
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neuroanat., 26 September 2017
Volume 11 - 2017 | https://doi.org/10.3389/fnana.2017.00083
This article is part of the Research TopicWhy have cortical layers? What is the function of layering? Do neurons in cortex integrate information across different layers?View all 15 articles
Studying neocortex and hippocampus in parallel, we are struck by the similarities. All three to four layered allocortices and the six layered mammalian neocortex arise in the pallium. All receive and integrate multiple cortical and subcortical inputs, provide multiple outputs and include an array of neuronal classes. During development, each cell positions itself to sample appropriate local and distant inputs and to innervate appropriate targets. Simpler cortices had already solved the need to transform multiple coincident inputs into serviceable outputs before neocortex appeared in mammals. Why then do phylogenetically more recent cortices need multiple pyramidal cell layers? A simple answer is that more neurones can compute more complex functions. The dentate gyrus and hippocampal CA regions—which might be seen as hippocampal antecedents of neocortical layers—lie side by side, albeit around a tight bend. Were the millions of cells of rat neocortex arranged in like fashion, the surface area of the CA pyramidal cell layers would be some 40 times larger. Even if evolution had managed to fold this immense sheet into the space available, the distances between neurones that needed to be synaptically connected would be huge and to maintain the speed of information transfer, massive, myelinated fiber tracts would be needed. How much more practical to stack the “cells that fire and wire together” into narrow columns, while retaining the mechanisms underlying the extraordinary precision with which circuits form. This demonstrably efficient arrangement presents us with challenges, however, not the least being to categorize the baffling array of neuronal subtypes in each of five “pyramidal layers.” If we imagine the puzzle posed by this bewildering jumble of apical dendrites, basal dendrites and axons, from many different pyramidal and interneuronal classes, that is encountered by a late-arriving interneurone insinuating itself into a functional circuit, we can perhaps begin to understand why definitive classification, covering every aspect of each neurone's structure and function, is such a challenge. Here, we summarize and compare the development of these two cortices, the properties of their neurones, the circuits they form and the ordered, unidirectional flow of information from one hippocampal region, or one neocortical layer, to another.
On his deathbed in 1934, Santiago Ramón y Cajal wrote to his last student, Rafael Lorente de Nó, continuing a life-long discussion: “the mouse is not a good choice for the study of cortical circuits because of its paucity of short-axon cells…”*.
*Ramón y Cajal S. Letter to Lorente. 1934. Courtesy of Dr Francisco Alvarez, translation by Rafael Yuste. Lorente de Nó, like many since, did not agree.
This section draws heavily upon many excellent reviews (Nadarajah and Parnavelas, 2002; López-Bendito and Molnár, 2003; Cheung et al., 2007; Molnár et al., 2012; Tabata et al., 2012; Evsyukova et al., 2013; Tan and Shi, 2013; Sekine et al., 2014; Hoerder-Suabedissen and Molnár, 2015; Kawauchi, 2015; Molnár and Hoerder-Suabedissen, 2016). (Montiel et al., 2016, Figure 1; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4832283/figure/cne23871-fig-0001/).
Principal cells, i.e., glutamatergic, spiny excitatory pyramidal and spiny stellate cells are generated in the ventricular zone (VZ) from asymmetrical division of progenitor radial glial cells (Miyata et al., 2001; Noctor et al., 2001) or basal progenitors in the subventricular zone (Noctor et al., 2004; Shitamukai et al., 2011; Wang et al., 2011). Post-mitotic neurones then move to the multipolar cell accumulation zone (MAZ) just above VZ. There they stay (1–3 days: Kitazawa et al., 2014), extending and retracting multiple fine processes (Tabata and Nakajima, 2003; Tabata et al., 2009), until they begin to move toward the intermediate zone (IZ) below the cortical plate (CP, future gray matter). In IZ, the neurones become bipolar and “climb” through the CP toward the marginal zone (MZ), using the process of a single radial glial cell as a scaffold (Rakic, 1972; http://rakiclab.med.yale.edu/research/; Kawauchi, 2015 Figures 1, 2, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4595654/figure/F1/; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4595654/figure/F2/). Their leading process becomes anchored in MZ, they part company with their radial glial partners and their somata are pulled up to lie beneath MZ, or CP (Nadarajah et al., 2001; Sekine et al., 2011; Kitazawa et al., 2014).
The earliest born pyramidal cells form the deepest layer, L6. As later born neurones migrate, they pass through L6, forming sequentially more superficial layers. Phylogenetically, development of an additional germinal layer, the subventricular zone (SVZ) coincides with the appearance of L2-4 and emergence of the mammalian six layered neocortex (Noctor et al., 2004; Wu et al., 2005); the layers of phylogenetically older, three layered cortices being considered equivalent to L1, L5, and L6. The primate goes further, adding an additional germinal layer, the outer subventricular zone (OSVZ) (Lukaszewicz et al., 2005), which in the Macaque results in correspondingly deeper supragranular layers (Hoerder-Suabedissen and Molnár, 2015; Montiel et al., 2016). (Molnár et al., 2006, Figures 5, 7, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1931431/figure/F5/; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1931431/figure/F7/).
Hippocampal CA regions are often considered to contain a single pyramidal cell layer, though developing CA regions also include neurones generated in SVZ (Kitazawa et al., 2014). Whether this population remains distinct from those arising in VZ is unclear. Likewise, whether there is an ordered, birth-date-dependent, inside-out layering of stratum pyramidale in hippocampal CA regions appears a matter for debate. However, while there may not be the wide range of pyramidal classes to be found in neocortex, CA1 pyramids are not all identical; to quote Lorente de Nò (1934) “There are two types of pyramids, superficial and deep ones. The superficial are arranged in one or two very dense rows. The deep pyramids are grouped into several less dense rows below.…”'
“Deep” refers to the earliest born cells whose migration terminates close to the germinal layers, adjacent to the ventricles, cells destined to lie adjacent to stratum oriens (Supplementary Figure 1. http://uclsop.net/interneuron-reconstruction/ca1-pyramid). Superficial pyramids, lying adjacent to the future stratum radiatum, are born 1–2 days later, contain the calcium binding protein, Calbindin (Cb), Zinc (Slomianka and Geneser, 1997) and reelin and are more commonly dye-coupled, one with another (indicative of electrical gap junctions) than pyramids devoid of Cb (Baimbridge et al., 1991; Mercer et al., 2006; Mercer, 2012 for review). They also express different transcription factors (similar to deep/superficial expression in neocortex: Britanova et al., 2005; Dobreva et al., 2006; Leone et al., 2008); the deep cells expressing Sox5 and the superficial cells, SatB2 (Slomianka et al., 2011) and Zbtb20 (Xie et al., 2010), which may control Cb-expression (Nielsen et al., 2010). Even in CA regions disrupted by mutations, like Reeler, pyramids maintain separate identities, forming distinct—if mislocated—layers. Later born cells spend longer in MAZ; regions of IZ devoid of cell bodies, become filled with axons after early born cells have passed through (Kitazawa et al., 2014) and connections with these axons may delay migration of later born multipolar neurones (Altman and Bayer, 1990b). Later born superficial pyramids fire earlier, with higher probability during sharp wave ripples, while deep pyramids more frequently exhibit place fields, fields that are more plastic. Deep pyramidal firing correlates more with specific landmarks, superficial with general context (Geiller et al., 2017, for review).
The CA3 hippocampal plate (HP, future stratum pyramidale) becomes apparent at E18 (rat), expanding to adopt its pronounced curved profile by E22 (Altman and Bayer, 1990b). This expansion presents long migration paths for neurones generated in VZ, especially those destined to lie near the dentate gyrus. Radial movement from the tangential migratory stream into developing CA3 stratum pyramidale is promoted by Math2 (transcription factor), while continued tangential migration toward the developing dentate gyrus is promoted by Prox-1 (Sugiyama et al., 2014). CA3 pyramids are—on average—born earlier than CA1 neurones (E16-E20); with those that will lie close to CA1 born first (Bayer, 1980; Altman and Bayer, 1990b,a). Like CA1 pyramids, newly generated CA3 pyramidal cells move from VZ to MAZ, becoming multipolar and waiting there longer than CA1 cells (Nakahira and Yuasa, 2005); possibly for innervation from dentate gyrus (Altman and Bayer, 1990b). That neurones born at the same time in dentate gyrus and CA regions, exhibit similar gene expression patterns and become preferentially connected with each other (Deguchi et al., 2011), has important implications for functional circuitry.
A distinct CA2 region, delineated by PCP4 immunostaining, is thought to emerge postnatally and to reach adult dimensions at P21 (San Antonio et al., 2014). Until relatively recently, rodent hippocampi were thought not to contain a CA2 region and further developmental detail has yet to materialize.
Future neocortical pyramids climb radially, up a single, straight, radial glial process to reach their final destination. Sister cells, resulting from divisions of a single progenitor, therefore come to lie in a narrow, radially oriented “column.” “The Radial Unit Hypothesis,” proposed by Rakic (1988) as the anatomical basis for neocortical columnar architecture (Mountcastle, 1957), states that the position of a neurone's precursor in VZ determines its final horizontal coordinates, while its birth date determines its radial position.
In contrast, sister CA pyramids become distributed horizontally, often across large areas of stratum pyramidale (Kitazawa et al., 2014; Sugiyama et al., 2014). The leading processes of radial glial cells that direct migration here are not always straight, or radially oriented, as in neocortex. In CA1, they often curve, to run almost parallel with layer boundaries (Nakahira and Yuasa, 2005). In addition, migrating multipolar neurones continue to extend and retract processes in HP, contacting several radial glial cells, selecting one and migrating along a different path, in a “zig-zag” manner (Nowakowski and Rakic, 1979; Kitazawa et al., 2014; Xu et al., 2014; Hayashi et al., 2015, for review).
This raises an interesting question about local pyramidal interconnectivity. Neocortical pyramidal cells preferentially innervate their sisters (Yu et al., 2009; Costa and Hedin-Pereira, 2010), which exhibit, for example, similar orientation preferences in primary visual cortex, V1 (Li et al., 2012). Electrical coupling may precede sister-to-sister chemical synapse-formation since this similarity in orientation preference is lost when gap junctions are blocked from P1-7, or Cx26 (connexin 26) mutated (Li et al., 2012). If similar orientation preferences do not result solely from another influence, such as preferential innervation of sister-cells by common afferent axons, the physical separation of sister neurones may be a significant factor in determining whether they “wire together.”
In both mature neocortex and CA regions, powerful electrical synapses form between closely neighboring pyramids (CA1, Baimbridge et al., 1991; CA1-3, neocortex, Mercer et al., 2006; Mercer, 2012); an average of 25% of steady state and 10% peak action potential (AP) voltage change transferring to the coupled cell. The resultant post-junctional “spikelets” can trigger overshooting APs. Quite unlike electrical junctions between interneurones (neocortex: Gibson et al., 1999; Tamás et al., 2000; Amitai et al., 2002; Simon et al., 2005; hippocampus: Fukuda and Kosaka, 2000; Meyer et al., 2002; Allen et al., 2011), these junctions form between somata and proximal apical dendrites; hence the very high electrical-coupling ratios. Vertically distributed neocortical sister-cells are, therefore, well positioned for such connections; horizontally distributed sister-CA pyramids are not (unless axon-axonic electrical junctions are also involved: Schmitz et al., 2001; Wang et al., 2010). However, other factors, such as the preferential innervation of CA1 pyramids by CA3 pyramids exhibiting similar gene expression patterns (Deguchi et al., 2011), may also contribute to the emergence of functionally related sister-cell groups across regions. Indeed, CA1 sister pyramids rarely develop electrical or chemical synapses with each other, but they do receive common input from nearby fast-spiking (FS, but not non-FS) interneurones and exhibit synchronous synaptic activity, indicative of common excitatory drive (Xu et al., 2014). During development, early born GABAergic “hub” neurones with long range connections (which later develop into projection interneurones: Picardo et al., 2011) facilitate such connectivity (Bonifazi et al., 2009; Villette et al., 2016). Spiny cells connected by chemical synapses receive common excitatory (Song et al., 2005; Yoshimura and Callaway, 2005; Kampa et al., 2006) and inhibitory inputs (Xu et al., 2014) and deliver coincident outputs, more frequently than unconnected cells, and input-convergence from electrically coupled pyramids via chemical synapses is high (5:11, Bannister and Thomson, 2007).
(Table 1; Cheung et al., 2007; Hoerder-Suabedissen and Molnár, 2012, 2013, 2015; Hayashi et al., 2015, for reviews).
Both the inside out, sequential formation of L2-L6 and the sequential generation of the different classes of pyramids destined for a single layer, ensure a shifting environment as new cells are born and begin to migrate. Distinct expression patterns of a large array of genes coding for transcription factors and regulators, growth factors, receptors, peptidase inhibitors, acetylation regulatory factors, glycoproteins, kinases, guidance-, signal-, adhesion-, and extracellular matrix- molecules, reelin, its receptors and their downstream signaling pathways, not to mention those genes for which no function has yet been found, have been identified in sub-populations of progenitors and differentiating neurones. The milieu into which a neurone is born, those it travels through as it migrates from VZ/SVZ, through MAZ, IZ and into CP/HP and where it eventually establishes itself, are both temporally and spatially regulated. One example is the postmitotic expression of Sox5 in subcortically projecting deep layer pyramids and Satb2 in corticocortically projecting, superficial layer cells (Slomianka et al., 2011). The latter, if induced to express Sox5 ectopically, lose their corticocortical projections and instead project subcortically (Arlotta et al., 2005; Alcamo et al., 2008; Britanova et al., 2008; Fishell and Hanashima, 2008, for review).
Like other layers, L6 contains several distinct classes of spiny, glutamatergic principal cells (Thomson, 2010 for review). The birth-dates of two broad groups are distinguished by their expression of latexin (carboxypeptidase-A inhibitor). Corticortical cells, which express latexin are born after corticothalamic cells which do not: E15 cf E14 (Arimatsu and Ishida, 2002). (Thomson and Lamy, 2007, Figure 5, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2518047/figure/F5/).
Only earlier born, corticothalamic pyramids receive direct thalamic input. One subclass of these upright cells, with apical dendritic tufts in L4, send narrow, ascending axonal arbors to L4 (and sometimes lower L3) where it terminates with characteristic short, drumstick-like side branches. These neurones project subcortically to “specific,” or primary sensory thalamic nuclei and to nucleus reticularis thalami (nRT, the thalamic inhibitory nucleus) (Zhang and Deschenes, 1998). All L6 corticothalamic pyramids fire with minimally accommodating/adapting, near tonic discharge and preferentially innervate GABAergic cells with consistently facilitating patterns of transmitter release (West et al., 2006). In neocortex, >90% of their synaptic boutons contact dendritic shafts of non-spiny neurones (White and Keller, 1987), including L4 (Tarczy-Hornoch et al., 1999; Beierlein et al., 2003) and L5 interneurones (Staiger et al., 1996). Despite their frequent innervation of parvalbumin (PV) interneurones (which receive depressing inputs from all other pyramidal classes), L6 corticothalamic pyramids elicited facilitating EPSPs (excitatory postsynaptic potentials) in all cell types studied, including ventroposterior, posterior medial thalamic and nRT neurones. This contrasts with the depressing EPSPs elicited by L5 pyramids in posterior medial thalamic nucleus (Reichova and Sherman, 2004).
The second corticothalamic subclass, more commonly found in deep L6, projects to both specific and non-specific thalamic regions such as PO (posterior thalamic group) The apical dendrites of these short, upright pyramids and their ascending axons typically terminate in upper L5. Neither subgroup of corticothalamic cells has long horizontal axon collaterals in the infragranular layers, all branches turn toward the pial surface.
In cats and primates, where L4 subdivisions are morphologically and functionally distinct, subclasses of corticothalamic cells are found, each with its apical dendritic branches and axonal ramifications restricted to a specific L4 sublayer (Lund, 1987; Wiser and Callaway, 1996). Further specificity was demonstrated by a study combining in vivo physiology and morphology in cat V1 (Hirsch et al., 1998b,a). L6 pyramidal simple cells (“simple” implying significant direct input from lateral geniculate nucleus, LGN and resembling “specific” thalamocortical pyramids) targeted L6 and/or L4, layers rich in simple cells. L6 complex cells (receiving integrated, rather than “specific” corticothalamic inputs), targeted L2/3 and L5, layers rich in complex cells.
In striking contrast to corticothalamic pyramidal cells, are the rapidly adapting, corticocortical L6 pyramids, which preferentially innervate other pyramidal cells with “depressing” synapses and display an array of morphologies (Mercer et al., 2005), short, upright pyramids whose apical dendrites terminate in L5, bipolar cells and inverted pyramids. All have long, horizontal axons confined to L5/6 (Zhang and Deschenes, 1998; Mercer et al., 2005), some crossing areal boundaries. Their pronounced spike accommodation/adaptation cannot be overcome by injecting larger square-wave current pulses; these only result in more rapid and profound soma/initial segment Na+ channel inactivation. However, a ramp-shaped current superimposed on the original threshold square-wave pulse, activates tonic firing of overshooting APs, probably originating at more distant axonal locations and propagated, or reflected passively, back to the soma (unpublished; Stuart et al., 1997; Colbert and Pan, 2002; Clark et al., 2005, for axonal spike-initiation).
The near tonically firing claustrum-projecting cells form the third major L6 pyramidal class, with long slender apical dendrites that reach L1 without forming a tuft there and a broad, axonal arbor confined to L5 and L6 (Katz, 1987). Like L6 corticocortical cells, claustrum-projecting pyramids preferentially innervate pyramids locally, with “depressing” synapses (Mercer et al., 2005).
L6 is often perceived as a predominantly thalamo-recipient layer, but only corticothalamic pyramids receive powerful, direct thalamic input. Nor do corticocortical cells receive powerful excitation from neighboring thalamo-recipient corticothalamic cells. Some descending inhibitory projections from L4 ramify in L6, but excitatory projections from superficial layers are often narrow and sparse. Binzegger et al. (2004, cat V1) estimated the numbers of synapses supplied to each layer by cortical and LGN relay neurones. When compared with estimates based on stereological analysis (Beaulieu and Colonnier, 1985), the estimates for excitatory synapses were within 10% for L2/3 and L5, but differed by 32% for L4 and 70% for L6. Many additional corticocortical, or subcortical inputs are required to account for the boutons in these thalamo-recipient layers. (Thomson, 2010, Figure 4; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2885865/figure/F4/).
In cats and primates most subplate (SP) neurones disappear during development; a few remaining in the underlying white matter as interstitial neurones (Kostovic and Rakic, 1980; Luskin and Shatz, 1985; Valverde et al., 1989; Naegele et al., 1991). However, in rodents, degeneration in SP is less dramatic and the SP becomes L6b (or L7) (Valverde et al., 1989; Ferrer et al., 1992).
The principal inputs to L5 (and to L5 pyramidal dendrites in L3) are local and more distant corticocortical projections. In turn, large L5 pyramidal cells project to many subcortical targets, including “non-specific” thalamic nuclei, superior colliculus, pons and spinal cord (targets depending on cortical region). Smaller L5 pyramids project to other cortical and subcortical regions and transcallosally, to contralateral neocortex.
Upper L5 contains the largest neocortical pyramids (only these approaching the size and spine densities of CA pyramids). In cat V1, the large cells that project to the colliculi, and/or the pons (Hallman et al., 1988), have thick apical dendrites with well-developed apical tufts in L1/2 and substantial basal dendritic arbors largely contained within L5. The largest, Betz cells (Betz, 1874), are found in motor cortex and project via the corticospinal tract to the spinal cord. Large L5 cells display a stereotypical “intrinsically burst-firing” behavior (Connors et al., 1982); the burst of two or more, high frequency spikes being superimposed on a well-developed depolarizing envelope, due to activation of a dendritic Ca2+ spike (Purpura and Shofer, 1965; Llinas, 1975; Larkum et al., 1999). The short interspike-interval train of two or more spikes, typical of rapidly adapting/accommodating neurones (smaller L5-, L6 corticocortical-, and some L4 pyramids), should not be confused with stereotypical bursts (though it often is); it is not superimposed upon, or triggered by a stereotypical depolarizing envelope and does not occur repetitively if the cell is held near spike threshold. Quasi burst-firing can also be elicited in adapting cells electrically coupled to intrinsic bursters (Mercer et al., 2006). The local axons of large L5 pyramids arborize almost exclusively within the deep layers while the smaller pyramids also project to the superficial layers (Larsen and Callaway, 2006).
A significant input to these large, intrinsically burst-firing pyramids comes from smaller L5 pyramids. Whether these smaller pyramids project to non-specific thalamus, or to other cortical regions was not determined. In adult rat L5, small adapting pyramids were 10 times more likely to innervate large burst-firing pyramids than vice versa (unpublished data: Thomson and West, 1993; Deuchars et al., 1994). Large L5 pyramidal cells that are close neighbors are, however, relatively densely interconnected (hit rate of 1:10: Markram, 1997). Large, but not small L5 pyramids, also receive a dense, highly focussed, input from deep L3 pyramids (hit rate > 1:4, Hübener et al., 1990; Thomson and Bannister, 1998, for reconstructions of cat L5 pyramids).
The apical dendrites of small-medium corticothalamic and corticocortical L5 pyramids are slender, rarely extend beyond L2/3 and have no significant apical tuft. L5 corticothalamic pyramids provide large boutons to non-specific thalamic regions from which they receive no reciprocal inputs, in contrast to L6 corticothalamic cells which are reciprocally connected with “specific” thalamic nuclei and deliver small boutons (Van Horn and Sherman, 2004). A separate population of smaller, shorter L5 pyramids projects transcallosally (Hübener et al., 1990; Kasper et al., 1994). Transcallosal cells are found in all layers except L1 (Kasper et al., 1994).
If we can assume that three layered cortices in some non-mammalian species do a perfectly good job, as far as the requirements of those animals are concerned, receiving e.g., sensory information in one layer (equivalent to mammalian L6) and integrating that information with signals from elsewhere, in the adjacent layer (equivalent to L5), which then sends instructions to other brain regions, we could ask why a presumed need for more complex and sophisticated organization and integration of that information could not have been achieved simply by expanding these two existing layers. Whether this was attempted in some long lost evolutionary dead-end, we may never know, we can only assume that such an attempt did not survive. Instead, a new germinal zone, SVC and three new layers (L2-L4) to which the SVC contributes spiny cells, were added. Interestingly, these new layers repeat the pattern established in deeper layers: peripheral input into L4, with integration within, and distribution from L2/3.
Layer 4 contains two broad classes of spiny excitatory cells. Typically, the basal dendrites of L4 pyramidal cells are contained within L4 with apical oblique dendrites in L2/3 (though they receive little or no input from local L3 pyramids) and an apical dendrite extending into L1, often forming a small tuft there. Spiny stellate cells lack an apical dendrite, most or all of their dendrites are confined to L4 (Lund, 1973). Perhaps the most striking distinguishing feature of L4 spiny neurones in rat and cat, especially when compared with the “chunkier” pyramids in adjacent layers, is their simple (Rojo et al., 2016) and delicate appearance (Bannister and Thomson, 2007).
Despite being a major thalamo-recipient layer, thalamocortical inputs to L4 contribute only 6% of the synapses onto spiny stellate neurones in cat V1 and up to 22.9% in mouse (Benshalom and White, 1986), terminating predominantly on dendritic spines via large en-passant boutons. In contrast, ascending L6 corticothalamic pyramidal axons, form synapses with small boutons, but provide 45% of the excitatory inputs onto L4 spiny cells (cat, primate, Lund et al., 1988, for review). In primate V1 axons from area MT terminate in L1, L4B, and L6. This contrasts with other so called “feedback” connections from “higher” visual areas terminating in L1; projections that might more meaningfully be termed “cognitive” or “attentional” feed-forward. In V2 they terminate primarily in L1 and L5 or L6 (Rockland and Knutson, 2000). A further 28% of the excitatory input to L4 spiny cells originates from within L4 (cat, Ahmed et al., 1994). Despite the small numbers of thalamocortical inputs, their large boutons provide secure, faithful transmission of early presynaptic spikes, albeit followed by pronounced presynaptically mediated depression. Thalamocortical synapses have three times more release sites than those of local circuit axons, with higher release probabilities, making the average thalamocortical connection several times more effective (Gil et al., 1999, mouse S1), at the start of a spike train.
The axons of L4 spiny neurones make dense, topographically precise ascending projections to L3 and sparse descending projections to upper L5 (rat, cat) (Valverde, 1976; Parnavelas et al., 1977; Feldman and Peters, 1978; Gilbert, 1983; Burkhalter, 1989) where they innervate pyramids and (less commonly) interneurones (Thomson et al., 2002). Pyramids and spiny stellates contribute to these projections and both provide relatively narrow, horizontal arbors within L4. In cat V1, some spiny cell axons make most of their synapses within L4, others form a larger proportion in L3 (Binzegger et al., 2004), a finding supported by morphometric analysis coupled with paired recordings in rat barrel cortex (Lübke et al., 2003) and one that correlates with distinct electrophysiological classes (below). The sparse projection, from L4 to L6, appears to originate predominantly with pyramids (unpublished).
The firing patterns of adult L4 pyramidal and/or spiny stellate cells correlated with distinct connectivity patterns. Stereotypical intrinsic bursts were rare. Around 60% displayed rapid spike accommodation and frequency adaptation (recoverable with a superimposed ramp) and innervated other L4 spiny cells. The remaining 40% produced a short train of 3–5 short interspike interval spikes, followed by a brief afterdepolarization, then a slow afterhyperpolarization upon which a spike-train of increasing interspike interval was superimposed. These cells preferentially innervated L3 pyramids (Bannister and Thomson, 2007). This raises interesting questions about patterns of synaptic input in vivo and how they might interact with the cells' inherent firing characteristics; tonic input to L3, phasic to neighboring L4 pyramids in reponse to maintained depolarization.
Connectivity ratios for pairs of L4 pyramids were relatively low (1:18 adult rat; 1:14 cat V1), with no selection for firing characteristics, and fell off extremely rapidly with increasing horizontal somatic separation. All identified synaptic contacts onto spiny cells (L4 and L3, rat and cat) were onto proximal primary, secondary, and tertiary, electrotonically compact basal dendrites, and all EPSPs were brief and depressing (Bannister and Thomson, 2007).
Layer 3 pyramidal cells are “typical” pyramids, with adapting firing patterns, well developed basal and apical oblique dendrites and an apical dendrite forming a tuft in L1. The largest are close to the L4 border. More superficial L2 cells are, naturally, very short with almost no apical dendrite. L3 pyramidal axons ramify densely in L2/3 delivering depressing inputs to other L3 pyramids with a hit rate of 1:3 that falls off only gradually with distance. Their main descending axons typically pass through L4 without branching to ramify in L5, in rat (Lorente de Nó, 1922; Burkhalter, 1989), cat (O'Leary, 1941; Gilbert and Wiesel, 1983; Kisvárday et al., 1986), and primate (Spatz et al., 1970; Lund et al., 1993; Yoshioka et al., 1994; Kritzer and Goldman-Rakic, 1995; Fujita and Fujita, 1996), where they innervate large L5 pyramids;. Somewhat surprisingly some deep L3 pyramids in mice have substantial axonal arbors within L4 (Larsen and Callaway, 2006). Their targets in L4 are of interest, because although L3 pyramids innervate L4 interneurones that have dendrites projecting into L3, they rarely, if ever excite L4 spiny cells in adult rat or cat (Bannister and Thomson, 2007).
An additional, distinctive firing pattern has been described in cat V1—chattering cells (Gray and McCormick, 1996). These neurones generate extremely fast intrinsic spike-bursts, with an intraburst firing rate up to 800 s−1 and a repeat rate of 20–70 s−1, in response to visual stimuli or suprathreshold current injection. During visual stimulation these cells exhibit pronounced oscillations in membrane potential that are largely absent at rest. All chattering cells recovered after dye-filling were typical L2/3 pyramidal neurones.
L3 receives a substantial trans-callosal input, larger than that to L5 (Porter and White, 1986) and pyramids in both layers project trans-callosally, often to topographically related cortical areas. In L2/3 of rat barrel cortex, 97% of the connections, both local and distant, made by L3 pyramidal axons are onto dendritic spines. This is a striking target preference (seen in all layers), when only 80% of all asymmetrical synapses in L3 are onto spines (White and Czeiger, 1991) and L6 corticothalamic cells preferentially innervate aspiny dendritic shafts in L4 and L6 (Elhanany and White, 1990). In primate visual, motor and somatosensory cortex, L3 (and to a lesser extent L5) cells also provide dense innervation of patches of cortex a few 100 μ wide and up to a few millimeters from the injection site within L1–3 (Levitt et al., 1993, 1994). In prefrontal cortex, a narrow stripe-like, rather than a patchy pattern is apparent (Levitt et al., 1993).
Deep L3 pyramids can also receive thalamocortical inputs from primary sensory thalamus, again, largely to proximal basal dendrites, though the further they are from the L4 border, the weaker this input becomes (White and Hersch, 1982). A major part of the projection from the pulvinar (the most caudal thalamic group, with roles in attention and oculomotor behavior) also terminates in L3 extrastriate visual areas (Rockland et al., 1999). The dense, focussed, excitatory input from L4 spiny cells onto L3 pyramids also terminates proximally, on first, second, or third order basal dendrites (Thomson et al., 2002; Thomson and Bannister, 2003), while the many inputs from other L3 pyramids are located more distally (mean 97 μm cf. 69 μm: Feldmeyer et al., 2002), on both basal and apical oblique dendritic branches. Proximal basal synapses result in taller, narrower EPSPs (excitatory postsynaptic potentials) than more distal inputs.
This relationship is partly due to the smoothing of current transfer over the length of a cable with resistance and capacitance (Rall, 1962) and partly to the activation of voltage-gated ion channels distributed with unique patterns of surface expression across somata, axons and dendrites of each class of neurone (Nusser, 2009, 2012, for reviews). Amongst the conductances whose density increases with distance from the soma, perhaps the most studied has been the rapidly inactivating K+ current, IA. The IA α-subunit Kv4.3 clusters in neocortical pyramidal dendrites and dendritic spines (Burkhalter et al., 2006) and IA density increases as INa decreases more distally in large L5 pyramidal basal dendrites (Kampa et al., 2006). In CA1 pyramidal dendrites, the density of Kv4.2 also increases along the soma-dendritic axis (Kerti et al., 2012), although the gradient was shallower than expected from dendritic recordings of IA (e.g., Hoffman et al., 1997; Sun et al., 2011; Nestor and Hoffman, 2012); a discrepancy that might result from involvement of other IA α-subunits, auxiliary subunits, or modulators of channel conductance.
In mouse L3 pyramids, selective blockade of Kv4.2/4.3 enhanced glu-EPSPs activated by focal glutamate-uncaging at single spines. It also promoted activation of fast, dendritic spikes by summed glu-EPSPs at proximal dendritic locations and of slower, all-or-none, stereotypical, depolarizing events at proximal-intermediate dendritic locations (A Biro, A Bremaud, A. Ruiz, unpublished). Without channel-blockers these additional events required near simultaneous activation at 7–8 closely neighboring locations. Such events would enhance responses of L3 cells to thalamocortical inputs. However, two excitatory synapses provided by any one presynaptic axon rarely, if ever innervate the same pyramidal dendrite, let alone 7 or 8. They distribute across the dendritic tree on different branches, albeit at similar electrotonic distances from the soma. How frequently 7–8 presynaptic terminals, each from 7 to 8 different presynaptic neurones, all impinging on a single dendritic compartment, are activated simultaneously in life, is difficult to predict. More distal inputs e.g., from other L3 neurones or cortical regions may lower the threshold for such events (Branco and Häusser, 2011), perhaps when attention to a behaviourally important input is required. In both mouse A1 and V1 L4, local circuit activation amplified and prolonged thalamocortical responses, without altering frequency or direction selectivity and with spectral range and tuning (auditory), or with frequency and direction selectivity (visual) preserved (Li et al., 2013a,b).
Both cellular and circuit properties appear to have developed to preserve the integrity of the signals arriving from the periphery. In L4, thalamocortical input arrives in proximal postsynaptic compartments that are near optimal for rapid, faithful transmission to soma/axon. The signals carrying this information may then be enhanced or suppressed in L3 and additional features, like direction in V1, computed there. However, the purity of salient feature representation in the direct thalamocortical signal is not compromised by excitation from other layers; from cells dealing with more highly integrated and processed information. The flow of excitatory input from the thalamus is unidirectional: from thalamus (and L6) to L4, L4 to L3, and L3 to L5 and transmitted thence to other cortical and subcortical regions. The strength of a response may be altered by coincident inputs from the recipient layer, from other layers, or regions; the response may be tuned, or suppressed by inhibition in L4 activated from elsewhere, but its fundamental integrity is preserved (Thomson et al., 2002; Thomson and Lamy, 2007, for review).
In this, the neocortical circuit is strongly reminiscent of hippocampus where dentate granule cells, activated by inputs from enthorinal cortex, send excitatory inputs to CA3, CA3 pyramids send excitatory inputs to CA2 and CA1 and CA1 pyramids project to different layers of the enthorinal cortex via the subiculum. CA1 pyramids do not project “back” to excite CA3 pyramidal cells. The CA1 neurones that innervate CA3 and dentate gyrus are not glutamatergic pyramidal cells, but GABAergic interneurones—“back projection cells” (below and Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/backprojection/). Some CA2 pyramids, as well as interneurones, do project “back” to CA3 (Figure 1; Mercer et al., 2007, 2012; Mercer, 2012), but their targets there have yet to be identified.
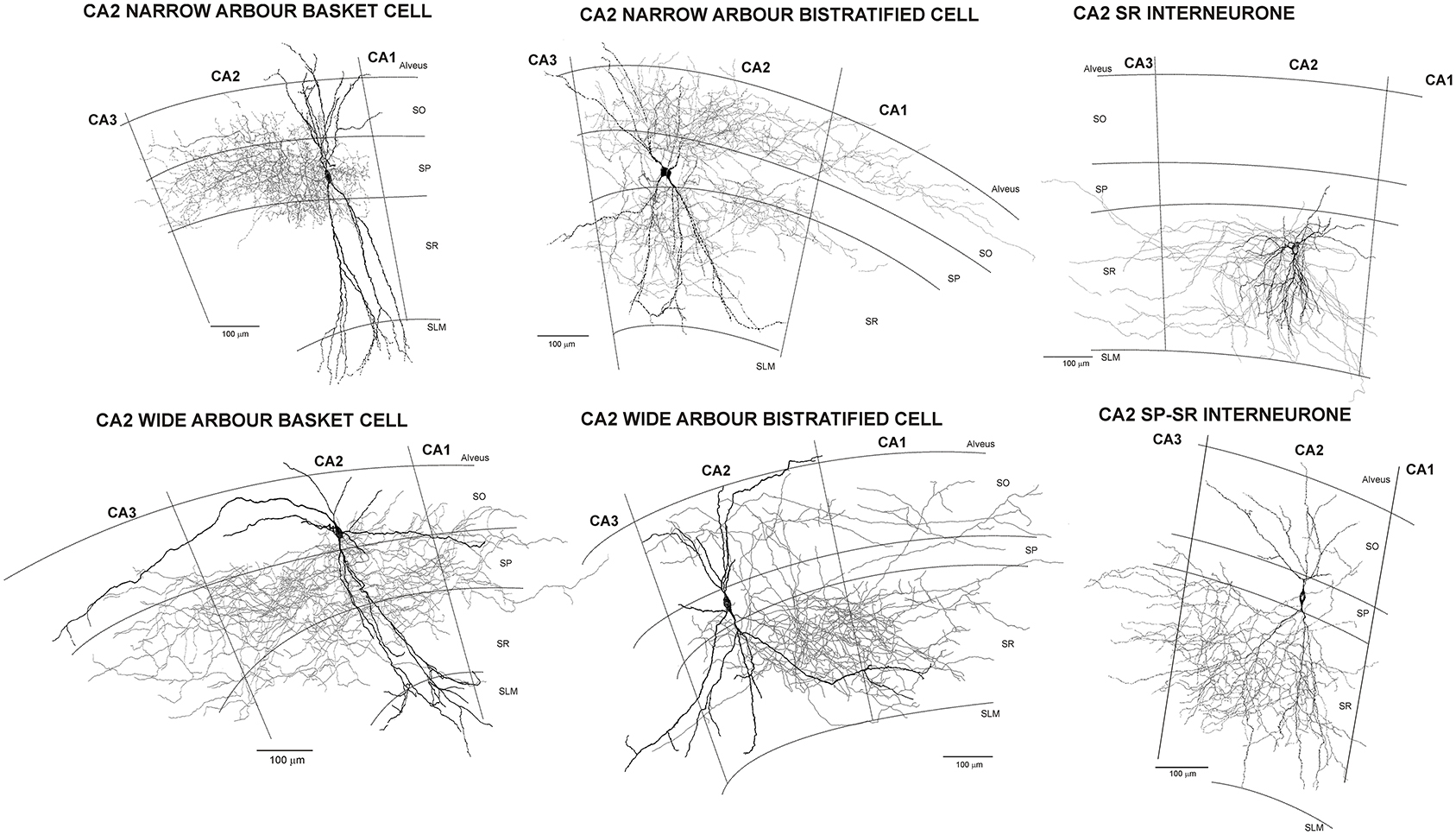
Figure 1. Reconstructions of CA2 interneurones filled during intracellular recordings in adult rat hippocampus (from Mercer et al., 2007, 2012). The largest population of interneurones recorded and filled in CA2 were basket cells. Like those in CA1, CA2 basket cells had dendrites that extended through stratum oriens, sometimes entering the alveus, and through stratum radiatum and into stratum lacunosum moleculare. Their axons arbourized extensively in stratum pyramidale and in some, also in very proximal stratum oriens and/or radiatum (wide axonal arbor basket cells). Two distinct subtypes of CA2 basket cells were identified. The first (CA2 narrow dendritic arbor basket cells) resembled those of CA1 with a narrow, aspiny dendritic arbor and axon confined to CA2. In contrast, both the axons and dendrites of the CA2 wide dendritic arbor basket cells, extended into all three CA-subfields and the horizontally oriented, distal dendritic branches were sparsely spiny. Similarly, two subtypes of CA2 bistratified cells were reported, CA2 narrow and wide dendritic arbor bistratified cells. The dendrites of both subtypes extended through stratum oriens and radiatum without entering stratum lacunosum moleculare, those of wide dendritic arbor cells extending further horizontally than is typical of CA1 bistratified cells and becoming sparsely spiny. Bistratified cell axons ramified in both CA2 and proximal CA1, but stopped abruptly at the CA2/CA3 border. The somata of CA2 SP-SR interneurones were found in stratum pyramidale and their dendrites extended to stratum oriens, branched extensively in stratum radiatum, rarely penetrated SLM, but often extended horizontally to CA1 and CA3. Their axons emerged from the soma and arbourized almost exclusively in stratum radiatum of CA2. The axons and dendrites of CA2 stratum radiatum, Reelin-immunopositive interneurones ramified predominantly in CA2 stratum radiatum, with a few axonal branches extending into neighboring regions.
One of the reasons such an elegant organization in neocortex has been difficult to accept, or even imagine (e.g., Binzegger et al., 2004) is the apparent chaos that results from neocortical layering, cf the discreet regional organization in hippocampus. In neocortical layers 2–6, there are somata, axons and apical and basal dendrites arising from many different classes of neurones whose somata reside in any of these layers. The inputs from other layers, from other cortical and subcortical areas may terminate neatly in specific layers, or sublayers, but what do they find there but a jumbled multiplicity of potential targets. To propose that these axons can seek out and connect only to specific targets amongst this confusion—not only to connect to certain subclasses of neurones, but to specific postsynaptic compartments belonging to those neurones—seemed quite preposterous.
It is, however, the case. Those of us not skilled in the art may view electron micrographs of the neocortical neuropil with a sense of horrified bewilderment, but axons and dendrites apparently know with whom they are destined to communicate and make it their business to find each other. We have come to accept that GABAergic interneuronal axons can find and innnervate very specific targets, eschewing all others in their path, so why have we assumed that excitatory axons make synaptic contacts indiscriminately, with any old neuronal element they happen to pass? (see also Markram et al., 2015). Different pyramidal classes are born on different embryonic days, express different combinations of gene products at different times during migration and differentiation, migrate through gradually changing chemical and physical environments, halt for different lengths of time en route and receive different incoming synapses. Neocortical pyramidal (unlike interneuronal) axons may often follow almost linear, class-specific trajectories, but their targets are more flexible—employing spines to sample the environment and twisting and bending to capture an attractive input.
We do not yet know which molecules are involved in this synaptic partner-identification; they are likely to be different at each class of synapse (defined by the subclasses of pre- and post-synaptic neurones). But we do know that each class of synapse, so defined, displays its own unique characteristics: specificity in transmitter(s) used, pre- and post-synaptic receptors inserted, frequency-dependent patterns of transmitter release, postsynaptic compartments involved and thereby the modulation of each input by cable and voltage-gated properties and by other nearby inputs.
There is considerable evidence that epigenetic cues are required for the final differentiation of neocortical neurones, still somewhat multi-potent on arrival. Obvious candidates for such cues are in-growing axons, particularly, perhaps, thalamocortical axons in primary sensory regions (López-Bendito and Molnár, 2003, for review).
Connections between the neocortex and subcortical structures course through the internal capsule, a thick fiber tract lying between the caudate nucleus and thalamus. Subplate neurones, diverse in site of origin, birth date, survival and gene expression, exhibit a range of morphologies and axonal projection patterns (Hoerder-Suabedissen and Molnár, 2013), including pioneer axons to the emerging internal capsule and commissural fibers of the early hippocampus (Sarnat and Flores-Sarnat, 2002). The subplate zone becomes a “waiting compartment” in which thalamocortical-, basal forebrain cholinergic-, callosal, commissural, and ipsilateral corticocortical-afferents cease growing until an appropriate environment or signal emerges.
Early born GABAergic cells migrate tangentially from their germinal zone in the LGE (lateral ganglionic eminence) and into the MGE (medial ganglionic eminence), forming a stream of cells between the MGE and globus pallidus (E11.5 to E14). These “corridor” cells form a permissive pathway through which thalamocortical axons can grow (López-Bendito et al., 2006) (Molnár et al., 2012: Figures 1 and 2; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4370206/figure/F1/; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5040712/figure/F2/). Otherwise chemical repellents and the structure of the PSPB (pallial-subpallial boundaries): high cell-density, and a radial glial fascicle running across the trajectory of thalamocortical axons, would hinder their onward growth toward the cortex. Cortigofugal axons may also assist the forward growth of thalamocortical axons through this barrier (Molnár et al., 1998; Molnár and Butler, 2002).
Like many cortical neurones, most thalamic neurones are born between E13 and E19 (rat), the LGN, for example, between E12 and E14. By E16/E17, nuclear differentiation in thalamus has begun and both neocortex and dorsal (specific) thalamus have started to generate prospective reciprocal connections. To reach their destinations, these axons must overcome and cross several emerging barriers, or boundary zones: the diencephalic-telencephalic (DTB) and (PSPB) form transient barriers to axon growth, but interestingly, also a route for early born migrating neurones that form the permissive corridor. A largely transient population of pioneering subplate neurones sends the first projections to the internal capsule (IC) and beyond; though the axons of other cortical neurones actually invade and innervate specific thalamic nuclei first. However, without subplate projections, thalamocortical axons cannot traverse the PSPB to enter the telencephalon. Moreover, subplate ablation at this time, prevents formation of ocular dominance columns, inhibition in L4 does not mature, barrels are disrupted and spindle activity abolished (Hoerder-Suabedissen and Molnár, 2015).
By P0, axons from L6 have reached the ventrobasal thalamic nucleus (primary somatosensory) and over the next 4 days they invade and form a barreloid pattern. Corticothalamic fibers do not, however, ramify within LGN until the eyes open and spontaneous activity begins. By E16-19 thalamocortical axons have accumulated in the subplate, but they also wait, extending horizontal collaterals that may facilitate reorganization of maps at a later date, until peripheral afferents innervate the appropriate dorsal thalamic nucleus (Molnár et al., 2012).
Here we see an important change in the forward growth of thalamocortical axons from the external route seen in lower vertebrates lacking a six layered cortex, where they run over the developing cortex, to the internal route of mammals, via the corpus callosum. The midline repellent, Slit2, redirects the migration of corridor neurones, switching thalamic axons from an external to a mammalian-specific internal path (Bielle et al., 2011). It is proposed that this switch allowed the neocortex to grow radially. Interestingly, the hippocampus is deep in the brain and bounded by dense fiber tracts. Perhaps it was not able to grow in this way.
Having accumulated in the subplate, the growth of thalamocortical axons into neocortex is prevented if SNARE-complex proteins, essential for AP-driven, Ca2+-dependent transmitter release (though not spontaneous, “miniatures,” Ramirez and Kavalali, 2011) are knocked out. With the arrival of thalamocortical axons, transient circuits form between thalamic axons, subplate and L4 neurones. Multiple interactions now control the growth of- and connections made by- incoming axons and the development of cortical neurones and circuits. For example, two extracellular molecules: NRN1 (Neuritin-1, a GPI-anchored neuronal protein that modulates neurite outgrowth) and VGF (a nerve growth factor), both manufactured by thalamic cells and transported to their neocortical terminals, promote L4 spiny stellate dendritic growth—selectively (Sato et al., 2012). Lhx2 promotes activity-dependent L4 dendritic growth toward thalamic afferents, by inducing the transcription factor BBtbd3 (Wang et al., 2017), while several neurotrophins are implicated in the critical stages during which precise thalamocortical connections are made (Ma et al., 2002; Yamamoto and Hanamura, 2005). As L4 and its thalamic inputs mature postnatally, spiny stellate cells receive a transient input from SOM interneurones in L5b, which themselves receive thalamic input. Development of thalamic input to spiny stellates is delayed in the absence of this transient input (Marques-Smith et al., 2016), while thalamic afferents are misdirected to inappropriate barrels when Proteoglycan-2 (PRG-1, a phospholipid- interacting molecule) is knocked out (Cheng et al., 2016).
As in neocortex, expression patterns demonstrate that pyramidal classes are predestined at E15.5 while they are still in IZ. For example, SCIP (POU domain transcription factor), is present in future CA1 pyramids (Frantz et al., 1994; Tole et al., 1997), while KA1 (GluR subunit) is expressed in future CA3 neurones (Wisden and Seeburg, 1993; Tole et al., 1997) and many regulators that control neurogenesis in neocortex also act here (Urbán and Guillemot, 2014).
From LII and LIII of the entorhinal cortex information from many subcortical structures is relayed to the hippocampus via the perforant path, providing powerful input to the molecular layer of the dentate gyrus and to distal apical dendritic tufts of CA1-3 pyramidal cells in stratum lacunosum moleculare. Mossy fibers project from dentate granule cells to CA3 stratum lucidum, innervating the most proximal apical dendrites of CA3 pyramids with huge boutons. In turn, CA3 pyramidal axons (Schaffer collaterals) project to stratum radiatum and oriens of CA1. The hippocampus also sends information to and receives inputs from subcortical regions: medial septum, cingulate gyrus, mammillary bodies, thalamus and amygdala as well as regions of association cortex.
The precise position of CA2 in this unidirectional trisynaptic pathway has been unveiled more recently (Chevaleyre and Piskorowski, 2016; Dudek et al., 2016; for reviews). LII of the entorhinal cortex provides strong, proximal excitation to CA2 pyramidal cells via dentate and mossy fiber synapses in stratum lucidum (Kohara et al., 2014). CA2 is also thought to receive direct input from LIII of the entorhinal cortex in stratum radiatum and lacunosum moleculare (Chevaleyre and Siegelbaum, 2010) in addition to Schaffer collaterals (Chevaleyre and Siegelbaum, 2010; Jones and McHugh, 2011). In turn, CA2 pyramids project preferentially to calbindin-negative, deep CA1 pyramids which lie adjacent to stratum oriens (Kohara et al., 2014). CA2 pyramids also project “back” to the supramammillary nucleus (Tamamaki et al., 1988; Cui et al., 2013) and in some cases, back to LII of the medial enthorinal cortex (Rowland et al., 2013).
During development, a projection from CA1 non-pyramidal cells to the medial septum, (hippocampo-septal pathway) (Supèr and Soriano, 1994) develops before the reverse, septo-hippocampal projection: E15 vs. E17 (mouse) (for parallel studies in rat and involvement of Cajal-Retzius cells: Ceranik et al., 1999, 2000). Chemo-repulsive semiphorins repel septal axons, promote growth cone collapse and may contribute to target selection; GABAergic septo-hippocampal fibers terminate preferentially on sema3C-expressing GABAergic interneurones, while cholinergic septo-hippocampal fibers terminate on sema3E- and sema3A-expressing CA pyramidal and dentate granule cells (Pascual et al., 2005).
By E17, LIII entorhinal axons are ramifying densely and exclusively in stratum lacunosum moleculare. Invasion of the dentate comes later, but by E19, the first entorhinal axons begin to ramify there, predominantly in the outer molecular layer (Supèr and Soriano, 1994). Commissural fibers first enter the contralateral hippocampus at E18 and arborize in stratum radiatum and oriens, along with the Schaffer collaterals. The earliest commissural fibers to enter the dentate gyrus are seen even later, at P2, terminating in the inner zone of the molecular layer and the hilus. Thus, as in neocortex, incoming pathways do not meander indiscriminately; they invade their ultimate target layers and regions from their earliest appearance, some following paths marked by early born, non-pyramidal neurones.
Again, a host of genes selectively expressed at different times, in different locations and in different cell classes in the developing hippocampus appear to contribute to its normal development. For examples, see Fazzari et al. (2010) for signaling with Nrg1(Neuregulin1, a ligand for ERBB3 and 4) and ErbB4 (receptor tyrosine kinase, an epidermal growth factor receptor); Silva et al. (2015), for LGI1 (Leucine-rich, glioma inactivated 1) in both neocortex and hippocampus; (Mingorance et al., 2004), for tempero-spatial patterns of Nogo expression and its debated involvement in perforant path development (Urbán and Guillemot, 2014).
Large numbers of calretinin-expressing (CR), bipolar or multipolar Cajal-Retzius neurones appear in the molecular layer of the developing CP, becoming distributed through all layers. Collaterals of their thick primary axon make synaptic contact first with pyramidal cells in emerging L6, then sequentially with pyramids in L5 to L2 (del Rio et al., 1995). In hippocampus they become densely innervated by afferent axons from entorhinal cortex, whose ramification in CA stratum lacunosum moleculare and dentate outer molecular layer is severely reduced if Cajal-Retzius cells are ablated. Up to 90% of these cells disappear during development; the remainder form a sparse population in adult neocortical L1 (del Rio et al., 1995), hippocampal stratum lacunosum moleculare and the dentate gyrus outer molecular layer (Del Río et al., 1997). Cajal-Retzius neurones produce GABA, possibly ACh, calmodulin, PV(parvalbumin) and CR and neuropeptides. They express important mediators of radial neuroblast migration and lamination of the cortical plate: Reelin (a secreted extracellular matrix protein, essential for the normal “inside-out” development of neocortical layering), Lis1 (a motor protein Dynein-regulator), and Dscam (Down syndrome cell adhesion molecule). In addition to forming the first intrinsic synaptic circuits of the cortical plate and its first afferent and efferent connections with subcortical structures, Cajal-Retzius neurones may contribute to ocular dominance column-formation, to regulation of neurogenesis, and to cortical repair (Sarnat and Flores-Sarnat, 2002, for review).
(Meyer and Wahle, 1988; Wonders and Anderson, 2006; Batista-Brito and Fishell, 2009; Vitalis and Rossier, 2011; Miyoshi et al., 2013; Li and Pleasure, 2014; Wamsley and Fishell, 2017, for reviews; Yavorska and Wehr, 2016, Figure 1, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5040712/figure/F1/; Batista-Brito and Fishell, 2009, Figure 3, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4465088/figure/F3/; Cauli et al., 2014, Figure 1, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4067953/figure/F1/; Jovanovic and Thomson, 2011, Figure 1, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3139172/figure/F1/; Brandão and Romcy-Pereira, 2015, Figure 1, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4412069/figure/F1/).
In humans, 65% of neocortical interneurones develop from Mash1-expressing progenitor cells of the VZ and SVC. Mash1 is a gene responsible for differentiation of GABAergic neurones and is also expressed in the subpallium (Letinic et al., 2002; Jakovcevski et al., 2011). In most mammals, however, the majority of GABAergic cortical interneurones are born in the subpallium, divisible into lateral (LGE), medial (MGE), and caudal (CGE) ganglionic eminences and preoptic area (POA). Interneurones expressing PV are born in ventral MGE (vMGE); those expressing SOM in dorsal MGE (dMGE); interneurones expressing the 5HT3 receptor (5HT3R, ionotropic serotonin receptor, Lee et al., 2010) plus cells variously expressing CR, CCK, VIP, SOM, PV, reelin and NPY (neuropeptide Y) are born in CGE (Lee et al., 2010). Finally, a mixed population of CR, CCK, VIP (vasoactive intestinal polypeptide), SOM, PV, reelin, and NPY cells are born in POA. Between E9.5 and E15.5, PV cells in vMGE, SOM cells in the dMGE and cells expressing reelin, SOM, CR, are born. 5HT3R cells are born later (E12.5–E15.5). The orphan nuclear receptor COUP-TFII is expressed in the CGE and in hippocampal interneurone-specific interneurones. It is required, with COUP-TFI, for caudal migration of cortical interneurones (Cauli et al., 2014), while activation of 5HT3AR promotes migration and appropriate positioning of CGE-derived reelin-cells (Murthy et al., 2014) (Yavorska and Wehr, Figure 2, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5040712/figure/F2/).
The interneurones then migrate tangentially toward the cortex. Corticofugal axons expressing TAG-1 (an axonal glycoprotein) provide a pathway for early-born MGE interneurones, while later-born interneurones migrate preferentially along axons lacking TAG-1 (McManus et al., 2004; Denaxa et al., 2011). Along two main migratory streams (in MZ and SVZ) they interact with soluble chemo-attractants and-repellents. For example, Cxcl12, interacting with its receptors, Cxcr4, Cxcr7, is a potent chemo-attractant for MGE-derived interneurones and required for normal positioning of these interneurones (Li et al., 2008; López-Bendito et al., 2008). Activation of GluRs and GABABRs, promotes tangential migration of interneurones into the cortex (Luhmann et al., 2015). Early born, SOMinterneurones, in receipt of strong thalamic input at this time, innervate PV interneurones and pyramids. These transient circuits promote maturation of thalamocortical input to PV interneurones (Tuncdemir et al., 2016; see above, for the influence of transient circuits involving thalamorecipient-SOM interneurones, on spiny stellate maturation). CGE-derived interneurones must insinuate themselves into the cortex even later, after many other interneurones are in place. To migrate properly and develop appropriate processes, they need network activity and, after P3, glutamate-release (De Marco García et al., 2011).
Each subpallial region expresses different combinations of transcription factors and both birth-date and -location influence the classes of interneurones generated. By P0, a large part of their fate has been defined by their own genetic programmes, but most interneurones arrive after pyramidal neurones and early interneurones have populated the cortex. Additional factors fine tune their structure and function: interactions with pyramidal cells influence their final positions, electrical activity regulates late acquisition of neurochemical identity and of soluble factors, which also influence chemical identity and thereby the relative proportions of interneuronal subtypes (Brandão and Romcy-Pereira, 2015, for review).
Many recent studies have used rodents—young enough for many neuronal properties still to be maturing. Neonatal voltage gated channels, transporters and receptors are replaced during the first few postnatal weeks, resulting in a dramatic—up to four fold—reduction in the time course of many electrophysiological events. This “juvenile” period is also a time of synapse proliferation and pruning, and the speed and complexity of short term synaptic dynamics (Thomson, 2000a,b, 2003) increase in parallel. Some of the ambiguity encountered in attempts to classify cortical interneurones could result from cells at different stages of maturity; a day or two at these ages could make quite a difference: Kvα1 (Butler et al., 1998); SK2 (Cingolani et al., 2002); Kv3.2 (Tansey et al., 2002); Kv3.1b (Du et al., 1996); speeding of AMPA-R-EPSPs, P8 cf P35; shortening of synaptically released glutamate waveform, P8-P18 (Cathala et al., 2003, 2005); GABAA-R α6-subunit expression, P7 cf P30 (Tia et al., 1996; time course of NMDA-R mediated EPSCs (Hestrin, 1992; Cathala et al., 2000); NMDA-R subunits (Farrant et al., 1994); switch from FLIP to FLOP GluR splice variants P8-14 (Monyer et al., 1991). (Batista-Brito and Fishell, 2009, Figure 5, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4465088/figure/F5/).
Moreover, reconstructions of “juvenile” cells typically demonstrate rather limited axonal ramification.
Studying a more restricted developmental stage might, therefore result in a “tidier” picture. However, cortical interneurones have to establish their own territories within a field of already established cortical layers, sublayers and regions; environments, moreover, that continue to change throughout development and in ways not entirely prescribed genetically. Following detailed studies of the crab stomatogastric ganglion, Marder and Prinz (2002) concluded that “…similar neuronal and network outputs can be produced by a number of different combinations of ion channels and synapse strengths. This suggests that individual neurons of the same class may each have found an acceptable solution to a genetically determined pattern of activity, and that networks of neurons in different animals may produce similar output patterns by somewhat variable underlying mechanisms….” It is perhaps not surprising, therefore, that while many properties are common to all members, where a given clearly definable subclass exists, others may be subject to variation and modification by the existing environment. It is only necessary to study the convoluted trajectories of interneuronal axons (and pyramidal dendritic branches) to appreciate how thoroughly they explore their environment for appropriate synaptic partners.
(Klausberger et al., 2003, for review) (Figure 1. Supplementary Figure 1 for 3D reconstructions of CA1 interneurones). (Klausberger and Somogyi, 2008, Figures 1,2, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4487503/figure/F1/; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4487503/figure/F2/; Bezaire and Soltesz, 2013, Figure 1, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3775914/figure/F1/; Markram et al., 2004).
Hippocampal interneurones are generated in much the same way and in the same regions as neocortical interneurones, though they take a more caudal path to their destination. They must also become integrated into an existing network, but the organization of that network, with only one principal cell layer and major pathways spatially separated, is more straightforward.
Two broad classes of interneurones target somata/proximal dendrites and axon initial segments of pyramidal cells, respectively. Many of their axonal branches become significantly—if sporadically—myelinated and their synaptic boutons are large and contain mitochondria; facilitating the fast, precisely timed, proximal inhibition they provide.
Basket cells “Baskets” of axons bearing large synaptic boutons that surrround principal cell somata were first described in cerebellum (Golgi, 1883, 1906) then elsewhere (Cajal, 1888, 1906; Lorente de Nó, 1922; Kritzer and Goldman-Rakic, 1995; Buhl et al., 1997; Ali et al., 1998; Tamás et al., 1998). A hippocampal interneurone destined to inhibit pyramidal somata has little choice but to innervate stratum pyramidale. Similarly, to sample all excitatory inputs controlling activity in its target cells, it extends its dendrites across all layers, from stratum oriens to lacunosum moleculare—an easily identifiable, classical CA basket cell. Some basket cell axons can also extend to proximal stratum oriens and stratum radiatum (wide arbor basket cells, Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/basket).
Three types of CA1 basket cells, the majority otherwise fairly similar in their appearance, are distinguished by immunoreactivity for PV, CCK/VIP; or CCK/VGLUT3 (Katona et al., 1999; Somogyi et al., 2004) and CB1R (type-1 cannabinoid receptor: Takács et al., 2015) (Pawelzik et al., 2002, for distributions of CA1 PV and CCK interneurones). CA1 CCK basket cells receive less synaptic input than PV baskets, with proportionally more inhibition, suggesting that they do indeed subserve different rôles (Mátyás et al., 2004) and unlike PV basket cells, whose cell bodies lie predominantly in stratum pyramidale, CCK basket somata are also found in stratum oriens and radiatum, i.e. their sampling of incoming information also has a different bias.
For neurones with such a similar overall structure and specific target preference, it is surprising perhaps that PV and CCK basket cells originate in different subpallial regions: PV interneurones in vMGE, CCK interneurones in POA or CGE and may be born later. PV interneurones (devoid of other common markers) are typically fast spiking (FS) and deliver fast IPSPs mediated by α1β2/3γ2-GABAARs to pyramids, while many CCK basket cells display adapting firing patterns, have broader action potentials (Pawelzik et al., 2002) and activate α2β2/3γ2-GABAARs on pyramids. The different pharmacologies of these receptors (hippocampus: Pawelzik et al., 1999, 2003; Thomson et al., 2000; neocortex: Ali and Thomson, 2008) and the behavioral effects of manipulating their efficacy (Möhler et al., 2002) suggest that PV baskets mediate pharmacological sedation and contribute to anti-convulsant therapies, CCK basket cells (and possibly axo-axonic cells, Nusser et al., 1996) promote anxiolysis (Möhler et al., 2002), while certain dendrite-preferring interneurones, acting on α5β1γ2-GABAARs (Pawelzik et al., 1999, 2003; Ali and Thomson, 2008) influence cognition (Rudolph and Möhler, 2014).
Chandelier, or Axo-axonic cells innervate pyramidal axon initial segments in deep stratum pyramidale and proximal oriens. Their cartridge bouton arrays are only partially coincident with basket cell axonal arbors (Buhl et al., 1994b). For chandeliers with somata in stratum pyramidale this and the often distinctive claw-like appearance of their apical dendritic terminal branches as they extend into stratum lacunosum moleculare assist their identification (Pawelzik et al., 2002) (Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/axo-axonic). For stratum oriens axo-axonic cells with horizontal dendrites, see Ganter et al. (2004).
At least nine classes of CA1 interneurones preferentially innervate pyramidal dendrites. Their termination zones suggest that each class selectively innervates dendritic regions also receiving a particular afferent pathway, or combination thereof. The names they have acquired often reflect this preference (Klausberger et al., 2003; Klausberger and Somogyi, 2008; Bezaire and Soltesz, 2013, for reviews). As a gross generalization, dendrite-targeting interneurones have finer, unmyelinated axons and smaller, mitochondria-poor synaptic boutons than proximally targeting cells. They display a range of firing patterns, but are rarely classical FS. Those that have horizontally oriented dendrites (OLM cells being a prime example), be they in stratum oriens, radiatum, or lacunosum moleculare, in CA1 or CA2, often display an adapting firing pattern and a pronounced “sag” current in responses to large hyperpolarizing current pulses, which can elicit rebound firing and many receive facilitating EPSPs from pyramids.
Perforant path associated cells Perforant path associated cells whose axons and dendrites are restricted to stratum lacunosum moleculare respond to perforant path input by inhibiting pyramidal apical dendritic tufts that are also in receipt of perforant path input. (CCK) (Vida et al., 1998; Pawelzik et al., 2002) (Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/ppa).
Bistratified cells have axonal arbors ramifying in stratum oriens and radiatum, but not in stratum pyramidale or lacunosum moleculare. Bistratified cells with somata in stratum pyramidale, have dendrites that span stratum oriens and radiatum. Those with cell bodies in stratum oriens, have horizontal dendrites confined to stratum oriens.The axons of both subtypes are associated with Schaffer collateral/commissural inputs to intermediate pyramidal dendrites via α5β1γ2-GABAARs (Pawelzik et al., 1999; Thomson et al., 2000; Thomson and Jovanovic, 2010 for review). (Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/bistratified).
(SOM, PV, CCK) (Buhl et al., 1994a, 1996; Halasy et al., 1996; Pawelzik et al., 2002; Klausberger et al., 2004; Baude et al., 2007).
Schaffer collateral-associated cells innervate the same regions as bistratified cells, but receive a different combination of inputs. Their somata lie close to the stratum radiatum-lacunosum moleculare border and their dendrites span both these layers and stratum oriens. In addition to Schaffer collateral and commissural input, therefore, these interneurones receive proximal input from perforant path, but restrict their influence to the termination regions of the Schaffer/commissural inputs (CCK: Vida et al., 1998; Pawelzik et al., 2002). (Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/sca).
Apical dendrite-innervating cells have axonal and dendritic spans similar to those of the Schaffer collateral-associated cells, but innervate the main apical dendritic trunks of pyramids, rather than their apical oblique branches (Klausberger et al., 2005; Klausberger, 2009) (CCK).
Oriens-lacunosum moleculare, or OLM cells (Cajal, 1911; Lacaille et al., 1987; Lacaille and Williams, 1990; Buckmaster et al., 1994; Blasco-Ibáñez and Freund, 1995), have horizontal thorny dendrites restricted to stratum oriens (in CA1) where they receive their most powerful drive from CA1 pyramids (Blasco-Ibáñez and Freund, 1995) with facilitating EPSPs (Ali and Thomson, 1998). In CA3, OLM dendrites also project into stratum radiatum, where local CA3 pyramidal axons also ramify. OLM cells do not, however, innervate stratum oriens, pyramidale, or radiatum. They send one or more long axons to stratum lacunosum moleculare, where they form a dense arbor in the perforant path termination zone and deliver fast IPSPs, almost invisible at the soma, but apparent in distal apical dendritic recordings (Hannelore Pawelzik, 1960-2004; Hannelore Pawelzik, unpublished) (Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/olm).
(SOM: Morrison et al., 1982; Kosaka et al., 1988; Kunkel and Schwartzkroin, 1988). (mGluR1α: Ferraguti et al., 2004) (up to one third express PV weakly: Ferraguti et al., 2004; Varga et al., 2012) one (metabotropic glutamate receptor 7, mGluR7, selectively expressed in excitatory boutons contacting OLM cells: Shigemoto et al., 1996).
(Jinno, 2009, Figure 1, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2718779/figure/F1/).
These are perhaps the group most difficult to classify and one of the smallest 4% of CA1 interneurones. Since the majority of reported cells in the following four classes have horizontally oriented dendrites confined to stratum oriens, it is probable that, like OLM cells, they receive strong excitatory input from CA1 pyramids and relay information about activity here to other regions. In addition to long distance projections, they have local axonal arbors in stratum oriens and radiatum.
(SOM; Jinno et al., 2007; Katona et al., 2017) PV possible: Ferraguti et al., 2004).
Oriens-retrohippocampal projection cells project to the subiculum. (SOM/Cb: Jinno et al., 2007; Klausberger and Somogyi, 2008). A range of subtypes project to subiculum, including an mGluR8-decorated, M2R-expressing, SOM-negative trilaminar cell (Ferraguti et al., 2005).
Double projection cells (Klausberger and Somogyi, 2008) project to the septum and subiculum (SOM/Cb, or CR). Some also express mGluR1α and/or NPY and up to 30% express PV weakly.
Back-projection cells (Sik et al., 1994; Katona et al., 2017, for in vivo filled cells) project to CA3 and/or dentate gyrus, sometimes crossing the fissure, which appears to be an impenetrable barrier to other neuronal processes. (PV, SOM, Cb-negative). (Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/backprojection).
Cb-septal projection cells project to the septum (SOM, Cb) (Gulyás et al., 2003).
Amygdala-projecting interneurones project from ventral CA1 stratum oriens, pyramidale and radiatum, to the amygdala (Lübkemann et al., 2015). (PV, Cb, SOM, NPY and/or CCK).
The neurogliaform family (Overstreet-Wadiche and McBain, 2015, for review).
Two classes have been described, which differ predominantly in the inputs they receive and the subcellular compartments they inhibit.
Neurogliaform cells are often found at the stratum radiatum-lacunosum moleculare border, with short, fine, often highly convoluted dendrites and a dense and spatially restricted axonal arbor, positioned to inhibit distal apical dendrites of pyramidal cells; (nNOS (neuronal nitric oxide synthase), NPY, α-actinin-2, COUP-TFII) (Price et al., 2005; Fuentealba et al., 2010).
Ivy cells are structurally similar to neurogliaform cells, but lie close to the stratum radiatum-pyramidale border where they inhibit proximal pyramidal compartments. Although the GABAARs activated by ivy cells demonstrate rapid kinetics via receptors also utilized at PV basket synapses (α1β2/3γ2, unpublished results), the proximal IPSPs elicited by ivy cells are very slow. This may be due to non-synaptic, as well as synaptic release of GABA, since what appear to be synaptic vesicles in these axons are not always apposed to postsynaptic specializations (Fuentealba et al., 2008a; see also Oláh et al., 2009; Armstrong et al., 2012, for review) (nNOS, NPY). (Supplementary Figure 1, http://uclsop.net/interneuron-reconstruction/ivy).
(Acsády et al., 1996; Freund and Buzsaki, 1996; Freund and Gulyas, 1997) (CR and/or VIP and COUP-TFII: Gulyas et al., 1999).
Interneurone-specific type I somata are found in stratum pyramidale. Their dendrites and axons span stratum oriens and radiatum. They innervate Cb interneurones, VIP-, but not PV- basket cells and other IS-1 interneurones (CR).
Interneurone-specific type II somata lie near the stratum lacunosum moleculare- radiatum border. Their dendites run horizontally in lacunosum moleculare. They innervate distal stratum radiatum, making multiple contacts with Cb, but not PV-dendrites (VIP).
Interneurone-specific type III somata are found in stratum pyramidale, their bipolar/bitufted dendrites span stratum oriens through radiatum to lacunosum moleculare. Their axons innervate stratum oriens, where they inhibit Cb and SOM/mGluRa, interneurones including OLM cells (Acsády et al., 1996) (CR, VIP and possibly nNOS).
We cannot leave hippocampal interneurones without mentioning, however briefly, the elegant experiments in which a neurone is recorded through different e.g., states, then filled juxta-cellularly and identified; studies that demonstrate distinctive patterns of firing in relation to network rhythms such as theta and sharp wave ripples, for each class of interneurone (Klausberger et al., 2003, 2004; Fuentealba et al., 2008b, 2010; Klausberger and Somogyi, 2008; Varga et al., 2012; Katona et al., 2017).
There are around twenty, more or less distinct, classifiable classes of interneurones in CA1. Although those in CA3 remain to be explored as thoroughly, there appears to be a similar variety. In CA2, much the same profile is seen, but some unique subclass features and a stratum pyramidale-stratum radiatum interneuronal class, specific to this region, have been demonstrated (Figure 1) (Mercer et al., 2007, 2012a,c).
Some of the distinguishing features used to classify hippocampal interneurones, such as topographical relationship to specific pathways, have not been systematically applied to neocortical interneurone classification. If we look at broad classes of GABAergic neurones, those, for example that express the same markers, we find a similar picture in hippocampus and neocortex. Nearly all neocortical interneurones also belong to three broad groups, 40% expressing PV, 30% SOM and 30% 5-HT3αR, with little overlap (Rudy et al., 2011). As a broad generalization, PV interneurones (expressing neither SOM, Cb, nor CR) display fast spiking (FS) behavior, innervate proximal regions of pyramidal cells, generate fast IPSPs mediated by α1β2/3γ2 GABAARs (Ali and Thomson, 2008) and receive depressing EPSPs from pyramids (excepting L6 corticothalamic pyramids). SOM cells including bipolar and bitufted neurones, display adapting or “burst-firing” behavior, innervate pyramidal dendrites with slower IPSPs (somatic recordings) and receive facilitating EPSPs from pyramids. 5HT3R cells displaying various non-FS behaviors are often relatively small cells with small overlapping axonal and dendritic trees (Lee et al., 2010). Expression of mRNA for certain voltage-gated ion channels clusters with three major calcium binding proteins, PV, Cb, and CR, and correlates with firing characteristics: fast IA K+ channel subunits in the PV cluster, that rapidly repolarize action potentials, reducing Na+-channel inactivation, would facilitate fast spiking behavior, while a T-type Ca2+ current in the Cb cluster that would support burst-firing behavior (Toledo-Rodriguez et al., 2004).
Neocortical proximally targeting interneurones include subclasses of basket and chandelier or axo-axonic cells, but with a far wider range of sizes, axonal and dendritic distributions, potential inputs and targets than in hippocampus.
Basket cells in neocortex have complex choices to make. Some of the pyramids that a neocortical basket cell is destined to control will receive excitatory input in several, or even in all layers, while some spiny cells, like inverted, or bipolar L6 corticocortical cells, or L4 spiny stellate cells, may receive inputs only in one. A neocortical basket cell must also choose which spiny cells it will inhibit - any or all pyramids in a given layer, or a specific subtype, perhaps one receiving only certain inputs. Some smaller basket cells have axonal arbors restricted to a single layer, or sublayer. Large basket cells often innervate more than one layer, though this choice is not indiscriminate; the axonal arbors are often restricted to two related layers, such as the two thalamorecipient layers, L4 and L6, or the integration layers, L3 and L5, with only unbranched collaterals passing through intermediate layers (e.g., L3 and L5: Lund, 1987; Lund et al., 1988; Buhl et al., 1997; L6 and L4: Lund, 1987; Lund et al., 1988; Thomson et al., 2002; Thomson and Bannister, 2003). These larger basket cells often have dendrites that extend over several layers and some, in cat and primate primary sensory regions, also generate long horizontal axonal branches that terminate in smaller, but equally dense arbors in more distant columns (Lund, 1987; Lund et al., 1988; Kritzer and Goldman-Rakic, 1995; Lund and Wu, 1997; Thomson et al., 2002; Thomson and Bannister, 2003).
Traditionally, as in hippocampus, neocortical basket cells have been found to stain either for PV, or CCK. However, neocortical basket cells have also been classified according to axonal branch length and angle, bouton frequency etc. and these parameters correlated with their potential to generate calcium binding proteins and neuropeptides (RT-PCR). “Small basket cells,” including “clutch cells” (Kisvárday et al., 1985) express mRNA for VIP and SOM or CCK and variously PV, Cb, or CR. Large basket cells express mRNA for PV or Cb and variously NPY or CCK. “Nest basket cells” express PV or Cb mRNA and approximately equal proportions mRNA for NPY, SOM, or CCK (Wang et al., 2002).
For quality reconstructions of identified neocortical basket cells and of other GABAergic interneurones: (Jones, 1975; Jones and Peters, 1984; Lund, 1987; Lund et al., 1988; Lund and Yoshioka, 1991; Lund and Wu, 1997; DeFelipe, 2002; Thomson et al., 2002; Thomson and Bannister, 2003; West et al., 2006) and for connections with these cells: (Somogyi et al., 1983; Kritzer and Goldman-Rakic, 1995; Buhl et al., 1996; Halasy et al., 1996; Tamás et al., 1997; Dantzker and Callaway, 2000; Thomson et al., 2002; Thomson and Bannister, 2003; West et al., 2006; Ali et al., 2007).
Chandelier or Axo-axonic cells (Inan and Anderson, 2014). Since the targets of chandelier cells are highly restricted—to pyramidal axon initial segments (Somogyi, 1977; Somogyi et al., 1982) and their function - to control pyramidal firing, is well documented (if somewhat controversial), we can assign part of their function according to the distribution of their cartridge synapses. The synapses made by some neocortical axo-axonic cells are restricted to a single layer, others to two physically separated, but related layers/sublayers. Cartridge synapses, on short, radially projecting collaterals make these cells easy to identify (Szentagothai and Arbib, 1974; Lund, 1987; Lund et al., 1988; Lund and Yoshioka, 1991; Kritzer and Goldman-Rakic, 1995; Lund and Wu, 1997; Thomson and Bannister, 2003). Although most studies identify PV as a predominant marker for chandelier cells, (DeFelipe et al., 1989; Kawaguchi and Kubota, 1997; Gonchar and Burkhalter, 1999) some primate and human L5/L6 chandeliers contain Cb (del Rio and DeFelipe, 1997) and a separate population of corticotrophin-containing cells has been described in primate, although relative proportions vary between species, layer and area (Lewis and Lund, 1990).
Neocortical dendrite-targeting interneurones may, like their hippocampal equivalents, sample only certain inputs and seek only those targets that receive specific inputs. We are inclined to suspect that they most probably do, in the face of little direct evidence.
Somatostatin (SOM) dendrite-targeting interneurones (Yavorska and Wehr, 2016), often bipolar or bitufted, have fine axons forming dense, vertically oriented arbors with small boutons, spanning one, two or more adjoining layers. They are typically adapting, or burst-firing, with broader APs than FS cells and receive facilitating inputs from pyramids (Deuchars and Thomson, 1995; Thomson et al., 1995; Thomson and Bannister, 2003), stronger inhibition from VIP interneurones than PV cells receive and deliver slower IPSPs than basket cells (somatic recordings), mediated by α5-subunit-containing-GABAARs (Ali and Thomson, 2008).
Martinotti cells from the dMGE, were first described as resident in L5, with a fine, dense axonal arbor extending to L1 and innervating pyramidal dendrites (Martinotti, 1889); leading some to claim, erroneously, any cell with a portion of axon drifting northwards as a “Martinotti.” They display “low threshold spiking” behavior (Kawaguchi and Kubota, 1996, 1997; Beierlein et al., 2000, 2003; Wang et al., 2004; Ma et al., 2006). This class is now agreed to include similar, but adapting/burst-firing SOM cells in superficial layers. However, L5/6 and L2/3/4 Martinotti cells do differ; two distinct populations are identifiable in GIN and X98 mice respectively (Ma et al., 2006), both populations including SOM/Cb and SOM/NPY cells and in mouse, SOM/Cb/CR or SOM/Cb/NPY (Ma et al., 2006).
Other, probably dendrite-targeting, SOM interneurones are less distinctive.
SOM cells in L4/5 of the X94 mouse did not express Cb or NPY,
SOM cells not labeled in X94, X98 or GIN lines express NPY, nNOS and SPR (substance P receptor) (Xu and Callaway, 2009).
VIP Bipolar/bitufted interneurones, from the CGE, also containing neither PV nor SOM, often show irregular spiking behavior, supported by an ID-like K+ current (Porter et al., 1998). Their slender axonal tree preferentially innervates fine/medium caliber dendrites of other VIP cells as well as pyramids (rat: Peters, 1990; Acsády et al., 1996; Staiger et al., 1996, 1997; mouse: Prönneke et al., 2015), with boutons often closely associated with asymmetrical synapses (rat: Hajós et al., 1988). They receive high probability, depressing inputs from pyramidal cells mediated by AMPA-Rs with fast kinetics (GluR1/2 flop: Porter et al., 1998), particularly strong inputs from deep layers and stronger inputs from distant cortical areas: basal nucleus of Meynert and thalamus (Wall et al., 2016) and from PV cells (Staiger et al., 1997) than other interneurones.
Double bouquet cells (Cajal, 1899b; DeFelipe et al., 2006) with somata in L2/3/4 have a distinctive, narrow, axonal arbor (“horse-tail”) descending to L6, in addition to a dense local arbor (often unstained in Golgi preparations). They contain VIP or CR, commonly display a “sag” in response to hyperpolazing current and a range of firing patterns including stuttering and adapting, but not classical FS (Prönneke et al., 2015).
(rat: VIP, Kawaguchi and Kubota, 1997 or CR, primate: Lund and Lewis, 1993).
Smaller VIP/CCK or VIP/CR cells (Kawaguchi and Kubota, 1997) probably include small basket cells, like Arcade cells, whose axon first ascends toward the pia, then turns south, to form a cone-shaped arbor (Jones, 1975) innervating somata and proximal dendrites.
Multipolar burst-firing dendrite-targeting cells which are strongly interconnected (electrically and chemically) and unusually express both PV and Cb, may form an additional VIP subclass (mouse, Blatow et al., 2003).
5HT3R cells include subsets of later born CCK, CR and NPY expressing neurones (Lee et al., 2010; Rudy et al., 2011), from the CGE.
Neurogliaform cells (Cajal, 1891; Lund and Yoshioka, 1991; Lund and Wu, 1997; Armstrong et al., 2012, for review) with dense, convoluted dendritic and axonal arbors display late-spiking behavior. As in hippocampus, non-synaptic, but AP-driven, vesicular release (in addition to synaptic) may account for the slow time course of the IPSPs, the presynaptic GABAergic inhibition and activation of extrasynaptic α4βxδ-GABAARs these cells elicit (Oláh et al., 2009).
NPY (Xu and Callaway, 2009), COUP-TFII (Fuentealba et al., 2010) 5HT3aR, but not VIP (Lee et al., 2010; Rudy et al., 2011).
COUP-TFII - Interneurone-specific interneurones? In rat hippocampus, COUP-TFII is expressed in neurogliaform cells and basket cells in stratum radiatum and by CR- and/or VIP- interneurone-specific-interneurones (Fuentealba et al., 2010). This member of the steroid/thyroid-receptor family is expressed in the dMGE and CGE, in the SVZ in humans and by interneurones, predominantly in L1-3. They do not co-express PV, SOM, or Cb, but half express CR (80%), a quarter reelin (VIP not tested). They display irregular or adapting firing patterns, exhibit a pronounced “sag” and innervate small dendritic shafts of both interneurones and pyramids (Human temporal cortex; Varga et al., 2015). Two classes of mouse L2/3 CR cells preferentially innervate interneurones: burst-firing, bipolar VIP/CR-cells and adapting, accommodating multipolar CR-cells and may be cortical equivalents of ISI-I and III respectively (Caputi et al., 2009).
Projection neurones: A small population (6–9%) of low threshold spiking SOM cells that also express NPY, nNOS and SPR form a distinct morphological class with long distance corticocortical or corticofugal projections (Yavorska and Wehr, 2016).
The similarities between hippocampal CA regions and neocortical layers are striking: their development, the classes of neurones which result and the unidirectional flow of excitation through the regions and layers, which preserves the integrity of original signals. The prominent differences may result from a need for a far larger number of often smaller and simpler principal neurones in neocortex to perform a wider range of sophisticated computations, while avoiding the inefficiency of long, myelinated “local circuit” connections. Stacking principal cells in columns maximizes efficiency. However, this new arrangement presents new challenges, both to axons and dendrites that must make appropriate connections, to interneurones that must infiltrate this apparent chaos and to neuroscientists trying to understand the circuitry. Within these columns, myriad neuronal compartments, belonging to many neuronal classes, lie side by side. How do the axons that ramify there, or those simply passing through, choose from amongst these targets and how do postsynaptic compartments know which to accept? Understanding the mechanisms already apparent in simpler cortices, but hitherto largely unexplained; mechanisms that ensure the rejection of inappropriate and the formation of appropriate connections, each with its own unique signature, is an exciting challenge for the future.
AM and AT wrote the manuscript and designed the figures.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The work of many years from our own laboratories and reported here was supported (in approximately chronological order) by the Wellcome Trust (1985–2000), the Medical Research Council (1984–2014), Novartis Pharma (1995–2005), the Engineering and Physical Sciences Research Council (COLAMN), EU Framework 6 (FACETS), Glaxo Smith Kline, the Biotechnology and Biological Sciences Research Council, the Physiological Society and the Human Brain Project, (European Commission FET Flagship) (2013-). Contributions made by lab-members who recorded, dye-filled and reconstructed neurones and analyzed data, are gratefully acknowledged: D. C. West, J. Deuchars, H. Pawelzik, D. I. Hughes, P. Ogun-Muyiwa, A. B Ali, J. Hahn, A. P. Bannister, O. T.Morris, S. Kirchhecker, H. McPhail K. Eastlake, H. Trigg, N. A. Botcher, J. E. Falck, S. Lange and Georgia Economides. Unpublished glutamate uncaging studies were performed by A. Biro A, Brémaud and A. Ruiz.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnana.2017.00083/full#supplementary-material
Acsády, L., Arabadzisz, D., and Freund, T. F. (1996). Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience 73, 299–315. doi: 10.1016/0306-4522(95)00610-9
Ahmed, B., Anderson, J. C., Douglas, R. J., Martin, K. A. C., and Nelson, J. C. (1994). Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J. Comp. Neurol. 341, 39–49. doi: 10.1002/cne.903410105
Alcamo, E. A., Chirivella, L., Dautzenberg, M., Dobreva, G., Fariñas, I., Grosschedl, R., et al. (2008). Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57, 364–377. doi: 10.1016/j.neuron.2007.12.012
Ali, A. B., and Thomson, A. M. (1998). Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. J. Physiol. 507 (Pt. 1), 185–199. doi: 10.1111/j.1469-7793.1998.185bu.x
Ali, A. B., and Thomson, A. M. (2008). Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb. Cortex 18, 1260–1271 doi: 10.1093/cercor/bhm160
Ali, A. B., Bannister, A. P., and Thomson, A. M. (2007). Robust correlations between action potential duration and the properties of synaptic connections in layer 4 interneurones in neocortical slices from juvenile rats and adult rat and cat. J. Physiol. 580, 149–169. doi: 10.1113/jphysiol.2006.124214
Ali, A. B., Deuchars, J., Pawelzik, H., and Thomson, A. M. (1998). CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J. Physiol. 507(Pt. 1), 201–217. doi: 10.1111/j.1469-7793.1998.201bu.x
Allen, K., Fuchs, E. C., Jaschonek, H., Bannerman, D. M., and Monyer, H. (2011). Gap junctions between interneurons are required for normal spatial coding in the hippocampus and short-term spatial memory. J. Neurosci. 31, 6542–6552. doi: 10.1523/JNEUROSCI.6512-10.2011
Altman, J., and Bayer, S. A. (1990a). Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J. Comp. Neurol. 301, 325–342.
Altman, J., and Bayer, S. A. (1990b). Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J. Comp. Neurol. 301, 343–364. doi: 10.1002/cne.903010303
Amitai, Y., Gibson, J. R., Beierlein, M., Patrick, S. L., Ho, A. M., Connors, B. W., et al. (2002). The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J. Neurosci. 22, 4142–4152.
Arimatsu, Y., and Ishida, M. (2002). Distinct neuronal populations specified to form corticocortical and corticothalamic projections from layer VI of developing cerebral cortex. Neuroscience 114, 1033–1045. doi: 10.1016/S0306-4522(02)00201-4
Arlotta, P., Molyneaux, B. J., Chen, J., Inoue, J., Kominami, R., and Macklis, J. D. (2005). Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221. doi: 10.1016/j.neuron.2004.12.036
Armstrong, C., Krook-Magnuson, E., and Soltesz, I. (2012). Neurogliaform and Ivy cells: a major family of nNOS expressing GABAergic neurons. Front. Neural Circuits 6:23. doi: 10.3389/fncir.2012.00023
Baimbridge, K. G., Peet, M. J., McLennan, H., and Church, J. (1991). Bursting response to current-evoked depolarization in rat CA1 pyramidal neurons is correlated with lucifer yellow dye coupling but not with the presence of calbindin-D28k. Synapse 7, 269–277. doi: 10.1002/syn.890070404
Bannister, A. P., and Thomson, A. M. (2007). Dynamic properties of excitatory synaptic connections involving layer 4 pyramidal cells in adult rat and cat neocortex. Cereb. Cortex 17, 2190–2203. doi: 10.1093/cercor/bhl126
Batista-Brito, R., and Fishell, G. (2009). The developmental integration of cortical interneurons into a functional network. Curr. Top. Dev. Biol. 87, 81–118. doi: 10.1016/S0070-2153(09)01203-4
Baude, A., Bleasdale, C., Dalezios, Y., Somogyi, P., and Klausberger, T. (2007). Immunoreactivity for the GABAA receptor alpha1 subunit, somatostatin and Connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb. Cortex 17, 2094–2107. doi: 10.1093/cercor/bhl117
Bayer, S. A. (1980). Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J. Comp. Neurol. 190, 87–114. doi: 10.1002/cne.901900107
Beaulieu, C., and Colonnier, M. (1985). A laminar analysis of the number of round-asymmetrical and flat-symmetrical synapses on spines, dendritic trunks, and cell bodies in area 17 of the cat. J. Comp. Neurol. 231, 180–189. doi: 10.1002/cne.902310206
Beierlein, M., Gibson, J. R., and Connors, B. W. (2000). A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat. Neurosci. 3, 904–910. doi: 10.1038/78809
Beierlein, M., Gibson, J. R., and Connors, B. W. (2003). Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 90, 2987–3000 doi: 10.1152/jn.00283.2003
Benshalom, G., and White, E. L. (1986). Quantification of thalamocortical synapses with spiny stellate neurons in layer IV of mouse somatosensory cortex. J. Comp. Neurol. 253, 303–314 doi: 10.1002/cne.902530303
Betz, W. (1874). Anatomischer Nachweis zweier Gehirncentra. Centralblatt für die Medizinischen Wissenschaften 12, 578-580, 595–599.
Bezaire, M. J., and Soltesz, I. (2013). Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 23, 751–785. doi: 10.1002/hipo.22141
Bielle, F., Marcos-Mondejar, P., Keita, M., Mailhes, C., Verney, C., Nguyen Ba-Charvet, K., et al. (2011). Slit2 activity in the migration of guidepost neurons shapes thalamic projections during development and evolution. Neuron 69, 1085–1098. doi: 10.1016/j.neuron.2011.02.026
Binzegger, T., Douglas, R., and Martin, K. (2004). A quantitative map of the circuit of cat primary visual cortex. J. Neurosci. 24, 8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004
Blasco-Ibáñez, J. M., and Freund, T. F. (1995). Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur. J. Neurosci. 7, 2170–2180.
Blatow, M., Rozov, A., Katona, I., Hormuzdi, S. G., Meyer, A. H., Whittington, M. A., et al. (2003). A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38, 805–817. doi: 10.1016/S0896-6273(03)00300-3
Bonifazi, P., Goldin, M., Picardo, M. A., Jorquera, I., Cattani, A., Bianconi, G., et al. (2009). GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424. doi: 10.1126/science.1175509
Branco, T., and Häusser, M. (2011). Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron 69, 885–892. doi: 10.1016/j.neuron.2011.02.006
Brandão, J. A., and Romcy-Pereira, R. N. (2015). Interplay of environmental signals and progenitor diversity on fate specification of cortical GABAergic neurons. Front. Cell Neurosci. 9:149. doi: 10.3389/fncel.2015.00149
Britanova, O., Akopov, S., Lukyanov, S., Gruss, P., and Tarabykin, V. (2005). Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS. Eur. J. Neurosci. 21, 658–668. doi: 10.1111/j.1460-9568.2005.03897.x
Britanova, O., De Juan Romero, C., Cheung, A., Kwan, K. Y., Schwark, M., Gyorgy, A., et al. (2008). Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57, 378–392. doi: 10.1016/j.neuron.2007.12.028
Buckmaster, P. S., Kunkel, D. D., Robbins, R. J., and Schwartzkroin, P. A. (1994). Somatostatin-immunoreactivity in the hippocampus of mouse, rat, guinea pig, and rabbit. Hippocampus 4, 167–180. doi: 10.1002/hipo.450040207
Buhl, E. H., Halasy, K., and Somogyi, P. (1994a). Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 8, 823–828. doi: 10.1038/368823a0
Buhl, E. H., Han, Z. S., Lörinczi, Z., Stezhka, V. V., Karnup, S. V., and Somogyi, P. (1994b). Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J. Neurophysiol. 71, 1289–1307.
Buhl, E. H., Szilágyi, T., Halasy, K., and Somogyi, P. (1996). Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus 6. 294–305. doi: 10.1002/(SICI)1098-1063(1996)6:3<294::AID-HIPO7>3.0.CO;2-N
Buhl, E. H., Tamas, G., Szilagyi, T., Stricker, C., Paulsen, O., and Somogyi, P. (1997). Effect, number and location of synapses made by single pyramidal cells onto aspiny interneurones of cat visual cortex. J. Physiol. 500, 689–713. doi: 10.1113/jphysiol.1997.sp022053
Burkhalter, A. (1989). Intrinsic connections of rat primary visual cortex: laminar organization of axonal projections. J. Comp. Neurol. 279, 171–186 doi: 10.1002/cne.902790202
Burkhalter, A., Gonchar, Y., Mellor, R. L., and Nerbonne, J. M. (2006). Differential expression of I(A) channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J. Neurosci. 26, 12274–12282. doi: 10.1523/JNEUROSCI.2599-06.2006
Butler, D. M., Ono, J. K., Chang, T., McCaman, R. E., and Barish, M. E. (1998). Mouse brain potassium channel beta1 subunit mRNA: cloning and distribution during development. J. Neurobiol. 34, 135–150. doi: 10.1002/(SICI)1097-4695(19980205)34:2<135::AID-NEU4>3.0.CO;2-3
Cajal, S. R. (1888). Estructura de los centros nerviosos de las ayes. Rev. Trim. Hist. Norm. Pat. 1, 1–10.
Cajal, S. R. (1891). “Sur la structure de l'ecorce cérébrale de quelques mammifères” [On the structure of the cerebral cortex in some mammals]. La Cellule 7, 123–176.
Cajal, S. R. (1899a). “Estudios sobre la corteza cerebral humana. I. Corteza visual” [Studies on the human cerebral cortex. I. Visual Cortex]. Rev. Trim. Micrográf. 4, 1–63.
Cajal, S. R. (1899b). Estudios sobre la corteza cerebral humana II: estructura de la corteza motriz del hombre y mamíferos superiores. Rev. Trim. Micrográf. Madrid 4, 117–200.
Cajal, S. R. (1904). Textura del Systema Nervioso del Hombre y los Vertebrados, Vol. 2. Madrid: Moya.
Cajal, S. R. (1911). Histologie du Systéme Nerveux de l'Homme et des Vertébrés, Vol. 2. Paris: Maloine.
Caputi, A., Rozov, A., Blatow, M., and Monyer, H. (2009). Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb. Cortex 19, 1345–1359. doi: 10.1093/cercor/bhn175
Cathala, L., Brickley, S., Cull-Candy, S., and Farrant, M. (2003). Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse. J. Neurosci. 23, 6074–6085. Erratum in: J. Neurosci. 2004 24:2343.
Cathala, L., Holderith, N. B., Nusser, Z., DiGregorio, D. A., and Cull-Candy, S. G. (2005). Changes in synaptic structure underlie the developmental speeding of AMPA receptor-mediated EPSCs. Nat. Neurosci. 8, 1310–1318. doi: 10.1038/nn1534
Cathala, L., Misra, C., and Cull-Candy, S. (2000). Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J. Neurosci. 20, 5899–5905.
Cauli, B., Zhou, X., Tricoire, L., Toussay, X., and Staiger, J. F. (2014). Revisiting enigmatic cortical calretinin-expressing interneurons. Front. Neuroanat. 8:52. doi: 10.3389/fnana.2014.00052
Ceranik, K., Deng, J., Heimrich, B., Lübke, J., Zhao, S., Förster, E., et al. (1999). Hippocampal Cajal-Retzius cells project to the entorhinal cortex: retrograde tracing and intracellular labelling studies. Eur. J. Neurosci. 11, 4278–4290. doi: 10.1046/j.1460-9568.1999.00860.x
Ceranik, K., Zhao, S., and Frotscher, M. (2000). Development of the entorhino-hippocampal projection: guidance by Cajal-Retzius cell axons. Ann. N.Y. Acad. Sci. 911, 43–54 doi: 10.1111/j.1749-6632.2000.tb06718.x
Cheng, J., Sahani, S., Hausrat, T. J., Yang, J. W., Ji, H., Schmarowski, N., et al. (2016). Precise somatotopic thalamocortical axon guidance depends on LPA-mediated PRG-2/Radixin Signaling. Neuron 92, 126–142. doi: 10.1016/j.neuron.2016.08.035
Cheung, A. F., Pollen, A. A., Tavare, A., DeProto, J., and Molnár, Z. (2007). Comparative aspects of cortical neurogenesis in vertebrates. J. Anat. 211, 164–176. doi: 10.1111/j.1469-7580.2007.00769.x
Chevaleyre, V., and Piskorowski, R. (2016). Hippocampal Area CA2: an Overlooked but Promising Therapeutic Target. Trends Mol. Med. 22, 645–655. doi: 10.1016/j.molmed.2016.06.007
Chevaleyre, V., and Siegelbaum, S. A. (2010). Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron 66, 560–572. doi: 10.1016/j.neuron.2010.04.013
Cingolani, L. A., Gymnopoulos, M., Boccaccio, A., Stocker, M., and Pedarzani, P. (2002). Developmental regulation of small-conductance Ca2+activated K+ channel expression and function in rat Purkinje neurons. J. Neurosci. 22, 4456–4467.
Clark, B. A., Monsivais, P., Branco, T., London, M., and Hausser, M. (2005). The site of action potential initiation in cerebellar Purkinje neurons. Nat. Neurosci. 8, 137–139. doi: 10.1038/nn1390
Colbert, C. M., and Pan, E. (2002). Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat. Neurosci. 5, 533–538. doi: 10.1038/nn0602-857
Connors, B. W., Gutnick, M. J., and Prince, D. A. (1982). Electrophysiological properties of neocortical neurons in vitro. J. Neurophysiol. 48, 1302–1320.
Costa, M. R., and Hedin-Pereira, C. (2010). Does cell lineage in the developing cerebral cortex contribute to its columnar organization? Front. Neuroanat. 4:26. doi: 10.3389/fnana.2010.00026
Cui, Z., Gerfen, C. R., and Young, W. S. (2013). Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol. 521, 1844–1866. doi: 10.1002/cne.23263
Dantzker, J. L., and Callaway, E. M. (2000). Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat. Neurosci. 3, 701–707. doi: 10.1038/76656
De Marco García, N. V., Karayannis, T., and Fishell, G. (2011). Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 472, 351–355. doi: 10.1038/nature09865
DeFelipe, J. (2002). Cortical interneurons: from Cajal to 2001. Prog. Brain Res. 136, 215–238. doi: 10.1016/S0079-6123(02)36019-9
DeFelipe, J., Ballesteros-Yáñez, I., Inda, M. C., and Muñoz, A. (2006). Double-bouquet cells in the monkey and human cerebral cortex with special reference to areas 17 and 18. Prog Brain Res. 154, 15–32. doi: 10.1016/S0079-6123(06)54002-6
DeFelipe, J., Hendry, S. H., and Jones, E. G. (1989). Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc. Natl. Acad. Sci. U.S.A. 86, 2093–2097. doi: 10.1073/pnas.86.6.2093
Deguchi, Y., Donato, F., Galimberti, I., Cabuy, E., and Caroni, P. (2011). Temporally matched subpopulations of selectively interconnected principal neurons in the hippocampus. Nat. Neurosci. 14, 495–504. doi: 10.1038/nn.2768
Del Río, J. A., Heimrich, B., Borrell, V., Förster, E., Drakew, A., Alcántara, S., et al. (1997). A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385, 70–74. doi: 10.1038/385070a0
del Rio, J. A., Martinez, A., Fonseca, M., Auladell, C., and Soriano, C. (1995). Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb. Cort. 5, 13–21.
del Rio, J., and DeFelipe, J. (1997). Colocalization of parvalbumin and calbindin D-28k in neurons including chandelier cells of the human temporal neocortex. J. Chem. Neuroanat. 12, 165–173. doi: 10.1016/S0891-0618(96)00191-3
Denaxa, M., Chan, C. H., Schachner, M., Parnavelas, J. G., and Karagogeos, D. (2011). The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development 128, 4635–4644.
Deuchars, J., and Thomson, A. M. (1995). Innervation of burst firing spiny interneurons by pyramidal cells in deep layers of rat somatomotor cortex: paired intracellular recordings with biocytin filling. Neuroscience. 69, 739–755. doi: 10.1016/0306-4522(95)00288-T
Deuchars, J., West, D. C., and Thomson, A. M. (1994). Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. J. Physiol. 478, 423–435. doi: 10.1113/jphysiol.1994.sp020262
Dobreva, G., Chahrour, M., Dautzenberg, M., Chirivella, L., Kanzler, B., Fariñas, I., et al. (2006). SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell 125, 971–986. doi: 10.1016/j.cell.2006.05.012
Du, J., Zhang, L., Weiser, M., Rudy, B., and McBain, C. J. (1996). Developmental expression and functional characterization of the potassium-channel subunit Kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J. Neurosci. 16, 506–518.
Dudek, S. M., Alexander, G., and Farris, S. (2016). Rediscovering area CA2: unique properties and functions. Nat. Rev. Neurosci. 17, 89–102. doi: 10.1038/nrn.2015.22
Elhanany, E., and White, E. L. (1990). Intrinsic circuitry: synapses involving the local axon collaterals of corticocortical projection neurons in the mouse primary somatosensory cortex. J. Comp. Neurol. 291, 43–54. doi: 10.1002/cne.902910105
Evsyukova, I., Plestant, C., and Anton, E. S. (2013). Integrative mechanisms of oriented neuronal migration in the developing brain. Annu. Rev. Cell Dev. Biol. 29, 299–353. doi: 10.1146/annurev-cellbio-101512-122400
Farrant, M., Feldmeyer, D., Takahashi, T., and Cull-Candy, S. G. (1994). NMDA-receptor channel diversity in the developing cerebellum. Nature 6469, 335–339. doi: 10.1038/368335a0
Fazzari, P., Paternain, A. V., Valiente, M., Pla, R., Luján, R., Lloyd, K., et al. (2010). Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464, 1376–1380. doi: 10.1038/nature08928
Feldman, M. L., and Peters, A. (1978). The forms of non-pyramidal cells in the visual cortex of the rat. J. Comp. Neurol. 179, 761–794. doi: 10.1002/cne.901790406
Feldmeyer, D., Lübke, J., Silver, R. A., and Sakmann, B. (2002). Synaptic connections between layer 4 spiny neurone- layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J. Physiol. 538, 803–822. doi: 10.1113/jphysiol.2001.012959
Ferraguti, F., Cobden, P., Pollard, M., Cope, D., Shigemoto, R., Watanabe, M., et al. (2004). Immunolocalization of metabotropic glutamate receptor 1alpha (mGluR1alpha) in distinct classes of interneuron in the CA1 region of the rat hippocampus. Hippocampus 14, 193–215. doi: 10.1002/hipo.10163
Ferraguti, F., Klausberger, T., Cobden, P., Baude, A., Roberts, J. D. B., Szucs, P., et al. (2005). Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J. Neurosci. 25, 10520–10536. doi: 10.1523/JNEUROSCI.2547-05.2005
Ferrer, I., Soriano, E., del Rio, J. A., Alcántara, S., and Auladell, C. (1992). Cell death and removal in the cerebral cortex during development. Prog. Neurobiol. 39, 1–43. doi: 10.1016/0301-0082(92)90029-E
Fishell, G., and Hanashima, C. (2008). Pyramidal neurons grow up and change their mind. Neuron 57, 333–338. doi: 10.1016/j.neuron.2008.01.018
Frantz, G., Bohner, A. P., Akers, R. M., and McConnell, S. K. (1994). Regulation of the POU domain gene SCIP during cerebral cortical development. J. Neurosci. 14, 472–485.
Freund, T., and Buzsaki, G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I
Freund, T., and Gulyas, A. (1997). Inhibitory control of GABAergic interneurons in the hippocampus. Can. J. Physiol. Pharmacol. 75, 479–487. doi: 10.1139/y97-033
Fuentealba, P., Begum, R., Capogna, M., Jinno, S., Marton, L., Csicsvari, J., et al. (2008a). Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron 57, 917–929. doi: 10.1016/j.neuron.2008.01.034
Fuentealba, P., Klausberger, T., Karayannis, T., Suen, W. Y., Huck, J., Tomioka, R., et al. (2010). Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J. Neurosci. 30, 1595–1609. doi: 10.1523/JNEUROSCI.4199-09.2010
Fuentealba, P., Tomioka, R., Dalezios, Y., Márton, L. F., Studer, M., Rockland, K., et al. (2008b). Rhythmically active enkephalin-expressing GABAergic cells in the CA1 area of the hippocampus project to the subiculum and preferentially innervate interneurons. J Neurosci. 28, 10017–10022. doi: 10.1523/JNEUROSCI.2052-08.2008
Fujita, I., and Fujita, T. (1996). Intrinsic connections in the macaque inferior temporal cortex. J. Comp. Neurol. 368, 467–486. doi: 10.1002/(SICI)1096-9861(19960513)368:4<467::AID-CNE1>3.0.CO;2-2
Fukuda, T., and Kosaka, T. (2000). Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J. Neurosci. 20, 1519–1528.
Ganter, P., Szücs, P., Paulsen, O., and Somogyi, P. (2004). Properties of horizontal axo-axonic cells in stratum oriens of the hippocampal CA1 area of rats in vitro. Hippocampus 14, 232–243. doi: 10.1002/hipo.10170
Geiller, T., Royer, S., and Choi, J. S. (2017). Segregated Cell Populations Enable Distinct Parallel Encoding within the Radial Axis of the CA1 Pyramidal Layer. Exp. Neurobiol. 26, 1–10. doi: 10.5607/en.2017.26.1.1
Gibson, J. R., Beierlein, M., and Connors, B. W. (1999). Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402, 75–79. doi: 10.1038/47035
Gil, Z., Connors, B. W., and Amitai, Y. (1999). Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron. 23, 385–397. doi: 10.1016/S0896-6273(00)80788-6
Gilbert, C. D. (1983). Microcircuitry of the visual cortex. Annu. Rev. Neurosci. 6, 217–247. doi: 10.1146/annurev.ne.06.030183.001245
Gilbert, C. D., and Wiesel, T. N. (1983). Clustered intrinsic connections in cat visual cortex. J. Neurosci. 3, 1116–1133.
Golgi, C. (1883). Sulla fina anatomia degli organi centrali del sistema nervoso IV. Sulla fina anatomia delle circonvoluzioni cerebellari. Rivista Sperimentale di Freniatria 9, 1–17.
Golgi, C. (1906). The Neuron Doctrine - Theory and Facts. Nobel Lecture. Amsterdam: Elsevier Publishing Company.
Gonchar, Y., and Burkhalter, A. (1999). Differential subcellular localization of forward and feedback interareal inputs to parvalbumin expressing GABAergic neurons in rat visual cortex. J. Comp. Neurol. 406, 346–360. doi: 10.1002/(SICI)1096-9861(19990412)406:3<346::AID-CNE4>3.0.CO;2-E
Gray, C. M., and McCormick, D. A. (1996). Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science 274:5284. doi: 10.1126/science.274.5284.109
Gulyás, A. I., Hájos, N., Katona, I., and Freund, T. F. (2003). Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur. J. Neurosci. 17, 1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x
Gulyas, A. I., Megias, M., Emri, Z., and Freund, T. F. (1999). Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J. Neurosci. 19, 10082–10097.
Hajós, F., Zillesm, K., Schleicher, A., and Kálmán, M. (1988). Types and spatial distribution of vasoactive intestinal polypeptide (VIP)-containing synapses in the rat visual cortex. Anat. Embryol. 178, 207–217. doi: 10.1007/BF00318224
Halasy, K., Buhl, E. H., Lörinczi, Z., Tamás, G., and Somogyi, P. (1996). Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus 6, 306–329. doi: 10.1002/(SICI)1098-1063(1996)6:3<306::AID-HIPO8>3.0.CO;2-K
Hallman, L. E., Schofield, B. R., and Lin, C. S. (1988). Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J. Comp. Neurol. 272, 149–160. doi: 10.1002/cne.902720111
Hayashi, K., Kubo, K., Kitazawa, A., and Nakajima, K. (2015). Cellular dynamics of neuronal migration in the hippocampus. Front Neurosci. Rev. 9:135. doi: 10.3389/fnins.2015.00135
Hestrin, S. (1992). Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357, 686–689. doi: 10.1038/357686a0
Hirsch, J. A., Alonso, J. M., Reid, R. C., and Martinez, L. M. (1998b). Synaptic integration in striate cortical simple cells. J. Neurosci. 18, 9517–9528.
Hirsch, J. A., Gallagher, C. A., Alonso, J. M., and Martinez, L. M. (1998a). Ascending projections of simple and complex cells in layer 6 of the cat striate cortex. J. Neurosci. 18, 8086–8094.
Hoerder-Suabedissen, A., and Molnár, Z. (2012). Morphology of mouse subplate cells with identified projection targets changes with age. J. Comp. Neurol. 520, 174–185. doi: 10.1002/cne.22725
Hoerder-Suabedissen, A., and Molnár, Z. (2013). Molecular diversity of early-born subplate neurons. Cereb. Cortex 23, 1473–1483. doi: 10.1093/cercor/bhs137
Hoerder-Suabedissen, A., and Molnár, Z. (2015). Development, evolution and pathology of neocortical subplate neurons. Nat. Rev. Neurosci. 16, 133–146. doi: 10.1038/nrn3915
Hoffman, D. A., Magee, J. C., Colbert, C. M., and Johnston, D. (1997). K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875. doi: 10.1038/43119
Hübener, M., Schwarz, C., and Bolz, J. (1990). Morphological types of projection neurons in layer 5 of cat visual cortex. J. Comp. Neurol. 301, 655–674. doi: 10.1002/cne.903010412
Inan, M., and Anderson, S. A. (2014). The chandelier cell, form and function. Curr. Opin. Neurobiol. 26, 142–148. doi: 10.1016/j.conb.2014.01.009
Jakovcevski, I., Mayer, N., and Zecevic, N. (2011). Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb. Cortex 8, 1771–1782. doi: 10.1093/cercor/bhq245
Jinno, S. (2009). Structural organization of long-range GABAergic projection system of the hippocampus. Front. Neuroanat. 3:13. doi: 10.3389/neuro.05.013.2009
Jinno, S., Klausberger, T., Marton, L. F., Dalezios, Y., Roberts, J. D., Fuentealba, P., et al. (2007). Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 27, 8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007
Jones, E. G. (1975). Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J. Comp. Neurol. 160, 205–267. doi: 10.1002/cne.901600204
Jones, M. W., and McHugh, T. J. (2011). Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci. 34, 526–553. doi: 10.1016/j.tins.2011.07.007
Jovanovic, J. N., and Thomson, A. M. (2011). Development of cortical GABAergic innervation. Front. Cell. Neurosci. 5:14. doi: 10.3389/fncel.2011.00014
Kampa, B. M., Letzkus, J. J., and Stuart, G. J. (2006). Cortical feed-forward networks for binding different streams of sensory information. Nat. Neurosci. 9, 1472–1473. doi: 10.1038/nn1798
Kasper, E. M., Larkman, A. U., Lübke, J., and Blakemore, C. (1994). Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J. Comp. Neurol. 339, 459–474 doi: 10.1002/cne.903390402
Katona, I., Sperlágh, B., Sík, A., Käfalvi, A., Vizi, E. S., Mackie, K., et al. (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558.
Katona, L., Micklem, B., Borhegyi, Z., Swiejkowski, D. A., Valenti, O., Viney, T., et al. (2017). Behavior-dependent activity patterns of GABAergic long-range projecting neurons in the rat hippocampus. Hippocampus 27, 359–377. doi: 10.1002/hipo.22696
Katz, L. C. (1987). Local circuitry of identified projection neurons in cat visual cortex brain slices. J. Neurosci. 7, 1223–1249.
Kawaguchi, Y., and Kubota, Y. (1996). Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 16, 2701–2715.
Kawaguchi, Y., and Kubota, Y. (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486. doi: 10.1093/cercor/7.6.476
Kawauchi, T. (2015). Cellullar insights into cerebral cortical development: focusing on the locomotion mode of neuronal migration. Front. Cell. Neurosci. 9:394. doi: 10.3389/fncel.2015.00394
Kerti, K., Lorincz, A., and Nusser, Z. (2012). Unique somato-dendritic distribution pattern of Kv4.2 channels on hippocampal CA1 pyramidal cells. Eur. J. Neurosci. 35, 66–75. doi: 10.1111/j.1460-9568.2011.07907.x
Kisvárday, Z. F., Martin, K. A. C., Freund, T. F., Maglóczky, Z. S., W.hitteridge, D., and Somogyi, P. (1986). Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Expl. Brain Res. 64, 541–552. doi: 10.1007/BF00340492
Kisvárday, Z. F., Martin, K. A., Whitteridge, D., and Somogyi, P. (1985). Synaptic connections of intracellularly filled clutch cells: a type of small basket cell in the visual cortex of the cat. J. Comp. Neurol. 241, 111–137. doi: 10.1002/cne.902410202
Kitazawa, A., Kubo, K., Hayashi, K., Matsunaga, Y., Ishii, K., and Nakajima, K. (2014). Hippocampal pyramidal neurons switch from a multipolar migration mode to a novel “climbing” migration mode during development. J. Neurosci. 34, 1115–1126. doi: 10.1523/JNEUROSCI.2254-13.2014
Klausberger, T. (2009). GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur. J. Neurosci. 30, 947–957. doi: 10.1111/j.1460-9568.2009.06913.x
Klausberger, T., and Somogyi, P. (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. doi: 10.1126/science.1149381
Klausberger, T., Magill, P., Marton, L., Roberts, J., Cobden, P., Buzsáki, G., et al. (2003). Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421:844–848. doi: 10.1038/nature01374
Klausberger, T., Márton, L. F., Baude, A., Roberts, J. D., Magill, P. J., and Somogyi, P. (2004). Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat. Neurosci. 7, 41–47. doi: 10.1038/nn1159
Klausberger, T., Marton, L. F., O'Neill, J., Huck, J. H., Dalezios, Y., Fuentealba, P., et al. (2005). Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. Neuroscience 25, 9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005
Kohara, K., Pignatelli, M., Rivest, A. J., Jung, H. Y., Kitamura, T., Suh, J., et al. (2014). Cell type-specific genetic and optogenetic tools reveal novel hippocampal CA2 circuits. Nat. Neurosci. 17, 269–279. doi: 10.1038/nn.3614
Kosaka, T., Wu, J. Y., and Benoit, R. (1988). GABAergic neurons containing somatostatin-like immunoreactivity in the rat hippocampus and dentate gyrus. Exp Brain Res. 71, 388–398. doi: 10.1007/BF00247498
Kostovic, I., and Rakic, P. (1980). Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J. Neurocytol. 9, 219–242. doi: 10.1007/BF01205159
Kritzer, M. F., and Goldman-Rakic, P. S. (1995). Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefontal cortex in the rhesus monkey. J. Comp. Neurol. 359, 131–143. doi: 10.1002/cne.903590109
Kunkel, D. D., and Schwartzkroin, P. A. (1988). Ultrastructural characterization and GAD co-localization of somatostatin-like immunoreactive neurons in CA1 of rabbit hippocampus. Synapse 2, 371–381. doi: 10.1002/syn.890020404
Lacaille, J. C., and Williams, S. (1990). Membrane properties of interneurons in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience 36, 349–359. doi: 10.1016/0306-4522(90)90431-3
Lacaille, J. C., Mueller, A. L., Kunkel, D. D., and Schwartzkroin, P. A. (1987). Local circuit interactions between oriens/alveus interneurones and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J. Neurosci. 7, 1979–1993.
Larkum, M. E., Zhu, J. J., and Sakmann, B. (1999). A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398, 338–341. doi: 10.1038/18686
Larsen, D. D., and Callaway, E. M. (2006). Development of layer-specific axonal arborizations in mouse primary somatosensory cortex. J. Comp. Neurol. 494, 398–414. doi: 10.1002/cne.20754
Lee, S., Hjerling-Leffler, J., Zagha, E., Fishell, G., and Rudy, B. (2010). The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 30, 16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010
Leone, D. P., Srinivasan, K., Chen, B., Alcamo, E., and McConnell, S. K. (2008). The determination of projection neuron identity in the developing cerebral cortex. Curr. Opin. Neurobiol. 18, 28–35. doi: 10.1016/j.conb.2008.05.006
Letinic, K., Zoncu, R., and Rakic, P. (2002). Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649. doi: 10.1038/nature00779
Levitt, J. B., Lewis, D. A., Yoshioka, T., and Lund, J. S. (1993). Topography of pyramidal neuron intrinsic connections in macaque monkey prefrontal cortex (areas 9 and 46). J. Comp. Neurol. 338, 360–376. doi: 10.1002/cne.903380304
Levitt, J. B., Yoshioka, T., and Lund, J. S. (1994). Intrinsic cortical connections in macaque visual area V2: evidence for interaction between different functional streams. J. Comp. Neurol. 342, 551–570. doi: 10.1002/cne.903420405
Lewis, D. A., and Lund, J. S. (1990). Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J. Comp. Neurol. 293, 599–615. doi: 10.1002/cne.902930406
Li, G., and Pleasure, S. J. (2014). The development of hippocampal cellular assemblies. Wiley Interdiscip. Rev. Dev. Biol. 3, 165–177. doi: 10.1002/wdev.127
Li, H., Han, Y. R., Bi, C., Davila, J., Goff, L. A., Thompson, K., et al. (2008). Functional differentiation of a clone resembling embryonic cortical interneuron progenitors. Dev. Neurobiol. 68, 1549–1564. doi: 10.1002/dneu.20679
Li, L. Y., Li, Y. T., Zhou, M., Tao, H. W., and Zhang, L. I. (2013a). Intracortical multiplication of thalamocortical signals in mouse auditory cortex. Nat. Neurosci. 16, 1179–1181. doi: 10.1038/nn.3493
Li, Y. T., Ibrahim, L. A., Liu, B. H., Zhang, L. I., and Tao, H. W. (2013b). Linear transformation of thalamocortical input by intracortical excitation. Nat. Neurosci. 16, 1324–1330. doi: 10.1038/nn.3494
Li, Y., Lu, H., Cheng, P. L., Ge, S., Xu, H., Shi, S. H., et al. (2012). Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature 486, 118–121. doi: 10.1038/nature11110
Llinas, R. (1975). “Electroresponsive properties of dendrites in central neurons,” in Advances in Neurology, Vol. 12, Physiology and Pathology of Dendrites, ed G. W. Kreutzberg (New York, NY: Raven Press), 1–13.
López-Bendito, G., and Molnár, Z. (2003). Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 4, 276–289. doi: 10.1038/nrn1075
López-Bendito, G., Cautinat, A., Sánchez, J. A., Bielle, F., Flames, N., Garratt, A. N., et al. (2006). Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell 125, 127–142. doi: 10.1016/j.cell.2006.01.042
López-Bendito, G., Sánchez-Alcañiz, J. A., Pla, R., Borrell, V., Pic,ó, E., Valdeolmillos, M., et al. (2008). Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J. Neurosci. 28, 1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008
Lorente de Nò, R. (1934). Studies on the structure of the cerebral cortex II. Continuation of the study of the ammonic system. J. Psychol. Neurol. 46, 113–177.
Lübke, J., Roth, A., Feldmeyer, D., and Sakmann, B. (2003). Morphometric analysis of the columnar innervation domain of neurons connecting layer 4 and layer 2/3 of juvenile rat barrel cortex. Cereb. Cortex 13, 1051–1063. doi: 10.1093/cercor/13.10.1051
Lübkemann, R., Eberhardt, J., Röhl, F. W., Janitzky, K., Nullmeier, S., Stork, O., et al. (2015). Identification and characterization of GABAergic projection neurons from ventral hippocampus to amygdala. Brain Sci. 5, 299–317. doi: 10.3390/brainsci5030299
Luhmann, H. J., Fukuda, A., and Kilb, W. (2015). Control of cortical neuronal migration by glutamate and GABA. Front. Cell. Neurosci. 94:4. doi: 10.3389/fncel.2015.00004
Lukaszewicz, A., Savatier, P., Cortay, V., Giroud, P., Huissoud, C., Berland, M., et al. (2005). G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 47, 353–364. doi: 10.1016/j.neuron.2005.06.032
Lund, J. S. (1973). Organization of neurons in the visual cortex area 17, of the monkey (Macaca mulatta). J. Comp. Neurol. 147, 455–496 doi: 10.1002/cne.901470404
Lund, J. S. (1987). Local circuit neurons of macaque monkey striate cortex: I. Neurons of laminae 4C and 5A. J. Comp. Neurol. 257, 60–92. doi: 10.1002/cne.902570106
Lund, J. S., and Lewis, D. A. (1993). Local circuit neurons of developing and mature macaque prefrontal cortex: golgi and immunocytochemical characteristics. J. Comp. Neurol. 328, 282–312. doi: 10.1002/cne.903280209
Lund, J. S., Hawken, M. J., and Parker, A. J. (1988). Local circuit neurons of macaque monkey striate cortex: II. Neurons of laminae 5B and 6. J. Comp. Neurol. 276, 1–29. doi: 10.1002/cne.902760102
Lund, J. S., and Wu, C. Q. (1997). Local Circuit Neurons of Macaque Monkey Striate Cortex: IV. Neurons of Laminae 1–3A. J. Comp. Neurol. 384, 109–126. doi: 10.1002/(SICI)1096-9861(19970721)384:1<109::AID-CNE7>3.0.CO;2-5
Lund, J. S., and Yoshioka, T. (1991). Local circuit neurons of macaque monkey striate cortex: III. Neurons of laminae 4B, 4A, and 3B. J. Comp. Neurol. 311, 234–258. doi: 10.1002/cne.903110206
Lund, J. S., Yoshioka, T., and Levitt, J. B. (1993). Comparison of intrinsic connectivity in different areas of macaque monkey cerebral cortex. Cereb. Cortex 3, 148–162. doi: 10.1093/cercor/3.2.148
Luskin, M. B., and Shatz, C. J. (1985). Neurogenesis of the cat's primary visual cortex. J. Comp. Neurol. 242, 611–631. doi: 10.1002/cne.902420409
Ma, L., Harada, T., Harada, C., Romero, M., Hebert, J. M., McConnell, S. K., et al. (2002). Neurotrophin-3 is required for appropriate establishment of thalamocortical connections. Neuron 36, 623–634. doi: 10.1016/S0896-6273(02)01021-8
Ma, Y., Hu, H., Berrebi, A. S., Mathers, P. H., and Agmon, A. (2006). Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J. Neurosci. 26, 5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006
Marder, E., and Prinz, A. A. (2002). Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays 24, 1145–1154. doi: 10.1002/bies.10185
Markram, H. (1997). A network of tufted layer 5 pyramidal neurons. Cereb. Cortex 7, 523–533. doi: 10.1093/cercor/7.6.523
Markram, H., Muller, E., Ramaswamy, S., Reimann, M. W., Abdellah, M., Sanchez, C. A., et al. (2015). Reconstruction and Simulation of Neocortical Microcircuitry. Cell 163, 456–492. doi: 10.1016/j.cell.2015.09.029
Markram, H., Toledo-Rodriguez, M., Wang, Y., Gupta, A., Silberberg, G., and Wu, C. (2004). Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807. doi: 10.1038/nrn1519
Marques-Smith, A., Lyngholm, D., Kaufmann, A. K., Stacey, J. A., Hoerder-Suabedissen, A., Becker, E. B., et al. (2016). A transient translaminar GABAergic interneuron circuit connects thalamocortical recipient layers in neonatal somatosensory cortex. Neuron 89, 536–549. doi: 10.1016/j.neuron.2016.01.015
Martinotti, C. (1889). Contributo allo studio della corteccia cerebrale, ed all'origine centrale dei nervi. Ann. Freniatr. Sci. Affini. 1, 14–381.
Mátyás, F., Freund, T. F., and Gulyás, A. I. (2004). Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur. J. Neurosci. 19, 1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x
McManus, M. F., Nasrallah, I. M., Gopal, P. P., Baek, W. S., and Golden, J. A. (2004). Axon mediated interneuron migration. J. Neuropathol. Exp. Neurol. 63, 932–941. doi: 10.1093/jnen/63.9.932
Mercer, A. (2012). Electrically coupled excitatory neurones in cortical regions. Brain Res. 1487, 192–197. doi: 10.1016/j.brainres.2012.03.069
Mercer, A., Bannister, A. P., and Thomson, A. M. (2006). Electrical coupling between pyramidal cells in adult cortical regions. Brain Cell. Biol. 35, 13–27. doi: 10.1007/s11068-006-9005-9
Mercer, A., Botcher, N. A., Eastlake, K., and Thomson, A. M. (2012a). SP-SR interneurones: a novel class of neurones of the CA2 region of the hippocampus. Hippocampus 22, 1758–1769. doi: 10.1002/hipo.22010
Mercer, A., Eastlake, K., Trigg, H. L., and Thomson, A. M. (2012). Local circuitry involving parvalbumin-positive basket cells in the CA2 region of the hippocampus. Hippocampus 22, 43–56. doi: 10.1002/hipo.20841
Mercer, A., Trigg, H. L., and Thomson, A. M. (2007). Characterization of neurons in the CA2 subfield of the adult rat hippocampus. J. Neurosci. 27, 7329–7338. doi: 10.1523/JNEUROSCI.1829-07.2007
Mercer, A., West, D. C., Morris, O. T., Kirchhecker, S., Kerkhoff, J. E., and Thomson, A. M. (2005). Excitatory connections made by presynaptic cortico-cortical pyramidal cells in layer 6 of the neocortex. Cereb. Cortex 15, 1485–1496. doi: 10.1093/cercor/bhi027
Meyer, A. H., Katona, I., Blatow, M., Rozov, A., and Monyer, H. (2002). In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J. Neurosci. 22, 7055–7064.
Meyer, G., and Wahle, P. (1988). Early postnatal development of cholecystokinin-immunoreactive structures in the visual cortex of the cat. J. Comp. Neurol. 276, 360–386. doi: 10.1002/cne.902760304
Mingorance, A., Fontana, X., Solé, M., Burgaya, F., Ureña, J. M., Teng, F. Y., et al. (2004). Regulation of Nogo and Nogo receptor during the development of the entorhino-hippocampal pathway and after adult hippocampal lesions. Mol. Cell. Neurosci. 26, 34–49. doi: 10.1016/j.mcn.2004.01.001
Miyata, T., Kawaguchi, A., Okano, H., and Ogawa, M. (2001). Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 31, 727–741. doi: 10.1016/S0896-6273(01)00420-2
Miyoshi, G., Machold, R. P., and Fishell, G. (2013). “Specification of GABAergic neocortical interneurons,” in Cortical Development: Neural Diversity and Neocortical Organization, eds R. Kageyama and T. Yamamori (Tokyo: Springer), 89–126. doi: 10.1007/978-4-431-54496-8_5
Möhler, H., Fritschy, J. M., and Rudolph, U. (2002). A new benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 300, 2–8. doi: 10.1124/jpet.300.1.2
Molnár, Z., Métin, C., Stoykova, A., Tarabykin, V., Price, D. J., Francis, F., et al. (2006). Comparative aspects of cerebral cortical development. Eur. J. Neurosci. 23, 921–934. doi: 10.1111/j.1460-9568.2006.04611.x
Molnár, Z., Adams, R., Goffinet, A. M., and Blakemore, C. (1998). The role of the first postmitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J. Neurosci. 18, 5746–5765.
Molnár, Z., and Butler, A. B. (2002). The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays 24, 530–541. doi: 10.1002/bies.10100
Molnár, Z., and Hoerder-Suabedissen, A. (2016). Regional scattering of primate subplate. Proc. Natl.Acad. Sci. U.S.A. 113, 9676–9678. doi: 10.1073/pnas.1611194113
Molnár, Z., Garel, S., López-Bendito, G., Maness, P., and Price, D. J. (2012). Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain. Eur. J. Neurosci. 35, 1573–1585. doi: 10.1111/j.1460-9568.2012.08119.x
Montiel, J. F., Vasistha, N. A., Garcia-Moreno, F., and Molnár, Z. (2016). From sauropsids to mammals and back: new approaches to comparative cortical development. J. Comp. Neurol. 524, 630–645. doi: 10.1002/cne.23871
Monyer, H., Seeburg, P. H., and Wisden, W. (1991). Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron 6, 799–810.
Morrison, J. H., Benoit, R., Magistretti, P. J., Ling, N., and Bloom, F. E. (1982). Immunohistochemical distribution of pro-somatostatin-related peptides in hippocampus. Neurosci. Lett. 34, 137–142. doi: 10.1016/0304-3940(82)90165-3
Mountcastle, V. B. (1957). Modality and topographic properties of single neurons of cat's somatic sensory cortex. J. Neurophysiol. 20, 408–434.
Murthy, S., Niquille, M., Hurni, N., Limoni, G., Frazer, S., Chameau, P., et al. (2014). Serotonin receptor 3A controls interneuron migration into the neocortex. Nat Commun. 5:5524. doi: 10.1038/ncomms6524
Nadarajah, B., and Parnavelas, J. G. (2002). Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci. 3, 423–432. doi: 10.1038/nrn845
Nadarajah, B., Brunstrom, J. E., Grutzendler, J., Wong, R. O., and Pearlman, A. L. (2001). Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 4, 143–150. doi: 10.1038/83967
Naegele, J. R., Barnstable, C. J., and Wahle, P. R. (1991). Expression of a unique 56-kDa polypeptide by neurons in the subplate of the developing cerebral cortex. Proc. Natl. Acad. Sci. U.S.A. 88, 330–334. doi: 10.1073/pnas.88.2.330
Nakahira, E., and Yuasa, S. (2005). Neuronal generation, migration, and differentiation in the mouse hippocampal primoridium as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J. Comp. Neurol. 483, 329–340. doi: 10.1002/cne.20441
Nestor, M. W., and Hoffman, D. A. (2012). Differential cycling rates of Kv4.2 channels in proximal and distal dendrites of hippocampal CA1 pyramidal neurons. Hippocampus 22, 969–980. doi: 10.1002/hipo.20899
Nielsen, J. V., Blom, J. B., Noraberg, J., and Jensen, N. A. (2010). Zbtb20-induced CA1 pyramidal neuron development and area enlargement in the cerebral midline cortex of mice. Cereb. Cortex 20, 1904–1914. doi: 10.1093/cercor/bhp261
Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S., and Kriegstein, A. R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720. doi: 10.1038/35055553
Noctor, S. C., Martínez-Cerdeño, V., Ivic, L., and Kriegstein, A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144. doi: 10.1038/nn1172
Nowakowski, R. S., and Rakic, P. (1979). The mode of migration of neurons to the hippocampus: a Golgi and electron microscopic analysis in foetal rhesus monkey. J. Neurocytol. 8, 697–718. doi: 10.1007/BF01206671
Nusser, Z. (2009). Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 32, 267–274. doi: 10.1016/j.tins.2009.01.003
Nusser, Z. (2012). Differential subcellular distribution of ion channels and the diversity of neuronal function. Curr. Opin. Neurobiol. 22, 366–371. doi: 10.1016/j.conb.2011.10.006
Nusser, Z., Sieghart, W., Benke, D., Fritschy, J. M., and Somogyi, P. (1996). Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc. Natl. Acad. Sci. U.S.A. 93, 11939–11944. doi: 10.1073/pnas.93.21.11939
Oláh, S., Füle, M., Komlósi, G., Varga, C., Báldi, R., Barzo, P., et al. (2009). Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461, 1278–1281. doi: 10.1038/nature08503
Overstreet-Wadiche, L., and McBain, C. J. (2015). Neurogliaform cells in cortical circuits. Nat. Rev. Neurosci. 16, 458–468. doi: 10.1038/nrn3969
Parnavelas, J. G., Lieberman, A. R., and Webster, K. E. (1977). Organization of neurons in the visual cortex, area 17, of the rat. J. Anat. 124, 305–322.
Pascual, M., Pozas, E., and Soriano, E. (2005). Role of class 3 semaphorins in the development and maturation of the septohippocampal pathway. Hippocampus 15, 184–202. doi: 10.1002/hipo.20040
Pawelzik, H., Bannister, A. P., Deuchars, J., Ilia, M., and Thomson, A. M. (1999). Modulation of bistratified cell IPSPs and basket cell IPSPs by pentobarbitone sodium, diazepam and Zn2+: dual recordings in slices of adult rat hippocampus. Eur. J. Neurosci. 11, 3552–3564. doi: 10.1046/j.1460-9568.1999.00772.x
Pawelzik, H., Hughes, D. I., and Thomson, A. M. (2002). Physiological and morphological diversity of immunocytochemically defined parvalbumin-and cholecystokinin-positive interneurones in CA1 of the adult rathippocampus. J. Comp. Neurol. 443, 346–367. doi: 10.1002/cne.10118
Pawelzik, H., Hughes, D. I., and Thomson, A. M. (2003). Modulation of inhibitory autapses and synapses on rat CA1 interneurones by GABAA receptor ligands. J. Physiol. 546, 701–716. doi: 10.1113/jphysiol.2002.035121
Peters, A. (1990). The axon terminals of vasoactive intestinal polypeptide (VIP)-containing bipolar cells in rat visual cortex. J. Neurocytol. 19, 672–685. doi: 10.1007/BF01188036
Picardo, M. A., Guigue, P., Bonifazi, P., Batista-Brito, R., Allene, C., Ribas, A., et al. (2011). Pioneer GABA cells comprise a subpopulation of hub neurons in the developing hippocampus. Neuron 71, 695–709. doi: 10.1016/j.neuron.2011.06.018
Porter, J. T., Cauli, B., Staiger, J. F., Lambolez, B., Rossier, J., and Audinat, E. (1998). Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur. J. Neurosci. 10, 3617–3628. doi: 10.1046/j.1460-9568.1998.00367.x
Porter, L. L., and White, E. L. (1986). Synaptic connections of callosal projection neurons in the vibrissal region of mouse primary motor cortex: an electron microscopic/horseradish peroxidase study. J. Comp. Neurol. 248, 573–587. doi: 10.1002/cne.902480409
Price, C. J., Cauli, B., Kovacs, E. R., Kulik, A., Lambolez, B., Shigemoto, R., et al. (2005). Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J. Neurosci. 25, 6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005
Prönneke, A., Scheuer, B., Wagener, R. J., Möck, M., Witte, M., and Staiger, J. F. (2015). Characterizing VIP Neurons in the Barrel Cortex of VIPcre/tdTomato Mice Reveals Layer-Specific Differences. Cereb, Cortex 25, 4854–4868. doi: 10.1093/cercor/bhv202
Purpura, D. P., and Shofer, R. J. (1965). Spike-generation in dendrites and synaptic inhibition in immature cerebral cortex. Nature 206, 833–834. doi: 10.1038/206833a0
Rakic, P. (1972). Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–83. doi: 10.1002/cne.901450105
Rakic, P. (1988). Specification of cerebral cortical areas. Science 241, 170–176. doi: 10.1126/science.3291116
Rall, W. (1962). Electrophysiology of a dendritic neuron model. Biophys. J. 2(2 Pt. 2), 145–167. doi: 10.1016/S0006-3495(62)86953-7
Ramirez, D. M., and Kavalali, E. T. (2011). Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr. Opin. Neurobiol. 21, 275–282. doi: 10.1016/j.conb.2011.01.007
Reichova, I., and Sherman, S. M. (2004). Somatosensory corticothalamic projections: distinguishing drivers from modulators. J. Neurophysiol. 92, 2185–2197. doi: 10.1152/jn.00322.2004
Retzius, G. (1893). The Cajal'schen cells of the cerebral cortex in humans and mammals. Biol. Unter. 5, 1–8.
Rockland, K. S., and Knutson, T. (2000). Feedback connections from area MT of the squirrel monkey to areas V1 and V2. J. Comp. Neurol. 425, 345–368. doi: 10.1002/1096-9861(20000925)425:3<345::AID-CNE2>3.0.CO;2-O
Rockland, K. S., Andresen, J., Cowie, R. J., and Robinson, D. L. (1999). Single axon analysis of pulvinocortical connections to several visual areas in the macaque. J. Comp. Neurol. 406, 221–250. doi: 10.1002/(SICI)1096-9861(19990405)406:2<221::AID-CNE7>3.0.CO;2-K
Rojo, C., Leguey, I., Kastanauskaite, A., Bielza, C., Larrañaga, P., DeFelipe, J., et al. (2016). Laminar Differences in Dendritic Structure of Pyramidal Neurons in the Juvenile Rat Somatosensory Cortex. Cereb. Cortex 26, 2811–2822. doi: 10.1093/cercor/bhv316
Rowland, D. C., Weible, A. P., Wickersham, I. R., Wu, H., Mayford, M., Witter, M. P., et al. (2013). Transgenically targeted rabies virus demonstrates a major monosynaptic projection from hippocampal area CA2 to medial entorhinal layer II neurons. J. Neurosci. 33, 14889–14898. doi: 10.1523/JNEUROSCI.1046-13.2013
Rudolph, U., and Möhler, H. (2014). GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 54, 483–507. doi: 10.1146/annurev-pharmtox-011613-135947
Rudy, B., Fishell, G., Lee, S., and Hjerling-Leffler, J. (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61. doi: 10.1002/dneu.20853
San Antonio, A., Liban, K., Ikrar, T., Tsyganovskiy, E., and Xu, X. (2014). Distinct physiological and developmental properties of hippocampal CA2 subfield revealed by using anti-Purkinje cell protein 4 (PCP4) immunostaining. J. Comp. Neurol. 522, 1333–1354. doi: 10.1002/cne.23486
Sarnat, H. B., and Flores-Sarnat, L. (2002). Role of Cajal-Retzius and subplate neurons in cerebral cortical development. Semin. Pediatr. Neurol. 9, 302–308. doi: 10.1053/spen.2002.32506
Sato, H., Fukutani, Y., Yamamoto, Y., Tatara, E., Takemoto, M., Shimamura, K., et al. (2012). Thalamus-derived molecules promote survival and dendritic growth of developing cortical neurons. J. Neurosci. 32, 15388–15402. doi: 10.1523/JNEUROSCI.0293-12.2012
Schmitz, D., Schuchmann, S., Fisahn, A., Draguhn, A., Buhl, E. H., Petrasch-Parwez, E., et al. (2001). Axo-axonal coupling. A novel mechanism for ultrafast neuronal communication. Neuron 31, 831–840. doi: 10.1016/S0896-6273(01)00410-X
Sekine, K., Honda, T., Kawauchi, T., Kubo, K., and Nakajima, K. (2011). The outermost region of the developing cortical plate is crucial for both the switch of the radial migration mode and the Dab1-dependent “inside-out” lamination in the neocortex. J. Neurosci. 31, 9426–9439. doi: 10.1523/JNEUROSCI.0650-11.2011
Sekine, K., Kubo, K., and Nakajima, K. (2014). How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex? Neurosci. Res. 86, 50–58. doi: 10.1016/j.neures.2014.06.004
Shigemoto, R., Kulik, A., Roberts, J. D., Ohishi, H., Nusser, Z., Kaneko, T., et al. (1996). Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 381, 523–525. doi: 10.1038/381523a0
Shitamukai, A., Konno, D., and Matsuzaki, F. (2011). Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci. 31, 3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011
Sik, A., Penttonen, M., Ylinen, A., and Buszáki, G. (1994). Inhibitory CAI-CA3-hilar region feedback in the hippocampus. Science 265, 1722–1724. doi: 10.1126/science.8085161
Silva, J., Sharma, S., and Cowell, J. K. (2015). Homozygous Deletion of the LGI1 Gene in Mice Leads to Developmental Abnormalities Resulting in Cortical Dysplasia. Brain Pathol. 25, 587–597. doi: 10.1111/bpa.12225
Simon, A., Oláh, S., Molnár, G., Szabadics, J., and Tamás, G. (2005). Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J. Neurosci. 25, 6278–6285. doi: 10.1523/JNEUROSCI.1431-05.2005
Slomianka, L., Amrein, I., Knuesel, I., Sørensen, J. C., and Wolfer, D. P. (2011). Hippocampal pyramidal cells: the reemergence of cortical lamination. Brain Struct. Funct. 216, 301–317. doi: 10.1007/s00429-011-0322-0
Slomianka, L., and Geneser, F. A. (1997). Postnatal development of zinc-containing cells and neuropil in the hippocampal region of the mouse. Hippocampus 7, 321–340.
Somogyi, J., Baude, A., Omori, Y., Shimizu, H. E., Mestikawy, S., Fukaya, M., et al. (2004). GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur. J. Neurosci. 19, 552–569. doi: 10.1111/j.0953-816X.2003.03091.x
Somogyi, P. (1977). A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Res. 136, 345–350. doi: 10.1016/0006-8993(77)90808-3
Somogyi, P., Freund, T. F., and Cowey, A. (1982). The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience 7, 2577–2607. doi: 10.1016/0306-4522(82)90086-0
Somogyi, P., Kisvárday, Z. F., Martin, K. A., and Whitteridge, D. (1983). Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience 10, 261–294. doi: 10.1016/0306-4522(83)90133-1
Song, S., Sjostrom, P. J., Reigl, M., Nelson, S., and Chklovskii, D. B. (2005). Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3:e68. doi: 10.1371/journal.pbio.0030068
Spatz, W. B., Tigges, J., and Tigges, M. (1970). Subcortical projections, cortical associations and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey. J. Comp. Neurol. 140, 155–174. doi: 10.1002/cne.901400203
Staiger, J. F., Freund, T. F., and Zilles, K. (1997). Interneurons immunoreactive for vasoactive intestinal polypeptide (VIP) are extensively innervated by parvalbumin-containing boutons in rat primary somatosensory cortex. Eur. J. Neurosci. 9, 2259–2268. doi: 10.1111/j.1460-9568.1997.tb01644.x
Staiger, J. F., Zilles, K., and Freund, T. F. (1996). Distribution of GABAergic elements postsynaptic to ventroposteromedial thalamic projections in layer IV of rat barrel cortex. Eur. J. Neurosci. 8, 2273–2285. doi: 10.1111/j.1460-9568.1996.tb01191.x
Stuart, G., Schiller, J., and Sakmann, B. (1997). Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. 505, 617–632. doi: 10.1111/j.1469-7793.1997.617ba.x
Sugiyama, T., Osumi, N., and Katsuyama, Y. (2014). A novel cell migratory zone in the developing hippocampal formation. J. Comp. Neurol. 522, 3520–3538. doi: 10.1002/cne.23621
Sun, W., Maffie, J. K., Lin, L., Petralia, R. S., Rudy, B., and Hoffman, D. A. (2011). DPP6 establishes the A-type K(+) current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 71, 1102–1115. doi: 10.1016/j.neuron.2011.08.008
Supèr, H., and Soriano, E. (1994). The organization of the embryonic and early postnatal murine hippocampus. II. Development of entorhinal, commissural, and septal connections studied with the lipophilic tracer DiI. J. Comp. Neurol. 344, 101–120. doi: 10.1002/cne.903440108
Szentagothai, J., and Arbib, M. A. (1974). Conceptual models of neural organization. Neurosci. Res. Program Bull. 12, 305–510.
Tabata, H., and Nakajima, K. (2003). Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci. 23, 9996–10001.
Tabata, H., Kanatani, S., and Nakajima, K. (2009). Differences of migratory behavior between direct progeny of apical progenitors and basal progenitors in the developing cerebral cortex. Cereb. Cortex 19, 2092–2105. doi: 10.1093/cercor/bhn227
Tabata, H., Yoshinaga, S., and Nakajima, K. (2012). Cytoarchitecture of mouse and human subventricular zone in developing cerebral neocortex. Exp Brain Res. 216, 161–168. doi: 10.1007/s00221-011-2933-3
Takács, V. T., Szőnyi, A., Freund, T. F., Nyiri, G., and Gulyás, A. I. (2015). Quantitative ultrastructural analysis of basket and axo-axonic cell terminals in the mouse hippocampus. Brain Struct. Funct. 220, 919–940. doi: 10.1007/s00429-013-0692-6
Tamamaki, N., Abe, K., and Nojyo, Y. (1988). Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Res. 452, 255–272. doi: 10.1016/0006-8993(88)90030-3
Tamás, G., Buhl, E. H., Lörincz, A., and Somogyi, P. (2000). Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat. Neurosci. 3, 366–371. doi: 10.1038/73936
Tamás, G., Buhl, E. H., and Somogyi, P. (1997). Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J. Physiol. 500, 715–738.
Tamás, G., Somogyi, P., and Buhl, E. H. (1998). Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J. Neurosci. 18, 4255–4270.
Tan, X., and Shi, S. H. (2013). Neocortical neurogenesis and neuronal migration. Wiley Interdiscip. Rev. Dev. Biol. 2, 443–459. doi: 10.1002/wdev.88
Tansey, E. P., Chow, A., Rudy, B., and McBain, C. J. (2002). Developmental expression of potassium-channel subunit Kv3.2 within subpopulations of mouse hippocampal inhibitory interneurons. Hippocampus 12, 137–148. doi: 10.1002/hipo.1104
Tarczy-Hornoch, K., Martin, A., Stratford, J., and Jack, J. J. (1999). Intracortical excitation of spiny neurons in layer 4 of cat striate cortex in vitro. Cereb. Cortex 9, 833–843. doi: 10.1093/cercor/9.8.833
Thomson, A. M. (2000a). Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 23, 305–312. doi: 10.1016/S0166-2236(00)01580-0
Thomson, A. M. (2000b). Molecular frequency filters at central synapses. Prog Neurobiol. 62, 159–196. doi: 10.1016/S0301-0082(00)00008-3
Thomson, A. M. (2003). Presynaptic frequency- and pattern-dependent filtering. J. Comput. Neurosci. 15, 159–202. doi: 10.1023/A:1025812808362
Thomson, A. M. (2010). Neocortical layer 6, a review. Front. Neuroanat. 4:13. doi: 10.3389/fnana.2010.00013
Thomson, A. M., and Bannister, A. P. (1998). Postsynaptic pyramidal target selection by descending layer III pyramidal axons: dual intracellular recordings and biocytin filling in slices of rat neocortex. Neuroscience 84, 669–683 doi: 10.1016/S0306-4522(97)00557-5
Thomson, A. M., and Bannister, A. P. (2003). Interlaminar Connections in the Neocortex. Cereb. Cortex 13, 5–14. doi: 10.1093/cercor/13.1.5
Thomson, A. M., and Jovanovic, J. N. (2010). Mechanisms underlying synapse-specific clustering of GABA(A) receptors. Eur. J. Neurosci. 31, 2193–2203. doi: 10.1111/j.1460-9568.2010.07252.x
Thomson, A. M., and Lamy, C. (2007). Functional maps of neocortical local circuitry. Front. Neurosci. 1, 19–42. doi: 10.3389/neuro.01.1.1.002.2007
Thomson, A. M., and West, D. C. (1993). Fluctuations in pyramid-pyramid EPSPs modified by presynaptic firing pattern and postsynaptic membrane potential using paired intracellular recordings in rat neocortex. Neuroscience 54, 329–346. doi: 10.1016/0306-4522(93)90256-F
Thomson, A. M., West, D. C., and Deuchars, J. (1995). Properties of single axon excitatory postsynaptic potentials elicited in spiny interneurons by action potentials in pyramidal neurons in slices of rat neocortex. Neuroscience 69, 727–738. doi: 10.1016/0306-4522(95)00287-S
Thomson, A. M., Bannister, A. P., Hughes, D. I., and Pawelzik, H. (2000). Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur. J. Neurosci. 12, 425–436. doi: 10.1046/j.1460-9568.2000.00915.x
Thomson, A. M., West, D. C., Wang, Y., and Bannister, A. P. (2002). Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labeling in vitro. Cereb. Cortex 12, 936–953. doi: 10.1093/cercor/12.9.936
Tia, S., Wang, J. F., Kotchabhakdi, N., and Vicini, S. (1996). Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor alpha 6 subunit. J. Neurosci. 16, 3630–3640.
Tole, S., Christian, C., and Grove, E. A. (1997). Early specification and autonomous development of cortical fields in the mouse hippocampus. Development 124, 4959–4970.
Toledo-Rodriguez, M., Blumenfeld, B., Wu, C., Luo, J., Attali, B., Goodman, P., et al. (2004). Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb. Cortex 14, 1310–1327. doi: 10.1093/cercor/bhh092
Tuncdemir, S. N., Wamsley, B., Stam, F. J., Osakada, F., Goulding, M., Callaway, E. M., et al. (2016). Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron 89, 521–535. doi: 10.1016/j.neuron.2015.11.020
Urbán, N., and Guillemot, F. (2014). Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front. Cell. Neurosci. 8:396. doi: 10.3389/fncel.2014.00396
Valverde, F. (1976). Aspects of cortical organization related to the geometry of neurons with intra-cortical axons. J. Neurocytol. 5, 509–529 doi: 10.1007/BF01175566
Valverde, F., Facal-Valverde, M. V., Santacana, M., and Heredia, M. (1989). Development and differentiation of early generated cells of sublayer VIb in the somatosensory cortex of the rat: a correlated Golgi and autoradiographic study. J. Comp. Neurol. 290, 118–140. doi: 10.1002/cne.902900108
Van Horn, S. C., and Sherman, S. M. (2004). Differences in projection patterns between large and small corticothalamic terminals. J. Comp. Neurol. 475, 406–415 doi: 10.1002/cne.20187
Varga, C., Golshani, P., and Soltesz, I. (2012). Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc. Natl. Acad. Sci. U.S.A. 109, 2726–2734. doi: 10.1073/pnas.1210929109
Varga, C., Tamas, G., Barzo, P., Olah, S., and Somogyi, P. (2015). Molecular and electrophysiological characterization of GABAergic interneurons expressing the transcription factor COUP-TFII in the adult human temporal cortex. Cereb. Cortex 25, 4430–4449. doi: 10.1093/cercor/bhv045
Vida, I., Halasy, K., Szinyei, C., Somogyi, P., and Buhl, E. H. (1998). Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J. Physiol. 506, 755–773. doi: 10.1111/j.1469-7793.1998.755bv.x
Villette, V., Guigue, P., Picardo, M. A., Sousa, V. H., Leprince, E., Lachamp, P., et al. (2016). Development of early-born γ-Aminobutyric acid hub neurons in mouse hippocampus from embryogenesis to adulthood. J. Comp. Neurol. 524, 2440–2461. doi: 10.1002/cne.23961
Vitalis, T., and Rossier, J. (2011). New insights into cortical interneurons development and classification: contribution of developmental studies. Dev. Neurobiol. 71, 34–44. doi: 10.1002/dneu.20810
Wall, N. R., De La Parra, M., Sorokin, J. M., Taniguchi, H., Huang, Z. J., and Callaway, E. M. (2016). Brain-wide maps of synaptic input to cortical interneurons. J. Neurosci. 36, 4000–4009. doi: 10.1523/JNEUROSCI.3967-15.2016
Wamsley, B., and Fishell, G. (2017). Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat. Rev. Neurosci. 18, 299–309. doi: 10.1038/nrn.2017.30
Wang, C. F., Hsing, H. W., Zhuang, Z. H., Wen, M. H., Chang, W. J., Briz, C. G., et al. (2017). Lhx2 Expression in postmitotic cortical neurons. Cell Rep. 4, 849–856. doi: 10.1016/j.celrep.2017.01.001
Wang, X., Tsai, J. W., LaMonica, B., and Kriegstein, A. R. (2011). A new subtype of progenitor cell in the mouse embryonic neocortex. Nat. Neurosci. 14, 555–561. doi: 10.1038/nn.2807
Wang, Y., Barakat, A., and Zhou, H. (2010). Electrotonic coupling between pyramidal neurons in the neocortex. PLoS ONE 5:e10253. doi: 10.1371/journal.pone.0010253
Wang, Y., Gupta, A., Toledo-Rodriguez, M., Wu, C. Z., and Markram, H. (2002). Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb. Cortex 12, 395–410. doi: 10.1093/cercor/12.4.395
Wang, Y., Toledo-Rodriguez, M., Gupta, A., Wu, C., Silberberg, G., Luo, J., et al. (2004). Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 561, 65–90. doi: 10.1113/jphysiol.2004.073353
West, D. C., Mercer, A., Kirchhecker, S., Morris, O. T., and Thomson, A. M. (2006). Layer 6 cortico-thalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb. Cortex 16, 200–211. doi: 10.1093/cercor/bhi098
White, E. L., and Czeiger, D. (1991). Synapses made by axons of callosal projection neurons in mouse somatosensory cortex: emphasis on intrinsic vonnections. J. Comp. Neurol. 303, 233–244. doi: 10.1002/cne.903030206
White, E. L., and Hersch, S. M. (1982). A quantitative study of thalamocortical and other synapses involving the apical dendrites of corticothalamic projection cells in mouse SmI cortex. J. Neurocytol. 11, 137–157. doi: 10.1007/BF01258009
White, E. L., and Keller, A. (1987). Intrinsic circuitry involving the local axon collaterals of corticothalamic projection cells in mouse SmI cortex. J. Comp. Neurol. 262, 13–26. doi: 10.1002/cne.902620103
Wisden, W., and Seeburg, P. H. (1993). A complex mosaic of high-affinity kainate receptors in rat brain. J. Neurosci. 13, 3582–3598.
Wiser, A. K., and Callaway, E. M. (1996). Contributions of individual layer 6 pyramidal neurons to local circuitry in the macaque primary visual cortex. J. Neurosci. 16, 2724–2739.
Wonders, C. P., and Anderson, S. A. (2006). The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687–696. doi: 10.1038/nrn1954
Wu, S. X., Goebbels, S., Nakamura, K., Nakamura, K., Kometani, K., Minato, N., et al. (2005). Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc. Natl. Acad. Sci. U.S.A. 102, 17172–17177. doi: 10.1073/pnas.0508560102
Xie, Z., Ma, X., Ji, W., Zhou, G., Lu, Y., Xiang, Z., et al. (2010). Zbtb20 is essential for the specification of CA1 field identity in the developing hippocampus. Proc. Natl. Acad. Sci. U.S.A. 107, 6510–6515. doi: 10.1073/pnas.0912315107
Xu, H. T., Han, Z., Gao, P., He, S., Li, Z., Shi, W., et al. (2014). Distinct lineage-dependent structural and functional organization of the hippocampus. Cell 157, 1552–1564. doi: 10.1016/j.cell.2014.03.067
Xu, X., and Callaway, E. M. (2009). Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J. Neurosci. 29, 70–85. doi: 10.1523/JNEUROSCI.4104-08.2009
Yamamoto, N., and Hanamura, K. (2005). Formation of the thalamocortical projection regulated differentially by BDNF- and NT-3-mediated signaling. Rev. Neurosci. 16, 223–231. doi: 10.1515/REVNEURO.2005.16.3.223
Yavorska, I., and Wehr, M. (2016). Somatostatin-expressing inhibitory interneurons in cortical circuits. Front. Neural Circuits 10:76. doi: 10.3389/fncir.2016.00076
Yoshimura, Y., and Callaway, E. M. (2005). Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat. Neurosci. 8, 1552–1559. doi: 10.1038/nn1565
Yoshioka, T., Levitt, J. B., and Lund, J. S. (1994). Independence and merger of thalamocortical channels within macaque monkey primary visual cortex: anatomy of interlaminar projections. Vis. Neurosci. 11, 1–23. doi: 10.1017/S0952523800002406
Yu, Y. C., Bultje, R. S., Wang, X., and Shi, S. H. (2009). Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504. doi: 10.1038/nature07722
Keywords: neocortex, hippocampus, pyramidal cells, interneurones, development, neuronal circuitry, neocortical columns
Citation: Mercer A and Thomson AM (2017) Cornu Ammonis Regions–Antecedents of Cortical Layers? Front. Neuroanat. 11:83. doi: 10.3389/fnana.2017.00083
Received: 01 June 2017; Accepted: 08 September 2017;
Published: 26 September 2017.
Edited by:
Javier DeFelipe, Cajal Institute (CSIC), SpainReviewed by:
Tamas F. Freund, Institute of Experimental Medicine (MTA), HungaryCopyright © 2017 Mercer and Thomson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audrey Mercer, YS5tZXJjZXJAdWNsLmFjLnVr
Alex M. Thomson, YWxleC50aG9tc29uQHVjbC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.