
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neuroanat. , 15 October 2021
Volume 15 - 2021 | https://doi.org/10.3389/fnana.2021.767330
This article is part of the Research Topic Brain Imaging for Glycobiology View all 7 articles
 Junko Nio-Kobayashi*
Junko Nio-Kobayashi* Tetsuya Itabashi
Tetsuya ItabashiGalectins are β-galactoside-binding lectins consisting of 15 members in mammals. Galectin-1,-3,-4,-8, and -9 are predominantly expressed in the central nervous system (CNS) and regulate various physiological and pathological events. This review summarizes the current knowledge of the cellular expression and role of galectins in the CNS, and discusses their functions in neurite outgrowth, myelination, and neural stem/progenitor cell niches, as well as in ischemic/hypoxic/traumatic injuries and neurodegenerative diseases such as multiple sclerosis. Galectins are expressed in both neurons and glial cells. Galectin-1 is mainly expressed in motoneurons, whereas galectin-3-positive neurons are broadly distributed throughout the brain, especially in the hypothalamus, indicating its function in the regulation of homeostasis, stress response, and the endocrine/autonomic system. Astrocytes predominantly contain galectin-1, and galectin-3 and−9 are upregulated along with its activation. Activated, but not resting, microglia contain galectin-3, supporting its phagocytic activity. Galectin-1,−3, and -4 are characteristically expressed during oligodendrocyte differentiation. Galectin-3 from microglia promotes oligodendrocyte differentiation and myelination, while galectin-1 and axonal galectin-4 suppress its differentiation and myelination. Galectin-1- and- 3-positive cells are involved in neural stem cell niche formation in the subventricular zone and hippocampal dentate gyrus, and the migration of newly generated neurons and glial cells to the olfactory bulb or damaged lesions. In neurodegenerative diseases, galectin-1,-8, and -9 have neuroprotective and anti-inflammatory activities. Galectin-3 facilitates pro-inflammatory action; however, it also plays an important role during the recovery period. Several ligand glycoconjugates have been identified so far such as laminin, integrins, neural cell adhesion molecule L1, sulfatide, neuropilin-1/plexinA4 receptor complex, triggering receptor on myeloid cells 2, and T cell immunoglobulin and mucin domain. N-glycan branching on lymphocytes and oligodendroglial progenitors mediated by β1,6-N-acetylglucosaminyltransferase V (Mgat5/GnTV) influences galectin-binding, modulating inflammatory responses and remyelination in neurodegenerative diseases. De-sulfated galactosaminoglycans such as keratan sulfate are potential ligands for galectins, especially galectin-3, regulating neural regeneration. Galectins have multitudinous functions depending on cell type and context as well as post-translational modifications, including oxidization, phosphorylation, S-nitrosylation, and cleavage, but there should be certain rules in the expression patterns of galectins and their ligand glycoconjugates, possibly related to glucose metabolism in cells.
Galectins are β-galactoside-binding animal lectins, consisting of 15 members in mammals. Galectins possess carbohydrate recognition domains (CRDs) consisting of approximately 130 amino acids, which contain seven conserved amino acids sequence required for carbohydrate recognition (Figure 1A). Galectins are structurally classified into three groups (Figure 1B) (Hirabayashi and Kasai, 1993): proto type (galectin-1,-2,-5,-7,-10,-11/15,-13,-14,-16), chimera type (galectin-3), and tandem-repeat type (galectin-4,-6,-8,-9,-12). Proto type galectins have one CRD and exist as dimers, whereas chimera type galectin-3 consists of one CRD and a non-lectin N-terminal peptide, inducing galectin-3 self-association to form a pentamer. Tandem-repeat type galectins contain two CRDs with different glycan-binding affinities (Figure 1B).
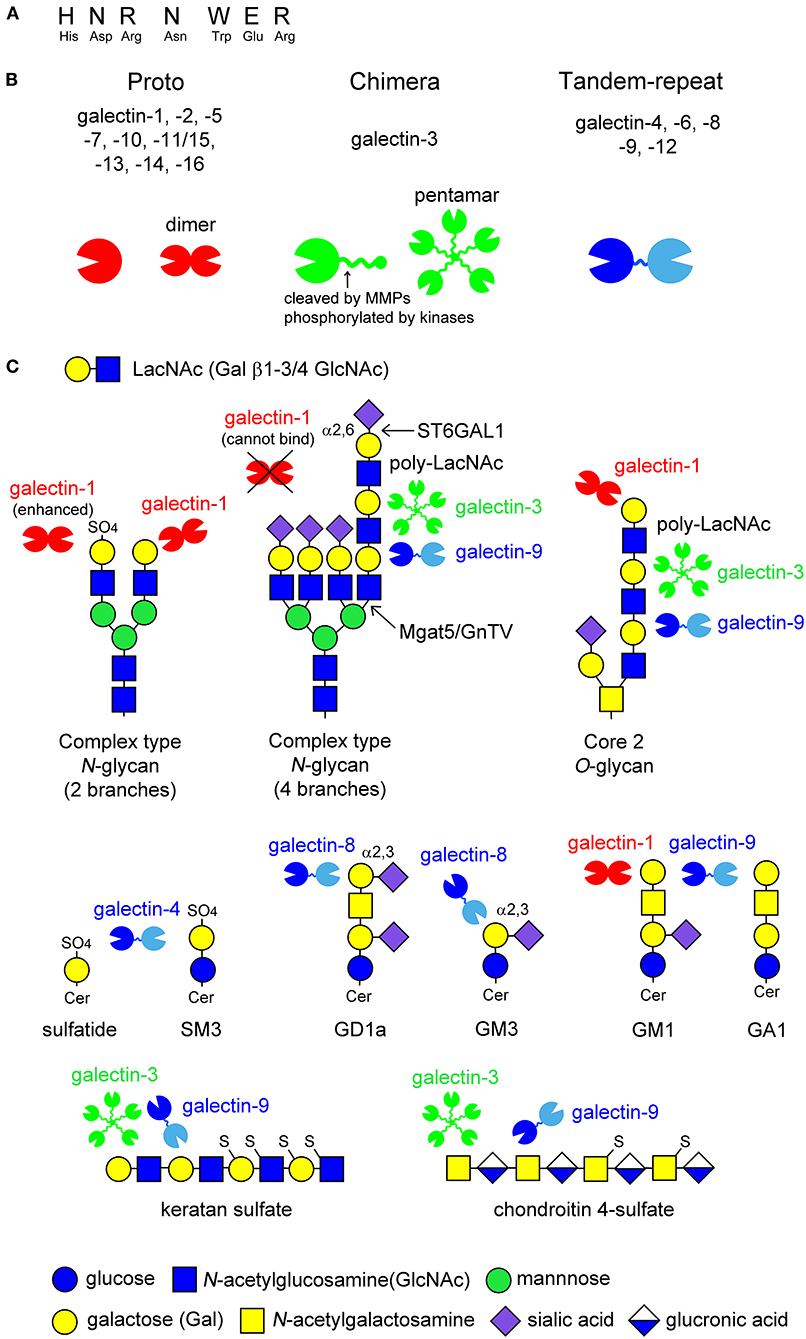
Figure 1. Schematic image of the structure of galectins and the ligand glycans. Galectins contain seven conserved amino acids sequence (A). Fifteen members of galectins are classified into three types: proto type, chimera type, and tandem-repeat type. Proto type galectins have one carbohydrate recognition domain (CRD) and exist as dimers, whereas chimera type galectin-3 consists of C-terminal CRD and non-lectin N-terminal peptide, which can be phosphorylated by kinases or cleaved by proteinases, such as matrix metalloproteinases (MMPs) (arrow in B). N-terminal peptide is involved in self-association to form pentamer. Tandem-repeat type galectins possess two CRDs with different glycan-binding properties, that are linked by short peptide (B). Known glycan structures for each galectin are shown in (C). Galectins recognize N-acetyllactosamine (LacNAc) unit consisting of galactose (Gal) β1-3/4 linked to N-acetylglucosamine (GlcNAc). The binding of galectins to LacNAc is enhanced by 3-O-sulfation (galectin-1) or the formation of poly-LacNAc units (galectin-3 and -9), while α2,6-sialylation on terminal Gal blocks galectin-1-binding. Galectin-4 binds to 3-O-sulfated glycolipids such as sulfatide and SM3. Galectin-8 and -9 have affinity to GD1a and GM3, and GM1 and GA1, respectively. The binding of galectin-1 to ganglioside GM1 seems to depend on its clustering on lipid raft. De-sulfated keratan sulfate and chondroitin 4-sulfate are potential ligands for galectin-3 and -9.
Galectins recognize β-linked galactose (Gal), such as N-acetyllactosamine (LacNAc), consisting of Gal β1-3/4 linked to N-acetylglucosamine (GlcNAc), in cell surface glycoproteins and glycolipids, as well as proteoglycans in the extracellular matrix (Figure 1C). The glycan-binding affinity for each galectin differs depending on the glycan structure and modification of the Gal residue by fucosylation, sialylation, and sulfation (Hirabayashi et al., 2002; Rabinovich and Toscano, 2009). Generally, the binding affinity of galectins is enhanced by increased branching of complex type N-glycans or a repeated LacNAc motif. For example, galectin-1 binds to the LacNAc motif on complex type N-glycans, whereas galectin-3 and -9 have a high affinity for multiple LacNAc units formed on the fourth branch of complex type N-glycans and core 2 type O-glycans (Figure 1C). Modification at the C6 position of Gal, such as α2,6-sialylation catalyzed by β-galactoside α2,6-sialyltransferase 1 (ST6GAL1), inhibits the binding of galectins, such as galectin-1, although galectin-3 and -9 sustains their binding affinity (Figure 1C). In contrast, 3-O-sulfation enhances the galectin-1 binding to LacNAc (Figure 1C). Galectin-4 binds to 3-O-sulfated galactose-carrying glycolipids such as sulfatide and SM3. Galectin-8 and -9 have a high affinity for GD1a and GM3, GM1 and GA1, respectively (Figure 1C). Galectin-1 is also reported to bind to ganglioside GM1; however, its binding seems to depend on its clustering in the membrane microdomains (Figure 1C). De-sulfated keratan sulfate and chondroitin 4-sulfate are also potential ligands for galectin-3 and -9 (Figure 1C).
Since galectins can bind to multiple glycoconjugate ligands in the body as well as microorganisms, they are involved in various physiological and pathological events, including cell proliferation, differentiation, apoptosis, autophagy, and signal transduction regulating development, immune responses, and cancer progression (Liu and Rabinovich, 2005; Yang et al., 2008; Cummings et al., 2017). Galectins exist in the cytoplasm, sensing membrane damage in the cell by the exposure of glycan chains of intraluminal glycans (Thurston et al., 2012; Jia et al., 2020). They are also secreted extracellularly via unknown mechanisms as leaderless secretory proteins because they lack a recognizable signal sequence for their transport into the classical endoplasmic reticulum-Golgi cargo trafficking machinery.
Galectins are broadly distributed in the mammalian body in tissue- and cell-specific manners (Nio-Kobayashi, 2017). Galectins are abundant in the gastrointestinal tract, immune system, and nervous system. This review summarizes the current knowledge of the expression patterns of each galectin in the central nervous system (CNS), and their functions in the regulation of neuronal and glial cell properties, as well as in the pathogenesis of neurodegenerative diseases.
Galectin-1,-3,-4,-8, and -9 are expressed in neurons in the CNS. Hynes et al. (1990) analyzed galectin-1 expression in embryonic and adult rat nervous systems using RNA blot and in situ hybridization analyses. Galectin-1 expression is found in primary sensory neurons in the dorsal root ganglion (DRG) as well as in motoneurons in the anterior horn of the spinal cord and the motor nuclei in the brain stem, including the facial motor nucleus, the trigeminal motor nucleus, and the nucleus ambiguus. In addition, the cerebellar dentate nucleus responsible for the planning, initiation, and control of voluntary movements, and sensory neurons, such as the trigeminal mesencephalic nucleus and the vestibular nuclei, also express galectin-1 (Figure 2A). Other brain regions, including the midbrain, cerebellum, and hypothalamus, contain a much lower expression of galectin-1 mRNA, whereas the cerebral cortex, hippocampus, and corpus striatum express barely detectable levels of galectin-1 mRNA. The galectin-1 mRNA expression is much higher in the DRG and spinal cord than in the brain and is detectable soon after neuronal differentiation (Hynes et al., 1990).
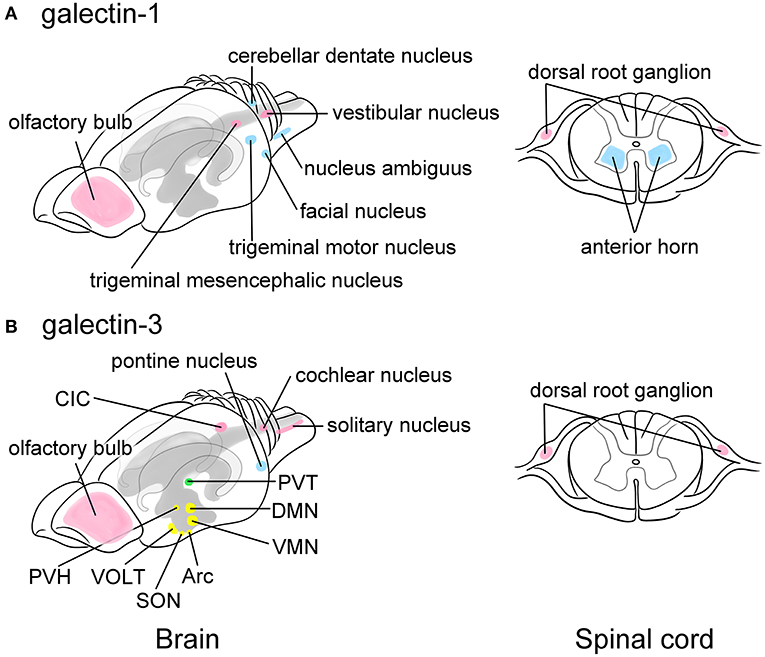
Figure 2. Schematic images showing the predominant localization of galectin-1 (A) and -3 (B) in neurons of the adult rat brain (left) and the spinal cord (right). Galectin-1 is predominantly localized to motoneurons or neurons controlling voluntary movements such as the facial nucleus, the trigeminal motor nucleus, the nucleus ambiguus, and the cerebellar dentate nucleus in the brain stem as well as the anterior horn of the spinal cord (blue in A). Certain sensory neurons, including the trigeminal mesencephalic nucleus and the vestibular nucleus in the brain and the neurons in the dorsal root ganglion (DRG) also contain galectin-1 (pink in A). Galectin-3 is abundant in the arcuate nucleus (Arc) and the dorsomedial nucleus (DMN), and moderately expressed in the ventromedial nucleus (VMN), the supraoptic nucleus (SON), and the paraventricular nucleus (PVH) in the hypothalamus that play central role in the regulation of appetite control, endocrine/autonomic system, and stress response (yellow in B). Galectin-3 is also present in the vascular organ of the lamina terminalis (VOLT), one of the sensory circumventricular organs (yellow in B). The paraventricular nucleus in the thalamus (PVT) related to the emotion also highly expresses galectin-3 (green in B). Other nuclei such as the central nucleus of inferior colliculus (CIC), the cochlear nucleus, and the solitary nucleus also express galectin-3 (pink in B), suggesting its role in the regulation of taste and hearing sensing. Similar to galectin-1, the primary sensory neurons in the DRG express galectin-3 (pink in B). The pontine nucleus regulating voluntary movements also contains abundant galectin-3 (blue in B). Olfactory bulb expresses both galectins (pink in A and B). Motoneurons and neurons involved in movement are represented in blue, sensory-related neurons are in pink, and the neurons regulating endocrine/autonomic functions are indicated in yellow, and nucleus related to higher brain function is labeled in green. The cerebral ventricles are indicated in gray and the hippocampus is bordered in light gray. This figure is created based on the in situ hybridization data by Hynes et al. (1990) for galectin-1 and immunohistochemical data by Yoo et al. (2017) for galectin-3. For detailed localization of galectins in the rat brain, please refer their manuscripts. For data of the mouse and human brain, please visit Allen Brain Map (https://portal.brain-map.org).
Although mRNA expression remains unclear in the rat brain, Yoo et al. (2017) immunohistochemically revealed that galectin-3 is broadly distributed in neurons of adult rat brain (Figure 2B), especially in the hypothalamus, which plays a central role in the regulation of endocrine/autonomic regulation, stress response, and appetite control. In the hypothalamus, the arcuate nucleus and the dorsomedial nucleus abundantly contain galectin-3. The supraoptic nucleus, the paraventricular nucleus, and the ventromedial nucleus in the hypothalamus also express moderate level of galectin-3. Notably, galectin-3 expression is found in the vascular organ of the lamina terminalis (VOLT), one of the sensory circumventricular organs (CVOs) (Figure 2B). The sensory CVOs, which lack the blood-brain barrier, consist of subfornical organs and area postrema in addition to VOLT, monitoring the conditions of both the blood and the cerebrospinal fluids, as well as various sensory stimulations such as light, smell, and noise. The paraventricular nucleus in the thalamus, which is involved in emotion-related behaviors, contains high level of galectin-3. Intense immunoreactivities for galectin-3 are found in the central nucleus of inferior colliculus and the cochlear nucleus, as well as in the solitary nucleus, suggesting its role in the regulation of auditory and taste sensation. The pontine nucleus involved in motor activity is also immunoreactive for galectin-3. In contrast, the subcortical nuclei responsible for controlling voluntary motor functions and the limbic system exhibited no galectin-3 immunoreactivity. Note that in the mouse brain galectin-3 mRNA is not likely expressed in neurons according to the data from Allen Brain Atlas (https://mouse.brain-map.org).
The galectin-4 expression in the rat brain was analyzed by western blot analysis (Stancic et al., 2012; Velasco et al., 2013). Galectin-4 is expressed in the rat brain during the early postnatal developmental stage, decreases on days 12–16 onwards, being consistent with the onset of myelination, and becomes undetectable in adults (Stancic et al., 2012). Galectin-4 is localized to the cerebral cortex, hippocampal formation, thalamus, and corpus callosum, where both neurons and oligodendrocytes are immunoreactive (Stancic et al., 2012; Velasco et al., 2013). Interestingly, galectin-4 displays a segmented distribution in axons of cultured mature neurons (Velasco et al., 2013), which is involved in the transport of axonal glycoproteins and promotion of axonal growth but prevention of myelination.
Galectin-8 is expressed in the thalamus, choroid plexus, and weakly in the hippocampus and cortex in mice. Galectin-8 is produced by primary cultured hippocampal neurons and protects them from damaging conditions such as nutrient deprivation, glutamate-induced excitotoxicity, hydrogen peroxide-induced oxidative stress, and amyloid β (Aβ) aggregation (Pardo et al., 2019). John and Mishra (2016) carried out mRNA transcriptomics of galectins using the Allen Brain Atlas in young adult mouse brains and microarray data of the human brain. Galectin-9 is broadly distributed in the mouse brain, including the cerebral cortex, olfactory bulb, basal ganglia, hippocampus, thalamus, hypothalamus, amygdala, cerebellum, and substantia nigra, whereas galectin-1 is almost ubiquitously expressed in the human brain. The distribution of galectin-8 is similar between mice and humans and is expressed in the limbic region related to learning, memory, and emotions, and the substantia nigra for motor movements. They also noted the expression of other galectins such as galectin-2,-7 and -12 in the mouse and human brain.
The olfactory bulb transmits smell information from the nose to the brain and receives information from the amygdala, neocortex, hippocampus, locus coeruleus and substantia nigra for odorant/chemical awareness, and predominantly express galectin-1 and -3 (Figure 2). Mahanthappa et al. (1994) first demonstrated the localization of galectin-1 in non-neuronal cells in the olfactory bulb of developing mice, suggesting its function in promoting olfactory axon fasciculation. Prof. Brian Key et al. have extensively studied the expression and function of galectin-1 and -3 in the olfactory system (Puche and Key, 1995; Puche et al., 1996; Tenne-Brown et al., 1998; St John and Key, 1999; Storan et al., 2004). In mice lacking the Lgals1 gene, which encodes galectin-1, a subset of primary sensory olfactory axons fails to project to their correct target sites in the caudal olfactory bulb (Puche et al., 1996). Galectin-1 is also expressed in periglomerular cells and granule cells in the ventromedial region of adult mice (St John and Key, 1999). In addition, galectin-4,-7, and -8 are expressed by primary olfactory axons as they grow from the nasal cavity to the olfactory bulb (Storan et al., 2004). Galectins in the olfactory system have neurite outgrowth-promoting activity and play a role in neuronal pathfinding through the rostral migratory stream from the neural stem/progenitor cell niche, as described below.
Glial cells also express multiple galectins. Astrocytes are supporting cells in the CNS, surround the blood-brain barrier and synapse, and proliferate following brain injury to form a glial scar (Figure 3A). Astrocytes predominantly contain galectin-1, and activated astrocytes express galectin-3 and -9 (Sasaki et al., 2004; Yang et al., 2012; Steelman et al., 2013). Sasaki et al. (2004) reported that galectin-1 promoted astrocyte differentiation, but inhibited proliferation. Galectin-1 is remarkably upregulated in activated astrocytes around ischemic infarct, inhibiting astrocyte proliferation to downregulate astrogliosis (Qu et al., 2011). Similarly, galectin-1 suppresses microglial activation and protects against neural damage caused by inflammation (Starossom et al., 2012). The galectin-1 expression is also found in activated astrocytes and activated microglia in the injured rat spinal cord (Gaudet et al., 2015). Galectin-9 is not expressed in astrocytes under normal conditions, whereas its expression is increased by tumor necrosis factor α (TNFα) as well as interleukin (IL)-1β and interferon γ (IFNγ) stimulation in primary cultured astrocytes (Steelman et al., 2013).
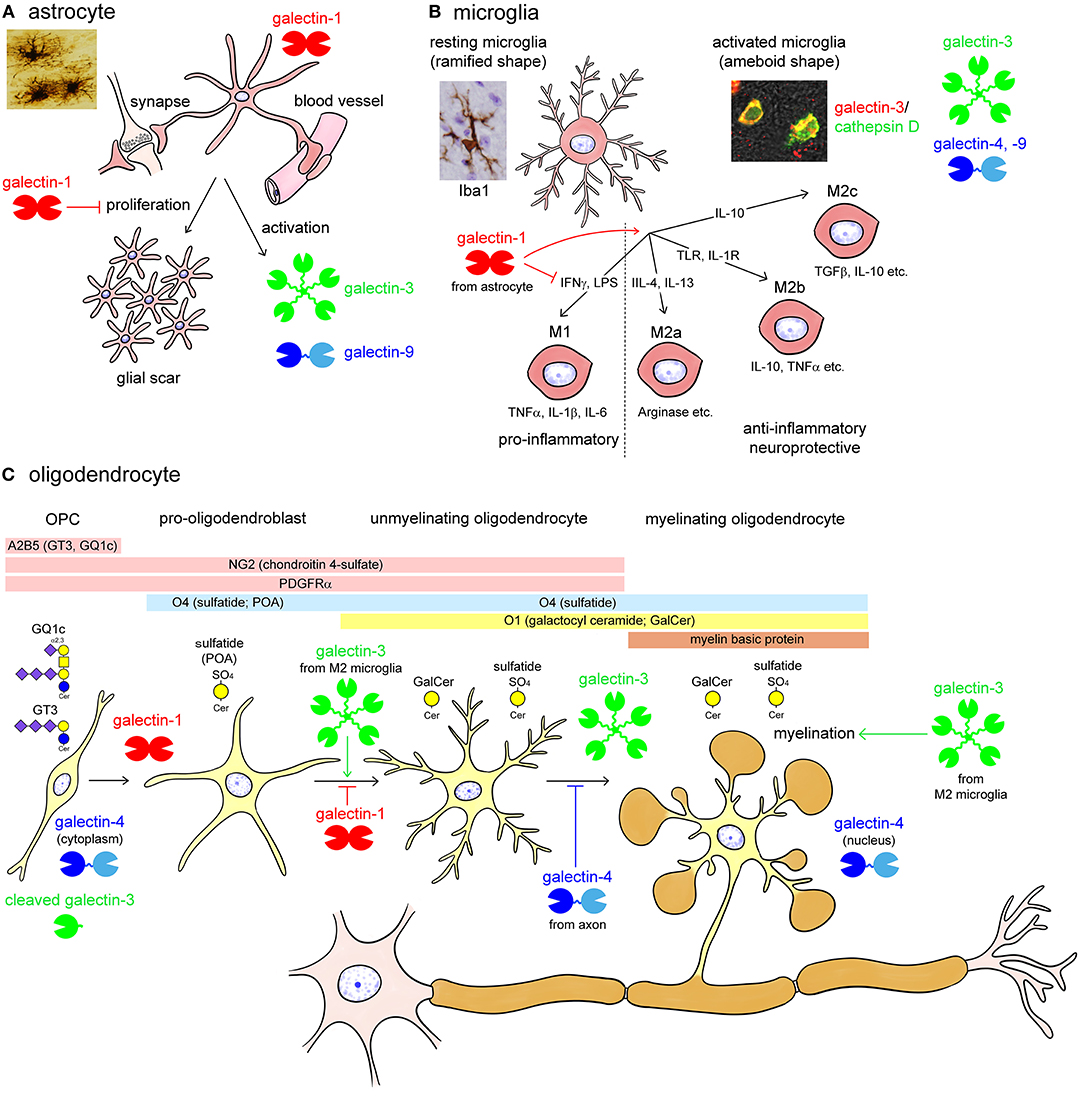
Figure 3. Summary images of galectin expression in glial cells (A; astrocytes, B; microglia, and C; oligodendrocytes) in the central nervous system (CNS). Astrocytes are supporting cells and surround the synapse and the blood vessels forming the blood-brain barrier in the CNS (A). Astrocytes predominantly contain galectin-1 and activated astrocytes express galectin-3 and -9 (A). Astrocytes proliferate in response to brain injury, forming a glial scar. Galectin-1 inhibits the proliferation and promotes differentiation of astrocytes, protecting neural function (A). Microglia is responsible for controlling neural inflammation (B). Resting microglia with elongated fine processes, so-called ramified-shaped microglia, activated by various stimuli including interferon γ (IFNγ), lipopolysaccharides (LPS), interleukin (IL)-4, IL-13, toll-like receptor (TLR) and IL-1 receptor stimulation, and IL-10 into pro-inflammatory M1 phenotype and anti-inflammatory, neuroprotective M2 (M2a, M2b, and M2c) phenotypes with ameboid shape (B). Activated microglia abundantly contain galectin-3, and also express galectin-4 and -9. On the contrary, galectin-1 de-activates M1 type microglia, facilitating the shift from M1 to M2 phenotype (B). Oligodendrocytes are myelinating cells in the CNS and are differentiated from oligodendrocyte precursor cells (OPC) reactive for monoclonal antibody (mAb) A2B5, which recognizes c-series gangliosides such as GT3 and GQ1c (C). OPCs are also immunoreactive for chondroitin 4-sulfate-recognizing NG2 antibody and express platelet-derived growth factor receptor α (PDGFRα) (C). Pro-oligodendroblasts contain sulfatide recognized by mAb O4 in addition to the OPC markers, NG2 and PDGFRα, and differentiated into oligodendrocytes, which contain galactosyl ceramide (GalCer) reactive for mAb O1. O4 recognizes sulfatide with short-chain fatty acids in pro-oligodendroblasts, called pro-oligodendroblast antigen (POA). Myelinating oligodendrocytes contain myelin basic protein forming myelin membrane around the axon. Galectin-1 is expressed in O1-negative OPC and pro-oligodendroblasts whereas galectin-3 is abundant in differentiated oligodendrocytes (C). Galectin-4 localizes to the cytoplasm of OPC whereas in the nuclei of oligodendrocytes (C). Galectin-1 and axonal galectin-4 inhibit the differentiation of oligodendrocytes; however, galectin-3, possibly from activated M2 type microglia, promotes oligodendrocyte differentiation and myelination (C). Note that galectin-3 expressed in OPC is cleaved by a matrix metalloproteinase. TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α.
Microglia are yolk sac-derived resident macrophages that play an important role in the regulation of immune responses in the CNS. Resting microglia show a ramified shape with elongated fine processes and disperse in the CNS (Figure 3B). Microglia are activated in response to CNS injury, and several phenotypes of activated microglia, also called ameboid-shaped microglia, have been reported so far: M1 type microglia produce pro-inflammatory cytokines such as TNFα, IL-1β, and IL-6. However, M2 (M2a, b, c) type microglia actively phagocytose cellular debris and secrete anti-inflammatory cytokines, including IL-10 and transforming growth factor β, as well as neurotropic molecules, such as insulin-like growth factor 1 (IGF1), promoting convergence of inflammation, synapse reconstitution, and remyelination (Figure 3B). Activated but not resting microglia contain abundant galectin-3. Galectin-3 is upregulated in both M1 and M2 type activated microglia (Figure 3B). Galectin-3 expression in microglia is enhanced by ischemic conditions or traumatic brain injury (Walther et al., 2000; Lalancette-Hébert et al., 2007; Yan et al., 2009; Venkatesan et al., 2010; Wesley et al., 2013; Yip et al., 2017; Rahimian et al., 2018; Wang et al., 2019). Treatment with trimethyltin, an organotin neurotoxicant, also induces galectin-3 expression in microglia and astrocytes in the hippocampal dentate gyrus (HDG) (Yang et al., 2012). Although galectin-3 has pro-inflammatory action, it also facilitates phagocytic activity and promotes neural recovery and post-ischemic tissue remodeling (Lalancette-Hébert et al., 2007). Galectin-9 is also reported to be increased in microglia following poly (I:C) stimulation (Steelman and Li, 2014). Although galectin-4 is virtually negligible in both microglia and astrocytes under normal conditions (Stancic et al., 2012), it is expressed in activated microglia in the brain and spinal cord of cuprizone-induced demyelination models and multiple sclerosis lesions (de Jong et al., 2018).
Oligodendrocytes are myelinating cells in the CNS equivalent to Schwann cells in the peripheral nervous system (PNS). Oligodendrocytes are produced from oligodendrocyte precursor cells (OPC), which are reactive for the monoclonal antibody (mAb) A2B5. This antibody recognizes c-series gangliosides such as GT3 and GQ1c (Farrer and Quarles, 1999; Saito et al., 2001). OPC are also immunoreactive for the chondroitin 4-sulfate-recognizing NG2 antibody, and express platelet-derived growth factor receptor α (PDGFRα). Pro-oligodendroblasts differentiated from OPC are immunoreactive for the mAb O4, which recognizes sulfatide, and differentiated into oligodendrocytes that contain galactosyl ceramide reactive for the mAb O1 (Sommer and Schachner, 1981; Bansal et al., 1992). Then, they are finally differentiated to myelinating oligodendrocytes, expressing myelin basic protein (MBP) to form myelin membranes around the axon (Figure 3C). Galectin-1 is expressed in A2B5/PDGFRα/O4-positive OPC and pro-oligodendroblasts but not in O1-positive oligodendrocytes in the primary culture of murine oligodendrocytes. In contrast, galectin-3 is upregulated during oligodendrocyte differentiation (Figure 3C) (Pasquini et al., 2011). Galectin-3 appears to be cleaved in OPC by matrix metalloproteinase 2 (MMP2), which results in a CRD peptide lacking the ability to form oligomers but having increased carbohydrate-binding activity (Figure 3C) (Ochieng et al., 1994; Gao et al., 2017). Both OPCs and mature oligodendrocytes contain galectin-4, with differential immunohistochemical localization: galectin-4 is present in the cytoplasm of OPC but accumulates in the nucleus of mature oligodendrocytes (Figure 3C) (Stancic et al., 2012). Extracellularly added galectin-3 promotes oligodendrocyte differentiation and myelination, whereas galectin-1 inhibits oligodendrocyte differentiation, suggesting that galectin-1 and -3 exert contrasting effects during the process of oligodendrocyte differentiation (Figure 3C) (Pasquini et al., 2011). Galectin-4 is released from neurons but not from oligodendrocytes, and inhibits myelination by binding to premyelinating oligodendrocytes, determining the timing of myelination (Figure 3C) (Stancic et al., 2012; Díez-Revuelta et al., 2017).
Galectin-1 and -3 are expressed in olfactory ensheathing cells; glial cells resemble Schwann cells in the PNS in ventromedial and lateral surfaces of the olfactory bulb in developing mice (Tenne-Brown et al., 1998; St John and Key, 1999). Ependymal cells in the subventricular zone (SVZ), where neural stem/progenitor cells are present, contain galectin-3 (Comte et al., 2011; Pasquini et al., 2011), and galectin-8 is expressed in the choroid plexus and secreted into the cerebrospinal fluid to protect neurons and suppress immune responses (Pardo et al., 2019).
Galectin-1,-3, and -4 are reported to be involved in the determination of axon fate, axon growth, guidance and regeneration, and polarized axonal glycoprotein transport (Figure 4A) (Higuero et al., 2017). Recombinant galectin-1 promotes neurite outgrowth of the cultured olfactory neuron (Puche et al., 1996). The oxidized form of galectin-1, which lacks lectin activity, stimulates neurite outgrowth (Outenreath and Jones, 1992; Horie et al., 1999; Inagaki et al., 2000), and there is convincing evidence showing that galectin-1 promotes neurite outgrowth and axonal regeneration in both the CNS and the PNS (Camby et al., 2006). Galectin-1 induces the repolymerization of F-actin in both growth cones and filopodium by inhibiting hydrogen oxide production and promoting axonal regeneration (Quintá et al., 2016). Similarly, microglial galectin-3 promotes neurite outgrowth in DRG explants (Pesheva et al., 1998), and phosphorylated galectin-3 promotes axon branching in cultured hippocampal neurons by local actin destabilization (Díez-Revuelta et al., 2010). Galectin-4 is sorted into discrete segments of the axonal membrane in a microtubule- and sulfatide-dependent manner and promotes axonal growth by clustering axon growth-promoting molecules, such as the neural cell adhesion molecule (NCAM) L1 (Velasco et al., 2013). Taken together, it is conceivable that galectin-1,-3, and -4 promote neurite outgrowth and branching, possibly binding to different partners, and post-translational modifications such as oxidization and phosphorylation are important for the neuroprotective function of galectins.
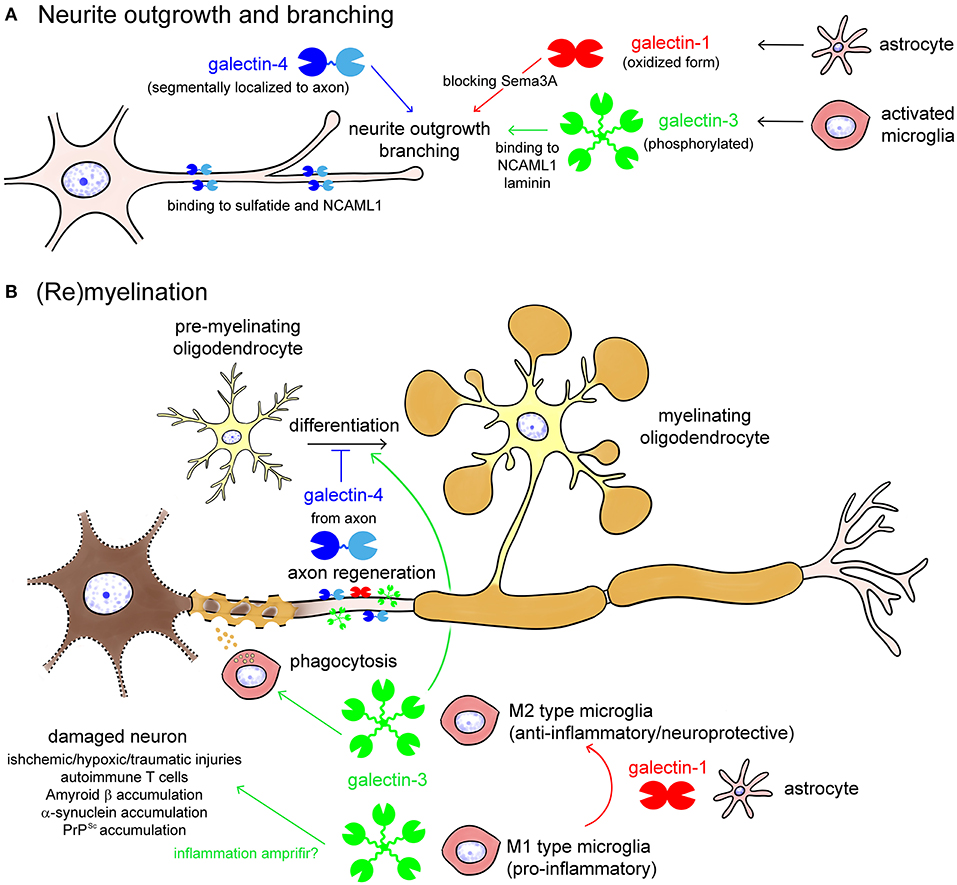
Figure 4. Galectins in neurite outgrowth and branching (A) as well as (re)myelination (B). Galectin-1 from astrocytes and galectin-3 from activated microglia promote neurite outgrowth and branching, by blocking Semaphorin 3A (Sema3A) pathway or binding to neural cell adhesion molecule L1 (NCAML1) and laminin, respectively (A). Galectin-4 is segmentary localized to the axon by binding to sulfatide and NCAML1, regulating axonal growth and branching (A). Oxidization and phosphorylation of galectin-1 and galectin-3, respectively, enhances neuroprotective activities of them (A). In damaged neurons, galectin-3 from M1 type microglia enhances inflammation, whereas galectin-3 from M2 type microglia promotes phagocytic cleaning and oligodendrocyte differentiation (B). Galectin-1, possibly from astrocytes, induces shift from the M1 to M2 phenotype of activated microglia and oligodendrocyte differentiation. Galectin-4 from regenerating axon inhibits maturation of pre-myelinating oligodendrocyte to determine the timing of (re)myelination.
Galectin-1,-3, and -4 are also involved in (re)myelination in different ways (Figure 4B) (Rinaldi et al., 2016a; Thomas and Pasquini, 2018; de Jong et al., 2020). Galectin-3 secreted from M2 type activated microglia accelerates phagocytic cleaning of damaged cell debris and oligodendrocyte differentiation, whereas galectin-4 suppresses (re)myelination until appropriate axonal regeneration is completed (Figure 4B). Galectin-1, possibly secreted from astrocytes, promotes (re)myelination via shifting microglia phenotype from M1 to M2 (Figure 4B).
Recent studies indicate that the adult brain contains neural stem cells that continuously generate new neurons as well as glial cells, such as oligodendrocytes. Neurogenesis in the adult brain can be observed in the SVZ and the subgranular layer of the HDG (Figure 5A). The SVZ of the lateral wall of the lateral ventricles in adult mice is composed of astrocytic neural stem cells (type B1 cells), astrocytes (type B2 cells), transit amplifying cells (type C cells), and neuroblasts (type A cells) (Figure 5B) (Doetsch et al., 1997). Type B1 cells are neural stem cells that contain glial fibrillary acidic protein (GFAP), which differentiate into type C cells, followed by type A neuroblasts to generate new neurons (Figure 5B). Produced neurons migrate to the olfactory bulb through glial tubes formed by astrocytes, namely the rostral migratory stream (RMS), and they differentiate into interneurons such as periglomerular cells and granule cells in the olfactory bulb (Figure 5A). The SVZ also provides newly generated neurons to the damaged brain regions, such as infarct lesions induced by middle cerebral artery occlusion.
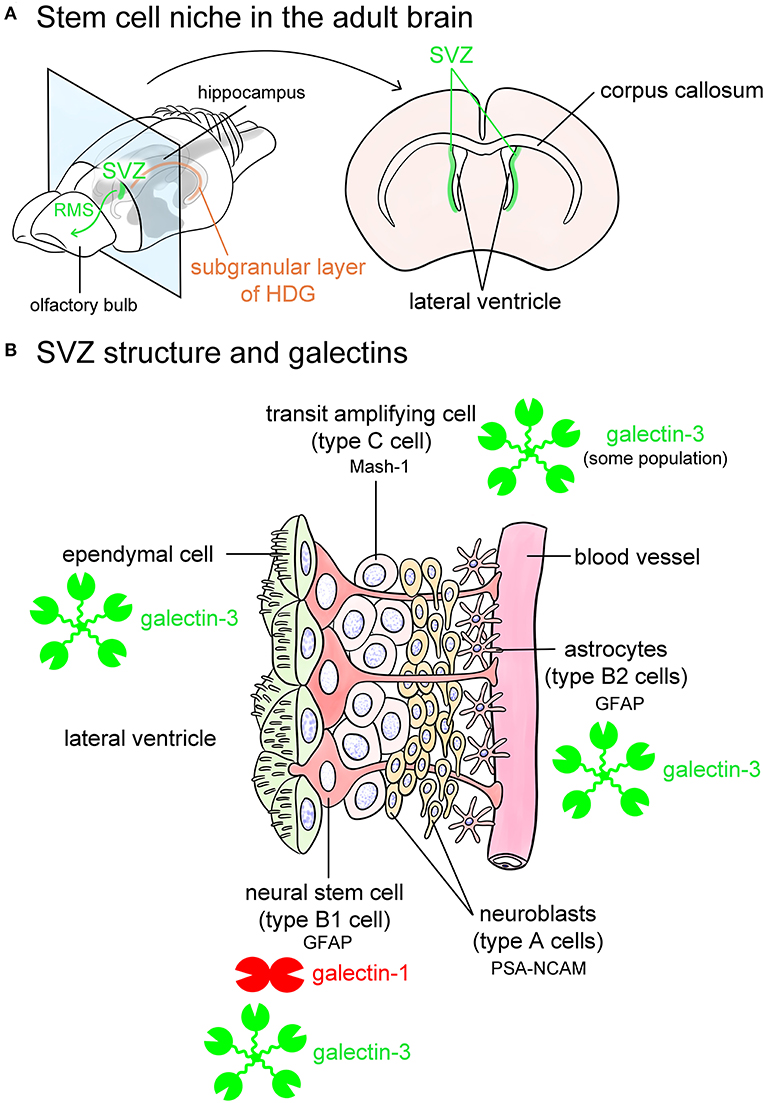
Figure 5. Galectins in the stem cell niches. Known stem cell niches in the adult brain are the subventricular zone (SVZ) (green in A) and subgranular layer of hippocampal dentate gyrus (HDG) (orange in A). The SVZ is located in the lateral wall of the lateral ventricles in the adult mouse brain (A). Newly produced neurons in the SVZ migrate through rostral migratory stream (RMS) to the olfactory bulb (green arrow in A) at where they differentiate into interneurons such as periglomerular cells and granule cells, and also migrate to damaged lesions. The SVZ beneath ependymal cells is composed of glial fibrillary acidic protein (GFAP)-positive astrocytic neural stem cells (type B1 cells), astrocytes (type B2 cells) located along the blood vessels, mammalian achaete-scute homolog 1 (Mash-1)-positive transit amplifying cells (type C cells), and neuroblasts (type A cells) (B). Type B1 neural stem cells express galectin-1, and possibly galectin-3. Some population of type C cells contains galectin-3 (B). Galectin-3 is also abundant in ependymal cells and type B2 astrocytes (B). Based on the researches by Sakaguchi et al. (2006), Comte et al. (2011), and Pasquini et al. (2011). PSA-NCAM, polysialylated form of neural cell adhesion molecule.
SVZ contains galectin-1 and -3 (Sakaguchi et al., 2006; Pasquini et al., 2011; Hillis et al., 2016; James et al., 2016). Galectin-1 is expressed in certain populations of GFAP-positive type B1 cells, namely neural stem cells in the SVZ (Sakaguchi et al., 2006). Comte et al. (2011) reported that galectin-3 is expressed in GFAP-positive astrocytes but not in neuroblasts in the SVZ. In contrast, Pasquini et al. (2011) insisted that galectin-3 immunoreactivity is negative in GFAP-positive type B1 neural stem cells. Galectin-3 is also abundant in RMS. Galectin-3 normally limits SVZ cell migration following cuprizone treatment (Hillis et al., 2016). Sirko et al. (2015) reported that both galectin-1 and -3 are increased in neural stem cells and reactive astrocytes in the lesioned brain following a stab wound injury. Neural stem cells in the HDG also contain galectin-1 and -3 (Kajitani et al., 2009; Imaizumi et al., 2011; Yang et al., 2012). Taken together, galectin-1 and -3 contribute to providing a stem cell niche and possibly play differential roles in a neural generation.
Galectin-1 and 3 play important roles in the pathogenesis of neuroinflammatory and neurodegenerative disorders such as multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Alzheimer's disease (AD), Parkinson's disease, Huntington's disease, and prion disease (Barake et al., 2020). Generally, galectin-1,-8, and -9 have anti-inflammatory and neuroprotective functions, but the role of galectin-3 is controversial. Galectin-3 facilitates pro-inflammatory action; however, it also plays an important role during the recovery period.
MS is a demyelinating neurodegenerative disease. Autoimmune T cells play an important role in the pathogenesis of MS. Several animal models for MS have been established, including experimental autoimmune encephalomyelitis (EAE), cuprizone or lysolecithin-induced demyelination models, and Theiler's murine encephalomyelitis virus (TMEV) infection model. The administration of recombinant galectin-1 improves both the clinical and histological scores of EAE (Offner et al., 1990). Galectin-1 has a neuroprotective role via anti-inflammatory action and regulates the microglial phenotype to stimulate re-myelination in EAE mouse model (Rinaldi et al., 2016b). On the contrary, the lack of galectin-3 improves EAE symptoms and reduces leukocyte infiltration, suggesting its pro-inflammatory function (Jiang et al., 2009). Galectin-3 may differentially contribute to the pathogenesis of EAE depending on cell type. In our experiment using a transfer EAE model in which autoimmune T cells were intravenously injected, both activated microglia and Schwann cells in the ventral but not dorsal roots become positive for galectin-3 whose expression patterns differ during progression and recovery periods: galectin-3 in phagocytic microglia elevates during symptom peaks while galectin-3 in Schwann cells appears during the recovery period (Itabashi et al., 2018). In a murine TMEV infection model, the loss of galectin-3 blocked an increase in chemokines after TMEV infection, reduced immune cell migration into the SVZ, reestablished SVZ proliferation, and increased the number of progenitors in the corpus callosum (James et al., 2016). The loss of galectin-8 increases T helper (Th)17 cells but decreases T regulatory cells, suggesting that galectin-8 eliminates activated Th17, but not Th1, and ameliorates EAE (Pardo et al., 2017). TNFα increases galectin-9 expression in astrocytes, inducing apoptosis of encephalitogenic T-cells (Steelman et al., 2013). Increased expression of galectin-1,-3,-8, and -9 has been confirmed in human MS lesions (Stancic et al., 2011), and the concentration of anti-galectin-1 autoantibodies is increased in the serum of MS patients (Lutomski et al., 1997). The cerebrospinal fluid of MS patients contains the anti-galectin-8 antibody, and its increase is related to relapse (Pardo et al., 2017).
ALS is a progressive neurological disorder in which motor neurons are selectively impaired in the brain and spinal cord. Galectin-1,-3, and -9 are increased in SOD1G93A mice, an animal model of ALS and the spinal cord, cerebrospinal fluids, and serum of ALS patients (Zhou et al., 2010; Lerman et al., 2012; Peters et al., 2015; Ashraf and Baeesa, 2018). Galectin-1 is found in a component of the neurofilamentous lesions in ALS patients (Kato et al., 2001) and localizes to swollen motor axons and astrocytes in SOD1G93A mice (Kobayakawa et al., 2015). Chang-Hong et al. (2005) reported that oxidized galectin-1 improves the clinical symptoms of SOD1G93A mice, suggesting its neuroprotective role. Galectin-3 is increased in microglia in the spinal cord of ALS patients and phagocytosing damaged γ-synuclein-positive neurons (Peters et al., 2015). Galectin-3-knockout mice have severe clinical significance, suggesting a protective role of galectin-3 in neural damage in ALS (Lerman et al., 2012).
AD is caused by abnormal accumulation of Aβ and tau proteins in the brain, damaging neurons and affecting gradual brain degeneration and atrophy. Galectin-3 is highly upregulated in microglia associated with Aβ plaques in the brains of AD patients and the familial AD model, 5xFAD mice (Boza-Serrano et al., 2019). Since single-nucleotide polymorphisms in the galectin-3-encoding LGALS3 gene being associated with an increased risk of AD and galectin-3 deletion decreasing Aβ burden in 5xFAD mice, whereas injected galectin-3 promotes aggregation of insoluble Aβ, galectin-3 may accelerate AD progression (Boza-Serrano et al., 2019). In Aβ-induced neuroinflammation in the hippocampus, galectin-3 expression is increased in microglia and astrocytes (Ramírez et al., 2019). In an animal model of AD, APP/PS1 mice, galectin-3 overexpression enhanced the accumulation of Aβ oligomerization (Tao et al., 2020). In mice lacking galectin-3, the Aβ-degrading enzyme neprilysin is increased, showing better acquisition and retention performance in the water maze (Tao et al., 2020). Galectin-3 is reported to be increased in the frontal lobe in association with Aβ oligomerization (Tao et al., 2020) and in the serum of AD patients (Wang et al., 2015). Galectin-8 regulates autophagy by binding to NDP52 (Thurston et al., 2012), and inhibition of galectin-8 promotes tau aggregation in cultured cells (Falcon et al., 2018).
Few reports show the involvement of galectins in Parkinson's and Huntington's diseases, which are caused by a loss of dopamine neurons in the substantia nigra, inducing progressive movement disorders such as tremor, stiff, and inflexible muscles. Galectin-1 plays an anti-inflammatory function and suppresses neuronal degeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease model (Li et al., 2020). Galectin-3 is involved in microglial activation by accumulating α-synuclein, which induces the loss of dopamine neurons (Boza-Serrano et al., 2014). The serum concentrations of galectin-3 and -4 are increased, but galectin-1 in the cerebrospinal fluids is decreased in patients with Parkinson's disease (Cengiz et al., 2019; Marques et al., 2019; Yazar et al., 2019). Galectin-3 is also increased in the serum of patients with Huntington's disease (Siew et al., 2019).
Prion diseases are infectious and fatal neurodegenerative diseases, including scrapie in sheep and goats, bovine spongiform encephalopathy in cows, and Creutzfeldt-Jakob disease in humans. Prion diseases are caused by the accumulation of PrPSc, which is an abnormal and relatively proteinase K-resistant isoform of cellular prion protein PrPC, inducing gradual neuron loss and astrocytosis, inducing transmissible spongiform encephalopathies. Galectin-3 is identified as one of the upregulated genes in prion disease (Riemer et al., 2004), which increased in activated microglia in the brains of scrapie-infected mice, contributing to disease development by regulating lysosomal function and autophagy (Jin et al., 2007; Mok et al., 2007). Galectin-1, especially the S-nitrosylated form of galectin-1, is upregulated in the brains of scrapie-infected rodents and human prion diseases, where astrocytes and neurons, but not microglia, are immunoreactive for galectin-1 (Guo et al., 2017).
Galectin-1 and -3 knockout mice exhibit impaired stress-coping and increased compulsive-like behavior, implying that galectin-1 and -3 are related to depression and obsessive-compulsive-like behaviors (Sartim et al., 2020). Serum galectin-3 level is elevated in association with higher levels of depressive symptoms (King et al., 2021). Recent studies noted an involvement of galectins, especially galectin-3 and -8 in schizophrenia (Kajitani et al., 2017; Petralia et al., 2021). Although information about galectins in mental illness such as depression, schizophrenia, and autism is limited, galectins are likely involved in etiology of these disease.
Several ligand glycoconjugates for galectins in the CNS have been identified and summarized in Table 1. They include molecules that regulate neurite outgrowth and branching, myelination, cell-extracellular matrix adhesion, and inflammatory responses.
Galectins are distributed both in neurons and glial cells, regulating their growth, differentiation, and function in different ways. They are also involved in the pathogenesis of neuroinflammatory and neurodegenerative diseases in the CNS. Generally, galectin-1,-8, and -9 act as neuroprotective and anti-inflammatory agents, whereas galectin-3 has multiple functions depending on the cell type and context. Galectin-3 is upregulated in activated microglia in both M1 and M2 phenotypes. Galectin-3 in M1 type microglia acts as pro-inflammatory, whereas galectin-3 in M2 type microglia facilitates phagocytic activity and neuroprotective and anti-inflammatory functions. Thus, the action of galectin-3 is often controversial, depending on disease status. Although we still do not know what determines the differential activity of galectin-3 on microglial function, it may be due to the differential glycoconjugate expression to which galectin-3 binds in different types of microglia or surrounding environmental cells and extracellular matrix. Galectin-3 may act as pattern recognition receptors (PRRs) such as TLRs that recognize pathogen-associated molecular pattern molecules (PAMPs) such as lipopolysaccharides (LPS) and damage-associated molecular pattern molecules (DAMPs) such as high-mobility group box-1 in innate immunity. Galectin-3 and -9 also act as DAMPs, and galectin-3 can bind to TLR4, participating in initial neuroinflammatory processes through secretion of pro-inflammatory cytokines such as TNFα, IL-1β, and IL-6 (Burguillos et al., 2015; Rahimian et al., 2021). Galectin-3 controls the severity of inflammation and induces the activation of NLRP3 inflammasome-dependent pathways in Huntington's disease and spinal cord injuries (Ren et al., 2019; Siew et al., 2019). Galectin-3 may also play a role in inflammation amplifiers in neurodegenerative diseases (Rabinovich et al., 2002) (Figure 4B). In contrast, galectin-3 influences microglial polarization to the M2 phenotype through the IL-4 receptor pathway, possibly by binding to the glycan on the IL-4 receptor (Rahimian et al., 2019, 2021). Although the underlying mechanism to define the function of galectin-3 in neurodegenerative disease is not fully understood, it may depend on the timing and context of disease status.
Post-translational modification of galectins influences the localization and function of galectins, especially galectin-1 and -3 (Gao et al., 2017). Galectin-3 consists of a C-terminal CRD and N-terminal peptide, required for oligomerization (Figure 1B). N-terminal peptides contain serine and tyrosine residues that are phosphorylated by kinases, such as casein kinase I, and the sites cleaved by several proteinases, such as MMPs. Galectin-3 usually exists as a monomer but can also oligomerize via self-association of the N-terminal domain to form pentamers in the presence of multivalent oligosaccharides containing terminal LacNAc. Galectin-3 crosslinks cell surface glycoproteins and extracellular matrix to mediate cell-to-cell and cell-to-matrix adhesion, as well as the trafficking and presentation of surface glycoproteins. Cleavage of the N-terminal domain by proteases results in the abrogation forming oligomer, whereas released CRD has a high glycan-binding affinity (Ochieng et al., 1994). Because galectin-3 appears to be cleaved in OPCs and OPCs contain a plant lectin PNA-positive glycans as the substrate to generate core 2 type O-glycans, in which elongation of poly-LacNAc is formed (Figure 1C) (Pasquini et al., 2011), galectin-3-glycan-binding seems to be enhanced in OPC without forming lattice on cellular membrane. In contrast, serine phosphorylation by casein kinase I reduces the glycan-binding activity of galectin-3, while it regulates the galectin-3 cellular distribution, which is important for its anti-apoptosis/anoikis activity (Gao et al., 2017). As mentioned above, phosphorylated galectin-3 promotes axon branching in cultured neurons (Díez-Revuelta et al., 2010). Oxidization, biologically induced by reactive oxygen species such as hydrogen peroxide, attenuates the glycan-binding ability of galectin-1. The promoting action of galectin-1 on neurite outgrowth is not dependent on its lectin properties (Inagaki et al., 2000). Tamura et al. (2015) reported that nitric oxide-caused S-nitrosylation of cysteine residues in CRD of galectin-2, which is intensely expressed in the stomach and small intestine, prevents the hydrogen peroxide-induced inactivation of glycan-binding activity. In prion diseases, S-nitrosylated galectin-1 is increased in scrapie-infected rodents and human prion diseases, where neuroprotection of galectin-1 does not appear to function efficiently (Guo et al., 2017). Since the oxidized form of galectin-1 lacking lectin activity promotes axonal regeneration at much lower concentrations at which galectin-1 exhibits lectin activity (Inagaki et al., 2000), S-nitrosylation of galectin-1, which protects against oxidization to maintain lectin activity, may attenuate its neuroprotective action in prion disease. Thus, local environment influences the glycan-binding ability of galectins and may change their functions.
As mentioned in the introduction, the affinity of galectins for glycan is enhanced by increased branching and repeated LacNAc motifs. Branching of complex type N-glycans is mediated by mannose N-acetylglucosaminyl transferases (Mgat1, 2, 4, 5/GnTI, II, IV, V) that transfer GlcNAc to core trimannoses on N-glycans via α1,3 or α1,6 linkages to form branches (Figure 1C). Mgat5/GnTV promotes fourth branching, where galectins, especially galectin-3, form galectin lattice by binding to poly-LacNAc units, regulating trafficking and presentation of cell surface proteins. Prof. James W. Dennis and Prof. Michael Demetriou have extensively investigated the immunosuppressive effect of Mgat5-mediated N-glycan branching and the formation of the galectin lattice (Demetriou et al., 2001). Differentiation of peripheral T cells into pro-inflammatory Th1 and Th17 cells is inhibited by the galectin lattice, which combines with T cell receptor regulation, and determines the risk of autoimmune diseases such as MS (Demetriou et al., 2001; Lee et al., 2007; Grigorian et al., 2011). In addition to immune regulation, the galectin lattice drives myelination by promoting the surface retention of PDGFRα in OPC, a receptor that plays a critical role in oligodendrogenesis (Sy et al., 2020). Mgat5-mediated branching and poly-LacNAc formation depend on GlcNAc availability in cells. Since oral administration of GlcNAc drives anti-inflammatory action as well as myelin repair, reducing axonal damage and enhancing functional recovery in EAE mice (Grigorian et al., 2011; Lee et al., 2019), GlcNAc is a new therapeutic supplement for MS patients. Oral GlcNAc administration in patients with MS is under clinical trial led by Dr. M. Demetriou's group.
It is well known that glycosaminoglycans such as chondroitin sulfate and keratan sulfates inhibit neurite outgrowth (Snow et al., 1990; Cole and McCabe, 1991), and chondroitinase ABC is a promising therapeutic agent for CNS regeneration. Keratan sulfate is abundantly located in the cartilage, cornea, and the brain, and contains a repeated LacNAc units (Figure 1C). Iwaki et al. (2008) demonstrated that de-sulfated keratan sulfates, as well as de-sulfated chondroitin/dermatan sulfates, are potential galectin ligands, especially galectin-3 and -9. Sulfation and sialylation on keratan sulfate in microglia influence the progression and recovery of spinal cord injuries, ALS, and AD (Ito et al., 2010; Hirano et al., 2013; Foyez et al., 2015; Ueno et al., 2015; Zhang et al., 2017). Deficiency of arylsulfatase A and B, that are associated with metachromatic leukodystrophy and Maroteaux-Lamy syndrome, respectively, increases sulfatides and sulfated chondroitin 4-sulfate in the CNS, attenuating oligodendrocyte differentiation and neural recovery (Zhang et al., 2014; Bhattacharyya et al., 2015; Pituch et al., 2015). Increased sulfation in glycosaminoglycans as well as glycolipids likely impairs glycan-binding of galectin-3, resulting in reduced its functions. Sulfotransferase and sulfatase activity may link to the galectin-3 action during neuroinflammation and neural regeneration. Because GlcNAc treatment enhances the galectin-3-glycan-binding via increasing N-glycan branching and improve (re)myelination by forming lattice on PGDFRα as mentioned above, it also would be a potential therapeutic strategy for genetic disorders such as metachromatic leukodystrophy with increased sulfation in glycosaminoglycans and glycolipids.
Because the availability of glucose and glutamine is related to de novo synthesis of UDP-GlcNAc required for N-glycan branching, thus influencing galectin lattice formation, there may be a link between energy conditions and galectin expression and glycan composition in each cell (Araujo et al., 2017). During ischemia, the brain is exposed to hypoglycemic and hypoxic conditions. In the hypoxic tumor microenvironment, inhibition of α3β1 integrin translocation to the plasma membrane, decreased 1,2-fucosylation of cell-surface glycans, and galectin overexpression was consequently observed by alteration of intracellular glucose metabolism from aerobic cellular respiration to anaerobic glycolysis (Silva-Filho et al., 2017). Although the mechanisms driving the change in glycosylation status and galectin expression are still unclear, the glucose availability and metabolism pathway are conceivable to be an essential key to elucidating the interactive changes in glycans and galectins. Although evidence showing the relationship between galectin expression and the glucose metabolism pathway, which leads to changes in glycan structure in cells, is limited, to revealing their connection will provide beneficial information to elucidate the function of galectins and the ligand glycoconjugates in the regulation of physiological and pathological events in the CNS.
Ligand glycoconjugates for galectins serve not only glycoproteins but also glycolipids as well as extracellular proteoglycans. Multiple galectins are involved in the regulation of physiological and pathological events in the CNS in different ways. In addition to galectins, other lectins, such as sialic acid-recognizing siglecs, are also involved in the pathogenesis of neurodegenerative diseases (Siddiqui et al., 2019; Puigdellívol et al., 2020). Therefore, it is necessary to understand the overall regulation of glycans and lectins. To find common or individual rules regulating cellular glycan-lectin properties will be beneficial to reveal the etiology and pathogenesis of neurodegenerative diseases and to establish new therapeutic strategies.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was funded by the Medtronic Japan External Research Institute Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Prof. Jun Hirabayashi, Nagoya University, and Prof. Sachiko Sato, Laval University, for their kind advice and beneficial information for writing up this review. The authors also appreciate Dr. Mayumi Tamura, Teikyo University, who kindly provided valuable information about S-nitrosylated galectins. We also thank Dr. Miwako Yamasaki, Hokkaido University, and Prof. Yoshio Bando, Akita University, for their kind help for editing the figures.
Al-Dalahmah, O., Soares, L. C., Nicholson, J., Draijer, S., Mundim, M., Lu, V. M., et al. (2020). Galectin-3 modulates postnatal subventricular zone gliogenesis. Glia 68, 435–450. doi: 10.1002/glia.23730
Araujo, L., Khim, P., Mkhikian, H., Mortales, C. L., and Demetriou, M. (2017). Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife 6:e21330. doi: 10.7554/eLife.21330
Ashraf, G. M., and Baeesa, S. S. (2018). Investigation of gal-3 expression pattern in serum and cerebrospinal fluid of patients suffering from neurodegenerative disorders. Front. Neurosci. 12:430. doi: 10.3389/fnins.2018.00430
Bansal, R., Stefansson, K., and Pfeiffer, S. E. (1992). Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J. Neurochem. 58, 2221–2229. doi: 10.1111/j.1471-4159.1992.tb10967.x
Barake, F., Soza, A., and González, A. (2020). Galectins in the brain: advances in neuroinflammation, neuroprotection and therapeutic opportunities. Curr. Opin. Neurol. 33, 381–390. doi: 10.1097/WCO.0000000000000812
Bhattacharyya, S., Zhang, X., Feferman, L., Johnson, D., Tortella, F. C., Guizzetti, M., et al. (2015). Decline in arylsulfatase B and increase in chondroitin 4-sulfotransferase combine to increase chondroitin 4-sulfate in traumatic brain injury. J. Neurochem. 134, 728–739. doi: 10.1111/jnc.13156
Boza-Serrano, A., Reyes, J. F., Rey, N. L., Leffler, H., Bousset, L., Nilsson, U., et al. (2014). The role of galectin-3 in α-synuclein-induced microglial activation. Acta Neuropatol. Commun. 2:156. doi: 10.1186/PREACCEPT-1285543917141325
Boza-Serrano, A., Ruiz, R., Sanchez-Varo, R., García-Revilla, J., Yang, Y., Jimenez-Ferrer, I., et al. (2019). Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer's disease. Acta Neuropathol. 138, 251–273. doi: 10.1007/s00401-019-02013-z
Burguillos, M. A., Svensson, M., Schulte, T., Boza-Serrano, A., Garcia-Quintanilla, A., Kavanagh, E., et al. (2015). Microglia-secreted galectin-3 acts as a toll-like receptor 4 ligand and contributes to microglial activation. Cell Rep. 10, 1626–1638. doi: 10.1016/j.celrep.2015.02.012
Camby, I., Mercier, M. L., Lefranc, F., and Kiss, R. (2006). Galectin-1: a small protein with major functions. Glycobiology 16, 137R-157R. doi: 10.1093/glycob/cwl025
Cengiz, T., Türkboylari, S., Gençler, O. S., and Anlar, Ö. (2019). The roles of galectin-3 and galectin-4 in the idiopatic Parkinson disease and its progression. Clin. Neurol. Neurosurg. 184:105373. doi: 10.1016/j.clineuro.2019.105373
Chang-Hong, R., Wada, M., Koyama, S., Kimura, H., Arawaka, S., Kawanami, T., et al. (2005). Neuroprotective effect of oxidized galectin-1 in a transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 194, 203–211. doi: 10.1016/j.expneurol.2005.02.011
Cole, G. J., and McCabe, C. F. (1991). Identification of a developmentally regulated keratan sulfate proteoglycan that inhibits cell adhesion and neurite outgrowth. Neuron 7, 1007–1018. doi: 10.1016/0896-6273(91)90345-Z
Comte, I., Kim, Y., Young, C. C., van der Harg, J. M., Hockberger, P., Bolam, P. J., et al. (2011). Galectin-3 maintains cell motility from the subventricular zone to the olfactory bulb. J. Cell Sci. 124, 2438–2447. doi: 10.1242/jcs.079954
Cummings, R. D., Liu, F. T., and Vasta, G. R. (2017). Galectins. Essentials of Glycobiology, 3rd edition. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
de Jong, C. G. H. M., Gabius, H. J., and Baron, W. (2020). The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis. Cell Mol. Life Sci. 77, 1289–1317. doi: 10.1007/s00018-019-03327-7
de Jong, C. G. H. M., Stancic, M., Pinxterhuis, T. H., van Horssen, J., van Dam, A. M., Gabius, H. J., et al. (2018). Galectin-4, a negative regulator of oligodendrocyte differentiation, is persistently present in axons and microglia/macrophages in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 77, 1024–1038. doi: 10.1093/jnen/nly081
Demetriou, M., Granovsky, M., Guaggin, S., and Dennis, J. W. (2001). Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glyosylation. Nature 409, 733–739. doi: 10.1038/35055582
Díez-Revuelta, N., Higuero, A. M., Velasco, S., Peñas-de-la-Iglesia, M., Gabius, H. J., and Abad-Rodríguez, J. (2017). Neurons define non-myelinated axon segments by the regulation of galectin-4-containing axon membrane domains. Sci. Rep. 7:12246. doi: 10.1038/s41598-017-12295-6
Díez-Revuelta, N., Velasco, S., André, S., Kaltner, H., Kübler, D., Gabius, H. J., et al. (2010). Phosphorylation of adhesion- and growth-regulatory human galectin-3 leads to the induction of axonal branching by local membrane L1 and ERM redistribution. J. Cell Sci. 123, 671–681. doi: 10.1242/jcs.058198
Doetsch, F., García-Verdugo, J. M., and Alvarez-Buylla, A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997
Falcon, B., Noad, J., McMahon, H., Randow, F., and Goedert, M. (2018). Galectin-8-mediated selective autophagy protects against seeded tau aggregation. J. Biol. Chem. 293, 2438–2451. doi: 10.1074/jbc.M117.809293
Farrer, R. G., and Quarles, R. H. (1999). GT3 and its O-acetylated derivative are the principal A2B5-reactive gangliosides in cultured O2A lineage cells and are down-regulated along with O-acetyl GD3 during differentiation to oligodendrocytes. J. Neurosci. Res. 57, 371–380. doi: 10.1002/(SICI)1097-4547(19990801)57:3<371::AID-JNR9>3.0.CO;2-O
Foyez, T., Takeda-Uchimura, Y., Ishigaki, S., Narentuya, Z., Hang, Z., Sobue, G., Kadomatsu, K., et al. (2015). Microglial keratan sulfate epitope elicits in central nervous tissues of transgenic model mice and patients with amyotrophic lateral sclerosis. Am. J. Pathol. 185, 3053–3065. doi: 10.1016/j.ajpath.2015.07.016
Gao, X., Liu, J., Liu, X., Li, L., and Zheng, J. (2017). Cleavage and phosphorylation: important post-translational modifications of galectin-3. Cancer Metastasis Rev. 36, 367–374. doi: 10.1007/s10555-017-9666-0
Gaudet, A. D., Sweet, D. R., Polinski, N. K., Guan, Z., and Popovich, P. G. (2015). Galectin-1 in injured rat spinal cord: implications for macrophage phagocytosis and neural repair. Mol. Cell Neurosci. 64, 84–94. doi: 10.1016/j.mcn.2014.12.006
Grigorian, A., Araujo, L., Naidu, N. N., Place, D. J., Choudhury, B., and Demetriou, M. (2011). N-acetylglucosamine inhibits T-helper 1(Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem. 286, 40133–40141. doi: 10.1074/jbc.M111.277814
Guo, Y. J., Shi, Q., Yang, X. D., Li, J. L., Ma, Y., Xiao, K., et al. (2017). Increases of galectin-1 and its S-nitrosylated form in the brain tissue of scrapie-infected rodent models and human prion disease. Mol. Neurobiol. 54, 3707–3716. doi: 10.1007/s12035-016-9923-1
Higuero, A. M., Díez-Revuelta, N., and Abad-Rodríguez, J. (2017). The sugar code in neuronal physiology. Histochem. Cell Biol. 147, 257–267. doi: 10.1007/s00418-016-1519-3
Hillis, J. M., Davies, J., Mundim, M. V., Al-Dalahmah, O., and Szele, F. G. (2016). Cuprizone demyelination induces a unique inflammatory response in the subventricular zone. J. Neuroinflamm. 13:190. doi: 10.1186/s12974-016-0651-2
Hirabayashi, J., Hashidate, T., Arata, Y., Nishi, N., Nakamura, T., Hirashima, M., et al. (2002). Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254. doi: 10.1016/S0304-4165(02)00311-2
Hirabayashi, J., and Kasai, K. (1993). The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3, 297–304. doi: 10.1093/glycob/3.4.297
Hirano, K., Ohgomori, T., Kobayashi, K., Tanaka, F., Matsumoto, T., Natori, T., et al. (2013). Ablation of keratan sulfate accelerates early phase pathogenesis of ALS. PLoS ONE 8:e66969. doi: 10.1371/journal.pone.0066969
Horie, H., Inagaki, Y., Sohma, Y., Nozawa, R., Okawa, K., Hasegawa, M., et al. (1999). Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J. Neurosci. 19, 9964–9974. doi: 10.1523/JNEUROSCI.19-22-09964.1999
Hynes, M. A., Gitt, M., Barondes, S. H., Jessell, T. M., and Buck, L. B. (1990). Selective expression of an endogenous lactose-binding lectin gene in subsets of central and peripheral neurons. J. Neurosci. 10, 1004–1013. doi: 10.1523/JNEUROSCI.10-03-01004.1990
Imaizumi, Y., Sakaguchi, M., Morishita, T., Ito, M., Poirier, F., Sawamoto, K., et al. (2011). Galectin-1 is expressed in early-type neural progenitor cells down-regulates neurogenesis in the adult hippocampus. Mol. Brain 4:7. doi: 10.1186/1756-6606-4-7
Inagaki, Y., Sohma, Y., Horie, H., Nozawa, R., and Kadoya, T. (2000). Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur. J. Biochem. 267, 2955–2964. doi: 10.1046/j.1432-1033.2000.01311.x
Itabashi, T., Arima, Y., Kamimura, D., Higuchi, K., Bando, Y., Takahashi-Iwanaga, H., et al. (2018). Cell- and stage-specific localization of galectin-3, a β-galactoside-binding lectin, in a mouse model of experimental autoimmune encephalomyelitis. Neurochem. Int. 118, 176–184. doi: 10.1016/j.neuint.2018.06.007
Ito, Z., Sakamoto, K., Imagama, S., Matsuyama, Y., Zhang, H., Hirano, K., et al. (2010). N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J. Neurosci. 30, 5937–5947. doi: 10.1523/JNEUROSCI.2570-09.2010
Iwaki, J., Minamisawa, T., Tateno, H., Kominami, J., Suzuki, K., Nishi, N., et al. (2008). Desulfated galactosaminoglycans are potential ligands for galectins: evidence from frontal affinity chromatography. Biochem. Biophys. Res. Commun. 373, 206–212. doi: 10.1016/j.bbrc.2008.05.190
James, R. E., Hillis, J., Adorján, I., Gration, B., Mundim, M. V., Iqbal, A. J., et al. (2016). Loss of galectin-3 decreases the number of immune cells in the subventricular zone and restores proliferation in a viral model of multiple sclerosis. Glia 64, 105–121. doi: 10.1002/glia.22906
Jia, J., Claude-Taupin, A., Gu, Y., Choi, S. W., Peters, R., Bissa, B., et al. (2020). Galectin-3 coordinates a cellular system for lysosomal repair and removal. Dev. Cell 52, 69–87. doi: 10.1016/j.devcel.2019.10.025
Jiang, H. R., Rasebi, Z. A., Mensah-Brown, E., Shahin, A., Xu, D., Goodyear, C. S., et al. (2009). Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J. Immunol. 182, 1167–1173. doi: 10.4049/jimmunol.182.2.1167
Jin, J. K., Na, Y. J., Song, J. H., Joo, H. G., Kim, S., Kim, J. I., et al. (2007). Galectin-3 expression is correlated with abnormal prion protein accumulation in murine scrapie. Neurosci. Lett. 420, 138–143. doi: 10.1016/j.neulet.2007.04.069
John, S., and Mishra, R. (2016). mRNA transcriptomics of galectins unveils heterogeneous organization in mouse and human brain. Front. Mol. Neurosci. 9:139. doi: 10.3389/fnmol.2016.00139
Kajitani, K., Nomaru, H., Ifuku, M., Yutsudo, N., Dan, Y., Miura, T., et al. (2009). Galectin-1 promotes basal and kainite-induced proliferation of neural progenitors in the dentate gyrus of adult mouse hippocampus. Cell Death Differ. 16, 417–427. doi: 10.1038/cdd.2008.162
Kajitani, K., Yanagimoto, K., and Nakabeppu, Y. (2017). Serum galectin-3, but not galectin-1, levels are elevated in schizophrenia: implications for the role of inflammation. Psychopharmacology 234, 2919–2927. doi: 10.1007/s00213-017-4683-9
Kato, T., Kurita, K., Seino, T., Kadoya, T., Horie, H., Wada, M., et al. (2001). Galectin-1 is a component of neurofilamentous lesions in sporadic and familial amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 282, 166–172. doi: 10.1006/bbrc.2001.4556
King, D. R., Salako, D. C., Arthur-Bentil, S. K., Rubin, A. E., Italiya, J. B., Tan, J. S., et al. (2021). Relationship between novel inflammatory biomarker galectin-3 and depression symptom severity in a large community-based sample. J. Affect. Disord. 281, 384–389. doi: 10.1016/j.jad.2020.12.050
Kobayakawa, Y., Sakumi, K., Kajitani, K., Kadoya, T., Horie, H., Kira, J. I., et al. (2015). Galectin-1 deficiency improves axonal swelling of motor neurons in SOD1(G93A) transgenic mice. Neuropathol. Appl. Neurobiol. 41, 227–244. doi: 10.1111/nan.12123
Lalancette-Hébert, M., Gowing, G., Simard, A., Weng, Y. C., and Kriz, J. (2007). Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007
Lee, S. U., Grigorian, A., Pawling, J., Chen, I. J., Gao, G., Mozaffar, T., et al. (2007). N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J. Biol. Chem. 282, 33725–33734. doi: 10.1074/jbc.M704839200
Lee, S. U., Li, C. F., Mortales, C. L., Pawling, J., Dennis, J. W., Grigorian, A., et al. (2019). Increasing cell permeability of N-acetylglucosamine via 6-acetylation enhances capacity to suppress T-helper 1 (TH1)/TH17 responses and autoimmunity. PLoS ONE 14:e0214253. doi: 10.1371/journal.pone.0214253
Lerman, B. J., Hoffman, E. P., Sutherland, M. L., Bouri, K., Hsu, D. K., Liu, F. T., et al. (2012). Deletion of galectin-3 exacerbates microglial activation and accelerates disease progression and demise in a SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Brain Behav. 2, 563–575. doi: 10.1002/brb3.75
Li, Y., Chen, N., Wu, C., Lu, Y., Gao, G., Duan, C., et al. (2020). Galectin-1 attenuates neurodegeneration in Parkinson's disease model by modulating microglial MAPK/IkB/NFkB axis through its carbohydrate recognition domain. Brain Behav. Immun. 83, 214–225. doi: 10.1016/j.bbi.2019.10.015
Liu, F. T., and Rabinovich, G. A. (2005). Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41. doi: 10.1038/nrc1527
Lutomski, D., Joubert-Caron, R., Lefebure, C., Salama, J., Belin, C., Bladier, D., et al. (1997). Anti-galectin-1 autoantibodies in serum of patients with neurological diseases. Clin. Chim. Acta 262, 131–138. doi: 10.1016/S0009-8981(97)06544-3
Mahanthappa, N. K., Cooper, D. N., Barondes, S. H., and Schwarting, G. A. (1994). Rat olfactory neurons can utilize the endogenous lectin, L-14, in a novel adhesion mechanism. Development 120, 1373–1384. doi: 10.1242/dev.120.6.1373
Marques, T. M., van Rumund, A., Bruinsma, I. B., Wessels, H. J. C. T., Gloerich, J., Esselink, R. A. J., et al. (2019). Cerebrospinal fluid galectin-1 levels discriminate patients with Parkinsonism from controls. Mol. Neurobiol. 56, 5067–5074. doi: 10.1007/s12035-018-1426-9
Mok, S. W. F., Riemer, C., Madela, K., Hsu, D. K., Liu, F. T., Gültner, S., et al. (2007). Role of galectin-3 in prion infections of the CNS. Biochem. Biophys. Res. Commun. 359, 672–678. doi: 10.1016/j.bbrc.2007.05.163
Nio-Kobayashi, J. (2017). Tissue- and cell-specific localization of galectins, β-galactose-binding animal lectins, and their potential functions in health and disease. Anat. Sci. Int. 92, 25–36. doi: 10.1007/s12565-016-0366-6
Ochieng, J., Fridman, R., Nangia-Makker, P., Kleiner, D. E., Liotta, L. A., Stetler-Stevenson, W. G., et al. (1994). Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 33, 14109–14014. doi: 10.1021/bi00251a020
Offner, H., Celnik, B., Bringman, T. S., Casentini-Borocz, D., Nedwin, G. E., and Vandenbark, A. A. (1990). Recombinant human beta-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 28, 177–184. doi: 10.1016/0165-5728(90)90032-I
Outenreath, R. L., and Jones, A. L. (1992). Influence of an endogenous lectin substrate on cultured dorsal root ganglion cells. J. Neurocytol. 21, 788–795. doi: 10.1007/BF01237904
Pardo, E., Barake, F., Godoy, J. A., Oyanadel, C., Espinoza, S., Metz, C., et al. (2019). Galectin-8 is a neuroprotective factor in the brain that can be neutralized by human autoantibodies. Mol. Neurobiol. 56, 7774–7788. doi: 10.1007/s12035-019-1621-3
Pardo, E., Cárcamo, C., Martín, R. U. S., Ciampi, E., Segovia-Miranda, F., Curkovic-Peña, C., et al. (2017). Galectin-8 as an immunosuppressor in experimental autoimmune encephalomyelitis and a target of human early prognostic antibodies in multiple sclerosis. PLoS ONE 12:e0177472. doi: 10.1371/journal.pone.0177472
Pasquini, L. A., Millet, V., Hoyos, H. C., Giannoni, J. P., Croci, D. O., Marder, M., et al. (2011). Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 18, 1746–1756. doi: 10.1038/cdd.2011.40
Pesheva, P., Kuklinski, S., Schmitz, B., and Probstmeier, R. (1998). Galectin-3 promotes neural cell adhesion and neurite growth. J. Neurosci. Res. 54, 639–654. doi: 10.1002/(SICI)1097-4547(19981201)54:5<639::AID-JNR9>3.0.CO;2-2
Peters, O. M., Shelkovnikova, T., Highley, J. R., Cooper-Knock, J., Hortobágyi, T., Troakes, C., et al. (2015). Gamma-synuclein pathology in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2, 29–37. doi: 10.1002/acn3.143
Petralia, M. C., Ciurleo, R., Bramanti, A., Bramanti, P., Saraceno, A., Mangano, K., et al. (2021). Transcriptomic data analysis reveals a down-expression of galectin-8 in schizophrenia hippocampus. Brain Sci. 11:973. doi: 10.3390/brainsci11080973
Pituch, K. C., Moyano, A. L., Lopez-Rosas, A., Marottoli, F. M., Li, G., Hu, C., et al. (2015). Dysfunction of platelet-derived growth factor receptor α (PDGFR α) represses the production of oligodendrocytes from arylsulfatase A-deficient multipotential neural precursor cells. J. Biol. Chem. 290, 7040–7053. doi: 10.1074/jbc.M115.636498
Puche, A. C., and Key, B. (1995). Identification of cells expressing galectin-1, a galactose-binding receptor, in the rat olfactory system. J. Comp. Neurol. 357, 513–523. doi: 10.1002/cne.903570403
Puche, A. C., Poirier, F., Hair, M., Bartlett, P. F., and Key, B. (1996). Role of galectin-1 in the developing mouse olfactory system. Dev. Biol. 179, 274–287. doi: 10.1006/dbio.1996.0257
Puigdellívol, M., Allendorf, D. H., and Brown, G. C. (2020). Sialylation and galectin-3 in microglia-mediated neuroinflammation and neurodegeneration. Front. Cell Neurosci. 14:162. doi: 10.3389/fncel.2020.00162
Qu, W. S., Wang, Y. H., Ma, J. F., Tian, D. S., Zhang, Q., Pan, D. J., et al. (2011). Galectin-1 attenuates astrogliosis-associated injuries and improves recovery of rats following focal cerebral ischemia. J. Neurochem. 116, 217–226. doi: 10.1111/j.1471-4159.2010.07095.x
Quintá, H. R., Pasquini, J. M., Rabinovich, G. A., and Pasquini, L. A. (2014). Glycan-dependent binding of galectin-1 to neuropilin-1 promotes axonal regeneration after spinal cord injury. Cell Death Differ. 21, 941–955. doi: 10.1038/cdd.2014.14
Quintá, H. R., Wilson, C., Blidner, A. G., González-Billault, C., Pasquini, L. A., Rabinovich, G. A., et al. (2016). Ligand-mediated galectin-1 endocytosis prevents intraneural H2O2 production promoting F-actin dynamics reactivation and axonal re-growth. Exp. Neurol. 283, 165–178. doi: 10.1016/j.expneurol.2016.06.009
Rabinovich, G. A., Baum, L. G., Tinari, N., Paganelli, R., Natoli, C., Liu, F. T., et al. (2002). Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 23, 313–320. doi: 10.1016/S1471-4906(02)02232-9
Rabinovich, G. A., and Toscano, M. A. (2009). Turning ‘sweet' on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352. doi: 10.1038/nri2536
Rahimian, R., Béland, L. C., and Kriz, J. (2018). Galectin-3: mediator of microglia responses in injured brain. Drug Discov. Today 23, 375–381. doi: 10.1016/j.drudis.2017.11.004
Rahimian, R., Béland, L. C., Sato, S., and Kriz, J. (2021). Microglia-derived galectin-3 in neuroinflammation; a bittersweet ligand? Med. Res. Rev. 41, 2582–2589. doi: 10.1002/med.21784
Rahimian, R., Lively, S., Abdelhamid, E., Lalancette-Hébert, M., Schlichter, L., Sato, S., et al. (2019). Delayed galectin-3-mediated reprogramming of microglia after stroke in protective. Mol. Neurobiol. 56, 6371–6385. doi: 10.1007/s12035-019-1527-0
Ramírez, E., Sánchez-Maldonado, C., Mayoral, M. A., Mendieta, L., Alatriste, V., Patricio-Martínez, A., et al. (2019). Neuroinflammation induced by the peptide amyloid-β (25-35) increase the presence of galectin-3 in astrocytes and microglia and impairs spatial memory. Neuropeptides 74, 11–23. doi: 10.1016/j.npep.2019.02.001
Ren, Z., Liang, W., Sheng, J., Xun, C., Xu, T., Cao, R., et al. (2019). Gal-3 is a potential biomarker for spinal cord injury and Gal-3 deficiency attenuates neuroinflammation through ROS/TXNIP/NLRP3 signaling pathway. Biosci. Rep. 39:BSR20192368. doi: 10.1042/BSR20192368
Riemer, C., Neidhold, S., Burwinkel, M., Schwarz, A., Schultz, J., Krätzschmar, J., et al. (2004). Gene expression profiling of scrapie-infected brain tissue. Biochem. Biophys. Res. Commun. 323, 556–564. doi: 10.1016/j.bbrc.2004.08.124
Rinaldi, M., Thomas, L., Mathieu, P., Carabias, P., Troncoso, M. F., Pasquini, J. M., et al. (2016b). Galectin-1 circumvents lysolecithin-induced demyelination through the modulation of microglial polarization/phagocytosis and oligodendroglial differentiation. Neurobiol. Dis. 96, 127–143. doi: 10.1016/j.nbd.2016.09.003
Rinaldi, M., Thomas, L., and Pasquini, L. A. (2016a). Galectin-1 in myelin repair. Oncotarget 7, 81979–81980. doi: 10.18632/oncotarget.13455
Saito, M., Kitamura, H., and Sugiyama, K. (2001). The specificity of monoclonal antibody A2B5 to c-series ganglioside. J. Neurochem. 78, 64–74. doi: 10.1046/j.1471-4159.2001.00365.x
Sakaguchi, M., Imaizumi, Y., Shingo, T., Tada, H., Hayama, K., Yamada, O., et al. (2010). Regulation of adult neural progenitor cells by galectin-1/beta1 integrin interaction. J. Neurochem. 113, 1516–1524. doi: 10.1111/j.1471-4159.2010.06712.x
Sakaguchi, M., Shingo, T., Shimazaki, T., Okano, H. J., Shiwa, M., Ishibashi, S., et al. (2006). A carbohydrate-binding protein, galectin-1, promotes proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA. 103, 7112–7117. doi: 10.1073/pnas.0508793103
Sartim, A. G., Sartim, M. A., Cummings, R. D., Dias-Baruffi, M., and Joca, S. R. (2020). Impaired emotional response to stress in mice lacking galectin-1 or galectin-3. Physiol. Behav. 220:112862. doi: 10.1016/j.physbeh.2020.112862
Sasaki, T., Hirabayashi, J., Manya, H., Kasai, K. I., and Endo, T. (2004). Galectin-1 induces astrocyte differentiation, which leads to production of brain-derived neurotrophic factor. Glycobiology 14, 357–363. doi: 10.1093/glycob/cwh043
Siddiqui, S. S., Matar, R., Merheb, M., Hodeify, R., Vazhappilly, C. G., Marton, J., et al. (2019). Siglecs in brain function and neurological disorders. Cells 8:1125. doi: 10.3390/cells8101125
Siew, J. J., Chen, H. M., Chen, H. Y., Chen, H. L., Chen, C. M., Soong, B. W., et al. (2019). Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington's disease. Nat. Commun. 10:3473. doi: 10.1038/s41467-019-11441-0
Silva-Filho, A. F., Sena, W. L. B., Lima, L. R. A., Carvalho, L. V. N., Pereira, M. C., Santos, L. G. S., et al. (2017). Glycobiology modifications in intratumoral hypoxia: the breathless side of glycans interaction. Cell Physiol. Biochem. 41, 1801–1829. doi: 10.1159/000471912
Sirko, S., Irmler, M., Gascón, S., Bek, S., Schneider, S., Dimou, L., et al. (2015). Astrocyte reactivity after brain injury-: the role of galectin-1 and 3. Glia 53, 2340–2361. doi: 10.1002/glia.22898
Snow, D. M., Lemmon, V., Carrino, D. A., Caplan, A. I., and Silver, J. (1990). Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp. Neurol. 109, 111–130. doi: 10.1016/S0014-4886(05)80013-5
Sommer, I., and Schachner, M. (1981). Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev. Biol. 83, 311–327. doi: 10.1016/0012-1606(81)90477-2
St John, J. A., and Key, B. (1999). Expression of galectin-1 in the olfactory nerve pathway of rat. Dev. Brain Res. 117, 171–178. doi: 10.1016/S0165-3806(99)00118-2
Stancic, M., Slijepcevic, D., Nomden, A., Vos, M. J., de Jonge, J. C., Sikkema, A. H., et al. (2012). Galectin-4, a novel neuronal regulator of myelination. Glia 60, 919–935. doi: 10.1002/glia.22324
Stancic, M., van Horssen, J., Thijssen, V. L., Gabius, H. J., van der Valk, P., Hoekstra, D., et al. (2011). Increased expression of distinct galectins in multiple sclerosis lesions. Neuropathol. Appl. Neurobiol. 37, 654–671. doi: 10.1111/j.1365-2990.2011.01184.x
Starossom, S. C., Mascanfroni, I. D., Imitola, J., Cao, L., Raddassi, K., Hernandez, S. F., et al. (2012). Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37, 249–263. doi: 10.1016/j.immuni.2012.05.023
Steelman, A. J., and Li, J. (2014). Astrocyte galectin-9 potentiates microglial TNF secretion. J. Neuroinflammation 11:144. doi: 10.1186/s12974-014-0144-0
Steelman, A. J., Smith, R. 3rd, Welsh, C. J., and Li, J. (2013). Galectin-9 protein is up-regulated in astrocytes by tumor necrosis factor and promotes encephalitogenic T-cell apoptosis. J. Biol. Chem. 288, 23776–23787. doi: 10.1074/jbc.M113.451658
Storan, M. J., Magnaldo, T., Biol-N'Garagba, M. C., Zick, Y., and Key, B. (2004). Expression and putative role of lactoseries carbohydrates present on NCAM in the rat primary olfactory pathway. J. Comp. Neurol. 475, 289–302. doi: 10.1002/cne.20167
Sy, M., Brandt, A. U., Lee, S. U., Newton, B. L., Pawling, J., Golzar, A., et al. (2020). N-acetylglucosamine drives myelination by triggering oligodendrocyte precursor cell differentiation. J. Biol. Chem. 295, 17413–17424. doi: 10.1074/jbc.RA120.015595
Tamura, M., Saito, M., Yamamoto, K., Takeuchi, T., Ohtake, K., Tateno, H., et al. (2015). S-nitrosylation of mouse galectin-2 prevents oxidative inactivation by hydrogen peroxide. Biochem. Biophys. Res. Commun. 457, 712–717. doi: 10.1016/j.bbrc.2015.01.055
Tao, C. C., Cheng, K. M., Ma, Y. L., Hsu, W. L., Chen, Y. C., Fuh, J. L., et al. (2020). Galectin-3 promotes Aβ oligomerization and Aβ toxicity in a mouse model of Alzheimer's disease. Cell Death Differ. 27, 192–209. doi: 10.1038/s41418-019-0348-z
Tenne-Brown, J., Puche, A. C., and Key, B. (1998). Expression of galectin-1 in the mouse olfactory system. Int. J. Dev. Biol. 42, 791–799.
Thomas, L., and Pasquini, L. A. (2018). Galectin-3-mediated glial crosstalk drives oligodendrocyte differentiation and (re)myelination. Front. Cell Neurosci. 12:297. doi: 10.3389/fncel.2018.00297
Thurston, T. L. M., Wandel, M. P., von Muhlinen, N., Foeglein, A., and Randow, F. (2012). Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414–418. doi: 10.1038/nature10744
Ueno, R., Miyamoto, K., Tanaka, N., Moriguchi, K., Kadomatsu, K., and Kusunoki, S. (2015). Keratan sulfate exacerbates experimental autoimmune encephalomyelitis. J. Neurosci. Res. 93, 1874–1880. doi: 10.1002/jnr.23640
Velasco, S., Díez-Revuelta, N., Hernández-Iglesias, T., Kaltner, H., André, S., Gabius, H. J., et al. (2013). Neuronal galectin-4 is required for axon growth and for the organization of axonal membrane L1 delivery and clustering. J. Neurochem. 125, 49–62. doi: 10.1111/jnc.12148
Venkatesan, C., Chrzaszcz, M., Choi, N., and Wainwright, M. S. (2010). Chronic upregulation of activate microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J. Neuroinflammation 7:32. doi: 10.1186/1742-2094-7-32
Walther, M., Kuklinski, S., Pesheva, P., Guntinas-Lichius, O., Angelov, D. N., Neiss, W. F., et al. (2000). Galectin-3 is upregulated in microglial cells in response to ischemic brain lesions, but not to facial nerve axotomy. J. Neurosci. Res. 61, 430-435. doi: 10.1002/1097-4547(20000815)61:4430::AID-JNR93.0.CO;2-3
Wang, Q., Gao, R., Wang, M., Chen, Q., Xiao, M., Li, Z., et al. (2019). Spatiotemporal expression patterns of galectin-3 in perinatal rat hypoxic-ischemic brain injury model. Neurosci. Lett. 711:134439. doi: 10.1016/j.neulet.2019.134439
Wang, X., Zhang, S., Lin, F., Chu, W., and Yue, S. (2015). Elevated galectin-3 levels in the serum of patients with Alzheimer's disease. Am. J. Alzheimers Dis. Other Demen. 30, 729–732. doi: 10.1177/1533317513495107
Wei, Q., Eviatar-Ribak, T., Miskimins, W. K., and Miskimins, R. (2007). Galectin-4 is involved in p27-mediated activation of the myelin basic protein promoter. J. Neurochem. 101, 1214–1223. doi: 10.1111/j.1471-4159.2007.04488.x
Wesley, U. V., Vemuganti, R., Ayvaci, E., and Dempsey, R. J. (2013). Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res. 1496, 1–9. doi: 10.1016/j.brainres.2012.12.008
Yan, Y. P., Lang, B. T., Vemuganti, R., and Dempsey, R. J. (2009). Galectin-3 mediates post-ischemic tissue remodeling. Brain Res. 1288, 116–124. doi: 10.1016/j.brainres.2009.06.073
Yang, M., Kim, J., Kim, T., Kim, S. H., Kim, J. C., Kim, J., et al. (2012). Possible involvement of galectin-3 in microglial activation in the hippocampus with trimethyltin treatment. Neurochem. Int. 61, 955–962. doi: 10.1016/j.neuint.2012.09.015
Yang, R. Y., Rabinovich, G. A., and Liu, F. T. (2008). Galectins: structure, function and therapeutic potential. Expert Rev. Mol. Med. 10:e17. doi: 10.1017/S1462399408000719
Yazar, H. O., Yazar, T., and Cihan, M. (2019). A preliminary data: evaluation of serum galectin-3 levels in patients with idiopathic Parkinson's disease. J. Clin. Neurosci. 70, 164–168. doi: 10.1016/j.jocn.2019.08.032
Yip, P. K., Carrillo-Jimenez, A., King, P., Vilalta, A., Nomura, K., Chau, C. C., et al. (2017). Galectin-3 released in response to traumatic brain injury acts as an alarmin orchestrating brain immune response and promoting neurodegeneration. Sci. Rep. 7:41689. doi: 10.1038/srep41689
Yoo, H. I., Kim, E. G., Lee, E. J., Hong, S. Y., Yoon, C. S., Hong, M. J., et al. (2017). Neuroanatomical distribution of galectin-3 in the adult rat brain. J. Mol. Histol. 48, 133–146. doi: 10.1007/s10735-017-9712-9
Zhang, X., Bhattacharyya, S., Kusumo, H., Goodlett, C. R., Tobacman, J. K., and Guizzetti, M. (2014). Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: dysregulation by ethanol. Glia 62, 259–271. doi: 10.1002/glia.22604
Zhang, Z., Takeda-Uhimura, Y., Foyez, T., Ohtake-Niimi, S., Narentuya Akatsu, H., et al. (2017). Deficiency of a sulfotransferase for sialic acid-modified glycans mitigates Alzheimer's pathology. Proc. Natl. Acad. Sci. U S A. 114, E2947–E2954. doi: 10.1073/pnas.1615036114
Keywords: galectin, glycan, neurodegenerative disease, Mgat5/GnTV, keratan sulfate, post-translational modification, glucose metabolism
Citation: Nio-Kobayashi J and Itabashi T (2021) Galectins and Their Ligand Glycoconjugates in the Central Nervous System Under Physiological and Pathological Conditions. Front. Neuroanat. 15:767330. doi: 10.3389/fnana.2021.767330
Received: 30 August 2021; Accepted: 17 September 2021;
Published: 15 October 2021.
Edited by:
Yoshihiro Akimoto, Kyorin University, JapanCopyright © 2021 Nio-Kobayashi and Itabashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junko Nio-Kobayashi, bmlvanVuQG1lZC5ob2t1ZGFpLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.