
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neuroanat. , 05 January 2016
Volume 9 - 2015 | https://doi.org/10.3389/fnana.2015.00162
A correction has been applied to this article in:
Corrigendum: Unexpected Variation in Neuroanatomy among Diverse Nematode Species
Nematodes are considered excellent models for understanding fundamental aspects of neuron function. However, nematodes are less frequently used as models for examining the evolution of nervous systems. While the habitats and behaviors of nematodes are diverse, the neuroanatomy of nematodes is often considered highly conserved. A small number of nematode species greatly influences our understanding of nematode neurobiology. The free-living species Caenorhabditis elegans and, to a lesser extent, the mammalian gastrointestinal parasite Ascaris suum are, historically, the primary sources of knowledge regarding nematode neurobiology. Despite differences in size and habitat, C. elegans and A. suum share a surprisingly similar neuroanatomy. Here, we examined species across several clades in the phylum Nematoda and show that there is a surprising degree of neuroanatomical variation both within and among nematode clades when compared to C. elegans and Ascaris. We found variation in the numbers of neurons in the ventral nerve cord and dye-filling pattern of sensory neurons. For example, we found that Pristionchus pacificus, a bacterial feeding species used for comparative developmental research had 20% fewer ventral cord neurons compared to C. elegans. Steinernema carpocapsae, an insect-parasitic nematode capable of jumping behavior, had 40% more ventral cord neurons than C. elegans. Interestingly, the non-jumping congeneric nematode, S. glaseri showed an identical number of ventral cord neurons as S. carpocapsae. There was also variability in the timing of neurodevelopment of the ventral cord with two of five species that hatch as second-stage juveniles showing delayed neurodevelopment. We also found unexpected variation in the dye-filling of sensory neurons among examined species. Again, sensory neuron dye-filling pattern did not strictly correlate with phylogeny. Our results demonstrate that variation in nematode neuroanatomy is more prevalent than previously assumed and recommend this diverse phylum for future “evo-devo-neuro” studies.
For the past 200 years, nematodes received significant attention from neurobiologists due to their relatively simple anatomy (reviewed in Chitwood and Chitwood, 1938). The nervous system of the nematode Caenorhabditis elegans consists of only 302 neurons in the adult hermaphrodite and remains the only nervous system to be completely reconstructed (White et al., 1986). In addition to C. elegans, the neuroanatomy of several parasitic and free-living (non-parasitic) species has been examined using both light and transmission electron microscopy (TEM).
The phylum Nematoda is currently divided into 12 Clades (Figure 1) (Holterman et al., 2006; van Megen et al., 2009). Significant divergence in neuroanatomy exists between nematodes in basal clades (class Enoplea; formerly Adenophorea) and those in higher clades (class Chromadorea; formerly Secernentea) (Sulston and Horvitz, 1977; Gans and Burr, 1994; Malakhov, 1994). However, within the higher clades (clades 8–12), which include C. elegans and other intensely studied species, the neuroanatomy is often considered highly conserved (Angstadt et al., 1989; Martin et al., 2002; Burr and Robinson, 2004; Kimber and Fleming, 2005; Hallem and Sternberg, 2008; Srinivasan et al., 2008).
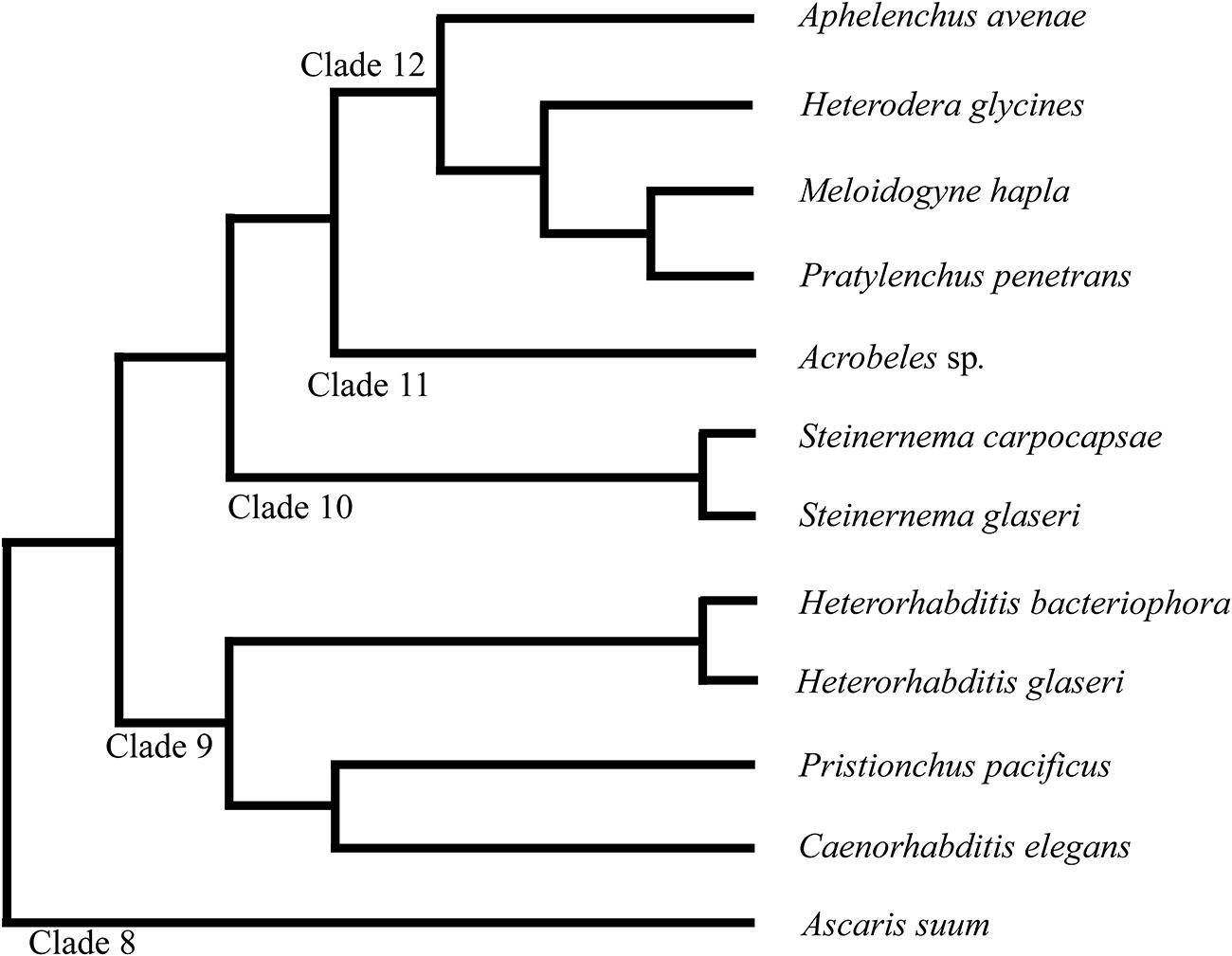
FIGURE 1. Phylogeny of nematode species discussed in this study. The phylum Nematoda is currently divided into 12 clades as discussed in van Megen et al. (2009). Branch lengths do not represent distance.
A classic example supporting a high degree of conservation of neuroanatomy among nematodes is the similarity in structure between the ventral nerve cords (VNC) of C. elegans (Clade 9) and the gastrointestinal parasitic nematode Ascaris suum (Clade 8). The VNC consists of a series of motor neurons that innervate body-wall muscles and regulate movement (White et al., 1976). While Ascaris adults are hundreds of times larger than C. elegans and inhabit an extremely different environment, the number of ventral cord neurons is remarkably similar. In C. elegans, 57 neurons in the VNC innervate 95 body-wall muscles (Sulston, 1976; White et al., 1976). In A. suum, 55 neurons in the VNC innervate approximately 50,000 muscle cells (Stretton, 1976; Stretton et al., 1978).
While the VNC anatomy of other nematode species has received little attention, several studies have examined the anatomy of anterior sensory neurons and pharyngeal neurons (Ward et al., 1975; Ashton and Schad, 1996; Endo, 1998; Li et al., 2001; Bumbarger et al., 2009; Ragsdale et al., 2009). In C. elegans, there is one pair of amphid sensilla each containing 12 sensory neurons. The anterior nervous system of C. elegans also contains six inner labial, six outer labial and four cephalic sensilla each with an invariant number of neurons (Ward et al., 1975). While the pattern of sensilla and underlying neurons is typically conserved among examined species, variations in the number, position and ultrastructure of the anterior nervous system are well documented (Endo, 1998; Ashton et al., 1999; Bumbarger et al., 2009; Ragsdale et al., 2009). Similarly, recent data demonstrated extensive differences in neuronal connectivity between the pharynxes of C. elegans and Pristionchus pacificus (Bumbarger et al., 2013), a Clade 9 nematode frequently used for evo-devo studies. These anatomical differences may underlie functional differences in feeding behavior between the two species (Chiang et al., 2006).
To elucidate the evolution of nematode nervous systems, we utilized Differential Interference Contrast (DIC) and fluorescence microscopy to examine the neuroanatomy of the VNC and sensory neurons in nematodes from clades 9 to 12 (Holterman et al., 2006; van Megen et al., 2009). We found unexpected variation in the number of putative neurons in the VNC as well as the dye-filling pattern of chemosensory neurons among several species of parasitic and free-living nematodes. The variability was found both within and among nematodes clades suggesting a dynamic evolution of nematode neuroanatomy. Furthermore, we found variation in the developmental timing of the VNC among nematode species. Our results suggest that nematodes represent a valuable resource for understanding the evolution of nervous systems.
Meloidogyne hapla was isolated by the senior author from infected tomato plants and identified using morphological characters. Pratylenchus penetrans was isolated by Dr. Terry Niblack (formerly University of Illinois). Both M. hapla and P. penetrans were cultured on monoxenic excised corn and tomato root cultures, respectively (Lauritis et al., 1983). Seeds for monoxenic cultures were surface sterilized and germinated on water agar. After germination, roots were excised and transferred to Gamborg’s agar (Gamborg et al., 1968; Rebois and Huettel, 1986). Heterodera glycines was received from the plant clinic at University of Illinois and maintained in a sandy loam soil on the soybean variety ‘Lee’ in the greenhouse. Aphelenchus avenae was originally isolated by the senior author from soil surrounding garlic plants and identified using morphological characters. A. avenae was cultured on ¼ strength Potato Dextrose Agar with the fungus Botrytis cinerea as previously described (De Soyza, 1973). The entomopathogenic species (Steinernema sp. and Heterorhabditis sp.) were received from Dr. Albrecht Koppenhöfer at Rutgers University and reared on living greater wax moth larvae Galleria mellonella (Carolina Biological Supply Company, Burlington, NC, USA; Kaya and Stock, 1997). Infective juveniles (IJs) of the four species were collected using White traps (White, 1927) and stored in cell culture flasks with water before DAPI staining. Two methods were used to collect non-IJ and adult stages of entomopathogenic nematodes. For S. carpocapsae, IJs were induced to recover and complete development on lipid agar plates as previously described (Wouts, 1981). For other EPNs, non-IJs were collected by dissecting open Galleria mellonella approximately 9 days after inoculation. This allows for sufficient time for IJs to recover and develop into mixed stages of non-IJs and adults. Acrobeles sp. (stain PS1156), C. elegans (strain N2), and P. pacificus (strain PS312) were received from the Caenorhabditis Genetics Center and cultured on NGM agar with Escherichia coli OP50 using standard methods (Brenner, 1974).
Pratylenchus penetrans, A. avenae, and M. hapla were extracted from Petri dishes using a Baermann funnel and washed three times with distilled water before fixation. Second stage juveniles (J2s) of H. glycines were extracted from soybean roots using sugar centrifugation and washed three times with distilled water (Jenkins, 1964). C. elegans, Acrobeles sp., and P. pacificus were washed from the Petri dishes and rinsed three times with M9 buffer (Brenner, 1974) to remove adhering bacteria before fixation. IJs of H. bacteriophora, H. megidis, S. carpocapsae, and S. glaseri were washed three times with distilled water before fixation.
Nematodes were fixed in 4% formaldehyde at 4°C overnight in microcentrifuge tubes. Following formaldehyde fixation, nematodes were washed three times with Phosphate buffered saline with Triton X-100 (PBST; 0.1% Triton) and incubated in methanol for at least 4 h. Nematodes were then washed three times with PBST and incubated in 0.2–0.5 μg/ ml of 4′, 6-diamidino-2-phenylindole (DAPI; Life technologies, Carlsbad, CA, USA) overnight in dark at room temperature. Nematodes were store at 4°C prior to examination. We were unable to distinguish the sex of H. glycines, M. hapla or young juveniles of P. penetrans. The gender of Steinernema was identified based on the shape of the gonad. Only hermaphrodites of C. elegans and P. pacificus, and females of Acrobeles sp. were examined. Between 10 and 30 animals were examined for each species. Putative neurons in the VNC were identified based on the size and morphology of the nuclei (Sulston, 1976; White et al., 1976). Counts of neuronal nuclei were made from immediately posterior of the retrovesicular ganglion (RVG) to immediately anterior of the preanal ganglion (PAG) (Figure 2). In cases where a cell could not be unambiguously identified as a neuron an independent count was made by a researcher blind to the species. If the cell identity was still in doubt, it was excluded from the total neuron count.
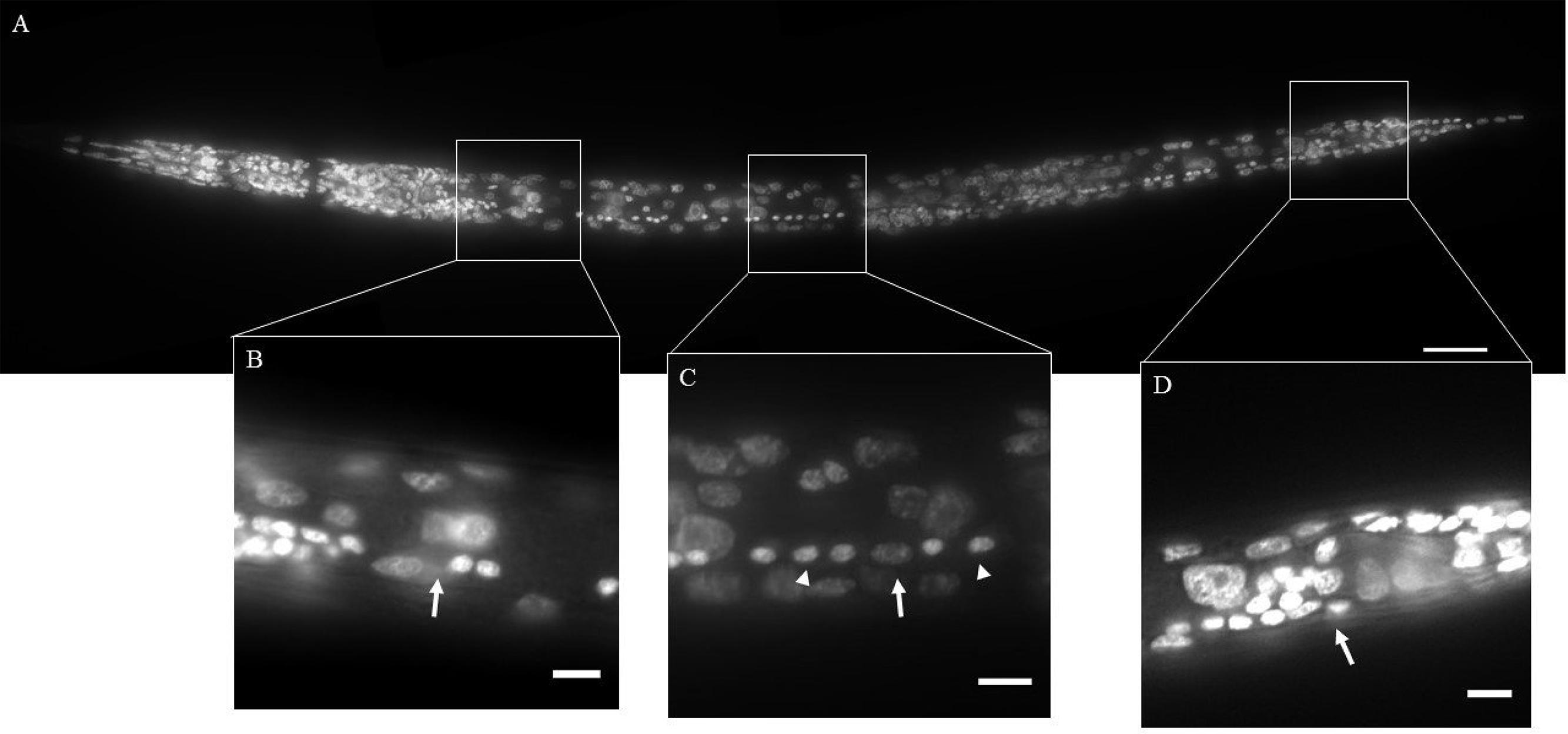
FIGURE 2. DAPI staining of wild-type Caenorhabditis elegans (Clade 9). The ventral nerve cord (VNC) consists of a line of motor neurons extending along the ventral midline from the retrovesicular ganglion (RVG) to the pre-anal ganglion (PAG). (A) Ventral view of DAPI stained animal. (B) Region surrounding end of RVG and anterior portion of VNC (arrow). (C) Part of the VNC showing neuronal (arrowheads) and hypodermal (arrow) nuclei. (D) Division between PAG and VNC (arrow). Inset scale bars = 5 μm.
A separate microwave fixation method was developed for the staining of J2 M. hapla. Nematodes were recovered from tomato root cultures and transferred to 0.2X Finney-Ruvkun buffer with 5% methanol and 2% formaldehyde (http://www.wormatlas.org/EMmethods/Antibodystaining.htm; Finney and Ruvkun, 1990). The fixation solution was placed in a 1 L ice bath in a household microwave with rotating turntable. A separate 1 L beaker of H2O was included as a heat sink. Nematodes were exposed to three separate 1 min irradiations at 30% power with a 30°C maximum temperature. Following fixation, nematodes were washed three times with PBST and then irradiated nine times in 0.2 μg/ml DAPI for 3–4 min at 30% power with a maximum temperature of 39°C.
Dye-filling was adapted from previously described methods (Tong and Bürglin, 2010). All nematodes were transferred into centrifuge tubes and prewashed 3 times with distilled water. Nematodes were incubated in 10 μg/ml of DiI (1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Life technologies, Carlsbad, CA, USA) and wrapped with aluminum foil on an orbital shaker for at least 2 h. Excess liquid was removed from the centrifuge tubes and nematodes were transferred onto 1.5% water agar for at least 1 h covered with foil to remove excess dye. Animals were then picked to agar pads amended with 20 mM levamisole for imaging with DIC and fluorescent microscopy (Shaham, 2006). For each species, more than 30 animals were examined. Images were acquired using a Zeiss M2 AxioImager with mechanized stage and Zen software. Z-projections were created using FIJI.
Synchronized A. avenae eggs were obtained by picking gravid A. avenae females into 5% M9 buffer (Stiernagle, 2006) for 1 h to lay eggs. Adults were then removed and the remaining eggs stored at 22°C until hatching (∼48 h). Immediately after hatching, J2s were transferred to 1/8 strength Potato Dextrose Agar with the fungus Phomopsis logicolla at 22°C. A. avenae nematodes were then examined at specified time points after hatching by mounting on a 5% agar pad with 20 mM levamisole and observed using DIC microscopy.
The VNC is an easily recognized series of neurons lying along the ventral cord of nematodes (Figure 2). In C. elegans, the VNC contains a series of 57 motorneurons lying between the RVG and the PAG (Sulston, 1976; White et al., 1976). Using DIC optics, neuronal nuclei in C. elegans are typically small, granular in appearance and lacking obvious nucleoli (Sulston, 1976; Yochem, 2006). Neurons are seen as highly condensed round fluorescent puncta following DAPI staining (Sulston, 1976).
The free-living nematode, P. pacificus (Clade 9) is used as a satellite nematode species for evolutionary studies (Sommer, 2005, 2006). Similar to C. elegans, P. pacificus typically feeds on bacteria. However, P. pacificus is also capable of predatory behavior toward other nematodes (Bumbarger et al., 2013). We found that the P. pacificus ventral cord contained approximately 20% fewer VNC neurons than C. elegans (Table 1; Figure 3A) suggesting that the number of neurons does not strictly correlate with phylogeny. Furthermore, this data indicates that evolved behaviors such as predation do not necessarily require an increase in the number of motorneurons. To test if other, more distantly related bacterial feeding nematodes, show even greater divergence in ventral cord neuron number, we examined the Clade 11 bacterial feeding nematode Acrobeles sp. Interestingly, Acrobeles sp. showed a nearly identical number of VNC neurons as C. elegans (Table 1; Figure 3F).
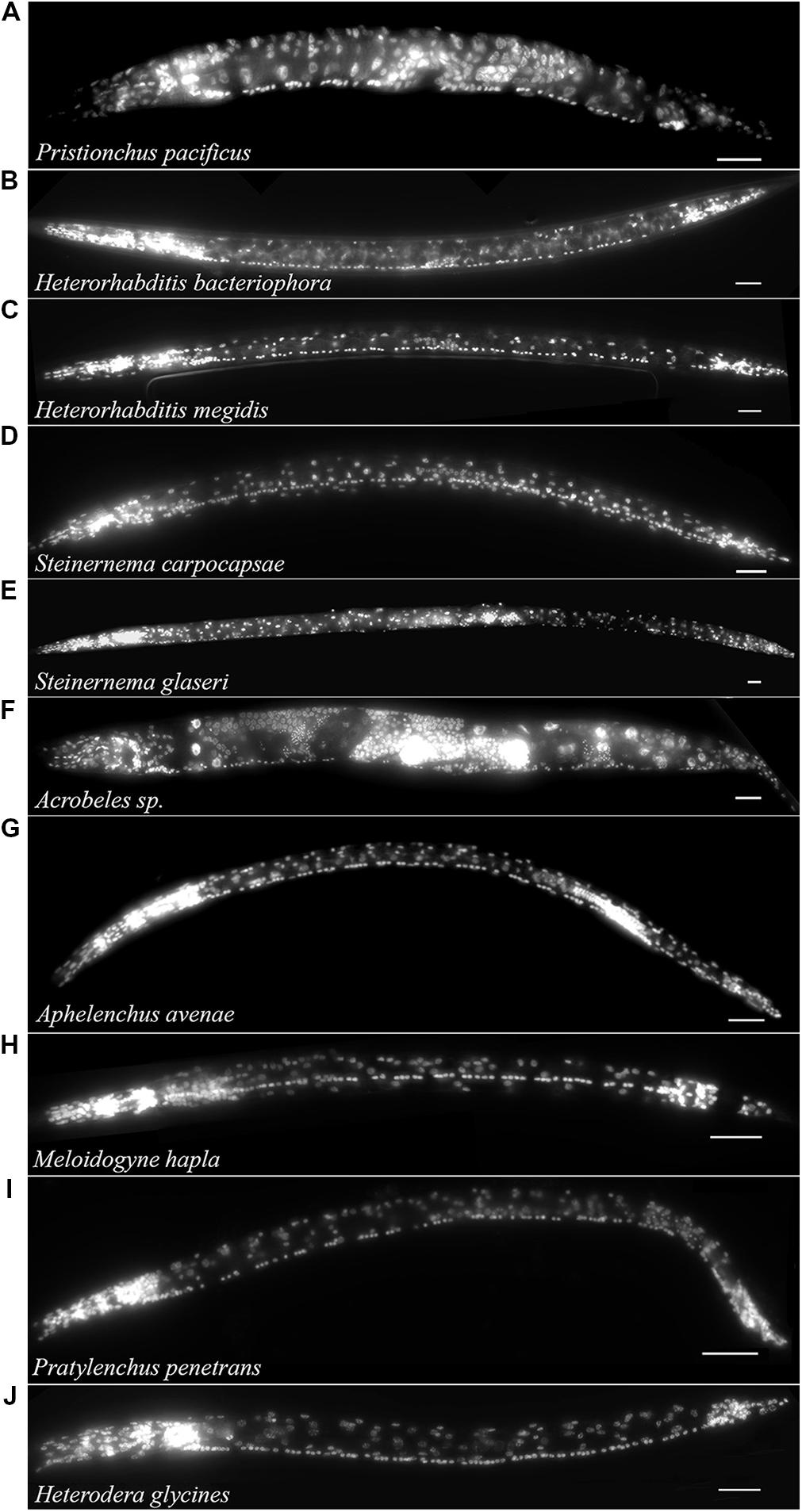
FIGURE 3. The VNC of nematodes in Clades 8–12 is highly variable. Fluorescent micrographs of individual nematode species fixed in formaldehyde and exposed to DAPI followed by imaging under fluorescent light. Species examined include: (A) Pristionchus pacificus hermaphrodite (Clade 9). (B) Heterorhabditis bacteriophora infective juvenile (Clade 9). (C) Heterorhabditis megidis infective juvenile (Clade 9). (D) Steinernema carpocapsae infective juvenile (Clade 10). (E) Steinernema glaseri infective juvenile (Clade 10). (F) Acrobeles sp. adult female (Clade 11). (G) Aphelenchus avenae J3 (Clade 12). (H) Meloidogyne hapla J2 (Clade 12). (I) Pratylenchus penetrans adult female (Clade 12). (J) Heterodera glycines J2 (Clade 12). Scale bar = 20 μm.
The entomopathogenic nematode genera Heterorhabditis sp. (Clade 9) and Steinernema sp. (Clade 10) infect a wide range of insect hosts. Though phylogenetically distinct, the two genera have similar lifestyles. Heterorhabditis sp. is more closely related to C. elegans than to Steinernema sp. (Clade 10). We found that two species of Heterorhabditis had 59 ventral cord neurons, similar to C. elegans (Table 1; Figure 3B). However, the ventral cord of S. carpocapsae contained 76 neurons (Table 1; Figure 3D); approximately 40% more than C. elegans and the largest number of VNC neurons among all examined species. S. carpocapsae is capable of an unusual jumping behavior wherein it stands on its tail, curls to form a loop and quickly extends to jump into the air (Reed and Wallace, 1965). We hypothesized that the increased number of neurons in S. carpocapsae evolved to allow for jumping behavior. To test this, we examined S. glaseri, another entomopathogenic species that does not exhibit jumping behavior. While slightly longer than S. carpocapsae, S. glaseri also had 76 neurons in the VNC (Table 1; Figure 3E). This data suggests either that jumping behavior does not specifically require additional motor neurons or that an ancestor to S. glaseri could jump and the additional neurons in S. glaseri are remnants of this ancestor. Steinernema sp. are male-female species. During the IJ stage sexes are easily distinguishable based on the shape of the gonad. However, we did not observe major differences in the number of VNC nuclei between sexes (data not shown).
Clade 12 contains nematodes with diverse life histories including fungal-feeding, plant-parasitic, and insect-parasitic species (van Megen et al., 2009). We examined four species in Clade 12 including one fungal-feeding species and three plant-parasitic species. There was no obvious correlation between the number of VNC neurons and food source or phylogeny. The fungal-feeding nematode Aphelenchus avenae and the plant-parasitic nematodes, Heterodera glycines and Meloidogyne hapla each had approximately 65 VNC neurons (Table 1; Figures 3G,H,J). However, the plant-parasitic nematode P. penetrans ventral cord contained fewer neurons than any other Clade 12 species (Table 1; Figure 3I). Pratylenchus is considered basal to Meloidogyne (van Megen et al., 2009). This data suggests either that there were multiple events leading to an increase in VNC number or that Pratylenchus underwent a loss in VNC neurons during evolution.
Caenorhabditis elegans does not hatch with a full set of VNC neurons. Following embryogenesis, C. elegans hatches as a J1 (equivalent to L1 in C. elegans nomenclature) with 15 VNC neurons (Sulston, 1976). During J1 development, additional precursor cells (P0–P12 cells) migrate into the ventral cord followed by multiple rounds of cell division to produce the final complement of 57 VNC neurons (Sulston, 1976). This post-embryonic development occurs entirely within the J1–J2 developmental period. To determine if post-embryonic VNC development during J1 development is conserved, we examined the VNC of three species that hatch as J2s rather than J1s. We examined the VNC of both newly hatched J2s and adult nematodes of P. pacificus (Clade 9), A. avenae (Clade 12) and P. penetrans (Clade 12). Both P. pacificus and A. avenae showed delayed development in the VNC. P. pacificus hatched as a J2 with 20 VNC neurons while the adult hermaphrodite has 45 VNC neurons (Table 1). Similarly, A. avenae hatched as a J2 with 26 VNC neurons, while the adult female has 66 (Table 1). Interestingly, the delayed development of the VNC was not conserved among all species that hatch as J2s. We found no apparent difference in the number of VNC neurons between newly hatched J2 and adult P. penetrans (Clade 12) (data not shown). These data suggest an independent evolution of neuronal heterochrony in nematodes.
The delayed development seen in A. avenae and P. pacificus may be due to a shift in development from the C. elegans-like J1 VNC development to a J2 VNC development. Alternatively, the delayed development may be due to a progressive increase in VNC neuron number from J1 to the adult stage. To test these options, we collected time-series data on the development of the VNC in A. avenae using DIC microscopy. Aphelenchus avenae is an easily cultured fungal feeding nematode, closely related to, and a possible transitional model for, plant-parasitic nematodes. We found that eggs developed from single-cell embryos to hatched J2s in approximately 48 h. We observed 26 neurons in the VNC of newly hatched J2s using DIC microscopy. Cell division occurs after hatching and during feeding. Immediately prior to the J3 molt (25–35 h after hatching), we observed the most intensive increase of neurons in the VNC. By 48 h after hatching, A. avenae had a fully developed VNC with 66 neurons (Table 1; Figures 3 and 4). Thus, the delayed development of the VNC in A. avenae is a shift from a C. elegans-like J1 developmental sequence to a J2 post-hatching developmental sequence.
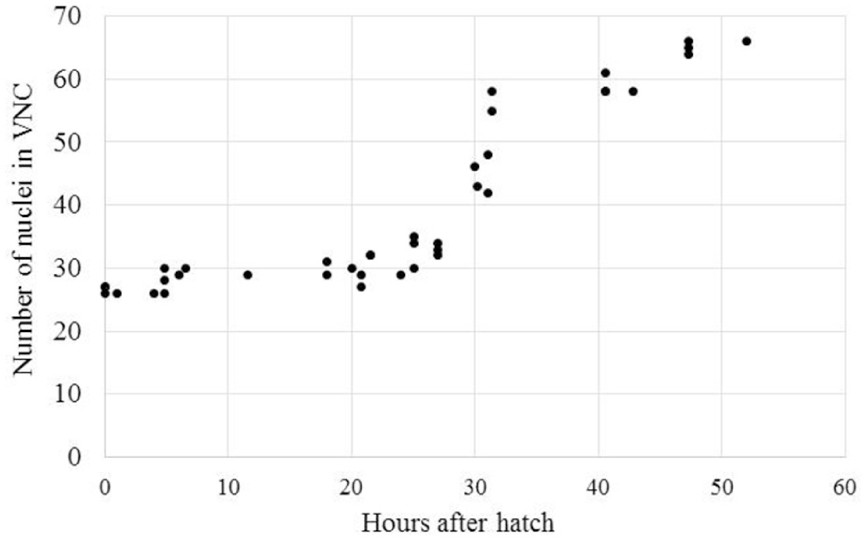
FIGURE 4. Aphelenchus avenae undergoes a post-hatch increase in the number of VNC neurons during J2. The number of VNC nuclei was examined with DIC microscopy in synchronized A. avenae nematodes at various time-points following hatch. The molt from J2 to J3 occurs at approximately 36 h after hatching. Each data point represents an individual animal.
We were interested if a similar divergence in neuronal properties could be detected using a dye-filling protocol common to C. elegans research. Specific ciliated sensory neurons in the head and tail of C. elegans will fluoresce following exposure to the lipophilic compound DiI (Hedgecock et al., 1985; Collet et al., 1998). In C. elegans, six pairs of amphid neurons in the head and two pairs of phasmid neurons in the tail can be stained using fluorescent dyes (DiI, DiO and FITC; Figure 5A; Hedgecock et al., 1985; Collet et al., 1998). Staining patterns can vary depending on the method. For example, under certain conditions six inner-labial sensory neurons can also be stained (Tong and Bürglin, 2010). While the precise mechanism is unknown, dye-filling is frequently used to indicate the structural integrity of specific sensory neurons in C. elegans (Perkins et al., 1986). For example, Srinivasan et al. (2008) used a comparative dye-filling approach to identify and investigate the function of a homologous sensory neuron among six free-living nematodes in Clades 9 and 10. They found a nearly identical dye-filling pattern among all tested nematodes, suggesting a high degree of conservation of nematode neuroanatomy. We expanded upon these results by testing additional species in Clades 9–12.

FIGURE 5. DiI-filling is highly variable among nematodes. Live nematodes were exposed to DiI for 2 h followed by repeated washes in water or buffer and then imaged with fluorescent microscopy. (A) Left Anterior of the Clade 9 nematode Caenorhabditis elegans dauer, arrowhead indicates amphid neurons. (A) Right Posterior of a Caenorhabditis elegans dauer, two pairs of phasmid neurons are shown. (B) Left Anterior of the Clade 10 nematode Steinernema carpocapsae female, arrow indicates inner labial neurons. (B) Right Posterior of a S. carpocapsae male, arrow indicates phasmid neurons and arrowheads indicate unidentified neurons. (C) Left Anterior of the Clade 11 nematode Acrobeles sp. female, arrow indicates amphid neurons and arrowhead indicates inner labial neurons. (C) Right Posterior of an Acrobeles sp. female, two pairs of phasmid neurons are shown. (D) Left Anterior of the Clade 12 nematode Aphelenchus avenae, pentagon indicates amphid neurons, arrow indicates cephalic neurons, and arrow head indicates inner labial neurons. (D) Right Posterior of an Aphelenchus avenae female, two pairs of phasmid neurons are shown. (E) Anterior of the Clade 12 nematode Pratylenchus penetrans female (ventral view), one pair of amphid neurons in the anterior of the nematode is shown. Scale bar = 10 μm for all images.
We initially attempted dye-filling on the entomopathogenic nematodes Heterorhabditis bacteriophora (Clade 9) and Steinernema carpocapsae (Clade 10). These entomopathogenic nematodes infect their hosts as IJs, a non-feeding developmentally arrested stage analogous to the C. elegans dauer stage. Following infection, the nematodes will resume development and feed until resources are depleted. The nematodes then reenter the IJ stage and disperse to find a new host. Interestingly, we did not observe any dye-filling in IJs of either species. Therefore, we isolated non-infective stages of these species using both in vitro and in vivo methods. We observed dye-filling in six putative IL2 orthologs in non-IJs and adults of S. carpocapsae (Figure 5B). Two pairs of putative phasmid neurons also dye-filled in the tail of males of S. carpocapsae (Figure 5B). Surprisingly, dye-filling of amphid neurons was only periodically observed in both of these insect-parasitic species. Based on these results, we examined the dauer stage of C. elegans. As previously shown, five of the six amphid neurons routinely dye-filled in C. elegans dauers (Figure 5A) (Peckol et al., 2001). However, we never observed IL2 dye-filling in C. elegans dauers using modified dye-filling protocols that routinely result in IL2 dye-filling in non-dauers. This lack of dye-filling may be due to modifications to cilia structure during dauer as previously shown by TEM (Albert and Riddle, 1983). Together these results demonstrate that development has a marked influence on sensory neuron properties.
Similar to our VNC data, we found significant variability in dye-filling among nematode clades. As discussed above, H. bacteriophora (Clade 9) and S. carpocapsae (Clade 10) showed different dye-filling patterns than C. elegans. However, the bacterial-feeding nematode Acrobeles sp. (Clade 11) showed an identical dye-filling pattern to C. elegans (Figure 5C). The fungal-feeding nematode A. avenae (Clade 12) also displayed a similar dye-filling pattern as C. elegans. Specifically, six putative IL2 orthologs and twelve putative amphid orthologs dye-filled in A. avenae (Figure 5D). In A. avenae, an additional four neurons were stained anterior to amphid neurons that we identified as cephalic neurons. Previous TEM data demonstrated that A. avenae females have two cephalic neurons (CEP1 and CEP2) in each sensillum (Ragsdale et al., 2009). The cilia of CEP1 in A. avenae are exposed to the environment, whereas CEP2 is embedded in the cuticle. Therefore, the dye-filling of cephalic neurons in A. avenae corresponds to the EM data. In C. elegans hermaphrodites, there is one cephalic neuron in each sensillum that is not exposed to the external environment and does not dye-fill (Hedgecock et al., 1985). However, C. elegans males, similar to A. avenae females, have two cephalic neurons. While these male-specific cephalic neurons (CEM) in C. elegans are exposed to the external environment, they do not dye-fill (Perkins et al., 1986).
Unlike the fungal feeding Clade 12 nematode A. avenae, the Clade 12 plant-parasitic nematodes displayed restricted dye-filling. In P. penetrans, only one pair of putative amphid neurons stained (Figure 5E). Based on axon morphology and nuclear position we identified these neurons as likely ADL orthologs. We observed only occasional and weak dye-filling in P. penetrans phasmid neurons (data not shown). Two plant-parasitic nematodes, H. glycines and M. hapla, did not show dye-filling in any neuron suggesting a possible modification of these neurons.
Nematodes have made substantial contributions to our understanding of the molecular basis of evolution. These studies have generally focused on non-neuronal structures such as vulva development and male-tail morphology (Fitch, 1997; Sommer, 2000). Fewer studies have utilized nematodes for examining the evolution of neurodevelopment and neuroanatomy. The lack of nematode “evo-neuro” studies may be due, in part, to the assumption that the neuroanatomy of nematodes is highly conserved. The few comparative studies have primarily utilized low-throughput TEM to dissect ultrastructural differences in the sensory or pharyngeal nervous systems of a handful of species. Here, we demonstrated that light microscopy can be used to observe differences in neuroanatomy and neurodevelopment across multiple species. Furthermore, we showed that neuroanatomical and neurodevelopmental differences among nematodes are more abundant than previously assumed.
We found differences in the number and developmental timing of the VNC among various nematode species. The VNC of C. elegans and A. suum consist of a series of motor neurons that regulate movement through coordinated excitation and inhibition of body wall muscles. Amazingly, these phylogenetically and ecologically separate species show a nearly identical number and pattern of cholinergic and GABAergic VNC neurons (Stretton, 1976; White et al., 1976; Stretton et al., 1978; Johnson and Stretton, 1985, 1987; Mclntire et al., 1993; Duerr et al., 2001). As we found that several species diverge from the C. elegans and Ascaris neuroanatomy, it will be useful to examine the neurotransmitter identity of VNC neurons in these species for conservation of the basic patterning.
While identification of neuronal nuclei in the VNC is relatively straightforward using DAPI fluorescence and DIC microscopy there are two potential causes of ambiguity and resulting variation. In some species, a clear demarcation did not exist between the VNC and the retrovesicular and PAGs. This may have led to some of the variation in the number of VNC neurons recorded among individuals within a species. A similar variability in the position of individual VNC neurons is seen in C. elegans (White et al., 1976). An additional source of variability within species may be due to misidentification of cell type. While cell types were usually unambiguous, occasionally nuclei could not be strictly categorized as neuronal or non-neuronal. This ambiguity occurred most frequently in earlier larval stages where nuclei were often immediately adjacent to one another. In these cases, we used a second observer, blind to the species, to categorize the nucleus. In cases where the nucleus type was ambiguous to both researchers, the nucleus in question was excluded from the count. Therefore, it is possible that some of our counts were underestimates of the true number of VNC neurons. It will be interesting to confirm these counts and conduct comparative connectomics on select species with TEM.
The variation in nematode neuroanatomy did not strictly correlate with phylogeny. While more closely related to C. elegans (Clade 9) than A. suum (Clade 8), P. pacificus (Clade 9) had 20% fewer VNC neurons than either species. Similarly, P. penetrans had 20% fewer VNC neurons than all other examined Clade 12 species (van Megen et al., 2009). The number of VNC neurons does appear to be conserved within individual genera as shown by Steinernema sp. and Heterorhabditis sp. Similarly, sensory neuron dye-filling did not correlate with phylogeny. While C. elegans (Clade 9) and Acrobeles sp. (Clade 11) show a nearly identical dye-filling pattern, the entomopathogenic nematode Heterorhabditis bacteriophora (Clade 9) showed striking differences. This variability in dye-filling is consistent with previous results showing only a single pair of amphid neurons dye-fill in the Clade 10 mammalian-parasitic nematode Parastrongyloides trichosuri (Zhu et al., 2011). Although the precise mechanism of dye-filling is unknown, we hypothesize that differential dye-filling patterns indicate ultrastructural or biochemical differences among sensory neurons. Alternatively, the sensilla pores may be blocked or filled with secretions in certain species (Perry, 1996). However, we think this alternative hypothesis less likely. Previous TEM studies on Heterodera glycines and Meloidogyne sp. clearly indicate that the amphids are exposed to the environment (Wergin and Endo, 1976; Endo, 1980). Heterodera glycines (Clade 12), which showed no dye-filling with DiI, was previously shown to undergo amphid dye-filling with fluorescein isothiocyanate (Winter et al., 2002). In C. elegans, certain environmentally exposed amphid neurons do not dye-fill (Hedgecock et al., 1985). Again, this implies that ultrastructural or biochemical differences in individual neurons may underlie species–specific dye-filling in our study. Finally, we demonstrate that there are developmental differences in dye-filling within individual species. In C. elegans dauers, changes in the ultrastructure of individual neurons likely result in altered dye-filling during this developmental stage (Albert and Riddle, 1983; Peckol et al., 2001; Schroeder et al., 2013).
In C. elegans, post-embryonic development of the VNC requires the migration and subsequent cell division of 12 sub-ventral precursor cells (P cells). Following migration, the P cells divide to form an anterior neuroblast cell (Pn.a) and a posterior cell (Pn.p) which either forms a hypodermal nucleus or a vulval precursor cell. Certain ancestors of the C. elegans Pn.a cell undergo programmed cell death eventually leading to the defined 57 VNC neurons. Previous lineage analysis in P. pacificus demonstrated that the non-vulva forming Pn.P cells undergo apoptosis prior to hatch (Sommer and Sternberg, 1996). As P. pacificus and C. elegans have an identical number of Pn.a neuroblast cells (Félix et al., 2000), it seems likely that additional apoptotic events occur in the P. pristionchus Pn.a lineage following migration. Similarly, with species showing additional neurons, it will be important to find if this results from the embryonic development of additional P cells or through extra rounds of cell division.
Heterochrony is defined as differences in the timing of developmental events and may play an important role in evolution (Gould, 1977; Alberch et al., 1979; Smith, 2003). The delayed development of neurons in the VNC of A. avenae (Clade 12) and P. pacificus (Clade 9) is suggestive of neuronal heterochrony. As P. penetrans (Clade 12), which also hatches as a J2, shows no post-hatch VNC development we propose that the heterochronic developmental events in A. avenae and P. pacificus arose independently. In C. elegans, several mutants have been isolated that result in altered timing of development events (Ambros and Horvitz, 1984; Ambros, 1988; Lee et al., 1993). The homologs of these heterochronic genes and microRNAs in C. elegans are present throughout the animal kingdom and may play roles in altered developmental timing (Moss and Tang, 2003).
Our data suggest that the neuroanatomy of nematodes is not as highly conserved as previously described. The evolution of nematode nervous systems has been relatively neglected, in part, due to previous assumptions of high anatomical conservation. It will be valuable to use higher resolution imaging techniques and antibody staining to define the neuronal subtypes found in these species. For example, Stretton et al. (1978) found that the VNC of A. suum consists of five repeating sets of neurons each containing 11 cells. Among these cells, they found seven neuron types based on synaptic connectivity (Stretton et al., 1978). In our study, we observed a possible repeating pattern in the VNC of M. hapla (Figure 3H). However, our methods do not allow for the identification of individual neuron types. TEM could be used to study the neuron types in order to classify repeated segments on select species. In addition, immunohistochemistry may be utilized to distinguish the neurotransmitter identity of individual cells. Using immunohistochemistry, Loer and Rivard (2007) found variation in the expression of serotonin among the VNC of male Rhabditid nematodes. It will be interesting to examine the VNC for immunoreactivity to neurotransmitters such as serotonin and GABA. Together with previous data demonstrating large-scale rewiring between the pharyngeal nervous systems of C. elegans and P. pacificus (Bumbarger et al., 2013), we propose that the phylum Nematoda represent a bountiful source of data for understanding the evolution of nervous systems. A close comparative examination of the nervous systems of parasitic nematodes may also lead to the development of species–specific control strategies.
ZH and NS conceived project and designed experiments. ZH, SB, and NS performed experiments and analyzed results. ZH, SB, and NS wrote the paper.
Funding for this research was originally provided as a postdoctoral fellowship to NS from the USDA (2010-65106-20587) while in the lab of Maureen Barr, supported by NIH (5R01DK59418). Current funding to NS is provided through the NIH (1R01GM111566) and the USDA NIFA Hatch program (ILLU-802-934).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Caenorhabditis Genetics Center, funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), and Albrecht Koppenhöfer for providing nematode strains. We thank Jayna Patel for providing technical assistance.
Alberch, P., Gould, S. J., Oster, G. F., and Wake, D. B. (1979). Size and shape in ontogeny and phylogeny. Paleobiology 5, 296–317.
Albert, P. S., and Riddle, D. L. (1983). Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J. Comp. Neurol. 219, 461–481. doi: 10.1002/cne.902190407
Ambros, V. (1988). “Genetic basis for heterochronic variation,” in Anonymous Heterochrony in Evolution, ed. M. L. McKinney (New York, NY: Springer), 269–285.
Ambros, V., and Horvitz, H. (1984). Heterochronic mutants of the nematode. Science 226, 409–416. doi: 10.1126/science.6494891
Angstadt, J. D., Donmoyer, J. E., and Stretton, A. O. (1989). Retrovesicular ganglion of the nematode Ascaris. J. Comp. Neurol. 284, 374–388. doi: 10.1002/cne.902840305
Ashton, F., Li, J., and Schad, G. (1999). Chemo-and thermosensory neurons: structure and function in animal parasitic nematodes. Vet. Parasitol. 84, 297–316. doi: 10.1016/S0304-4017(99)00037-0
Ashton, F. T., and Schad, G. A. (1996). Amphids in Strongyloides stercoralis and other parasitic nematodes. Parasitol. Today 12, 187–194. doi: 10.1016/0169-4758(96)10012-0
Bumbarger, D. J., Riebesell, M., Rödelsperger, C., and Sommer, R. J. (2013). System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes. Cell 152, 109–119. doi: 10.1016/j.cell.2012.12.013
Bumbarger, D. J., Wijeratne, S., Carter, C., Crum, J., Ellisman, M. H., and Baldwin, J. G. (2009). Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (nematoda: Rhabditida). J. Comp. Neurol. 512, 271–281. doi: 10.1002/cne.21882
Burr, A., and Robinson, A. F. (2004). “Locomotion behaviour,” in Nematode Behaviour, eds R. Gaugler and A. L. Bilgrami (Cambridge, MA: CABI Publishing), 25–62.
Chiang, J. T., Steciuk, M., Shtonda, B., and Avery, L. (2006). Evolution of pharyngeal behaviors and neuronal functions in free-living soil nematodes. J. Exp. Biol. 209, 1859–1873. doi: 10.1242/jeb.02165
Chitwood, B. G., and Chitwood, M. B. (1938). An Introduction to Nematology. Baltimore, MD: University Park Press.
Collet, J., Spike, C. A., Lundquist, E. A., Shaw, J. E., and Herman, R. K. (1998). Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics 148, 187–200.
De Soyza, K. (1973). Energetics of Aphelenchus avenae in monoxenic culture. Proc. Helminth. Soc. Wash. 40, 1–10.
Duerr, J. S., Gaskin, J., and Rand, J. B. (2001). Identified neurons in C. elegans coexpress vesicular transporters for acetylcholine and monoamines. Am. J. Physiol. Cell Physiol. 280, 1616–1622.
Endo, B. Y. (1980). Ultrastructure of the anterior neurosensory organs of the larvae of the soybean cyst nematode, Heterodera glycines. J. Ultrastruct. Res. 72, 349–366. doi: 10.1016/S0022-5320(80)90070-2
Endo, B. Y. (1998). Atlas on Ultrastructure of Infective Juveniles of the Soybean Cyst Nematode, Heterodera Glycines, Vol. 711. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service, Agriculture Handbook, 220.
Félix, M., De Ley, P., Sommer, R. J., Frisse, L., Nadler, S. A., Thomas, W. K., et al. (2000). Evolution of vulva development in the Cephalobina (Nematoda). Dev. Biol. 221, 68–86. doi: 10.1006/dbio.2000.9665
Finney, M., and Ruvkun, G. (1990). The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 63, 895–905. doi: 10.1016/0092-8674(90)90493-X
Fitch, D. H. (1997). Evolution of male tail development in rhabditid nematodes related to Caenorhabditis elegans. Syst. Biol. 46, 145–179.
Gamborg, O. L., Miller, R., and Ojima, K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50, 151–158. doi: 10.1016/0014-4827(68)90403-5
Gans, C., and Burr, A. (1994). Unique locomotory mechanism of Mermis nigrescens, a large nematode that crawls over soil and climbs through vegetation. J. Morphol. 222, 133–148. doi: 10.1002/jmor.1052220203
Hallem, E. A., and Sternberg, P. W. (2008). Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 105, 8038–8043. doi: 10.1073/pnas.0707469105
Hedgecock, E. M., Culotti, J. G., Thomson, J. N., and Perkins, L. A. (1985). Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111, 158–170. doi: 10.1016/0012-1606(85)90443-9
Holterman, M., van der Wurff, A., van den Elsen, S., van Megen, H., Bongers, T., Holovachov, O., et al. (2006). Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 23, 1792–1800. doi: 10.1093/molbev/msl044
Jenkins, W. (1964). A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 48, 692.
Johnson, C. D., and Stretton, A. O. (1985). Localization of choline acetyltransferase within identified motoneurons of the nematode Ascaris. J. Neurosci. 5, 1984–1992.
Johnson, C. D., and Stretton, A. O. (1987). GABA-immunoreactivity in inhibitory motor neurons of the nematode Ascaris. J. Neurosci. 7, 223–235.
Kaya, H. K., and Stock, S. (1997). “Techniques in insect nematology,” in Manual of Techniques in Insect Pathology, ed. L. Lacey (San Diego, CA: Academic Press Inc.), 281–324.
Kimber, M., and Fleming, C. (2005). Neuromuscular function in plant parasitic nematodes: a target for novel control strategies? Parasitology 131, S129–S142. doi: 10.1017/S0031182005009157
Lauritis, J. A., Rebois, R. V., and Graney, L. S. (1983). Development of Heterodera glycines Ichinohe on soybean, Glycine max (L.) Merr., under gnotobiotic conditions. J. Nematol. 15, 272–281.
Lee, R. C., Feinbaum, R. L., and Ambros, V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854. doi: 10.1016/0092-8674(93)90529-Y
Li, J., Zhu, X., Ashton, F. T., Gamble, H. R., and Schad, G. A. (2001). Sensory neuroanatomy of a passively ingested nematode parasite, Haemonchus contortus: amphidial neurons of the third-stage larva. J. Parasitol. 87, 65–72. doi: 10.1645/0022-3395(2001)087[0065:SNOAPI]2.0.CO;2
Loer, C. M., and Rivard, L. (2007). Evolution of neuronal patterning in free-living rhabditid nematodes I: sex-specific serotonin-containing neurons. J. Comp. Neurol. 502, 736–767. doi: 10.1002/cne.21288
Malakhov, V. V. (1994). Nematodes Structure, Development, Classification, and Phylogeny. London: Smithsonian Institution Press.
Martin, R. J., Purcell, J., Robertson, A., and Valkanov, M. (2002). “Neuromuscular organisation and control in nematodes,” in The Biology of Nematodes, ed. D. L. Lee (Boca Raton, FL: CRC Press), 321–344.
Mclntire, S. L., Jorgensen, E., and Horvitz, H. R. (1993). Genes required for GABA function in Caenorhabditis elegans. Nature 364, 334–337. doi: 10.1038/364334a0
Moss, E. G., and Tang, L. (2003). Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev. Biol. 258, 432–442. doi: 10.1016/S0012-1606(03)00126-X
Peckol, E. L., Troemel, E. R., and Bargmann, C. I. (2001). Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 98, 11032–11038. doi: 10.1073/pnas.191352498
Perkins, L. A., Hedgecock, E. M., Thomson, J. N., and Culotti, J. G. (1986). Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487. doi: 10.1016/0012-1606(86)90314-3
Perry, R. N. (1996). Chemoreception in plant parasitic nematodes. Annu. Rev. Phytopathol. 34, 181–199. doi: 10.1146/annurev.phyto.34.1.181
Ragsdale, E. J., Ngo, P. T., Crum, J., Ellisman, M. H., and Baldwin, J. G. (2009). Comparative, three-dimensional anterior sensory reconstruction of Aphelenchus avenae (nematoda: Tylenchomorpha). J. Comp. Neurol. 517, 616–632. doi: 10.1002/cne.22170
Rebois, R. V., and Huettel, R. N. (1986). Population dynamics, root penetration, and feeding behavior of Pratylenchus agilis in monoxenic root cultures of corn, tomato, and soybean. J. Nematol. 18, 392–397.
Reed, E., and Wallace, H. (1965). Leaping locomotion by an insect-parasitic nematode. Nature 206, 210–211. doi: 10.1038/206210a0
Schroeder, N. E., Androwski, R. J., Rashid, A., Lee, H., Lee, J., and Barr, M. M. (2013). Dauer-specific dendrite arborization in C. elegans is regulated by KPC-1/Furin. Curr. Biol. 23, 1527–1535. doi: 10.1016/j.cub.2013.06.058
Shaham, S. (2006). “Methods in cell biology,” in WormBook, ed. The C. elegans Research Community (Pasadena, CA: WormBook). doi: 10.1895/wormbook.1.49.1
Smith, K. K. (2003). Time’s arrow: heterochrony and the evolution of development. Int. J. Dev. Biol. 47, 613–622.
Sommer, R. J. (2000). Evolution of nematode development. Curr. Opin. Genet. Dev. 10, 443–448. doi: 10.1016/S0959-437X(00)00110-6
Sommer, R. J. (2005). “Evolution of development in nematodes related to C. elegans,” in WormBook, ed. The C. elegans Research Community (Pasadena, CA: WormBook). doi: 10.1895/wormbook.1.46.1
Sommer, R. J. (2006). “Pristionchus pacificus,” in WormBook, ed. The C. elegans Research Community (Pasadena, CA: WormBook). doi: 10.1895/wormbook.1.124.1
Sommer, R. J., and Sternberg, P. W. (1996). Apoptosis and change of competence limit the size of the vulva equivalence group in Pristionchus pacificus: a genetic analysis. Curr. Biol. 6, 52–59. doi: 10.1016/S0960-9822(02)00421-9
Srinivasan, J., Durak, O., and Sternberg, P. W. (2008). Evolution of a polymodal sensory response network. BMC Biol. 6:52. doi: 10.1186/1741-7007-6-52
Stiernagle, T. (2006). “Maintenance of C. elegans,” in WormBook, ed. The C. elegans Research Community (Pasadena, CA: WormBook). doi: 10.1895/wormbook.1.101.1
Stretton, A. O. (1976). Anatomy and development of the somatic musculature of the nematode Ascaris. J. Exp. Biol. 64, 773–788.
Stretton, A. O., Fishpool, R. M., Southgate, E., Donmoyer, J. E., Walrond, J. P., Moses, J. E., et al. (1978). Structure and physiological activity of the motoneurons of the nematode Ascaris. Proc. Natl. Acad. Sci. U.S.A. 75, 3493–3497. doi: 10.1073/pnas.75.7.3493
Sulston, J. E. (1976). Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275, 287–297. doi: 10.1098/rstb.1976.0084
Sulston, J. E., and Horvitz, H. R. (1977). Post-embryonic cell lineages of the nematode. Caenorhabditis elegans. Dev. Biol. 56, 110–156.
Tong, Y., and Bürglin, T. R. (2010). Conditions for dye-filling of sensory neurons in Caenorhabditis elegans. J. Neurosci. Methods 188, 58–61. doi: 10.1016/j.jneumeth.2010.02.003
van Megen, H., van den Elsen, S., Holterman, M., Karssen, G., Mooyman, P., Bongers, T., et al. (2009). A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 11, 927–950. doi: 10.1163/156854109X456862
Ward, S., Thomson, N., White, J. G., and Brenner, S. (1975). Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160, 313–337. doi: 10.1002/cne.901600305
Wergin, W. P., and Endo, B. Y. (1976). Ultrastructure of a neurosensory organ in a root-knot nematode. J. Ultrastruct. Res. 56, 258–276. doi: 10.1016/S0022-5320(76)90002-2
White, G. F. (1927). A method for obtaining infective nematode larva from cultures. Science 66, 302–303. doi: 10.1126/science.66.1709.302-a
White, J. G., Southgate, E., Thomson, J. N., and Brenner, S. (1976). The structure of the ventral nerve cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275, 327–348. doi: 10.1098/rstb.1976.0086
White, J. G., Southgate, E., Thomson, J. N., and Brenner, S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340. doi: 10.1098/rstb.1986.0056
Winter, M., McPherson, M., and Atkinson, H. (2002). Neuronal uptake of pesticides disrupts chemosensory cells of nematodes. Parasitology 125, 561–565.
Wouts, W. M. (1981). Mass production of the entomogenous nematode Heterorhabditis heliothidis (Nematoda: Heterorhabditidae) on artificial media. J. Nematol. 13, 467–469.
Yochem, J. (2006). “Nomarski images for learning the anatomy, with tips for mosaic analysis,” in WormBook, ed. The C. elegans Research Community (Pasadena, CA: WormBook). doi: 10.1895/wormbook.1.100.1
Keywords: invertebrate, amphid, phasmid, Pratylenchus, Meloidogyne, Heterodera, Heterorhabditis, heterochrony
Citation: Han Z, Boas S and Schroeder NE (2016) Unexpected Variation in Neuroanatomy among Diverse Nematode Species. Front. Neuroanat. 9:162. doi: 10.3389/fnana.2015.00162
Received: 09 October 2015; Accepted: 30 November 2015;
Published: 05 January 2016.
Edited by:
Agustín González, Universidad Complutense de Madrid, SpainReviewed by:
Veronica Martinez Cerdeño, Institute for Pediatric Regenerative Medicine, USACopyright © 2016 Han, Boas and Schroeder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathan E. Schroeder, bmVzQGlsbGlub2lzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.