- 1 Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, Jackson, MS, USA
- 2 Department of Physiology and Biophysics, Howard University College of Medicine, Washington, DC, USA
Cells in the locus coeruleus (LC) constitute the sole source of norepinephrine (NE) in the brain and change their discharge rates according to vigilance state. In addition to its well established role in vigilance, NE affects synaptic plasticity in the postnatal critical period (CP) of development. One form of CP synaptic plasticity affected by NE results from monocular occlusion, which leads to physiological and cytoarchitectural alterations in central visual areas. Selective suppression of rapid eye movement sleep (REMS) in the CP kitten enhances the central effects of monocular occlusion. The mechanisms responsible for heightened cortical plasticity following REMS deprivation (REMSD) remain undetermined. One possible mediator of an increase in plasticity is continuous NE outflow, which presumably persists during extended periods of REMSD. Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the synthesis of NE and serves as a marker for NE-producing cells. We selectively suppressed REMS in kittens for 1 week during the CP. The number and size of LC cells expressing immunoreactivity to tyrosine hydroxylase (TH-ir) was assessed in age-matched REMS-deprived (RD)-, treatment–control (TXC)-, and home cage-reared (HCC) animals. Sleep amounts and slow wave activity (SWA) were also examined relative to baseline. Time spent in REMS during the study was lower in RD compared to TXC animals, and RD kittens increased SWA delta power in the latter half of the REMSD period. The estimated total number of TH-ir cells in LC was significantly lower in the RD than in the TXC kittens and numerically lower than in the HCC animals. The size of LC cells expressing TH-ir was greatest in the HCC group. HCC cells were significantly larger than TH-ir cells in the RD kittens. These data are consistent with presumed reduction in NE in forebrain areas, including visual cortex, caused by 1 week of REMSD.
Introduction
Located in the mesopontine brainstem, neurons in the locus coeruleus (LC) are the primary source of cortical norepinephrine (NE; Jones and Yang, 1985; Gu, 2002). Involvement of the noradrenergic neurons of the LC in regulation of the major vigilance states is well established (Siegel, 2005; Pace-Schott and Hobson, 2002; Fuller et al., 2006). During waking, these cells fire at a high rate (Jouvet, 1972; Hobson et al., 1975). They increase their waking firing rate further in response to salient (Aston-Jones et al., 1991) or noxious stimuli (Abercrombie and Jacobs, 1987), and electrical stimulation of LC neurons enhances the reactivity of neocortex (Aston-Jones et al., 1991; Foote et al., 1991). LC cells therefore appear to participate actively in the mechanisms mediating arousal as well as in the attendant processing of sensory information (Foote et al., 1991).
Locus coeruleus cells fire more slowly in slow wave sleep (SWS) than in waking and, by virtue of their active inhibition by cholinergic inputs to brainstem GABAergic sites, are virtually silent in rapid eye movement sleep (REMS; Jacobs, 1986; Pace-Schott and Hobson, 2002). On the other hand, presynaptic activation of LC cells induces an increase in the mRNA of tyrosine hydroxylase (TH) in NE cells. Microdialysis studies have shown that release of NE in both LC and amygdala is correlated with activity of the NE cells in LC: greatest in wake and least in REMS (Shouse et al., 2001a,b; Park, 2002). This pattern of activity of LC neurons supports the belief that NE plays a role in a REMS-gating mechanism (Hobson et al., 1975). During REMS deprivation (REMSD), LC neurons never stop discharging. Accordingly, during suppression of REMS (and its associated increase in wake time), NE output never entirely ceases. This may be true irrespective of how long REMSD prevails.
Recent evidence in critical period (CP) animals demonstrates that REMSD amplifies the form of developmentally regulated synaptic plasticity resulting from monocular occlusion (Hubel and Wiesel, 1963; Wiesel and Hubel, 1963). Selective elimination of REMS in CP kittens enhances the physiological and cytoarchitectural alterations in central visual areas caused by monocular occlusion (Oksenberg et al., 1996; Shaffery et al., 1998). However, the means by which this plasticity is enhanced has not been determined. One possible mechanism is an increase in NE expression in visual cortex. A functional elevation in NE output from LC cells may develop as wake time accumulates during REMSD (Porkka-Heiskanen et al., 1995).
Endogenous production of NE is limited by the synthesizing enzyme, TH. TH mRNA expression as well as NE concentration increase in adult rats after 3 days of REMSD carried out by the small-platform method (Porkka-Heiskanen et al., 1995). A recent report indicates that pedestal REMSD also increases the size of TH immunoreactive (TH-ir) cells in LC (Majumdar and Mallick, 2003). Cytomorphic changes in LC TH-ir cells have not been described, however, in developing kittens under conditions of extended REMSD. In the present study, we utilized stereological methods to obtain non-biased estimates of the number and size of TH-ir neurons within the LC of CP kittens selectively deprived of REMS for 1 week by computer-controlled cage-shaking (RD group). We evaluated the findings relative to a treatment–control (TXC) group and to a cohort of non-treated, home cage-reared (HCC) animals.
Materials and Methods
Procedures were carried out in conformity with NIH guidelines and were approved by the local Internal Review Board for the Care and Use of Animals. Every effort was made to minimize animal suffering. We used the smallest number of animals needed for statistically reliable data. The study animals were the offspring of pregnant, random-source cats (of wild-type genetics) that were obtained by the University of Mississippi’s Laboratory Animal Facility. Seven kittens from two litters were assigned randomly to the RD (n = 4) or TXC (n = 3) conditions. In one RD animal, the LC could not be recovered and is not included in the stereology results but was evaluated for sleep stages. Age-matched kittens from two additional litters were reared with their mothers and selected for the HCC group (n = 6), which received no sleep treatment during the experimental period. Several kittens used in this study had been monocularly deprived. Effects of REMSD on visual system development in these kittens will be described in a separate report (Shaffery et al., in preparation).
All treated kittens were housed with their mothers and were on a 12:12-h light–dark schedule until surgery on postnatal day (PN) 34 or 35. The surgical procedure was carried out according to previously published protocols described here briefly (Oksenberg et al., 1996; Shaffery et al., 1998; Hogan et al., 2001). Animals were anesthetized with pentobarbital (40 mg/kg i.p.) or isoflurane (1.0%) anesthesia and implanted under sterile conditions with an array of standard sleep-recording electrodes. Three stainless-steel screws were tapped into the skull to record the electrocorticogram (ECoG). Two small-gage, multi-stranded, stainless-steel wire electrodes were inserted and sutured bilaterally into the trapezius muscle to obtain a nuchal electromyogram (EMG). The electrodes were routed to a plastic connector and fixed to the skull with dental acrylic. The scalp was sewn in place to surround the connector. After recovery from surgery, all animals were monitored until able to move about on their own. They were then returned to their home cages in the company of their mothers for 5–6 days until randomized to a treatment group. During the surgical procedure, four animals (two from each of the TXC and RD groups) had an opaque PVC occluder, shaped as a contact lens, placed between the skin and muscle of the right eyelid (Spiro and Kolbert, 1974). The remaining animals were not visually manipulated. Inasmuch as monocular occlusion is not known to affect LC unit activity, we did not anticipate any effect upon LC TH-ir.
In the PN40–49 period, animals were housed in individual sleep-monitoring cages (45 cm × 35 cm × 30 cm) in separate, temperature-controlled, soundproofed, video-monitored recording chambers (1 m × 1 m × 3 m). To control for light experience in the wake state, the cage-shaking protocol was conducted in total darkness. Following our previously reported protocol, each of the seven days of the REMSD period (which was also the shaker-control period) contained three 1.33 h “wake–break” intervals (Oksenberg et al., 1996; Shaffery et al., 1998). During these breaks, animals were removed from the recording chambers, allowed free movement, and remained awake under normal room light (2000 lux). As a result, every animal received exactly 4 h of waking-light experience per day independent of individual differences in their total sleep and wake times in the dark. Four hours of light approximates the spontaneous, average waking-light exposure of kittens at this age in a laboratory setting (Jouvet-Mounier et al., 1970; McGinty et al., 1977; Ursin and Sterman, 1981). Electrophysiological recording in the dark chambers was conducted continuously during two 5-h periods and one 10-h period. Each of the three, daily recording sessions was followed by a wake–break interval. Water was always accessable, but food and social interaction with littermates were available only during the non-recording times.
In the sleep-monitoring cage within each chamber, an animal’s electrodes were attached through a connector on its head. The connector wires led to a slip-ring commutator (Airflyte, Bayonne, NJ, USA) counterbalanced by a swing-arm and from there to a shielded electrical cable that terminated at the recording- and REMSD-actuating equipment in an adjacent room. The first day, PN40, was devoted to a 24-h adaptation period in the recording chambers. The kittens remained on their home cage 12L/12D schedule during the adaptation day. The next day, PN41, constituted a baseline day in a stationary cage. Undisturbed sleep–wake electrophysiology was recorded on the experimental schedule described above. The kittens were then subjected to either the systematic REMSD or TXC experimental protocols during the period from PN42 through 49 (see below). Early on day PN50, kittens were sacrificed under deep pentobarbital anesthesia (80 mg/kg, i.p.). Each animal was perfused transcardially, first with cold physiological saline and then with 4% paraformaldehyde with 0.1 M L-lysine and 0.01 M sodium periodate in 0.1 M phosphate buffer, pH 7.4 (McLean and Nakane, 1974). The brains were removed and processed for immunohistochemistry (see Immunohistochemistry, below).
Our computer-controlled system for detection as well as deprivation of REMS was modified from a previously reported technique (Shaffery et al., 1990; Hogan et al., 2001). The system reliably identifies REMS on the basis of individually determined, minimum-amplitude threshold criteria configured from each kitten’s digitized ECoG and EMG recordings. Pursuant to standardized scoring criteria, 3 s of minimum amplitudes on the ECoG and EMG channels signal onset of REMS. The computer immediately activates the motor of a horizontally and rotary-moving shaker-platform that supports the attached recording cage. The cage moves at about 6 Hz for 0.5–1.5 s. The sudden agitation of the cage quickly awakens the sleeping animal, abruptly terminating REMS. Following these provoked arousals, animals typically fall back into SWS after short but variable intervals of wake time. Stage REMS spontaneously reappears generally after 10–30 min of SWS.
Each REMSD kitten is quasi-yoked to a TXC kitten in a separate chamber. The TXC animal receives the same number and intensity of shakes (spaced 3–4 s apart) experienced by the experimental kitten in a given half-hour. The “control” shakes, however, are delivered only within the last 5 min of the half-hour. In our earlier REMSD protocol, which utilized a fully yoked-control design, REMS in the shaker-control animals was found to be reduced to 62% of baseline values (Shaffery et al., 1990). In contrast, the quasi-yoked protocol used in this study permitted REMS and SWS times in the shaker-control animals to remain at baseline percentages.
Seyle’s Stress Indicators
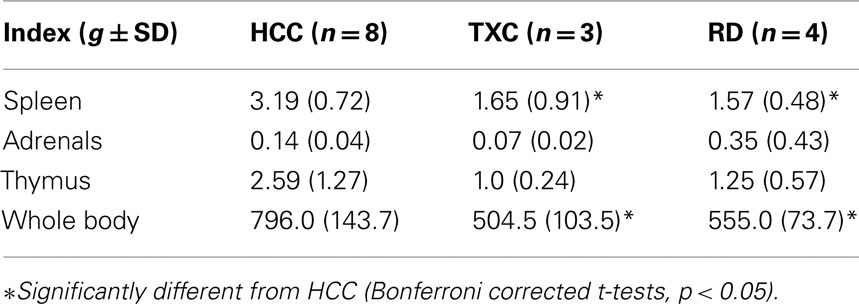
At sacrifice (PN49–50), body weight was obtained. Spleen, thymus, and adrenal glands were removed and stored in formalin fixative until weighed to furnish an index of stress. Single factor ANOVA (p < 0.05) determined group differences in final body weight and in the weights of the individual organs across the groups (Selye, 1936, 1950).
Sleep Stage Analysis
Digitized ECoG and EMG recordings were visually scored in 15 s epochs (by standard criteria, adapted for kittens in this laboratory from published criteria for adults (Ursin and Sterman, 1981)) as either Wake, REMS, or SWS (non-REMS). Time in each state was tabulated for every hour of every study day. Statistical analyses of sleep and wake state amounts were performed on four, 24-h periods: baseline day (PN40), and first (PN42), middle (PN44–45), and final (PN48) days of the sleep-perturbation period.
Power Spectral Analysis
Power spectral analysis was carried out on 10, targeted, 15-s epochs of digitized SWS ECoG data from all recorded kittens on each of the analyzed days (c.f. Joho et al., 1999). Five, 15-s epochs of SWS were taken on days P41, P45, and P48 from the second hour of sleep-recording after both morning and evening “wake–breaks.” Each set was constituted from the first, five, artifact-free, 15-sec epochs encountered that consisted solely of SWS. Epochs immediately preceded or followed by an epoch exhibiting a state-change were omitted from the analyses. A subset of 30, non-overlapping, 4 s periods, drawn equally from morning and afternoon tracings, was selected from each day’s 10 epochs of uncontaminated SWS. These 4 s periods were subjected to a Fast Fourier Transform (FFT) algorithm (Microcal Origin 6.1; Hamming window) to generate their power spectra at 0.25 Hz resolution. For each animal, a measure of SWS slow wave activity (SWA) was calculated as the mean absolute power density (in μV2/Hz) in the delta frequency band (0.7–4.4 Hz) from the mean of power densities in all 0.25 Hz bins divided by 4 to express power as μV2/Hz.
Immunohistochemistry
After perfusion, brains were rapidly removed, blocked, cryoprotected in 30% sucrose in phosphate buffered saline (PBS), frozen in powdered dry ice, and stored at −80°C until processing. Brains were serially sectioned on a cryostat microtome into 60 μm coronal slices through the entire brainstem.
Every fifth section of LC was selected and processed for TH expression, using diaminobenzidine (DAB) immunohistochemistry. Free-floating sections were quenched in 0.3% hydrogen peroxide for 30 min at room temperature, washed in 0.3% Triton X-100/PBS (3 × 10 min), and incubated in 5% normal goat serum (NGS) in PBS. Sections were then incubated overnight in polyclonal rabbit anti-TH primary antibody (1:3000 dilution, Chemicon, Temecula, CA, USA) for 16–24 h at 4°C. The following day, sections were washed in 0.3% Triton X-100/PBS (3 × 10 min) and incubated for 2 h with goat anti-rabbit biotinylated IgG antibody (1:400 Vector Laboratories, Burlingame, CA, USA). Following another rinse in 0.3% Triton X-100/PBS, sections were incubated for 2 h in Avidin–Biotin Complex, rinsed in PBS, and stained with nickel chloride-enhanced DAB until a strong color reaction was observed. Tissues were finally rinsed, mounted, air-dried, and cover-slipped with Permount (Fisher Scientific, Atlanta, GA, USA).
Non-Biased, Quantitative Stereology
We used the optical fractionator method, which systematically samples regions of histological interest, as published previously (Gundersen, 1988; Gundersen et al., 1988; Long et al., 1998; Mouton et al., 2002). Estimates of the number of TH-immunopositive cells on both sides of the entire LC were derived from cell counts in every fifth serial section following a first, randomly chosen, section. Counting frames and sampling-grid sizes were determined to accomplish counting a minimum of 200 cells in each half of the LC, employing magnification from a 60× objective (1.4 numerical aperture). The bilateral values were averaged for each individual for final analysis. All data collection, employing Stereo Investigator software (MicroBrightfield, Inc., Colchester, VT, USA), was carried out under double-blind conditions. The system hardware included an X–Y–Z motorized stage; Optronics color-video camera interfaced to a Nikon E800 microscope; high-resolution video card; focus-measurement encoder (providing 0.25 μm resolution of absolute, microscope-stage, focus position); and personal computer and monitor. This system generates an unbiased estimate of the number of cells within the LC. Concurrent use of the Nucleator method furnishes unbiased estimates of cell size. The cell size estimate is based upon the mean lengths (measured by the software) of four, randomly orientated radii extending from a visualized nucleolus to the edge of the cell body (Gundersen, 1988; Gundersen et al., 1988; Kroustrup et al., 1988).
Statistics
Statistical analyses were performed with commercially available statistical software, SPSS (SPSS, Inc., Chicago, IL, USA). A repeated-measures, multivariate ANOVA model that employed Recording Day and State as within-subject variables and treatment group (RD vs. TXC) as the between-subjects variable, analyzed treatment effects in terms of amounts of the several vigilance states across discrete days in the experimental sequence. Detection of significant interaction effects was followed by univariate ANOVAs that defined recording day as the within-subject variable and a priori specific contrast to determine differences in the course of the protocol. Results were considered significant at p < 0.05.
To detect changes in delta spectral power during the recording period, we analyzed delta power values during the baseline, middle, and end days. Two-factor, repeated-measures ANOVAs were employed, with treatment group as the between-subjects and recording day as the within-subject factors. We adopted a 95% confidence level criterion for significance (p < 0.05). Significance in the two-way ANOVA was pursued with one-way ANOVAs to assess differences in delta power in each group independently across the three analyzed days. Post hoc analyses included Bonferroni corrected t-tests to determine directional relationships between the means on the days when one-way ANOVAs were significant (p < 0.05).
Results
REM Sleep Deprivation
During baseline, percent time in all three vigilance states was similar in TXC and RD animals (Figure 1). Repeated-measures ANOVA of group-by-day effects for each of the vigilance states demonstrated a difference in REMS proportions in the two groups, but no significant SWS or WAKE differences were uncovered (REMS, F = 604.8, p = 0.0001; WAKE, F = 2.25, NS; SWS, F = 0.2, NS). The RD group experienced a reduction in REMS to 2% of recording time on the first day and to 11% on the sixth day (Figure 1). REMS was reduced overall by 80% from baseline values in the RD animals. Early in the REMSD period, the amount of lost REMS tended to be replaced by WAKE. Toward the end of REMSD, the trend in the direction of compensatory WAKE time progressively gave way to a non-significant increase in SWS.
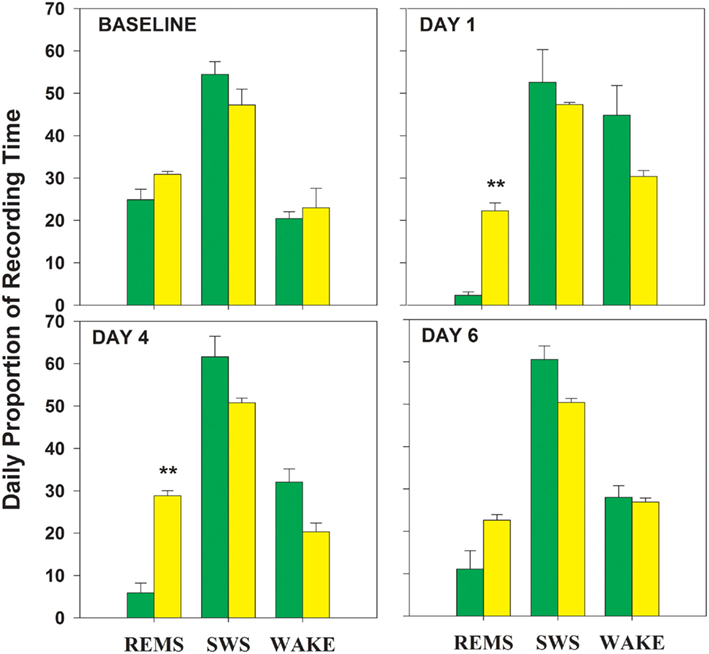
Figure 1. Proportion of time spent in each of three vigilance states is graphed for the four 24 h periods analyzed within the 8-day experimental protocol (baseline day and 7 shaker days). On each graph, the percent times spent in REMS, rapid eye movement sleep; SWS, slow wave sleep, and waking (WAKE) are plotted. RD = green bars; TXC = yellow bars; **significantly different from corresponding TXC mean; p = 0.001 (Bonferroni corrected post hoc t-tests).
Slow Wave Activity
Rapid eye movement sleep deprivation affected SWA (delta frequency band, 0.7–4.4 Hz) power during SWS. No difference in delta power was present in the baseline recordings of the two shaker groups (RD and TXC). Tests for specific contrasts determined that by the end of the study both groups displayed cumulative changes in SWA (Figure 2) but in opposite directions (F = 10.58, df = 1.5, p = 0.028). SWA trended higher in the RD group on the third day of REMSD and elevated significantly by the sixth day relative to baseline (F = 7.13, df = 2.10, p = 0.012). Shaker-control animals showed only a slight reduction in SWA that did not achieve statistical significance at any point in the cage-shaking period (Figure 2). Differences between the two groups serially increased on the third and sixth days, but the disparities did not reach significance on either day (p = 0.051; p = 0.058, respectively; Bonferroni corrected, post hoc t-tests).
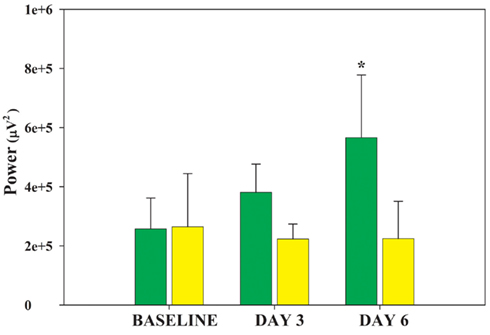
Figure 2. Changes in ECoG delta power by group. Average delta power during 30 artifact-free, 4 s periods of SWS is plotted for each group on the baseline day and shaker days 3 and 6. The DAY by GROUP interaction is significant (F = 7.13, df = 2.10, p = 0.012). The RD group (green bars) successively increases delta power whereas the TXC group (yellow bars) first shows slightly decreased delta power that later levels off. Post hoc comparisons between the two groups do not reach statistical significance on any single day, but trended toward differences between each other on DAY 3 and DAY6 (p = 0.051, p = 0.056, respectively; Bonferroni corrected post hoc t-tests). The rising delta power in the RD group’s SWS achieved significance in its difference from baseline on DAY6 (*p = 0.047).
TH-ir Stereological Data
Stereological analysis furnished unbiased estimates of total area and volume of the LC as well as of size, number, and density of TH-ir cells within the nucleus (Figure 3). The effect of REMSD on these measures reached significance for total number of TH-ir cells (Figure 4, F = 4.3, df = 2.9, p = 0.049),indicating that RD animals had fewer TH-ir cells than TXC animals (post hoc Bonferroni corrected t-tests, p = 0.05). Cell number in the HCC group was numerically larger than in animals subjected to REMSD. The HCC value, however, did not differ significantly from cell number in either RD or TXC kittens (Figure 4).
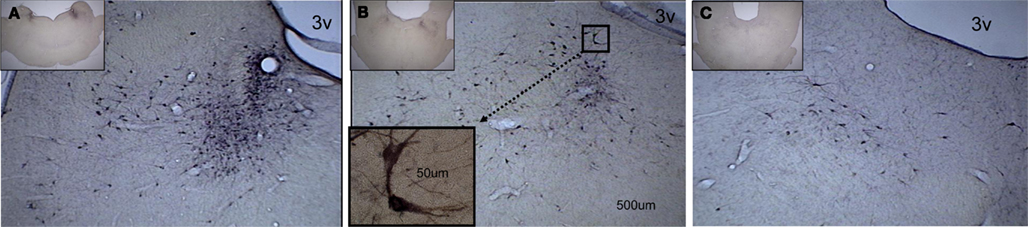
Figure 3. A caudal-to-rostral subset of photomicrographs, taken from the complete series of midbrain coronal sections, showing darkly stained cell bodies evenly distributed throughout the medial–lateral aspect of the locus coeruleus. The low-power (2.5×) insert on each photomicrograph indicates the caudal-to-rostral level of the higher-power (10×) enlargement. (A) Higher densities of TH-ir cells are found at the more caudal sites. The scale bar is the same on three of the photomicrographs and applies to the high-power (10×) picture. (B) At this level, fewer TH-ir cells are seen. The insert shows a higher-power (63×) view of a pair of TH-ir cells. Their location in the 10× section is indicated by the dotted line connecting the two boxes. (C) The most rostral section has the fewest TH-ir cells. 3v designates the third ventricle.

Figure 4. Estimated total number of TH-ir cells in the locus coeruleus in the three kitten groups. Values are based on fractionator stereological probe data, which provide statistically non-biased estimates of total number of cells in an area of interest. Home cage-reared (HCC)-, shaker-control (TXC)-, and RD groups are indicated on the abscissa. HCC = red bar; TXC = yellow bar; RD = green bar; * = different from TXC, p = 0.024 (Bonferroni post hoc corrected t-test).
Rapid eye movement sleep deprivation also affected mean size of TH-ir cells in the LC (F = 4.816, df = 2.9, p = 0.038). Cells were significantly smaller in the RD group than in the HCC animals (Bonferroni corrected t-test, p = 0.039). The TXC group’s cells were almost as large as the cells of the HCC animals, but, unlike the latter, their mean size could not be significantly differentiated from the RD group’s soma profile (Figure 5).
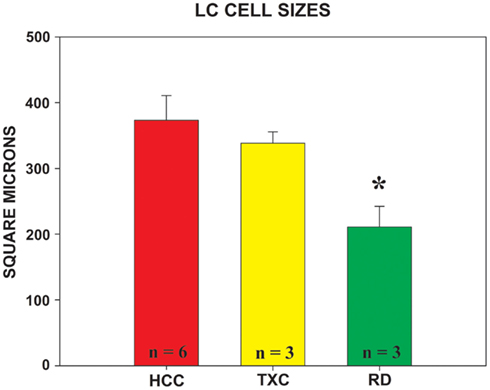
Figure 5. Mean area of TH-ir cells measured in the locus coeruleus in each group. The nucleator probe, utilized in conjunction with the fractionator, provides the estimated value of cell size. Group designations are as given in Figure 4. HCC = red bar; TXC = yellow bar; RD = green bar;* = different from HCC, p = 0.039 (Bonferroni post hoc corrected t-test).
Seyle’s Organ-Weight Stress Assay
One-way ANOVA assessed the effects of treatments on adrenal, thymus, spleen, and whole body weights at the time of sacrifice (Table 1). Treatment effects were found for spleen (F = 9.55, df = 2, 12, p = 0.003), thymus (F = 3.88,df = 2.12, p = 0.05), and body (F = 8.62, df = 2.12, p = 0.005) weights. The group effect was not significant for adrenal weight. Post hoc analysis showed that, although RD and TXC animals did not differ from each other on any of the weight measures, both groups’ spleen and body weights (only) could be significantly distinguished from those in the HCC animals (Bonferroni corrected t-tests, p < 0.05).
Discussion
Sleep Architecture
The computer-based REMSD system effectively and selectively reduced REMS. The specificity of our current system for REMSD, which utilizes rotatory shakers is comparable to our previously described, vertical-action REMSD shakers and considerably quieter (Hogan et al., 2001). During the 7-day, cage-shaking procedure, SWA in the TXC group was slightly, though not significantly, reduced from baseline levels. RD animals, in contrast, exhibited a significant increase in delta power during SWS epochs as deprivation proceeded.
Several studies in humans and rats have demonstrated a gradual reduction in SWA after both total sleep deprivation and selective REMSD (Beersma et al., 1990; Endo et al., 1997, 1998). The increase in SWA that we found during the course of REMSD arguably reflects a compensatory (for lost total sleep) SWA-deepening process. This is also indicated by the progressive increase in SWA in the RD kittens during the latter half of the sleep-perturbation sequence.
TH-ir Cytoarchitecture
Seven days of REMSD appeared to affect the cytoarchitecture of TH-ir neurons in kitten LC. Reductions in mean number and size of LC TH-ir cells were found. Cell number in RD animals was lower than in the HCC or TXC groups, but only significantly lower than the latter. (TXC group cell number was not significantly higher than the number in HCC animals.)
The average size of TH-ir cells also varied across groups. TXC animals tended to have larger soma profile areas than RD animals. Only the HCC group showed significantly larger cells than RD animals, though TXC and HCC cell sizes were not statistically distinguishable. Our data does not clearly implicate a particular mechanism mediating the effects of REMSD upon either LC cell number or size, but different regulation mechanisms affecting cell size and number are suggested by our findings.
During development in rats, separate subsets of LC cells express TH-ir, whereas TH-ir is revealed in older rats in only specific portions of LC populated primarily by small cells (Bezin et al., 1994). Though both TH-ir cell size and number were smaller in our REMS-deprived kittens, the significant differences in the two measures varies among the groups. The outcomes generally indicate that changes in number of LC TH-ir cells accrue from mechanisms distinct from those influencing cell size. Smaller LC cells expressing TH-ir in REMS-deprived animals could signify either loss of larger LC cells during REMSD, or, alternatively, reduction in TH-ir expression in still-extant large cells. A third possibility is that available TH is ultimately depleted as REMSD progresses, contributing also to reduced size of TH-releasing cells.
Cell growth within the LC of developing rats is dynamic (Bezin et al., 1994, 2000). NE cells in rat LC are smallest at birth, reach maximum size just prior to weaning (PN14), and by PN60 are only slightly larger than at birth (Bezin et al., 1994; Saito et al., 1996). Our finding of smaller TH-ir cell size in RD compared to HCC animals is consistent with the possibility that maturation and growth of LC cells are developmentally delayed by REMSD. Previous studies also suggest relative retardation of CNS maturational processes after REMSD (Shaffery et al., 2002, 2006). Our TXC animals, though frequently perturbed, were not deprived of REMS, and no delay in size maturation of their TH-ir cells size was apparent.
Though selective loss of larger cells in RD animals may have occurred, the greater variability observed in mean LC cell size in these kittens (Figure 5, see SEMs) suggests that not all of the large cells were lost; rather, an overall shift toward predominantly smaller cells still expressing TH-ir may have taken place. This fits with the observed smaller cell size found after REMSD.
A recent report in adult rats demonstrated that the size of TH-ir cells in LC is larger in REMS-deprived (single platform-over-water technique) rats compared to non-treated animals (Majumdar and Mallick, 2003). This evidence is contrary to our finding of small cell size after shaker-produced REMSD. We speculate that this apparent inconsistency arises from a number of sources: first, the studies were in different species and at different ages. Our study is the first, to our knowledge, to present cytomorphic measurements for TH-ir cells in kitten LC; second, the two studies did not employ the same REMSD technique. The shaker system of REMSD has been shown to be less stressful than the pedestal method. For example, animals that are REMS-deprived on pedestals typically lose or do not gain weight in the course of the deprivation, whereas shaker RD animals gain weight at the same rate as TXC animals (see below; Hogan et al., 1998); third, the adult rat study did not randomize the selection of slides used to obtain the cytomorphometric data. In contrast, we adopted the standard stereological approach and employed random-regular sampling to choose specific slides and representative areas to size and count TH-ir cells, as required to ensure non-biased estimates (Gundersen, 1988; Gundersen et al., 1988; West et al., 1991; That this difference in technique accounts in part for the differences in the data is not at all certain.) Nevertheless, the non-biased stereological approach strengthens confidence in the validity of our findings. Whether the species, age, REMSD–stress differences, or other factors explain the divergent TH-ir cell size findings in the two studies requires further investigation.
The number of cells expressing TH-ir in our kittens may be subject to several additional contingencies. REMSD increases time awake (Oksenberg et al., 1996; Hogan et al., 2001). During waking, LC cells continue to fire rather than ceasing discharge as in REMS (Mallick et al., 1990). The cells’ uninterrupted activity during a 7-day REMSD protocol may eventually cause a decrease in firing rate (Porkka-Heiskanen et al., 1995). This might be reflected in reduced numbers of cells expressing TH-ir. An earlier report has afforded evidence that TH expression, measured by blotting techniques, correlates linearly with the number of TH-ir cells in LC (Debure et al., 1992). Accordingly, differences in mean number of cells between the experimental groups in our study may have resulted from depletion of TH-ir in the RD kittens or from an increase in TH-ir production in the TXC animals. The latter effect would not be surprising because of increased LC cell activity in the TXC kittens due to stress induced by the “make-up” shakes during waking.
Though experimental evidence is presently lacking concerning the influence of protracted periods of REMSD on LC unit firing, slowing of firing rate of “REM sleep-off” LC cells during brief REMSD periods has been reported. Such slowing should eventuate in less expression of TH in some or all LC cells (Mallick et al., 1990). An extended REMSD period, occasioning long-term, slowed LC discharge, could account for the smaller number of TH-ir cells observed in our REMS-deprived animals. The number of TH-ir cells in the RD group was only significantly lower relative to the TXC group, which tended to show a larger number than both other groups.
In the present study, the spleen- and body weight data from the TXC and RD animals suggest that both groups experienced some stress. Other observations suggest that the largest cell number (found in the TXC kittens) may be accounted for by greater stress than the RD kittens. That stress increases LC cell activity is well studied (Lehnert et al., 1998; Kawahara et al., 2000; Asbach et al., 2001; Valentino and Van, 2001), and we have shown, as determined by preference for saccharine, that shaker-perturbed TXC animals may experience more stress than shaker-REMS-deprived animals (Shaffery et al., 2003). In war veterans with post-traumatic stress disorder (PTSD) who experience extreme stress, a loss of LC neurons was reported (Bracha et al., 2005). These data in humans may be affected by chronic disease and intervening medications but, the cell loss suggests more extreme stress than experienced during REMSD.
The relative reduction in TH-ir expression we observed in the LC of REMSD animals (i.e., smaller and fewer observed cells), we speculate, reflects a progressive decrease in the firing of LC cells after a period of (probably) increased discharge earlier in the 7-day REMSD sequence. It is reasonable to suppose that greater recorded wake time due to REMSD-provoked arousals would lead to an overall increase in NE expression in forebrain sites despite the relative reduction in LC unit activity (c.f. Mallick et al., 1990). Inasmuch as NE modulates synaptic plasticity during development of visual cortex (Kasamatsu and Pettigrew, 1976; Bear and Singer, 1986; Imamura and Kasamatsu, 1991; Brocher et al., 1992; Kirkwood et al., 1999), and suppression of NE curtails developmental plasticity-facilitating effects (Bear et al., 1983; Bear and Singer, 1986; Brocher et al., 1992), increased cortical NE may be an important element in the enhanced plasticity of geniculocortical cells found during monocular occlusion and REMSD (Oksenberg et al., 1996; Shaffery et al., 1998).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by: NS31720 and NS39407-03S1.
References
Abercrombie, E. D., and Jacobs, B. L. (1987). Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J. Neurosci. 7, 2837–2843.
Asbach, S., Schulz, C., and Lehnert, H. (2001). Effects of corticotropin-releasing hormone on locus coeruleus neurons in vivo: a microdialysis study using a novel bilateral approach. Eur. J. Endocrinol. 145, 359–363.
Aston-Jones, G., Chiang, C., and Alexinsky, T. (1991). Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog. Brain Res. 88, 501–520.
Bear, M. F., Paradiso, M. A., Schwartz, M., Nelson, S. B., Carnes, K. M., and Daniels, J. D. (1983). Two methods of catecholamine depletion in kitten visual cortex yield different effects on plasticity. Nature 302, 245–247.
Bear, M. F., and Singer, W. (1986). Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320, 172–176.
Beersma, D. G., Dijk, D. J., Blok, C. G., and Everhardus, I. (1990). REM sleep deprivation during 5 hours leads to an immediate REM sleep rebound and to suppression of non-REM sleep intensity. Electroencephalogr. Clin. Neurophysiol. 76, 114–122.
Bezin, L., Marcel, D., Debure, L. I., Ginovart, N., Rousset, C., Pujol, J. F., and Weissmann, D. (1994). Postnatal development of the tyrosine hydroxylase-containing cell population within the rat locus coeruleus: topological organization andphenotypic plasticity. J. Neurosci. 14, 7486–7501.
Bezin, L., Marcel, D., Desgeorges, S., Pujol, J. F., and Weissmann, D. (2000). Singular subsets of locus coeruleus neurons may recover tyrosine hydroxylase phenotype transiently expressed during development. Brain Res. Mol. Brain Res. 76, 275–281.
Bracha, H. S., Garcia-Rill, E., Mrak, R. E., and Skinner, R. (2005). Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J. Neuropsychiatry Clin. Neurosci. 17, 503–509.
Brocher, S., Artola, A., and Singer, W. (1992). Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 573, 27–36.
Debure, L. I., Moyse, E., Fevre-Montange, M., Hardin, H., Belin, M. F., Rousset, C., Pujol, J. F., and Weissmann, D. (1992). Somatotopic organization of tyrosine hydroxylase expression in the rat locus coeruleus: long term effect of RU24722. Brain Res. 581, 19–32.
Endo, T., Roth, C., Landolt, H. P., Werth, E., Aeschbach, D., Achermann, P., and Borbely, A. A. (1998). Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am. J. Physiol. 274, R1186–R1194.
Endo, T., Schwierin, B., Borbely, A. A., and Tobler, I. (1997). Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 66, 97–110.
Foote, S. L., Berridge, C. W., Adams, L. M., and Pineda, J. A. (1991). Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog. Brain Res. 88, 521–532.
Fuller, P. M., Gooley, J. J., and Saper, C. B. (2006). Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythms 21, 482–493.
Gu, Q. (2002). Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, 815–835.
Gundersen, H. J., Bagger, P., Bendtsen, T. F., Evans, S. M., Korbo, L., Marcussen, N., Moller, A., Nielsen, K., Nyengaard, J. R., and Pakkenberg, B. (1988). The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96, 857–881.
Hobson, J. A., McCarley, R. W., and Wyzinski, P. W. (1975). Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189, 55–58.
Hogan, D., Roffwarg, H. P., Li, Z., and Shaffery, J. P. (1998). Calcium-binding protein immunoreactive neurons in central visual pathways are changed by one week of rapid eye-movement sleep (REMS) deprivation in kittens. Abstr. Soc. Neurosci. 24, 563.5.
Hogan, D., Roffwarg, H. P., and Shaffery, J. P. (2001). The effects of 1 week of REM sleep deprivation on parvalbumin and calbindin immunoreactive neurons in central visual pathways of kittens. J. Sleep Res. 10, 285–296.
Hubel, D. H., and Wiesel, T. N. (1963). Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017.
Imamura, K., and Kasamatsu, T. (1991). Ocular dominance plasticity restored by NA infusion to aplastic visual cortex of anesthetized and paralyzed kittens. Exp. Brain Res. 87, 309–318.
Jacobs, B. L. (1986). Single unit activity of locus coeruleus neurons in behaving animals. Prog. Neurobiol. 27, 183–194.
Joho, R. H., Ho, C. S., and Marks, G. A. (1999). Increased gamma- and decreased delta-oscillations in a mouse deficient for a potassium channel expressed in fast-spiking interneurons. J. Neurophysiol. 82, 1855–1864.
Jones, B. E., and Yang, T. Z. (1985). The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 242, 56–92.
Jouvet, M. (1972). The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb. Physiol. 64, 165–307.
Jouvet-Mounier, D., Astic, L., and Lacote, D. (1970). Ontogenesis of the states of sleep in the rat, cat and guinea pig during the first postnatal month. Dev. Psychobiol. 2, 216–239.
Kasamatsu, T., and Pettigrew, J. D. (1976). Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science 194, 206–209.
Kawahara, H., Kawahara, Y., and Westerink, B. H. (2000). The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur. J. Pharmacol. 387, 279–286.
Kirkwood, A., Rozas, C., Kirkwood, J., Perez, F., and Bear, M. F. (1999). Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J. Neurosci. 19, 1599–1609.
Kroustrup, J. P., Gundersen, H. J., and Vaeth, M. (1988). Stereological analysis of three-dimensional structure organization of surfaces in multiphase specimens: statistical methods and model- inferences. J. Microsc. 149(Pt 2), 135–152.
Lehnert, H., Schulz, C., and Dieterich, K. (1998). Physiological and neurochemical aspects of corticotropin-releasing factor actions in the brain: the role of the locus coeruleus. Neurochem. Res. 23, 1039–1052.
Long, J. M., Kalehua, A. N., Muth, N. J., Hengemihle, J. M., Jucker, M., Calhoun, M. E., Ingram, D. K., and Mouton, P. R. (1998). Stereological estimation of total microglia number in mouse hippocampus. J. Neurosci. Methods 84, 101–108.
Majumdar, S., and Mallick, B. N. (2003). Increased levels of tyrosine hydroxylase and glutamic acid decarboxylase in locus coeruleus neurons after rapid eye movement sleep deprivation in rats. Neurosci. Lett. 338, 193–196.
Mallick, B. N., Siegel, J. M., and Fahringer, H. (1990). Changes in pontine unit activity with REM sleep deprivation. Brain Res. 515, 94–98.
McGinty, D. J., Stevenson, M., Hoppenbrouwers, T., Harper, R. M., Sterman, M. B., and Hodgman, J. (1977). Polygraphic studies of kitten development: sleep state patterns. Dev. Psychobiol. 10, 455–469.
McLean, I. W., and Nakane, P. K. (1974). Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 22, 1077–1083.
Mouton, P. R., Long, J. M., Lei, D. L., Howard, V., Jucker, M., Calhoun, M. E., and Ingram, D. K. (2002). Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 956, 30–35.
Oksenberg, A., Shaffery, J. P., Marks, G. A., Speciale, S. G., Mihailoff, G., and Roffwarg, H. P. (1996). Rapid eye movement sleep deprivation in kittens amplifies LGN cell-size disparity induced by monocular deprivation. Brain Res. Dev. Brain Res. 97, 51–61.
Pace-Schott, E. F., and Hobson, J. A. (2002). The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 3, 591–605.
Park, S. P. (2002). In vivo microdialysis measures of extracellular norepinephrine in the rat amygdala during sleep-wakefulness. J. Korean Med. Sci. 17, 395–399.
Porkka-Heiskanen, T., Smith, S. E., Taira, T., Urban, J. H., Levine, J. E., Turek, F. W., and Stenberg, D. (1995). Noradrenergic activity in rat brain during rapid eye movement sleep deprivation and rebound sleep. Am. J. Physiol. 268, R1456–R1463.
Saito, N., Shimada, M., Kitahama, K., and Maeda, T. (1996). Postnatal development of adrenergic terminals in rat locus coeruleus, with special reference to growth of noradrenergic neurons. Brain Res. Dev. Brain Res. 96, 241–248.
Selye, H. (1950). Stress and the general adaptation syndrome. Br. Med. J. (Clin. Res. Ed.) 4667, 1383–1392.
Shaffery, J. P., Hoffmann, R., and Armitage, R. (2003). The neurobiology of depression: perspectives from animal and human sleep studies. Neuroscientist 9, 82–98.
Shaffery, J. P., Lopez, J., Bissette, G., and Roffwarg, H. P. (2006). Rapid eye movement sleep deprivation revives a form of developmentally regulated synaptic plasticity in the visual cortex of post-critical period rats. Neurosci. Lett. 391, 96–101.
Shaffery, J. P., Marks, G. A., Speciale, S. G., and Roffwarg, H. P. (1990). REM-sleep deprivation on a vibrating platform: automating a standard technique. J. Sleep Res. 19, 110.
Shaffery, J. P., Oksenberg, A., Marks, G. A., Speciale, S. G., Mihailoff, G., and Roffwarg, H. P. (1998). REM sleep deprivation in monocularly occluded kittens reduces the size of cells in LGN monocular segment. Sleep 21, 837–845.
Shaffery, J. P., Sinton, C. M., Bisset, G., Roffwarg, H. P., and Marks, G. A. (2002). Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience 110, 431–443.
Shouse, M. N., Staba, R. J., Ko, P. Y., Saquib, S. F., and Farber, P. R. (2001a). Monoamines and seizures: microdialysis findings in locus ceruleus and amygdala before and during amygdala kindling. Brain Res. 892, 176–192.
Shouse, M. N., Staba, R. J., Saquib, S. F., and Farber, P. R. (2001b). Long-lasting effects of feline amygdala kindling on monoamines, seizures and sleep. Brain Res. 892, 147–165.
Siegel, J. M. (2005). “REM sleep,” in Principles and Practice of Sleep Medicine, eds M. H. Kryger, T. Roth, and W. C. Dement (Philadelphia: Elsevier Saunders), 120–135.
Spiro, R. H., and Kolbert, G. S. (1974). A new technique for functional visual deprivation. Electroencephalogr. Clin. Neurophysiol. 37, 654–656.
Ursin, R., and Sterman, M. B. (1981). A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat. Los Angeles: Brain Information Service.
Valentino, R. J., and Van, E. (2001). Bockstaele, opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl.) 158, 331–342.
West, M. J., Slomianka, L., and Gundersen, H. J. (1991). Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497.
Keywords: norepinephrine, critical period, fast Fourier transforms, stereology, depression
Citation: Shaffery JP, Allard JS, Manaye KF and Roffwarg HP (2012) Selective rapid eye movement sleep deprivation affects cell size and number in kitten locus coeruleus. Front. Neur. 3:69. doi: 10.3389/fneur.2012.00069
Received: 20 February 2012; Paper pending published: 04 March 2012;
Accepted: 10 April 2012; Published online: 15 May 2012.
Edited by:
Larry Sanford, Eastern Virginia Medical School, USAReviewed by:
Edgar Garcia-Rill, University of Arkansas for Medical Sciences, USAChris Ward, University of Houston-Clear Lake, USA
Copyright: © 2012 Shaffery, Allard, Manaye and Roffwarg. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: James P. Shaffery, Division of Neurobiology and Behavior Research, Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, 2500 N. State Street, Jackson, MS 39216-4505, USA. e-mail:anNoYWZmZXJ5QHBzeWNoaWF0cnkudW1zbWVkLmVkdQ==

 Joanne S. Allard2
Joanne S. Allard2