- 1 Human Sleep Research Program, Center for Health Sciences, SRI International, Menlo Park, CA, USA
- 2 School of Psychological Sciences, The University of Melbourne, Parkville, VIC, Australia
- 3 Brain Function Research Group, School of Physiology, University of the Witwatersrand, Johannesburg, South Africa
Stimuli presented during sleep can produce an evoked EEG delta wave referred to as a K-complex. These responses occur when large numbers of cortical cells burst fire in a synchronized manner. Large amplitude synchronized scalp responses require that the CNS contain large numbers of healthy neurons that are interconnected with highly functional white matter pathways. The P2, N550, and P900 components of the evoked K-complex are sensitive measures of normal healthy brain aging, showing a decrease in amplitude with age. N550 and P900 amplitudes are also reduced in recently detoxified alcoholics, most dramatically over frontal scalp regions. The present study tested the hypothesis that the amplitude of K-complex related evoked potential components would increase with prolonged abstinence. Fifteen alcoholics (12 men) were studied twice, separated by a 12 month period, during which time they were followed with monthly phone calls. Subjects were aged between 38 and 60 years at their first study. They had on average a 29.3 ± 6.7 year drinking history and had been abstinent for between 54 and 405 days at initial testing. Evoked K-complexes were identified in the EEG and averaged to enable measurement of the P2, N550 and P900 peaks. Data were collected from seven scalp sites (FP1, FP2, Fz, FCz, Cz, CPz, and Pz). N550 and P900 amplitudes were significantly higher after 12 months of abstinence and an improvement of at least 5 μV occurred in 12 of the 15 subjects. N550 and P900 also showed highly significant site by night interactions with the largest increases occurring over prefrontal and frontal sites. The data indicate that the sleep evoked response may provide a sensitive marker of brain recovery with abstinence from alcohol.
Introduction
Alcoholism is a major health problem with an estimated lifetime prevalence in the United States of approximately 14% (American-Psychiatric-Association, 2000). Sleep disturbances are ubiquitous in those suffering from alcohol abuse and dependence (Brower, 2003), last long after successful detoxification and have been viewed as a potential pathway to relapse, when alcohol is consumed to facilitate sleep onset (Roehrs et al., 1999). Quantitative markers of sleep physiology may therefore be indicative of severity of alcoholism pathology and may have a causal relationship to heightened relapse risk.
The most consistent sleep finding (Benca et al., 1992) in detoxified alcoholics is a reduction in delta activity measured either as SWS (Allen and Wagman, 1975; Rundell et al., 1975; Wagman and Allen, 1975; Williams and Rundell, 1981; Othmer et al., 1982; Gillin et al., 1990; Le Bon et al., 1997; Drummond et al., 1998; Irwin et al., 2000, 2002; Gann et al., 2001; Feige et al., 2007), or slow wave activity (SWA; Irwin et al., 2000, 2002; Colrain et al., 2009a). Deficient SWS has also been shown to be a predictor of relapse (Brower, 2003). The averaged KC that can be evoked by a stimulus provides a novel experimentally controlled probe of delta EEG production. Its amplitude (N550) is a marker of the ability of the underlying cortex to produce a synchronized response, and thus an indicator of its functional integrity (Finelli et al., 2001; Crowley et al., 2002, 2004, 2005; Colrain, 2005; Nicholas et al., 2006). N550 and P900 amplitudes and KC incidence are reduced in alcoholics (Colrain et al., 2009b) in a manner that represents an exacerbation of the normal aging effects on these components (Crowley et al., 2002, 2004; Colrain et al., 2010), and is more pronounced at frontal sites.
In addition to being reduced in alcoholics, the N550 has been shown to be correlated with periods of abstinence across subjects but was not related to family history of alcoholism (Colrain et al., 2011). It therefore appears to act as a state marker of the pharmacological effects of alcohol use in those with alcohol dependence, and leads to a hypothesis that within individuals, longitudinal data should show increased N550 and P900 amplitudes with abstinence. The hypothesis is further supported by evidence that brain structure demonstrates changes with abstinence (Cardenas et al., 2007) as do aspects of alcoholism-related cognitive and motor decline (Fein and Mcgillivray, 2007; Yeh et al., 2007), although to varying extents and with variable trajectories (Sullivan and Pfefferbaum, 2005).
The present study sought to determine whether 12 months of abstinence is associated with functional recovery of delta generating ability in sleep EEG.
Materials and Methods
Subjects
The present data are drawn from 15 alcohol-dependent subjects (12 men) who had been studied previously (Colrain et al., 2009b). All were followed with monthly phone calls and then restudied approximately 12 months after their initial laboratory visit (mean 394 ± 48 days). Potential participants in the initial study underwent medical and psychiatric screening that included a structured alcohol history (Pfefferbaum et al., 1988) and structured clinical interview (SCID; First et al., 1994). All alcoholic participants met DSM-IV criteria for alcohol dependence, and none had clinically significant sleep disorders based on polysomnographic screening. Eighty-four subjects completed the initial study, 42 with alcohol dependence (27 men) and 42 controls (19 men). The 15 subjects studied a second time after 12 months of abstinence were aged between 38 and 60 years at the time of the first study (mean 52.1 ± 7.2 year). They had on average a 29.3 ± 6.7 year drinking history and had been abstinent for between 54 and 405 days at initial testing (mean 165.3 ± 107.7). Their estimated lifetime alcohol consumption (mean 1283 ± 814 kg) ranged between 603 and 3811 kg for the men and 621 and 1384 kg for the women in the group.
Monthly phone calls and a detailed drinking history taken at the 12 month visit revealed that 13 of the subjects had remained totally abstinent. Of the remaining two, one had a single drink 262 days prior to follow-up, and the other a single drinking occasion 300 days prior to follow-up.
General Laboratory Protocol
The protocol for eliciting sleep evoked potentials is outlined in detail in (Colrain et al., 2009b). Briefly, in both the initial and 12 month follow-up studies subjects slept in the laboratory and were exposed to a standard sleep evoked potential protocol. Auditory stimuli consisted of 1000 Hz pure tones presented for 50 ms (2 ms rise time) at 80 dB(A), presented binaurally via E-A-RTONE 3A insert earphones, with a random inter stimulus interval of between 15 and 30 s. A minimum of 200 stimuli were presented in stable stage 2 sleep across a night in the laboratory.
EEG and evoked potential data were recorded from seven scalp sites adapted from the international 10/20 system (FP1, FP2, Fz, FCz, Cz, CPz, Pz), using Grass gold-plated 10 mm electrodes. Two channels of electro-oculogram (EOG) and a submental electromyogram (EMG) were also recorded. All EEG channels were referenced to linked earlobes. Raw data were acquired with a sampling rate of 1 kHz using Neuroscan Synamps® amplifiers and stored for offline analysis using Neuroscan Scan™ software. Signals were continuously displayed in real-time and filtered optimally for visual recognition of sleep stages. Raw EEG data were epoched, time-locked to tone stimuli, and corrected for baseline differences across the pre-stimulus period.
Evoked KCs were defined as per our standard laboratory practice (Crowley et al., 2002, 2004; Nicholas et al., 2002a,b; Colrain et al., 2009b, 2010, 2011) using data from Cz and Fz. The negative peak of the KC had to occur between 400 and 900 ms. after the tone, but no amplitude criterion was used. Epochs containing movement artifacts observed in EOG or EMG channels were discarded. All KC responses within stage 2 sleep were then averaged for each subject to produce an averaged KC evoked potential at all sites.
Component identification was achieved using Scan™ software. The P2 component was defined as the most positive peak between 100 and 300 ms post stimulus, the N550 as the most negative value between 400 and 900 ms post stimulus and the P900 as the most positive peak between 800 and 1200 ms post stimulus. Amplitudes were then determined relative to the average of the pre-stimulus baseline. All peaks were determined within all electrode sites.
Statistical Analysis
Data are shown as mean (SD). Repeated measures analysis of variance was conducted to determine whether there were effects of time (initial vs. follow-up) and electrode site (FP1, FP2, Fz, FCz, Cz, CPz, Pz) or time by site interactions. When the assumption of sphericity was violated, degrees of freedom were adjusted using the Greenhouse–Geisser correction, but original degrees of freedom are reported. Relationships between changes in peak amplitude and measures of drinking were evaluated using bivariate correlation analysis.
Results
Grand mean waveforms for all subjects at initial assessment and 12 month follow-up are presented in Figure 1.
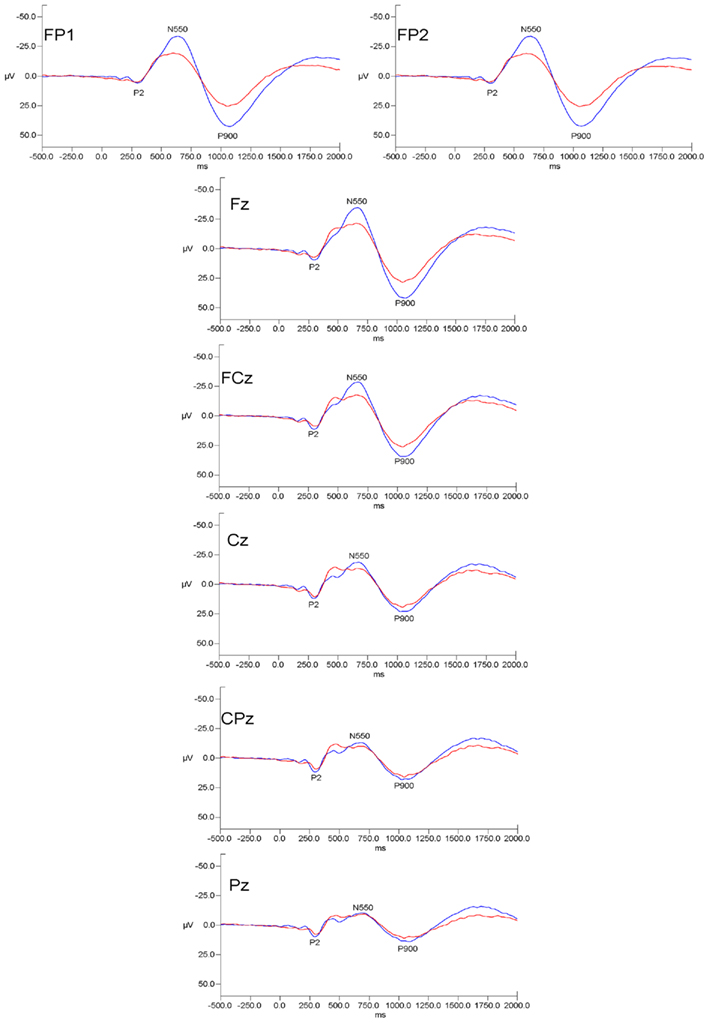
Figure 1. Grand mean evoked potential waveforms for alcoholics at initial assessment (red lines) and at 12 month follow-up (blue lines) for the seven measured electrode sites. Data are presented with negative voltages up the Y axis. The pattern of results indicates that initial-follow-up differences were prominent over frontal and frontal–polar sites but were diminished at more posterior scalp locations.
N550 amplitude was significantly larger at follow-up than at the initial assessment (F(1,12) = 10.631, p < 0.01). The follow-up amplitude at Fz was at least 5 μV larger than that at initial assessment in 12 of the 15 subjects. It displayed a significant effect of electrode site (F(6,72) = 18.055, p < 0.001), with the typical anterior to posterior gradient. There was a significant time by electrode site interaction (F(6,72) = 7.310, p = 0.005) indicating that the increase in amplitude occurred only at the anterior sites (see Figure 2). N550 latency showed no time, site or time by site interaction effects.
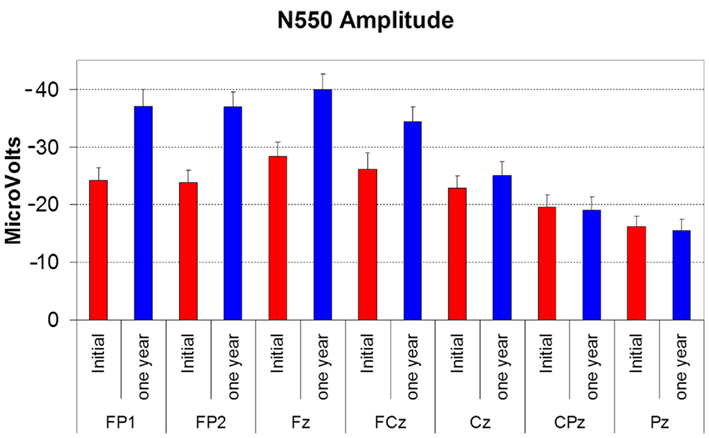
Figure 2. Histogram showing the amplitude of N550 (in microvolts) at initial assessment (red bars) and at 12 month follow-up (blue bars) at each electrode site. Error bars reflect SD. Note that for this figure increasing negative values are represented as traveling up the Y axis.
P900 amplitude displayed the same effects as seen for N550 amplitude, with effects of time (F(1,12) = 8.099, p = 0.015), electrode site (site F(6,72) = 25.218, p < 0.001), and time by site interaction (F(6,72) = 8.444, p = 0.003). P900 was larger at anterior sites, larger overall at follow-up, with the increase being more prominent at anterior sites (see Figure 3). P900 latency did not vary with time or electrode site.
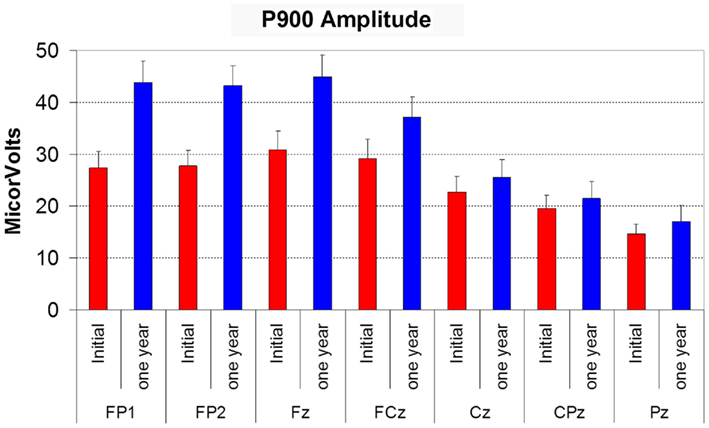
Figure 3. Histogram showing the amplitude of P900 (in microvolts) at initial assessment (red bars) and at 12 month follow-up (blue bars) at each electrode site. Error bars reflect SD.
P2 amplitude displayed a significant effect of electrode site (F(6,72) = 11.020, p < 0.01) with the typical vertex maximum on both occasions (see Figure 4). Follow-up was not significantly different to the initial recording, and there was no time by site interaction. P2 latency also did not vary with time or electrode site.
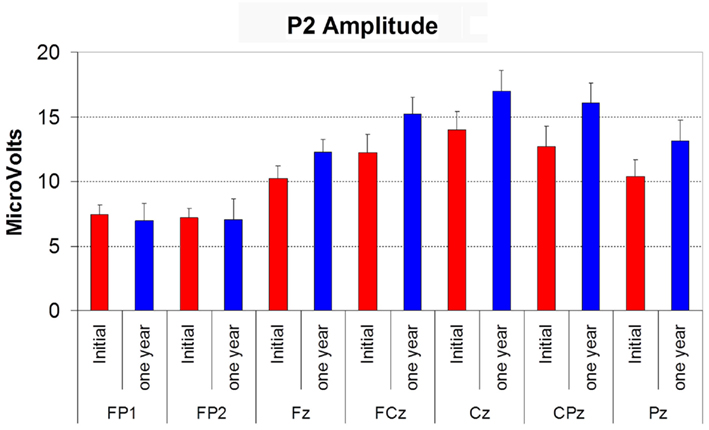
Figure 4. Histogram showing the amplitude of P2 (in microvolts) at initial assessment (red bars) and at 12 month follow-up (blue bars) at each electrode site. Error bars reflect SD.
The magnitude of recovery in N550 amplitude at Fz did not correlate with total estimated lifetime alcohol consumption, the period of abstinence prior to the initial assessment, or the number of years spent drinking. The same negative findings were apparent when only the male subjects were included in the analysis.
Discussion
The results show promising initial support for sleep evoked response as a marker of brain recovery with abstinence. Despite small numbers, highly significant results were found showing recovery in N550 and P900 amplitudes at electrode sites in which we have previously shown alcohol dependence-related reductions (Colrain et al., 2009b) and significant relations to MRI measures of brain gray matter volume (Colrain et al., 2011).
It should be emphasized that this apparent partial recovery in delta generation, while a useful functional marker of brain change should not be interpreted as recovery of sleep. Sleep problems are highly persistent even in long-term abstinent alcoholics and are commonly found to be predictive of relapse (Brower, 2003). We have previously shown that variations in prior length sobriety are not significantly predictive of increased Pittsburgh sleep quality index (PSQI; Buysse et al., 1989) score, decreased SWS, and increased REM in abstinent alcoholics, within the observed range of prior abstinence of between 30 and 719 days (Colrain et al., 2009a).
Abstinence is associated with recovery of structural brain tissue: white matter volume increases with abstinence (Shear et al., 1994; O’Neill et al., 2001) and declines further with relapse (Pfefferbaum et al., 1995). There is less evidence for improvements in gray matter volume (GMV), although Pfefferbaum et al. (1995) reported a trend for increased GMV at 30 days of abstinence. Whole brain tissue volume increases with abstinence (Parks et al., 2002; Gazdzinski et al., 2005; Bartsch et al., 2007), with some evidence that the increase may be greater in alcoholics who are also smokers compared to non-smokers (Yeh et al., 2007). There is some evidence for a good proportion of the tissue volume increase to occur within the first month or so following drinking cessation (Parks et al., 2002) and for rapid reversal of brain volume gains with resumption of drinking (Gazdzinski et al., 2005). The most extensive study of volumetric changes with abstinence was reported by Cardenas et al. (2007). Abstainers showed between 7 and 12% faster recovery in a number of brain regions. This recovery may underlie the apparent recovery in N550 amplitude given the recent finding of GMV volume accounting for approximately 20% of the variance in N550 amplitude in control and alcoholic subjects (Colrain et al., 2011).
The study has limitations in terms of the number of subjects observed, and the absence of data from alcoholics who relapsed. Attempts to conduct monthly follow-up by phone were made for all 42 alcoholics from the initial study (Colrain et al., 2009b) with success in 39 cases. Of these 14 relapsed and 25 maintained abstinence. A feature of the disease is that while drinking, alcoholics are unlikely to reliably attend the laboratory, so the study is limited to those abstainers who were sufficiently motivated to re-attend. Nothing definitive can thus be said regarding the possibility that relapsing alcoholics might also have shown a recovery if studied, and more extensive longitudinal assessment of relapsing and abstaining alcoholics is required to address this possibility. Cross-sectional data show N550 and P900 amplitude as lower in older subjects, with regression analysis showing a 1.5 μV decrease per year in N550 at Fz (Colrain et al., 2010). As indicated above, 12 of the 15 subjects displayed at least a 5 μV increase at their 12 month follow-up.
Drinking cessation can herald structural and functional brain recovery: ventricular volume can shrink, frontal gray matter volume can increase, and improved function can accompany positive changes in brain structure. Recovery of delta generation with abstinence has implications for cognitive function in abstinent alcoholics. Delta activity is considered beneficial and with processes of long-term facilitation and disfacilitation of neural synapses (Tononi and Cirelli, 2006) and there is growing evidence to show it is related to memory consolidation (Poe et al., 2010).
While accepting their limitations, we suggest that the present data are the first to show suggestive evidence of recovery of delta generating ability in recovering alcoholics. We hypothesize that such recovery is mediated by improvements in both white matter integrity and GMV.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by NIH grants AA020565, AA014211.
References
Allen, R., and Wagman, A. (1975). “Do sleep patterns relate to the desire for alcohol?” in Alcohol Intoxication and Withdrawal, ed. M. M. Grosss (New York: Plenum Press), 495–508.
American-Psychiatric-Association. (2000). Diagnostic and Statistical Manual for Psychiatric Disorders IV Text Revision. Washington, DC: American Psychiatric Press.
Bartsch, A. J., Homola, G., Biller, A., Smith, S. M., Weijers, H. G., Wiesbeck, G. A., Jenkinson, M., De Stefano, N., Solymosi, L., and Bendszus, M. (2007). Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 130, 36–47.
Benca, R. M., Obermeyer, W. H., Thisted, R. A., and Gillin, J. C. (1992). Sleep and psychiatric disorders. A meta-analysis. Arch. Gen. Psychiatry 49, 651–668.
Buysse, D. J., Reynolds, C. F. III, Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213.
Cardenas, V. A., Studholme, C., Gazdzinski, S., Durazzo, T. C., and Meyerhoff, D. J. (2007). Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34, 879–887.
Colrain, I., Turlington, S. R., and Baker, F. C. (2009a). The impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep 32, 1341–1352.
Colrain, I. M., Crowley, K. E., Nicholas, C. L., Padilla, M., and Baker, F. C. (2009b). The impact of alcoholism on sleep evoked Delta frequency responses. Biol. Psychiatry 66, 177–184.
Colrain, I. M., Crowley, K. E., Nicholas, C. L., Afifi, L., Baker, F. C., Padilla, M., Turlington, S. R., and Trinder, J. (2010). Sleep evoked delta frequency responses show a linear decline in amplitude across the adult lifespan. Neurobiol. Aging 31, 874–883.
Colrain, I. M., Sullivan, E. V., Rohlfing, T., Baker, F. C., Nicholas, C. L., Padilla, M. L., Chanraud, S., Pitel, A. L., and Pfefferbaum, A. (2011). Independent contributions of cortical gray matter, aging, sex and alcoholism to K-complex amplitude evoked during sleep. Sleep 34, 787–795.
Crowley, K., Sullivan, E. V., Adalsteinsson, E., Pfefferbaum, A., and Colrain, I. M. (2005). Differentiating pathologic delta from healthy physiologic delta in patients with Alzheimer disease. Sleep 28, 865–870.
Crowley, K. E., Trinder, J., and Colrain, I. M. (2002). An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J. Sleep Res. 11, 129–140.
Crowley, K. E., Trinder, J., and Colrain, I. M. (2004). Evoked K-complex generation: the impact of sleep spindles and age. Clin. Neurophysiol. 115, 471–476.
Drummond, S. P., Gillin, J. C., Smith, T. L., and Demodena, A. (1998). The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol. Clin. Exp. Res. 22, 1796–1802.
Feige, B., Scaal, S., Hornyak, M., Gann, H., and Riemann, D. (2007). Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol. Clin. Exp. Res. 31, 19–27.
Fein, G., and Mcgillivray, S. (2007). Cognitive performance in long-term abstinent elderly alcoholics. Alcohol. Clin. Exp. Res. 31, 1788–1799.
Finelli, L. A., Borbely, A. A., and Achermann, P. (2001). Functional topography of the human nonREM sleep electroencephalogram. Eur. J. Neurosci. 13, 2282–2290.
First, M. B., Spitzer, R. L., Gibbon, M., and Williams, J. B. W. (1994). Structured Clinical Interview for Axis I DSM IV Disorders. New York: New York State Psychiatric Institute.
Gann, H., Feige, B., Hohagen, F., Van Calker, D., Geiss, D., and Dieter, R. (2001). Sleep and the cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biol. Psychiatry 50, 383–390.
Gazdzinski, S., Durazzo, T. C., and Meyerhoff, D. J. (2005). Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 78, 263–273.
Gillin, J., Smith, T., Irwin, M., Kripke, D., and Schuckit, M. (1990). EEG sleep studies in “pure” primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biol. Psychiatry 27, 477–488.
Irwin, M., Gillin, J. C., Dang, J., Weissman, J., Phillips, E., and Ehlers, C. L. (2002). Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol. Psychiatry 51, 632–641.
Irwin, M., Miller, C., Gillin, J. C., Demodena, A., and Ehlers, C. L. (2000). Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol. Clin. Exp. Res. 24, 1376–1384.
Le Bon, O., Verbanck, P., Hoffmann, G., Murphy, J., Staner, L., De Groote, D., Mampunza, S., Den Dulk, A., Vacher, C., Kornreich, C., and Pelc, I. (1997). Sleep in detoxified alcoholics: impairment of most standard sleep parameters and increased risk for sleep apnea, but not for myoclonias – a controlled study. J. Stud. Alcohol 58, 30–36.
Nicholas, C. L., Sullivan, E. V., Pfefferbaum, A., Trinder, J., and Colrain, I. M. (2002a). The effects of alcoholism on auditory evoked potentials during sleep. J. Sleep Res. 11, 247–253.
Nicholas, C. L., Trinder, J., and Colrain, I. M. (2002b). Increased production of evoked and spontaneous K-complexes following a night of fragmented sleep. Sleep 25, 882–887.
Nicholas, C. L., Trinder, J., Crowley, K. E., and Colrain, I. M. (2006). The impact of slow wave sleep proximity on evoked K-complex generation. Neurosci. Lett. 404, 127–131.
O’Neill, J., Cardenas, V. A., and Meyerhoff, D. J. (2001). Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol. Clin. Exp. Res. 25, 1673–1682.
Othmer, O., Dauhaday, W., Goodin, D., Levine, W., Malarkey, W., Freemon, F., and Halikas, J. (1982). Sleep and growth hormone secretion in adults. J. Clin. Psychiatry 43, 411–414.
Parks, M. H., Dawant, B. M., Riddle, W. R., Hartmann, S. L., Dietrich, M. S., Nickel, M. K., Price, R. R., and Martin, P. R. (2002). Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol. Clin. Exp. Res. 26, 1368–1380.
Pfefferbaum, A., Rosenbloom, M., Crusan, K., and Jernigan, T. L. (1988). Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol. Clin. Exp. Res. 12, 81–87.
Pfefferbaum, A., Sullivan, E. V., Mathalon, D. H., Shear, P. K., Rosenbloom, M. J., and Lim, K. O. (1995). Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol. Clin. Exp. Res. 19, 1177–1191.
Poe, G. R., Walsh, C. M., and Bjorness, T. E. (2010). Cognitive neuroscience of sleep. Prog. Brain Res. 185, 1–19.
Roehrs, T., Papineau, K., Rosenthal, L., and Roth, T. (1999). Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology 20, 279–286.
Rundell, O., Williams, H., and Lester, B. (1975). “Sleep in alcoholic patients: longitudinal findings,” in Alcohol Intoxication and Withdrawal, ed. M. M. Gross (New York: Plenum Press), 389–402.
Shear, P. K., Jernigan, T. L., and Butters, N. (1994). Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol. Clin. Exp. Res. 18, 172–176.
Sullivan, E. V., and Pfefferbaum, A. (2005). Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl.) 180, 583–594.
Tononi, G., and Cirelli, C. (2006). Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62.
Wagman, A., and Allen, R. (1975). “Effects of alcohol ingestion and abstinence on slow wave sleep of alcoholics,” in Alcohol Intoxication and Withdrawal, ed. M. M. Gross (New York: Plenum Press), 453–466.
Williams, H. L., and Rundell, O. H. Jr. (1981). Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol. Clin. Exp. Res. 5, 318–325.
Keywords: alcoholism, N550, K-complex, abstinence
Citation: Colrain IM, Padilla ML and Baker FC (2012) Partial recovery of alcohol dependence-related deficits in sleep evoked potentials following 12 months of abstinence. Front. Neur. 3:13. doi: 10.3389/fneur.2012.00013
Received: 08 December 2011;
Paper pending published: 30 December 2011;
Accepted: 17 January 2012;
Published online: 06 February 2012.
Edited by:
Michael Czisch, Max Planck Institute of Psychiatry, GermanyReviewed by:
Timo Partonen, University of Helsinki, FinlandPeter Halasz, Hungarian Sleep Society, Hungary
Copyright: © 2012 Colrain, Padilla and Baker. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Ian M. Colrain, Human Sleep Research Program, SRI International, 333 Ravenswood Avenue, Menlo Park, CA 94025, USA. e-mail:aWFuLmNvbHJhaW5Ac3JpLmNvbQ==

 Mayra L. Padilla1 and Fiona C. Baker2,3
Mayra L. Padilla1 and Fiona C. Baker2,3