
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Neuroanat., 22 May 2015
Volume 9 - 2015 | https://doi.org/10.3389/fnana.2015.00062
 Juan Nacher1,2,3*
Juan Nacher1,2,3* Luca Bonfanti4,5*
Luca Bonfanti4,5*A commentary has been posted on this article:
Response: New neurons from old beliefs in the adult piriform cortex? A Commentary on: “Occurrence of new neurons in the piriform cortex”
A commentary on
Occurrence of new neurons in the piriform cortex
by Yuan, T.-F., Liang,Y.-X., and So, K.-F. (2015) Front. Neuroanat. 8:167. doi: 10.3389/fnana.2014.00167
In a recent mini-review (Yuan et al., 2015), support is given to the idea that neurons are generated during adulthood in the mammalian piriform cortex (PC), their periventricular origin being also discussed. It is known since long time that a subpopulation of cortical layer II cells in the adult PC of rodents express immature neuronal markers such as polysialylated NCAM (PSA-NCAM; Seki and Arai, 1991; Bonfanti et al., 1992) and doublecortin (DCX; Nacher et al., 2002). These immature neurons have been found in most mammals studied so far, their occurrence being restricted to the paleocortex in rodents (Seki and Arai, 1991; Bonfanti et al., 1992; Nacher et al., 2002), and extended to neocortical areas in species with increasing brain size and gyrencephaly, e.g., rabbits, cats, and primates (Bonfanti and Nacher, 2012). The fact that these cortical cells express markers of immaturity which are usually present in the young neurons produced within adult neurogenic sites (Bonfanti and Theodosis, 1994; Brown et al., 2003), suggested the possibility that they might be newly generated. Across the years, different laboratories undertook 5-bromo-2-deoxyuridine (BrdU) pulse-chase experiments to test this hypothesis. A first group of studies reported that some of the cells in the PC layer II have been generated during adulthood (Bernier et al., 2002; Pekcec et al., 2006; Shapiro et al., 2007a,b). However, the number of recently generated neurons was extremely low, they appeared to have a transient existence, and their precise location in the PC was not properly indicated. In parallel, many other studies did not find evidence for the incorporation of new neurons in the PC of adult rats (Nacher et al., 2002; Gomez-Climent et al., 2008), rabbits (Luzzati et al., 2009), and cats (Varea et al., 2011). These latter studies by no means exclude the possibility of adult neurogenesis in this area; yet, for their relevance, they should be reported in any review article dealing with such controversial issue. By contrast, in their mini-review Yuan et al. deliberately chose to ignore these articles, together with those that have provided solid evidence for the embryonic origin of most of these cells (Gomez-Climent et al., 2008), suggesting that they represent a “reservoir” of non-newly generated, immature neurons which maintain features of structural plasticity throughout life (Bonfanti and Nacher, 2012).
In addition, they omit several important data. First of all, the well-established fact that PSA-NCAM and DCX can also be expressed by non-newly generated cells, which perform other forms of structural plasticity (Bonfanti, 2006; Bonfanti and Nacher, 2012). When discussing the fate of the PC immature neurons they omit that these cells progressively disappear as aging progresses (Abrous et al., 1997; Murphy et al., 2001; Xiong et al., 2008; Varea et al., 2009). Additionally, they cite the work by Nacher et al. (2004) as describing reduction in PC neurogenesis after chronic stress, when only PSA-NCAM expression was studied herein. Again, the expression of PSA-NCAM by cells of the adult brain is mistaken for the occurrence of adult neurogenesis. Finally, they make statements which are not supported by the current literature, e.g., by affirming that many of the immature neurons can be classified as neurogliaform cells. The small cells expressing immature neuronal markers in the PC were erroneously classified as neurogliaform cells, an interneuronal subtype (Price et al., 2005), but later phenotypic analysis clearly demonstrated that they were not mature interneurons (Gomez-Climent et al., 2008).
Concerning the putative fate of these cells, the Authors only cite work by Bedard and Parent (2004) and Klempin et al. (2011), indicating that these studies found some DCX+ cells coexpressing interneuronal markers. They claim that both studies used DCX-GFP mice lineage tracing, which only was used by Klempin et al. (2011). This study has to be taken cautiously because, as acknowledged by the authors, a fraction of GFP-expressing cells did not express DCX protein. The study of Bedard and Parent is focused on olfactory bulb neurogenesis in humans, and does not contain any reference to adult neurogenesis in the PC. Here again the Authors decide to ignore the more solid evidence regarding the putative nature and fate of the PC immature neurons, which revealed that many of them have the typical structure of PC principal cells (Gomez-Climent et al., 2008) and express molecules exclusively found in pallial-derived excitatory neurons, another aspect playing against an origin from the SVZ (Gomez-Climent et al., 2008; Luzzati et al., 2009). Yuan et al. also mention the possibility that the recently generated neurons differentiate into pyramidal neurons, citing the work by Guo et al. (2010). However, this study (as also reported in Rivers et al., 2008) proposed that these neurons could be generated by NG2+ local glial progenitors which have ceased to proliferate at least since the first postnatal month, then undergoing maturation (Rivers et al., 2008; Guo et al., 2010); but technical artifacts might have led to misinterpretation of the results, as acknowledged by the same Authors (Richardson et al., 2011). A complex set of experiments carried out in these studies clearly excluded that new neurons can reach the PC through migration from the SVZ neurogenic site. In spite of these data, completely omitted by Yuan et al., a schematic figure of their mini-review represents the putative newlyborn PC neurons as coming from the SVZ through a migratory stream. The image is described as modified from Klempin et al. (2011), yet, it is not the graphics but the significance of the figure itself which has been modified, since a non-neurogenic PC in the original article by Klempin et al., has been now turned into a neurogenic region (Figure 1).
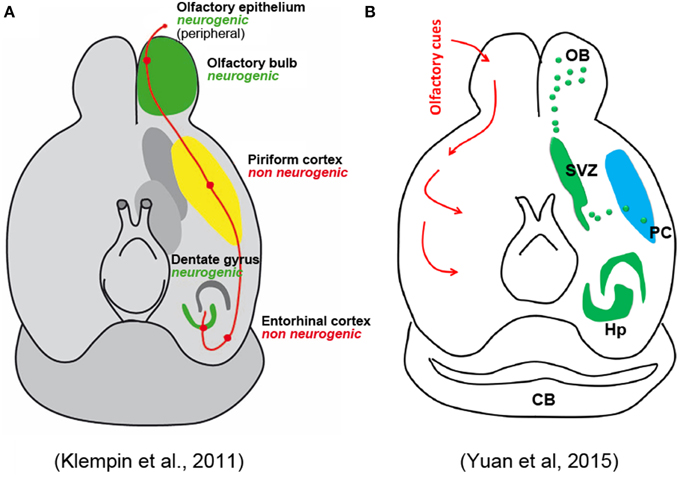
Figure 1. Schematic representation of olfactory pathways and relative neurogenic and non-neurogenic areas in the adult brain of rodents. (A) Original figure by Klempin et al. (2011); (B) figure in Yuan et al. (2015) reported as “modified” from the Klempin paper. Note that the non-neurogenic piriform cortex in (A) has became neurogenic in (B) with newlyborn cells coming from the SVZ through a caudal migratory stream.
On the whole, the article by Yuan et al. fails to acknowledge that some of the published evidence for this phenomenon is disputable, that many papers in the literature denied it, and that, if existent, it appears to be a very restricted/unusual event (Bonfanti and Peretto, 2011), thus leaving the reader with the false impression that most of the PC immature neurons might be generated postnatally.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abrous, D. N., Montaron, M. F., Petry, K. G., Rougon, G., Darnaudery, M., Le Moal, M., et al. (1997). Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res. 744, 285–292. doi: 10.1016/S0006-8993(96)01115-8
Bedard, A., and Parent, A. (2004). Evidence of newly generated neurons in the human olfactory bulb. Brain Res. Dev. Brain Res. 151, 159–168. doi: 10.1016/j.devbrainres.2004.03.021
Bernier, P. J., Bedard, A., Vinet, J., Levesque, M., and Parent, A. (2002). Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. U.S.A. 99, 11464–11469. doi: 10.1073/pnas.172403999
Bonfanti, L. (2006). PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 80, 129–164. doi: 10.1016/j.pneurobio.2006.08.003
Bonfanti, L., and Nacher, J. (2012). New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: the case of cortical layer II immature neurons. Prog. Neurobiol. 98, 1–15. doi: 10.1016/j.pneurobio.2012.05.002
Bonfanti, L., Olive, S., Poulain, D. A., and Theodosis, D. T. (1992). Mapping ofthe distribution of polysialylated neural cell adhesion moleculethroughout the central nervous system of the adult rat: an immunohistochemicalstudy. Neuroscience 49, 419–436. doi: 10.1016/0306-4522(92)90107-D
Bonfanti, L., and Peretto, P. (2011). Adult neurogenesis in mammals – a theme with many variations. Eur. J. Neurosci. 34, 930–950. doi: 10.1111/j.1460-9568.2011.07832.x
Bonfanti, L., and Theodosis, D. T. (1994). Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience 62, 291–305. doi: 10.1016/0306-4522(94)90333-6
Brown, J. P., Couillard-Despres, S., Cooper-Kuhn, C. M., Winkler, J., Aigner, L., and Kuhn, H. G. (2003). Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10. doi: 10.1002/cne.10874
Gomez-Climent, M. A., Castillo-Gomez, E., Varea, E., Guirado, R., Blasco-Ibanez, J. M., Crespo, C., et al. (2008). A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb. Cortex 18, 2229–2240. doi: 10.1093/cercor/bhm255
Guo, F., Maeda, Y., Ma, J., Xu, J., Horiuchi, M., Miers, L., et al. (2010). Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J. Neurosci. 30, 12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010
Klempin, F., Kronenberg, G., Cheung, G., Kettenmann, H., and Kempermann, G. (2011). Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE 6:e25760. doi: 10.1371/journal.pone.0025760
Luzzati, F., Bonfanti, L., Fasolo, A., and Peretto, P. (2009). DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb. Cortex 19, 1028–1041. doi: 10.1093/cercor/bhn145
Murphy, K. J., Fox, G. B., Foley, A. G., Gallagher, H. C., O'Connell, A., Griffin, A. M., et al. (2001). Pentyl-4-yn-valproic acid enhances both spatial and avoidance learning, and attenuates age-related NCAM-mediated neuroplastic decline within the rat medial temporal lobe. J. Neurochem. 78, 704–714. doi: 10.1046/j.1471-4159.2001.00411.x
Nacher, J., Alonso-Llosa, G., Rosell, D., and McEwen, B. (2002). PSA-NCAM expression in the piriform cortex of the adult rat. Modulation by NMDA receptor antagonist administration. Brain Res. 927, 111–121. doi: 10.1016/S0006-8993(01)03241-3
Nacher, J., Pham, K., Gil-Fernandez, V., and McEwen, B. S. (2004). Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience 126, 503–509. doi: 10.1016/j.neuroscience.2004.03.038
Pekcec, A., Loscher, W., and Potschka, H. (2006). Neurogenesis in the adult rat piriform cortex. Neuroreport 17, 571–574. doi: 10.1097/00001756-200604240-00003
Price, C. J., Cauli, B., Kovacs, E. R., Kulik, A., Lambolez, B., Shigemoto, R., et al. (2005). Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J. Neurosci. 25, 6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005
Richardson, W. D., Young, K. M., Tripathi, R. B., and McKenzie, I. (2011). NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70, 661–673. doi: 10.1016/j.neuron.2011.05.013
Rivers, L. E., Young, K. M., Rizzi, M., Jamen, F., Psachoulia, K., Wade, A., et al. (2008). PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401. doi: 10.1038/nn.2220
Seki, T., and Arai, Y. (1991). Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat. Embryol. 184, 395–401. doi: 10.1007/BF00957900
Shapiro, L. A., Ng, K. L., Kinyamu, R., Whitaker-Azmitia, P., Geisert, E. E., Blurton-Jones, M., et al. (2007a). Origin, migration and fate of newly generated neurons in the adult rodent piriform cortex. Brain Struct. Funct. 212, 133–148. doi: 10.1007/s00429-007-0151-3
Shapiro, L. A., Ng, K. L., Zhou, Q. Y., and Ribak, C. E. (2007b). Olfactory enrichment enhances the survival of newlyborn cortical neurons in adult mice. Neuroreport 18, 981–985. doi: 10.1097/WNR.0b013e3281532bc1
Varea, E., Belles, M., Vidueira, S., Pastor, A. M., and Nacher, J. (2011). PSA-NCAM is expressed in immature, but not recently generated, neurons in the adult cat cerebral cortex layer II. Front. Neurosci. 5:17. doi: 10.3389/fnins.2011.00017
Varea, E., Castillo-Gomez, E., Gomez-Climent, M. A., Guirado, R., Blasco-Ibañez, M., Crespo, C., et al. (2009). Differential evolution of PSA-NCAM expression during aging of the rat telencephalon. Neurobiol. Aging 5, 808–818. doi: 10.1016/j.neurobiolaging.2007.08.016
Xiong, K., Luo, D. W., Patrylo, P. R., Luo, X. G., Struble, R. G., Clough, R. W., et al. (2008). Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: partial colocalization with mature interneuron markers. Exp. Neurol. 211, 271–282. doi: 10.1016/j.expneurol.2008.02.003
Keywords: adult neurogenesis, piriform cortex, structural plasticity, PSA-NCAM, doublecortin
Citation: Nacher J and Bonfanti L (2015) New neurons from old beliefs in the adult piriform cortex? A Commentary on: “Occurrence of new neurons in the piriform cortex.” Front. Neuroanat. 9:62. doi: 10.3389/fnana.2015.00062
Received: 18 March 2015; Accepted: 06 May 2015;
Published: 22 May 2015.
Edited by:
Ricardo Insausti, University of Castilla–La Mancha, SpainReviewed by:
Alino Martinez-Marcos, University of Castilla–La Mancha, SpainCopyright © 2015 Nacher and Bonfanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Nacher,nacher@uv.es;
Luca Bonfanti,luca.bonfanti@unito.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.