
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neuroenergetics , 16 August 2010
Volume 2 - 2010 | https://doi.org/10.3389/fnene.2010.00016
This article is part of the Research Topic Neurovascular imaging View all 25 articles
Neurovascular research has made significant strides toward understanding how the brain neurovascular unit accomplishes rapid and spatial increases in blood flow following neuronal activation. Among the experimental models used, the in vitro brain slice preparation provides unique information revealing the potential signals and cellular mechanisms involved in functional hyperemia. The most crucial limitation of this model, however, is the lack of intraluminal pressure and flow in the vessels being studied. Moreover, differences in basal vascular tone have led to varied interpretations regarding the polarity of vascular responses following neuron-to-glial stimulation. Given the complexity of astrocyte-induced neurovascular responses, we propose the use of a modified in vitro brain slice preparation, where intraluminal arteriolar pressure and flow are retained. Throughout this review, we discuss the advantages and disadvantages to be considered when using brain slices for neurovascular studies. Potential ways to overcome the current limitations are proposed.
In this article we review and discuss recent advances in our understanding of neurovascular communication, and address the advantages and disadvantages of using the in vitro brain slice preparation as a tool to study neurovascular coupling (NVC) in the brain. A number of groups have used this in vitro model, and data obtained from their studies (Fergus et al., 1996; Zonta et al., 2003b; Cauli et al., 2004; Hamel, 2004; Mulligan and MacVicar, 2004; Lovick et al., 2005; Filosa et al., 2006; Metea and Newman, 2006; Blanco et al., 2008) have provided seminal work to the field, highlighting the neuronal and astrocytic signaling pathways underlying NVC. A caveat to the data presented below, is however, that NVC mechanism are brain-region specific and dependent on the constituents forming the neurovascular unit (NVU) under study.
Blood supply is carried to the brain by extracerebral and intracerebral arteries/arterioles. Extracerebral arteries branch off into pial arterioles that surround the surface of the brain and then penetrate the brain parenchyma at right angles as penetrating or parenchymal arterioles (Edvinsson and MacKenzie, 2002). Parenchymal arterioles in turn give rise to an extensive capillary network, the distribution of which is associated to the metabolic demands of the neuronal microenvironment (Edvinsson and MacKenzie, 2002). The neurovascular control of the cerebral circulation varies, depending on the location and caliber of the vessels. In general, extracerebral vessels are innervated by peripheral nerves (extrinsic innervation) originating from the superior cervical, sphenopalatine, otic, internal carotid, and trigeminal ganglia (Gulbenkian et al., 2001). Intracerebral parenchymal microvessels are primarily regulated by local interneurons and neuronal terminals from a central origin (intrinsic innervation) such as the basal forebrain, raphe, and locus coeruleus (Iadecola, 1998; Gulbenkian et al., 2001; Hamel, 2006). These arterioles are also regulated by surrounding glial cells and, to some extent, by peripheral nerves that penetrate the brain parenchyma (Goadsby and Edvinson, 2002).
Much of the brain infrastructure is needed to provide neurons with the proper delivery of oxygen and glucose, given the limited energy reserves of the brain. To accomplish this, the brain possesses two fundamental mechanisms namely, autoregulation and functional hyperemia (Iadecola and Nedergaard, 2007) which are tightly controlled by the intrinsic properties of the vascular cells and by signals released from the various cell types that make up the NVU. The activation of these mechanisms warrants constant blood flow and increased oxygen and glucose delivery under conditions of intense neuronal stimulation.
At the cellular level, the cells that make up the NVU mainly include vascular cells [endothelial cells (EC), pericytes and vascular smooth muscle cells], neuronal terminals or varicosities, astrocytes with their corresponding specialized endfeet processes, and microglia. The interaction between these various cell types aid in the establishment of the blood brain barrier and the control of cerebral blood flow. Cell-to-cell interactions are futher supported by structural components such as gap junctions (Simard et al., 2003; Figueroa and Duling, 2009) and anchoring proteins (e.g., integrins; del Zoppo, 2010) as well as by the functional activation of various ion channels [e.g., aquaporin 4, K+ channels, transient receptor potential (TRP) channels (Brayden et al., 2008)] strategically expressed on membranes of the cells of the NVU. These associations are crucial for the structural integrity and functional capacity of the NVU. On a larger scale, further control is provided by networks of cells occupying defined brain regions. For example, specialized gap junctions link astrocytes to one another forming a syncytium (Theis et al., 2005). These glial networks efficiently modulate the activity of neuronal populations (Giaume et al., 2010). In addition, the intimate anatomical association of astrocytic processes with synapses and blood vessels places them in an ideal position to integrate neuronal activity with changes in vascular dynamics. An example of such a coordinated event was shown by Xu et al. (2008) providing evidence that the glia limitans plays an important role in upstream cerebral vasodilation following neuronal stimulation or hypercapnia-induced pial arteriolar dilation (Xu et al., 2004).
While astrocytic endfeet processes occupy a significant portion of the abluminal surface of the vessel wall (Kacem et al., 1998; Simard et al., 2003), other innervations such as neuronal terminals and varicosities of various origins have also been described, suggesting direct neurovascular regulation. For example, basal forebrain nitric oxide synthase (NOS) neurons (Tong and Hamel, 2000) and dopaminergic neurons have been shown to make close contacts with cerebral microvessels or with astrocytic endfeet (Krimer et al., 1998). Cauli et al. (2004) provided structural and functional evidence for the involvement of GABAergic interneurons containing vasoactive mediators such as NO. Clearly, specialized contact areas such as the space between neuronal terminals or varicosities and astrocytic endfeet with the vascular wall provide structural evidence for the intricacy of the signaling mechanisms underlying NVC in the brain and the dynamic interactions between neuronal, glial and vascular networks. The in vitro brain slice preparation constitutes an ideal model to study cell-to-cell communication since it possesses all of the constituents of the NVU with the addition of local circuits that allow for the precise stimulation of discrete neuronal and/or astrocytic targets while simultaneously monitoring the activity of the vascular cells.
In addition to the above structural observations, strong functional evidence supports an important role for neurons in NVC. The release of neurotransmitters (e.g., glutamate and GABA) has been associated with vasomotor responses both in vivo and in vitro (Drake and Iadecola, 2007). A direct vasomotor effect from neuronally derived signals has been suggested for dopaminergic, serotonergic, norepinephrine and acetylcholine fibers as well as for neuronally derived neuropeptides such as substance P, neurotensin, vasoactive intestinal peptide (VIP), somatostatin and neuropeptide Y (for review see, Hamel, 2006; Drake and Iadecola, 2007). For example, in hippocampal slices Fergus et al. (1995) showed evidence that NO contributes to basal vascular tone and that CGRP dilated parenchymal arterioles. Interestingly, the magnitude of the vascular response to CGRP was dependent on the degree of arteriolar tone, suggesting an interplay between these signals (Fergus et al., 1995). In slices perfused with a thromboxane A2 agonist (U46619) to induce arteriolar tone, NMDA produced vasodilation via an NO-dependent mechanism (Lovick et al., 1999; Brown et al., 2000). Vasodilation of cortical arterioles has also been associated with the activation of cholinergic fibers (Vaucher and Hamel, 1995; Vaucher et al., 1997) and NO-containing neurons (Tong and Hamel, 2000). Tong and Hamel (2000) suggested that the anatomical interaction between the cholinergic and nitrergic systems may serve to potentiate vascular responses via acetylcholine’s influence on NO signaling (Tong and Hamel, 2000). Cauli et al. (2004) showed that stimulation of single interneurons expressing VIP or NOS mediated vasodilation, whereas stimulation of interneurons expressing somatostatin and neuropeptide Y produced microvessel constriction. Microinjection of dopamine produced constriction of ∼50% of microvessels (Krimer et al., 1998).
As has been the case with neurons, evidence exists that astrocyte-derived signals can also trigger vascular responses and play a pivotal role in the signaling mechanisms involved in NVC (Takano et al., 2006; Carmignoto and Gomez-Gonzalo, 2009). Astrocytic activation results in the release of vasoactive agents (Carmignoto and Gomez-Gonzalo, 2009; Koehler et al., 2009) capable of inducing localized vasodilation (in vitro an in vivo) and/or vasoconstriction (in vitro only) of parenchymal arterioles. Astrocytes respond to an increase in synaptic activity with a rise in intracellular Ca2+ (Cornell-Bell et al., 1990; Aguado et al., 2002). If the strength of synaptic activity is high, it triggers the propagation of a Ca2+ wave that travels to nearby microvessels (Zonta et al., 2003b; Fellin and Carmignoto, 2004; Filosa et al., 2004). This latter step is critical for astrocyte-induced vasodilation (Zonta et al., 2003b; Filosa et al., 2004). Furthermore, astrocytes synthesize and release a number of vasoactive substances such as NO (Wiencken and Casagrande, 1999; Li et al., 2003), prostacyclins, prostaglandins, epoxyeicosatrienoic acids (EETs), glutamate, adenosine, and ATP (Harder et al., 2002; Li et al., 2003; Simard et al., 2003; Zonta et al., 2003a,b; Anderson et al., 2004; Takano et al., 2006) making them key candidates in NVC.
Despite significant progress in our understanding of the signaling events involved in NVC and the contributing role of neurons and astrocytes, recent findings have raised new challenges to the field. For example, while the physiological significance of neuronal and astrocyte-induced vasodilatory responses is clear, it is still not apparent what is the physiological significance for stimulus-induced vasoconstriction at the site of neuronal activation (Girouard et al.; Cauli et al., 2004; Mulligan and MacVicar, 2004; Metea and Newman, 2006; Rancillac et al., 2006). More importantly, based on functional and structural observations, it has been proposed that vessel-to-glia or vessel-to-neuron signaling may play an important role in brain information processing (Moore and Cao, 2008), highlighting the importance of vascular-derived signals and raising the question as to whether information within the NVU flows in a bi-directional manner ( Kozlov et al., 2006; Paton et al., 2007; Moore and Cao, 2008; del Zoppo, 2010).
Under physiological conditions and in response to intraluminal pressure, arterioles display myogenic tone (i.e., constrictions independent of innervation). The mechanisms for this tone-dependent constriction have been extensively studied, and are underlined by an interplay between ion channels that increase intracellular Ca2+ (e.g., VDCC, TRPV4) (Knot and Nelson, 1998; Inoue et al., 2004; Zhang et al., 2009; Ma et al., 2010) as well as opening of K+ channels (e.g., BK, IK and SK) that oppose Ca2+-dependent mechanisms leading to hyperpolarization and vasodilation (Nelson et al., 1995; Wrzosek, 2009). The expression of these channels varies depending upon the cell type (e.g., EC or VSMC) and the location and caliber of the vessel. In a recent review Dunn and Nelson (2010) discusses the implication that K+ channel activation/inactivation has on VSMC tone.
In addition to the intrinsic properties of the VSMC, vascular tone is regulated by EC signaling. EC release vasodilating (e.g., NO, endothelium-derived hyperpolarizing factor, prostanoids) and vasoconstricting (e.g., endothelin, TXA2, prostaglandin) substances (Andresen et al., 2006; Feletou and Vanhoutte, 2009). Moreover, EC express an array of ion channels that, when activated, can directly or indirectly alter the membrane potential (Vm) of the VSMC, establishing EC-to-VSMC coupling. In excised parenchymal arterioles, Cipolla et al. (2009) showed the contribution of SK and IK channel activity (expressed in EC) on vascular tone. Direct interactions between ECs and astrocytes have also been proposed, providing structural and functional evidence for bi-directional communication between these cells (Leybaert et al., 2004). At the capillary level, Peppiat demonstrated pericyte constriction following electrical stimulation, purinergic activation, or GABA blockers, likely exemplifying an additional form of bi-directional communication between pericytes and EC and a form by which pericytes may control blood flow perfusion to capillary beds (Peppiatt and Attwell, 2004). Based on the intrinsic mechanisms controlling vascular tone and the intricate structural arrangement of the NVU, one can say that VSMCs are sandwitched from the luminal and abluminal side by functionally dynamic cells (ECs and astrocytes). Interestingly, many of the pathways described in the endothelium (Feletou et al., 2008; Feletou and Vanhoutte, 2009), that are known to play a major role in vascular tone have been observed in astrocytes. Figure 1 illustrates the various vasoactive signaling pathways described in the endothelium and also observed in astrocytes. What then would be the physiological consequences of such a structural arrangement?
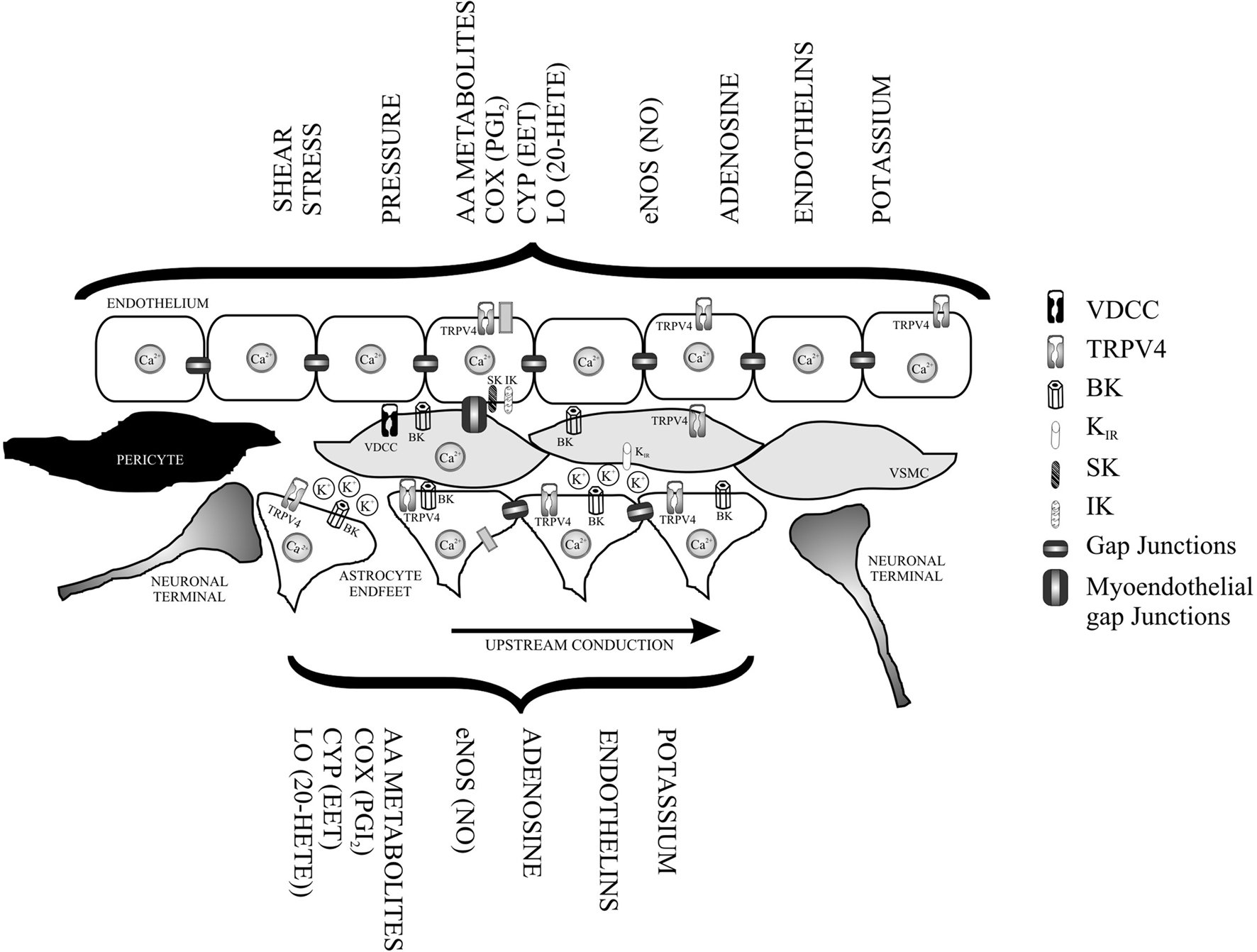
Figure 1. Functional overlapping mechanism from the luminal (endothelium) and abluminal side of a parenchymal arteriole. In addition to direct neuronal-vessel interactions vascular smooth muscle cells (VSMC) are tonically influenced by agonists and/or mechanically activated pathways from the endothelium and from signals released by astrocytic processes also in direct contact with the vessels. The presence of polarized and discrete ion channel types in the layer formed by endothelial cells and astrocytes, provides functional further support for bi-directional communication between these cells and VSMC, as well as, for an electrotonic arrangement capable of modulating vascular tone and conduction of signals over long distances along the microvessel. VDCC (voltage-dependent Ca2+ channels), TRPV4 (transient receptor potential vanilloid), BK (Ca2+-activated voltage-dependent K+ channel), IK (intermediate conductance Ca2+ activated K+ channel), SK (small conductance Ca2+ activated K+ channel), and Kir (inwardly rectifying K+ channel).
As is the case in EC-to-VSMC signaling, the close association between astrocytes to VSMCs may relate to the need to maintain or control vascular tone within optimal levels. To achieve this, astrocytes would need to constantly monitor hemodynamic changes and locally release vasoactive factors that adjust vascular diameter and maintain CBF constant. If astrocytes contribute to the tonic regulation of vascular tone, it may explain why functional hyperemia studies do not call for an important role for astrocytes and may provide a new meaning to astrocyte-induced vasoconstriction. While the role for astrocytes in functional hyperemia may be indeed relevant, their vasodilatory actions (Takano et al., 2006) may be modulatory depending on the neuronal pathways activated or degree of stimulation. In other words, it is possible that the vasoconstrictive role of astrocytes is primarily linked to their contribution to the re-establishment of vasomotor tone following neuronal stimulation or tone adjustments following hemodynamic-induced vascular responses to sudden blood pressure changes as occurs during autoregulation (Harder et al., 2002).
In an elegant study, Gordon et al. (2008) suggested that the polarity of the vascular response to glia-derived vasoactive signals is coupled to oxygen concentration levels, which in turn determine the metabolic state of the tissue. Oxygen levels modulate extracellular lactate and adenosine concentrations (Gordon et al., 2008). Increased lactate inhibits the activity of prostaglandin E2 (PGE2) transporters, raising extracellular PGE2 levels and thus favoring vasodilatory responses when PO2 levels are low (Chan et al., 2002). On the other hand, the authors suggested that vasoconstriction responses occur under hyperoxic conditions, where lactate levels are low and extracellular PGE2 availability is decreased (Gordon et al., 2008). Lindauer et al. (2010) recently challenged these findings by showing that in anesthetized rats, CBF responses to electrical forepaw stimulation or cortical spreading depression under conditions of hyperbaric oxygenation (absence of hemoglobin deoxygenation) were independent of O2 levels.
Likewise, a study by Metea and Newman (2006) in the retina showed that astrocytes induce both vasodilation and vasoconstriction, but in their case the polarity of the response was dependent on NO availability. While NO inhibition leads to vasodilation, NO availability favored vasoconstriction (Metea and Newman, 2006). The later observation was attributed to NO interactions with arachidonic acid (AA) metabolites favoring vasoconstriction through the potential NO inhibiting effects on cytochrome P450 (Udosen et al., 2003) and decreased downstream formation of the vasodilating metabolites, EETs (Metea and Newman, 2006). In support of these findings, Rancillac et al. (2006) showed that glutamate-induced NO released from cerebellar slices resulted in vasoconstriction of microvessels preconstricted with U46619. The vasoconstriction response was also dependent on the synthesis of prostanoids and endothelin. Given the strong evidence for NO inhibition of 20-HETE formation (Alonso-Galicia et al., 1997) and the ability of the AA metabolites, EETs and 20-HETEs, to modulate the activity of large conductance Ca2+-activated K+ (BK) channels in vascular cells (Feletou, 2009) and astrocytes (Gebremedhin et al., 2003, 2005) one possibility for NO-mediated constriction could be tonic EET-dependent BK activation in astrocytic endfeet, resulting in K+ accumulation and VSMC depolarization. A role for K+ channel-induced vasodilation and vasoconstriction was recently discussed by Girouard et al. (2010) (see below).
In a previous study, we showed that BK channel activation in astrocytic endfeet contributes to vasodilatory responses in parenchymal arterioles (Filosa et al., 2006). Moreover, our group also showed that the magnitude of the K+-induced vasodilatory response was correlated to the degree of tone of the arteriole, highlighting how changes to the intrinsic properties of the VSMCs alters their sensitivity to a given signal (Blanco et al., 2008). In addition, Girouard et al. (2010) recently showed that at the same level of tone, opposite type vascular responses could be evoked by varying K+ levels at the gliovascular space. Girouard’s study further incorporates the importance of upstream signaling events to vascular responses. The magnitude of the Ca2+ change in the astrocyte determined the amount of K+ efflux from the endfeet. It is suggested that low or modest changes in K+ levels activate inwardly rectifying (Kir) channels in VSMCs (Filosa et al., 2006) leading to vasodilation whereas high K+ levels at the gliovascular space cause VSMC depolarization and thus vasoconstriction. These studies suggest that in order to accurately interpret stimulus-induced vascular responses, the cells at the NVU must be close to physiological conditions including the steady-state of the EC and VSMCs, the resting Vm of the VSMCs, and the Ca2+ dynamic in all of the cells.
Undoubtedly, NO signaling plays a central role in NVC studies; however, the precise cellular sources and downstream targets for NO are largely unknown. While NO is believed to be the predominant signal in the cerebellum (Yang et al., 1999; Yang et al., 2000), findings from the cortex would suggest NO action in NVC are modulatory (Lindauer et al., 1999). In brain slices, NOS inhibition with L-NNA induced constriction of parenchymal arteriole, suggesting a tonic vasodilatory influence (Fergus et al., 1996), and neuronal stimulation-induced vasodilatory responses are in part mediated by NO derived from a neuronal origin (Lovick et al., 1999; Brown et al., 2000). Kitaura et al. (2007) showed that inhibiton of nNOS significantly decreased vasodilatory responses to stimulation while de Labra et al. (2009) recently suggested that stimulation-induced hemodynamic changes were associated with both eNOS and nNOS activity, depending on the degree of stimulation. In addition, de Labra suggested that NO from a vascular or glial source had a more prominent role in low frequency-induced vascular responses, while NO from a neuronal source played a more prominent role during intense stimulation. Data from these studies would suggest a possible mechanism by which the degree of stimulation may recruit the participation of different elements of the NVU, or networks, in orchestrating vascular responses.
Altogether, the ability of NO to interfere with AA signaling pathways in astrocytes (Metea and Newman, 2006), vascular tone (Feletou et al., 2008), neuronal activity (Ferraro and Sardo, 2004) and signaling events that affect many of the constituents of the NVU (i.e., K+ channel activity) brings into question whether interference with NO signaling alters the basal activity of the cells making up the NVU, disrupting, in turn, bi-directional steady-state communication pathways that optimize vascular responses to neuronally derived signals. Evidence supporting a role for astrocyte-derived NO in controlling vascular tone was provided by Chisari et al. (2004); co-cultures of basilar artery with activated LPS glia showed changes in vascular tone induced by NO. Moreover, because NO alters Ca2+ activity in astrocytes (Bal-Price et al., 2002), it is possible that endothelial-derived NO contributes to basal astrocytic activity. Future studies addressing the dynamic interactions between vascular cells and astrocytes in a bi-directional manner will help elucidate how interfering with these pathways may result in variable vascular responses following neuronal excitability.
Albeit these important observations and given confounding differences in the data obtained by various groups, a clearer understanding of NVC mechanisms may be possible if an improved and better standardized brain slice preparation is developed. Ideally, this preparation should include important hemodynamic variables such as intraluminal pressure and/or flow and take into consideration the levels of cellular activity at rest.
While numerous efforts have been made to maintain neuronal and glial viability in brain slices (Collingridge, 1995; Brahma et al., 2000; Hájos and Mody, 2009), little consideration has been made to ensure penetrating cerebral arterioles are maintained near physiological conditions when using this preparation. To this end, experts in the microcirculation field have provided extensive reports, albeit on excised arterioles, on the criteria needed to obtain proper vascular responses, and how changes to these variables could account for significant alterations in vascular reactivity and function (Ngai and Winn, 1995). However, only one study (Lovick et al., 2005) attempted to mimic these conditions in the brain slice preparation.
In an attempt to mimic physiological conditions, the solutions used to perfuse brain slices have been adjusted to include much of the constituents of the cerebrospinal fluid (CSF) (Reid et al., 1988; Hájos and Mody, 2009). While these solutions have proven to be successful in achieving reliable electrophysiological and imaging recordings from both neurons and astrocytes, a closer look at some of the major constituents of the artificial CSF (aCSF) is needed. For example, glucose concentrations used by most researchers (10–25 mM) far exceed that of the actual CSF (∼2.5 mM) (Silver and Erecinska, 1994). In addition, the use of 95% O2 has also been shown to result in different neuronal excitability levels and in the production of superoxide (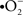 ) and cell death (D’Agostino et al., 2007; Hájos and Mody, 2009). Moreover, based on a study by Gordon et al. (2008), tissue PO2 levels may also have a crucial impact on the physiological response of vascular cells.
) and cell death (D’Agostino et al., 2007; Hájos and Mody, 2009). Moreover, based on a study by Gordon et al. (2008), tissue PO2 levels may also have a crucial impact on the physiological response of vascular cells.
Another important player affected by O2 levels is NO. Given that NO production is dependent on O2 availability (Kojima et al., 2001) and its presence influences cell function and NVC outcomes, caution should be taken when interpreting results where these variables are not carefully controlled or monitored. So what would be the ideal aCSF solution when performing NVC experiments in brain slices? While more data is needed to better address this question, perfusing slices with a 20% O2 solution might be closer to physiological conditions and is unlikely to induce cell death (D’Agostino et al., 2007). In a recent study, Ledo et al. (2010) simultaneously measured NO and O2 levels 200 μm deep into the slice using an aCSF solution bubbled with 95% O2–5% CO2; under these conditions O2 levels were around 57.3 ± 38.2 μM. To our knowledge the outcome of 20% O2 levels on NO availability at different depths of the brain slice has not been explored and would need further consideration, given the steep O2 gradient from the surface of the slice to deeper layers (∼200 μm) (Ledo et al., 2005). In addition, an even closer physiological approach would be to perfuse parenchymal arterioles with a solution that mimics blood constituents, establishing suitable PO2 gradients at the NVU before and during intense neuronal activation protocols are used. A similar approach has been extensively used in the renal physiology field where a renal arteriole is perfused with normal blood and hemodynamic variables are measured under different conditions (Casellas and Navar, 1984; Casellas and Moore, 2003). An approach of this nature would allow the exploration of further questions such as the effect vascular-derived signals have on resting astrocytic and neuronal function.
Altogether, these observations call for a more optimized in vitro model to dissect the underlying signaling mechanisms of NVC. While we strongly believe brain slices to be an ideal in vitro model, we suggest the inclusion of the following criteria: (1) optimal neuronal and astrocytic viability/activity and (2) optimal physiological conditions for the arteriole under investigation. As previously shown, brain slices should be kept under conditions that prevent cell swelling to maintain the viability of the neurons and astrocytes. In addition, the penetrating cerebral arterioles need to be exposed to an intraluminal solution that maximizes the viability of the vascular cells. Ideally, arterioles should be pressurized to allow for the development of myogenic tone. The presence of myogenic tone should be used as an indication that vascular cells have achieved optimal resting activity, the state at which vascular responses would be comparable to those under in vivo conditions. Using an in vitro preparation in which all the components of the NVU are brought to a closer physiological condition will provide more reliable interpretations of the dynamic interactions between vascular cells, astrocytes, and neurons in the brain.
Here we highlight previously reported experimental paradigms from which successful arteriolar myogenic tone and flow can be achieved in penetrating cerebral arterioles removed from the brain or from brain slices (Dacey and Duling, 1982; Ngai and Winn, 1995; Cipolla et al., 2004; Lovick et al., 2005). To this end, penetrating arterioles can be cannulated at one end and pressurized under no-flow conditions (Ngai and Winn, 1995). Following a successful cannulation, the arteriole is slowly pressurized in a stepwise manner until an intraluminal pressure between 20 and 40 mmHg is achieved and the vessel shows spontaneous tone (Dacey and Duling, 1982, 1984; Ngai and Winn, 1995). This latter step is typically conducted at 35°C to further stimulate tone (Ngai and Winn, 1995). Arterioles are allowed to equilibrate to their steady-state pressure before experimental protocols are executed. To maintain intraluminal pressure constant, the distal end of the arteriole is clamped and pressure is continuously monitored from a pressure transducer connected to a computer. In addition to intraluminal pressure, using the same cannula approach, flow can be introduced into parenchymal arterioles and its effects incorporated into experimental questions (Lovick et al., 2005). As previously performed in renal tubules, fluorescence recovery after photobleaching can be used to measure mean axial velocity of the intraluminal perfusate (Flamion et al., 1991) within the penetrating arteriole and thus provide a measurement of flow rate, a parameter not well investigated, but likely key in NVC-associated responses. For example, flow rate (shear stress) can induce NO production from the endothelium, changing the steady-state diameter (tone) of the arteriole and possibly vascular reactivity to vasoactive signals released by astrocytes and/or neurons (Ngai and Winn, 1995). Thus, one important question still to be determined is whether, under different hemodynamic conditions, cerebral arterioles would behave similarly to glial/neuronal-derived signals. An additional question, and suggested by the recently proposed hemo-neuronal hypothesis (Moore and Cao, 2008), is whether different hemodynamic conditions can alter neuronal excitability in the brain. Significant advances to this already reliable in vitro model will help expand our understanding of the cellular events underlying NVC in the brain.
Contrary to the disadvantages mentioned above, the brain slice preparation offers a number of advantages including: the presence of all of the cellular constituents comprising the NVU, the use of low-dose pharmacological agents to better define the cellular mechanisms underlying NVC, the unlimited access to brain regions of interest, and the simultaneous monitoring of the activity of multiple cell types. Moreover, the brain slice preparation allows for the study of cell-cell communication such as mechanisms underlying intracellular conduction throughout the astrocytic network and/or vascular networks and detailed mechanisms such as the spread of electrical conduction between cells with major emphasis on ECs.
In summary, further consideration and improvements are indeed needed to better address the signaling mechanisms underlying NVC in the brain. While in vivo approaches provide the ultimate clues to the understanding of how the brain communicates with the surrounding microcirculation, studies in brain slices have provided seminal observations on the cellular events underlying functional hyperemia in the brain. An optimized in vitro model will help address future unexplored questions such as the importance of studying NVC in different brain regions (inaccessible with current in vivo techniques) where the microenvironment of the NVC is drastically different.
The above observations also suggest that NVC mechanisms are driven by a multiplicity of signals and conditions. However, among future questions to address is the physiological function of neuronal-induced vs. astrocyte-induced vascular responses. Based on in vivo findings, a current suggestion is that increases in CBF in response to neuronal stimulation is mainly driven by neuronal networks (Rossier, 2009) with vasoconstriction distant to the site of activation (Devor et al., 2007). If this were the case, what is the role of astrocytes in NVC signaling? One possibility is that astrocytes contribute to basal CBF regulation or in the spatial spread of stimulus-dependent CBF activation to upstream vessels (Xu et al., 2008). Clearly, astrocytes possess vasoconstrictive and vasodilatory signals; however, in vivo studies have only associated their activation to vasodilation (Takano et al., 2006). We suggest that the vasoconstrictive action of these cells may be involved in the local maintenance of vascular tone, which plays an important role in determining the degree and efficiency of vascular responses to neuronally derived signals. Moreover, we predict that under basal conditions, VSMCs are under tonic influences by EC and astrocyte-derived signals, which are overwritten by neuronal signals following increases in neuronal activity. Accordingly, astrocytes may participate in autoregulatory mechanisms and also help re-establish vascular tone following hyperemic responses (Harder et al., 2002; Rossier, 2009).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
I thank Drs. Javier Stern and Laura Gonzalez Bosc for their comments on the manuscript. This work was funded by the National Heart, Lung and Blood Institute (R01HL089067) and the American Heart Association (0535231N).
Aguado, F., Espinosa-Parrilla, J. F., Carmona, M. A., and Soriano, E. (2002). Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J. Neurosci. 22, 9430–9444.
Alonso-Galicia, M., Drummond, H. A., Reddy, K. K., Falck, J. R., and Roman, R. J. (1997). Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension 29, 320–325.
Anderson, C. M., Bergher, J. P., and Swanson, R. A. (2004). ATP-induced ATP release from astrocytes. J. Neurochem. 88, 246–256.
Andresen, J., Shafi, N. I., and Bryan, R. M. Jr. (2006). Endothelial influences on cerebrovascular tone. J. Appl. Physiol. 100, 318–327.
Bal-Price, A., Moneer, Z., and Brown, G. C. (2002). Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia 40, 312–323.
Blanco, V. M., Stern, J. E., and Filosa, J. A. (2008). Tone-dependent vascular responses to astrocyte-derived signals. Am. J. Physiol. Heart Circ. Physiol. 294, H2855–2863.
Brahma, B., Forman, R. E., Stewart, E. E., Nicholson, C., and Rice, M. E. (2000). Ascorbate inhibits edema in brain slices. J. Neurochem. 74, 1263–1270.
Brayden, J. E., Earley, S., Nelson, M. T., and Reading, S. (2008). Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin. Exp. Pharmacol. Physiol. 35, 1116–1120.
Brown, L. A., Key, B. J., and Lovick, T. A. (2000). Fluorescent imaging of nitric oxide production in neuronal varicosities associated with intraparenchymal arterioles in rat hippocampal slices. Neurosci. Lett. 294, 9–12.
Carmignoto, G., and Gomez-Gonzalo, M. (2009). The contribution of astrocyte signaling to neurovascular coupling. Brain Res Rev.
Casellas, D., and Moore, L. C. (2003). The juxtamedullary nephron preparation. Methods Mol. Med. 86, 413–427.
Casellas, D., and Navar, L. G. (1984). In vitro perfusion of juxtamedullary nephrons in rats. Am. J. Physiol. 246, F349–F358.
Cauli, B., Tong, X. K., Rancillac, A., Serluca, N., Lambolez, B., Rossier, J., and Hamel, E. (2004). Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 24, 8940–8949.
Chan, B. S., Endo, S., Kanai, N., and Schuster, V. L. (2002). Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am. J. Physiol. Renal Physiol. 282, F1097–F1102.
Chisari, M., Salomone, S., Laureanti, F., Copani, A., and Sortino, M. A. (2004). Modulation of cerebral vascular tone by activated glia: involvement of nitric oxide. J. Neurochem. 91, 1171–1179.
Cipolla, M. J., Li, R., and Vitullo, L. (2004). Perivascular innervation of penetrating brain parenchymal arterioles. J. Cardiovasc. Pharmacol. 44, 1–8.
Cipolla, M. J., Smith, J., Kohlmeyer, M. M., and Godfrey, J. A. (2009). SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40, 1451–1457.
Collingridge, G. L. (1995). The brain slice preparation: a tribute to the pioneer Henry McIlwain. J. Neurosci. Methods 59, 5–9.
Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S., and Smith, S. J. (1990). Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473.
D’Agostino, D. P., Putnam, R. W., and Dean, J. B. (2007). Superoxide (*O2−) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J. Neurophysiol. 98, 1030–1041.
Dacey, R. G. Jr., and Duling, B. R. (1982). A study of rat intracerebral arterioles: methods, morphology, and reactivity. Am. J. Physiol. 243, H598–H606.
Dacey, R. G. Jr., and Duling, B. R. (1984). Effect of norepinephrine on penetrating arterioles of rat cerebral cortex. Am. J. Physiol. 246, H380–H385.
de Labra, C., Rivadulla, C., Espinosa, N., Dasilva, M., Cao, R., and Cudeiro, J. (2009). Different sources of nitric oxide mediate neurovascular coupling in the lateral geniculate nucleus of the cat. Front. Syst. Neurosci. 3, 9.
del Zoppo, G. J. (2010). The neurovascular unit in the setting of stroke. J. Intern. Med. 267, 156–171.
Devor, A., Tian, P., Nishimura, N., Teng, I. C., Hillman, E. M., Narayanan, S. N., Ulbert, I., Boas, D. A., Kleinfeld, D., and Dale, A. M. (2007). Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J. Neurosci. 27, 4452–4459.
Drake, C. T., and Iadecola, C. (2007). The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 102, 141–152.
Dunn, K. M., and Nelson, M. T. (2010). Potassium channels and neurovascular coupling. Circ. J. 74, 608–616.
Edvinsson, L. M., and MacKenzie, E. T. (2002). General and Comparative Anatomy of the Cerebral Circulation, 2nd Edn. New York: Lippincott Williams and Wilkins.
Feletou, M. (2009). Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br. J. Pharmacol. 156, 545–562.
Feletou, M., Tang, E. H., and Vanhoutte, P. M. (2008). Nitric oxide the gatekeeper of endothelial vasomotor control. Front. Biosci. 13, 4198–4217.
Fellin, T., and Carmignoto, G. (2004). Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J. Physiol. (Lond.) 559, 3–15.
Fergus, A., Jin, Y., Thai, Q. A., Kassell, N. F., and Lee, K. S. (1995). Vasodilatory actions of calcitonin gene-related peptide and nitric oxide in parenchymal microvessels of the rat hippocampus. Brain Res. 694, 78–84.
Fergus, A., Jin, Y., Thai, Q. A., Kassell, N. F., and Lee, K. S. (1996). Tonic protein kinase C-mediated vasoconstriction is unmasked when nitric oxide synthase is inhibited in cerebral microvessels. Neuroscience 74, 927–934.
Figueroa, X. F., and Duling, B. R. (2009). Gap junctions in the control of vascular function. Antioxid. Redox Signal. 11, 251–266.
Filosa, J. A., Bonev, A. D., and Nelson, M. T. (2004). Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 95, e73–e81.
Filosa, J. A., Bonev, A. D., Straub, S. V., Meredith, A. L., Wilkerson, M. K., Aldrich, R. W., and Nelson, M. T. (2006). Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 9, 1397–1403.
Flamion, B., Bungay, P. M., Gibson, C. C., and Spring, K. R. (1991). Flow rate measurements in isolated perfused kidney tubules by fluorescence photobleaching recovery. Biophys. J. 60, 1229–1242.
Gebremedhin, D., Narayanan, J., and Harder, D. R. (2005). Role of astrocytic KCa channel openings in the glutamate receptor mediated release of EETs from rat brain astrocytes. FASEB J. 19, A1257.
Gebremedhin, D., Yamaura, K., Zhang, C., Bylund, J., Koehler, R. C., and Harder, D. R. (2003). Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J. Neurosci. 23, 1678–1687.
Giaume, C., Koulakoff, A., Roux, L., Holcman, D., and Rouach, N. (2010). Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 11, 87–99.
Girouard, H., Bonev, A. D., Hannah, R. M., Meredith, A., Aldrich, R. W., and Nelson, M. T. (2010). Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. U.S.A. 107, 3811–3816.
Goadsby, P. J., and Edvinson, L. (2002). Neurovascular Control of the Cerebral Circulation, 2nd Edn. New York: Lippincott Williams and Wilkins.
Gordon, G. R., Choi, H. B., Rungta, R. L., Ellis-Davies, G. C., and MacVicar, B. A. (2008). Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749.
Gulbenkian, S., Uddman, R., and Edvinsson, L. (2001). Neuronal messengers in the human cerebral circulation. Peptides 22, 995–1007.
Hájos, N., and Mody, I. (2009). Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J. Neurosci. Methods 183, 107–113.
Hamel, E. (2004). Cholinergic modulation of the cortical microvascular bed. Prog. Brain Res. 145, 171–178.
Hamel, E. (2006). Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol. 100, 1059–1064.
Harder, D. R., Zhang, C., and Gebremedhin, D. (2002). Astrocytes function in matching blood flow to metabolic activity. News Physiol. Sci. 17, 27–31.
Iadecola, C. (1998). Neurogenic control of the cerebral microcirculation: is dopamine minding the store? Nat. Neurosci. 1, 263–265.
Iadecola, C., and Nedergaard, M. (2007). Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376.
Inoue, R., Morita, H., and Ito, Y. (2004). Newly emerging Ca2+ entry channel molecules that regulate the vascular tone. Expert Opin. Ther. Targets 8, 321–334.
Kacem, K., Lacombe, P., Seylaz, J., and Bonvento, G. (1998). Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia 23, 1–10.
Kitaura, H., Uozumi, N., Tohmi, M., Yamazaki, M., Sakimura, K., Kudoh, M., Shimizu, T., and Shibuki, K. (2007). Roles of nitric oxide as a vasodilator in neurovascular coupling of mouse somatosensory cortex. Neurosci. Res. 59, 160–171.
Knot, H. J., and Nelson, M. T. (1998). Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol. (Lond.) 508(Pt 1), 199–209.
Koehler, R. C., Roman, R. J., and Harder, D. R. (2009). Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 32, 160–169.
Kojima, H., Hirata, M., Kudo, Y., Kikuchi, K., and Nagano, T. (2001). Visualization of oxygen-concentration-dependent production of nitric oxide in rat hippocampal slices during aglycemia. J. Neurochem. 76, 1404–1410.
Kozlov, A. S., Angulo, M. C., Audinat, E., and Charpak, S. (2006). Target cell-specific modulation of neuronal activity by astrocytes. Proc. Natl. Acad. Sci. U.S.A. 103, 10058–10063.
Krimer, L. S., Muly, E. C. 3rd, Williams, G. V., and Goldman-Rakic, P. S. (1998). Dopaminergic regulation of cerebral cortical microcirculation. Nat. Neurosci. 1, 286–289.
Ledo, A., Barbosa, R., Cadenas, E., and Laranjinha, J. (2010). Dynamic and interacting profiles of *NO and O2 in rat hippocampal slices. Free Radic. Biol. Med. 48, 1044–1050.
Ledo, A., Barbosa, R. M., Gerhardt, G. A., Cadenas, E., and Laranjinha, J. (2005). Concentration dynamics of nitric oxide in rat hippocampal subregions evoked by stimulation of the NMDA glutamate receptor. Proc. Natl. Acad. Sci. U.S.A. 102, 17483–17488.
Leybaert, L., Cabooter, L., and Braet, K. (2004). Calcium signal communication between glial and vascular brain cells. Acta Neurol. Belg. 104, 51–56.
Li, N., Sul, J. Y., and Haydon, P. G. (2003). A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J. Neurosci. 23, 10302–10310.
Lindauer, U., Leithner, C., Kaasch, H., Rohrer, B., Foddis, M., Fuchtemeier, M., Offenhauser, N., Steinbrink, J., Royl, G., Kohl-Bareis, M., and Dirnagl, U. (2010). Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J. Cereb. Blood Flow Metab. 30, 757–768.
Lindauer, U., Megow, D., Matsuda, H., and Dirnagl, U. (1999). Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am. J. Physiol. 277, H799–H811.
Lovick, T. A., Brown, L. A., and Key, B. J. (1999). Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience 92, 47–60.
Lovick, T. A., Brown, L. A., and Key, B. J. (2005). Neuronal activity-related coupling in cortical arterioles: involvement of astrocyte-derived factors. Exp. Physiol. 90, 131–140.
Ma, X., Qiu, S., Luo, J., Ma, Y., Ngai, C. Y., Shen, B., Wong, C. O., Huang, Y., and Yao, X. (2010) Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler. Thromb. Vasc. Biol. 30, 851–858.
Metea, M. R., and Newman, E. A. (2006). Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 26, 2862–2870.
Moore, C. I., and Cao, R. (2008). The hemo-neural hypothesis: on the role of blood flow in information processing. J. Neurophysiol. 99, 2035–2047.
Mulligan, S. J., and MacVicar, B. A. (2004). Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199.
Nelson, M. T., Cheng, H., Rubart, M., Santana, L. F., Bonev, A. D., Knot, H. J., and Lederer, W. J. (1995). Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637.
Ngai, A. C., and Winn, H. R. (1995). Modulation of cerebral arteriolar diameter by intraluminal flow and pressure. Circ. Res. 77, 832–840.
Paton, J. F., Waki, H., Abdala, A. P., Dickinson, J., and Kasparov, S. (2007). Vascular-brain signaling in hypertension: role of angiotensin II and nitric oxide. Curr. Hypertens. Rep. 9, 242–247.
Rancillac, A., Rossier, J., Guille, M., Tong, X. K., Geoffroy, H., Amatore, C., Arbault, S., Hamel, E., and Cauli, B. (2006). Glutamatergic control of microvascular tone by distinct GABA neurons in the cerebellum. J. Neurosci. 26, 6997–7006.
Reid, K. H., Edmonds, H. L. Jr., Schurr, A., Tseng, M. T., and West, C. A. (1988). Pitfalls in the use of brain slices. Prog. Neurobiol. 31, 1–18.
Silver, I. A., and Erecinska, M. (1994). Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci. 14, 5068–5076.
Simard, M., Arcuino, G., Takano, T., Liu, Q. S., and Nedergaard, M. (2003). Signaling at the gliovascular interface. J. Neurosci. 23, 9254–9262.
Takano, T., Tian, G. F., Peng, W., Lou, N., Libionka, W., Han, X., and Nedergaard, M. (2006). Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267.
Theis, M., Sohl, G., Eiberger, J., and Willecke, K. (2005). Emerging complexities in identity and function of glial connexins. Trends Neurosci. 28, 188–195.
Tong, X. K., and Hamel, E. (2000). Basal forebrain nitric oxide synthase (NOS)-containing neurons project to microvessels and NOS neurons in the rat neocortex: cellular basis for cortical blood flow regulation. Eur. J. Neurosci. 12, 2769–2780.
Udosen, I. T., Jiang, H., Hercule, H. C., and Oyekan, A. O. (2003). Nitric oxide-epoxygenase interactions and arachidonate-induced dilation of rat renal microvessels. Am. J. Physiol. Heart Circ. Physiol. 285, H2054–H2063.
Vaucher, E., and Hamel, E. (1995). Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J. Neurosci. 15, 7427–7441.
Vaucher, E., Linville, D., and Hamel, E. (1997). Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience 79, 827–836.
Wiencken, A. E., and Casagrande, V. A. (1999). Endothelial nitric oxide synthetase (eNOS) in astrocytes: another source of nitric oxide in neocortex. Glia 26, 280–290.
Wrzosek, A. (2009). Endothelium as target for large-conductance calcium-activated potassium channel openers. Acta Biochim. Pol. 56, 393–404.
Xu, H. L., Koenig, H. M., Ye, S., Feinstein, D. L., and Pelligrino, D. A. (2004). Influence of the glia limitans on pial arteriolar relaxation in the rat. Am. J. Physiol. Heart Circ. Physiol. 287, H331–H339.
Xu, H. L., Mao, L., Ye, S., Paisansathan, C., Vetri, F., and Pelligrino, D. A. (2008). Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am. J. Physiol. Heart Circ. Physiol. 294, H622–H632.
Yang, G., Chen, G., Ebner, T. J., and Iadecola, C. (1999). Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am. J. Physiol. 277, R1760–R1770.
Yang, G., Huard, J. M., Beitz, A. J., Ross, M. E., and Iadecola, C. (2000). Stellate neurons mediate functional hyperemia in the cerebellar molecular layer. J. Neurosci. 20, 6968–6973.
Zhang, D. X., Mendoza, S. A., Bubolz, A. H., Mizuno, A., Ge, Z. D., Li, R., Warltier, D. C., Suzuki, M., and Gutterman, D. D. (2009). Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53, 532–538.
Zonta, M., Sebelin, A., Gobbo, S., Fellin, T., Pozzan, T., and Carmignoto, G. (2003a). Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J. Physiol. (Lond.) 553, 407–414.
Keywords: astrocytes, calcium, neurovascular coupling, vascular tone, arteriole, potassium, nitric oxide, brain slice
Citation: Filosa JA (2010) Vascular tone and neurovascular coupling: considerations toward an improved in vitro model. Front. Neuroenerg. 2:16. doi: 10.3389/fnene.2010.00016
Received: 01 March 2010;
Paper pending published: 08 April 2010;
Accepted: 28 June 2010;
Published online: 16 August 2010
Edited by:
Anna Devor, University of California San Diego, USAReviewed by:
Jean Rossier, Ecole Superieure de Physique et de Chimie Industrielles, FranceCopyright: © 2010 Filosa. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Jessica A. Filosa, Medical College of Georgia, CA 2092, 1120 15th Street, Augusta, GA 30912, USA. e-mail:amZpbG9zYUBtY2cuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.