
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 15 June 2012
Sec. Sleep Disorders
Volume 3 - 2012 | https://doi.org/10.3389/fneur.2012.00095
This article is part of the Research TopicNew insight into mechanisms of injury and disease in sleep apnea syndromeView all 9 articles
The causes of obstructive sleep apnea (OSA) are multifactorial. Neural injury affecting the upper airway muscles due to repetitive exposure to intermittent hypoxia and/or mechanical strain resulting from snoring and recurrent upper airway closure have been proposed to contribute to OSA disease progression. Multiple studies have demonstrated altered sensory and motor function in patients with OSA using a variety of neurophysiological and histological approaches. However, the extent to which the alterations contribute to impairments in upper airway muscle function, and thus OSA disease progression, remains uncertain. This brief review, primarily focused on data in humans, summarizes: (1) the evidence for upper airway sensorimotor injury in OSA and (2) current understanding of how these changes affect upper airway function and their potential to change OSA progression. Some unresolved questions including possible treatment targets are noted.
In the upper airway there are changes in sensation, muscle properties, and neural drive in patients with obstructive sleep apnea (OSA). These changes are loosely termed airway remodeling and may adversely affect upper airway function during sleep. Changes in nerve and muscle properties may result from vibration through snoring, hypoxia, or both.
The extent to which snoring and hypoxia exacerbate the disease and lead to important damage is unresolved. In this review, some of the more convincing evidence for and against upper airway remodeling in which data have been acquired in both OSA patients and non-OSA controls is highlighted. We review the pathophysiological evidence under three separate headings: (1) anatomical remodeling of the upper airway muscles, (2) efferent changes, and (3) afferent changes (see Figure 1). This encompasses evidence from a variety of neurophysiological approaches including histological, electrophysiological, and physiological studies. The function of upper airway reflexes and tongue force/fatigue characteristics in OSA vs. non-OSA subjects is also briefly reviewed. Finally, we discuss how upper airway remodeling and neural injury might contribute to upper airway closure during sleep.
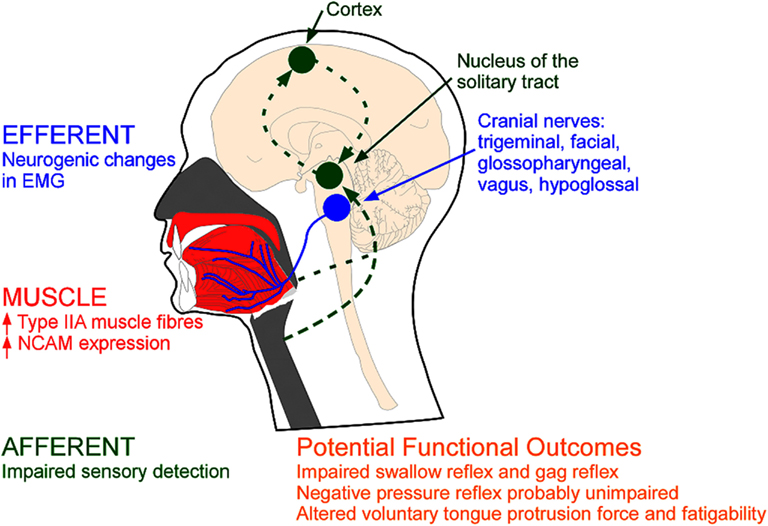
Figure 1. Types of evidence for neuromuscular pathology in OSA. MUSCLE: Remodelling may be reflected via anatomical changes within the upper airway muscles (red); EFFERENT: changes in the electromyogram (EMG) of the upper airway muscles innervated via the cranial nerves (blue); AFFERENT: changes in sensory pathways (green). NCAM=neural cell adhesion molecule.
Several histological studies support the presence of upper airway remodeling in patients with OSA (Tables 1 and 2). Broadly, this includes changes in: muscle fiber type, direction of muscle fibers, and anatomical arrangement of nerve fiber terminals. Sixteen studies are summarized in Table 2. Most of these studies did not rule out OSA in the healthy non-OSA/control groups (full polysomnography evaluations are reported in only four studies). Thus, the reported magnitude of these changes may be an underestimation. The upper airway muscles are composed of a variety of fast and slow muscle fiber types (Table 1) with conventional myosin heavy chain (MHC) isoforms and three primary MHC phenotypes and two subtypes (in order of prevalence for the adult genioglossus muscle in non-OSA subjects MHCIIA > MHCI-IIX > MHCI > MHCI-IIA > MHCIIX; Daugherty et al., 2012). A limitation of biopsies is that they are obtained from a limited area and may not represent the characteristics of the whole muscle. Nonetheless, a number of studies have shown an increase in the percentage of Type IIA muscle fibers in the order of ∼15% in several upper airway muscles in OSA patients including the uvula, genioglossus, medium pharyngeal constrictor, and palatopharyngeus (see Table 1; Smirne et al., 1991; Ferini-Strambi et al., 1998; Carrera et al., 1999; Series et al., 2000; Lindman and Stal, 2002; c.f. Friberg et al., 1998a). Type IIA muscle fibers are fast twitch fibers that can use both aerobic and anaerobic metabolism. While the cause for this increase in fiber type remains unknown, the change is likely to reflect training. Repetitive loading with regular nightly eccentric contractions (neural drive advancing the tongue anteriorly with negative airway pressure pulling the tongue posteriorly) combined with hypoxia may lead to fiber type alterations in the upper airway muscles indicative of an endurance trained muscle (Hildebrand et al., 1991; Pette and Staron, 2001). However, studies to definitively isolate the effects of training on upper airway muscle fiber type have not been done.
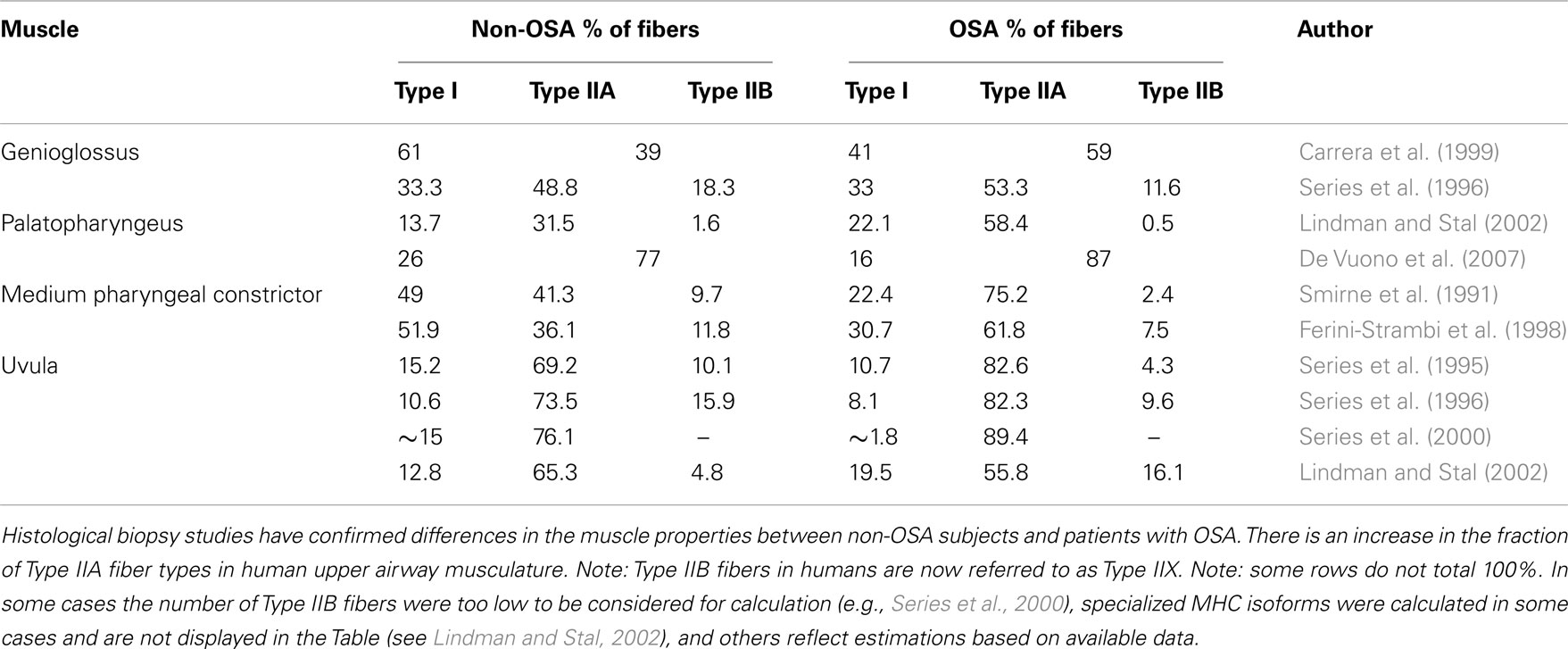
Table 1. Percentage of Type I, Type IIA, and Type IIB muscle fibers in non-OSA and patients with OSA.
Anatomical changes consistent with upper airway muscle remodeling in OSA in adults include: increased prevalence of angulated muscle fibers, increased diameter of muscle fibers (Smirne et al., 1991; Ferini-Strambi et al., 1998; Series et al., 2000; Sauleda et al., 2003; Svanborg, 2005; De Vuono et al., 2007; Stål et al., 2009), atrophied muscles fibers (Edstrom et al., 1992; Friberg et al., 1998a), changes in capillary density and mitochondria content (Stål et al., 2009; Stål and Johansson, 2012), fiber type grouping (Edstrom et al., 1992; Ferini-Strambi et al., 1998; see Table 2), and increased neural cell adhesion molecule (N-CAM) expression (Boyd et al., 2004). However, one study found similarly widespread neurogenic changes in children both with and without OSA (De Vuono et al., 2007). Thus, questions remain as to what degree of chronic partial denervation is “normal” for upper airway muscles.
Snoring is a key feature of OSA. The vibration induced by snoring and the mechanical strain caused by repetitive upper airway closure is associated with inflammatory changes to soft tissue structures of the upper airway (Berger et al., 2002; Boyd et al., 2004). The local inflammation increases the thickness of the surrounding tissues and may narrow the upper airway to exacerbate obstructive apneas (Rubinstein, 1995; Sekosan et al., 1996; Berger et al., 2002). Patients with severe snoring have soft palate swelling in the morning that recedes while awake (Sekosan et al., 1996).
There is also increased fat in and around the muscles of the upper airway in patients with OSA (Horner et al., 1989; Stauffer et al., 1989; Schwartz et al., 1991; Zohar et al., 1998b; Berger et al., 2002). The importance of increased fat is highlighted by the observation that weight loss in obese OSA patients can eliminate airway obstruction. While infiltration of fat is not a result of neural changes, it will alter the dynamics of the upper airway. With increased intramuscular fat, contractile performance may change via altered loading of individual muscle fibers and altered “passive” properties (Busha et al., 2002). Given that some of the tension developed in one part of the muscle can be transmitted via shear links to other parts of the muscle (Kjaer, 2004), increased fat may alter the force distribution across muscle fibers in OSA patients. Nevertheless, maximal voluntary tongue protrusion force is the same, or increased in OSA patients (e.g., Mezzanotte et al., 1992; Mortimore et al., 2000; Eckert et al., 2011).
In addition to changes in the soft tissues and muscle fibers, there are also electrophysiological changes in the muscles of patients with OSA (Table 3). During wakefulness patients with OSA have higher levels of multiunit electromyographic activity (EMG) recorded in the upper airway muscles compared to healthy control subjects (Mezzanotte et al., 1992, 1996; Fogel et al., 2001; c.f. Series et al., 2009). The apparent increase in drive was ascribed to a neural compensation for a narrow upper airway. This concept became widely accepted. The neural compensation was thought to be analogous to that in diaphragm and other obligatory inspiratory muscles of patients with chronic obstructive pulmonary disease where more motor units are recruited and their average discharge frequencies are higher (De Troyer et al., 1997; Gorman et al., 2005) because of the increased resistive load. Recent studies indicate that motor unit discharge frequencies also increase with higher neural drive to the genioglossus (Bailey et al., 2007; Saboisky et al., 2010). However, there is currently no strong evidence to support an overall increase in the discharge frequencies, or recruitment of additional motor units in the genioglossus in OSA during wakefulness (Saboisky et al., 2007, 2012). During the pre-inspiratory phase of the respiratory cycle the overall excitability of the genioglossus motoneuron pool appears to be increased in OSA patients vs. controls. The timing of the initial activation of phasic inspiratory motor units is earlier (Saboisky et al., 2007). Similarly, during late expiration, the conduction times of motor evoked potentials elicited via transcranial magnetic stimulation of the motor cortex are shorter in the genioglossus in OSA patients than in controls (Wang et al., 2010). Thus, while there is currently no evidence for increased discharge frequencies or additional recruitment of motor units there are quantifiable changes in the timing of neural drive to genioglossus in OSA. Therefore, how do the new findings fit with these earlier studies?
An alternative explanation is that the elevated EMG in the upper airway muscles in patients with OSA is secondary to neurogenic remodeling. This is characterized as chronic partial denervation of muscle fibers, with reinnervation of the orphaned muscle fibers by collateral sprouting of surviving motor axons (Boyd et al., 2004; Gonzalez-Forero et al., 2004). The overall duration of motor unit potentials increase, often with increased amplitude (Bertorini et al., 1994; Preston and Shapiro, 2002). Recent investigations using single motor unit techniques have shown that the motor unit potentials of upper airway muscles in OSA patients are larger in area, longer in duration, and more complex (Svanborg, 2005; Saboisky et al., 2007, 2012; see also Podnar and Dolenc Groselj, 2010). These changes could contribute to the increased multiunit EMG in OSA. Thus, active remodeling may help to preserve the functional capacity of the muscles. However, the presence of denervation and subsequent axonal sprouting may lead to changes in fine motor control such as speech (Goldshtein et al., 2011). An additional potential problem with the initial reports of increased multiunit EMG in OSA (awake) is that values were normalized to the EMG produced during a maximal volitional task and may not be comparable between OSA patients and controls.
If the anatomically deeper upper airway motor axons are affected by vibration, sensory afferents, closer to the airway surface, should also be impaired. However, the evidence supporting sensory nerve impairment in OSA is less convincing than that for motor nerves. If sensory nerves are affected, this may impair normal reflex mechanisms which contribute to upper airway function.
A variety of techniques have revealed potential sensory impairments in OSA. Two-point discrimination and detection of vibration, airflow, and temperature for the upper airway are all altered in OSA patients (see Table 4). The ability to detect and rate the size of inspiratory resistive loads to breathing in patients with OSA is impaired (McNicholas et al., 1984; Tun et al., 2000; c.f. Clerk et al., 1994). However, cognitive processing is important for these measurements and they may be independently affected by sleepiness and prior respiratory loading. Airway edema is present in untreated OSA patients and may impair upper airway sensation. Thus, separating the relative contribution of airway edema and sleepiness and cognitive processing vs. sensory nerve injury is difficult. One strategy to overcome some of these confounders is to repeat measurements after an acute period of continuous positive airway pressure (CPAP) treatment. Here, edema and sleepiness are likely to resolve more rapidly than potential nerve injury changes. In support of a role of long-term sensory injury in OSA, one study revealed only partial resolution of oropharynx sensation with vibration detection and two-point discrimination after 4 months of CPAP therapy (Kimoff et al., 2001). However, in another study, impaired sensation to loaded breathing was corrected after 2 weeks of CPAP therapy (Tun et al., 2000). In untreated OSA patients breathing responses to respiratory stimuli measured during wakefulness vary. Impaired respiratory load detection is associated with a reduction in the ventilatory response to hypercapnia (McNicholas et al., 1984). Conversely, the ability to adjust breathing (e.g., respiratory timing and minute ventilation) in response to respiratory loading may be unaltered in OSA (Hlavac et al., 2007).
Cortical evoked potentials can provide information about peripheral and central transmission as well as cortical processing of sensory information. Delays in the timing and/or reductions in amplitude of the early components of evoked potentials may reflect afferent impairment. The early parameters do not require a perceptual decision and are less affected by sleepiness. Measured during wakefulness, brainstem auditory evoked responses do not appear to be abnormal in OSA (e.g., Mosko et al., 1981; Karnaze et al., 1984). Similarly, the latency and amplitude of the early N1 component of the cortical evoked response to auditory stimuli are not altered in OSA patients (Afifi et al., 2003; Vakulin et al., 2012).
Of relevance to the upper airway, several studies have examined respiratory-related evoked potentials (RREPs) during wakefulness in OSA. Consistent with impaired sensory transmission to respiratory stimuli, two studies revealed a reduction in the amplitude but not the latency of the early RREP components to brief negative pressures during inspiration (Akay et al., 2003) and expiration (Grippo et al., 2011). However, other studies have shown no difference in P1 amplitude or latency (reflecting the arrival of the sensory information to the cortex) of the RREP to inspiratory occlusions and negative pressures in OSA patients (Gora et al., 2002; Afifi et al., 2003; Donzel-Raynaud et al., 2009; Eckert et al., 2011). During sleep, P1 does not appear to be different between OSA patients and controls (Gora et al., 2002; Afifi et al., 2003). However, the amplitude of the latter N550 component (reflecting sensory processing) is reduced in the OSA patients (Gora et al., 2002; Afifi et al., 2003).
While recordings of peripheral sensory and motor nerve potentials are affected by obesity, several findings consistent with sensory neuropathy have been observed in nerves innervating limb muscles and organs in patients with OSA (Mayer et al., 1999; Fanfulla et al., 2000; Lüdemann et al., 2001; Dziewas et al., 2007). OSA patients show evidence of a mild axonal neuropathy in the sural, median, and ulnar nerves (Mayer et al., 1999; Dematteis et al., 2001; Lüdemann et al., 2001; Dziewas et al., 2007). Two studies report slower sensory conduction and smaller amplitude responses (Mayer et al., 1999; Fanfulla et al., 2000). In addition, it is notable that comparable changes have been observed for the compound muscle action potential in the median, sural, and ulnar nerves (Mayer et al., 1999; Lüdemann et al., 2001; Dziewas et al., 2007). This finding occurs when controlling for the increased body mass index in OSA (Dziewas et al., 2007). Thus, intermittent hypoxia may mediate systemic neural changes in OSA that are not isolated to the upper airway.
In summary, these findings reveal varying levels of sensory impairment to certain sensory stimuli in OSA. While the precise pathophysiological role of sensory impairment remains uncertain, sensory testing using calibrated airflow has been proposed as a screening test for OSA (Dematteis et al., 2005). There may also be different populations of OSA patients; those with sensory impairments and those patients without (Nguyen et al., 2005). Thus, while sensory impairments may play a key role to obstructions in some patients with OSA, neuromuscular, and anatomical influences may be more important in others.
The integrity of the swallowing reflex is impaired in OSA patients and snorers compared to controls (Table 5). A greater bolus volume is required to elicit the swallowing reflex, swallowing onset latency is delayed, and bolus leakage throughout the pharynx occurs more frequently in OSA patients and snorers compared to controls (Zohar et al., 1998a; Teramoto et al., 1999; Jäghagen et al., 2000; Levring Jaghagen et al., 2003; Valbuza et al., 2011b). Inhibition of inspiration associated with swallowing is also less pronounced in OSA (Teramoto et al., 1999). While these changes would favor aspiration laryngeal penetration was uncommon. This indicates that alterations in swallowing are predominantly subclinical in OSA. CPAP may improve subclinical swallowing dysfunction in OSA (Okada et al., 2000). Palatal reflexes to tactile stimuli such as the gag reflex are diminished, particularly in severe OSA (Valbuza et al., 2011a). The extent to which these changes are caused by sensory impairments or efferent changes and whether or not they influence OSA severity remains unknown.
Conversely, the upper airway negative pressure reflex, crucial for maintaining airway patency, does not appear to be impaired in OSA patients during wakefulness. Studies that were not optimally designed to measure reflex responses have shown reduced EMG activation in the palatal muscles (Mortimore and Douglas, 1997), comparable genioglossus EMG activation to high levels of negative pressure (−20 cm H2O), and elevated genioglossus EMG activity to moderate suction pressures (−10 to −13 cm H2O; Berry et al., 2003). However, a recent study using sensitive neurophysiological techniques found no differences in the timing or the amplitude of the genioglossus and tensor palatini negative pressure reflex during wakefulness between OSA patients and controls (Eckert et al., 2011).
Maximum voluntary force of the tongue protuders is comparable (Mezzanotte et al., 1992; Mortimore et al., 2000; Busha et al., 2002; Blumen et al., 2004) or increased (Shepherd et al., 2006; Eckert et al., 2011) in OSA patients. While the maximal force generating capacity in the tongue protuders does not appear to be impaired in OSA, whether or not they are more vulnerable to fatigue remains uncertain. Fatigability, quantified as time to task failure during sustained isometric tongue protrusion tasks at 30, 50, and 80% of maximum, were not different between OSA patients and controls (Mortimore et al., 2000; Blumen et al., 2004). Conversely, time to task failure during an intermittent isometric tongue protrusion task (5 s on 5 s off at 70% maximal force) occurred approximately twice as quickly in OSA patients vs. controls (Eckert et al., 2011). Similarly, recovery of maximal force capacity following submaximal isometric tongue protrusion tasks was prolonged in OSA patients (Blumen et al., 2004). How various voluntary tongue protrusion measures during wakefulness relate to upper airway patency during sleep and OSA severity remains unclear. In an earlier study, maximal tongue protrusion force positively correlated with OSA severity, albeit it only weakly (r2 = 0.04), whereas fatigability to a submaximal isometric sustained task did not (Mortimore et al., 2000). In a subsequent study, there was no correlation between maximal tongue protrusion force and OSA severity (Shepherd et al., 2006).
Remodeling of the upper airway muscles occurs in OSA. The most commonly reported finding is an increase in the proportion of Type IIA muscle fibers, a change which is consistent with muscle training. Changes in multiunit EMG are difficult to interpret. Recent single motor unit studies reveal chronic partial denervation in OSA. Upper airway sensory impairments to a range of stimuli also occur in OSA. However, it remains challenging to differentiate the relative contribution of afferent neural injury vs. the confounding influences of OSA (e.g., edema) to primary impairments in sensation.
Given that long-term vibration causes neurogenic changes in limb muscles (Takeuchi et al., 1986; Dahlin and Lundborg, 2001), snoring and its mechanical effects are likely to contribute to the observed upper airway anatomical, efferent, and afferent changes. However, intermittent hypoxia may also impair peripheral nerves in OSA. Thus, there is the potential for synergistic effects to mediate upper airway remodeling in OSA. Some of the changes in muscle properties and sensory impairments resolve, at least in part, with CPAP treatment (e.g., Carrera et al., 1999; Mayer et al., 1999; Tun et al., 2000; Kimoff et al., 2001; Nguyen et al., 2005; Dziewas et al., 2007).
The evidence for a link between upper airway remodeling and the integrity of upper airway reflexes is limited. The swallow and gag reflexes are impaired in patients with OSA. However, the existing data does not support a link between the presence of swallowing impairment and OSA severity (Jäghagen et al., 2000) and the potential for an impaired gag reflex to influence OSA severity is uncertain. Other subtle alterations, such as changes in speech, may also occur in OSA. Importantly, there is no strong evidence to suggest that key protective reflexes involved in maintaining upper airway patency are impaired in OSA. In addition, the ability of the tongue to generate volitional protrusion force is similar or enhanced in OSA. However, despite the consistent observation of increased Type IIA fibers in the upper airway muscles of OSA patients, which would be predicted to increase resistance to muscle fatigue, the upper airway muscles may be more prone to fatigue. However, the laboratory task used to measure performance does not exactly mimic the repetitive movements associated with intermittent obstructions in OSA.
Given the current uncertainty as to the links between changes in sensorimotor function and the upper airway in OSA and sleep-disordered breathing, how novel therapeutic targets might modify these upper airway pathophysiological changes is unclear. Nonetheless, recent studies have demonstrated that novel exercise training techniques (e.g., didgeridoo playing and a battery of tasks designed by a speech pathologist) can reduce OSA severity (e.g., Puhan et al., 2006; Guimarães et al., 2009). Recent studies using implantable devices to electrically stimulate the genioglossus muscle during sleep have shown reductions in OSA severity (e.g., Eastwood et al., 2011; Oliven, 2011). However, consistent with the varying causes of OSA, treatment response varies between patients (Dotan et al., 2011; Eastwood et al., 2011). The upper airway muscles are stronger and have an increased proportion of Type IIA muscle fibers in OSA. These findings suggest that the upper airway muscles are highly trained by chronic overnight loading and/or hypoxia. Nonetheless, targeted exercise tasks performed during wakefulness may further enhance these changes and potentially lead to improved neuromechanical performance during sleep. The use of some of the measurement techniques outlined in this review to study the effects before and after training would help address some of this uncertainty and may provide novel therapeutic targets.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Afifi, L., Guilleminault, C., and Colrain, I. M. (2003). Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir. Physiol. Neurobiol. 136, 221–234.
Akay, M., Leiter, J. C., and Daubenspeck, J. A. (2003). Reduced respiratory-related evoked activity in subjects with obstructive sleep apnea syndrome. J. Appl. Physiol. 94, 429–438.
Bailey, E. F., Rice, A. D., and Fuglevand, A. J. (2007). Firing patterns of human genioglossus motor units during voluntary tongue movement. J. Neurophysiol. 97, 933–936.
Bassiouny, A., Nasr, S., Mashaly, M., Ayad, E., Qotb, M., and Atef, A. (2009). Electron microscopy study of peripheral nerves in the uvulae of snorers and obstructive sleep apnoea patients. J. Laryngol. Otol. 123, 203–207.
Berger, G., Gilbey, P., Hammel, I., and Ophir, D. (2002). Histopathology of the uvula and the soft palate in patients with mild, moderate, and severe obstructive sleep apnea. Laryngoscope 112, 357–363.
Berry, R. B., White, D. P., Roper, J., Pillar, G., Fogel, R. B., Stanchina, M., and Malhotra, A. (2003). Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J. Appl. Physiol. 94, 1875–1882.
Bertorini, T. E., Stalberg, E., Yuson, C. P., and Engel, W. K. (1994). Single-fiber electromyography in neuromuscular disorders: correlation of muscle histochemistry, single-fiber electromyography, and clinical findings. Muscle Nerve 17, 345–353.
Blumen, M. B., De La Sota, A. P., Quera-Salva, M. A., Frachet, B., Chabolle, F., and Lofaso, F. (2004). Tongue mechanical characteristics and genioglossus muscle EMG in obstructive sleep apnoea patients. Respir. Physiol. Neurobiol. 140, 155–164.
Boyd, J. H., Petrof, B. J., Hamid, Q., Fraser, R., and Kimoff, R. J. (2004). Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 170, 541–546.
Busha, B. F., Strobel, R. J., and England, S. J. (2002). The length-force relationship of the human genioglossus in patients with obstructive sleep apnea. Respir. Physiol. Neurobiol 130, 161–168.
Carrera, M., Barbe, F., Sauleda, J., Tomas, M., Gomez, C., and Agusti, A. G. (1999). Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am. J. Respir. Crit. Care Med. 159, 1960–1966.
Clerk, A. A., Dunan, S. R., and Guilleminault, C. (1994). Load detection in subjects with sleep-induced upper airway obstruction. Am. J. Respir. Crit. Care Med. 149, 727–730.
Dahlin, L. B., and Lundborg, G. (2001). Vibration-induced hand problems: role of the peripheral nerves in the pathophysiology. Scand. J. Plast. Reconstr. Surg. Hand. Surg. 35, 225–232.
Daugherty, M., Luo, Q., and Sokoloff, A. J. (2012). Myosin heavy chain composition of the human genioglossus muscle. J. Speech Lang. Hear. Res. 55, 609–625.
De Troyer, A., Leeper, J. B., McKenzie, D. K., and Gandevia, S. C. (1997). Neural drive to the diaphragm in patients with severe COPD. Am. J. Respir. Crit. Care Med. 155, 1335–1340.
De Vuono, I. M., Zanoteli, E., Oliveira, A. S., Fujita, R. R., Pignatari, S. S., Pizarro, G. U., Pradelle-Hallinan, M. L., and Moreira, G. A. (2007). Histological analysis of palatopharyngeal muscle from children with snoring and obstructive sleep apnea syndrome. Int. J. Pediatr. Otorhinolaryngol. 71, 283–290.
Dematteis, M., Levy, P., and Pepin, J. L. (2005). A simple procedure for measuring pharyngeal sensitivity: a contribution to the diagnosis of sleep apnoea. Thorax 60, 418–426.
Dematteis, M., Pepin, J. L., Jeanmart, M., Deschaux, C., Labarre-Vila, A., and Levy, P. (2001). Charcot-Marie-Tooth disease and sleep apnoea syndrome: a family study. Lancet 357, 267–272.
Donzel-Raynaud, C., Redolfi, S., Arnulf, I., Similowski, T., and Straus, C. (2009). Abnormal respiratory-related evoked potentials in untreated awake patients with severe obstructive sleep apnoea syndrome. Clin. Physiol. Funct. Imaging 29, 10–17.
Dotan, Y., Golibroda, T., Oliven, R., Netzer, A., Gaitini, L., Toubi, A., and Oliven, A. (2011). Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur. Respir. J. 38, 338–347.
Dziewas, R., Schilling, M., Engel, P., Boentert, M., Hor, H., Okegwo, A., Ludemann, P., Ringelstein, E. B., and Young, P. (2007). Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J. Neurol. Neurosurg. Psychiatr. 78, 295–297.
Eastwood, P. R., Barnes, M., Walsh, J. H., Maddison, K. J., Hee, G., Schwartz, A. R., Smith, P. L., Malhotra, A., McEvoy, R. D., Wheatley, J. R., O’Donoghue, F. J., Rochford, P. D., Churchward, T., Campbell, M. C., Palme, C. E., Robinson, S., Goding, G. S., Eckert, D. J., Jordan, A. S., Catcheside, P. G., Tyler, L., Antic, N. A., Worsnop, C. J., Kezirian, E. J., and Hillman, D. R. (2011). Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 34, 1479–1486.
Eckert, D. J., Lo, Y. L., Saboisky, J. P., Jordan, A. S., White, D. P., and Malhotra, A. (2011). Sensori-motor function of the upper airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J. Appl. Physiol. 111, 1644–1653.
Edstrom, L., Larsson, H., and Larsson, L. (1992). Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnoea: a muscle biopsy study. J. Neurol. Neurosurg. Psychiatr. 55, 916–920.
Fanfulla, F., Malaguti, S., Montagna, T., Salvini, S., Bruschi, C., Crotti, P., Casale, R., and Rampulla, C. (2000). Erectile dysfunction in men with obstructive sleep apnea: an early sign of nerve involvement. Sleep 23, 775–781.
Ferini-Strambi, L. J., Smirne, S., Moz, U., Sferrazza, B., and Iannaccone, S. (1998). Muscle fibre type and obstructive sleep apnea. Sleep Res. Online 1, 24–27.
Fogel, R. B., Malhotra, A., Giora, P., Edwards, J. K., Beauregard, J., Shea, S. A., and White, D. P. (2001). Genioglossal activation in paitents with obstructive sleep apnea versus control subjects. Am. J. Respir. Crit. Care Med. 164, 2025–2030.
Friberg, D., Ansved, T., Borg, K., Carlsson-Nordlander, B., Larsson, H., and Svanborg, E. (1998a). Histological indications of a progressive snorers disease in an upper airway muscle. Am. J. Respir. Crit. Care Med. 157, 586–593.
Friberg, D., Gazelius, B., Lindblad, L. E., and Nordlander, B. (1998b). Habitual snorers and sleep apnoics have abnormal vascular reactions of the soft palatal mucosa on afferent nerve stimulation. Laryngoscope 108, 431–436.
Friberg, D., Gazelius, B., Hokfelt, T., and Nordlander, B. (1997). Abnormal afferent nerve endings in the soft palatal mucosa of sleep apnoics and habitual snorers. Regul. Pept. 71, 29–36.
Goldshtein, E., Tarasiuk, A., and Zigel, Y. (2011). Automatic detection of obstructive sleep apnea using speech signals. IEEE Eng. Med. Biol. Mag. 58, 1373–1382.
Gonzalez-Forero, D., Portillo, F., Sunico, C. R., and Moreno-Lopez, B. (2004). Nerve injury reduces responses of hypoglossal motoneurones to baseline and chemoreceptor-modulated inspiratory drive in the adult rat. J. Physiol. 557, 991–1011.
Gora, J., Trinder, J., Pierce, R., and Colrain, I. M. (2002). Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 166, 1225–1234.
Gorman, R. B., McKenzie, D. K., Butler, J. E., Tolman, J. F., and Gandevia, S. C. (2005). Diaphragm length and neural drive after lung volume reduction surgery. Am. J. Respir. Crit. Care Med. 172, 1259–1266.
Grippo, A., Carrai, R., Romagnoli, I., Pinto, F., Fanfulla, F., and Sanna, A. (2011). Blunted respiratory-related evoked potential in awake obstructive sleep apnoea subjects: a NEP technique study. Clin. Neurophysiol. 122, 1562–1568.
Guilleminault, C., Li, K., Chen, N. H., and Poyares, D. (2002). Two-point palatal discrimination in patients with upper airway resistance syndrome, obstructive sleep apnea syndrome, and normal control subjects. Chest 122, 866–870.
Guimarães, K. C., Drager, L. F., Genta, P. R., Marcondes, B. F., and Lorenzi-Filho, G. (2009). Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 179, 962–966.
Hildebrand, I. L., Sylven, C., Esbjornsson, M., Hellstrom, K., and Jansson, E. (1991). Does chronic hypoxaemia induce transformations of fibre types? Acta Physiol. Scand. 141, 435–439.
Hlavac, M. C., Catcheside, P. G., Adams, A., Eckert, D. J., and McEvoy, R. D. (2007). The effects of hypoxia on load compensation during sustained incremental resistive loading in patients with obstructive sleep apnea. J. Appl. Physiol. 103, 234–239.
Horner, R. L., Mohiaddin, R. H., Lowell, D. G., Shea, S. A., Burman, E. D., Longmore, D. B., and Guz, A. (1989). Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur. Respir. J. 2, 613–622.
Jäghagen, E. L., Berggren, D., and Isberg, A. (2000). Swallowing dysfunction related to snoring: a videoradiographic study. Acta. Otolaryngol. 120, 438–443.
Karnaze, D., Gott, P., Mitchell, F., and Loftin, J. (1984). Brainstem auditory evoked potentials are normal in idiopathic sleep apnea. Ann. Neurol. 15, 406.
Kimoff, R. J., Sforza, E., Champagne, V., Ofiara, L., and Gendron, D. (2001). Upper airway sensation in snoring and obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 164, 250–255.
Kjaer, M. (2004). Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84, 649–698.
Levring Jaghagen, E., Franklin, K. A., and Isberg, A. (2003). Snoring, sleep apnoea and swallowing dysfunction: a videoradiographic study. Dentomaxillofac. Radiol. 32, 311–316.
Lindman, R., and Stal, P. S. (2002). Abnormal palatopharyngeal muscle morphology in sleep-disordered breathing. J. Neuro. Sci. 195, 11–23.
Lüdemann, P., Dziewas, R., Soros, P., Happe, S., and Frese, A. (2001). Axonal polyneuropathy in obstructive sleep apnoea. J. Neurol. Neurosurg. Psychiatr. 70, 685–687.
Mayer, P., Dematteis, M., Pépin, J. L., Wuyam, B., Veale, D., Vila, A., and Lévy, P. (1999). Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am. J. Respir. Crit. Care Med. 159, 213–219.
McNicholas, W. T., Bowes, G., Zamel, N., and Phillipson, E. A. (1984). Impaired detection of added inspiratory resistance in patients with obstructive sleep apnea. Am. Rev. Respir. Dis. 129, 45–48.
Mezzanotte, W. S., Tangel, D. J., and White, D. P. (1992). Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J. Clin. Invest. 89, 1571–1579.
Mezzanotte, W. S., Tangel, D. J., and White, D. P. (1996). Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am. J. Respir. Crit. Care Med. 153, 1880–1887.
Mortimore, I. L., Bennett, S. P., and Douglas, N. J. (2000). Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J. Sleep Res. 9, 389–393.
Mortimore, I. L., and Douglas, N. J. (1997). Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am. J. Respir. Crit. Care Med. 156, 867–873.
Mosko, S. S., Pierce, S., Holowach, J., and Sassin, J. F. (1981). Normal brain stem auditory evoked potentials recorded in sleep apneics during waking and as a function of arterial oxygen saturation during sleep. Electroencephalogr. Clin. Neurophysiol. 51, 477–482.
Nguyen, A. T., Jobin, V., Payne, R., Beauregard, J., Naor, N., and Kimoff, R. J. (2005). Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep 28, 585–593.
Okada, S., Ouchi, Y., and Teramoto, S. (2000). Nasal continuous positive airway pressure and weight loss improve swallowing reflex in patients with obstructive sleep apnea syndrome. Respiration 67, 464–466.
Oliven, A. (2011). Treating obstructive sleep apnea with hypoglossal nerve stimulation. Curr. Opin. Pulm. Med. 17, 419–424.
Paulsen, F. P., Steven, P., Tsokos, M., Jungmann, K., Muller, A., Verse, T., and Pirsig, W. (2002). Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 166, 501–509.
Pette, D., and Staron, R. S. (2001). Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 115, 359–372.
Podnar, S., and Dolenc Groselj, L. (2010). “Neuropathic changes of the genioglossus muscle in patients with snoring and obstructive sleep apnoea,” in 20th Congress of the European Sleep Research Society, Lisbon.
Preston, D. C., and Shapiro, B. E. (2002). Needle electromyography. Fundamentals, normal and abnormal patterns. Neurol. Clin. 20, 361–396.
Puhan, M. A., Suarez, A., Lo Cascio, C., Zahn, A., Heitz, M., and Braendli, O. (2006). Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. Br. Med. J. (Clin. Res. Ed.) 332, 266–270.
Ramchandren, S., Gruis, K. L., Chervin, R. D., Lisabeth, L. D., Concannon, M., Wolfe, J., Albers, J. W., and Brown, D. L. (2010). Hypoglossal nerve conduction findings in obstructive sleep apnea. Muscle Nerve 42, 257–261.
Rubinstein, I. (1995). Nasal inflammation in patients with obstructive sleep apnea. Laryngoscope 105, 175–177.
Saboisky, J. P., Butler, J. E., McKenzie, D. K., Gorman, R. B., Trinder, J. A., White, D. P., and Gandevia, S. C. (2007). Neural drive to human genioglossus in obstructive sleep apnoea. J. Physiol. 585, 135–146.
Saboisky, J. P., Jordan, A. S., Eckert, D. J., White, D. P., Trinder, J. A., Nicholas, C. L., Gautam, S., and Malhotra, A. (2010). Recruitment and rate-coding strategies of the human genioglossus muscle. J. Appl. Physiol. 109, 1939–1949.
Saboisky, J. P., Stashuk, D. W., Hamilton-Wright, A., Carusona, A. L., Campana, L. M., Trinder, J., Eckert, D. J., Jordan, A. S., McSharry, D. G., White, D. P., Nandedkar, S., David, W. S., and Malhotra, A. (2012). Neurogenic changes in the upper airway of obstructive sleep apnea patients. Am. J. Respir. Crit. Care Med. 185, 322–329.
Sauleda, J., Garcia-Palmer, F. J., Tarraga, S., Maimo, A., Palou, A., and Agusti, A. G. (2003). Skeletal muscle changes in patients with obstructive sleep apnoea syndrome. Respir. Med. 97, 804–810.
Schwartz, A. R., Gold, A. R., Schubert, N., Stryzak, A., Wise, R. A., Permutt, S., and Smith, P. L. (1991). Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 144, 494–498.
Sekosan, M., Zakkar, M., Wenig, B. L., Olopade, C. O., and Rubinstein, I. (1996). Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope 106, 1018–1020.
Series, F., Cote, C., Simoneau, J. A., Gelinas, Y., St Pierre, S., Leclerc, J., Ferland, R., and Marc, I. (1995). Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J. Clin. Invest. 95, 20–25.
Series, F., Simoneau, J. A., and St Pierre, S. (2000). Muscle fiber area distribution of musculus uvulae in obstructive sleep apnea and non-apneic snorers. Int. J. Obes. Relat. Metab. Disord. 24, 410–415.
Series, F., Wang, W., and Similowski, T. (2009). Corticomotor control of the genioglossus in awake OSAS patients: a transcranial magnetic stimulation study. Respir. Res. 10, 74.
Series, F. J., Simoneau, S. A., St Pierre, S., and Marc, I. (1996). Characteristics of the genioglossus and musculus uvulae in sleep apnea hypopnea syndrome and in snorers. Am. J. Respir. Crit. Care Med. 153, 1870–1874.
Shepherd, K. L., Jensen, C. M., Maddison, K. J., Hillman, D. R., and Eastwood, P. R. (2006). Relationship between upper airway and inspiratory pump muscle force in obstructive sleep apnea. Chest 130, 1757–1764.
Smirne, S., Iannaccone, S., Ferini-Strambi, L., Comola, M., Colombo, E., and Nemni, R. (1991). Muscle fibre type and habitual snoring. Lancet 337, 597–599.
Stål, P. S., and Johansson, B. (2012). Abnormal mitochondria organisation and oxidative activity in palate muscles of long-term snorers with obstructive sleep apnea. Respiration 83, 407–417.
Stål, P. S., Lindman, R., and Johansson, B. (2009). Capillary supply of the soft palate muscles is reduced in long-term habitual snorers. Respiration 77, 303–310.
Stauffer, J. L., Buick, M. K., Bixler, E. O., Sharkey, F. E., Abt, A. B., Manders, E. K., Kales, A., Cadieux, R. J., Barry, J. D., and Zwillich, C. W. (1989). Morphology of the uvula in obstructive sleep apnea. Am. Rev. Respir. Dis. 140, 724–728.
Sunnergren, O., Brostrom, A., and Svanborg, E. (2011). Soft palate sensory neuropathy in the pathogenesis of obstructive sleep apnea. Laryngoscope 121, 451–456.
Svanborg, E. (2005). Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir. Physiol. Neurobiol. 147, 263–272.
Takeuchi, T., Futatsuka, M., Imanishi, H., and Yamada, S. (1986). Pathological changes observed in the finger biopsy of patients with vibration-induced white finger. Scand. J. Work Environ. Health 12, 280–283.
Teramoto, S., Sudo, E., Matsuse, T., Ohga, E., Ishii, T., Ouchi, Y., and Fukuchi, Y. (1999). Impaired swallowing reflex in patients with obstructive sleep apnea syndrome. Chest 116, 17–21.
Tun, Y., Hida, W., Okabe, S., Kikuchi, Y., Kurosawa, H., Tabata, M., and Shirato, K. (2000). Inspiratory effort sensation to added resistive loading in patients with obstructive sleep apnea. Chest 118, 1332–1338.
Vakulin, A., Catcheside, P. G., Baulk, S. D., Antic, N. A., Van Den Heuvel, C. J., Banks, S., and McEvoy, R. D. (2012). Auditory evoked potentials remain abnormal after CPAP treatment in patients with severe obstructive sleep apnoea. Clin. Neurophysiol. 123, 310–317.
Valbuza, J. S., De Oliveira, M. M., Conti, C. F., Prado, L. B., Carvalho, L. B., and Do Prado, G. F. (2011a). Oropharyngeal examination as a predictor of obstructive sleep apnea: pilot study of gag reflex and palatal reflex. Arq. Neuropsiquiatr. 69, 805–808.
Valbuza, J. S., De Oliveira, M. M., Zancanella, E., Conti, C. F., Prado, L. B., Carvalho, L. B., and Do Prado, G. F. (2011b). Swallowing dysfunction related to obstructive sleep apnea: a nasal fibroscopy pilot study. Sleep Breath. 15, 209–213.
Wang, W., Kang, J., and Kong, D. (2010). The central motor conductivity of genioglossus in obstructive sleep apnea. Respirology 15, 1209–1214.
Woodson, B. T., Garancis, J. C., and Toohill, R. J. (1991). Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope 101, 1318–1322.
Keywords: sleep apnea, upper airway muscles, neuropathy, myopathy, upper airway physiology, upper airway reflexes
Citation: Saboisky JP, Butler JE, Gandevia SC and Eckert DJ (2012) Functional role of neural injury in obstructive sleep apnea. Front. Neur. 3:95. doi: 10.3389/fneur.2012.00095
Received: 23 April 2012; Paper pending published: 13 May 2012;
Accepted: 28 May 2012; Published online: 15 June 2012.
Edited by:
Pierre-Charles Neuzeret, Lyon Neuroscience Research Center, FranceReviewed by:
Takashi Ono, Tokyo Medical and Dental University, JapanCopyright: © 2012 Saboisky, Butler, Gandevia and Eckert. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Danny J. Eckert, Neuroscience Research Australia, PO Box 1165 Randwick, Sydney, NSW 2031, Australia. e-mail:ZC5lY2tlcnRAbmV1cmEuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.