
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Neurol. , 21 December 2012
Sec. Neurodegeneration
Volume 3 - 2012 | https://doi.org/10.3389/fneur.2012.00180
This article is part of the Research Topic The Cell and Molecular Biology of Neurodegenerative diseases View all 13 articles
This article is a commentary on:
Unraveling 50-Year-Old Clues Linking Neurodegeneration and Cancer to Cycad Toxins: Are microRNAs Common Mediators?
A commentary on
Unraveling 50-year-old clues linking neurodegeneration and cancer to cycad toxins: are microRNAs common mediators?
by Spencer, P., Fry, R. C., and Kisby, G. E. (2012). Front. Gene. 3:192. doi: 10.3389/fgene.2012.00192
The Western Pacific amyotrophic lateral sclerosis and parkinsonism-dementia complex (ALS-PDC) has been described as a Rosetta Stone that bears the essential clue to understanding the etiopathogenesis of related neurodegenerative diseases. The three clinical forms (ALS, atypical parkinsonism with dementia, and dementia alone) have a single pathology (polyproteinopathy, notably tauopathy), just as the Rosetta Stone is inscribed with three distinct scripts bearing a common message. As recently discussed (Kisby and Spencer, 2011), studies of ALS-PDC in the three geographically separate and genetically distinct island populations (Chamorros on Guam; Japanese in Honshu Island's Kii Peninsula; and Papuan New Guineans in Irian Jaya, Indonesia) show:
Whereas, genotoxin-induced DNA damage is rapidly repaired in non-nervous tissue (i.e., cycling cells), this can persist in the brain because some DNA-repair mechanisms are weakly expressed in post-mitotic cells. MAM-induces O6-methylguanine (O6-mG) DNA damage, promutagenic lesions that induce uncontrolled mitoses (tumorigenesis) in mouse epithelial tissues and widespread degeneration in the developing murine brain. O6-mG lesions are clearly responsible for the MAM-induced neuronal loss because the pathology is greater in mice that lack the DNA-repair enzyme O6-mG methyltransferase (Mgmt−/−) and reduced or absent in mice that overexpress MGMT (Kisby and Spencer, 2011). Motor deficits in these mouse mutants are consistent with the extent of DNA damage. In sum, these findings suggest DNA damage is an initial event that leads to brain pathology (Figure 1).
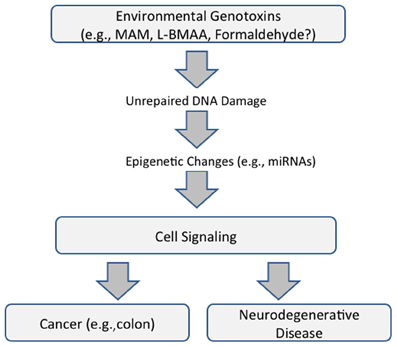
Figure 1. Proposed common pathway underlying cancer and neurodegenerative disease that is mediated by DNA damage and epigenetic changes (modified from Spencer et al., 2012).
MGMT activity is very low in the young adult human brain and may be absent in mature nerve cells. Mgmt−/− mice, an animal model of the young adult human brain, develop persistent brain O6-mG DNA lesions following a single dose of MAM. In the days following MAM treatment, these DNA lesions modulate key brain cell-signaling pathways that are also perturbed in human neurological disease, notably Alzheimer's disease, Parkinson's disease, and inherited and sporadic forms of ALS. Pathway analysis of MAM-modulated genes that are anchored to DNA damage reveals links with human cancer, genetic disorders, and skin and hair development (Kisby et al., 2011a). Several of these cell-signaling pathways continued to be modulated in the brain of Mgmt−/− mice 6 months later, with de novo expression changes of numerous genes involving olfaction (Kisby et al., 2011b).
These findings emphasize the relationship between acquired brain tissue DNA damage, modulation of cell-signaling pathways, and the induction of early and persistent molecular changes that lead to neuronal demise. They also reveal important relationships between seemingly disparate diseases—cancer and neurodegeneration—the phenotype of MAM-induced DNA damage being determined, respectively, by the presence or absence of the proliferative capacity of target tissues. The genotoxic properties of MAM and formaldehyde, a common metabolite of MAM and L-BMAA, also involve non-coding RNAs with functional roles in both neurodegeneration and cancer (Spencer et al., 2012) (Figure 1).
While proof is lacking that cycad toxin(s) trigger ALS-PDC, the results of recent biochemical and systems biology studies, coupled with the absence of known mutations in related neurodegenerative disorders, encourage further efforts to examine the molecular and cellular actions of MAM and L-BMAA. These studies not only highlight the response of the brain to unrepaired DNA damage-induced by a genotoxin (e.g., alkylating agent) as a potential initiator of a neurodegenerative process, they also provide a foundation for understanding whether such effects can lead to persistent changes at the protein level (e.g., tau and synuclein), including the erroneous incorporation of foreign amino acids, a subject of recent interest. The ability of MAM to perturb synuclein and several other classes of brain proteins (e.g., calcium homeostasis, mitochondrial and RNA processing) is consistent with this hypothesis (Kisby et al., 2006).
Kisby, G. E., Fry, R. C., Lasarev, M. R., Bammler, T. K., Beyer, R. P., Churchwell, M., et al. (2011a). The cycad genotoxin MAM modulates brain cellular pathways involved in neurodegenerative disease and cancer in a DNA damage-linked manner. PLoS ONE 6:e20911. doi: 10.1371/journal.pone.0020911
Kisby, G., Palmer, V., Lasarev, M., Fry, R., Iordanov, M., Magun, E., et al. (2011b). Does the cycad genotoxin MAM implicated in Guam ALS-PDC induce disease-relevant changes in mouse brain that includes olfaction? Commun. Integr. Biol. 4, 731–734.
Kisby, G. E., and Spencer, P. S. (2011). Is neurodegenerative disease a long-latency response to early-life genotoxin exposure? Int. J. Environ. Res. Public Health 8, 3889–3921.
Kisby, G. E., Standley, M., Park, T., Olivas, A., Fei, S., Jacob, T., et al. (2006). Proteomic analysis of the genotoxicant methylazoxymethanol (MAM) induced changes in the developing cerebellum. J. Proteome Res. 5, 2656–2665.
Citation: Spencer PS, Fry RC, Palmer VS and Kisby GE (2012) Western Pacific ALS-PDC: a prototypical neurodegenerative disorder linked to DNA damage and aberrant proteogenesis? Front. Neur. 3:180. doi:10.3389/fneur.2012.00180
Received: 29 November 2012; Accepted: 04 December 2012;
Published online: 21 December 2012.
Edited by:
Thomas M. Durcan, Mcgill University, CanadaReviewed by:
Thomas M. Durcan, Mcgill University, CanadaCopyright © 2012 Spencer, Fry, Palmer and Kisby. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence:c3BlbmNlckBvaHN1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.