- 1Department of Neurology, Leiden University Medical Center, Leiden, Netherlands
- 2Division of Image Processing (LKEB), Department of Radiology, Leiden University Medical Center, Leiden, Netherlands
- 3Percuros B.V., Department of Developmental Bio-Engineering, University of Twente, Enschede, Netherlands
- 4Department of Radiation Oncology, Netherlands Cancer Institute, Amsterdam, Netherlands
- 5Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands
- 6In-Vivo-NMR Laboratory, Max Planck Institute for Metabolism Research, Cologne, Germany
- 7Intelligent Systems Group, Faculty of Electrical Engineering, Mathematics and Computer Science, Delft University of Technology, Delft, Netherlands
Data—Summary and Highlights
In this data report we make available to the community a highly variable longitudinal MRI mouse brain data set of ischemic lesion after transient middle cerebral artery occlusion (tMCAo). Together with the provided semi-automated and automated segmentations, these data can be used to further improve the method proposed by Mulder et al. (2017) and also to serve as a benchmark for comparison between different approaches to segment ischemic lesions in MRI mouse brain data. It can also be used to develop and validate algorithms that further classify the stroke area into core and penumbra.
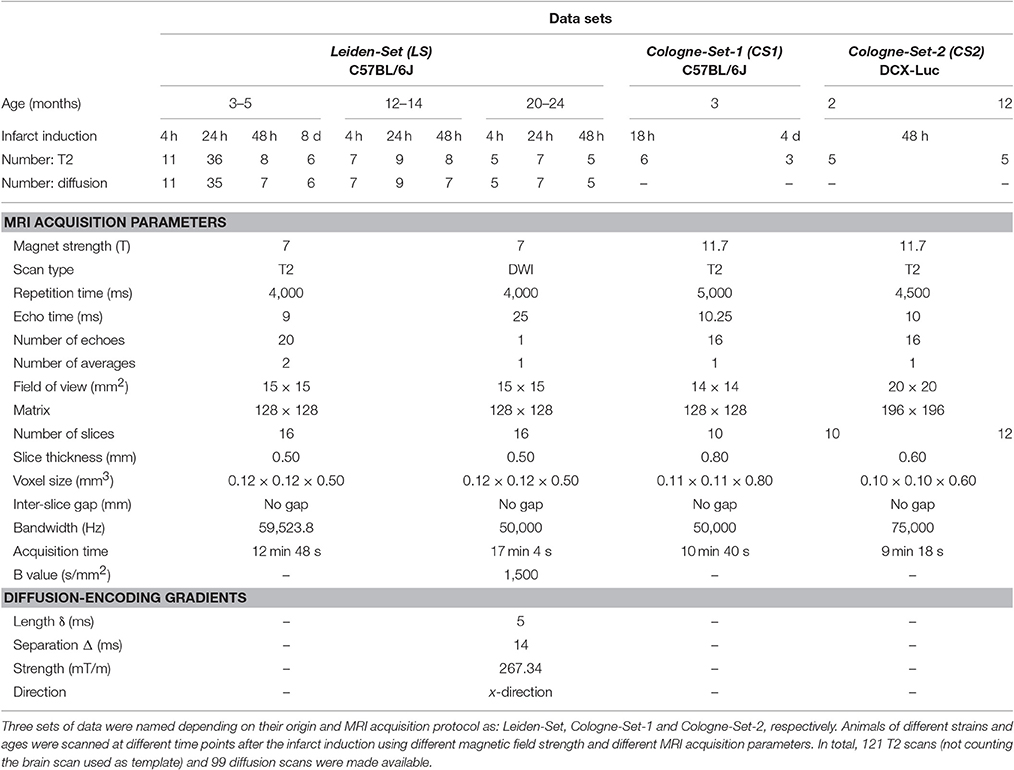
• The data were collected from mice: (i) of different ages, (ii) of two different strains, (iii) at different time points after the ischemic infarct induction, (iv) from two laboratories, (v) using two different MRI systems, and (vi) using three different sets of acquisition parameters.
• Segmentations of the ischemic lesions are provided as well. These were obtained by: (i) two observers using a semi-automated method and (ii) using the novel automated segmentation approach described by Mulder et al. (2017).
• Type/format of data: raw files, MetaImage files, text/Excel files, analyzed data.
• The following set of images associated with each of the 121 scans is included: raw Bruker MRI data (reference scan, T2 scan with all echoes, calculated T2-weighted map), automated segmentations of the ischemic lesions and semi-automated segmentations by two observers.
• For 99 of these scans, an accompanying set of Bruker MR diffusion maps, containing the Diffusion-Weighted Image (DWI) and calculated Apparent Diffusion Coefficient (ADC) maps, is included.
• Acquisition hardware: small-animal Bruker MRI systems (7 T and 11.7 T).
• Experimental set-up: infarct was induced in male mice of different age and background, using the tMCAo model. After that, MRI scans at different time points after infarct induction were acquired.
• Data sources: Leiden, Netherlands; Cologne, Germany.
• Data accessibility: all related data sets (121 T2 scans + template + 99 diffusion scans) were deposited in the public Dryad Digital Repository (https://doi.org/10.5061/dryad.1m528).
Experimental Design, Materials and Methods1
Animals and MRI data
Male WT mice were subdivided into three main sets, respectively labeled as: “Leiden-Set”, “Cologne-Set-1”, “Cologne-Set-2”, depending on the city of origin and acquisition protocol.
Leiden-Set: C57BL/6J mice (n = 58) were further subdivided into three age groups (3- to 5-, 12- to 14- and 20- to 24-month-old). Mice were repeatedly scanned at different time points: 4 h, 24 h, 48 h and 8 d after infarct induction; see “Experimental Infarct Model” section.
Cologne-Set-1: C57BL/6J mice (n = 6; 3-month-old) were scanned at 18 h and 4 d after infarct induction.
Cologne-Set-2: Transgenic mice expressing luciferase under doublecortin control (DCX-Luc, Couillard-Després et al., 2008) (n = 10; 2- and 12-month-old) were scanned at 48 h after infarct induction.
Animals were housed with littermates, in a temperature-controlled environment, with food and water ad libitum. All animal experiments performed at the Leiden University Medical Center (LUMC) were approved by the local committee for animal health, ethics, and research of LUMC. All animal experiments conducted at the Max Plank Institute for Metabolism Research in Cologne were performed in accordance with the German Animal Welfare Act and approved by the local authorities (Landesamt für Naturschutz, Umwelt und Verbraucherschutz NRW).
Scans were performed with small-animal Bruker MRI systems using a Multi-Slice Multi-Echo sequence protocol. Animals from the Leiden-Set were scanned at 7 T (Pharmascan, Bruker BioSpin, Ettlingen, Germany), whilst animals from the Cologne-Sets were scanned at 11.7 T (Biospec 11.7 T/16, Bruker BioSpin). Quantitative T2 and ADC maps were calculated from the raw data using Paravision 5.1 software (Bruker Pharmascan) for the Leiden-Set and IDL software was used to calculate the quantitative T2 maps of the Cologne-Sets.
Table 1 shows a complete overview of all scans, together with a summary of the main imaging acquisition parameters.
Experimental Infarct Model
Infarcts were induced using a modified transient middle cerebral artery occlusion (tMCAo) model first described by Longa et al. (1989). Mice were anesthetized using isoflurane (3% induction, 1.5% maintenance) in 70% pressurized air and 30% O2. Painkiller carprofen (5 mg/kg, s.c.; Carporal, 50 mg/mL, AST Farma BV, Oudewater, Netherlands) was given before surgery. During surgery, the mouse body temperature was maintained at around 37°C using a rectal probe and feedback system. During the surgical procedure, a silicone-coated nylon monofilament (7017PK5Re; Doccol Company, Redlands, CA, USA) was inserted into the right common carotid artery and advanced via the internal carotid artery and circle of Willis to eventually block the middle cerebral artery (MCA) at its origin (decreasing blood flow substantially in the MCA territory, in the right hemisphere) and the skin was sutured. During the occlusion period, the mouse was allowed to wake up in a temperature-controlled incubator (V1200; Peco Services Ltd., Brough, UK). After 30 min of occlusion, the mouse was re-anesthetized in order to remove the suture and withdraw the monofilament to allow reperfusion. After surgery, the animal was allowed to recover for 2 h in the incubator to maintain body temperature at around 37°C, with easy access to food and water.
Ischemic Lesion Segmentation
The novel method developed to segment the ischemic lesion in mouse brains in an automated fashion is described in detail in Mulder et al. (2017) and is available for download from the Software Downloads section of our webpage2. The same manuscript describes how the semi-automated segmentations (by two observers) used for validation of the automated algorithm were obtained. In “Description of the Files Associated with the Template Image” section, the files associated with the template brain image used as a part of the automated segmentation framework are described.
Description of All Scan Names and Corresponding MRI Bruker Folders
All scans are named according to the following convention: “[SetName]_[SubjectNumber]_[TimeAfterStrokeOnset]”.
Examples of scan names (SN):
– LS_m53_24h
– CS1_m8_19h
– CS2_m4_2d
Bruker 2dseq data-files can be loaded using ImageJ3 with the Paravision 5.1 Bruker plug-in4 installed. In case of the Leiden-Set, the reference scan is located in the “\1\pdata\1” folder, the echoes and the calculated quantitative T2 maps are located in the “\2\pdata\1” and the “\2\pdata\2” folders, respectively. In case of the Cologne-Set-1 and Cologne-Set-2, the echoes and T2 maps are located in the “\1\pdata\1” and the “\1\pdata\2” folders, respectively.
In case of the diffusion scans in the Leiden-Set, the reference scan is the same as the T2 reference scan and is located in the “\1\pdata\1” folder, the DWI maps (per effective B-value) are located in the “\3\pdata\1” folder and the calculated quantitative ADC maps: signal intensity (SI), standard deviation of SI, diffusion constant (mm2/seq), standard deviation of diffusion constant, standard deviation of the fitting curve, are located in the “\3\pdata\2” folder.
Inside the Bruker folders, all scans of each set were subdivided according to the different age groups.
Description of the File Names That Correspond to the Different Types of Data Made Available for Each Subject/Scan
In addition to the Bruker MRI data files, corresponding echoes, T2 maps, automated ischemic lesion segmentations and the semi-automated ones obtained by two observers (IM, SdJ) are provided in MetaImage format. In addition, the semi-automated segmentations are also provided in a format readable by the ROI manager of ImageJ. Each file was named according to the following convention (here “SN” = “ScanName”): “[SN]_[TypeOfDataDescriptor]”:
– Echoes (NR = 1…20 for Leiden-Set or NR = 1…16 for Cologne-Sets): SN_echoe_NR(.mhd/.raw)
– T2 maps: SN_T2map(.mhd/.raw)
– Automated segmentations: SN_AUTOMATED(.mhd/.raw)
– Semi-automated segmentations:
◦ ImageJ: SN_RoiSet_IAM.zip, SN_RoiSet_SdJ.zip
◦ MetaImage: SN_MANUAL_IAM(.mhd/.raw), SN_MANUAL_SdJ(.mhd/.raw)
Description of the Files Associated with the Template Image
All files associated with a single scan of the mouse, 24 h after stroke onset (belonging to the 3- to 5-month-old mice age group) that was used as a template/reference image in the registration step of the automated ischemic lesion approach proposed by Mulder et al. (2017), are provided in MetaImage format. The manually drawn labels were propagated to each data set and used to initialize the level-set-based segmentation. The sum of all the 20 echoes and the whole brain mask were used during the registration step as the fixed image and fixed mask, respectively:
– Sum-of-the-20-echoes image: Template_24h_SUM20echoes(.mhd/.raw)
– Whole brain mask: Template_24h_WholeBrainMask(.mhd/.raw)
– Echoes (NR = 1…20): Template_24h_echoe_NR(.mhd/.raw)
– T2 map: Template_24h_T2map(.mhd/.raw)
– Whole brain label: Template_24h_Label_1(.mhd/.raw)
– Ipsilateral hemisphere label: Template_24h_Label_2(.mhd/.raw)
– Ventricles label: Template_24h_Label_3(.mhd/.raw)
– Periventricular zone label: Template_24h_Label_4(.mhd/.raw)
Author Contributions
Conceived and designed the study: IM, AK, OD, MH, BL and AvdM. Performed animal experiments, tMCAo surgery and acquired MRI data: IM. Performed semi-automated ischemic lesion segmentation: IM and SdJ. Developed the algorithm for automated ischemic lesion segmentation in MRI mouse brain data after tMCAo occlusion and performed the automated segmentation: AK and OD. Analyzed segmentations and wrote the manuscript: IM, AK and OD. Commented on the manuscript: IM, AK, OD, MW, MH, BL and AvdM.
Funding
The authors acknowledge funding from the: Dutch Heart Foundation (2011T055; MW), ZonMW Veni grant (MW), Dutch Brain Foundation (F2014(1)-22; MW), Centre for Medical Systems Biology (CMSB) in the framework of the Netherlands Genomics Initiative (NGI) (AvdM), FP7 EUROHEADPAIN (No. 602633; AvdM), Marie Curie IAPP Program BRAINPATH (No. 612360; AK, AvdM and MH), FP7/2007-2013 under grant agreement No. 604102—Human Brain Project (AK, AvdM and BL) and Dutch Technology Foundation STW (as part of the STW project 12721: “Genes in Space” under the IMAGENE perspective program; OD).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^This section was partially adapted from Mulder et al. (2017).
References
Couillard-Després, S., Winner, B., Plötz, S., Wiedermann, D., Aigner, R., Bogdahn, U., et al. (2008). In vivo optical imaging of neurogenesis: watching new neurons in the intact brain. Mol. Imaging 7, 28–34. doi: 10.2310/7290.2008.0004
Longa, E. Z., Weinstein, P. R., and Carlson, S. (1989). Reversible middle cerebral artery occlusion without craniotomy in rats. Stroke 20, 84–91. doi: 10.1161/01.STR.20.1.84
Keywords: ischemic stroke, MRI, mouse, data collection, brain
Citation: Mulder IA, Khmelinskii A, Dzyubachyk O, de Jong S, Wermer MJH, Hoehn M, Lelieveldt BPF and van den Maagdenberg AMJM (2017) MRI Mouse Brain Data of Ischemic Lesion after Transient Middle Cerebral Artery Occlusion. Front. Neuroinform. 11:51. doi: 10.3389/fninf.2017.00051
Received: 23 December 2016; Accepted: 25 July 2017;
Published: 06 September 2017.
Edited by:
Pedro Antonio Valdes-Sosa, Joint China-Cuba Laboratory for Frontier Research in Translational Neurotechnology, ChinaReviewed by:
Nianming Zuo, Institute of Automation (CAS), ChinaEvelio Gonzalez, Cuban Neuroscience Center, Cuba
Copyright © 2017 Mulder, Khmelinskii, Dzyubachyk, de Jong, Wermer, Hoehn, Lelieveldt and van den Maagdenberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oleh Dzyubachyk, by5kenl1YmFjaHlrQGx1bWMubmw=
†These authors have contributed equally to this work.
 Inge A. Mulder
Inge A. Mulder Artem Khmelinskii2,3,4†
Artem Khmelinskii2,3,4† Oleh Dzyubachyk
Oleh Dzyubachyk Sebastiaan de Jong
Sebastiaan de Jong Boudewijn P. F. Lelieveldt
Boudewijn P. F. Lelieveldt