- Computational Physiology Laboratory, Department of Psychology, Cornell University, Ithaca, NY, USA
Concentration invariance—the capacity to recognize a given odorant (analyte) across a range of concentrations—is an unusually difficult problem in the olfactory modality. Nevertheless, humans and other animals are able to recognize known odors across substantial concentration ranges, and this concentration invariance is a highly desirable property for artificial systems as well. Several properties of olfactory systems have been proposed to contribute to concentration invariance, but none of these alone can plausibly achieve full concentration invariance. We here propose that the mammalian olfactory system uses at least six computational mechanisms in series to reduce the concentration-dependent variance in odor representations to a level at which different concentrations of odors evoke reasonably similar representations, while preserving variance arising from differences in odor quality. We suggest that the residual variance then is treated like any other source of stimulus variance, and categorized appropriately into “odors” via perceptual learning. We further show that naïve mice respond to different concentrations of an odorant just as if they were differences in quality, suggesting that, prior to odor categorization, the learning-independent compensatory mechanisms are limited in their capacity to achieve concentration invariance.
Introduction
In natural environments, odorants (analytes) can vary over many orders of magnitude in concentration—from ripe fruit or carrion in close proximity to the subtle scents of a trail of secretions or distant prey. In order to recognize known odors across the ranges of concentration at which they may be encountered, the olfactory system must in some way achieve concentration invariance in its odor representations, somehow separating concentration-dependent effects from information representing odor quality so that the odor source can be correctly identified.
Intensity invariance is a common problem across sensory systems, largely because the physical properties of the external environment vary to a much wider extent than the limited dynamic ranges of primary sensory receptors are able to capture. However, the problem is particularly acute in chemosensory modalities. Like all sensory receptors, primary chemosensors exhibit broad receptive fields that respond differentially to changes in intensity (concentration) as well as to changes in stimulus quality. Additionally, however, increasing odorant concentrations also recruit novel, lower-affinity ligand-receptor interactions that can interfere in unpredictable ways with existing interactions. The net effect is that, in addition to relatively predictable monotonic changes in receptor activation levels, concentration changes affect odor representations in unpredictable ways that are indistinguishable from changes in odor quality. Indeed, these effects essentially are changes in odor quality, as they arise from qualitative changes in the pattern of ligand-receptor interactions across the olfactory epithelium (Figure 1); interestingly, some odorants are perceived to shift in quality more than others when presented at different concentrations (Gross-Isseroff and Lancet, 1988; Johnson and Leon, 2000; Wright et al., 2005). The problem of olfactory concentration invariance consequently has been of considerable and persistent interest (Gross-Isseroff and Lancet, 1988; Duchamp-Viret et al., 1990; Bhagavan and Smith, 1997; Cleland and Linster, 2002; Cleland and Narla, 2003; Stopfer et al., 2003; Cleland et al., 2007; Uchida and Mainen, 2007).
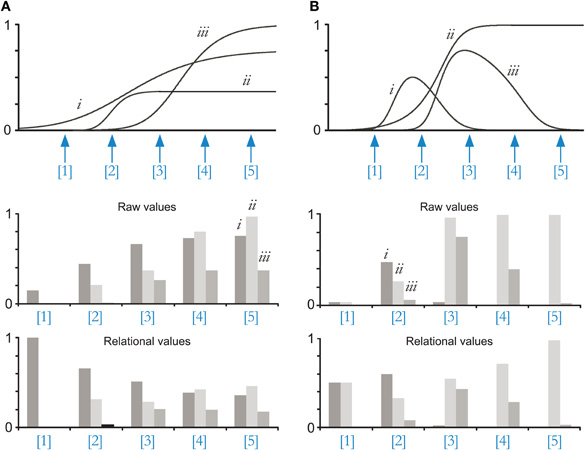
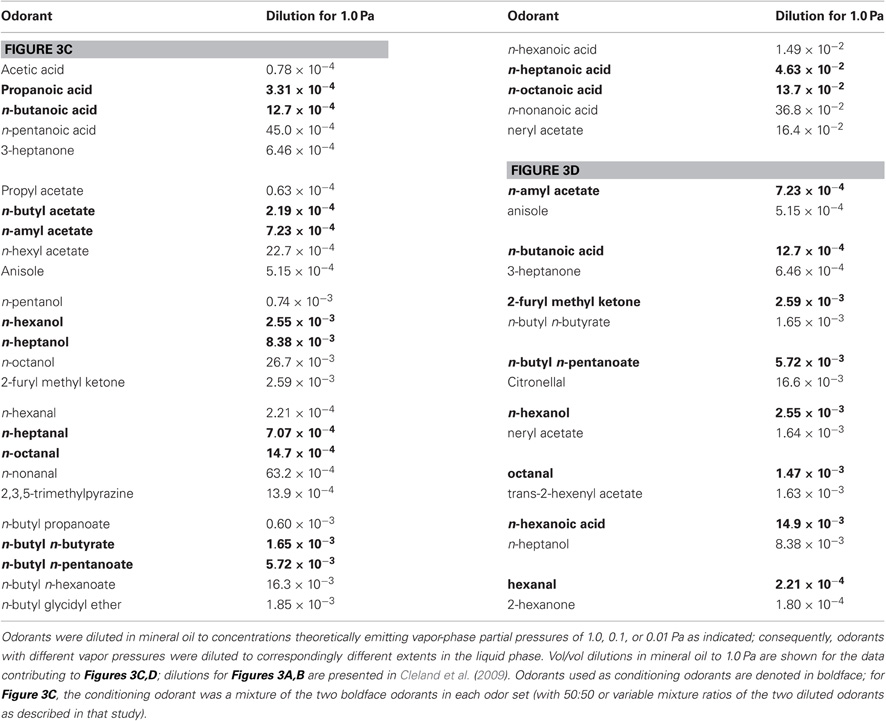
Figure 1. Depiction of the problem of concentration invariance. (A) Simple models of concentration invariance are predicated upon the principle that increases in concentration generate predictably monotonic increases in the activation levels of all sensitive receptors. The broad aggregate dose-response curves of glomeruli, hypothesized to combine inputs from similarly tuned OSNs that exhibit different half-activation concentrations owing to differences in receptor reserve, can in principle extend this quasi-linear range and thereby improve the similarity of relational representations of odorants across concentrations. Top panel. In a computational model of ligand-receptor interactions, three ORs are activated by three odotopes of an odorant presented at a range of concentrations (five of which are labeled: [1]–[5]). Dose-response curves that do not rise to a maximum value of 1 connote odotopes that are partial agonists for their cognate ORs. Ligand-receptor interaction i exhibits a glomerular Hill equivalent [exponent of the population dose-response function; (Cleland and Linster, 1999)] of 0.2, yielding a quasi-linear dose-response range extending across roughly five orders of magnitude in concentration. Interactions ii and iii exhibit somewhat higher—i.e., less extreme—Hill equivalents in this example and hence have steeper, narrower dose-response curves. As a result of these broadened curves, the relational representation of the odorant across concentrations is recognizable to some degree across modest concentration ranges. Middle panel. Primary odor representations at five concentrations, directly read as activation levels at each of the three OR interactions depicted (identified on graph of concentration [5]). Lower panel. Data from the middle panel, divisively normalized so that the activity resulting from each odor presentation sums to a constant. Odor representations at concentrations [4] and [5], and to some extent [3], are reasonably similar. This similarity across concentrations will improve if the quasi-linear ranges of OR interactions ii and iii are extended to resemble that of interaction i. (B) Top panel. Allosteric and other non-competitive interactions, even low-affinity interactions, can render dose-response profiles at individual ORs non-monotonic, generating variance that cannot be resolved by broadening glomerular intensity tuning ranges. Adding low-affinity non-competitive interactions to the model generated clearly non-monotonic dose-response profiles for odotopes i and iii. Middle panel. Primary odor representations at five concentrations, directly read as activation levels at each of the three OR interactions depicted (identified on graph of concentration [2]). Lower panel. Data from the middle panel, divisively normalized so that the activity resulting from each odor presentation sums to a constant. Odor representations are unrecognizable across even similar concentrations, even after normalization.
Animals, including humans, are able to recognize many odors across reasonably wide ranges of concentration, and this capacity is critical for the utility of artificial noses as well. What algorithms underlie this capability of biological olfactory systems, and how can they be adapted to artificial systems? To date, several alternatives have been explored, many with considerable merit, though none plausibly achieve the nominal goal of concentration invariance in its entirety. We here argue that true concentration invariance in chemosensory systems is not achievable, and instead outline a practical if imperfect solution to the problem that is effective in biological systems and calls for specific design elements in biomimetic artificial systems. Specifically, the unpredictable effects of concentration-dependent variance at the ligand-receptor interface produce a lossy, and therefore irreversible, transformation in the representation of a given odor across concentrations that is indistinguishable from the effects of quality-dependent variance. Rather than attempt solely to unravel the respective effects of concentration and quality on odor representations, we propose that a series of design features in the vertebrate olfactory system serve to (a) extend the capacity of the system to represent variance within a monotonic, quasi-linear regime, (b) utilize this capacity to reduce the magnitude of variance attributable to concentration changes when possible, and finally (c) categorize the range of odorant representations attributable to concentration series together via the same learning process by which variance in odor quality is categorically grouped so as to form odors. In essence, different concentrations of a given odorant are treated as a range of reasonably similar odors that can come to be interpreted as the same odor via learning. This proposed series of design features includes, in order: (1) adaptive sampling behaviors, (2) expansion of the quasi-linear range of olfactory receptor dose-response curves via receptor reserve and axonal convergence, (3) compression of this broad intensity tuning range into the modest dynamic range of single neurons, (4) dynamical matching of pre and postsynaptic dose-response curves at the first synapse in order to optimize the detection of small changes in the chemosensory environment, (5) relational normalization, the first stage at which there is competition among different chemosensors, and finally (6) generalization of the odor representation across concentrations by categorical learning.
Theoretical Foundations
Principles of Odor Ligand-Receptor Interaction
Primary olfactory sensory neurons (OSNs) in the vertebrate nasal cavity each canonically express one or a few species of odorant receptor proteins (ORs). In mice, there are over 1000 different functional odorant receptors expressed (Mombaerts, 2004), and millions of OSNs in the nasal cavity. The thousands of OSNs that express any given OR complement are distributed around the nasal cavity (Schoenfeld and Cleland, 2005, 2006), but their axonal projections converge and arborize together to form discrete target locations on the superficial olfactory bulb known as glomeruli (Figure 2). Setting aside the fact that glomeruli are anatomically duplicated—most glomeruli are replicated on the medial and lateral aspects of each olfactory bulb, yielding four glomeruli in total per OR per animal; (Schoenfeld and Cleland, 2005)—there is a direct correspondence between a given glomerulus, the OR complement expressed by its constituent OSNs, and the primary chemoreceptive field expressed by that OR complement (Belluscio et al., 2002; Treloar et al., 2002).
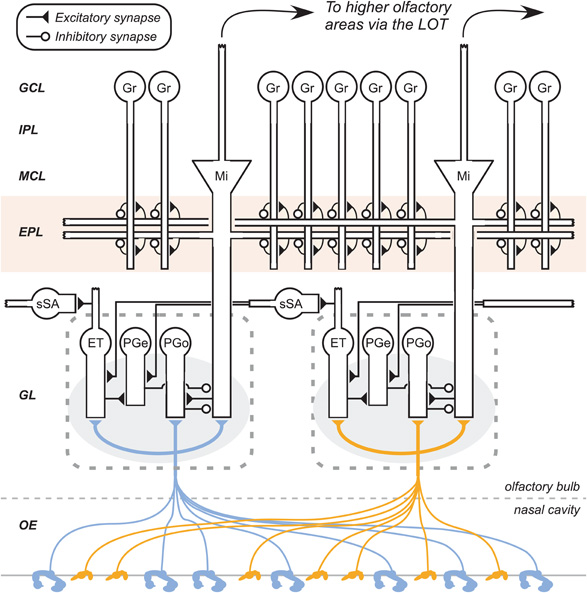
Figure 2. Circuit diagram of the mammalian olfactory bulb (two glomeruli shown, with corresponding postglomerular circuitry). The axons of olfactory sensory neurons (OSNs) expressing the same odorant receptor type (denoted by the shape and color of the receptor) converge together to form glomeruli (shaded ovals) on the surface of the olfactory bulb. Multiple classes of olfactory bulb neuron also innervate each glomerulus. Glomerular interneuron classes are heterogeneous, and include olfactory nerve-driven periglomerular cells (PGo), external tufted cell-driven periglomerular cells (PGe), and multiple subtypes of external tufted cells (ET). Superficial short-axon cells (sSA) are not associated with specific glomeruli but project broadly and laterally within the deep glomerular layer, interacting with glomerular interneurons. Principal neurons include mitral cells (Mi), which interact via reciprocal connections in the external plexiform layer (EPL) with the dendrites of inhibitory granule cells (Gr), thereby receiving recurrent and lateral inhibition. Middle/deep tufted cells, another class of olfactory bulb principal neurons, are not depicted. OE, olfactory epithelium (in the nasal cavity); GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; IPL, internal plexiform layer; GCL, granule cell layer. Filled triangles denote excitatory (glutamatergic) synapses; open circles denote inhibitory (GABAergic) synapses. Speckles surrounding OSN terminals connote volume-released GABA and dopamine approaching presynaptic GABAB and dopamine D2 receptors. Figure adapted from (Cleland, 2010).
The chemoreceptive fields of individual ORs are measurable (Araneda et al., 2000), but ultimately unknowable in practice for computational purposes. A complete functional characterization of an OR would require not only an exhaustive list of strong and moderate agonists, but also a comparably exhaustive characterization of odorants acting as weak agonists, antagonists, and non-competitive allosteric modulators that would by their presence impair that OR's capacity to respond to odorant agonists. Interfering ligands in this sense need not be odorants arising from distinct sources, or even different molecules within odor mixtures (which comprise most natural odors), but also can arise from multiple receptor binding sites presented by individual odorant molecules. In the relatively unregulated chemical environment to which the nasal cavity is subjected, these complex pharmacological interactions can substantially impact the net activation levels of OSNs, and therefore alter the primary odor representation.
Odor Space
As the competitive and non-competitive ligand-receptor interactions underlying primary olfactory transduction are unknowably complex, models of olfactory similarity space—commonly referred to as “odor space” and analogous to the one-dimensional space of pitch similarity in audition or the two-dimensional space of retinotopic location in early vision—cannot be usefully based on the physicochemical properties of odorants. Whereas in audition the tonotopic distribution of frequency sensitivities across the lengths of the cochlea or cochlear nucleus subdivisions can be mapped with respect to physical frequency itself (Luo et al., 2009), the analogous relationship in olfaction between OSN activation levels and the physicochemical properties of odorant molecules cannot practically be systematically measured. However, the pattern of OSN activation levels itself is a perfectly valid, albeit species-specific, foundational metric on which to base analyses of odor similarity. In this metric, any given olfactory representation (at a point in time) is uniquely defined as the pattern of levels of activation across each of the ∼1000 (in mice) ORs, with no reference made to the many possible configurations of agonists, antagonists, and allosteric site ligands that could underlie that pattern. (Indeed, there is by definition no way for the olfactory system to distinguish among different ligand configurations that result in the same pattern of input activity, so from the perspective of olfactory coding no information is lost). Moreover, the elemental odor stimulus is defined here not as an odorant molecule or molecular epitope per se, but rather an odotope, here defined as “the net effect of a given odorant molecule on a single type of odorant receptor” without direct correspondence to molecular structural features (Cleland, 2008). Any changes in the pattern of chemoreceptor activation consequently are treated equally, whether deriving from differences in concentration or in ligand complement.
In this framework, odor space is concretely defined as having a dimensionality equal to the number of different ORs—in mice, roughly 1000—because each OR can in principle be activated independently of any other. Conveniently, these dimensions directly correspond to olfactory bulb glomeruli and hence can be directly observed using various experimental techniques (Friedrich and Korsching, 1997; Belluscio and Katz, 2001; Leon and Johnson, 2003; Fletcher et al., 2009). Theoretically, any given odor representation can at a given point in time be described by an n-dimensional vector, where n is the number of different ORs in the system, and the magnitude in each dimension ranges from 0 (no activity) to 1 (maximal activation). However, the utility of this vector model is limited by the problem of variance.
Variance
Variance in stimulus quality is inescapable. Even subsequent presentations of the same odorant under experimentally controlled conditions will not evoke exactly the same pattern of neural activity. Rather, each of these different evoked activity patterns constitutes a different n-dimensional vector; however, these vectors can be bound together into a common perceptual quality by virtue of their overall similarity. That is, odor representations are not single vectors, but n-dimensional clouds of vectors in odor space with characteristic sizes and shapes. The probabilistic boundaries that define the size and shape of such clouds define the region within which a meaningful odor (such as “apple”) will be recognized irrespective of within-category variability (e.g., cultivar, ripeness, temperature, growing-season variables). Outside of these boundaries, an odor stimulus will be judged as to some degree different from that representation in its quality or implications. Quantitatively, these clouds constitute n-dimensional probability density functions (PDFs) that correspond to what might be termed odors, as distinct from odorants: learned ranges of chemosensory activation patterns that convey the same meaning. Importantly, the degree of tolerable variance in each dimension is an integral part of the odor representation; large changes in some dimensions may be included in the same odor representation whereas small differences in other dimensions may indicate a different odor with different implications.
These odor PDFs can be behaviorally measured using generalization gradients [Figure 3; (Shepard, 1987; Linster and Hasselmo, 1999; Tenenbaum and Griffiths, 2001; Cleland et al., 2002)]. Olfactory generalization gradients are systematically regulated by learning, and directly measure how progressively increasing dissimilarity among odors yields a corresponding decline in animals' expectation of similar outcomes (Daly et al., 2001; Wilson and Stevenson, 2006; Cleland et al., 2009; Fernandez et al., 2009). Olfactory generalization gradients in vertebrates also are regulated by extrinsic neuromodulation within olfactory bulb and piriform cortex (Linster and Cleland, 2002; Wilson et al., 2004; Mandairon et al., 2006; Mandairon and Linster, 2009); the level of activity in ascending neuromodulatory inputs reflects behavioral state and may underlie animals' capacity to alter the perception of similarity in accordance with task demands.
Figure 3. Olfactory generalization gradients in mice. (A) Associative generalization from a conditioned odorant stimulus (CS) to a series of four sequentially similar odorants (S1-S4) plus one structurally and perceptually dissimilar control odorant (D). Presenting all odorants at a higher concentration (theoretical vapor-phase partial pressure of 1.0 Pa; black line) yielded a steeper, narrower generalization gradient than did identical training with low-concentration odorants (0.01 Pa; gray line), reflecting the learning-theoretic principle that higher CS salience supports greater learning. Twelve training trials were administered prior to testing. Figure adapted from Cleland et al., (2009). (B) Increasing the number of training trials (CS-reward pairings) prior to testing progressively increased perseverance and sharpened associative generalization gradients. 3×: three training trials; 6×: six training trials; 12×: 12 training trials. Figure adapted from Cleland et al., (2009). (C) Generalization gradients adapt to the variance of the conditioning odor. The high-variance conditioning group (see Methods) generalized fully across the range of CS variability (no difference in digging times between 50:50 and either C4 or C5; Welch test, t(46.36) = 0.444, p = 0.659; t(47.43) = 0.854, p = 0.398, respectively), whereas the low-variance group clearly distinguished both C4 and C5 from the 50:50 odor mixture CS (significant differences in digging times; Welch test, t(56.87) = 2.583, p = 0.012; t(43.45) = 3.314, p = 0.002, respectively). (D) Mice perceive sufficiently different concentrations of novel odorants as distinct odors. One group of mice was conditioned to an odorant CS at a high concentration (1.0 Pa; black line, test concentrations in Pa listed in Roman font on x-axis) and tested on two lower concentrations of that odorant as well as a dissimilar control odorant (D) at 1.0 Pa. A second group was conditioned to the same odorant CS at a low concentration (0.01 Pa; gray line, test concentrations in Pa listed in italic font on x-axis) and tested on two higher concentrations of that odorant as well as a dissimilar control odorant (D) at 0.01 Pa. Both groups treated the test odorant that was two orders of magnitude higher or lower in concentration as a distinct odor, roughly comparable in similarity to a structurally dissimilar odorant D. See the Learning-Dependent Construction of Odor Representations section for analysis details. Odor sets and vol/vol dilutions are detailed in Table 1. In all figures, error bars denote the standard error of the mean.
Learning About Odor Variance
Multiple types of olfactory generalization gradient can be used to measure the sizes and shapes of odor representations (Cleland et al., 2002). In non-associative generalization gradients [also known as cross-habituation or spontaneous discrimination gradients; (Cleland et al., 2002; Mandairon et al., 2006)], an animal is repeatedly presented with an odorant until its investigation time drops to an asymptotic minimum. Presentation of highly dissimilar odorants will still evoke a full investigation response, whereas odorants similar to the habituated odorant evoke partial responses. The function of investigation time with respect to odorant similarity defines the probabilistic boundary of the odor representation. Associative generalization gradients are similar in principle, but are measured by conditioning an animal to work (dig) for a reward when an odor cue is delivered. Presentation of odorants dissimilar to the conditioning odorant evokes no conditioned response, whereas presentation of perceptually similar odorants elicits a digging response that declines in perseverance as the odorant cue becomes more dissimilar from the conditioning odorant [Figures 3A,B; (Linster and Hasselmo, 1999; Cleland et al., 2002, 2009)]. The function of perseverance (e.g., digging time) with respect to odorant similarity yields a generalization gradient that delineates the consequential region (Shepard, 1987) of the underlying odor representation.
Critically, the shape of olfactory generalization gradients is modified by learning. Classical learning determinants such as conditioned stimulus salience and unconditioned stimulus valence systematically modify associative generalization gradients, as do changes in the numbers of training trials prior to testing (Cleland and Narla, 2003; Cleland et al., 2009). That is: increased learning in these studies corresponds to progressively sharper generalization gradients (Figures 3A,B; adapted from Cleland et al., 2009). Considering these generalization gradients as PDFs of odor quality—that is, maps of gradually declining probability that increasingly dissimilar odor stimuli have the same implications as the conditioned odorant—this learning effect directly reflects the statistical principle that the standard error of the mean is reduced as the number of samples increases. In a broader sense, learned generalization gradients reflect the fundamental principle of sensory representation described above: generalization gradients are reflections of perceptual learning, in which the olfactory system progressively adapts its internal representation of odor space, through experience, to the statistics of odor encounters and consequences in the world (Wilson and Stevenson, 2006; Fernandez et al., 2009; Wright et al., 2009).
A corollary of this principle is that the different primary odor representations arising from the range of odor qualities that correspond to a meaningful common odor such as “apple” should be bound together into the same odor PDF so as to comprise a common percept. In a generalization framework, this connotes a region of full generalization across the range of odor qualities that exhibits no significant dissimilarity-dependent reduction in the operant response. This principle of generalization across similar stimulus qualities in a continuous metric space has been theoretically described in a Bayesian framework (Tenenbaum and Griffiths, 2001). We tested the predictions of this theory in a study in which two groups of mice were trained to the same degree with the same mean conditioned odorant, but with different degrees of variance in odor quality across training trials. As predicted, we observed that the full-generalization window of the olfactory generalization gradient expanded to contain all of the conditioned variance, and that generalization began to fall off just outside of the boundaries of stimulus quality defined by the training experience (Figure 3C).
Addressing the Problem of Concentration Invariance
Ideally, changes in odorant concentration simply would increase the activity of all sensitive receptors proportionally, such that subsequent processing could easily extract the portion of stimulus variance attributable to odor quality differences. This, of course, is not the case. The sigmoidal dose-response relationships of ligand-receptor interactions have a limited quasi-linear range, but also long asymptotic tails at the extremes such that concentration changes alter the relational pattern of receptor activation on which odor quality representations are based (Figure 1A). Given that the quasi-linear range of ligand-receptor interactions is strictly limited by mass action law (Cleland and Linster, 1999), these distorting effects on relational representations are substantial. Moreover, the Hill coefficients of individual odorant receptors are often substantially greater than unity (Firestein et al., 1993), exacerbating this problem; in such neurons, the range between a concentration evoking 10% activation and that evoking 90% activation (EC10–90) can be substantially less than two orders of magnitude. In intact olfactory epithelia, the median EC05–95 dynamic range has been estimated at 0.3–1.2 log units (Rospars et al., 2000). Finally, the emergence of lower-affinity ligands interacting with activated receptors can alter dose-response curves in the same way as would a novel odor component, altering effective ligand potencies and even rendering dose-response functions non-monotonic if allosteric or other non-competitive interactions arise [Figure 1B; discussed further in (Cleland and Linster, 1999; Cleland, 2008)]. The inevitable consequence of these physical principles is that, while a substantial fraction of the variance in odor representations attributable to odor concentration can be minimized, changing odor concentrations nevertheless results in significant residual representational differences that are indistinguishable from changes in odorant quality. This predicts that naïve animals will respond to different odor concentrations as if they were different odorants, which indeed is the case in both mice (Figure 3D) and honeybees (Choudhary, 2009).
The second problem of concentration is common among sensory systems: the fact that the narrow dynamic range of ligand-receptor interactions, as constrained by mass action law, is far more limited than the range of external environmental properties that are to be measured. Indeed, to be able to resolve large differences in concentration at all is a substantial feat of biological engineering, as no individual sensor is capable of resolving such a broad concentration range. Moreover, if multiple different sensors (olfactory receptor types) with differing affinities for a given ligand were employed systematically to resolve such a broad concentration range, their otherwise unrelated chemoreceptive fields would render odor quality and concentration irrevocably intertwined, such that little or none of the variance attributable to concentration could be identified as such. While this remains a problem, it is made considerably more tractable in olfaction by the early processing strategies outlined in the Broadening of the Aggregate Dose-Response Curves of Glomeruli, Intensity Compression at the First Synapse, and Adaptation to Background Odor Intensity sections below.
In sum, as briefly outlined above, the solution of the biological olfactory system to the problem of concentration is likely to involve multiple coordinated processing mechanisms, which we propose include the following: (1) constraining stimulus variance somewhat by behaviorally regulating the concentrations presented to the olfactory epithelium, (2) substantially extending the quasi-linear dynamic range of each individual olfactory receptor class via receptor reserve and axonal convergence, thereby reducing the distorting effects of concentration on relational representations, and (3) compressing the absolute range of intensities exhibited by OSN populations within each glomerulus to match the dynamic ranges of postsynaptic second-order neurons. This intensity compression stage collapses the expanded quasi-linear dynamic range generated at the glomerulus (Broadening of the Aggregate Dose-Response Curves of Glomeruli, below), thereby eliminating a substantial fraction of the variance attributable to concentration (Intensity Compression at the First Synapse, below). Processing at this stage also appears to include (4) an adaptive component that adjusts to stable odor backgrounds so as to emphasize changes in the chemosensory environment rather than always being dominated by the strongest stimuli, and (5) an additional, competitive stage of normalization across the input (glomerular) layer of olfactory bulb. The remaining concentration-dependent variance, while substantially reduced in magnitude, cannot be differentiated from quality-dependent variance and, we here propose, (6) is perceived as quality variance until and unless the animal learns to categorize this variance into a single odorant.
Adaptive Sampling Behaviors
Animals are capable of modulating the intensity of sniffing behavior, potentially increasing the concentration of weak odorants and limiting the intensity of strong odors in the nasal cavity by regulating the depth and frequency of inhalation (Verhagen et al., 2007, but see Teghtsoonian et al., 1978), and possibly even manipulating odor quality to a limited extent by altering the deposition pattern of odorants onto regions of the nasal mucosa that are differentially enriched with particular ORs (Schoenfeld and Cleland, 2006). Control over sniffing may be particularly important for limiting the access of highly intense odors to the nose, as the capacity for differentiation among odors is sharply reduced when odorants are extremely strong. The regulation of sampling behaviors (sniffing, antennal flicking) and their role in perception is an ongoing field of research (Koehl, 2006; Carey et al., 2009; Shusterman et al., 2011; Wesson et al., 2011).
Broadening of the Aggregate Dose-Response Curves of Glomeruli
Broadening the dose-response curves of olfactory ligand-receptor interactions until the quasi-linear portions of their concentration tuning ranges extend across multiple orders of magnitude would enable a substantial fraction of concentration-dependent variance in odor representations to be quasi-linearized, such that it subsequently could be selectively removed from the representation by some form of normalization. However, the breadth of the quasi-linear region [EC10–90; (Cleland and Linster, 1999)] of individual ligand-receptor binding curves is limited by mass action law to less than two orders of magnitude; moreover, the reported Hill coefficients of isolated olfactory receptor neurons (Firestein et al., 1993) suggest that many of these dose-response relationships are even narrower than they need be (based on a minimum Hill coefficient of unity).
The olfactory system appears to have utilized convergence to engineer a solution to this conundrum. Thousands of individual OSNs that express the same odorant receptor complement project their axons convergently onto single locations on the surface of the olfactory bulb to form glomeruli [indeed, a substantial fraction of the glomerular volume is made up of their axonal arborizations; (Kosaka et al., 2001; Nawroth et al., 2007)]. These convergent OSNs share the same receptive field for quality, but may be differently attuned for ranges of concentration via differences in receptor reserve (Zhu, 1993) such that the net dose-response curve of the convergent population can be greatly extended so as to span several orders of magnitude (Cleland and Linster, 1999). Indeed, aggregate glomerular dose-response curves, as assessed by imaging studies, can span several orders of magnitude (Friedrich and Korsching, 1997; Wachowiak et al., 2002).
This substantial broadening of glomerular dose-response relationships prior to the first synapse, when followed by a normalization process, can greatly reduce the magnitude of the variance that is attributable to concentration (Figure 1A). Theoretical calculations of dissimilarities among representations of the same odorant across even small concentration differences suggest that concentration-invariant perception would not be remotely possible if the broadening of the quasi-linear range of these glomerular activation functions did not enable the extraction of much of this concentration-dependent variance component (Cleland et al., 2007). Nevertheless, this process is imperfect in its linearity and also contributes nothing towards resolution of the problem of allosteric and noncompetitive interactions (Figure 1B).
Intensity Compression at the First Synapse
The broadened aggregate dose-response relationships observed in OB glomeruli enable olfactory stimuli to be encoded over broad ranges of concentration. However, as this broad dynamic range greatly exceeds the dynamic range of any one ligand-receptor relationship, it must be compressed if the full dose-response relationship is to be encodable by individual postsynaptic neurons such as mitral, tufted, and periglomerular cells (or, potentially, across small groups of such cells). Indeed, mitral and tufted cells are able to differentially respond to concentration changes across reasonably wide concentration ranges (Hamilton and Kauer, 1989; Wellis et al., 1989; Duchamp-Viret et al., 1990; Nagayama et al., 2004).
Compression of the absolute range of input intensity into a manageable dynamic range appears to be mediated in large part by feedback inhibition onto the presynaptic terminals of OSNs, mediated by presynaptic GABAB receptors that reduce calcium influx into terminals and thereby reduce transmitter release (Nawroth et al., 2007; Pirez and Wachowiak, 2008). OSN activity monosynaptically excites a subclass of GABAergic periglomerular interneurons [PGo cells; Figure 2; (Shao et al., 2009)] and indirectly excites another subclass of GABAergic periglomerular cells (PGe cells), both of which release GABA in the vicinity of OSN presynaptic terminals (as well as onto the dendrites of mitral cells). The resulting presynaptic inhibition substantially constrains the output of convergent OSN populations. Notably, the magnitude (weight) of this presynaptic inhibition is consistent across both weak and strong levels of afferent excitation (Pirez and Wachowiak, 2008), and is not observably activity-dependent, at least on the timescales studied to date. In principle, stable presynaptic feedback inhibition is precisely the computation required to compress a monotonic dose-response relationship into a narrower dynamic range, appropriate for the graded excitation of postsynaptic neurons. An important outstanding question is the degree to which the level (as opposed to the weight) of this presynaptic feedback inhibition is determined independently for each glomerulus versus how much it may be regulated by an average level of activation computed across many glomeruli. PGo-mediated inhibition would clearly generate the former, whereas PGe-mediated inhibition, evoked in large part via excitation by external tufted (ET) cells, may to some extent mediate the latter (Cleland et al., 2007). This latter possibility should not be confused with presynaptically mediated center-surround inhibition among glomeruli, which has been clearly ruled out (Pirez and Wachowiak, 2008).
Adaptation to Background Odor Intensity
In addition to GABAB receptors, OSN terminals presynaptically express D2-type dopamine receptors (Nickell et al., 1991), which respond to transmitter released by a dopaminergic subset of periglomerular neurons (Halasz et al., 1981; Gall et al., 1987; Toida et al., 2000). Like the GABAB receptors with which they are co-expressed, presynaptic D2 receptors are inhibitory (Hsia et al., 1999; Berkowicz and Trombley, 2000; Ennis et al., 2001; Davila et al., 2003), reducing the release of glutamate from OSN terminals in glomeruli. An important functional difference between these GABAB-ergic and dopaminergic feedback loops, however, is that the weight of dopaminergic feedback appears to be regulated by a running average level of afferent activation over a timescale of many minutes to hours. Specifically, sharply reducing odor stimulus levels through naris occlusion reduces dopamine levels in olfactory bulb (Brunjes et al., 1985), but also, on a somewhat longer timescale, reduces the expression of tyrosine hydroxylase in the dopaminergic periglomerular cells of the rat and mouse olfactory bulbs (Baker, 1990; Stone et al., 1990; Baker et al., 1993; Cho et al., 1996). As tyrosine hydroxylase is the rate-limiting enzyme for dopamine synthesis, reduced expression levels reflect reductions in the weight of dopaminergic feedback inhibition, suggesting a feedback-regulated system that gravitates toward a stable homeostatic level of activity. By this model, if input to a given glomerulus is consistently weak over many minutes or hours, dopaminergic feedback inhibition will be weakened until some homeostatic target level of average afferent activity is detected among postsynaptic neurons. Similarly, if high levels of presynaptic activity persist over a similar timescale, dopaminergic feedback inhibition will gradually increase in strength until the average level of postsynaptic activity is reduced to this same homeostatic baseline. The utility of such a mechanism is that the system adapts to stable background odor levels, effectively re-zeroing with respect to any background such that subtle changes in odor can be detected even within a strongly odorous environment. In essence, this imposes a timescale on olfactory perception that privileges changing signals over static background, analogous to a Pacinian corpuscle or the perception of visual motion. Additionally, this adaptive mechanism is likely to help keep the absolute range of total OSN output levels within a glomerulus adjusted to the absolute dynamic range for input to postsynaptic neurons, assuming that the homeostatic set point for OSN output is appropriate to this purpose.
This model predicts that manipulating dopamine D2 receptor activation levels in the olfactory bulb would affect the perceived concentration of odorants, which is indeed the case (Wei et al., 2006; Escanilla et al., 2009). Briefly, administration of D2 receptor agonists, either systemically or via direct infusion into olfactory bulb, reduced rats' performance levels in a concentration-sensitive odor discrimination task, whereas D2 antagonists improved their performance levels, both in a dose-dependent manner. Blocking D2 receptors also mimics the effects of olfactory deprivation on the activity of mitral/tufted cells (Wilson and Sullivan, 1995).
Relational Normalization
Normalization of outputs from all glomeruli with respect to the total activity level across the olfactory bulb in principle can preserve the relative levels of activity among activated glomeruli while keeping the total bulbar activation level roughly constant, generating the first competitive interaction among different glomerular columns and thereby forming a relational odor representation (Cleland et al., 2007). Competitive normalization, unlike intensity compression as described above, can generate non-monotonic dose-response functions in second-order neurons (e.g., mitral cells) as increasingly active glomerular columns outcompete less strongly activated columns, such that mitral cells innervating the outcompeted columns become less strongly activated, or even inhibited, as concentrations rise (Cleland and Sethupathy, 2006; Cleland, 2010). Indeed, increasing odorant concentrations do not generally increase spike rates in mitral cells monotonically; rather, mitral cell responses vary across concentrations in more complex ways, sometimes transitioning from excitation to inhibition, or vice versa, as odorant concentrations rise (Wellis et al., 1989; Chalansonnet and Chaput, 1998). Importantly, net activity among olfactory bulb neurons changes considerably less across concentrations than does that in OSNs, and raw maps of (predominantly presynaptic) glomerular activation in response to odorant presentation predict the perceptual similarity of odorants far less well than do the same maps after global normalization (Cleland et al., 2007). Moreover, global normalization is an important requirement for some models of olfactory contrast enhancement (Cleland and Sethupathy, 2006).
Evidence for a cellular or network mechanism that can mediate global normalization in the olfactory bulb is incomplete. A measure of constitutive GABA release from granule cells may help dampen mitral cell responses, although granule cells' inhibitory synapses onto mitral cells are electrotonically distant from the primary dendrite in which mitral cell spikes can be initiated, and there is no evidence that lateral inhibition mediated by granule cells would be sufficiently broad and unbiased to globally normalize afferent activation patterns. Indeed, measurements of intercolumnar inhibitory efficacies in olfactory bulb suggest a sparse and highly specific lateral inhibitory map (Fantana et al., 2008). Alternatively, theoretical modeling of a lateral excitatory network in the deep glomerular layer (Figure 2; ET and sSA cells) has illustrated a mechanism by which global normalization of bulbar activity could be effected postsynaptically, utilizing an anatomically center-surround connectivity matrix (Aungst et al., 2003) to generate a uniform level of inhibition proportional to the total input activity across the olfactory bulb and delivered onto mitral cells (Cleland et al., 2007; Cleland, 2010). The potential interactions between this GABAA-dependent mechanism and the presynaptic feedback inhibition mechanisms described above remain to be explored, both theoretically and experimentally.
Learning-Dependent Construction of Odor Representations
The work described above suggests several circuit mechanisms, working sequentially and in concert, that can substantially reduce the variance in odor representations attributable to concentration—e.g., that would be generated by different concentrations of the same odorant. However, they do not and cannot suffice to provide true concentration invariance in odor representations. We here propose that the remaining variance in odor representations generated by different concentrations of the same odorant is treated in the same way as variance deriving from changes in odor quality. That is: different concentrations of the same odorant are treated as different odors until and unless the animal learns that they have similar implications and categorizes them together into a common odor representation, as depicted for ranges of similar odorants in Figure 3C.
This model makes two predictions. First, it predicts that naïve animals will perceive different concentrations of the same odorant as different, and will generalize between them partially or not at all. Second, in order for these different concentrations to be bound together efficiently by learning, the prior processing stages described above presumably must have removed enough concentration-dependent variance for their representations to be at least somewhat similar. (The validity of this second prediction may vary depending on the specific odorants in question—notably, pentanal and 2-hexanone have been reported to change in perceptual quality to humans given modest concentration changes, whereas pentanoic acid, methyl pentanoate, and pentanol do not; Johnson and Leon, 2000). We here show that different odor concentrations are treated by naïve mice as if they were different odors, and that the degree of perceptual similarity decreases with increasing concentration differences (Figure 3D; see Methods). Specifically, for the 1.0 Pa conditioned stimulus (black line), independent-measures analysis ofvariance (ANOVA) demonstrated a significant difference in digging time among the four trials, F(3,116) = 7.964, p < 0.001. Comparing the 1.0 Pa CS to the 0.01 Pa test odorant using Tukey's honestly significant difference (HSD) criterion demonstrated a significant decline in perseverance, p = 0.040. Similarly, for animals conditioned with an 0.01 Pa CS (gray line), ANOVA again demonstrated a significant difference in digging time among the four trials, F(3,112) = 3.353, p = 0.022. Comparing the 0.01 Pa CS to the 1.0 Pa test odorant confirmed a significant decline in perseverance (Tukey's HSD, p = 0.035). Thus, after conditioning to an odor CS, animals treated an odorant presented at two orders of magnitude higher or lower concentration as a distinct odor. Moreover, the magnitude of the decline in generalization across these two orders of magnitude in concentration is roughly comparable to the degree of difference between the CS and a highly dissimilar control odorant D, indicating that odorants presented at these two different concentrations can be perceived as differently as two quite different odorants.
Summary and Conclusions
Concentration invariance in the olfactory system is important in that odors from natural sources vary substantially in concentration from any given vantage point, and there is obvious benefit to being able to identify such odors irrespective of this variance. Indeed, several sequential, coordinated mechanisms in the olfactory system appear to be able to reduce the impact of concentration-based changes in odor representations substantially. However, unavoidable non-linearities in signal processing and the particular problem of interference from lower-affinity ligands render complete concentration invariance unachievable. In this behavioral paradigm, the remaining concentration-dependent effects on primary odor representations are indistinguishable from quality-dependent changes that signal the presence of different odors, and indeed modestly different concentrations of a given odorant are perceived as different odors by naïve animals. However, after this preprocessing cascade, moderately different odorant concentrations are not perceived as enormously different in quality, as presynaptic imaging studies would suggest, but rather as modestly different in quality; tenfold concentration differences remain quite perceptually similar, whereas one hundred-fold concentration differences approach asymptotic dissimilarity. Critically, odor representations that are at least moderately similar in perceptual quality (i.e., ranges of odor qualities in odor space) and that predict the same outcome can be grouped together through learning.
Methods
Behavioral Procedures
Olfactory generalization gradients were measured in mice according to established procedures (Cleland et al., 2002, 2009). Briefly, age-matched cohorts of male CD-1 mice (outbred strain; Charles River Laboratories, Wilmington, MA) were shaped (trained to dig for rewards in response to odor cues) from five to eight weeks of age and subsequently employed in experiments. Mice were maintained on a shifted 12L:12D cycle; all behavioral training was conducted during their dark cycle (9:00 a.m.–9:00 p.m.). Water was continuously available; mice were food-deprived for up to 18 hours preceding each session to motivate them to obtain sucrose rewards. Mice were fed immediately after an experimental session, and were not deprived of food on two subsequent days. All procedures were performed under the auspices of a protocol approved by the Cornell University Institutional Animal Care and Use Committee.
During each conditioning trial, two sand-filled dishes were placed in a chamber; one contained a sucrose reward and was scented with the conditioned odorant CS, whereas the other contained no reward and no odorant. Each trial began when the mouse entered the chamber, at which point it encountered the dishes and was allowed to dig in both dishes until it retrieved the reward. The mouse was then removed for a one minute intertrial interval, during which the dishes were prepared for the next trial. After the training trials were complete (12 training trials, except for Figure 3B), the test trials were begun. In the test trials, one dish was scented either with the CS odorant or with one of a series of similar or dissimilar test odorants, whereas the other contained no odorant, and neither dish contained any reward. The amount of time that a mouse spent digging in the scented sand (perseverance) served as the dependent variable. The duration of test trials was one minute, whereas conditioning trials ended after mice recovered the sucrose reward (up to a maximum of one minute). Intertrial intervals were one minute long, and test trials began directly after the completion of the conditioning trials. In all cases, data are aggregates of multiple separate odor sets averaged together to ensure that results are not specific to a given odorant series (Table 1), and testing orders were randomized and counterbalanced.
Figures 3A,B are adapted from (Cleland et al., 2009); experimental details can be found therein. All odorants included in Figure 3B were presented at 0.01 Pa. In the experiment comprising Figure 3C, two groups of mice were assembled, a low-variance conditioning group and a high-variance conditioning group. A homologous series of four odorants (C3–C6, arbitrary labels) plus a dissimilar odorant D were employed; all odorants were presented at 1.0 Pa. Mice were given 12 training trials with a conditioned odorant, which was a mixture of odorants C4 and C5. Specifically, the low-variance group was trained on a 50:50 mixture of C4 and C5 for all 12 trials, whereas the high-variance group was trained on six different C4:C5 mixture ratios centered on but not including 50:50 (specifically: 95:5, 80:20, 60:40, 40:60, 20:80, 5:95, each presented twice in a randomized and counterbalanced order). Both groups then were tested using an identical set of six test odorants: the 50:50 mixture, unmixed odorants C4 and C5, the structurally similar odorants C3 and C6, and a dissimilar control odorant D.
In the experiment comprising Figure 3D, two groups of mice were conditioned to an odorant CS over 12 training trials and then tested using that same odorant at three different concentrations as well as with a structurally different odorant presented at the same vapor-phase concentration as the conditioned odorant. Critically, one group was trained on a relatively high concentration (1.0 Pa; see Odor Sets and Dilutions below) and tested on lower concentrations, whereas the other was trained on a relatively low concentration (0.01 Pa) and tested on higher concentrations. This enabled us to rule out an alternative interpretation, derived from work in honeybees (Pelz et al., 1997), in which the higher-concentration odorant might simply comprise a superior exemplar of the odorant, such that animals' responses to a higher-concentration odorant would be stronger than those to a lower concentration irrespective of which concentration had been explicitly conditioned. Note that the absolute level of conditioning to the lower concentration is less than that to the higher concentration, as expected (compare to Figure 3A), so the two generalization gradients are considered separately.
Odor Sets and Dilutions
Multiple odor sets were used to enable counterbalancing among subjects and ensure that results were not dependent on the use of specific odor sets. All mice in a cohort were tested using every odor set employed in the corresponding study. Each odor set consisted of a homologous series of 2–5 structurally similar, unbranched aliphatic odorant molecules plus one structurally dissimilar odorant used as a control; one study also utilized a binary mixture of two structurally adjacent odorants, whereas another utilized multiple concentrations of single conditioned odorants (see Behavioral Procedures above; Table 1). Vapor pressures of pure odorants were estimated with the Hass-Newton equation as implemented in ACD/Boiling Point and Vapor Pressure Calculator (version 4.5; Advanced Chemistry Development, Toronto, ON, Canada); pure odorants were diluted in mineral oil to concentrations theoretically emitting vapor-phase partial pressures of 1.0, 0.1, or 0.01 Pa as indicated. Solvent surface effects and other non-linearities were neglected. These dilutions should be considered a reduction in the variance of odor concentrations rather than true gas-phase concentration matching as could be achieved by gas chromatographic measurements. Odorants were diluted at least 18 hours in advance of each experiment to ensure an even distribution of odorant within the mineral oil solvent. These procedures have been utilized in previous studies (Cleland et al., 2002, 2009; Cleland and Narla, 2003).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Kafui Nutakor and Matthew A. Haber for assistance with behavioral experiments. This research was supported by NIDCD grant DC012249.
References
Araneda, R. C., Kini, A. D., and Firestein, S. (2000). The molecular receptive range of an odorant receptor. Nat. Neurosci. 3, 1248–1255.
Aungst, J. L., Heyward, P. M., Puche, A. C., Karnup, S. V., Hayar, A., Szabo, G., and Shipley, M. T. (2003). Centre-surround inhibition among olfactory bulb glomeruli. Nature 426, 623–629.
Baker, H. (1990). Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neuroscience 36, 761–771.
Baker, H., Morel, K., Stone, D. M., and Maruniak, J. A. (1993). Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 614, 109–116.
Belluscio, L., and Katz, L. C. (2001). Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J. Neurosci. 21, 2113–2122.
Belluscio, L., Lodovichi, C., Feinstein, P., Mombaerts, P., and Katz, L. C. (2002). Odorant receptors instruct functional circuitry in the mouse olfactory bulb. Nature 419, 296–300.
Berkowicz, D. A., and Trombley, P. Q. (2000). Dopaminergic modulation at the olfactory nerve synapse. Brain Res. 855, 90–99.
Bhagavan, S., and Smith, B. H. (1997). Olfactory conditioning in the honey bee, Apis mellifera: effects of odor intensity. Physiol. Behav. 61, 107–117.
Brunjes, P. C., Smith-Crafts, L. K., and McCarty, R. (1985). Unilateral odor deprivation: effects on the development of olfactory bulb catecholamines and behavior. Brain Res. 354, 1–6.
Carey, R. M., Verhagen, J. V., Wesson, D. W., Pirez, N., and Wachowiak, M. (2009). Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J. Neurophysiol. 101, 1073–1088.
Chalansonnet, M., and Chaput, M. A. (1998). Olfactory bulb output cell temporal response patterns to increasing odor concentrations in freely breathing rats. Chem. Senses 23, 1–9.
Cho, J. Y., Min, N., Franzen, L., and Baker, H. (1996). Rapid down-regulation of tyrosine hydroxylase expression in the olfactory bulb of naris-occluded adult rats. J. Comp. Neurol. 369, 264–276.
Choudhary, A. F. (2009). Olfactory Perceptual Invariance in the Honeybee: A Psychophysical Approach. PhD dissertation, Newcastle University, Newcastle-upon-Tyne, UK.
Cleland, T. A. (2008). “The construction of olfactory representations,” in Mechanisms of Information Processing in the Brain: Encoding of Information in Neural Populations, eds C. Holscher and M. Munk (Cambridge, UK: Cambridge University Press), 247–280.
Cleland, T. A., Johnson, B. A., Leon, M., and Linster, C. (2007). Relational representation in the olfactory system. Proc. Natl. Acad. Sci. U.S.A. 104, 1953–1958.
Cleland, T. A., and Linster, C. (1999). Concentration tuning mediated by spare receptor capacity in olfactory sensory neurons: a theoretical study. Neural. Comput. 11, 1673–1690.
Cleland, T. A., and Linster, C. (2002). How synchronization properties among second-order sensory neurons can mediate stimulus salience. Behav. Neurosci. 116, 212–221.
Cleland, T. A., Morse, A., Yue, E. L., and Linster, C. (2002). Behavioral models of odor similarity. Behav. Neurosci. 116, 222–231.
Cleland, T. A., and Narla, V. A. (2003). Intensity modulation of olfactory acuity. Behav. Neurosci. 117, 1434–1440.
Cleland, T. A., Narla, V. A., and Boudadi, K. (2009). Multiple learning parameters differentially regulate olfactory generalization. Behav. Neurosci. 123, 26–35.
Cleland, T. A., and Sethupathy, P. (2006). Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci. 7, 7.
Daly, K. C., Chandra, S., Durtschi, M. L., and Smith, B. H. (2001). The generalization of an olfactory-based conditioned response reveals unique but overlapping odour representations in the moth Manduca sexta. J. Exp. Biol. 204, 3085–3095.
Davila, N. G., Blakemore, L. J., and Trombley, P. Q. (2003). Dopamine modulates synaptic transmission between rat olfactory bulb neurons in culture. J. Neurophysiol. 90, 395–404.
Duchamp-Viret, P., Duchamp, A., and Sicard, G. (1990). Olfactory discrimination over a wide concentration range. Comparison of receptor cell and bulb neuron abilities. Brain Res. 517, 256–262.
Ennis, M., Zhou, F. M., Ciombor, K. J., Aroniadou-Anderjaska, V., Hayar, A., Borrelli, E., Zimmer, L. A., Margolis, F., and Shipley, M. T. (2001). Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J. Neurophysiol. 86, 2986–2997.
Escanilla, O., Yuhas, C., Marzan, D., and Linster, C. (2009). Dopaminergic modulation of olfactory bulb processing affects odor discrimination learning in rats. Behav. Neurosci. 123, 828–833.
Fantana, A. L., Soucy, E. R., and Meister, M. (2008). Rat olfactory bulb mitral cells receive sparse glomerular inputs. Neuron 59, 802–814.
Fernandez, P. C., Locatelli, F. F., Person-Rennell, N., Deleo, G., and Smith, B. H. (2009). Associative conditioning tunes transient dynamics of early olfactory processing. J. Neurosci. 29, 10191–10202.
Firestein, S., Picco, C., and Menini, A. (1993). The relation between stimulus and response in olfactory receptor cells of the tiger salamander. J. Physiol. 468, 1–10.
Fletcher, M. L., Masurkar, A. V., Xing, J., Imamura, F., Xiong, W., Nagayama, S., Mutoh, H., Greer, C. A., Knopfel, T., and Chen, W. R. (2009). Optical imaging of postsynaptic odor representation in the glomerular layer of the mouse olfactory bulb. J. Neurophysiol. 102, 817–830.
Friedrich, R. W., and Korsching, S. I. (1997). Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 18, 737–752.
Gall, C. M., Hendry, S. H., Seroogy, K. B., Jones, E. G., and Haycock, J. W. (1987). Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J. Comp. Neurol. 266, 307–318.
Gross-Isseroff, R., and Lancet, D. (1988). Concentration-dependent changes of perceived odor quality. Chem. Senses 13, 191–204.
Halasz, N., Johansson, O., Hokfelt, T., Ljungdahl, A., and Goldstein, M. (1981). Immunohistochemical identification of two types of dopamine neuron in the rat olfactory bulb as seen by serial sectioning. J. Neurocytol. 10, 251–259.
Hamilton, K. A., and Kauer, J. S. (1989). Patterns of intracellular potentials in salamander mitral/tufted cells in response to odor stimulation. J. Neurophysiol. 62, 609–625.
Hsia, A. Y., Vincent, J. D., and Lledo, P. M. (1999). Dopamine depresses synaptic inputs into the olfactory bulb. J. Neurophysiol. 82, 1082–1085.
Johnson, B. A., and Leon, M. (2000). Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J. Comp. Neurol. 422, 496–509.
Koehl, M. A. (2006). The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem. Senses 31, 93–105.
Kosaka, K., Aika, Y., Toida, K., and Kosaka, T. (2001). Structure of intraglomerular dendritic tufts of mitral cells and their contacts with olfactory nerve terminals and calbindin-immunoreactive type 2 periglomerular neurons. J. Comp. Neurol. 440, 219–235.
Leon, M., and Johnson, B. A. (2003). Olfactory coding in the mammalian olfactory bulb. Brain Res. Brain Res. Rev. 42, 23–32.
Linster, C., and Cleland, T. A. (2002). Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw. 15, 709–717.
Linster, C., and Hasselmo, M. E. (1999). Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol. Behav. 66, 497–502.
Luo, F., Wang, Q., Farid, N., Liu, X., and Yan, J. (2009). Three-dimensional tonotopic organization of the C57 mouse cochlear nucleus. Hear. Res. 257, 75–82.
Mandairon, N., Ferretti, C. J., Stack, C. M., Rubin, D. B., Cleland, T. A., and Linster, C. (2006). Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur. J. Neurosci. 24, 3234–3244.
Mandairon, N., and Linster, C. (2009). Odor perception and olfactory bulb plasticity in adult mammals. J. Neurophysiol. 101, 2204–2209.
Mombaerts, P. (2004). Genes and ligands for odorant, vomeronasal and taste receptors. Nat. Rev. Neurosci. 5, 263–278.
Nagayama, S., Takahashi, Y. K., Yoshihara, Y., and Mori, K. (2004). Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J. Neurophysiol. 91, 2532–2540.
Nawroth, J. C., Greer, C. A., Chen, W. R., Laughlin, S. B., and Shepherd, G. M. (2007). An energy budget for the olfactory glomerulus. J. Neurosci. 27, 9790–9800.
Nickell, W. T., Norman, A. B., Wyatt, L. M., and Shipley, M. T. (1991). Olfactory bulb DA receptors may be located on terminals of the olfactory nerve. Neuroreport 2, 9–12.
Pelz, C., Gerber, B., and Menzel, R. (1997). Odorant intensity as a determinant for olfactory conditioning in honeybees: roles in discrimination, overshadowing and memory consolidation. J. Exp. Biol. 200, 837–847.
Pirez, N., and Wachowiak, M. (2008). In vivo modulation of sensory input to the olfactory bulb by tonic and activity-dependent presynaptic inhibition of receptor neurons. J. Neurosci. 28, 6360–6371.
Rospars, J. P., Lansky, P., Duchamp-Viret, P., and Duchamp, A. (2000). Spiking frequency versus odorant concentration in olfactory receptor neurons. Biosystems 58, 133–141.
Schoenfeld, T. A., and Cleland, T. A. (2005). The anatomical logic of smell. Trends Neurosci. 28, 620–627.
Schoenfeld, T. A., and Cleland, T. A. (2006). Anatomical contributions to odorant sampling and representation in rodents: zoning in on sniffing behavior. Chem. Senses 31, 131–144.
Shao, Z., Puche, A. C., Kiyokage, E., Szabo, G., and Shipley, M. T. (2009). Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J. Neurophysiol. 101, 1988–2001.
Shepard, R. N. (1987). Toward a universal law of generalization for psychological science. Science 237, 1317–1323.
Shusterman, R., Smear, M. C., Koulakov, A. A., and Rinberg, D. (2011). Precise olfactory responses tile the sniff cycle. Nat. Neurosci. 14, 1039–1044.
Stone, D. M., Wessel, T., Joh, T. H., and Baker, H. (1990). Decrease in tyrosine hydroxylase, but not aromatic L-amino acid decarboxylase, messenger RNA in rat olfactory bulb following neonatal, unilateral odor deprivation. Brain Res. Mol. Brain Res. 8, 291–300.
Stopfer, M., Jayaraman, V., and Laurent, G. (2003). Intensity versus identity coding in an olfactory system. Neuron 39, 991–1004.
Teghtsoonian, R., Teghtsoonian, M., Berglund, B., and Berglund, U. (1978). Invariance of odor strength with sniff vigor: an olfactory analogue to size constancy. J. Exp. Psychol. Hum. Percept. Perform. 4, 144–152.
Tenenbaum, J. B., and Griffiths, T. L. (2001). Generalization, similarity, and Bayesian inference. Behav. Brain Sci. 24, 629–640; discussion 652–791.
Toida, K., Kosaka, K., Aika, Y., and Kosaka, T. (2000). Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb—IV. Intraglomerular synapses of tyrosine hydroxylase-immunoreactive neurons. Neuroscience 101, 11–17.
Treloar, H. B., Feinstein, P., Mombaerts, P., and Greer, C. A. (2002). Specificity of glomerular targeting by olfactory sensory axons. J. Neurosci. 22, 2469–2477.
Uchida, N., and Mainen, Z. F. (2007). Odor concentration invariance by chemical ratio coding. Front. Syst. Neurosci. 1 :3. doi: 10.3389/neuro.06.003.2007
Verhagen, J. V., Wesson, D. W., Netoff, T. I., White, J. A., and Wachowiak, M. (2007). Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat. Neurosci. 10, 631–639.
Wachowiak, M., Cohen, L. B., and Zochowski, M. R. (2002). Distributed and concentration-invariant spatial representations of odorants by receptor neuron input to the turtle olfactory bulb. J. Neurophysiol. 87, 1035–1045.
Wei, C. J., Linster, C., and Cleland, T. A. (2006). Dopamine D(2) receptor activation modulates perceived odor intensity. Behav. Neurosci. 120, 393–400.
Wellis, D. P., Scott, J. W., and Harrison, T. A. (1989). Discrimination among odorants by single neurons of the rat olfactory bulb. J. Neurophysiol. 61, 1161–1177.
Wesson, D. W., Varga-Wesson, A. G., Borkowski, A. H., and Wilson, D. A. (2011). Respiratory and sniffing behaviors throughout adulthood and aging in mice. Behav. Brain Res. 223, 99–106.
Wilson, D. A., Fletcher, M. L., and Sullivan, R. M. (2004). Acetylcholine and olfactory perceptual learning. Learn. Mem. 11, 28–34.
Wilson, D. A., and Stevenson, R. J. (2006). Learning to Smell: Olfactory Perception from Neurobiology to Behavior. Baltimore, MD: Johns Hopkins University Press.
Wilson, D. A., and Sullivan, R. M. (1995). The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J. Neurosci. 15, 5574–5581.
Wright, G. A., Choudhary, A. F., and Bentley, M. A. (2009). Reward quality influences the development of learned olfactory biases in honeybees. Proc. Biol. Sci. 276, 2597–2604.
Wright, G. A., Thomson, M. G., and Smith, B. H. (2005). Odour concentration affects odour identity in honeybees. Proc. Biol. Sci. 272, 2417–2422.
Keywords: learning, odor representations, categorization, generalization, olfactory bulb, concentration invariance, mice, computational neuroscience
Citation: Cleland TA, Chen ST, Hozer KW, Ukatu HN, Wong KJ and Zheng F (2012) Sequential mechanisms underlying concentration invariance in biological olfaction. Front. Neuroeng. 4:21. doi: 10.3389/fneng.2011.00021
Received: 03 October 2011; Paper pending published: 16 November 2011;
Accepted: 19 December 2011; Published online: 05 January 2012.
Edited by:
Thomas Nowotny, University of Sussex, UKReviewed by:
Maxim Bazhenov, University of California, USABarbara Webb, University of Edinburgh, UK
Copyright: © 2012 Cleland, Chen, Hozer, Ukatu, Wong and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Thomas A. Cleland, Computational Physiology Laboratory, Department of Psychology, Cornell University, Ithaca, NY 14853, USA. e-mail:dGFjMjlAY29ybmVsbC5lZHU=

 Szu-Yu T. Chen
Szu-Yu T. Chen