- 1 Instituto de Fisiología, Biología Molecular y Neurociencias, Consejo Nacional de Investigaciones Científicas y Técnicas, University of Buenos Aires, Buenos Aires, Argentina
- 2 Department of Neurobiology and Developmental Sciences, Center for Translational Neuroscience, University of Arkansas for Medical Sciences, Little Rock, AR, USA
This review considers recent evidence showing that cells in three regions of the reticular activating system (RAS) exhibit gamma band activity, and describes the mechanisms behind such manifestation. Specifically, we discuss how cells in the mesopontine pedunculopontine nucleus (PPN), intralaminar parafascicular nucleus (Pf), and pontine subcoeruleus nucleus dorsalis (SubCD) all fire in the beta/gamma band range when maximally activated, but no higher. The mechanisms behind this ceiling effect have been recently elucidated. We describe recent findings showing that every cell in the PPN have high-threshold, voltage-dependent P/Q-type calcium channels that are essential, while N-type calcium channels are permissive, to gamma band activity. Every cell in the Pf also showed that P/Q-type and N-type calcium channels are responsible for this activity. On the other hand, every SubCD cell exhibited sodium-dependent subthreshold oscillations. A novel mechanism for sleep–wake control based on well-known transmitter interactions, electrical coupling, and gamma band activity is described. The data presented here on inherent gamma band activity demonstrates the global nature of sleep–wake oscillation that is orchestrated by brainstem–thalamic mechanism, and questions the undue importance given to the hypothalamus for regulation of sleep–wakefulness. The discovery of gamma band activity in the RAS follows recent reports of such activity in other subcortical regions like the hippocampus and cerebellum. We hypothesize that, rather than participating in the temporal binding of sensory events as seen in the cortex, gamma band activity manifested in the RAS may help stabilize coherence related to arousal, providing a stable activation state during waking and paradoxical sleep. Most of our thoughts and actions are driven by pre-conscious processes. We speculate that continuous sensory input will induce gamma band activity in the RAS that could participate in the processes of pre-conscious awareness, and provide the essential stream of information for the formulation of many of our actions.
Coherence and Frequency
The reticular activating system (RAS) controls sleep and waking, and fight vs. flight responses. While this system provides signals that modulate our sleep–wake states, it also serves to help us respond to the world around us. For example, strong stimuli simultaneously activate ascending RAS projections to the thalamus and then the cortex and cause arousal, and also activate descending projections that influence the spinal cord in the form of postural changes in tone resulting from the startle response, as well as trigger locomotor events in fight vs. flight responses. During sleep, the same system is responsible for the relative lack of sensory awareness during slow wave sleep (SWS), as well as the atonia of paradoxical sleep that prevents us from acting out our dreams. This system also modulates the activity of virtually every other system in the CNS. Growing evidence suggests that the control of sleep and waking is a fundamental property of neuronal networks and prior activity within each network (Krueger et al., 2008), and that intrinsic properties of neurons in multiple regions modulate sleep auto-regulation, i.e., suggesting that sleep is neither a passive nor an active phenomenon (Kumar, 2010). Two major elements determining the activity of large assemblies of neurons such as in the electroencephalogram (EEG) are coherence and frequency. Coherence is the term for how groups of neurons, firing in coordination, can create a signal that is mirrored instantaneously and precisely by other groups of neurons across the brain. These transient episodes of coherence across different parts of the brain may be an electrical signature of thought and actions. A recent discovery demonstrated the presence of electrical coupling in three nuclei of the RAS, a mechanism that allows groups of neurons to fire synchronously. That mechanism was addressed in a recent review that described for the first time the presence of electrical coupling in the RAS, and how that mechanism is modulated by the stimulant modafinil, which increases electrical coupling, to drive coherence at higher frequencies to induce arousal (Garcia-Rill et al., 2008a). Briefly, modafinil increases electrical coupling and, since most coupled neurons in the RAS are GABAergic, the coupling decreases input resistance, decreasing activity, and GABA release, thus disinhibiting efferents to all other systems. This disinhibition leads to overall higher coherence in activity, i.e., during sleep and arousal, in the RAS (Garcia-Rill et al., 2007, 2008a; Heister et al., 2007), and thalamocortical systems (Urbano et al., 2007). Because increased coupling in GABAergic neurons will lead to decreased GABA release, the tendency will be to disinhibit most other transmitter systems, leading to increased excitation, especially during waking. However, if modafinil increases electrical coupling, it should enable better coherence at all frequencies, during waking and even during sleeping, after its effects are waning, during sleeping. Recently, use-dependent plasticity, which has been extensively described for chemical synapses, was described for reticular thalamic nucleus electrical synapses in the form of long-term depression (Haas et al., 2011), making knowledge about electrical coupling in the RAS even more compelling.
The other face of large-scale activity is frequency of firing, especially of ensemble activity, that is also essential to the neural encoding process. The present review is based on another major discovery, the presence of gamma band activity in the same RAS nuclei. Very recent data suggest that many, perhaps all, of the neurons in these three RAS regions fire at gamma band frequency when maximally activated, but no higher. These results now suggest that brainstem regions not only can generate, but are capped at, such frequencies, which is surprising because gamma band activity was first described in the cortex and is presumably involved in consciousness, learning, and memory. This is less surprising when one considers that gamma band activity has been described in other subcortical regions like thalamus, hippocampus, and cerebellum. The goal then becomes one to identify the mechanisms behind gamma band activity in the RAS. We will first address the classical role of gamma band activity, the presence and mechanisms behind gamma band activity in subcortical brain regions, then turn to the mechanisms behind gamma band activity in the RAS, and finally speculate on the potential role of such activity appearing at brainstem levels, in a very old, phylogenetically speaking, region such as the RAS.
Gamma Band Activity
During waking and paradoxical sleep, the EEG shows low amplitude, high frequency activity at beta/gamma frequencies (∼20–30/30–90 Hz; Steriade and McCarley, 1990; Buzsáki and Draguhn, 2004). Gamma oscillations appear to participate in sensory perception, problem solving, and memory (Eckhorn et al., 1988; Gray and Singer, 1989; Jones, 2007; Palva et al., 2009; Philips and Takeda, 2009; Voss et al., 2009), and coherence at these frequencies may occur at cortical or thalamocortical levels (Llinas et al., 1991; Singer, 1993). Indeed, synchronous gamma band activation among thalamocortical networks (Llinas et al., 2002), and in other neuronal groups (i.e., hippocampal and striatal afferents and efferents) is thought to contribute to the merger, or “binding,” of information originating from separate regions (Llinás and Paré, 1991). Conversely, gamma oscillation deficits have been suggested as a pathophysiologic feature of diseases like schizophrenia and Alzheimer’s disease (Steriade and Llinás, 1988; Ribary et al., 1991; Stam et al., 2002; Woo et al., 2010).
Gamma oscillations emerge from the dynamic interaction between intrinsic neuronal and synaptic properties of thalamocortical networks (Steriade and Llinás, 1988; Steriade and McCarley, 1990). Cortical gamma band generation can be influenced by subcortical structures like the hippocampus and cerebellum (Soteropoulos and Baker, 2006; Sirota et al., 2008). The neuronal networks behind such activity include the presence of inhibitory cortical interneurons with intrinsic membrane potential oscillatory activity in the gamma range (Steriade and Llinás, 1988; Llinas et al., 1991; Steriade, 1999), many of which are electrically coupled (Gibson et al., 1999), as well as of fast rhythmic bursting pyramidal neurons (also electrically coupled; Cunningham et al., 2004). At the thalamic level, thalamocortical excitatory neurons have intrinsic properties needed to generate subthreshold gamma band membrane potential oscillations (Pedroarena and Llinás, 1997).
While cortical interneurons can generate membrane potential gamma oscillations through the activation of voltage-dependent, persistent sodium channels (Llinas et al., 1991), and metabotropic glutamate receptors (Whittington et al., 1995), in thalamocortical neurons, the mechanism responsible for gamma band activity involves high-threshold P/Q-type voltage-gated calcium channels located in the dendrites (Pedroarena and Llinás, 1997). Moreover, the same intrinsic properties mediating gamma band oscillations are present in the thalamus of several vertebrate species, indicating considerable evolutionary conservation (Llinás and Steriade, 2006).
Voltage-gated calcium channel involvement in gamma band generation is particularly important. Indeed, calcium channels are known to play a pivotal role in determining intrinsic properties and synaptic transmission throughout the central nervous system (Katz and Miledi, 1965; Llinas and Hess, 1976; Caterall, 1988; Llinas, 1988; Llinas et al., 2007). P/Q-type channels (also known as Cav2.1 channels) are present widely in the brain (Hillman et al., 1991; Uchitel et al., 1992; Jones, 2007; Llinas et al., 2007). N-type calcium channels are found in the rat auditory brainstem, are restricted to the early postnatal period, and are replaced by P/Q-type channels later in development (Westenbroek et al., 1992; Iwasaki and Takahashi, 1998). Immunocytochemical techniques have demonstrated the presence of N-type channels in brainstem structures (Shen et al., 2004). Importantly, P/Q-type mutant mice have deficient gamma band activity in the EEG, abnormal sleep–wake states, ataxia, are prone to seizures (low frequency synchrony), and die by 3 weeks of age (Llinas et al., 2007).
Gamma Band in Subcortical Regions
Both the hippocampus and cerebellum have the intrinsic and synaptic properties necessary to generate gamma band oscillatory activity. Hippocampal oscillatory activity in the gamma range (30–60 Hz) has been extensively described to be functional associated with entorhinal cortex afferents (Charpak et al., 1995). Interestingly, neurons located in the entorhinal cortex can also oscillate at gamma band frequencies, suggesting a key role for such afferents in maintaining hippocampal gamma oscillations (Chrobak and Buzsáki, 1998). Recently, gamma band activity in the CA1 area was divided into fast (>5 Hz) and slow (∼25–60 Hz) frequency components that differentially couple CA1 and CA3 subfields, respectively (Colgin et al., 2009). Such differences have been proposed to “bind” CA1 fast gamma oscillations with very high frequency activity from entorhinal cortex (in charge of providing information about object and place recognition in rodents Bussey et al., 1999), whereas CA1 slow gamma oscillations would be locked to the slower frequencies present in the CA3 area in charge of memory storage (Colgin et al., 2009; Colgin and Moser, 2010).
Similarly, a peak in gamma band power has been described in the Purkinje cell layer around the apex of the lobule, and to a lower extent in distal white matter, has been described (Lang et al., 2006; Middleton et al., 2008). GABA-A but not glutamate receptors are critical for gamma oscillation generation in Purkinje cells (Lang et al., 2006). Cortico-cerebellar coherence at gamma frequencies is evident in monkeys during performance of a manual precision grip task (Soteropoulos and Baker, 2006), and cerebello-thalamic activity is synchronized with neocortical activity at gamma frequencies (Timofeev and Steriade, 1997). Finally, it has been proposed that both cerebellar and thalamocortical networks might oscillate at the same frequencies to enable information exchange among these brain areas (Middleton et al., 2008).
Given the wealth of information on gamma band activity in cortical, thalamic, cerebellar, and hippocampal systems, would the presence of gamma band activity in the RAS seem unexpected?
Gamma in the RAS – the PPN
The pedunculopontine nucleus (PPN) is most active during waking and paradoxical sleep (Steriade and McCarley, 1990). The PPN is the arm of the RAS that modulates ascending projections through the thalamus (modulating arousal) and descending projections through the pons and medulla (modulating posture and locomotion), and is composed of different populations of cholinergic, glutamatergic, and GABAergic neurons (Wang and Morales, 2009). The PPN contains three basic cell types based on in vitro intrinsic membrane properties (Leonard and Llinas, 1990; Kamondi et al., 1992; Takakusaki and Kitai, 1997). Type I PPN neurons display calcium-mediated low threshold spikes (LTS) following a return from hyperpolarization. Type II PPN neurons have a hyperpolarization activated potassium current (IA) which delays the return to baseline following a hyperpolarizing current step. Type III PPN neurons have both LTS and IA currents. Extracellular recordings of PPN neurons in vivo identified six categories of thalamic-projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital (PGO) wave generation (Steriade et al., 1990a). Some of these neurons had low rates of spontaneous firing (<10 Hz), but most had high rates of tonic firing in the beta/gamma range (20–80 Hz). PPN neurons increase firing during rapid eye movement (REM) sleep (“REM-on”), or both waking and REM sleep (“Wake/REM-on”), but decrease during SWS (Sakai et al., 1990; Steriade et al., 1990b, 1991; Datta and Siwek, 2002), suggestive of increased excitation only during activated states. Stimulation of the PPN potentiated the appearance of fast (20–40 Hz) oscillations in the cortical EEG, outlasting stimulation by 10–20 s (Steriade et al., 1991). Injections of glutamate into the PPN increased waking and paradoxical sleep (Datta et al., 2001a), while injections of the glutamatergic receptor agonist N-methyl-D-aspartic acid (NMDA) increased only waking (Datta et al., 2001b), and injections of the glutamatergic receptor agonist kainic acid (KA) increased only paradoxical sleep Datta, 2002). In addition, the PPN has recently been found to exhibit electrical coupling (Garcia-Rill et al., 2007), along with groups of cells in the Pf, that indirectly participates in cortical activation, and in the Subcoeruleus nucleus dorsalis (SubCD), that participates in paradoxical, or REM sleep regulation (Garcia-Rill et al., 2007, 2008a; Heister et al., 2007). These findings suggest that PPN outputs can indirectly induce gamma band activity at the level of the cortex, but does it do so by triggering such activity in its ascending targets such as the Pf, or by driving these regions with such activity? Does the PPN have the mechanisms necessary for generating its own gamma band activity?
A recent study was the first to report that all or most PPN cells fired maximally at gamma band frequency when depolarized using current steps (Figures 1A,B; Simon et al., 2010). A later study tested the hypothesis that, in the presence of tetrodotoxin (TTX, to block action potential generation by blockade of sodium channels) and fast synaptic blockers (APV to block NMDA receptors, CNQX to block AMPA/KA receptors, gabazine to block GABA receptors, and strychnine to block glycine receptors), the remaining oscillatory activity observed in PPN neurons during current clamp square pulse depolarization was due to activation of intrinsic membrane properties in the form of voltage-dependent calcium channels (Kezunovic et al., 2011). Due to the extensive activation of potassium channels during rapid depolarization using square steps, PPN neurons could not be depolarized beyond −25 mV. However, the use of 1–2 s-long depolarizing current ramps was successful in gradually changing the membrane potential from resting values up to 0 mV. Thus, square pulses generated smaller amplitude gamma oscillations in all three types of PPN neurons compared to the amplitude of the oscillations generated by ramps (Figure 1C). All PPN neurons tested exhibited both low (theta and alpha, 4–12 Hz) frequency and beta/gamma band (>20 Hz) frequencies. Gamma band oscillations were visible between −25 and −5 mV somatic membrane voltage range, and were absent at membrane potentials below −30 mV or above 0 mV, i.e., at the voltage levels of high-threshold calcium channels. All three types of PPN neurons exhibited statistically significant higher power spectrum amplitudes for gamma band oscillations when a depolarizing ramp was used (Figure 1C) compared to square wave steps (Kezunovic et al., 2011).
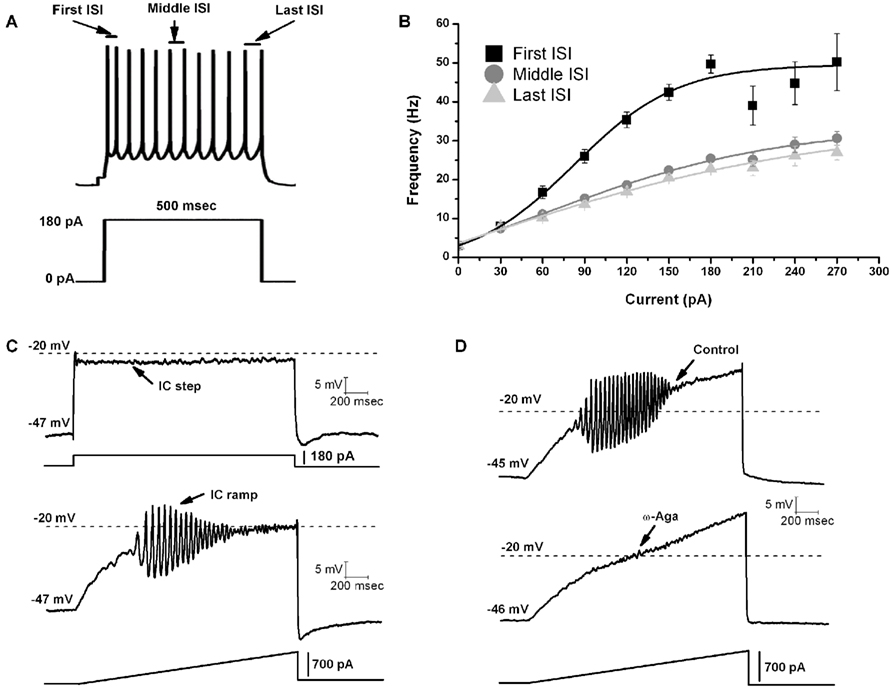
Figure 1. Gamma band activity in whole-cell recorded PPN neurons. (A) Maximal AP frequency was measured using nine current steps (each step was 500 ms in duration with an increase of 30 pA per step with the last step at 270 pA, and a 2.5 s delay between steps). The record shown in (A) is a representative response to a 180 pA step. Note the location of the first, middle, and last intespike intervals (ISI) used to analyze the firing frequency of PPN neurons. (B) Graph showing the average of the first (black line), middle (dark gray line), and last (light gray line) ISI (converted to frequency) of PPN neurons. Note that the firing frequency of PPN neurons did not increase linearly but instead plateaued at gamma frequency. (C) Representative membrane potential responses to depolarizing 2 s intracellular (IC) square steps (above) and responses to depolarizing 2 s ramps (below) for the same neuron obtained in the presence of synaptic blockers and TTX. (D) Representative 2 s long ramps before (above) and after (below) the application of ω-Aga (200 nM). Note the elimination of oscillations by the P/Q-type channel blocker ω-Aga.
Using a voltage clamp configuration, no clear oscillatory currents were observed at holding potentials below −30 mV. However, clear beta/gamma band oscillations were observed in the power spectra at both −20 and −10 mV holding potentials. The highest gamma band power amplitudes were observed at a −10 mV holding potential (Kezunovic et al., 2011). The amplitudes of the peaks in the power spectrum were reduced after series resistance compensation (40–60%; i.e., to reduce any space clamp problems during recordings), suggesting that PPN neuronal oscillatory activity in voltage clamp was generated in neuronal compartments distant from the soma, as previously shown for thalamic neurons (Pedroarena and Llinás, 1997). That study on thalamic neurons used calcium imaging to determine that the oscillations induced by such depolarization were generated in the dendrites, which presumably required high current application to the soma in order to depolarize the dendrites. Interestingly, the N-type blocker ω-conotoxin-GVIA (ω-CgTX) only reduced gamma band oscillation amplitude and power spectrum, while the P/Q-type blocker ω-agatoxin-IVA (ω-Aga) totally abolished them (Figure 1D). Moreover, bath pre-application (>30 min) of ω-Aga prevented PPN neurons from oscillating at gamma band. Furthermore, application of ω-Aga and ω-CgTX together, completely blocked all the oscillations in another group of cells tested. These results showed that both voltage-dependent N- and P/Q-type calcium channels mediated the depolarizing phase of gamma band oscillations in the PPN nucleus. However, only P/Q-type channels appeared to be essential for gamma oscillation generation. Moreover, voltage clamp results suggested that calcium channels might be located distally to the somata, in PPN dendritic compartments (Kezunovic et al., 2011), similar to thalamic neurons (Pedroarena and Llinás, 1997).
The average oscillation frequency of PPN neurons (age 8–13 days) was in the beta range at 23 ± 1 Hz when perfused with synaptic blockers and TTX only. However, when carbachol (CAR) was added, the average frequency of gamma oscillations was significantly higher and in the gamma range (47 ± 2 Hz, p < 0.001; Table 1). These oscillations were blocked by application of ω-Aga. Furthermore, different cell types did not show differential oscillatory frequencies after CAR exposure. Type II cells still showed generally lower amplitude (but not frequency) oscillations than type I or III cells. Interestingly, analysis of the power spectrum of oscillatory activity of all cells at different frequency ranges (from theta to gamma) showed that CAR significantly reduced the power amplitude at theta, alpha, and beta frequencies, while there was no difference in average amplitude of oscillations at gamma frequency (Kezunovic et al., 2011).
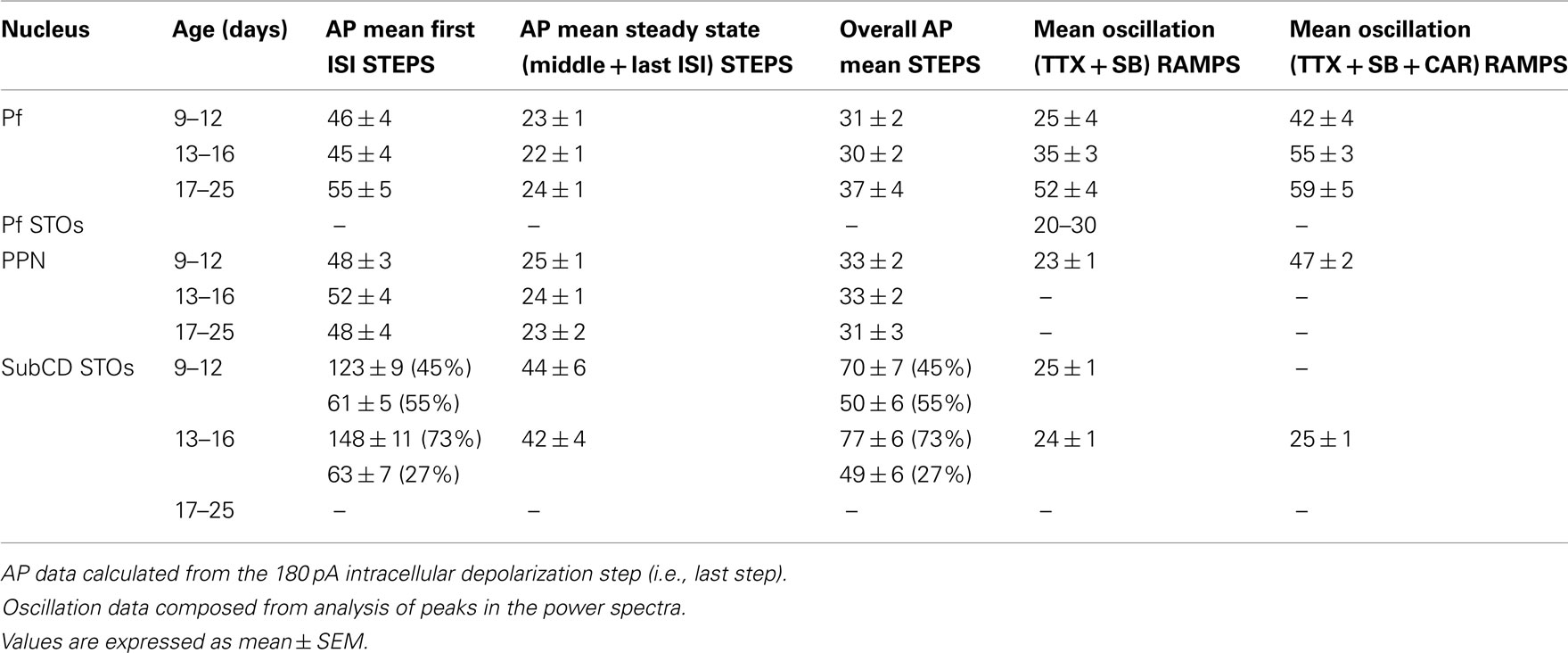
Table 1. Frequency in Hz of induced action potentials (AP) during STEPS, membrane oscillations during RAMPS, and after CAR.
These results (a) confirmed the presence of gamma band activity previously reported in PPN neurons (Simon et al., 2010), and (b) identified mechanisms underlying the generation of gamma band oscillations in the PPN (Kezunovic et al., 2011). It is also the first time that the potential roles of high voltage-activated P/Q-type calcium and voltage-gated delayed rectifier-like potassium channels have been described in oscillations in PPN cells. There is now little doubt that PPN neurons also have the ability to manifest gamma band activity that is enhanced by CAR, such as that reported in cortical, thalamic, hippocampal, and cerebellar cells. Moreover, PPN neurons appear to oscillate at gamma band through P/Q-, and N-type calcium channels, as well as voltage-gated, delayed rectifier-like, potassium channels, suggesting that multiple mechanisms may modulate these cells to fire at gamma band frequencies.
Gamma in the RAS –the Pf
The Pf is a component of the intralaminar thalamus, which is traditionally considered a part of the “non-specific” thalamocortical (TC) system. Pf cells differ from typical “specific” TC neurons in morphology, electrophysiological properties and some synaptic connections. Pf neurons have long, sparsely branching processes in their proximal dendrites instead of compact bushy primary dendrites like TC relay cells (Deschenes et al., 1996a,b). TC relay neurons are present throughout the “specific” and some “non-specific” thalamic nuclei, and are bushy, multidendritic cells with stereotypical intrinsic properties, i.e., bistable states of tonic vs. bursting patterns of activity due to the ubiquitous incidence of LTS mediated by T-currents (Llinás and Steriade, 2006). These authors considered this mechanism essential to inducing cortical synchronization of high frequency rhythms during waking and REM sleep (tonic pattern), and synchronization of low frequency rhythms during SWS (LTS + Ih oscillations). However, our previous electrophysiological studies demonstrated that “non-specific” Pf cells exhibited reduced incidence of calcium-mediated LTS currents (Phelan et al., 2005), compared to “specific” TC neurons (Llinas and Jahnsen, 1982; Jahnsen and Llinas, 1984a,b). It was recently reported that the Pf provides different patterned inputs to distinct striatal targets (Lacey et al., 2007). These findings suggest the possibility that Pf neurons may play a different role in the modulation of thalamocortical activity compared to “specific” TC neurons.
As one of the targets of the cholinergic arm of the RAS, the intralaminar thalamus receives dense projections with symmetrical and asymmetrical terminals from the PPN and laterodorsal tegmental (LDT) nuclei (Erro et al., 1999; Kha et al., 2000; Capozzo et al., 2003; Kobayashi and Nakamura, 2003), which participate in the modulation of cortical arousal, sleep–wake cycles, and sensory awareness (Llinás and Paré, 1991; Steriade et al., 1991; Van der Werf et al., 2002). In addition, Pf neurons are involved in maintaining the state of consciousness and selective attention in primates (Minamimoto and Kimura, 2002; Raeva, 2006), and they also receive vagal input and participate in motor control, as well as pain modulation (Ito and Craig, 2005). High frequency spike bursts recur rhythmically at ∼40 Hz during wakefulness and sleep in intralaminar thalamocortical cells (Steriade et al., 1993). These authors stated, “One of the most remarkable characteristics of these cells was the firing of high frequency bursts during natural states of wakefulness and REM sleep. At this time, this is the only known thalamic cell class exhibiting such behavior during brain-activated states” (Steriade et al., 1993).
To determine the maximal firing frequency of Pf neurons, a recent study used steps of increasing current amplitudes in current clamp mode (Kezunovic et al., 2012). Figure 2A shows that the frequency of firing increased with increasing current steps but plateaued at gamma band frequencies, as in the PPN. The initial firing frequency was higher than during the middle or end of the current step but still within the gamma range (Figure 2B). The firing frequency of action potentials (APs) was compared across three age groups 9–12, 13–16, and 17–25 day old. Statistical analysis revealed that the first interstimulus intervals (ISIs) of the 17–25 day old group steps showed significantly higher firing rates compared to the 9–12 day old group. Interestingly, no statistical significance was found for the middle and the last ISIs between these two age groups. Also, there was no statistically significant difference between the mean AP frequency in 9–12 vs. 13–16 vs. 17–25 day cells (Kezunovic et al., 2012). Square steps generated smaller amplitude (and power of) oscillations (Figures 2C,D) compared to the amplitude and power of the oscillations generated by current ramps in the same cell (Figures 2C,D). Statistical analysis showed that the amplitude of the oscillations generated by the ramps was significantly higher than that generated by the current steps. Oscillations were visible between −25 and −5 mV somatic membrane voltage range, and were absent at membrane potentials below −30 or above 0 mV. Power spectrum analysis of the membrane oscillations induced during ramp recordings revealed that 60% of the cells exhibited the highest peak of oscillatory activity at the gamma range, while some 38% of cells had the highest peak at beta frequency. However, those cells showing frequencies below gamma band were typically at earlier ages (Kezunovic et al., 2012; Table 1).
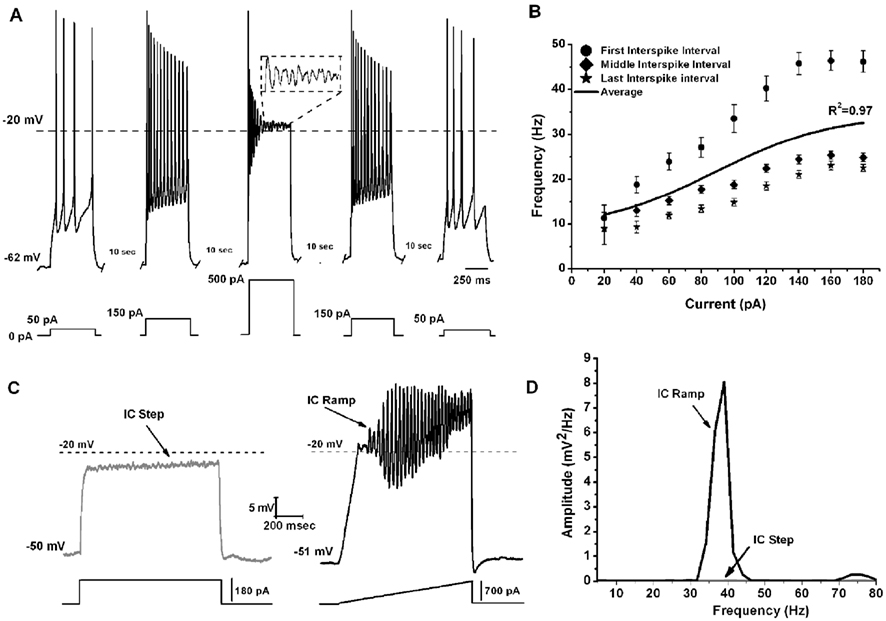
Figure 2. Gamma band activity in whole-cell recorded Pf neurons. (A) Representative pyramidal depolarizing steps (five steps 500 ms long and 10 s apart) with 500 pA step in the middle showing APs being replaced by oscillations at higher depolarizing levels (magnified view in dashed square). Note that the firing frequency of the APs returned to the usual firing rate when lower current steps were used (150, and 50 pA, respectively). (B) Graph showing the average of the first (circle), middle (diamond), and last (star) ISI (converted to frequency) of Pf neurons. The black line shows the average instantaneous firing frequency during the entire current step. Note that firing frequency did not increase linearly with increasing current during the last few steps (140–180 pA), but instead plateaued at gamma frequency. (C) Representative membrane potential responses to depolarizing 1 s square steps (left, gray record) and responses to depolarizing 1 s ramps (right, black record) for the same Pf neuron. (D) Power spectrum corresponding to the records shown in (C).
These authors also showed that, early in the development of Pf neurons (9–16 days), their maximal oscillatory frequency began at lower ranges (alpha and beta), and gradually plateaued at gamma range with age (Figures 3A,B, black line). However, CAR could increase the firing frequency of younger cells to the gamma range, and their frequency would eventually plateau within the gamma range, but no higher (Figures 3A,B, gray line). In the oldest age group (17–25 day), CAR did not significantly increase frequency beyond the gamma range, suggesting these older cells were already “capped” at gamma band frequencies (Figure 3B). In the Pf, the P/Q-type channel blocker ω-Aga totally abolished gamma band oscillations. Figure 3C shows control, blockade, and recovery of oscillations in a representative Pf neuron. Figure 3D shows the power spectrum of the oscillations during control, blockade, and recovery. The effect of the toxin was reversed during washout, which consisted of the same extracellular solution as in the control condition, including synaptic blockers and TTX. Moreover, bath pre-application (>30 min) of ω-Aga prevented Pf neurons from oscillating at higher frequencies. Interestingly, the N-type blocker ω-CgTX only reduced gamma band oscillation amplitude as evident in the power spectrum. The ω-CgTX effect was also reversed during washout and the amplitude of gamma band oscillations returned to almost the same level as in the control. Bath pre-application of ω-CgTX did not prevent Pf neurons from oscillating. However, the amplitude of those oscillations was significantly lower than in control conditions. These results showed that both voltage-dependent P/Q- and N-type calcium channels may mediate the depolarizing phase of gamma band oscillations in the Pf nucleus. However, only P/Q-type channels appeared to be essential, while N-type channels were only permissive, for gamma band oscillation generation. The specific calcium channel blockers had the same type of blocking effect on Pf neuron oscillatory activity regardless of age (Kezunovic et al., 2012).
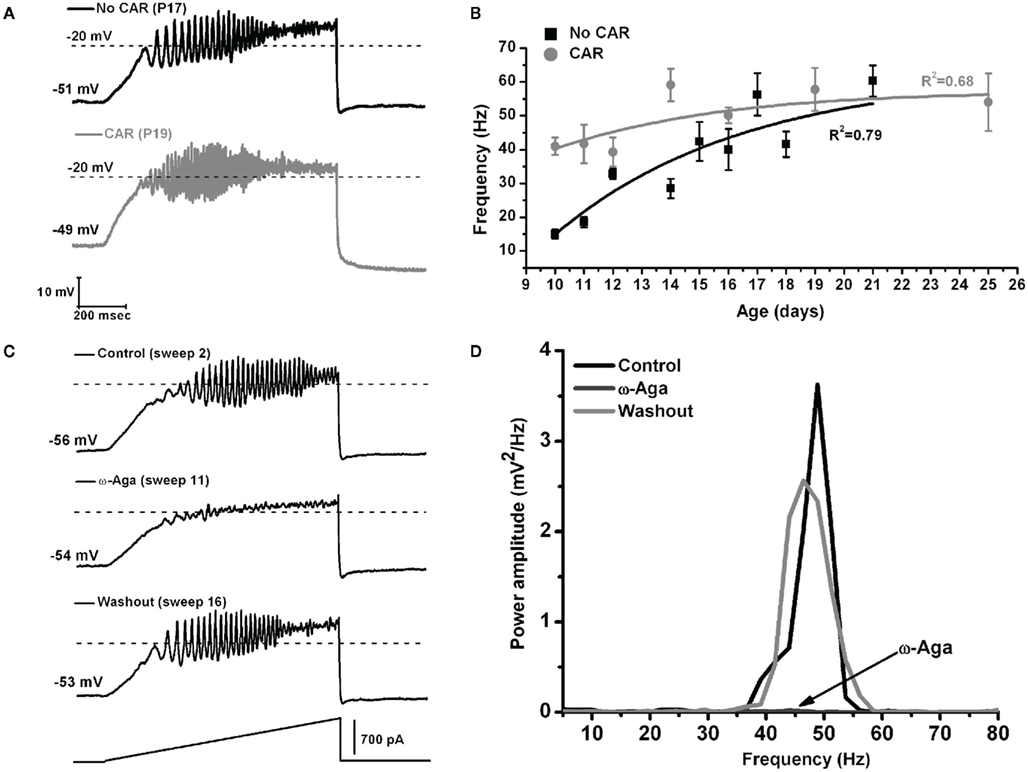
Figure 3. The effect of carbachol on the oscillatory frequency of Pf neurons. (A) Representative membrane potential oscillations of a Pf neuron without CAR (black record, above) and in the presence of CAR (gray record, below) in the extracellular solution, obtained during 1 s long ramps. (B) Graph showing the distribution of mean firing frequencies of Pf neurons recorded without (black squares), and with CAR in the extracellular solution (gray squares) during the developmental period used in this study (days 9–25). Black (No CAR) and gray (CAR) lines represent the best-fit lines of the corresponding data. (C) Representative membrane potential oscillations obtained during 1 s long ramps before application of the specific calcium channel blocker (top record), after bath application of ω-Aga (200 nM; middle record), and after washout (bottom record). Note the elimination of oscillations by the P/Q-type channel blocker ω-Aga, and the return of the oscillatory activity, after only 5 min of washout. (D) Power spectrum of the records shown in (C).
Current–Voltage (I–V) curves were obtained for both T-type and high-threshold current components. The authors plotted current density values (pA/pF) against voltage measured at the beginning and at the end of square steps. T-type calcium channel mediated currents peaked at −40 mV while high-threshold P/Q- and N-type mediated currents presented maximum values at −10 mV holding potential. Interestingly, the peak (holding potential −40 mV) T-type current density values were significantly increased after postnatal day 12, without affecting the peak density of P/Q- and N-type currents. Indeed, average I–V values from 12–25 day Pf cells showed an increase in T-type current density without changing high-threshold current density (Kezunovic et al., 2012).
In such structures as the striatum and subthalamic nucleus, depolarizing current steps linearly increase firing frequency to >500 and >250 Hz, respectively (Azouz et al., 1997; Barraza et al., 2009), but do not plateau. In monkey and rat prefrontal cortex, basket cells also do not plateau, almost linearly increasing firing frequency (Povysheva et al., 2008). On the other hand, pyramidal cells in mouse neocortex plateau below gamma band frequency, even after application of very high amplitude current steps (600–1000 pA; Zhou et al., 2010). However, the results described above show that when Pf neurons are depolarized with increasing current steps, they fire initially at high frequency, but the firing frequency then plateaus at gamma frequency (30–60 Hz). This plateau in firing rate was observed in all Pf cells. The main difference between the previous PPN study (Simon et al., 2010; Kezunovic et al., 2011), and the later study on Pf cells (Kezunovic et al., 2012), is that PPN neurons did not show the high frequency instantaneous rate at the beginning of the current steps. That is, PPN neurons fired at ∼50 Hz initially and then at ∼30–40 Hz during the rest of the step, while Pf cells fired at ∼80 Hz initially, then at ∼50–60 Hz for the remainder of the step. Thus, Pf neurons may provide slightly differing initial signaling than PPN neurons, but both ensure the maintenance of gamma band activity when maximally activated. No previous study used depolarizing pulses to determine maximal or close to maximal firing frequencies and membrane oscillations in Pf neurons. The more recent results demonstrate that all Pf neurons exhibit gamma band activity when activated, as well as gamma band membrane oscillations (Kezunovic et al., 2012). In addition, electrical and pharmacological activation of the Pf induced gamma band population responses. These results suggest that the Pf may generate gamma band activity when maximally activated, and perhaps impart gamma band activity on its targets. This newly discovered intrinsic membrane property of Pf neurons represents a novel mechanism for the induction of activated states such as waking by Pf efferents.
Gamma in the RAS – the SubCD
Rapid eye movement sleep is distinguished from other states by low amplitude, high frequency EEG activity, muscle atonia, and PGO waves in cats (P waves in the rat; Aserinsky and Kleitman, 1953; Datta et al., 1998). Nuclei located in the pons, including the SubCD, are critical for generation of this state (Mouret et al., 1967; Marks et al., 1980; Baghdoyan et al., 1984; Yamamoto et al., 1990; Datta et al., 1998; Boissard et al., 2002). The SubCD is most active during REM sleep (Boissard et al., 2002; Datta et al., 2009), and injection of the non-specific cholinergic agonist CAR or the glutamate receptor agonist kainic acid (KA) into this area induced a REM sleep like state with muscle atonia and PGO waves (Mitler and Dement, 1974; Baghdoyan et al., 1987; Vanni-Mercier et al., 1989; Yamamoto et al., 1990; Boissard et al., 2002). Lesion of this area produced REM sleep without muscle atonia or P waves (Mouret et al., 1967; Sanford et al., 1994; Mavanji et al., 2004; Karlsson et al., 2005), or diminished REM sleep Lu et al., 2006). The SubCD receives afferent input from several nuclei, including cholinergic, and perhaps glutamatergic, afferents from the PPN and LDT (Mitani et al., 1988; Shiromani et al., 1988; Datta et al., 1999; Boissard et al., 2003). The SubCD projects to many areas, including the thalamus, hippocampus, pons, and medulla (Datta and Hobson, 1994; Datta et al., 2004).
A recent study showed that some neurons in the SubCD fired APs at frequencies above gamma band (>100 Hz) at the beginning of a stimulus, but all neurons fired maximally (plateaued) at beta/gamma band following the initial portion of the current step (Simon et al., 2011a). Voltage and sodium channel-dependent subthreshold oscillations appear to be involved in generating this activity (Figure 4). Subthreshold oscillations were isolated using APV, CNQX, GBZ, and STR to block fast inhibitory and excitatory spontaneous synaptic activity. At membrane potentials below AP threshold (Figure 4A, −48 mV), subthreshold oscillations were observed and persisted at membrane potentials above AP threshold, where they were evident between APs (Figure 4A, −43 mV). Subthreshold oscillations were also observed following inactivation of sodium channels underlying APs (Figure 4A, −40 mV), suggesting the existence of two populations of voltage-gated sodium channels, one related to AP generation and the other related to subthreshold oscillations (Simon et al., 2011a).
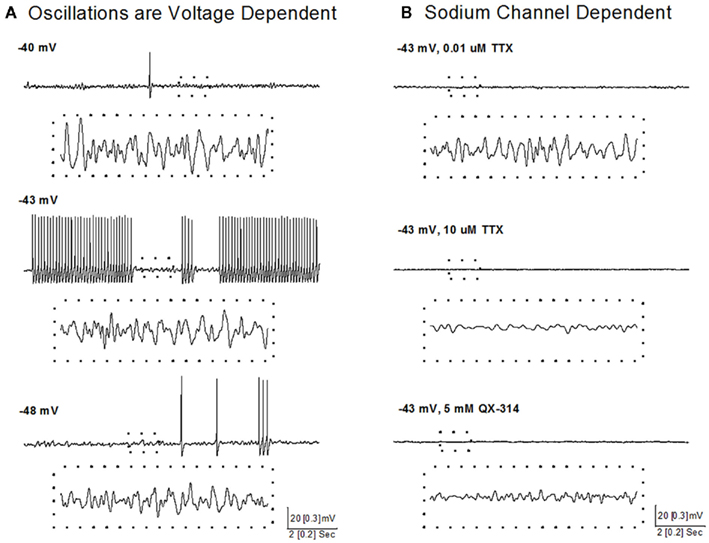
Figure 4. Voltage and sodium-dependent subthreshold oscillations in SubCD neurons. (A) Subthreshold oscillations were isolated using APV, CNQX, GBZ, and STR, and were observed at membrane potentials below AP threshold (bottom record, −48 mV), between APs at membrane potentials above AP threshold (middle record, −43 mV), and following inactivation of sodium channels underlying APs by further depolarization (top record, −40 mV). The dotted boxes include 1 s of recordings (from upper records) that are shown at higher resolution (dotted box records). (B) Low concentration TTX (0.01 μM, top record) blocked sodium channels responsible for AP generation, but subthreshold oscillations were still observed, which were then blocked by high concentration of TTX (10 μM, middle record). In another cell, addition of QX-314 (5 mM) to the intracellular solution also blocked the subthreshold oscillations (bottom record). The dotted boxes include 1 s of recordings (from upper records) that are shown at higher resolution (dotted box records).
A sodium-dependent mechanism was revealed using TTX, an extracellular sodium channel blocker, and QX-314, an intracellular sodium channel blocker. Low concentrations of TTX (0.01 μM, Figure 4B, top) completely blocked AP generation and reduced the power of gamma band oscillations but did not abolish subthreshold oscillations, while high concentrations of TTX (10 μM, Figure 4B, middle) completely blocked the remaining subthreshold gamma oscillations. QX-314 in the intracellular recording solution blocked both APs and subthreshold gamma oscillations (Figure 4B, bottom). These results suggest that beta/gamma frequency, sodium-dependent subthreshold oscillations may underlie the gamma frequency AP firing of SubCD neurons (Simon et al., 2011b).
Previous studies in the SubCD reported the presence of gamma firing frequency in vivo and in vitro following depolarizing pulses (Datta and Hobson, 1994; Brown et al., 2006). However, no previous study demonstrated the ability of all SubCD neurons to fire maximally at or above gamma frequency, or the presence of two distinct populations of SubCD neurons that fire at different frequencies at the beginning of a current step, but plateau at gamma frequency following the initial burst of APs. Furthermore, some neurons showed membrane oscillations in the gamma range, which were not affected when APs were blocked by low concentrations of TTX, but were blocked by high concentrations of TTX, and thus appeared to be sodium channel-dependent subthreshold oscillations. In single cell recordings, application of KA did not significantly increase the frequency of membrane subthreshold oscillations at specific frequencies, CAR increased activity in the beta/gamma range, and NMDA increased activity in the mid and high gamma range. In addition, population responses in the SubCD showed that NMDA increased activity at almost all frequencies, while CAR and KA induced specific peaks in the gamma range. These results suggest that the SubCD can generate beta (and to a lesser extent gamma) band activity via subthreshold oscillations, and thereby may impart such activity on its targets when maximally activated (Simon et al., 2011a). Interestingly, a previous study reported the presence of subthreshold oscillations in type II PPN cells (Takakusaki and Kitai, 1997), and these oscillations were maximal in the beta range. Table 1 shows that subthreshold oscillations in PPN and SubCD appear to peak at similar frequencies in the beta range, while P/Q-type calcium channel-mediated oscillations in the PPN and Pf appear to peak at similar frequencies in the gamma range. It is not clear if such a difference is present such that gamma band predominates during waking (when PPN and Pf are active) vs. beta band predominating during REM sleep (when PPN and SubCD are active).
Extracellular recordings from cells in the PGO wave generating site in cats recorded the presence of “PGO-on” cells, which increased their firing rates before the first PGO wave until the end of REM sleep, but had low firing rates during waking and SWS without PGO waves (Datta and Hobson, 1994). These cells discharged high frequency spike bursts (>500 Hz) during PGO-related states and also fired tonically at 25–100 Hz. Perhaps the cells recorded in vitro which showed high frequency bursts of APs during the beginning of current steps could be putative “PGO-on” cells in the rat (Simon et al., 2011a). These studies did not observe activity greater than 300 Hz, but perhaps this could be attributed to a species difference between rodents and cats, or between in vitro and in vivo recordings.
Developmental Changes
In humans, REM sleep decreases from approximately 8 h in the newborn to 1 h in the adult (Roffwarg et al., 1966). A similar decrease in REM sleep occurs in the rat during postnatal days 10–30 (Jouvet-Mounier et al., 1970), with the majority of changes occurring between 10 and 15 days (Garcia-Rill et al., 2008a). A number of developmental changes, such as a decrease in electrical coupling, have been observed during this transition period in brainstem nuclei that control arousal states (Garcia-Rill et al., 2008a). In the PPN, a developmental decrease in the contribution of the NMDA receptor and developmental increase in the contribution of the KA receptor was observed following electrical stimulation-induced glutamate input (Simon et al., 2011a,b). These changes were also observed following bath application in different cell types (randomly selected vs. thalamic-projecting). KA bath application produced an increase in the paired-pulse ratio (PPR) and a decrease in the frequency of miniature EPSCs (mEPSCs), suggesting that presynaptic kainate autoreceptors may decrease the probability of synaptic glutamate input. The increase in KA function is in keeping with studies described above showing that injection of KA into the PPN of adult rats preferentially induced increases in REM sleep (Simon et al., 2010). In contrast, NMDA application produced no changes in the PPR or mEPSCs (Simon et al., 2011b).
On the one hand, no such developmental changes in glutamate receptor function appear present in the Pf. However, the shift from NMDA to KA receptor function appears to be more prominent in the SubCD. A marked reduction in the effects of the NMDA receptor was coupled to a marked decrease in the effects of the KA receptor in these neurons (Simon et al., 2012). These results suggest that, in keeping with findings reviewed above, the SubCD is mainly involved in REM sleep control, and some of the developmental changes in REM sleep may be reflected in these shifts in glutamatergic receptor function. The increase in gamma band activity, especially in the PPN (Simon et al., 2010; Kezunovic et al., 2011) and Pf (Kezunovic et al., 2012), during the developmental decrease in REM sleep tends to suggest that these changes are not related to REM sleep but rather to waking. However, no change in gamma band activity appeared present in the subthreshold oscillations of SubCD cells, and these neurons may be more related to REM sleep than to waking (Simon et al., 2011b). A summary of the mechanisms underlying gamma band activity in the PPN, Pf and SubCD is provided in Figure 5.
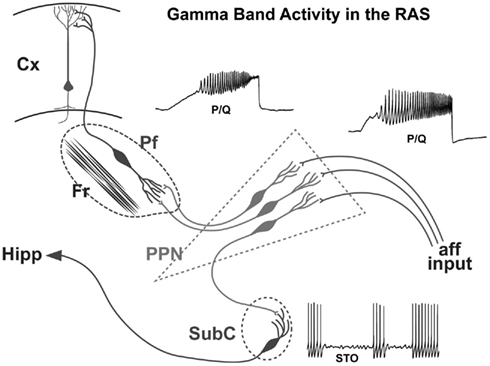
Figure 5. Wiring diagram and mechanisms behind gamma band activity in the RAS. Afferent input that originates from collateral activation of the RAS by sensory systems activates the dendrites of PPN neurons. The presence of inputs to dendritic P/Q- and N-type calcium channels set off oscillations at gamma band that influence firing frequency. The output of the PPN descends to the Subcoeruleus (SubC), presumably activating these neurons that have sodium-dependent subthreshold oscillations. These cells in turn influence downstream systems involved in the atonia of REM sleep, and ascending systems that may participate in the consolidation of memories such as the hippocampus (Hipp). The output of the PPN also ascends to the intralaminar thalamus, especially the parafascicular nucleus (Pf), activating its dendrites to oscillate at gamma frequency via P/Q- and N-type calcium channels. These cells in turn project to the cortex, particularly to upper cortical layers where the non-specific thalamic inputs terminate, to activate cortical neurons. Once cortical, hippocampal, and cerebellar cells are activated, the generation and maintenance of gamma band activity in the brain can more easily be maintained.
Table 1 summarizes the changes observed in the Pf, PPN, and SubCD across development and between CAR exposed and unexposed cells. These results suggest that SubCD neurons do not significantly change the frequency of the sodium-dependent subthreshold oscillations early in development or after exposure to CAR. In fact, these frequencies are in the beta range and remain at these rates following CAR (Simon et al., 2011b). Similarly, subthreshold oscillations in PPN neurons also appear to remain in the beta range (Takakusaki and Kitai, 1997). However, both PPN and Pf neurons exhibit high-threshold calcium channel-dependent oscillations that are P/Q- and N-type channel-dependent (Kezunovic et al., 2011, 2012). The firing rates of PPN and Pf cells are in the beta range early (9–12 days, before major changes in REM sleep) and middle ages (13–16 days, at the start of the decrease in REM sleep) in development, but PPN and Pf cells increase in firing to the gamma range after 17 days, during the greatest developmental decrease in REM sleep, and are driven at similar rates by CAR, but still remain within the gamma range after exposure to CAR. Unfortunately, recordings of the frequencies of oscillations in PPN and SubCD cells after 17 days have not been made to determine if these also increase in frequency or remain in the beta/gamma range later in development.
In summary, these results suggest that subthreshold oscillations in the RAS tend to cycle in the beta range, whether present in PPN or SubCD neurons. This suggests that, since both nuclei are involved in the modulation of REM sleep, this will be the “preferred” frequency during that state. [Note that subthreshold oscillations in cortical neurons cycle in the gamma range (Llinas, 1988; Llinas et al., 1991)] On the other hand, high-threshold calcium channel-dependent oscillations in the RAS tend to cycle in the gamma range, whether they are in PPN of Pf neurons. This suggests that, since both nuclei are involved in the modulation of arousal and waking, this will be the “preferred” frequency of that state, especially later in development and under the influence of cholinergic input.
A Gamma-Making Machine
There is little doubt that the RAS is akin to a gamma-making machine. Because gamma waves can occur during slow wave states and anesthesia, a close relation to consciousness has been questioned (Vanderwolf, 2000). It was suggested that consciousness is associated with continuous gamma band activity, as opposed to an interrupted pattern of activity (Vanderwolf, 2000). The original description of the RAS specifically suggested that it participates in tonic or continuous arousal (Moruzzi and Magoun, 1949), and lesions of this region were found to eliminate tonic arousal (Watson et al., 1974). This raises the question of how a circuit can maintain such rapid, recurrent activation. Expecting a circuit of 5 or 10 synapses to reliably relay 20–60 Hz cycling without failing is unrealistic. Without the intrinsic properties afforded by rapidly oscillating channels, such as those described recently for PPN, Pf, and subthreshold oscillations in SubCD, beta/gamma band activity could not be maintained. The combination of channels capable of fast oscillations and circuitry that involves activating these channels probably are both required for the maintenance of gamma band activity (Llinas, 1988; Llinas et al., 1991, 2002, 2007; Kezunovic et al., 2011). The group of RAS nuclei as a whole, in which every cell in every nucleus exhibits beta/gamma band activity, then becomes a gamma-making machine. We speculate that it is the continued activation of the RAS during waking and REM sleep that allows the maintenance of the background of gamma activity necessary to support a state capable of reliably assessing the world around us on a continuous basis.
One question that arises is, what is the role of other wake-promoting regions in the brain? For example, the lateral hypothalamic orexin system has been proposed to parallel the arousing activity of the RAS given its characteristic excitation of neuronal populations to which it projects, including the RAS, thalamus, and cortex. However, recent evidence suggests that the orexin system must activate the RAS before waking ensues. de Lecea et al. (1998), one of the discoverers of orexin in the hypothalamus, developed an optogenetic approach to selectively activate these cells. By genetically or via viral vector inserting a rhodopsin cation channel into orexin neurons, stimulation with blue light was found to depolarize these neurons selectively (Adamantidis et al., 2010). In rather complex new studies, his lab has engineered animals that have such rhodopsin cation channels in orexin and in noradrenergic locus coeruleus (LC) neurons (Carter et al., 2010). Their findings showed that light activation of orexin neurons requires several seconds of stimulation and has a latency ∼20 s to induce waking, implying that its output must travel elsewhere before it awakens, i.e., has an indirect effect. When they light activated LC cells, the animals awoke immediately, but if LC was inactivated, orexin neuron stimulation failed to awaken the animals. These results suggest that orexin neurons must first affect their descending RAS target, the LC, in order to manifest a waking effect. That is, the lateral hypothalamic system may act through the RAS to elicit arousal. Interestingly, de Lecea’s findings in light stimulating dopamine neurons bearing rhodopsin channels showed that selective dopaminergic neuron activation does not lead to waking or arousal (Carter and de Lecea, 2011).
Potential Functions of Gamma in the RAS
It is important to link these in vitro findings on gamma band activity to in vivo results, as well as to clinical evidence. Are there measurable outcomes of PPN output that can be quantitatively assessed in vivo? Winn has pioneered the use of PPN lesions to disturb performance in adult rats. They recently found that the PPN is involved during learning of both simple and complex tasks, and that different parts of the PPN are involved in motivational and/or response initiation processes, while other parts of the PPN are involved in response control (Wilson et al., 2009). In the freely moving rat, the P13 potential is an auditory evoked potential recorded maximally at the vertex at a latency of 11–14 ms following a click stimulus (Skinner et al., 2004). The P13 potential has three main characteristics: (1) it is present during waking and REM sleep, but not SWS, indicating that the generator of this response is functionally related to states of arousal, i.e., when PPN is most active and when gamma band activity is highest, (2) the response is blocked by the cholinergic receptor antagonist scopolamine, indicating that it is mediated, at least in part, by cholinergic neurons, and (3) it undergoes rapid habituation at stimulation rates greater than 2 Hz, indicating a low security, reticular type of pathway. Considering that the PPN is a REM sleep/wake “on” nucleus that contains cholinergic neurons and rapidly habituates to sensory stimuli, the PPN has been implicated as a likely site of generation of the P13 potential. This is supported by studies showing that injection of inhibitory agents into the PPN decreased the amplitude of the vertex-recorded P13 potential (Garcia-Rill and Skinner, 2001). This waveform is relayed to the cortex through the ILT, including the Pf. The amplitude of the P13 potential was increased in a dose-dependent fashion by either oral or intracranial injection into the PPN of the stimulant modafinil, an effect blocked by gap junction blockers (Beck et al., 2008). The P13 potential is therefore a valuable tool for assessing PPN output in vivo.
The human equivalent of the P13 potential appears to be the P50 potential, which is a midlatency auditory, click stimulus-evoked, response recorded maximally at the vertex that occurs at a latency of 40–70 ms (Erwin and Buchwald, 1986a,b; Buchwald et al., 1991). The P50 potential, like the P13 potential in the rodent, has three main characteristics, (a) it is present during waking and REM sleep, but not during deep SWS (i.e., it is sleep state-dependent, occurring during cortical EEG synchronization of fast oscillations, mainly in the low gamma band range (20–60 Hz); but not during cortical synchronization of slow oscillations, <10 Hz), (b) is blocked by the cholinergic antagonist scopolamine (i.e., may be mediated, at least in part, by cholinergic neurons), and (c) undergoes rapid habituation at stimulation rates greater than 2 Hz (i.e., is not manifested by a primary afferent pathway, but perhaps by multi-synaptic, low security synaptic elements of the RAS; Erwin and Buchwald, 1986a,b; Buchwald et al., 1991). The P50 potential, but none of the earlier latency primary auditory evoked potentials, diminishes, and disappears with progressively deep stages of sleep and then reappears during REM sleep (Erwin and Buchwald, 1986a). Recent studies established the localization of the magnetic equivalent of the P50 potential using magnetoencephalography (MEG) to record the M50 response (Garcia-Rill et al., 2008b). These MEG studies suggest that the manifestation of the M50 response in the region of the vertex may be more than a volume-conducted potential, but the exact neurological substrate for the M50 response still needs to be identified. This response peaked in a fronto-parietal region around the vertex that overlaps with the region found to elicit a significant change in cortical blood flow after deep brain stimulation of the PPN in the human (Ballanger et al., 2009).
As stated above, the PPN has become a target for deep brain stimulation for the treatment of Parkinson’s disease (PD; Androulidakis et al., 2008; Ballanger et al., 2009; Mazzone et al., 2010). In a recent study, self-paced movements generated theta and beta frequency activity in the PPN (Tsang et al., 2010). These authors proposed that different frequencies being generated by the PPN were dependent on the presence of dopaminergic medication, since beta desynchronization was observed in the OFF state, but beta synchronization occurred in the ON state. To date, no reports of gamma band activity in the human PPN have been forthcoming, however, our animal studies are in agreement with this proposition. We reported that rat PPN population responses were differentially induced by the cholinergic agent CAR compared to KA compared to NMDA (Simon et al., 2010). CAR induced a dose-dependent increase in beta/gamma activity, KA induced increases at beta and gamma, while NMDA induced increases at theta through gamma frequencies. That is, we concluded that it is the neurotransmitter profile being activated that may modulate the various frequencies being generated by the PPN (Simon et al., 2010; Kezunovic et al., 2011).
Sub- or Pre-Conscious Binding?
What is the role of gamma band activity in the RAS? People often act in order to meet desired goals, and feel that conscious will is the cause of their behavior. Scientific research suggests otherwise. Under some conditions, actions are initiated even though we are unconscious of the goal. Libet et al. (1983) was the first to show that when people consciously set a goal to engage in a behavior, their conscious will to act starts out unconsciously. Libet recorded the readiness potential (RP), a negative DC shift present long before the execution of a voluntary movement, in people asked to move voluntarily, and were also asked to subjectively time the will to move and the subjective timing of the onset of movement. (Note: the RP is maximal over the vertex, the same region described above where the P50 potential and M50 response are maximal, and where PPN deep brain stimulation produces the greatest changes in cortical blood flow. It is still not clear why the RP is localized to the region of the vertex. Moreover, studies to determine if the changes in blood flow after PPN deep brain stimulation, in activity during the M50 response, and the RP overlap other than being localized to a similar region. A testable hypothesis is that this may be a region that preferentially receives ILT output, and might thus be involved in the manifestation of PPN output resulting from PPN activation, in the appearance of the M50 response resulting from auditory evoked arousal responses, and/or in the build-up of activity resulting from integration of pre-conscious activity leading to a voluntary movement). The initial and later phases of the RP preceded the consciously determined will to move by hundreds of milliseconds. The authors concluded that cerebral initiation of spontaneous, freely voluntary acts can begin unconsciously, before there is any subjective awareness that a decision to act was initiated cerebrally. Even simple movements appear to be generated subconsciously, and the conscious sense of volition comes later (Hallett, 2007). This review described the details of studies showing that voluntary movements can be triggered with stimuli that are not perceived, that movement may well occur prior to the apparent planning of the movement, and that not only the sense of willing the movement, but also the sense of the movement having occurred, happens before the actual movement (Hallett, 2007). Another recent review described work on how even the pursuit of complex goals operates outside of conscious awareness (Custers and Aarts, 2010). These authors demonstrated that under some conditions, actions are initiated even though we are unconscious of complex goals to be attained or their motivating effects on behavior. This is clinically relevant when considering the results of studies on patients who appeared to be minimally conscious and were asked to perform two imagery tasks (Cruse et al., 2011). Different patterns of hemodynamic responses using fMRI were detected with each task, and some of the patients were able to elicit “yes” or “no” responses by generating either imagery signal. These studies suggest that even minimally conscious individuals who are unable to elicit an overt behavioral response can still use mental imagery to communicate complex ideas (Cruse et al., 2011).
So how does sub- or pre-conscious awareness arise? An attractive model of conscious perception is based on the presence of gamma band activity in the cortex. During activated states (waking and paradoxical sleep), EEG responses are characterized by low amplitude, high frequency oscillatory activity in the gamma band range (∼30–90 Hz). Gamma frequency oscillations have been proposed to participate in sensory perception, problem solving, and memory, and it has been suggested that such coherent events occur at cortical or thalamocortical levels (Eckhorn et al., 1988; Gray and Singer, 1989; Jones, 2007; Palva et al., 2009; Philips and Takeda, 2009; Voss et al., 2009). The mechanisms behind such activity, as described above, include the presence of inhibitory GABAergic cortical interneurons that exhibit intrinsic oscillatory activity in the gamma band frequency, many of which are electrically coupled, as well as of fast rhythmic bursting pyramidal neurons that are also electrically coupled. That is, the rhythm is generated initially and maintained, not by the circuit alone, but by the combination of a specific circuit AND the intrinsic membrane properties of cortical neurons.
Cognition has been proposed to arise from “specific” thalamocortical projections that carry the content of conscious experience, interacting with “non-specific” thalamocortical projections that carry the context of conscious events (Llinas et al., 1999). The cortical sites peaking at gamma band frequency via “specific” thalamocortical projections are thought to reverberate and summate with coincident “non-specific” gamma band activity. This summation, along with the coherence provided by electrical coupling, is proposed to provide global binding. Binding is the mechanism whereby the different aspects of a sensation, say, color, motion, shape, all different aspects of sensation, are combined into a unified perceptual experience. Disturbance in this mechanism results in “thalamocortical dysrhythmia,” and is thought to be involved in a number of neurological and psychiatric disorders (Llinas et al., 1999).
Again, how does “sub- or pre-conscious awareness” occur? Is there a mechanism, perhaps based on intrinsic oscillatory activity, that generates a similar process but at subcortical levels? As we saw above, gamma band activity has been reported in the hippocampus and cerebellum. Now, three nuclei that are part of the RAS all exhibit electrical coupling, providing a novel mechanism for sleep–wake control based on coherence driven by electrical coupling (Garcia-Rill et al., 2007). Moreover, virtually ALL neurons in these nuclei, regardless of cell or transmitter type, exhibit gamma band activity generated by intrinsic membrane properties. Regardless of depolarizing level or input, these cells are “pegged” to fire at gamma band frequency (30–60 Hz; Simon et al., 2010, 2011b, 2012; Kezunovic et al., 2011, 2012). This is a very unique property. Taken together, these results suggest that a similar mechanism to that in the cortex for achieving temporal coherence at high frequencies is present in the PPN, and perhaps its subcortical targets such as the Pf and SubCD nuclei. We suggested that gamma band activity generated in the PPN may help stabilize coherence related to arousal, providing a stable activation state during waking and paradoxical sleep (Simon et al., 2010; Kezunovic et al., 2011). Our overall hypothesis here is that sensory input will induce gamma band activity in the RAS that could participate in pre-conscious awareness. The RAS seems the ideal site for pre-conscious awareness since it is phylogenetically conserved, modulates sleep/wake cycles, the startle response, and fight-or-flight responses that include changes in muscle tone and locomotion.
Concluding Remarks
Functionally, the process of pre-conscious awareness would need to be fairly continuous during waking in order to provide the sensory foundation for planned behavior. This “stream of pre-consciousness” would need to begin upon waking, and it has been shown that increases in blood flow in the thalamus and brainstem begin within 5 min of waking, but as much as 15 min elapse before cortical changes in blood flow are observed (Balkin et al., 2002). This is more surprising considering that daily waking follows the last REM sleep episode of the night, during which frontal lobe blood flow is low (Garcia-Rill, 2010). This low blood flow is thought to be the reason why dream content is accepted at face value, because the brain is essentially “hypofrontal” (Garcia-Rill and Skinner, 2001; Skinner et al., 2004). Upon waking, significant increases in the brainstem and thalamus precede restoration of blood flow to the frontal lobes (Balkin et al., 2002). The importance of subcortical structures in the determination of states of awareness is being growingly emphasized. In his recent book, Damasio (2010) proposed that the brainstem is critical to the formulation of the self, which is critical to the formulation of feelings. The fact that we awaken as ourselves, despite low levels of frontal cortical blood flow, supports the view that subcortical structures are essential to this process.
Cortical function has been proposed to take place through reentrant signaling or reverberating oscillations (Olafspons et al., 1989). We assume that a similar process of cycling is present within brainstem–thalamus interactions, given the reciprocal nature of projections between the PPN and intralaminar thalamus, and between the PPN and the SubCD. Much additional investigation will be needed to substantiate this speculation, which is proposed only as a starting point for discussion of the nature of this potential process. From a pathological point of view, a similar process to thalamocortical dysrhythmia could occur at subcortical levels, in which the timing of brainstem–thalamus oscillations would be disturbed. The P50 potential is dysregulated in a number of neurological and psychiatric disorders (Garcia-Rill and Skinner, 2001; Skinner et al., 2004). The deficits produced by such dysregulation could be severe, perhaps resulting in the release of automatic behaviors such as fixed action patterns and tics, in addition to arousal and sensory gating deficits. That is, the deficits could arise from a lack of cortical regulation of these automatisms, resulting in involuntary movements, unregulated arousal, and exaggerated fight vs. flight responses. We are only beginning to explore the subcortical involvement of pre-conscious awareness in a number of disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by P20 RR020146. In addition, Dr. Urbano is a fellow of the John Simon Guggenheim Memorial Foundation (http://www.gf.org/fellows/17153-francisco-urbano) and is supported by FONCyT, Agencia Nacional de Promoción Científica y Tecnológica (http://www.ifibyne.fcen.uba.ar/new/): BID 1728 OC.AR. PICT 2007-1009, PICT 2008-2019, and PIDRI-PRH 2007.
Abbreviations
aCSF, artificial cerebrospinal fluid; AHP, after hyperpolarization; AP, action potential; CAR, carbachol; CL, centrolateral nucleus; Cx 36, connexin 36; EEG, electroencephalogram; GABA, gamma amino-butyric acid; GLUT, glutamate; IA, hyperpolarization activated current; Ik, delayed rectifier-like potassium current; ILT, intralaminar thalamus; KA, kainic acid; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; LTS, low threshold spikes; NMDA, N-methyl-D-aspartic acid; PD, Parkinson’s disease; Pf, parafascicular nucleus; PGO, ponto-geniculo-occipital; PPN, pedunculopontine nucleus; RAS, reticular activating system; REM, rapid eye movement; SubCD, subcoeruleus nucleus dorsalis; SWS, slow-wave sleep; TC, thalamocortical; TRN, thalamic relay neurons; TTX, tetrodotoxin.
References
Adamantidis, A., Carter, M. C., and de Lecea, L. (2010). Optogenetic deconstruction of sleep-wake circuitry in the brain. Front. Mol. Neurosci. 2:31. doi: 10.3389/neuro.02.031.2009
Androulidakis, M. S., Mazzone, P., Litvak, V., Penny, W., Dileone, M., Gaynor, L. M., Tisch, S., Di Lazzaro, V., and Brown, P. (2008). Oscillatory activity in the pedunculopontine area of patients with Parkisnson’s disease. Exp. Neurol. 211, 59–66.
Aserinsky, E., and Kleitman, N. (1953). Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118, 273–274.
Azouz, R., Gray, C. M., Nowak, L. G., and McCormick, D. A. (1997). Physiological properties of inhibitory interneurons in cat striate cortex. Cereb. Cortex 7, 534–545.
Baghdoyan, H. A., Rodrigo-Angulo, M. L., McCarley, R. W., and Hobson, J. A. (1984). Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 306, 39–52.
Baghdoyan, H. A., Rodrigo-Angulo, M. L., McCarley, R. W., and Hobson, J. A. (1987). A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 414, 245–261.
Balkin, T. J., Braun, A. R., Wesensten, N. J., Jeffries, K., Varga, M., Baldwin, P., Belenky, G., and Herscovitch, P. (2002). The process of awakening: a PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain 125, 2308–2319.
Ballanger, B., Lozano, A. M., Moro, E., van Eimeren, T., Hamani, C., Chen, R., Cilia, R., Houle, S., Poon, Y. Y., Lang, A. E., and Strafella, A. P. (2009). Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: a [(15)O] H2O PET study. Hum. Brain Mapp. 30, 3901–3909.
Barraza, D., Kita, H., and Wilson, C. J. (2009). Slow spike frequency adaptation in neurons of the rat subthalamic nucleus. J. Neurophysiol. 102, 3689–3697.
Beck, P., Odle, A., Wallace-Huitt, T., Skinner, R. D., and Garcia-Rill, E. (2008). Modafinil increases arousal determined by P13 potential amplitude; an effect blocked by gap junction antagonists. Sleep 31, 1647–1654.
Boissard, R., Fort, P., Gervasoni, D., Barbagli, B., and Luppi, P. H. (2003). Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur. J. Neurosci. 18, 1627–1639.
Boissard, R., Gervasoni, D., Schmidt, M. H., Barbagli, B., Fort, P., and Luppi, P. H. (2002). The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur. J. Neurosci. 16, 1959–1973.
Brown, R. E., Winston, S., Basheer, R., Thakkar, M. M., and McCarley, R. W. (2006). Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience 143, 739–755.
Buchwald, J. S., Rubinstein, E. H., Schwafel, J., and Strandburg, R. J. (1991). Midlatency auditory evoked responses: differential effects of a cholinergic agonist and antagonist. Electroencephalogr. Clin. Neurophysiol. 80, 303–309.
Bussey, T. J., Muir, J. L., and Aggleton, J. P. (1999). Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J. Neurosci. 19, 495–502.
Buzsáki, G., and Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929.
Capozzo, A., Florio, T., Cellini, R., Moriconi, U., and Scarnati, E. (2003). The pedunculopontine nucleus projection to the parafascicular nucleus of the thalamus: an electrophysiological investigation in the rat. J. Neural Transm. 110, 733–747.
Carter, M. E., and de Lecea, L. (2011). Optogenetic investigation of neural circuits in vivo. Trends Mol. Med. 17, 197–206.
Carter, M. E., Yizhar, O., Chikahisa, S., Nguyen, H., Adamantidis, A., Nishino, S., Deisseroth, K., and de Lecea, L. (2010). Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533.
Caterall, W. A. (1988). Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium 24, 307–323.
Charpak, S., Paré, D., and Llinás, R. R. (1995). The entorhinal cortex entrains fast CA1 hippocampal oscillations in the anaesthetized guinea-pig: role of the monosynaptic component of the perforant path. Eur. J. Neurosci. 7, 1548–1557.
Chrobak, J. J., and Buzsáki, G. (1998). Gamma oscillations in the entorhinal cortex of the freely behaving rat. J. Neurosci. 18, 388–398.
Colgin, L. L., Denninger, T., Fyhn, M., Hafting, T., Bonnevie, T., Jensen, O., Moser, M. B., and Moser, E. I. (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357.
Colgin, L. L., and Moser, E. I. (2010). Gamma oscillations in the hippocampus. Physiology 25, 319–329.
Cruse, D., Chennu, S., Chatelle, C., Beckinstein, T. A., Fernandez-Espejo, D., Pickard, J. D., Laureys, S., and Owen, A. M. (2011). Bedside detection of awareness in the vegetative state: a cohort study. Lancet 378, 2088–2094.
Cunningham, M. O., Whittington, M. A., Bibbig, A., Roopun, A., LeBeau, F. E., Vogt, A., Monyer, H., Buhl, E. H., and Traub, R. D. (2004). A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc. Natl. Acad. Sci. U.S.A. 101, 7152–7157.
Custers, R., and Aarts, H. (2010). The unconscious will: how the pursuit of goals operates outside of conscious awareness. Science 329, 47–50.
Datta, S. (2002). Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J. Neurophysiol. 87, 1790–1798.
Datta, S., and Hobson, J. A. (1994). Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J. Neurophysiol. 71, 95–109.
Datta, S., Mavanji, V., Ulloor, J., and Patterson, E. H. (2004). Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J. Neurosci. 24, 1416–1427.
Datta, S., Patterson, E. H., and Siwek, D. F. (1999). Brainstem afferents of the cholinoceptive pontine wave generation sites in the rat. Sleep Res. Online 2, 79–82.
Datta, S., Patterson, E. H., and Spoley, E. E. (2001a). Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J. Neurosci. Res. 66, 109–116.
Datta, S., Spoley, E. E., and Patterson, E. H. (2001b). Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R752–R759.
Datta, S., and Siwek, D. F. (2002). Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res. 70, 79–82.
Datta, S., Siwek, D. F., Patterson, E. H., and Cipolloni, P. B. (1998). Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse 30, 409–423.
Datta, S., Siwek, D. F., and Stack, E. C. (2009). Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neuroscience 163, 397–414.
de Lecea, L., Kilduff, T. S., Peyron, C., Gao, X., Foye, P. E., Danielson, P. E., Fukuhara, C., Battenberg, E. L., Gautvik, V. T., Bartlett, F. S. II., Frankel, W. N., van den Pol, A. N., Bloom, F. E., Gautvik, K. M., and Sutcliffe, J. G. (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A. 95, 322–327.
Deschenes, M., Bourassa, J., Doan, V. D., and Parent, A. (1996a). A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur. J. Neurosci. 8, 329–343.
Deschenes, M., Bourassa, J., and Parent, A. (1996b). Striatal and cortical projections of single neurons from the central lateral thalamic nucleus in the rat. Neuroscience 72, 679–687.
Eckhorn, R., Bauer, R., Jordan, W., Brosch, M., Kruse, W., Munk, M., and Reitboeck, H. J. (1988). Coherent oscillations: a mechanism of feature linking in the visual system? Biol. Cybern. 60, 121–130.
Erro, E., Lanciego, J. L., and Gimenez-Amaya, J. M. (1999). Relationships between thalamostriatal neurons and pedunculopontine projections to the thalamus: a neuroanatomical tract-tracing study in the rat. Exp. Brain Res. 127, 162–170.
Erwin, R. J., and Buchwald, J. S. (1986a). Midlatency auditory evoked responses: differential recovery cycle characteristics. Electroencephalogr. Clin. Neurophysiol. 64, 417–423.
Erwin, R. J., and Buchwald, J. S. (1986b). Midlatency auditory evoked responses: differential effects of sleep in the human. Electroencephalogr. Clin. Neurophysiol. 65, 383–392.
Garcia-Rill, E. (2010). “Reticular activating system,” in The Neuroscience of Sleep, eds R. Stickgold, and M. Walker (London: Academic Press), 133–138.
Garcia-Rill, E., Charlesworth, A., Heister, D., Ye, M., and Hayar, A. (2008a). The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep 31, 673–690.
Garcia-Rill, E., Moran, K., Garcia, J., Findley, W. M., Walton, K., Strotman, B., and Llinas, R. R. (2008b). Magnetic sources of the M50 response are localized to frontal cortex. Clin. Neurophysiol. 119, 388–398.
Garcia-Rill, E., Heister, D. S., Ye, M., Charlesworth, A., and Hayar, A. (2007). Electrical coupling: novel mechanism for sleep-wake control. Sleep 30, 1405–1414.
Garcia-Rill, E., and Skinner, R. D. (2001). “The sleep state-dependent P50 midlatency auditory evoked potential,” in Sleep Medicine, eds T. L. Lee-Chiong, M. A. Carskadon, and M. J. Sateia (Philadelphia: Hanley & Belfus), 697–704.
Gibson, J. R., Beierlein, M., and Connors, B. W. (1999). Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402, 75–79.
Gray, C. M., and Singer, W. (1989). Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. U.S.A. 86, 1698–1702.
Haas, J. S., Zavala, B., and Landisman, C. E. (2011). Activity-dependent long-term depression of electrical synapses. Science 334, 389–393.
Hallett, M. (2007). Volitional control of movement: the physiology of free will. Clin. Neurophysiol. 118, 1179–1192.
Heister, D. S., Hayar, A., Charlesworth, A., Yates, C., Zhou, Y., and Garcia-Rill, E. (2007). Evidence for electrical coupling in the SubCoeruleus (SubC) nucleus. J. Neurophysiol. 97, 3142–3147.
Hillman, D., Chen, S., Aung, T. T., Cherksey, B., Sugimori, M., and Llinas, R. R. (1991). Localiation of P-type calcium channels in the central nervous system. Proc. Natl. Acad. Sci. U.S.A. 88, 7076–7080.
Ito, S., and Craig, A. D. (2005). Vagal-evoked activity in the parafascicular nucleus of the primate thalamus. J. Neurophysiol. 94, 2976–2982.
Iwasaki, S., and Takahashi, T. (1998). Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J. Physiol. (Lond.) 509, 419–442.
Jahnsen, H., and Llinas, R. R. (1984a). Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J. Physiol. (Lond.) 349, 205–226.
Jahnsen, H., and Llinas, R. R. (1984b). Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J. Physiol. (Lond.) 349, 227–247.
Jones, E. G. (2007). Calcium channels in higher-level brain function. Proc. Natl. Acad. Sci. U.S.A. 14, 17903–17904.
Jouvet-Mounier, D., Astic, L., and Lacote, D. (1970). Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev. Psychobiol. 2, 216–239.
Kamondi, A., Williams, J., Hutcheon, B., and Reiner, P. (1992). Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J. Neurophysiol. 68, 1359–1372.
Karlsson, K. A., Gall, A. J., Mohns, E. J., Seelke, A. M., and Blumberg, M. S. (2005). The neural substrates of infant sleep in rats. PLoS Biol. 3, e143. doi: 10.1371/journal.pbio.0030143
Katz, B., and Miledi, R. (1965). The effect of calcium on acetylcholine release from motor nerve terminals. Proc. R. Soc. Lond. B Biol. Sci. 161, 483–495.
Kezunovic, N., Hyde, J., Simon, C., Urbano, F. J., William, D. K., and Garcia-Rill, E. (2012). Gamma band activity in the developing parafascicular nucleus. J. Neurophysiol. 107, 772–784.
Kezunovic, N., Urbano, F. J., Simon, C., Hyde, J., Smith, K., and Garcia-Rill, E. (2011). Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN). Eur. J. Neurosci. 34, 404–415.
Kha, H. T., Finkelstein, D. I., Pow, D. V., Lawrence, A. J., and Horne, M. K. (2000). Study of projections from the entopeduncular nucleus to the thalamus of the rat. J. Comp. Neurol. 426, 366–377.
Kobayashi, S., and Nakamura, Y. (2003). Synaptic organization of the rat parafascicular nucleus, with special reference to its afferents from the superior colliculus and the pedunculopontine tegmental nucleus. Brain Res. 980, 80–91.
Krueger, J. M., Rector, D. M., Roy, S., van Dongen, H. P., Belenky, G., and Panksepp, J. (2008). Sleep as a fundamental property of neuronal assemblies. Nat. Rev. Neurosci. 9, 910–919.
Kumar, V. M. (2010). Sleep is neither a passive nor an active phenomenon. Sleep Biol. Rhythms 8, 163–169.
Lacey, C. J., Bolam, J. P., and Magill, P. J. (2007). Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J. Neurosci. 27, 4374–4384.
Lang, E. J., Sugihara, I., and Llinás, R. R. (2006). Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J. Physiol. (Lond) 571, 101–120.
Leonard, C. S., and Llinas, R. R. (1990). “Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the contol of REM sleep,” in Brain Cholinergic Systems, eds M. Steriade and Biesold D. (Oxford: Oxford Science), 205–223.
Libet, B., Gleason, C. A., Wright, E. W., and Pearl, D. K. (1983). Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain 106, 623–642.
Llinas, R., and Jahnsen, H. (1982). Electrophysiology of mammalian thalamic neurones in vitro. Nature 297, 406–408.
Llinas, R. R. (1988). The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242, 1654–1664.
Llinas, R. R., Choi, S., Urbano, F. J., and Shin, H.-S. (2007). Gamma band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc. Natl. Acad. Sci. U.S.A. 104, 17819–17824.
Llinas, R. R., Grace, A. A., and Yarom, Y. (1991). In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc. Natl. Acad. Sci. U.S.A. 88, 897–901.
Llinas, R. R., and Hess, R. (1976). Tetrodotoxin-resistant dendritic spikes in avian Purkine cells. Proc. Natl. Acad. Sci. U.S.A. 73, 2520–2523.
Llinas, R. R., Leznik, E., and Urbano, F. J. (2002). Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc. Natl. Acad. Sci. U.S.A. 99, 449–454.
Llinas, R. R., Ribary, U., Jeanmonod, D., Kronberg, E., and Mitra, P. P. (1999). Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U.S.A. 96, 15222–15227.
Llinás, R. R., and Steriade, M. (2006). Bursting of thalamic neurons and states of vigilance. J. Neurophysiol. 95, 3297–3308.
Lu, J., Sherman, D., Devor, M., and Saper, C. B. (2006). A putative flip-flop switch for control of REM sleep. Nature 441, 589–594.
Marks, G. A., Farber, J., and Roffwarg, H. P. (1980). Metencephalic localization of ponto-geniculo-occipital waves in the albino rat. Exp. Neurol. 69, 667–677.
Mavanji, V., Ulloor, J., Saha, S., and Datta, S. (2004). Neurotoxic lesions of phasic pontine-wave generator cells impair retention of 2-way active avoidance memory. Sleep 27, 1282–1292.
Mazzone, P., Scarnati, E., and Garcia-Rill, E. (2010). Commentary: the pedunculopontine nucleus: clinical experience, basic questions and future directions. J. Neural Transm. 118, 1391–1396.
Middleton, S. J., Racca, C., Cunningham, M. O., Traub, R. D., Monyer, H., Knöpfel, T., Schofield, I. S., Jenkins, A., and Whittington, M. A. (2008). High-frequency network oscillations in cerebellar cortex. Neuron 58, 763–774.
Minamimoto, T., and Kimura, M. (2002). Participation of the thalamic CM-Pf complex in attentional orienting. J. Neurophysiol. 87, 3090–3101.
Mitani, A., Ito, K., Hallanger, A. E., Wainer, B. H., Kataoka, K., and McCarley, R. W. (1988). Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat. Brain Res. 451, 397–402.
Mitler, M. M., and Dement, W. C. (1974). Cataplectic-like behavior in cats after micro-injections of carbachol in pontine reticular formation. Brain Res. 68, 335–343.
Moruzzi, G., and Magoun, H. W. (1949). Brainstem reticular formation and activation. Electroencephalogr. Clin. Neurophysiol. 1, 455–473.
Mouret, J., Delorme, F., and Jouvet, M. (1967). Lesions of the pontine tegmentum and sleep in rats. C R Seances Soc. Biol. Fil. 161, 1603–1606.
Olafspons, J., Gally, G., Reeke, J., and Edelman, G. (1989). Reentrant signaling among simulated neuronal groups leads to coherency in their oscillatory activity. Proc. Natl. Acad. Sci. U.S.A. 86, 7265–7269.
Palva, S., Monto, S., and Palva, J. M. (2009). Graph properties of synchronized cortical netwoks during visual working memory maintenance. Neuroimage 49, 3257–3268.
Pedroarena, C., and Llinás, R. R. (1997). Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc. Natl. Acad. Sci. U.S.A. 94, 724–728.
Phelan, K. D., Mahler, H. R., Deere, T., Cross, C. B., Good, C., and Garcia-Rill, E. (2005). Postnatal maturational properties of rat parafascicular thalamic neurons recorded in vitro. Thalamus Relat. Syst. 1, 1–25.
Philips, S., and Takeda, Y. (2009). Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int. J. Psychophysiol. 73, 350–354.
Povysheva, N. V., Zaitsev, A. V., Rotaru, D. C., Gonzalez-Burgos, G., Lewis, D. A., and Krimer, L. S. (2008). Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J. Neurophysiol. 100, 2348–2360.
Raeva, S. N. (2006). The role of the parafascicular complex (CM-Pf) of the human thalamus in the neuronal mechanisms of selective attention. Neurosci. Behav. Physiol. 36, 287–295.
Ribary, U., Ioannides, A. A., Singh, K. D., Hasson, R., Bolton, J. P., Lado, F., Mogilner, A., and Llinás, R. R. (1991). Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc. Natl. Acad. Sci. U.S.A. 88, 11037–11041.
Roffwarg, H. P., Muzio, J. N., and Dement, W. C. (1966). Ontogenetic development of the human sleep-dream cycle. Science 152, 604–619.
Sakai, K., El Mansari, M., and Jouvet, M. (1990). Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 527, 213–223.
Sanford, L. D., Morrison, A. R., Mann, G. L., Harris, J. S., Yoo, L., and Ross, R. J. (1994). Sleep patterning and behaviour in cats with pontine lesions creating REM without atonia. J. Sleep Res. 3, 233–240.
Shen, W., Hernandez-Lopes, S., Tkatch, T., Held, E., and Surmeier, J.. (2004). Kv1.2-containing K+ channels regulate subthreshold excitability of striatal medium spiny neurons. J. Neurophysiol. 91, 1337–1349.
Shiromani, P. J., Armstrong, D. M., and Gillin, J. C. (1988). Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci. Lett. 95, 19–23.
Simon, C., Hayar, A., and Garcia-Rill, E. (2011a). Responses of developing pedunculopontine neurons to glutamate receptor agonists. J. Neurophysiol. 105, 1918–1931.
Simon, C., Kezunovic, N., Williams, D. K., Urbano, F. J., and Garcia-Rill, E. (2011b). Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons. Am. J. Physiol. Cell Physiol. 301, C327–C335.
Simon, C., Hayar, A., and Garcia-Rill, E. (2012). Developmental changes in glutamatergic fast synaptic neurotransmission in the dorsal subcoeruleus nucleus. Sleep (in press).
Simon, C., Kezunovic, N., Ye, M., Hyde, J., Hayar, A., Williams, D. K., and Garcia-Rill, E. (2010). Gamma band unit and population responses in the pedunculopontine nucleus. J. Neurophysiol. 104, 463–474.
Singer, W. (1993). Synchronization of cortical activity and its putative role in informtion processing and learning. Annu. Rev. Physiol. 55, 349–374.
Sirota, A., Montgomery, S., Fujisawa, S., Isomura, Y., Zugaro, M., and Buzsáki, G. (2008). Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697.
Skinner, R. D., Miyazato, H., and Garcia-Rill, E. (2004). “Arousal mechanisms related to posture and movement. I. Ascending modulation,” in Brain Mechanisms for the Integration of Posture and Movement, Vol. 143, Progress in Brain Research: Brain Mechanisms for the Integration of Posture and Movement, eds S. Mori, D. G. Stuart, and M. Wiesendanger, 291–298.
Soteropoulos, D. S., and Baker, S. N. (2006). Cortico-cerebellar coherence during a precision grip task in the monkey. J. Neurophysiol. 95, 1194–1206.
Stam, C. J., van Cappellen van Walsum, A. M., Pijnenburg, Y. A., Berendse, H. W., de Munck, J. C., Scheltens, P., and van Dijk, B. W. (2002). Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J. Clin. Neurophysiol. 19, 562–574.
Steriade, M. (1999). “Cellular substrates of oscillations in corticothalamic systems during states of vigilance,” in Handbook of Behavioral State Control. Cellular and Molecular Mechanisms, eds R. Lydic, and H. A. Baghdoyan (New York: CRC Press), 327–347.
Steriade, M., Curro Dossi, R., Paré, D., and Oakson, G. (1991). Fast oscillations (20-40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc. Natl. Acad. Sci. U.S.A. 88, 4396–4400.
Steriade, M., Curro-Dossi, R., and Contreras, D. (1993). Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (∼40Hz) spike-bursts at ∼1000Hz during waking and rapid eye movement sleep. Neuroscience 56, 1–9.
Steriade, M., Datta, S., Paré, D., Oakson, G., and Curro Dossi, R. (1990a). Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 10, 2541–2559.
Steriade, M., Paré, D., Datta, S., Oakson, G., and Curro Dossi, R. (1990b). Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J. Neurosci. 10, 2560–2579.
Steriade, M., and Llinás, R. R. (1988). The functional states of the thalamus and the associated neuronal interplay. Physiol. Rev. 68, 649–742.
Steriade, M., and McCarley, R. W. (1990). Brainstem Control of Wakefulness and Sleep. New York: Plenum Press.
Takakusaki, K., and Kitai, S. T. (1997). Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience 78, 771–794.
Timofeev, I., and Steriade, M. (1997). Fast (mainly 30-100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. J. Physiol. (Lond.) 504, 153–168.
Tsang, E. W., Hamani, C., Moro, E., Mazzella, F., Poon, Y. Y., Lozano, A. M., and Chen, R. (2010). Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology 75, 950–959.
Uchitel, O. D., Protti, D. A., Sanchez, V., Cherkesey, B. D., Sugimori, M., and Llinas, R. R. (1992). P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc. Natl. Acad. Sci. U.S.A. 89, 3330–3333.
Urbano, F. J., Leznik, E., and Llinas, R. (2007). Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc. Natl. Acad. Sci U.S.A. 104, 12554–12559.
Van der Werf, Y. D., Witter, M. P., and Groenewegen, H. J. (2002). The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 39, 107–140.
Vanderwolf, C. H. (2000). What is the significance of gamma wave activity in the pyriform cortex? Brain Res. 877, 125–133.
Vanni-Mercier, G., Sakai, K., Lin, J. S., and Jouvet, M. (1989). Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch. Ital. Biol. 127, 133–164.
Voss, U., Holzmann, R., Tuin, I., and Hobson, J. A. (2009). Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep 32, 1191–1200.
Wang, H. L., and Morales, M. (2009). Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 29, 340–358.
Watson, R. T., Heilman, K. M., and Miller, B. D. (1974). Neglect after mesencephalic reticulr formation lesions. Neurology 24, 294–298.
Westenbroek, R. E., Hell, J. W., Warner, C., Dubel, S. J., Snutch, T. P., and Catterall, W. A. (1992). Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron 9, 1099–1115.
Whittington, M. A., Traub, R. D., and Jefferys, J. G. (1995). Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615.
Wilson, D. I. G., MacLaren, D. A. A., and Winn, P. (2009). Bar pressing for food: differential consequences of lesions to anterior versus posterior pedunculopontine. Behav. Neurosci. 30, 504–513.
Woo, T. U., Spencer, K., and McCarley, R. W. (2010). Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv. Rev. Psychiatry 8, 173–189.
Yamamoto, K., Mamelak, A. N., Quattrochi, J. J., and Hobson, J. A. (1990). A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: spontaneous and drug-induced neuronal activity. Neuroscience 39, 295–304.
Keywords: arousal, calcium channels, gamma band, subthreshold oscillations
Citation: Urbano FJ, Kezunovic N, Hyde J, Simon C, Beck P and Garcia-Rill E (2012) Gamma band activity in the reticular activating system. Front. Neur. 3:6. doi: 10.3389/fneur.2012.00006
Received: 17 November 2011; Accepted: 06 January 2012;
Published online: 31 January 2012.
Edited by:
V. Mohan Kumar, Sree Chitra Tirunal Institute for Medical Sciences and Technology, IndiaReviewed by:
Subimal Datta, Boston University School of Medicine, USAPeter Halasz, Hungarian Sleep Society, Hungary
Pablo Torterolo, Universidad de la República, Uruguay
Copyright: © 2012 Urbano, Kezunovic, Hyde, Simon, Beck and Garcia-Rill. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Edgar Garcia-Rill, Department of Neurobiology, Center for Translational Neuroscience, University of Arkansas for Medical Sciences, 4301 West Markham Street, Slot 847, Little Rock, AR 72205, USA. e-mail:Z2FyY2lhcmlsbGVkZ2FyQHVhbXMuZWR1

 Nebojsa Kezunovic2
Nebojsa Kezunovic2
