain undifferentiated in the glial scar region, and these NG2 cells contribute to axon regeneration failure by expressing CSPGs and forming synaptic structures that prevent further axonal growth. Similar to astrocytes, NG2 cells may also contribute to neuroinflammation, which remains an area that has been underappreciated in the field (Figure 2). Therefore, NG2 cells share similarities and differences with astrocytes as a part of the glial scar, which present novel mechanisms that may be targeted to promote repair after SCI.
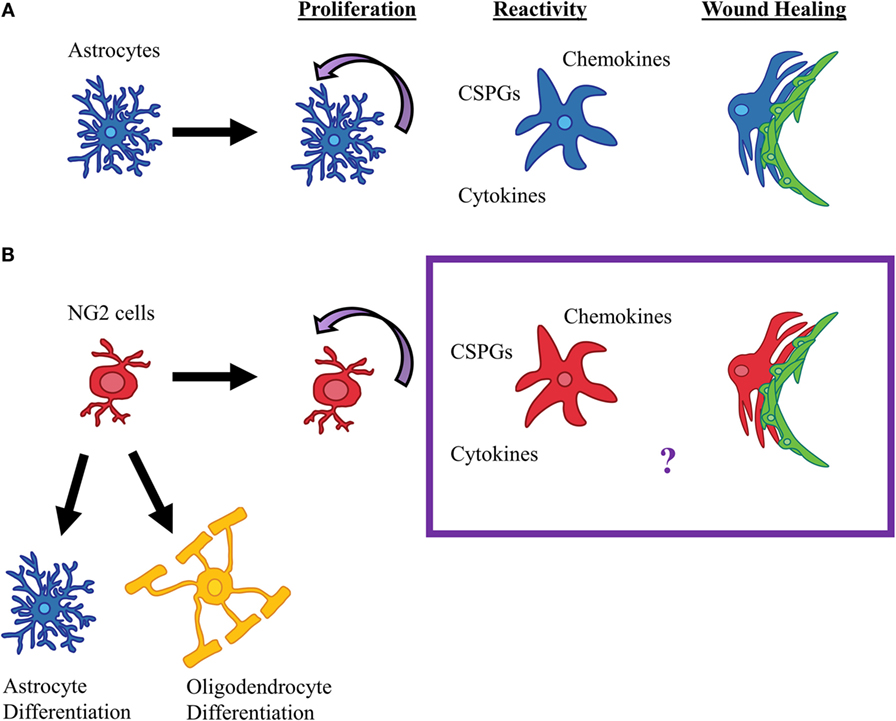
Figure 2. Reactive gliosis after SCI. (A) After SCI in mice and rats, astrocytes proliferate; secrete cytokines, chemokines, and CSPGs; and form glial-fibrotic borders. (B) It is known that NG2 cells proliferate, differentiate into oligodendrocytes and astrocytes, and contribute to scar formation after SCI, however, whether NG2 cells contribute to the glial scar by secreting cytokines or contribute to the wound healing process is currently unknown.
AH and JL selected the content and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Wolfgang Pita-Thomas for helpful comments.
This study was funded by NINDS R01NS081040, R21NS082835, U.S. Army W81XWH131007715, The Miami Project to Cure Paralysis, and the Buoniconti Fund. AH was funded by the Lois Pope LIFE Fellows Program.
1. Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med (1997) 3:73–6. doi:10.1038/nm0197-73
2. McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci (2001) 21:3392–400.
3. Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol (1993) 59:75–89.
4. Cao Q, Zhang YP, Iannotti C, Devries WH, Xu XM, Shields CB, et al. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol (2005) 191(Suppl 1):S3–16. doi:10.1016/j.expneurol.2004.08.026
5. Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol (2005) 192:384–93. doi:10.1016/j.expneurol.2004.11.033
6. Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol (2005) 486:373–83. doi:10.1002/cne.20517
7. Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia (2005) 50:247–57. doi:10.1002/glia.20176
8. Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia (2007) 55:698–711. doi:10.1002/glia.20491
9. Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci (2009) 29:6722–33. doi:10.1523/JNEUROSCI.4538-08.2009
10. Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell (2010) 7:470–82. doi:10.1016/j.stem.2010.07.014
11. Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, et al. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci U S A (2013) 110:4075–80. doi:10.1073/pnas.1210293110
12. Crawford AH, Tripathi RB, Foerster S, Mckenzie I, Kougioumtzidou E, Grist M, et al. Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am J Pathol (2016) 186(3):511–6. doi:10.1016/j.ajpath.2015.11.005
13. Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci (2008) 11:1392–401. doi:10.1038/nn.2220
14. Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci (2007) 25:1711–24. doi:10.1111/j.1460-9568.2007.05390.x
15. Almad A, Sahinkaya FR, Mctigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics (2011) 8:262–73. doi:10.1007/s13311-011-0033-5
16. Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta (2011) 1812:184–93. doi:10.1016/j.bbadis.2010.09.010
17. Franklin RJ, Goldman SA. Glia disease and repair-remyelination. Cold Spring Harb Perspect Biol (2015) 7:a020594. doi:10.1101/cshperspect.a020594
18. Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci (1994) 14:4716–30.
19. Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci (1994) 14:7616–28.
20. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci (2004) 24:2143–55. doi:10.1523/JNEUROSCI.3547-03.2004
21. Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci (2013) 33:12870–86. doi:10.1523/JNEUROSCI.2121-13.2013
22. Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med (2005) 202:145–56. doi:10.1084/jem.20041918
23. Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun (2010) 24:540–53. doi:10.1016/j.bbi.2009.11.007
24. Rhodes KE, Raivich G, Fawcett JW. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience (2006) 140:87–100. doi:10.1016/j.neuroscience.2006.01.055
25. Sahinkaya FR, Milich LM, Mctigue DM. Changes in NG2 cells and oligodendrocytes in a new model of intraspinal hemorrhage. Exp Neurol (2014) 255:113–26. doi:10.1016/j.expneurol.2014.02.025
26. Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol (2014) 7:a020602. doi:10.1101/cshperspect.a020602
27. Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci (2013) 16:668–76. doi:10.1038/nn.3390
28. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci (2004) 5:146–56. doi:10.1038/nrn1326
29. Cregg JM, Depaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol (2014) 253:197–207. doi:10.1016/j.expneurol.2013.12.024
30. Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, et al. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol (2014) 254:109–20. doi:10.1016/j.expneurol.2014.01.013
31. Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol Dis (2015) 74:114–25. doi:10.1016/j.nbd.2014.10.024
32. Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science (2011) 333:238–42. doi:10.1126/science.1203165
33. Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci (2013) 33:13882–7. doi:10.1523/JNEUROSCI.2524-13.2013
34. Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, et al. NG2 and phosphacan are present in the astroglial scar after human traumatic spinal cord injury. BMC Neurol (2009) 9:32. doi:10.1186/1471-2377-9-32
35. Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci (1987) 7:2721–31.
36. Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci (1987) 7:2737–44.
37. Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res (1996) 43:299–314. doi:10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E
38. Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci (2002) 22:2792–803.
39. McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol (2006) 65:406–20. doi:10.1097/01.jnen.0000218447.32320.52
40. Levine J. The reactions and role of NG2 glia in spinal cord injury. Brain Res (2015) 1638(Pt B):199–208. doi:10.1016/j.brainres.2015.07.026
41. Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci (2008) 28:10434–42. doi:10.1523/JNEUROSCI.2831-08.2008
42. Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, et al. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A (2005) 102:18183–8. doi:10.1073/pnas.0506535102
43. Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia (2009) 57:270–85. doi:10.1002/glia.20755
44. Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia (2001) 34:296–310. doi:10.1002/glia.1063
45. Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci (2001) 2:840–3. doi:10.1038/35097593
46. Pozniak CD, Langseth AJ, Dijkgraaf GJ, Choe Y, Werb Z, Pleasure SJ. Sox10 directs neural stem cells toward the oligodendrocyte lineage by decreasing suppressor of fused expression. Proc Natl Acad Sci U S A (2010) 107:21795–800. doi:10.1073/pnas.1016485107
47. Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron (2010) 68:668–81. doi:10.1016/j.neuron.2010.09.009
48. Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development (2011) 138:745–53. doi:10.1242/dev.047951
49. Hackett AR, Lee DH, Dawood A, Rodriguez M, Funk L, Tsoulfas P, et al. STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiol Dis (2016) 89:10–22. doi:10.1016/j.nbd.2016.01.017
50. Honsa P, Pivonkova H, Dzamba D, Filipova M, Anderova M. Polydendrocytes display large lineage plasticity following focal cerebral ischemia. PLoS One (2012) 7:e36816. doi:10.1371/journal.pone.0036816
51. Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, et al. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res (2008) 86:3494–502. doi:10.1002/jnr.21862
52. Birey F, Aguirre A. Age-dependent netrin-1 signaling regulates NG2+ glial cell spatial homeostasis in normal adult gray matter. J Neurosci (2015) 35:6946–51. doi:10.1523/JNEUROSCI.0356-15.2015
53. Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia (1998) 22:161–70. doi:10.1002/(SICI)1098-1136(199802)22:2<161::AID-GLIA7>3.0.CO;2-A
54. Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci (2014) 17:1518–27. doi:10.1038/nn.3815
55. Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science (2014) 344:1252304. doi:10.1126/science.1252304
56. Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol (1992) 118:889–900. doi:10.1083/jcb.118.4.889
57. Tripathi RB, McTigue DM. Chronically increased ciliary neurotrophic factor and fibroblast growth factor-2 expression after spinal contusion in rats. J Comp Neurol (2008) 510:129–44. doi:10.1002/cne.21787
58. Zai LJ, Yoo S, Wrathall JR. Increased growth factor expression and cell proliferation after contusive spinal cord injury. Brain Res (2005) 1052:147–55. doi:10.1016/j.brainres.2005.05.071
59. Whittaker MT, Zai LJ, Lee HJ, Pajoohesh-Ganji A, Wu J, Sharp A, et al. GGF2 (Nrg1-beta3) treatment enhances NG2+ cell response and improves functional recovery after spinal cord injury. Glia (2012) 60:281–94. doi:10.1002/glia.21262
60. Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One (2011) 6:e27000. doi:10.1371/journal.pone.0027000
61. Furusho M, Roulois AJ, Franklin RJ, Bansal R. Fibroblast growth factor signaling in oligodendrocyte-lineage cells facilitates recovery of chronically demyelinated lesions but is redundant in acute lesions. Glia (2015) 63:1714–28. doi:10.1002/glia.22838
62. Kasai M, Jikoh T, Fukumitsu H, Furukawa S. FGF-2-responsive and spinal cord-resident cells improve locomotor function after spinal cord injury. J Neurotrauma (2014) 31:1584–98. doi:10.1089/neu.2009.1108
63. Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron (1996) 17:229–43. doi:10.1016/S0896-6273(00)80155-5
64. Cannella B, Hoban CJ, Gao YL, Garcia-Arenas R, Lawson D, Marchionni M, et al. The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Natl Acad Sci U S A (1998) 95:10100–5. doi:10.1073/pnas.95.17.10100
65. Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev (2009) 23:1571–85. doi:10.1101/gad.1806309
66. Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM. Abrogation of beta-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci (2014) 34:10285–97. doi:10.1523/JNEUROSCI.4915-13.2014
67. Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development (1994) 120:143–53.
68. Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol Cell Neurosci (1996) 8:146–56. doi:10.1006/mcne.1996.0053
69. Ishibashi T, Lee PR, Fields RD. Leukemia inhibitory factor regulates the timing of oligodendrocyte development and myelination in the postnatal optic nerve. J Neurosci Res (2009) 87:3343–55. doi:10.1002/jnr.22173
70. Kuhlmann T, Remington L, Cognet I, Bourbonniere L, Zehntner S, Guilhot F, et al. Continued administration of ciliary neurotrophic factor protects mice from inflammatory pathology in experimental autoimmune encephalomyelitis. Am J Pathol (2006) 169:584–98. doi:10.2353/ajpath.2006.051086
71. Steelman AJ, Zhou Y, Koito H, Kim S, Payne HR, Lu QR, et al. Activation of oligodendroglial Stat3 is required for efficient remyelination. Neurobiol Dis (2016) 91:336–46. doi:10.1016/j.nbd.2016.03.023
72. Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci (2001) 4:1116–22. doi:10.1038/nn738
73. Madsen PM, Motti D, Karmally S, Szymkowski DE, Lambertsen KL, Bethea JR, et al. Oligodendroglial TNFR2 mediates membrane TNF-dependent repair in experimental autoimmune encephalomyelitis by promoting oligodendrocyte differentiation and remyelination. J Neurosci (2016) 36:5128–43. doi:10.1523/JNEUROSCI.0211-16.2016
74. Novrup HG, Bracchi-Ricard V, Ellman DG, Ricard J, Jain A, Runko E, et al. Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice. J Neuroinflammation (2014) 11:159. doi:10.1186/s12974-014-0159-6
75. Molofsky AV, Deneen B. Astrocyte development: a guide for the perplexed. Glia (2015) 63:1320–9. doi:10.1002/glia.22836
76. Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol (2016) 8:a020453. doi:10.1101/cshperspect.a020453
77. Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature (1983) 303:390–6. doi:10.1038/303390a0
78. Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development (2008) 135:145–57. doi:10.1242/dev.004895
79. Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol (2008) 4:19–26. doi:10.1017/S1740925X09000015
80. Komitova M, Serwanski DR, Lu QR, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia (2011) 59:800–9. doi:10.1002/glia.21152
81. Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science (1997) 278:477–83. doi:10.1126/science.278.5337.477
82. Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci (1998) 18:3620–9.
83. Fukuda S, Kondo T, Takebayashi H, Taga T. Negative regulatory effect of an oligodendrocytic bHLH factor OLIG2 on the astrocytic differentiation pathway. Cell Death Differ (2004) 11:196–202. doi:10.1038/sj.cdd.4401332
84. Setoguchi T, Kondo T. Nuclear export of OLIG2 in neural stem cells is essential for ciliary neurotrophic factor-induced astrocyte differentiation. J Cell Biol (2004) 166:963–8. doi:10.1083/jcb.200404104
85. Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci (1997) 17:4112–20.
86. Hampton DW, Asher RA, Kondo T, Steeves JD, Ramer MS, Fawcett JW. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci (2007) 26:3024–35. doi:10.1111/j.1460-9568.2007.05940.x
87. Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci (2011) 31:6053–8. doi:10.1523/JNEUROSCI.5524-09.2011
88. Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science (1999) 284:479–82.
89. Bugga L, Gadient RA, Kwan K, Stewart CL, Patterson PH. Analysis of neuronal and glial phenotypes in brains of mice deficient in leukemia inhibitory factor. J Neurobiol (1998) 36:509–24.
90. Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, et al. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci (1999) 19:5429–34.
91. Zhu X, Zuo H, Maher BJ, Serwanski DR, Loturco JJ, Lu QR, et al. Olig2-dependent developmental fate switch of NG2 cells. Development (2012) 139:2299–307. doi:10.1242/dev.078873
92. Bresnahan JC. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J Neurol Sci (1978) 37:59–82. doi:10.1016/0022-510X(78)90228-9
93. Bunge MB, Holets VR, Bates ML, Clarke TS, Watson BD. Characterization of photochemically induced spinal cord injury in the rat by light and electron microscopy. Exp Neurol (1994) 127:76–93. doi:10.1006/exnr.1994.1082
94. Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell (2010) 6:578–90. doi:10.1016/j.stem.2010.04.002
95. Bartus K, Galino J, James ND, Hernandez-Miranda LR, Dawes JM, Fricker FR, et al. Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain (2016) 139(Pt 5):1394–416. doi:10.1093/brain/aww039
96. Doucette R. PNS-CNS transitional zone of the first cranial nerve. J Comp Neurol (1991) 312:451–66. doi:10.1002/cne.903120311
97. Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol (2003) 182:399–411. doi:10.1016/S0014-4886(03)00087-6
98. McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci (1999) 19:10778–88.
99. Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, et al. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci (2000) 20:2427–38.
100. Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci (2002) 22:2225–36.
101. Crespo D, Asher RA, Lin R, Rhodes KE, Fawcett JW. How does chondroitinase promote functional recovery in the damaged CNS? Exp Neurol (2007) 206:159–71. doi:10.1016/j.expneurol.2007.05.001
102. Asher RA, Morgenstern DA, Properzi F, Nishiyama A, Levine JM, Fawcett JW. Two separate metalloproteinase activities are responsible for the shedding and processing of the NG2 proteoglycan in vitro. Mol Cell Neurosci (2005) 29:82–96. doi:10.1016/j.mcn.2005.02.001
103. Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci (2006) 26:4729–39. doi:10.1523/JNEUROSCI.3900-05.2006
104. Petrosyan HA, Hunanyan AS, Alessi V, Schnell L, Levine J, Arvanian VL. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J Neurosci (2013) 33:4032–43. doi:10.1523/JNEUROSCI.4702-12.2013
105. Lee JK, Chow R, Xie F, Chow SY, Tolentino KE, Zheng B. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J Neurosci (2010) 30:10899–904. doi:10.1523/JNEUROSCI.2269-10.2010
106. Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci (2013) 33:15350–61. doi:10.1523/JNEUROSCI.2510-13.2013
107. Anderson MA, Burda JE, Ren Y, Ao Y, O’shea TM, Kawaguchi R, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature (2016) 532:195–200. doi:10.1038/nature17623
108. Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci (2006) 26:3829–39. doi:10.1523/JNEUROSCI.4247-05.2006
109. Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci (2003) 23:9276–88.
110. Williams RR, Henao M, Pearse DD, Bunge MB. Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant (2015) 24:115–31. doi:10.3727/096368913X674657
111. de Castro R Jr, Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol (2005) 192:299–309. doi:10.1016/j.expneurol.2004.11.027
112. Filous AR, Tran A, Howell CJ, Busch SA, Evans TA, Stallcup WB, et al. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci (2014) 34:16369–84. doi:10.1523/JNEUROSCI.1309-14.2014
113. Han SB, Kim H, Skuba A, Tessler A, Ferguson T, Son YJ. Sensory axon regeneration: a review from an in vivo imaging perspective. Exp Neurobiol (2012) 21:83–93. doi:10.5607/en.2012.21.3.83
114. Son YJ. Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration? Neural Regen Res (2015) 10:346–8. doi:10.4103/1673-5374.153672
115. Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature (2000) 405:187–91. doi:10.1038/35012083
116. Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, et al. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci (2010) 30:255–65. doi:10.1523/JNEUROSCI.3705-09.2010
117. Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, et al. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci (2011) 31:4569–82. doi:10.1523/JNEUROSCI.4638-10.2011
118. Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma (2001) 18:351–9. doi:10.1089/08977150151071035
119. Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol (2009) 182:2628–40. doi:10.4049/jimmunol.0802954
120. Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia (2014) 62:452–67. doi:10.1002/glia.22616
121. Choi SS, Lee HJ, Lim I, Satoh J, Kim SU. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One (2014) 9:e92325. doi:10.1371/journal.pone.0092325
122. Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, et al. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol (2014) 274:53–61. doi:10.1016/j.jneuroim.2014.06.009
123. Mills Ko E, Ma JH, Guo F, Miers L, Lee E, Bannerman P, et al. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J Neuroinflammation (2014) 11:105. doi:10.1186/1742-2094-11-105
124. Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci (2013) 16:1401–8. doi:10.1038/nn.3505
125. Tirotta E, Ransohoff RM, Lane TE. CXCR2 signaling protects oligodendrocyte progenitor cells from IFN-gamma/CXCL10-mediated apoptosis. Glia (2011) 59:1518–28. doi:10.1002/glia.21195
Keywords: OPCs, oligodendrocytes, scar formation, astroglial scar, oligodendrocyte progenitor cells, axon regeneration
Citation: Hackett AR and Lee JK (2016) Understanding the NG2 Glial Scar after Spinal Cord Injury. Front. Neurol. 7:199. doi: 10.3389/fneur.2016.00199
Received: 12 September 2016; Accepted: 31 October 2016;
Published: 15 November 2016
Edited by:
Mattias K. Sköld, Uppsala University, SwedenReviewed by:
Linda Noble, University of California San Francisco, USACopyright: © 2016 Hackett and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae K. Lee, amxlZTIyQG1lZC5taWFtaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.