
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neuroanat. , 24 November 2022
Volume 16 - 2022 | https://doi.org/10.3389/fnana.2022.1028546
This article is part of the Research Topic The Four Streams of the Prefrontal Cortex View all 10 articles
Smith and Lane have suggested a model of emotion processing with at least three stations: areas like the amygdala, which process discrete body features areas like the anterior insula, which process whole-body patterns and areas like the medial prefrontal cortex, which process emotion concepts. Ben Shalom and Bonneh have suggested a model of the prefrontal cortex, in which medial BA 9 integrates emotional states, and lateral BA 9 performs selection/inhibition on these states. Taken together, the current paper suggests a pathway for emotion processing with at least four stations: areas like the amygdala, which process discrete body features areas like the anterior insula, which process whole-body patterns, medial BA 9 which integrates emotion concepts, and lateral BA 9, which performs selection/inhibition on these concepts. Following the existing literature, it then suggest that there is a significant involvement of the amygdala in psychopathy (Blair), of the anterior insula in alexithymia (Bird), of the medial BA 9 in deficits in somatosensory discrimination (Ben Shalom), and of lateral BA 9 in emotional impulsivity (Ronel).
The current paper can be seen as either an extension of Smith and Lane (2015) model of emotional processing, or as an application of Ben Shalom and Bonneh (2019) model of the prefrontal cortex. Either way, one ends up with a pathway of four stations: the amygdala, insula, medial prefrontal cortex, and lateral prefrontal cortex. Smith and Lane (2015) model of emotion processing talks about three types of emotion representations: Stage 1 (discrete body features), such as in the posterior insula, and presumably the amygdala; Stage 2 (whole body patterns), such as the anterior insula; and Stage 3 (emotion concepts), such as in the medial prefrontal cortex. In other words, it proposes a pathway with at least three consecutive stations: the amygdala, the anterior insula, and the medial prefrontal cortex (Figure 1).
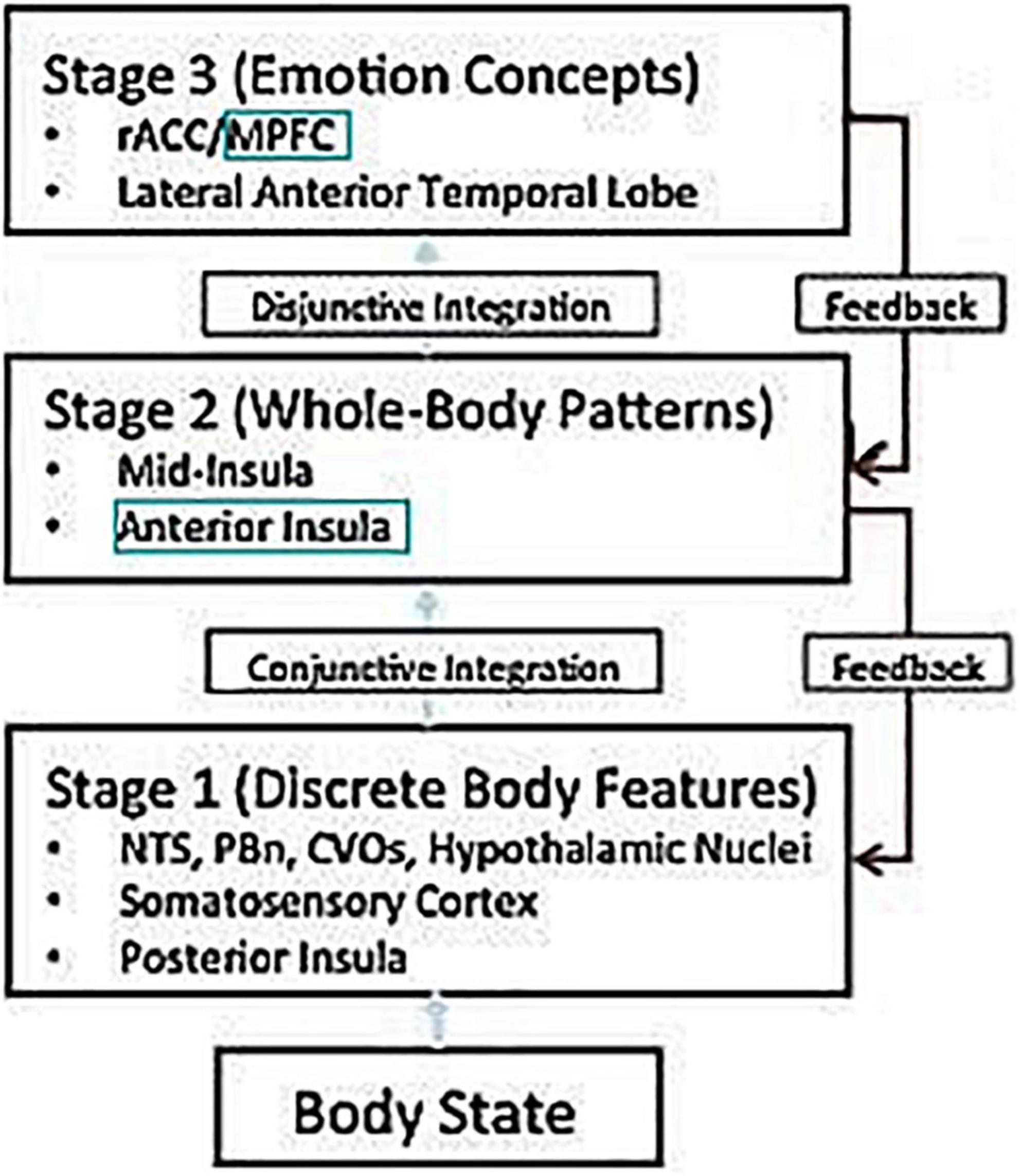
Figure 1. Adapted from Smith and Lane (2015). NTS, nucleus of the solitary tract; PBn, parabrachial nucleus; CVOs, circumventricular organs; rACC, rostral ACC; MPFC, medial prefrontal cortex.
Ben Shalom and Bonneh (2019) suggest a model of the narrow prefrontal cortex (BA 8, 9, 10, 11) in terms of two divisions: horizontal and vertical. But while their horizontal division is traditional (medial vs. lateral), their vertical division is new: four streams of information, from dorsal to ventral (motor, emotion, memory, and sensory). Within each stream, the medial prefrontal cortex integrates basic cognitive objects, while the lateral prefrontal cortex performs selection/inhibition on these objects. In other words, it proposes a pathway with at least two consecutive stations: the medial prefrontal cortex, and the lateral prefrontal cortex (Figure 2).
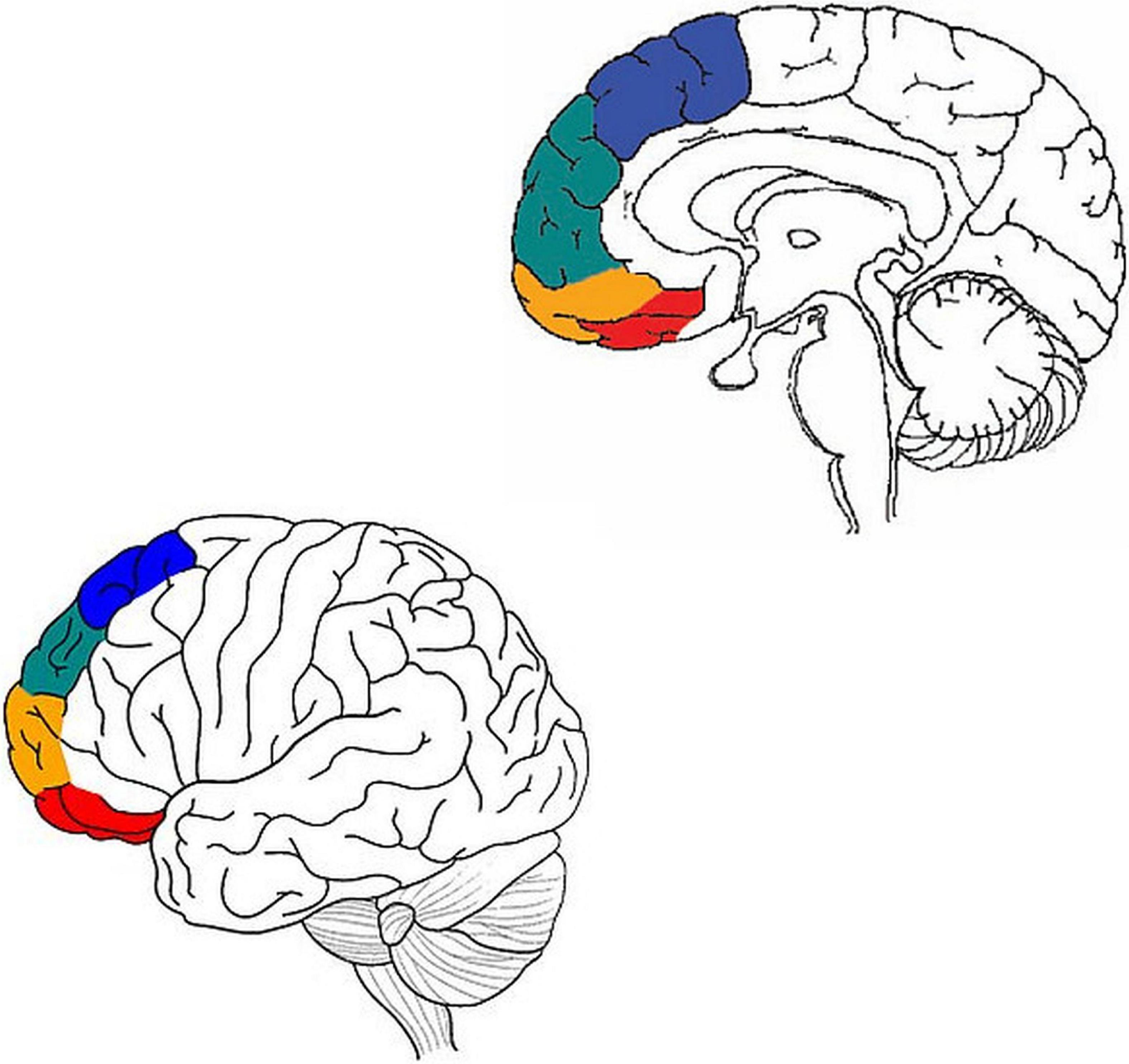
Figure 2. Adapted from Ben Shalom (2009) and Ronel (2018). Motor processing in blue, emotion in green, memory in orange, and sensory in red.
Putting these two models together, one gets a pathway with at least four consecutive stations: Stage 1 (discrete body features), such as in the posterior insula, and presumably the amygdala; Stage 2 (whole body patterns), such as in the anterior insula; Stage 3 (integration of emotion concepts) such as medial BA 9; and Stage 4 (selection/inhibition of emotion concepts), in lateral BA 9.
Psychopathy is a personality disorder characterized by an emotional dysfunction (reduced guilt and empathy) whose antecedents can be identified in a subgroup of young people showing severe antisocial behavior (Hare, 2003). Even though we now know that it correlates with dysfunction in several brain regions (De Brito et al., 2021), it is still accepted that a major defining feature of the disorder is dysfunction of the amygdala (Blair, 2008; Marsh et al., 2013): the amygdala is involved in the formation of both positive and negative stimulus associations. Individuals with psychopathy show impairment in stimulus reinforcement learning (whether positive or negative), which is crucial for learning that some social things are bad to do. As such, these individuals are more likely to learn to use antisocial strategies to achieve their goals. In addition, the reduced amygdala responsivity leads to reduced empathy. Finally, the impairment in positive stimulus learning may relate to the reduced attachment reported in this disorder (Hare, 2003); individuals with psychopathy may find their carers to be less positive stimuli and thus be less motivated to seek their company.
Alexithymia has been described as a subclinical phenomenon marked by difficulties in identifying and describing feelings and difficulties in distinguishing feelings from the bodily sensations of emotion (Bird et al., 2010). The argument for connecting alexithymia to dysfunction of the anterior insula comes from both functional and structural sources (Smith et al., 2020). In terms of _function_, alexithymia is associated with reduced anterior insula activation on several emotional tasks, such as when rating the emotional valence of stimuli from the International Affective Pictures System (Silani et al., 2008), or when observing either emotional facial expressions (Kano et al., 2003; Reker et al., 2010) or the sight of others in pain (Bird et al., 2010; Feldmanhall et al., 2013). In terms of _structure_, alexithymia is associated with reduced anterior insula volume (Borsci et al., 2009; Ihme et al., 2013; Bernhardt et al., 2014), and reduced coherence of the structural connections of the anterior insula. A recent study (Hogeveen et al., 2016) found _acquired_ alexithymia following damage to the anterior insula.
In contrast, there is considerable evidence that the medial prefrontal cortex is involved in the processing of basic conscious feelings. For example, Phan et al. (2002) reviewed 55 PET and fMRI studies of the processing of basic conscious feelings (happiness, fear, anger, sadness, and disgust), and concluded the following: that while every basic feeling has its own associated areas, the one area that was in common to all of them was the medial prefrontal cortex (BA 9/10). Thus, a problem with medical BA 9 would lead to impaired emotion concepts, and a difficulty in reading the anterior insula body maps, even if the body maps themselves are in fact intact.
But the deficit is probably even more general. For example, somatosensory discrimination relates to the discrimination capacities of the tactile and proprioceptive modalities, derived from somatosensory information regarding touch, pressure, vibration, temperature, texture, pain, and the location and movement of body parts (Bröring et al., 2008).
A recent scoping review (Zetler et al., 2019) found that most studies of people with ASD (a disorder proposed to involve the medial prefrontal cortex, Ben Shalom, 2009; Uddin, 2011) showed atypical somatosensory discrimination, especially among young children. In other words, a difficulty in discriminating basic feelings can be a special case of a difficulty in discriminating body states, whether they are emotional or not.
Finally, there is much evidence supporting a relation between lateral BA 9 and emotional impulsivity, or, more generally, emotion regulation. One piece of evidence comes from studies of addiction, which is often assumes to be related to emotional impulsivity. For example, a study by Chen and Mo (2017) compared regional homogeneity in nicotine addicts and control participants. The nicotine addicts had lower regional homogeneity values in a prefrontal area whose peak coordinates were in lateral BA 9. Similarly, a post-mortem analysis of individuals with alcohol use disorder demonstrated that DNA methylation alterations in the lateral BA 9 are associated with (and might result in) increased risk of alcohol use disorders (Wang et al., 2016). Another piece of evidence comes from the study of emotion regulation strategies such as reappraisal and suppression (Ronel, 2018): Compared to passive viewing conditions, both reappraisal (Xiong et al., 2013; Hallam et al., 2014; Rabinak et al., 2014), as well as suppression (Hallam et al., 2014), were found to show greater brain activation in lateral BA 9. In addition, two different meta-analyses have been used to examine fMRI studies of emotion regulation. Buhle et al. (2014) found that reappraisal consistently activated lateral BA 9; Frank et al. (2014) found that such reappraisal was accompanied by increased activation in lateral BA 9 together with reduced activation in the amygdala.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
DB wrote the manuscript, contributed to the article, and approved the submitted version.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ben Shalom, D. (2009). The medial prefrontal cortex and integration in autism. Neuroscientist 15, 589–598.
Ben Shalom, D., and Bonneh, Y. S. (2019). Editorial: The medial prefrontal cortex and integration in ASD and typical cognition. Front. Hum. Neurosci. 13:74. doi: 10.3389/fnhum.2019.00074
Bernhardt, B. C., Valk, S. L., Silani, G., Bird, G., Frith, U., and Singer, T. (2014). Selective disruption of sociocognitive structural brain networks in autism and alexithymia. Cereb. Cortex 24, 3258–3267. doi: 10.1093/cercor/bht182
Bird, G., Silani, G., Brindley, R., White, S., Frith, U., and Singer, T. (2010). Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain 133, 1515–1525. doi: 10.1093/brain/awq060
Blair, R. J. (2008). The amygdala and ventromedial prefrontal cortex: Functional contributions and dysfunction in psychopathy. Philos. Trans. R. Soc. Lond. Series B. Biol. Sci. 363, 2557–2565.
Borsci, G., Boccardi, M., Rossi, R., Rossi, G., Perez, J., Bonetti, M., et al. (2009). Alexithymia in healthy women: A brain morphology study. J. Affect. Disorder. 114, 208–215.
Bröring, T., Rommelse, N., Sergeant, J., and Scherder, E. (2008). Sex differences in tactile defensiveness in children with ADHD and their siblings. Dev. Med. Child Neurol. 50, 129–133. doi: 10.1111/j.1469-8749.2007.02024.x
Buhle, J. T., Silvers, J. A., Wager, T. D., Lopez, R., Onyemekwu, C., Kober, H., et al. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex 24, 2981–2990.
Chen, H., and Mo, S. (2017). Regional homogeneity changes in nicotine addicts by resting-state fMRI. PLoS One 12:e0170143. doi: 10.1371/journal.pone.0170143
De Brito, S. A., Forth, A. E., Baskin-Sommers, A. R., Brazil, I. A., Kimonis, E. R., Pardini, D., et al. (2021). Psychopathy. Nat. Rev. Dis. Primers 7:49.
Feldmanhall, O., Dalgleish, T., and Mobbs, D. (2013). Alexithymia decreases altruism in real social decisions. Cortex 49, 899–904. doi: 10.1016/j.cortex.2012.10.015
Frank, D. W., Dewitt, M., Hudgens-Haney, M., Schaeffer, D. J., Ball, B. H., Schwarz, N. F., et al. (2014). Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 45, 202–211.
Hallam, G. P., Webb, T. L., Sheeran, P., Miles, E., Niven, K., Wilkinson, I. D., et al. (2014). The neural correlates of regulating another person’s emotions: An exploratory fMRI study. Front. Hum. Neurosci. 8:376. doi: 10.3389/fnhum.2014.00376
Hare, R. D. (2003). Hare Psychopathy Checklist-Revised (PCL-R), 2nd Edn. Toronto: Multi Health Systems.
Hogeveen, J., Bird, G., Chau, A., Krueger, F., and Grafman, J. (2016). Acquired alexithymia following damage to the anterior insula. Neuropsychologia 82, 142–148. doi: 10.1016/j.neuropsychologia.2016.01.021
Ihme, K., Dannlowski, U., Lichev, V., Stuhrmann, A., Grotegerd, D., Rosenberg, N., et al. (2013). Alexithymia is related to differences in gray matter volume: A voxel-based morphometry study. Brain Res. 1491, 60–67.
Kano, M., Fukudo, S., Gyoba, J., Kamachi, M., Tagawa, M., Mochizuki, H., et al. (2003). Specific brain processing of facial expressions in people with alexithymia: An H2 15O-PET study. Brain 126, 1474–1484. doi: 10.1093/brain/awg131
Marsh, A. A., Finger, E. C., Fowler, K. A., Adalio, C. J., Jurkowitz, I. T., Schechter, J. C., et al. (2013). Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J. Child Psychol. Psychiatry 54, 900–910. doi: 10.1111/jcpp.12063
Phan, K. L., Wager, T., Taylor, S. F., and Liberzon, I. (2002). Functional neuro-anatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348. doi: 10.1006/nimg.2002.1087
Rabinak, C. A., MacNamara, A., Kennedy, A. E., Angstadt, M., Stein, M. B., Liberzon, I., et al. (2014). Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress. Anxiety 31, 851–861. doi: 10.1002/da.22243
Reker, M., Ohrmann, P., Rauch, A. V., Kugel, H., Bauer, J., Dannlowski, U., et al. (2010). Individual differences in alexithymia and brain response to masked emotion faces. Cortex 46, 658–667.
Ronel, Z. (2018). The Lateral Prefrontal Cortex and Selection/Inhibition in ADHD. Front. Hum. Neurosci. 12:65. doi: 10.3389/fnhum.2018.00065
Silani, G., Bird, G., Brindley, R., Singer, T., Frith, C., and Frith, U. (2008). Levels of emotional awareness and autism: An fMRI study. Social Neurosci. 3, 97–112.
Smith, R., and Lane, R. D. (2015). The neural basis of one’s own conscious and unconscious emotional states. Neurosci. Biobehav. Rev. 57, 1–29. doi: 10.1016/j.neubiorev.2015.08.003
Smith, R., Steklis, H. D., Steklis, N. G., Weihs, K. L., and Lane, R. D. (2020). The evolution and development of the uniquely human capacity for emotional awareness: A synthesis of comparative anatomical, cognitive, neurocomputational, and evolutionary psychological perspectives. Biol. Psychol. 154:107925. doi: 10.1016/j.biopsycho.2020.107925
Uddin, L. Q. (2011). The self in autism: An emerging view from neuroimaging. Neurocase 17, 201–208. doi: 10.1080/13554794.2010.509320
Wang, F., Xu, H., Zhao, H., Gelernter, J., and Zhang, H. (2016). DNA co-methylation modules in postmortem prefrontal cortex tissues of European Australians with alcohol use disorders. Sci. Rep. 6:19430. doi: 10.1038/srep19430
Xiong, K., Zhang, Y., Qiu, M., Zhang, J., Sang, L., Wang, L., et al. (2013). Negative emotion regulation in patients with posttraumatic stress disorder. PLoS One 8:e81957. doi: 10.1371/journal.pone.0081957
Keywords: amygdala, insula, mPFC, LPFC, emotion
Citation: Ben Shalom D (2022) The amygdala–insula–medial prefrontal cortex–lateral prefrontal cortex pathway and its disorders. Front. Neuroanat. 16:1028546. doi: 10.3389/fnana.2022.1028546
Received: 26 August 2022; Accepted: 09 November 2022;
Published: 24 November 2022.
Edited by:
Basilis Zikopoulos, Boston University, United StatesReviewed by:
Casimiro Cabrera Abreu, Queens University, CanadaCopyright © 2022 Ben Shalom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorit Ben Shalom, ZG9yaXRiQGJndS5hYy5pbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.