
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 27 December 2011
Sec. Neuro-Otology
volume 2 - 2011 | https://doi.org/10.3389/fneur.2011.00088
This article is part of the Research Topic New Understanding of Plasticity in the Vestibular System: From Bench to Bed Side View all 12 articles
 Andrew A. McCall
Andrew A. McCall Bill J. Yates*
Bill J. Yates*Bilateral loss of vestibular inputs affects far fewer patients than unilateral inner ear damage, and thus has been understudied. In both animal subjects and human patients, bilateral vestibular hypofunction (BVH) produces a variety of clinical problems, including impaired balance control, inability to maintain stable blood pressure during postural changes, difficulty in visual targeting of images, and disturbances in spatial memory and navigational performance. Experiments in animals have shown that non-labyrinthine inputs to the vestibular nuclei are rapidly amplified following the onset of BVH, which may explain the recovery of postural stability and orthostatic tolerance that occurs within 10 days. However, the loss of the vestibulo-ocular reflex and degraded spatial cognition appear to be permanent in animals with BVH. Current concepts of the compensatory mechanisms in humans with BVH are largely inferential, as there is a lack of data from patients early in the disease process. Translation of animal studies of compensation for BVH into therapeutic strategies and subsequent application in the clinic is the most likely route to improve treatment. In addition to physical therapy, two types of prosthetic devices have been proposed to treat individuals with bilateral loss of vestibular inputs: those that provide tactile stimulation to indicate body position in space, and those that deliver electrical stimuli to branches of the vestibular nerve in accordance with head movements. The relative efficacy of these two treatment paradigms, and whether they can be combined to facilitate recovery, is yet to be ascertained.
Disorders of the vestibular system are common. Dizziness is a symptom that frequently results from vestibular disorders and affects up to 36% of the population (Gopinath et al., 2009). More specifically, up to 7% of people experience vertigo related to a vestibular disorder within their lifetime (Neuhauser and Lempert, 2009). Bilateral partial or complete vestibular loss, sometimes referred to as bilateral vestibular hypofunction (BVH) or Dandy’s (1941) syndrome, is less often identified than unilateral vestibular loss as a cause of dizziness; however, it remains a significant clinical problem. In a review of the office records of over 6000 patients from an academic dizziness practice, 4% of patients were diagnosed with BVH (Zingler et al., 2009).
A variety of conditions can produce BVH, and in many cases the cause of the disease is unknown. Table 1 lists causes of BVH; the three most common etiologies that have been documented are exposure to ototoxic antibiotics, Menière’s disease, and encephalitis (Rinne et al., 1998; Gillespie and Minor, 1999; Zingler et al., 2009). It is also likely that a variety of autoimmune disorders cumulatively result in an appreciable fraction of cases of BVH (Rinne et al., 1998; Gillespie and Minor, 1999; Zingler et al., 2009). While BVH resulting from autoimmune disorders (Hughes et al., 1984), ototoxic antibiotics (Reiter et al., 2011), and traumatic injury such as blast exposure (Akin and Murnane, 2011) can develop quickly, that resulting from Menière’s disease usually develops slowly over time, as the disease typically first manifests on one side, with involvement of the contralateral ear in a subset of patients after a number of years (Sumi et al., 2011). The occurrence of BVH is sometimes associated with cerebellar ataxia, which may be a distinct syndrome that is associated with an impaired visually enhanced vestibulo-ocular reflex (Szmulewicz et al., 2011).
Even following complete loss of labyrinthine inputs, some of the signs and symptoms resulting from BVH diminish over time. Other clinical problems, however, are permanent. This review describes and contrasts the short- and long-term consequences of BVH, as well as the possible neural mechanisms that mediate the process of compensation. Although a large number of reviews have addressed compensation following a unilateral labyrinthectomy (e.g., Dieringer, 1995; Vidal et al., 1998; Curthoys, 2000; Gliddon et al., 2005; Cullen et al., 2009; Dutia, 2010), far less is known about recovery following the complete loss of vestibular inputs. Nonetheless, some existing information does provide insights into the mechanisms underlying this process, which will be discussed along with the shortfalls in the data. In addition, this review evaluates new clinical tools and strategies that may aid patients with BVH.
Research on animals has provided a better opportunity than clinical studies to understand compensation following the bilateral loss of vestibular inputs, as lesions can be created at a prescribed time and the effects on behavior or physiological responses can be studied systematically. Macpherson and colleagues documented the effects of a bilateral labyrinthectomy on postural stability in cats (Thomson et al., 1991; Inglis and Macpherson, 1995; Stapley et al., 2006; Macpherson et al., 2007). The animals were severely impaired for the first 2 days after lesions, after which they could stand unsupported on a tilt platform and walk in a staggering fashion (Thomson et al., 1991). Within a week, animals could jump to and from a chair, ataxia was profoundly reduced, and locomotion speeds were much faster (Thomson et al., 1991). Although limb muscle responses to linear translations had normal patterning after the loss of vestibular inputs, hypermetria was present for the first 10 days (Inglis and Macpherson, 1995). These observations show that a rapid compensation process occurs during the first 7–10 days following the removal of labyrinthine signals, which then slows considerably. However, some postural deficits were enduring. For example, balance was permanently destabilized when the head was turned (Thomson et al., 1991; Stapley et al., 2006), due to the fact that at peak yaw head velocity the lesioned cats produced an unexpected burst in extensors of the contralateral limbs that thrust the body to the ipsilateral side (Stapley et al., 2006). The magnitude of the counterproductive limb extension was largest during the first few days after lesions, but the response remained present when the experiment was discontinued ∼40 days after the removal of vestibular inputs.
Other groups have also examined the effects of a bilateral labyrinthectomy on postural responses. It was demonstrated that limb extension during falling, which is critical for normal landing, is permanently lost following a bilateral labyrinthectomy (Watt, 1976). However, righting responses did recover over time (Igarashi and Guitierrez, 1983). In addition, there were permanent impairments in the ability to keep to a straight course in darkness, although veering was minimal when visual cues were present (Marchand and Amblard, 1990). In another study, tonic activity of some trunk muscles, including the abdominal musculature, remained elevated for the entire 30-day recording period following a bilateral labyrinthectomy (Cotter et al., 2001), although muscle activity was highest during the first week following lesions.
Postural alterations that place the long axis of the body below the heart, such as head-up tilts in quadrupeds or standing in man, tend to produce a reduction in venous return to the heart (Yavorcik et al., 2009) that requires rapid responses of the autonomic nervous system to avoid an alteration in blood pressure (Rushmer, 1976; Hall, 2011). The responses include vasoconstriction in the portion of the body below the heart to prevent peripheral blood pooling (Wilson et al., 2006; Yavorcik et al., 2009). The top panel of Figure 1B illustrates that in a vestibular-intact animal, blood flow to the hindlimb decreased below basal levels within 10 s of a sudden 60° head-up tilt. However at the onset of the tilt, blood flow to the hindlimb increased because of the effects of gravity; this increased blood flow would have persisted if vasoconstriction did not occur (Wilson et al., 2006; Yavorcik et al., 2009). As a consequence of the autonomic nervous system responses during large head-up rotations, blood pressure remains relatively stable during the postural alteration (see Figure 1A; Jian et al., 1999).
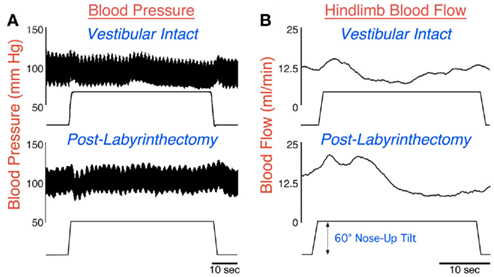
Figure 1. Arterial blood pressure (A) and femoral artery blood flow (B) recorded in a conscious cat during a 60° head-up tilt before (top) and a few days subsequent (bottom) to a combined bilateral labyrinthectomy and vestibular neurectomy. When the labyrinth was intact (top), blood pressure remained relatively stable during the head-up rotation. Although femoral artery blood flow initially increased during the movement due to the effects of gravity, flow quickly dropped due to peripheral vasoconstriction. However, following the removal of vestibular inputs (bottom), a drop in blood pressure occurred at the onset of the head-up rotation. In addition, the gravity-induced increase in blood flow in the femoral artery was larger and more prolonged, because peripheral vasoconstriction was delayed. Data in (A) from Jian et al. (1999); data in (B) from Wilson et al. (2006).
Following a bilateral labyrinthectomy, the attenuation in hindlimb blood flow that ordinarily occurs during 60° head-up rotations was delayed and diminished (Wilson et al., 2006; Yavorcik et al., 2009), as shown in the bottom panel of Figure 1B. In addition, blood pressure became unstable at the onset of head-up tilts (Jian et al., 1999), as illustrated in the bottom panel of Figure 1A. However, these deficits were only prominent for a week after the loss of vestibular inputs, at which time blood pressure was stable during postural alterations (Jian et al., 1999). A caveat is that the animals could have expected to be tilted quite often when restrained in the rotating device, such that they were particularly vigilant during the experimental sessions. Animals may not always maintain such a high level of attention to environmental cues regarding body position in space outside of laboratory conditions. Thus, BVH could result in a long lasting deficit in correcting blood pressure, but this deficit only becomes apparent when the level of alertness diminishes.
Eye movements in response to head rotations performed in the dark are permanently abolished in animals following a bilateral labyrinthectomy (Baarsma and Collewijn, 1974; Barmack et al., 1980; Waespe and Wolfensberger, 1985; Waespe et al., 1992). The rapid component of eye movements during head rotations in a lighted environment is also lost, but the slow component driven by visual inputs persists (Baarsma and Collewijn, 1974; Barmack et al., 1980; Waespe and Wolfensberger, 1985). Although eye movements triggered by a slowly moving visual field (optokinetic responses) were retained following the loss of labyrinthine inputs (Baarsma and Collewijn, 1974; Barmack et al., 1980), optokinetic after nystagmus was eliminated (Cohen et al., 1973; Waespe and Wolfensberger, 1985). These observations show that unlike postural and autonomic responses that recover rapidly following the loss of labyrinthine inputs, vestibular-related eye movements are permanently affected.
Following chemical damage of both labyrinths, rodents have impaired navigational abilities and diminished spatial memory (Blair and Sharp, 1995; Stackman and Herbert, 2002; Wallace et al., 2002; Russell et al., 2003a; Smith et al., 2005; Baek et al., 2010). Furthermore, the spatially related modulation of activity of neurons believed to be critical for spatial cognition, including place cells in the hippocampus (Russell et al., 2003b) and head direction cells in the lateral mammillary nucleus, postsubiculum, and anterior thalamic nuclei (Stackman and Taube, 1997; Taube, 1998; Muir et al., 2009; Shinder and Taube, 2010), is lost following bilateral vestibular lesions. The effects of removal of labyrinthine inputs on navigational abilities showed little recovery even when animals were tested 14 months after injury (Baek et al., 2010). Thus, the cognitive deficits produced by BVH do not appear to dissipate over time.
Although an initial report indicated that the firing rate of vestibular nucleus neurons is depressed for a prolonged period following a bilateral labyrinthectomy (Ryu and McCabe, 1976), more recent studies showed that spontaneous activity of vestibular nucleus units returns within hours following bilateral elimination of labyrinthine inputs, and is nearly identical to prelesion levels within less than a week (Waespe et al., 1992; Ris and Godaux, 1998; Miller et al., 2008). For example, Figure 2 compares the spontaneous activity and firing regularity (coefficient of variation, the SD of interval between spikes divided by mean interval between spikes) of neurons in the inferior and caudal medial vestibular nuclei of a conscious cat before and in the first week after a combined labyrinthectomy and vestibular neurectomy (Miller et al., 2008). The firing rates before and after elimination of vestibular inputs [30 ± 2 (SEM) vs. 32 ± 2 spikes/s, respectively], as well as the coefficient of variation of firing rates (0.78 ± 0.05 vs. 0.76 ± 0.05), were virtually identical in the two populations. Ris and Godaux (1998) conducted a longitudinal study of firing rates of vestibular nucleus neurons in conscious guinea pigs before and at 1 h, 1 day, and 1 week after a bilateral vestibular neurectomy. In control animals, no silent vestibular nucleus units could be detected; 53% of the cells were inactive at 1–5 h after a bilateral labyrinthectomy, and ∼35% were inactive at 1 day after lesions. By a week after elimination of labyrinthine signals, no silent neurons could be observed in the vestibular nuclei (Ris and Godaux, 1998).
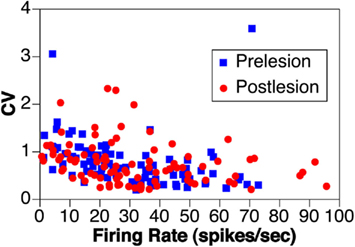
Figure 2. The rate and coefficient of variation (CV) of spontaneous activity of vestibular nucleus neurons before (prelesion) and in the first week following (postlesion) removal of vestibular inputs through a bilateral vestibular neurectomy in one animal. Data from Miller et al. (2008).
Although vestibular nucleus neurons are insensitive to horizontal rotations following the removal of labyrinthine inputs (Ris and Godaux, 1998), the firing rates of some cells can be modulated by 15° tilts in vertical planes (Yates et al., 2000; Miller et al., 2008). In conscious cats, such response modulation was uncommon (7/168 neurons recorded in three animals; Miller et al., 2008); however, 18/67 neurons recorded from the vestibular nuclei of decerebrate cats that had undergone a combined bilateral labyrinthectomy and vestibular neurectomy over a month previously responded to vertical rotations (Yates et al., 2000). The response properties of vestibular nucleus neurons to vertical rotations in animals lacking labyrinthine inputs are illustrated in Figure 3. Figure 3A compares the vector orientations for responses to vertical tilts in animals lacking vestibular inputs to those observed in labyrinth-intact decerebrate and conscious animals tested using the same tilt table. In both decerebrate and conscious cats lacking vestibular inputs, the response vector orientations of most neurons were near the pitch plane (mean deviation from the pitch axis of 15°). In contrast, in labyrinth-intact cats, the response vector orientations were much nearer the roll axis; the mean vector deviation from the pitch axis was 50° in conscious animals (Miller et al., 2008) and 57° in decerebrate animals (Jian et al., 2002). The differences in response vector orientations between labyrinth-intact and labyrinthectomized animals were shown to be significantly different (p < 0.01) using a non-parametric one-way ANOVA (Kruskal–Wallis test). Figure 3B illustrates the dynamic properties of the responses of vestibular nucleus neurons to vertical tilts in labyrinthectomized animals. The response gain for units was relatively constant across stimulus frequencies, whereas the response phase was near stimulus position at low frequencies, and lagged position slightly at higher frequencies. Such properties are consistent with the responses being elicited by graviceptive inputs. In contrast, a large fraction of neurons in the vestibular nuclei of labyrinth-intact cats have responses to vertical tilts that are similar to those of semicircular canals: the response gain increases with advancing stimulus position and the response phase is near stimulus velocity (Jian et al., 2002; Miller et al., 2008). The marked differences in the responses to tilt of vestibular nucleus neurons in labyrinth-intact and labyrinthectomized animals show that the activity recorded in the latter group is not due to failure to eliminate inputs from the inner ear. Responses to vertical rotations were also recordable in bilaterally labyrinthectomized animals from additional regions of the central nervous system that receive vestibular inputs, particularly the cerebellar fastigial nucleus (Yates and Miller, 2009).
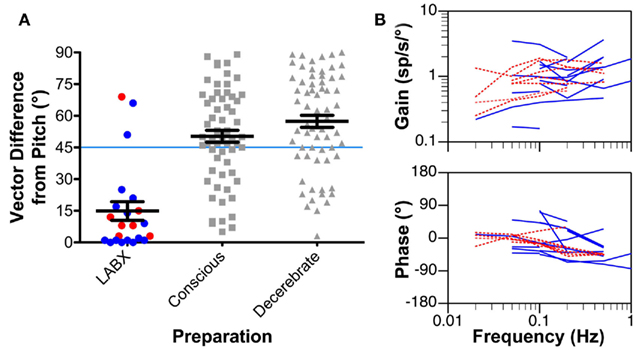
Figure 3. (A) The difference from the pitch axis in the response vector orientations for vestibular nucleus neurons determined using rotations in vertical planes. Response vector orientations aligned with the pitch axis have a vector difference of 0°, whereas those aligned with the roll axis have a vector difference of 90°. Left column: response vector orientations determined for neurons recorded in animals with a bilateral vestibular labyrinthectomy (LABX). Blue symbols represent data from decerebrate animals, and red symbols indicate findings from conscious cats. Middle column: response vector orientations determined in labyrinth-intact conscious animals. Right column: response vector orientations determined in labyrinth-intact decerebrate animals. Horizontal lines indicate mean values, and error bars designate one SEM. The response vector orientations for most neurons in animals lacking vestibular inputs were aligned near the pitch axis, whereas those in decerebrate and conscious labyrinth-intact animals were nearer the roll axis. (B) Bode plots indicating the response dynamics of neurons in animals lacking labyrinthine inputs. Response gain and phase are plotted with respect to stimulus position. Blue lines represent data from decerebrate animals, and red lines indicate findings from conscious cats. Data from Yates et al. (2000); Miller et al. (2008).
The rapid restoration of vestibular nucleus neuronal activity following a bilateral labyrinthectomy is likely due to an increase in the relative influence of non-labyrinthine excitatory inputs to the vestibular nuclei. The injection of retrogradely transported tracers into the inferior and caudal medial vestibular nuclei showed that this area receives direct inputs from several areas of the nervous system that process non-labyrinthine inputs, including the spinal gray matter, prepositus hypoglossi, pontomedullary reticular formation, inferior olivary nucleus, lateral reticular nucleus, medullary raphe nuclei, the spinal and principal trigeminal nuclei, and the facial nucleus (Jian et al., 2005). Other anatomical studies showed that the caudal regions of the inferior and medial vestibular nuclei receive direct inputs from primary afferent fibers entering the cervical spinal cord (Bankoul et al., 1995). In addition, neurons throughout the vestibular nucleus complex receive direct and polysynaptic inputs from cerebral cortex (Wilson et al., 1999). The firing rate of a majority of neurons in the medial, inferior, and lateral vestibular nuclei is affected by stimulation of somatosensory afferents from the limbs (Fredrickson et al., 1966; Wilson et al., 1966; Rubin et al., 1977; Jian et al., 2002); most neurons were excited by limb inputs. The excitability of some cells was also altered by activation of visceral afferents (Jian et al., 2002). However, the influences of limb afferents on vestibular nucleus neuronal activity are not ubiquitous. For example, few neurons that mediate vestibulo-ocular reflexes respond to stimulation of limb nerves (Rubin et al., 1978). Vestibulo-ocular neurons are concentrated in the rostral portion of the vestibular nucleus complex, in the superior and rostral medial and lateral nuclei (Graybiel and Hartwieg, 1974; Gacek, 1977, 1979a,b). Since afferents from the spinal cord only provide heavy inputs to the caudal regions of the vestibular nuclei (Rubertone and Haines, 1982; McKelvey-Briggs et al., 1989; Bankoul et al., 1995), the paucity of somatosensory influences on vestibulo-ocular units is not surprising. Thus, the non-labyrinthine inputs that are responsible for restoration of vestibular nucleus neuronal activity following bilateral damage to the inner ear may differ along a rostral–caudal gradient. For the caudal portions of the vestibular nuclei (caudal medial, inferior, and lateral nuclei), ascending inputs from the spinal cord could play a major role in regulating neuronal excitability. In contrast, descending inputs from cerebral cortex and thalamus could play a larger role in regulating the excitability of neurons in the rostral vestibular nuclei (superior and rostral medial nuclei).
As noted in Section “Eye Movements and Oscillopsia,” body rotations in the sagittal plane modulate the activity of some vestibular nucleus neurons in animals lacking labyrinthine inputs. Caudal vestibular nucleus neurons become more sensitive to somatosensory and visceral stimulation subsequent to a bilateral labyrinthectomy, suggesting that their postural-related responses after removal of vestibular inputs could be due to these inputs (Jian et al., 2002). This notion is supported by the observation that the responses were abolished by spinalization (Cotter et al., 2004). These findings suggest that non-labyrinthine sensory inputs generated by body movement, such as those related to stretch of muscles, brushing of the skin, or movement of the viscera, can elicit responses of vestibular nucleus neurons that reflect body position in space. As such, these findings raise the prospect that recovery of behavioral and physiological responses after BVH is due to substitution of non-labyrinthine for labyrinthine inputs in the central vestibular system.
Some deficits produced by bilateral damage to the inner ear in animals, such as loss of reflexive eye movements during head rotations (Baarsma and Collewijn, 1974; Barmack et al., 1980; Waespe and Wolfensberger, 1985; Waespe et al., 1992) and reduced navigational abilities (Baek et al., 2010), do not diminish over time. However, rapid recovery occurs for other consequences of bilateral loss of vestibular inputs, particularly impaired postural stability (Thomson et al., 1991) and inability to maintain stable blood pressure during postural alterations (Jian et al., 1999). It thus is useful to consider the similarities and differences between these responses to gain insights into the process of compensation. As illustrated in Figure 4, one difference between the deficits that dissipate and those that do not is the region of the vestibular nucleus complex where they are mediated. Vestibulo-ocular reflexes are mainly elicited by neurons in the rostral portion of the vestibular nucleus complex (Graybiel and Hartwieg, 1974; Gacek, 1977, 1979a,b), as are cognitive responses that are dependent on labyrinthine inputs (Brown et al., 2005; Shinder and Taube, 2010). In contrast, many of the neurons that control balance (Nyberg-Hansen and Mascitti, 1964; Petras, 1967; Peterson et al., 1978; Carleton and Carpenter, 1983; Carpenter, 1988) and influence blood pressure (Uchino et al., 1970; Yates et al., 1993; Kerman and Yates, 1998) are located caudally in the vestibular nucleus complex. In addition, the major components of vestibulo-ocular reflexes are dependent on inputs from semicircular canals (Money and Scott, 1962; Suzuki and Cohen, 1964, 1966; Baker et al., 1982; Hess et al., 2000; Sadeghi et al., 2009; Yakushin et al., 2011). Although data are limited, at least some cognitive responses related to vestibular inputs also appear to require inputs from semicircular canals (Muir et al., 2009). In contrast, while the properties of vestibulo-spinal reflexes are altered by canal plugging, which inactivates the semicircular canals but not the otolith organs, postural stability mainly requires otolithic inputs (Money and Scott, 1962; Watt, 1976; Schor and Miller, 1981). Similarly, vestibular system influences on the sympathetic nervous system are mainly related to signals from the otolith organs (Yates and Miller, 1994). Thus, following a bilateral labyrinthectomy, compensation occurs for responses that are elicited by otolith organ inputs processed by the caudal portion of the vestibular nucleus complex, but not for responses elicited predominantly by semicircular canal inputs that are processed by the superior and rostral medial vestibular nuclei. This dichotomy is at least partly related to the fact that otolithic inputs tend to target more caudal regions of the vestibular nuclear complex (Dickman and Angelaki, 2002; Newlands and Perachio, 2003; Newlands et al., 2003).
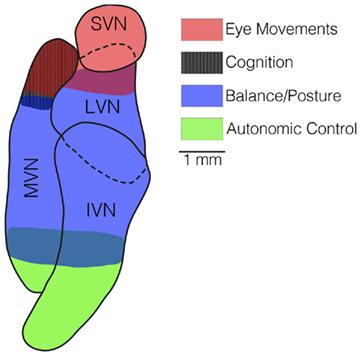
Figure 4. A horizontal section through the vestibular nucleus complex in the cat, showing the locations of the majority of neurons that mediate vestibulo-ocular reflexes (red shading), spatial cognition (black shading), balance (blue shading), and autonomic responses (green shading). Neurons that elicit eye movements and participate in spatial cognition are located rostrally in the vestibular nucleus complex, mainly in the superior vestibular nucleus (SVN) and rostral portion of the medial vestibular nucleus (MVN). In contrast, neurons responsible for vestibulo-spinal and vestibulo-autonomic responses are located more caudally, in the lateral vestibular nucleus (LVN), inferior vestibular nucleus (IVN), and caudal portion of the medial vestibular nucleus (MVN).
Since a variety of sensory systems provide graviceptive signals to the central nervous system (Mittelstaedt, 1992, 1995, 1996; Mittelstaedt and Mittelstaedt, 1996; Balaban and Yates, 2004), it is not surprising that these inputs can be substituted at least partially for those from the otolith organs. Some of these graviceptive inputs are conveyed to the vestibular nuclei (see “Cognitive deficits”), and are likely responsible for the modulation of vestibular nucleus neuronal activity that can be detected during body rotations in the pitch plane conducted following a bilateral vestibular neurectomy (Yates et al., 2000; Miller et al., 2008). Bilateral lesions placed in the caudal region of the vestibular nuclei result in a permanent deficit in cardiovascular system responses during postural alterations (Mori et al., 2005), as well as deficits in postural control that are more severe and long lasting than those resulting from a bilateral labyrinthectomy (unpublished observations). These findings suggest that the vestibular nuclei participate in the “sensory substation” that facilitates recovery of postural stability and movement-related cardiovascular adjustments following the loss of labyrinthine inputs. However, the specific role that the central vestibular system plays in the compensation process is yet to be determined.
Analysis of longitudinal compensation in humans with BVH, in contrast to animals with experimentally generated damage to the inner ear, has been limited by a lack of access to BVH patients early in the compensation process. Most studies investigating the effects of BVH describe findings years after the vestibular loss occurred and presumably the dynamic compensatory process had long been completed. The postural effects of BVH therefore have just been studied in humans who previously underwent the dominant period of vestibular compensation; consequently, the literature only describes the end result of BVH on posture (see “Postural stability”). Studies on eye movements (see “Eye Movements and Oscillopsia”) and cognition (see “Cognitive deficits”) are limited by the same constraint.
Humans with BVH are able to maintain normal stance in the light, although when the support surface they stand upon moves, falls are much more likely to occur (Nashner et al., 1982; Mergner et al., 2009). When the support surface is systematically tilted in the eyes closed condition, BVH subjects either sway with the platform movement (at lower peak tilt angular velocity) or fall (at higher peak tilt angular velocity; Maurer et al., 2006). Tandem Romberg stance (one foot in front of the other) is a challenging posture to maintain: control subjects without assistance are able to maintain the posture in light or dark, but subjects with BVH fall in both conditions (Lackner et al., 1999). The elevated fall risk presented by unstable support surfaces and challenging postural positions translates to increased risk of fall and injury in the daily lives of BVH patients.
Patterning of postural muscular responses following perturbations is altered in BVH patients, but the origin and organization of the muscular responses that occur in this patient population is debatable. It has been proposed that balance-correcting responses are elicited by somatosensory signals from the trunk or about the hip joint, which are then modulated by inputs from the ankles, knees, and the vestibular system (Horstmann and Dietz, 1990; Allum and Honegger, 1998). Other investigators have proposed that somatosensory signals originating about the ankle may be the primary drivers of postural responses (Nashner et al., 1982; Horak and Nashner, 1986; Horak et al., 1990). To add to the complexity of deciphering muscular control of posture, direction of perturbation is an important factor in maintenance of posture. Humans are more unstable when subjected to perturbations in the roll plane then when they are tilted in the pitch plane (Carpenter et al., 2001). Hip and trunk muscles are activated during roll motion, while pitch perturbations mainly involve an activation of leg muscles (Henry et al., 1998; Gruneberg et al., 2005; Allum et al., 2008). BVH subjects have been shown to demonstrate increased paraspinal muscular activity in response to toes up pitch rotations (Allum et al., 2001). The normal lower body response to roll tilt of a support surface also involves uphill leg flexion and downhill leg extension; BVL subjects have a reduction of this compensatory leg motion (Allum et al., 2008). Quasi-static roll perturbations, which are believed to be detected mainly via proprioceptors rather than otolithic graviceptors, are perceived at low thresholds in both normal subjects and BVH patients suggesting that proprioceptive inputs are satisfactory for maintenance up upright posture in relatively adynamic conditions (Teasdale et al., 1999; Bringoux et al., 2002). The relative role such proprioceptive inputs play in more dynamic conditions, such as during ambulation, remains undefined.
Dependence on other sensory systems, namely proprioceptive and visual, is widely regarded as the means that permits BVH patients to maintain upright posture. Some data suggest that a transition occurs months to years following the onset of BVH from a visually dominant postural control paradigm to a more proprioceptive guided paradigm, although longitudinal studies within individuals are lacking (Bles et al., 1983). Modeling of human posture indicates that normal subjects are able to reweigh sensory data from visual, proprioceptive, and vestibular inputs in response to changes in stimulus amplitude (i.e., non-linear changes in gain and phase occur in response to changes in amplitude of stimuli; Peterka, 2002). Subjects with BVH are not able to reweigh sensory information in this fashion. Some BVH subjects utilize a balance strategy dependent upon an increase in stiffness, which results in decreased sensory error, but necessitates a higher energy expenditure to maintain (Peterka, 2002). Subjective visual vertical (SVV) estimates during rotation of the visual field or placement of the body in the lateral position are more deviated in BVH patients compared with normal subjects, suggesting that vestibular inputs normally serve to influence or supersede less accurate proprioceptive or visual postural information (Bronstein et al., 1996). Alternatively, exaggeration of error in SVV when BVH subjects are tilted onto their sides could suggest that signals from proprioceptive pathways are enhanced following vestibular loss. As balance control strategies are not nearly as critical for preventing falls in the supine position, errors in estimated verticality in this position are less clinically relevant than if such errors were to occur with upright stance. Enhancement or disinhibition of proprioceptive pathways may be important to maintenance of upright posture in BVH. For example, the enhanced pattern of paraspinal muscular activity in toes up pitch rotations is opposite to that seen in patients with proprioceptive loss, suggesting that proprioceptive responses may be enhanced in BVH (Allum et al., 2001). Alternative sensory input in the form of putative trunk graviceptors may be another important factor in postural control following loss of vestibular input (Mittelstaedt, 1998).
Humans with BVH exhibit eye movement abnormalities. BVH results in markedly diminished or absent vestibulo-ocular reflexes (VOR) bilaterally, such that measurement of the VOR during clinical vestibular testing is used to diagnose BVH (Brown et al., 2001; Goebel et al., 2009; Jen, 2009). Responses to head thrust testing, which assesses the high-frequency VOR, are also abnormal in BVH (Halmagyi and Curthoys, 1988; Jorns-Haderli et al., 2007; Zingler et al., 2008). Over time, there does not appear to be recovery of VOR function regardless of the etiology of BVH (Baloh et al., 2001; Zingler et al., 2008). Optokinetic after nystagmus is also severely reduced or abolished in BVH (Zee et al., 1976; Ireland and Jell, 1982; Bles et al., 1984; Hain and Zee, 1991). Most groups have found smooth pursuit to be undisturbed in BVH (Kasai and Zee, 1978; Leigh et al., 1987; Waterston et al., 1992), although a slight potentiation of smooth pursuit in BVH has been reported (Bockisch et al., 2004).
Since the VOR is believed to stabilize vision during head motion, its absence in BVH requires compensatory mechanisms to optimize visual input. Mechanisms postulated to participate in this compensatory process include enhancement of the cervico-ocular reflex, central preprogramming of eye movements, and increase in the optokinetic response (Kasai and Zee, 1978; Bronstein and Hood, 1986; Huygen et al., 1989, 1991; Waterston et al., 1992). The cervico-ocular reflex, which is triggered by inputs from neck muscles, is normally of small magnitude in humans, but its gain increases following bilateral vestibular loss particularly when the head is moved at low frequency (∼0.1 Hz; Bronstein and Hood, 1986; Huygen et al., 1991). Central preprogramming of eye movements necessitates that the subject is aware of where the next target will be located or what head movement will take place; in this situation, subjects with BVH more accurately identify the visual target (Herdman et al., 2001). However, many head movements and choices for gaze direction in daily life are not predictable and this strategy would not be useful in many circumstances. Efference copy of motor commands may also facilitate accurate targeting during gaze shifts (Maurer et al., 1998), but it is unclear whether this mechanism provides for compensation in BVH patients. In some low frequency head movement circumstances, optokinetic responses can also contribute to compensatory eye movements in BVH patients (Leigh et al., 1992).
It is known that the generation of compensatory eye movements in patients with BVH requires a functioning cerebellum (Bronstein et al., 1991; Waterston et al., 1992). In the converse situation, individuals with cerebellar deficiency and intact vestibular systems have an elevated VOR gain, show an inappropriate direction of the slow phase of eye movements orthogonal to the axis of rotation, and display abnormalities during cervical vestibulo-ocular myogenic potential testing thought to be analogous to an increase in vestibulocollic reflex gain (Shaikh et al., 2011). Therefore, the cerebellum may be responsible for potentiating a number of pathways for compensation in BVH.
Oscillopsia is the perception of blurring or movement of the environment when the head is placed in motion during activities such as turning the head to the side or ambulating (Schubert et al., 2004). Oscillopsia is a common symptom in BVH, but it is not ubiquitous (McGath et al., 1989; Sargent et al., 1997). Oscillopsia is thought to occur in BVH because slip of visual targets across the retina occurs in the absence of a functioning corrective VOR. Dynamic visual acuity during ambulation is reduced in BVH, which is likely related to retinal slip (Lambert et al., 2010). Tolerance to retinal slip, rather than corrective eye movements, appears to be important for adaptation to oscillopsia. Subjective reporting of the degree of oscillopsia is negatively correlated with the amount of retinal slip (Grunfeld et al., 2000). Similarly, visual motion detection thresholds are reduced in BVH patients compared with controls (Shallo-Hoffmann and Bronstein, 2003). It is likely that adaptive compensatory mechanisms are responsible for the reduced sensitivity to visual field motion, although the pathways involved remain to be elucidated.
Patients with vestibular loss are often noted to suffer cognitive deficits such as difficulty concentrating or being in a “brain fog,” and patients with BVH are likely not an exception (Hanes and McCollum, 2006). Although cognitive deficits of this nature are sometimes difficult to study, several important cognitive effects have been demonstrated in humans with BVH, chiefly impaired spatial learning and memory and, less directly, impairment in dual tasking.
Humans with bilateral vestibular loss have impaired spatial learning and spatial memory deficits. BVH subjects had poor performance on the virtual Morris water task, which tests spatial learning and memory in the absence of somatosensory or vestibular cues (Schautzer et al., 2003). Anatomic and physiologic data support these findings and indicate that the hippocampus, a brain region known to be involved in spatial learning and memory, is responsible for the deficiencies (Brandt et al., 2005; Jahn et al., 2009; Smith et al., 2010b; Viard et al., 2011). MRI testing showed that BVH subjects have a 17% reduction in hippocampal volume (Brandt et al., 2005), and functional MRI studies demonstrated that these patients also have decreased activity within the anterior hippocampus (Jahn et al., 2009). Memory and navigation deficits in BVH patients were limited to spatial problems; general memory was found to be intact (Brandt et al., 2005). Although there is some suggestion that the right cerebral cortex is dominant for spatial orientation, right unilateral vestibular loss subjects only displayed subtle differences in task performance compared with subjects with left unilateral loss, and there is no hippocampal atrophy in unilateral vestibular loss subjects (Hufner et al., 2007; Jahn et al., 2009). Taken together, these data suggest that spatial learning and memory are dependent upon the presence of function of at least one labyrinth; without any functional labyrinthine input, deficits of spatial learning and memory arise.
Patients with vestibular loss have also been shown to have difficulty with dual mental tasks, such as performing arithmetic along with orienting or balance tasks (Yardley et al., 2001, 2002). These dual task studies mostly included patients with unilateral vestibular loss; a few patients with BVH were also studied but were not segregated, such that drawing specific conclusions on dual tasking in BVH is not currently possible. As such, further study of multitask performance is warranted in BVH patients.
Spontaneous recovery of vestibular function rarely occurs in BVH patients (Brandt et al., 2010); treatment strategies therefore necessarily rely upon coping with existing vestibular function (in the case of partial loss) or providing alternative means to present information normally sensed by the vestibular system to the brain (Minor, 1998). The mainstay of treatment for BVH is vestibular physical therapy. Treatment in an active vestibular rehabilitation program was shown in a double blind placebo-controlled study to improve dynamic gait stability considerably among patients with BVH (Krebs et al., 1993). Similarly, dynamic visual acuity has been shown to improve with vestibular physical therapy in BVH (Herdman et al., 2007). Such improvements should translate readily to improvements in quality of life amongst treatment responders.
Improvement with vestibular physical therapy has been shown to occur in approximately 50% of patients with BVH; however, this leaves a substantial population that does not benefit (Shepard and Telian, 1995; Gillespie and Minor, 1999; Brown et al., 2001). Risk factors for poor response to vestibular physical therapy include multiple medical comorbidities, slowly progressive bilateral vestibular loss, and increased severity of BVH (Gillespie and Minor, 1999). A further understanding of the compensatory process in BVH may lead to explanations about the variability of recovery in BVH and suggest new treatment strategies.
Investigators continue to search for new ways to refine balance function in patients suffering from BVH. Sensory substitution, converting information normally encoded by one system (in this case, the vestibular system) into information that can be detected with another sensory modality (such as visual cues or sensory tactile information), has been one approach taken to improve stance and gait in BVH patients. Sensory substitution in BVH is a pragmatic approach because evidence suggests that patients with BVH already may use a form of it – visual sensory substitution. While undergoing optokinetic stimulation, fMRI data from BVH patients demonstrates increased activity within visual and oculomotor areas in comparison with normal subjects, suggesting that BVH individuals use visual flow to assist with balance (Dieterich et al., 2007). Furthermore, BVH subjects have been shown to have better postural stability when permitted to use visual cues (Buchanan and Horak, 2002; Horak, 2010). Non-supportive light touch can also substitute as an earth vertical reference point and stabilize posture (Creath et al., 2002, 2008). Auditory biofeedback has also been used as a form of vestibular sensory substitution by notifying patients about the degree of postural sway through auditory cues (Dozza et al., 2007, 2011).
Several devices are under investigation to assist in sensory substitution for BVH. The most studied sensory substitution device delivers body tilt information through a vibrotactile somatosensory feedback system attached to the torso that has been shown to reduce postural sway and decrease falls (Kentala et al., 2003; Wall and Kentala, 2005). Another sensory substitution system involves wearing a head mounted vibrotactile device. This system has undergone phase 1 clinical testing and was shown to significantly reduce instability in BVH patients as measured by dynamic posturography (Goebel et al., 2009). Another device provides electrotactile feedback to the tongue based upon head position relative to gravity (Barros et al., 2010).
An alternative approach to sensory substitution based prosthetics involves direct electrical stimulation of vestibular sensory nerves with an implanted vestibular prosthetic device. Single channel and multichannel head mounted devices are currently under study in animals (Della Santina et al., 2005, 2007; Merfeld et al., 2007; Lewis et al., 2010; Dai et al., 2011). The foundation of modern vestibular prosthesis work comes from experiments in which precise eye movements were elicited through electrical stimulation of branches of the vestibular nerve (Suzuki et al., 1964, 1969). Current vestibular prostheses typically include electrodes within the semicircular canals that deliver electrical stimulation to individual ampullary nerves. The electrical stimuli are controlled by measurements from accelerometers that detect head rotation. An underlying premise for this therapy is that the animal or human with the implant will adapt to the firing rate of ampullary nerves induced by the device, and use this information to elicit appropriate compensatory eye movements. In guinea pigs, this acclimation process was shown to occur rapidly. Importantly, acclimation to chronic baseline stimulation did not interfere with generation of eye movements when electrical stimulation was used to modify the activity of particular vestibular nerves (Merfeld et al., 2006). Longer-term adaptation studies in the squirrel monkey showed that an electrically evoked VOR is synchronized with head motion after 3 months of chronic stimulation (Merfeld et al., 2007). One important concern regarding electrical stimulation is the problem of current spread from the intended ampullary nerve to other nearby ampullary nerves, resulting in cross stimulation. VOR adaptation studies have shown that over the first week following prosthesis activation in chinchillas, erroneous VOR eye movements caused by current spread rapidly diminish, such that eye movements more closely approximate those expected based upon head motion (Dai et al., 2011). To date, one human has been implanted with an electrically based vestibular prosthesis. The surgery involved placing a single electrode from a modified cochlear implant adjacent to the posterior ampullary nerve in a patient with BVH and bilateral deafness. Experiments using the device demonstrated that nystagmus diminished over time, and that eye movements were elicited in accordance with the amplitude and frequency of stimulation (Guyot et al., 2011).
Since we are at the forefront of investigation of vestibular prostheses in humans, many questions remain. It is unclear whether such prostheses will stabilize oscillopsia by accurately encoding head motion in space. Similarly, it remains to be reported whether electrical stimulation of ampullary nerves improves balance control in patients or animal subjects with BVH. Galvanic or caloric stimulation of the vestibular system of normal humans has been shown to improve memory (spatial, verbal, and facial recall; Bachtold et al., 2001; Wilkinson et al., 2008; Smith et al., 2010a). Whether electrical vestibular stimulation will affect cognitive processing in BVH patients remains unknown. It is also unclear whether vestibular prostheses will alter the sensory substitution that ordinarily occurs in BVH patients, and whether sensory substitution and electrical stimulation devices can be effectively used in combination in particular subjects.
Very little is known about the compensatory process in humans with BVH because virtually all studies of the effects of this condition on posture, visual stability, and cognition have been conducted in patients long into the disease process. Longitudinal studies in humans that commence upon the acute bilateral loss of vestibular function would be particularly enlightening, but are inherently challenging or impossible to perform, since access to patients with acute onset BVH is rare. To understand the compensatory processes that have occurred, we can only make conclusions based upon the end functional result. Although it is clear that the effects of BVH on posture, eye movements, and spatial learning and memory are long lasting, it is unknown how much improvement in signs and symptoms occurs over time. Another important open question is, what neural systems contribute to recovery of these responses? It is tempting to infer about compensation for BVH from what is known about recovery following unilateral vestibular lesions, but such thinking may be spurious. As an example, patients with unilateral vestibular loss performed better on balance measures when they relied on their remaining vestibular information rather than on visual or somatosensory information. In contrast, subjects with BVH would necessarily have to depend on visual and somatosensory signals (Horak, 2010). A more pragmatic approach is to draw conclusions from compensation studies in experimental animals, learn how they compensate from the onset of BVH, and determine where we may intervene to improve compensation. As noted above, the consequences of elimination of vestibular inputs are similar in animal subjects and human patients, such that studies in animals will likely provide insights that can be translated to clinical medicine.
Experiments on animal subjects with induced BVH have shown that non-labyrinthine inputs rapidly restore resting activity in the vestibular nuclei (Waespe et al., 1992; Ris and Godaux, 1998; Miller et al., 2008) and can modulate this neural activity during some changes in body position (Yates et al., 2000; Miller et al., 2008). An important line of investigation is to determine whether non-labyrinthine effects on activity in the vestibular system can be strengthened following BVH, and whether the information can be effectively utilized to compensate for deficits. Experiments in animals could also be used to determine whether a combination of prosthetic devices, such as combined stimulation of ampullary nerves and somatosensory receptors, may be more effective in facilitating recovery following bilateral damage to the inner ear than a therapy that activates one sensory modality. A promising finding is that some strategies thought to be compensatory for BVH are plastic and modifiable over time. COR gain and phase readily and appropriately change in BVH subjects wearing magnifying or reducing lenses (Heimbrand et al., 1996). If we are able to identify other compensatory strategies in humans, whether they utilize proprioceptive, visual, or other inputs, we may ultimately be able to modify these signals for therapeutic use.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors’ work related to this manuscript is supported by National Institutes of Health grant R01-DC00693 to Bill J. Yates.
Akin, F. W., and Murnane, O. D. (2011). Head injury and blast exposure: vestibular consequences. Otolaryngol. Clin. North Am. 44, 323–334, viii.
Allum, J. H., Bloem, B. R., Carpenter, M. G., and Honegger, F. (2001). Differential diagnosis of proprioceptive and vestibular deficits using dynamic support-surface posturography. Gait Posture 14, 217–226.
Allum, J. H., and Honegger, F. (1998). Interactions between vestibular and proprioceptive inputs triggering and modulating human balance-correcting responses differ across muscles. Exp. Brain Res. 121, 478–494.
Allum, J. H., Oude Nijhuis, L. B., and Carpenter, M. G. (2008). Differences in coding provided by proprioceptive and vestibular sensory signals may contribute to lateral instability in vestibular loss subjects. Exp. Brain Res. 184, 391–410.
Baarsma, E., and Collewijn, H. (1974). Vestibulo-ocular and optokinetic reactions to rotation and their interaction in the rabbit. J. Physiol. 238, 603–625.
Bachtold, D., Baumann, T., Sandor, P. S., Kritos, M., Regard, M., and Brugger, P. (2001). Spatial- and verbal-memory improvement by cold-water caloric stimulation in healthy subjects. Exp. Brain Res. 136, 128–132.
Baek, J. H., Zheng, Y., Darlington, C. L., and Smith, P. F. (2010). Evidence that spatial memory deficits following bilateral vestibular deafferentation in rats are probably permanent. Neurobiol. Learn. Mem. 94, 402–413.
Baker, J., Goldberg, J., Peterson, B., and Schor, R. (1982). Oculomotor reflexes after semicircular canal plugging in cats. Brain Res. 252, 151–155.
Balaban, C. D., and Yates, B. J. (2004). “Vestibulo-autonomic interactions: a teleologic perspective,” in Anatomy and Physiology of the Central and Peripheral Vestibular System, eds S. M. Highstein, R. R. Fay, and A. N. Popper (Heidelberg: Springer), 286–342.
Baloh, R. W., Enrietto, J., Jacobson, K. M., and Lin, A. (2001). Age-related changes in vestibular function: a longitudinal study. Ann. N. Y. Acad. Sci. 942, 210–219.
Bankoul, S., Goto, T., Yates, B. J., and Wilson, V. J. (1995). Cervical primary afferent input to vestibulospinal neurons projecting to the cervical dorsal horn: an anterograde and retrograde tracing study in the cat. J. Comp. Neurol. 353, 529–538.
Barmack, N. H., Pettorossi, V. E., and Erickson, R. G. (1980). The influence of bilateral labyrinthectomy on horizontal and vertical optokinetic reflexes in the rabbit. Brain Res. 196, 520–524.
Barros, C. G., Bittar, R. S., and Danilov, Y. (2010). Effects of electrotactile vestibular substitution on rehabilitation of patients with bilateral vestibular loss. Neurosci. Lett. 476, 123–126.
Blair, H. T., and Sharp, P. E. (1995). Anticipatory head direction signals in anterior thalamus: evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. J. Neurosci. 15, 6260–6270.
Bles, W., De Jong, J. M., and Rasmussens, J. J. (1984). Postural and oculomotor signs in labyrinthine-defective subjects. Acta Otolaryngol. Suppl. 406, 101–104.
Bles, W., Vianney De Jong, J. M., and De Wit, G. (1983). Compensation for labyrinthine defects examined by use of a tilting room. Acta Otolaryngol. 95, 576–579.
Bockisch, C. J., Straumann, D., Hess, K., and Haslwanter, T. (2004). Enhanced smooth pursuit eye movements in patients with bilateral vestibular deficits. Neuroreport 15, 2617–2620.
Brandt, T., Huppert, T., Hufner, K., Zingler, V. C., Dieterich, M., and Strupp, M. (2010). Long-term course and relapses of vestibular and balance disorders. Restor. Neurol. Neurosci. 28, 69–82.
Brandt, T., Schautzer, F., Hamilton, D. A., Bruning, R., Markowitsch, H. J., Kalla, R., Darlington, C., Smith, P., and Strupp, M. (2005). Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 128, 2732–2741.
Bringoux, L., Schmerber, S., Nougier, V., Dumas, G., Barraud, P. A., and Raphel, C. (2002). Perception of slow pitch and roll body tilts in bilateral labyrinthine-defective subjects. Neuropsychologia 40, 367–372.
Bronstein, A. M., and Hood, J. D. (1986). The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res. 373, 399–408.
Bronstein, A. M., Mossman, S., and Luxon, L. M. (1991). The neck-eye reflex in patients with reduced vestibular and optokinetic function. Brain 114 (Pt 1A), 1–11.
Bronstein, A. M., Yardley, L., Moore, A. P., and Cleeves, L. (1996). Visually and posturally mediated tilt illusion in Parkinson’s disease and in labyrinthine defective subjects. Neurology 47, 651–656.
Brown, J. E., Card, J. P., and Yates, B. J. (2005). Polysynaptic pathways from the vestibular nuclei to the lateral mammillary nucleus of the rat: substrates for vestibular input to head direction cells. Exp. Brain Res. 161, 47–61.
Brown, K. E., Whitney, S. L., Wrisley, D. M., and Furman, J. M. (2001). Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope 111, 1812–1817.
Buchanan, J. J., and Horak, F. B. (2002). Vestibular loss disrupts control of head and trunk on a sinusoidally moving platform. J. Vestib. Res. 11, 371–389.
Carleton, S. C., and Carpenter, M. B. (1983). Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 278, 29–51.
Carpenter, M. B. (1988). Vestibular nuclei: afferent and efferent projections. Prog. Brain Res. 76, 5–15.
Carpenter, M. G., Allum, J. H., and Honegger, F. (2001). Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp. Brain Res. 140, 95–111.
Cohen, B., Uemura, T., and Takemori, S. (1973). Effects of labyrinthectomy on optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). Int. J. Equilib. Res. 3, 88–93.
Cotter, L. A., Arendt, H. E., Cass, S. P., Jian, B. J., Mays, D. F. 2nd, Olsheski, C. J., Wilkinson, K. A., and Yates, B. J. (2004). Effects of postural changes and vestibular lesions on genioglossal muscle activity in conscious cats. J. Appl. Physiol. 96, 923–930.
Cotter, L. A., Arendt, H. E., Jasko, J. G., Sprando, C., Cass, S. P., and Yates, B. J. (2001). Effects of postural changes and vestibular lesions on diaphragm and rectus abdominis activity in awake cats. J. Appl. Physiol. 91, 137–144.
Creath, R., Kiemel, T., Horak, F., and Jeka, J. J. (2002). Limited control strategies with the loss of vestibular function. Exp. Brain Res. 145, 323–333.
Creath, R., Kiemel, T., Horak, F., and Jeka, J. J. (2008). The role of vestibular and somatosensory systems in intersegmental control of upright stance. J. Vestib. Res. 18, 39–49.
Cullen, K. E., Minor, L. B., Beraneck, M., and Sadeghi, S. G. (2009). Neural substrates underlying vestibular compensation: contribution of peripheral versus central processing. J. Vestib. Res. 19, 171–182.
Dai, C., Fridman, G. Y., Chiang, B., Davidovics, N. S., Melvin, T. A., Cullen, K. E., and Della Santina, C. C. (2011). Cross-axis adaptation improves 3D vestibulo-ocular reflex alignment during chronic stimulation via a head-mounted multichannel vestibular prosthesis. Exp. Brain Res. 210, 595–606.
Dandy, W. E. (1941). The surgical treatment of Meniere’s disease. Surg. Gynecol. Obstet. 72, 421–425.
Della Santina, C., Migliaccio, A., and Patel, A. (2005). Electrical stimulation to restore vestibular function development of a 3-d vestibular prosthesis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 7, 7380–7385.
Della Santina, C. C., Migliaccio, A. A., and Patel, A. H. (2007). A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-d vestibular sensation. IEEE Trans. Biomed. Eng. 54, 1016–1030.
Dickman, J. D., and Angelaki, D. E. (2002). Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J. Neurophysiol. 88, 3518–3533.
Dieringer, N. (1995). “Vestibular compensation”: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog. Neurobiol. 46, 97–129.
Dieterich, M., Bauermann, T., Best, C., Stoeter, P., and Schlindwein, P. (2007). Evidence for cortical visual substitution of chronic bilateral vestibular failure (an fMRI study). Brain 130, 2108–2116.
Dozza, M., Chiari, L., Peterka, R. J., Wall, C., and Horak, F. B. (2011). What is the most effective type of audio-biofeedback for postural motor learning? Gait Posture 34, 313–319.
Dozza, M., Horak, F. B., and Chiari, L. (2007). Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp. Brain Res. 178, 37–48.
Dutia, M. B. (2010). Mechanisms of vestibular compensation: recent advances. Curr. Opin. Otolaryngol. 18, 420–424.
Fredrickson, J. M., Schwarz, D., and Kornhuber, H. H. (1966). Convergence and interaction of vestibular and deep somatic afferents upon neurons in the vestibular nuclei of the cat. Acta Otolaryngol. 61, 168–188.
Gacek, R. R. (1977). Location of brain stem neurons projecting to the oculomotor nucleus in the cat. Exp. Neurol. 57, 725–749.
Gacek, R. R. (1979b). Location of trochlear vestibuloocular neurons in the cat. Exp. Neurol. 66, 692–706.
Gillespie, M. B., and Minor, L. B. (1999). Prognosis in bilateral vestibular hypofunction. Laryngoscope 109, 35–41.
Gliddon, C. M., Darlington, C. L., and Smith, P. F. (2005). GABAergic systems in the vestibular nucleus and their contribution to vestibular compensation. Prog. Neurobiol. 75, 53–81.
Goebel, J. A., Sinks, B. C., Parker, B. E. Jr., Richardson, N. T., Olowin, A. B., and Cholewiak, R. W. (2009). Effectiveness of head-mounted vibrotactile stimulation in subjects with bilateral vestibular loss: a phase 1 clinical trial. Otol. Neurotol. 30, 210–216.
Gopinath, B., Mcmahon, C. M., Rochtchina, E., and Mitchell, P. (2009). Dizziness and vertigo in an older population: the Blue Mountains prospective cross-sectional study. Clin. Otolaryngol. 34, 552–556.
Graybiel, A. M., and Hartwieg, E. A. (1974). Some afferent connections of the oculomotor complex in the cat: an experimental study with tracer techniques. Brain Res. 81, 543–551.
Gruneberg, C., Duysens, J., Honegger, F., and Allum, J. H. (2005). Spatio-temporal separation of roll and pitch balance-correcting commands in humans. J. Neurophysiol. 94, 3143–3158.
Grunfeld, E. A., Morland, A. B., Bronstein, A. M., and Gresty, M. A. (2000). Adaptation to oscillopsia: a psychophysical and questionnaire investigation. Brain 123 (Pt 2), 277–290.
Guyot, J. P., Sigrist, A., Pelizzone, M., and Kos, M. I. (2011). Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann. Otol. Rhinol. Laryngol. 120, 143–149.
Hain, T. C., and Zee, D. S. (1991). Abolition of optokinetic afternystagmus by aminoglycoside ototoxicity. Ann. Otol. Rhinol. Laryngol. 100, 580–583.
Halmagyi, G. M., and Curthoys, I. S. (1988). A clinical sign of canal paresis. Arch. Neurol. 45, 737–739.
Hanes, D. A., and McCollum, G. (2006). Cognitive-vestibular interactions: a review of patient difficulties and possible mechanisms. J. Vestib. Res. 16, 75–91.
Heimbrand, S., Bronstein, A. M., Gresty, M. A., and Faldon, M. E. (1996). Optically induced plasticity of the cervico-ocular reflex in patients with bilateral absence of vestibular function. Exp. Brain Res. 112, 372–380.
Henry, S. M., Fung, J., and Horak, F. B. (1998). Control of stance during lateral and anterior/posterior surface translations. IEEE Trans. Rehabil. Eng. 6, 32–42.
Herdman, S. J., Hall, C. D., Schubert, M. C., Das, V. E., and Tusa, R. J. (2007). Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch. Otolaryngol. Head Neck Surg. 133, 383–389.
Herdman, S. J., Schubert, M. C., and Tusa, R. J. (2001). Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch. Otolaryngol. Head Neck Surg. 127, 1205–1210.
Hess, B. J., Lysakowski, A., Minor, L. B., and Angelaki, D. E. (2000). Central versus peripheral origin of vestibuloocular reflex recovery following semicircular canal plugging in rhesus monkeys. J. Neurophysiol. 84, 3078–3082.
Horak, F. B. (2010). Postural compensation for vestibular loss and implications for rehabilitation. Restor. Neurol. Neurosci. 28, 57–68.
Horak, F. B., and Nashner, L. M. (1986). Central programming of postural movements: adaptation to altered support-surface configurations. J. Neurophysiol. 55, 1369–1381.
Horak, F. B., Nashner, L. M., and Diener, H. C. (1990). Postural strategies associated with somatosensory and vestibular loss. Exp. Brain Res. 82, 167–177.
Horstmann, G. A., and Dietz, V. (1990). A basic posture control mechanism: the stabilization of the centre of gravity. Electroencephalogr. Clin. Neurophysiol. 76, 165–176.
Hufner, K., Hamilton, D. A., Kalla, R., Stephan, T., Glasauer, S., Ma, J., Bruning, R., Markowitsch, H. J., Labudda, K., Schichor, C., Strupp, M., and Brandt, T. (2007). Spatial memory and hippocampal volume in humans with unilateral vestibular deafferentation. Hippocampus 17, 471–485.
Hughes, G. B., Kinney, S. E., Barna, B. P., and Calabrese, L. H. (1984). Practical versus theoretical management of autoimmune inner ear disease. Laryngoscope 94, 758–767.
Huygen, P. L., Verhagen, W. I., and Nicolasen, M. G. (1991). Cervico-ocular reflex enhancement in labyrinthine-defective and normal subjects. Exp. Brain Res. 87, 457–464.
Huygen, P. L., Verhagen, W. I., Theunissen, E. J., and Nicolasen, M. G. (1989). Compensation of total loss of vestibulo-ocular reflex by enhanced optokinetic response. Acta Otolaryngol. Suppl. 468, 359–364.
Igarashi, M., and Guitierrez, O. (1983). Analysis of righting reflex in cats with unilateral and bilateral labyrinthectomy. ORL J. Otorhinolaryngol. Relat. Spec. 45, 279–289.
Inglis, J. T., and Macpherson, J. M. (1995). Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J. Neurophysiol. 73, 1181–1191.
Ireland, D. J., and Jell, R. M. (1982). Optokinetic after-nystagmus in man after loss or reduction of labyrinthine function – a preliminary report. J. Otolaryngol. 11, 86–90.
Jahn, K., Wagner, J., Deutschlander, A., Kalla, R., Hufner, K., Stephan, T., Strupp, M., and Brandt, T. (2009). Human hippocampal activation during stance and locomotion: fMRI study on healthy, blind, and vestibular-loss subjects. Ann. N. Y. Acad. Sci. 1164, 229–235.
Jen, J. C. (2009). Bilateral vestibulopathy: clinical, diagnostic, and genetic considerations. Semin. Neurol. 29, 528–533.
Jian, B. J., Acernese, A. W., Lorenzo, J., Card, J. P., and Yates, B. J. (2005). Afferent pathways to the region of the vestibular nuclei that participates in cardiovascular and respiratory control. Brain Res. 1044, 241–250.
Jian, B. J., Cotter, L. A., Emanuel, B. A., Cass, S. P., and Yates, B. J. (1999). Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J. Appl. Physiol. 86, 1552–1560.
Jian, B. J., Shintani, T., Emanuel, B. A., and Yates, B. J. (2002). Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp. Brain Res. 144, 247–257.
Jorns-Haderli, M., Straumann, D., and Palla, A. (2007). Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J. Neurol. Neurosurg. Psychiatr. 78, 1113–1118.
Kasai, T., and Zee, D. S. (1978). Eye-head coordination in labyrinthine-defective human beings. Brain Res. 144, 123–141.
Kentala, E., Vivas, J., and Wall, C. III. (2003). Reduction of postural sway by use of a vibrotactile balance prosthesis prototype in subjects with vestibular deficits. Ann. Otol. Rhinol. Laryngol. 112, 404–409.
Kerman, I. A., and Yates, B. J. (1998). Regional and functional differences in the distribution of vestibulosympathetic reflexes. Am. J. Physiol. 275, R824–R835.
Krebs, D. E., Gill-Body, K. M., Riley, P. O., and Parker, S. W. (1993). Double-blind, placebo-controlled trial of rehabilitation for bilateral vestibular hypofunction: preliminary report. Otolaryngol. Head Neck Surg. 109, 735–741.
Lackner, J. R., Dizio, P., Jeka, J., Horak, F., Krebs, D., and Rabin, F. (1999). Precision contact of the fingertip reduces postural sway of individuals with bilateral vestibular loss. Exp. Brain Res. 126, 459–466.
Lambert, S., Sigrist, A., Delaspre, O., Pelizzone, M., and Guyot, J. P. (2010). Measurement of dynamic visual acuity in patients with vestibular areflexia. Acta Otolaryngol. 130, 820–823.
Leigh, R. J., Sawyer, R. N., Grant, M. P., and Seidman, S. H. (1992). High-frequency vestibuloocular reflex as a diagnostic tool. Ann. N. Y. Acad. Sci. 656, 305–314.
Leigh, R. J., Sharpe, J. A., Ranalli, P. J., Thurston, S. E., and Hamid, M. A. (1987). Comparison of smooth pursuit and combined eye-head tracking in human subjects with deficient labyrinthine function. Exp. Brain Res. 66, 458–464.
Lewis, R. F., Haburcakova, C., Gong, W., Makary, C., and Merfeld, D. M. (2010). Vestibuloocular reflex adaptation investigated with chronic motion-modulated electrical stimulation of semicircular canal afferents. J. Neurophysiol. 103, 1066–1079.
Macpherson, J. M., Everaert, D. G., Stapley, P. J., and Ting, L. H. (2007). Bilateral vestibular loss in cats leads to active destabilization of balance during pitch and roll rotations of the support surface. J. Neurophysiol. 97, 4357–4367.
Marchand, A. R., and Amblard, B. (1990). Early sensory determinants of locomotor speed in adult cats: I. Visual compensation after bilabyrinthectomy in cats and kittens. Behav. Brain Res. 37, 215–225.
Maurer, C., Mergner, T., Becker, W., and Jurgens, R. (1998). Eye-head coordination in labyrinthine-defective humans. Exp. Brain Res. 122, 260–274.
Maurer, C., Mergner, T., and Peterka, R. J. (2006). Multisensory control of human upright stance. Exp. Brain Res. 171, 231–250.
McGath, J. H., Barber, H. O., and Stoyanoff, S. (1989). Bilateral vestibular loss and oscillopsia. J. Otolaryngol. 18, 218–221.
McKelvey-Briggs, D. K., Saint-Cyr, J. A., Spence, S. J., and Partlow, G. D. (1989). A reinvestigation of the spinovestibular projection in the cat using axonal transport techniques. Anat. Embryol. (Berl.) 180, 281–291.
Merfeld, D. M., Gong, W., Morrissey, J., Saginaw, M., Haburcakova, C., and Lewis, R. F. (2006). Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans. Biomed. Eng. 53, 2362–2372.
Merfeld, D. M., Haburcakova, C., Gong, W., and Lewis, R. F. (2007). Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans. Biomed. Eng. 54, 1005–1015.
Mergner, T., Schweigart, G., Fennell, L., and Maurer, C. (2009). Posture control in vestibular-loss patients. Ann. N. Y. Acad. Sci. 1164, 206–215.
Miller, D. M., Cotter, L. A., Gandhi, N. J., Schor, R. H., Cass, S. P., Huff, N. O., Raj, S. G., Shulman, J. A., and Yates, B. J. (2008). Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp. Brain Res. 188, 175–186.
Mittelstaedt, H. (1992). Somatic versus vestibular gravity reception in man. Ann. N. Y. Acad. Sci. 656, 124–139.
Mittelstaedt, H. (1995). Evidence of somatic graviception from new and classical investigations. Acta Otolaryngol. Suppl. 520, 186–187.
Mittelstaedt, H. (1998). Origin and processing of postural information. Neurosci. Biobehav. Rev. 22, 473–478.
Mittelstaedt, M. L., and Mittelstaedt, H. (1996). The influence of otoliths and somatic graviceptors on angular velocity estimation. J. Vestib. Res. 6, 355–366.
Money, K. E., and Scott, J. W. (1962). Functions of separate sensory receptors of nonauditory labyrinth of the cat. Am. J. Physiol. 202, 1211–1220.
Mori, R. L., Cotter, L. A., Arendt, H. E., Olsheski, C. J., and Yates, B. J. (2005). Effects of bilateral vestibular nucleus lesions on cardiovascular regulation in conscious cats. J. Appl. Physiol. 98, 526–533.
Muir, G. M., Brown, J. E., Carey, J. P., Hirvonen, T. P., Della Santina, C. C., Minor, L. B., and Taube, J. S. (2009). Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. J. Neurosci. 29, 14521–14533.
Nashner, L. M., Black, F. O., and Wall, C. III. (1982). Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J. Neurosci. 2, 536–544.
Neuhauser, H. K., and Lempert, T. (2009). Vertigo: epidemiologic aspects. Semin. Neurol. 29, 473–481.
Newlands, S. D., and Perachio, A. A. (2003). Central projections of the vestibular nerve: a review and single fiber study in the Mongolian gerbil. Brain Res. Bull. 60, 475–495.
Newlands, S. D., Vrabec, J. T., Purcell, I. M., Stewart, C. M., Zimmerman, B. E., and Perachio, A. A. (2003). Central projections of the saccular and utricular nerves in macaques. J. Comp. Neurol. 466, 31–47.
Nyberg-Hansen, R., and Mascitti, T. A. (1964). Sites and mode of termination of fibers of the vestibulospinal tract in the cat. an experimental study with silver impregnation methods. J. Comp. Neurol. 122, 369–383.
Peterka, R. J. (2002). Sensorimotor integration in human postural control. J. Neurophysiol. 88, 1097–1118.
Peterson, B. W., Maunz, R. A., and Fukushima, K. (1978). Properties of a new vestibulospinal projection, the caudal vestibulospinal tract. Exp. Brain Res. 32 287–292.
Petras, J. M. (1967). Cortical, tectal and tegmental fiber connections in the spinal cord of the cat. Brain Res. 6, 275–324.
Reiter, R. J., Tan, D. X., Korkmaz, A., and Fuentes-Broto, L. (2011). Drug-mediated ototoxicity and tinnitus: alleviation with melatonin. J. Physiol. Pharmacol. 62, 151–157.
Rinne, T., Bronstein, A. M., Rudge, P., Gresty, M. A., and Luxon, L. M. (1998). Bilateral loss of vestibular function: clinical findings in 53 patients. J. Neurol. 245, 314–321.
Ris, L., and Godaux, E. (1998). Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J. Neurophysiol. 80, 2352–2367.
Rubertone, J. A., and Haines, D. E. (1982). The vestibular complex in a prosimian primate (Galago senegalensis): morphology and spinovestibular connections. Brain Behav. Evol. 20, 129–155.
Rubin, A. M., Liedgren, S. C., Odkvist, L. M., Milne, A. C., and Fredrickson, J. M. (1978). Labyrinthine and somatosensory convergence upon vestibulo-ocular units. Acta Otolaryngol. 85, 54–62.
Rubin, A. M., Liedgren, S. R., Odkvist, L. M., Milne, A. C., and Fredrickson, J. M. (1977). Labyrinthine input to the vestibular nuclei of the awake cat. Acta Otolaryngol. 84, 328–337.
Russell, N. A., Horii, A., Smith, P. F., Darlington, C. L., and Bilkey, D. K. (2003a). Bilateral peripheral vestibular lesions produce long-term changes in spatial learning in the rat. J. Vestib. Res. 13, 9–16.
Russell, N. A., Horii, A., Smith, P. F., Darlington, C. L., and Bilkey, D. K. (2003b). Long-term effects of permanent vestibular lesions on hippocampal spatial firing. J. Neurosci. 23, 6490–6498.
Ryu, J. H., and McCabe, B. F. (1976). Central vestibular compensation. Effect of the bilateral labyrinthectomy on neural activity in the medial vestibular nucleus. Arch. Otolaryngol. 102, 71–76.
Sadeghi, S. G., Goldberg, J. M., Minor, L. B., and Cullen, K. E. (2009). Effects of canal plugging on the vestibuloocular reflex and vestibular nerve discharge during passive and active head rotations. J. Neurophysiol. 102, 2693–2703.
Sargent, E. W., Goebel, J. A., Hanson, J. M., and Beck, D. L. (1997). Idiopathic bilateral vestibular loss. Otolaryngol. Head Neck Surg. 116, 157–162.
Schautzer, F., Hamilton, D., Kalla, R., Strupp, M., and Brandt, T. (2003). Spatial memory deficits in patients with chronic bilateral vestibular failure. Ann. N. Y. Acad. Sci. 1004, 316–324.
Schor, R. H., and Miller, A. D. (1981). Vestibular reflexes in neck and forelimb muscles evoked by roll tilt. J. Neurophysiol. 46, 167–178.
Schubert, M. C., Tusa, R. J., Grine, L. E., and Herdman, S. J. (2004). Optimizing the sensitivity of the head thrust test for identifying vestibular hypofunction. Phys. Ther. 84, 151–158.
Shaikh, A. G., Marti, S., Tarnutzer, A. A., Palla, A., Crawford, T. O., Straumann, D., Carey, J. P., Nguyen, K. D., and Zee, D. S. (2011). Ataxia telangiectasia: a “disease model” to understand the cerebellar control of vestibular reflexes. J. Neurophysiol. 105, 3034–3041.
Shallo-Hoffmann, J., and Bronstein, A. M. (2003). Visual motion detection in patients with absent vestibular function. Vision Res. 43, 1589–1594.
Shepard, N. T., and Telian, S. A. (1995). Programmatic vestibular rehabilitation. Otolaryngol. Head Neck Surg. 112, 173–182.
Shinder, M. E., and Taube, J. S. (2010). Differentiating ascending vestibular pathways to the cortex involved in spatial cognition. J. Vestib. Res. 20, 3–23.
Smith, P. F., Darlington, C. L., and Zheng, Y. (2010a). Move it or lose it–is stimulation of the vestibular system necessary for normal spatial memory? Hippocampus 20, 36–43.
Smith, P. F., Geddes, L. H., Baek, J. H., Darlington, C. L., and Zheng, Y. (2010b). Modulation of memory by vestibular lesions and galvanic vestibular stimulation. Front. Neurol. 1:141.doi: 10.3389/fneur.2010.00141
Smith, P. F., Horii, A., Russell, N., Bilkey, D. K., Zheng, Y., Liu, P., Kerr, D. S., and Darlington, C. L. (2005). The effects of vestibular lesions on hippocampal function in rats. Prog. Neurobiol. 75, 391–405.
Stackman, R. W., and Herbert, A. M. (2002). Rats with lesions of the vestibular system require a visual landmark for spatial navigation. Behav. Brain Res. 128, 27–40.
Stackman, R. W., and Taube, J. S. (1997). Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J. Neurosci. 17, 4349–4358.
Stapley, P. J., Ting, L. H., Kuifu, C., Everaert, D. G., and Macpherson, J. M. (2006). Bilateral vestibular loss leads to active destabilization of balance during voluntary head turns in the standing cat. J. Neurophysiol. 95, 3783–3797.
Sumi, T., Watanabe, I., Tsunoda, A., Nishio, A., Komatsuzaki, A., and Kitamura, K. (2011). Longitudinal study of 29 patients with Meniere’s disease with follow-up of 10 years or more. Acta Oto-Laryngologica, in press.
Suzuki, J. I., and Cohen, B. (1964). Head, eye, body and limb movements from semicircular canal nerves. Exp. Neurol. 10, 393–405.
Suzuki, J. I., and Cohen, B. (1966). Integration of semicircular canal activity. J. Neurophysiol. 29, 981–995.
Suzuki, J. I., Cohen, B., and Bender, M. B. (1964). Compensatory eye movements induced by vertical semicircular canal stimulation. Exp. Neurol. 9, 137–160.
Suzuki, J. I., Goto, K., Tokumasu, K., and Cohen, B. (1969). Implantation of electrodes near individual vestibular nerve branches in mammals. Ann. Otol. Rhinol. Laryngol. 78, 815–826.
Szmulewicz, D. J., Waterston, J. A., Macdougall, H. G., Mossman, S., Chancellor, A. M., Mclean, C. A., Merchant, S., Patrikios, P., Halmagyi, G. M., and Storey, E. (2011). Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video-oculographic diagnosis. Ann. N. Y. Acad. Sci. 1233, 139–147.
Taube, J. S. (1998). Head direction cells and the neurophysiological basis for a sense of direction. Prog. Neurobiol. 55, 225–256.
Teasdale, N., Nougier, V., Barraud, P. A., Bourdin, C., Debu, B., Poquin, D., and Raphel, C. (1999). Contribution of ankle, knee, and hip joints to the perception threshold for support surface rotation. Percept. Psychophys. 61, 615–624.
Thomson, D. B., Inglis, J. T., Schor, R. H., and Macpherson, J. M. (1991). Bilateral labyrinthectomy in the cat: motor behaviour and quiet stance parameters. Exp. Brain Res. 85, 364–372.
Uchino, Y., Kudo, N., Tsuda, K., and Iwamura, Y. (1970). Vestibular inhibition of sympathetic nerve activities. Brain Res. 22 195–206.
Viard, A., Doeller, C. F., Hartley, T., Bird, C. M., and Burgess, N. (2011). Anterior hippocampus and goal-directed spatial decision making. J. Neurosci. 31, 4613–4621.
Vidal, P. P., De Waele, C., Vibert, N., and Muhlethaler, M. (1998). Vestibular compensation revisited. Otolaryngol. Head Neck Surg. 119, 34–42.
Waespe, W., Schwarz, U., and Wolfensberger, M. (1992). Firing characteristics of vestibular nuclei neurons in the alert monkey after bilateral vestibular neurectomy. Exp. Brain Res. 89, 311–322.
Waespe, W., and Wolfensberger, M. (1985). Optokinetic nystagmus (OKN) and optokinetic after-responses after bilateral vestibular neurectomy in the monkey. Exp. Brain Res. 60, 263–269.
Wall, C. III, and Kentala, E. (2005). Control of sway using vibrotactile feedback of body tilt in patients with moderate and severe postural control deficits. J. Vestib. Res. 15, 313–325.
Wallace, D. G., Hines, D. J., Pellis, S. M., and Whishaw, I. Q. (2002). Vestibular information is required for dead reckoning in the rat. J. Neurosci. 22, 10009–10017.
Waterston, J. A., Barnes, G. R., Grealy, M. A., and Luxon, L. M. (1992). Coordination of eye and head movements during smooth pursuit in patients with vestibular failure. J. Neurol. Neurosurg. Psychiatr. 55, 1125–1131.
Watt, D. G. (1976). Responses of cats to sudden falls: an otolith-originating reflex assisting landing. J. Neurophysiol. 39, 257–265.
Wilkinson, D., Nicholls, S., Pattenden, C., Kilduff, P., and Milberg, W. (2008). Galvanic vestibular stimulation speeds visual memory recall. Exp. Brain Res. 189, 243–248.
Wilson, T. D., Cotter, L. A., Draper, J. A., Misra, S. P., Rice, C. D., Cass, S. P., and Yates, B. J. (2006). Vestibular inputs elicit patterned changes in limb blood flow in conscious cats. J. Physiol. 575, 671–684.
Wilson, V. J., Kato, M., Thomas, R. C., and Peterson, B. W. (1966). Excitation of lateral vestibular neurons by peripheral afferent fibers. J. Neurophysiol. 29, 508–529.
Wilson, V. J., Zarzecki, P., Schor, R. H., Isu, N., Rose, P. K., Sato, H., Thomson, D. B., and Umezaki, T. (1999). Cortical influences on the vestibular nuclei of the cat. Exp. Brain Res. 125, 1–13.
Yakushin, S. B., Kolesnikova, O. V., Cohen, B., Ogorodnikov, D. A., Suzuki, J. I., Della Santina, C. C., Minor, L. B., and Raphan, T. (2011). Complementary gain modifications of the cervico-ocular (COR) and angular vestibulo-ocular (aVOR) reflexes after canal plugging. Exp. Brain Res. 210, 583–594
Yardley, L., Gardner, M., Bronstein, A., Davies, R., Buckwell, D., and Luxon, L. (2001). Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J. Neurol. Neurosurg. Psychiatr. 71, 48–52.
Yardley, L., Papo, D., Bronstein, A., Gresty, M., Gardner, M., Lavie, N., and Luxon, L. (2002). Attentional demands of continuously monitoring orientation using vestibular information. Neuropsychologia 40, 373–383.
Yates, B. J., Jakus, J., and Miller, A. D. (1993). Vestibular effects on respiratory outflow in the decerebrate cat. Brain Res. 629, 209–217.
Yates, B. J., Jian, B. J., Cotter, L. A., and Cass, S. P. (2000). Responses of vestibular nucleus neurons to tilt following chronic bilateral removal of vestibular inputs. Exp. Brain Res. 130, 151–158.
Yates, B. J., and Miller, A. D. (1994). Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J. Neurophysiol. 71, 2087–2092.
Yates, B. J., and Miller, D. M. (2009). Integration of nonlabyrinthine inputs by the vestibular system: role in compensation following bilateral damage to the inner ear. J. Vestib. Res. 19, 183–189.
Yavorcik, K. J., Reighard, D. A., Misra, S. P., Cotter, L. A., Cass, S. P., Wilson, T. D., and Yates, B. J. (2009). Effects of postural changes and removal of vestibular inputs on blood flow to and from the hindlimb of conscious felines. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1777–R1784.
Zee, D. S., Yee, R. D., and Robinson, D. A. (1976). Optokinetic responses in labyrinthine-defective human beings. Brain Res. 113, 423–428.
Zingler, V. C., Weintz, E., Jahn, K., Huppert, D., Cnyrim, C., Brandt, T., and Strupp, M. (2009). Causative factors, epidemiology, and follow-up of bilateral vestibulopathy. Ann. N. Y. Acad. Sci. 1164, 505–508.
Keywords: bilateral vestibular hypofunction, balance disorder, oscillopsia, postural control, vestibulo-ocular reflex, vestibular–autonomic response
Citation: McCall AA and Yates BJ (2011) Compensation following bilateral vestibular damage. Front. Neur. 2:88. doi: 10.3389/fneur.2011.00088
Received: 13 October 2011;
Paper pending published: 23 November 2011;
Accepted: 12 December 2011;
Published online: 27 December 2011.
Edited by:
Kenna Peusner, George Washington University, USAReviewed by:
Maurizio Versino, Pavia University, ItalyCopyright: © 2011 McCall and Yates. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Bill J. Yates, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Suite 500, Pittsburgh, PA 15213, USA. e-mail:YnlhdGVzQHBpdHQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.