- 1Division of Nephrology, 2nd Department of Propaedeutic Internal Medicine, Attikon University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
- 2Department of Nephrology, University Hospital of Ioannina, Faculty of Medicine, University of Ioannina, Ioannina, Greece
- 3Clinic of Nephrology and Renal Transplantation, Laiko General Hospital, School of Health Sciences, National and Kapodistrian University of Athens, Athens, Greece
- 4Department of Nephrology, School of Health Sciences, Democritus University of Thrace, Alexandroupolis, Greece
- 5Department of Nephrology and Renal Transplantation, University Hospital of Patras, Patras, Greece
- 6Department of Nephrology, Peripheral General Hospital Athens Giorgos Gennimatas, Athens, Greece
- 72nd Department of Nephrology, AHEPA University Hospital, Medical school, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 84th Department of Internal Medicine, Attikon University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
- 9Department of Rheumatology, University Hospital of Ioannina, Faculty of Medicine, University of Ioannina, Ioannina, Greece
- 10Department of Nephrology, Aretaieio Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Introduction: This study aimed to describe the clinical course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with glomerular diseases (GDs) and its impact on the probability of relapse.
Methods: Patients with biopsy-proven GD and positive PCR test for SARS-CoV-2 from glomerular clinics across Greece were studied retrospectively. Those who received the GD diagnosis after the SARS-CoV-2 vaccination or coronavirus disease 2019 (COVID-19) or ended in ESKD prior to infection were excluded. Demographics, histopathological diagnoses, past medical history, immunosuppression, and GD activity status were recorded.
Results: A total of 219 patients with GDs and documented SARS-CoV-2 infection were included. The mean time from the diagnostic kidney biopsy to SARS-CoV-2 infection was 67.6 ( ± 59.3) months. Among the participants, 82.5% had been vaccinated against SARS-CoV-2 with three doses (range: 2.5–3) without subsequent GD reactivation in 96.2% of them. Twenty-two patients (10%) were hospitalized for COVID-19 and one (0.5%) required mechanical ventilation. Four (1.8%) died due to COVID-19 and one (0.5%) had long COVID-19 symptoms. Among patients in remission prior to SARS-CoV-2 infection, 22 (11.2%) experienced a GD relapse within 2.2 (range: 1.5–3.7) months from the diagnostic test. The relapse-free survival after COVID-19 was significantly shorter for patients with minimal change disease, pauci-immune glomerulonephritis, and focal segmental glomerulosclerosis. No difference was observed in the relapse-free survival post-COVID-19 based on the history of SARS-CoV-2 vaccination.
Conclusions: SARS-CoV-2 infection appears to have a symptomatic but uncomplicated sequence in vaccinated patients with GDs, with a significant impact on the clinical course of GD, associated with an increased probability of relapse in certain histopathological types.
Introduction
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, over 700 million confirmed cases and over 6.9 million deaths have been reported worldwide (1, 2). Certain populations, including older patients and those with chronic conditions, appear to be at a higher risk for severe coronavirus disease 2019 (COVID-19) (3, 4). Glomerular diseases can result from inherited or acquired disorders and can manifest in a variety of ways, ranging in severity from asymptomatic urinary abnormalities to acute kidney injury, nephrotic syndrome, or rapidly progressive glomerulonephritis leading to end-stage kidney disease (ESKD). The diagnosis is generally made through a kidney biopsy revealing the underlying pathology. The severity of the disease is related to the nature and type of the immunologic process causing the disease and to the degree and extent of inflammation (5). Yet, patients with glomerular diseases (GDs) are known to be at a higher risk for severe infections due to an underlying immune dysfunction and renal impairment and as a consequence of long-term immunosuppressive therapy (6). Therefore, there is significant treatment-related morbidity in patients with GDs, associated with an increased incidence rate of severe infections (7). Treatment with high-dose oral corticosteroids was identified as the most important contributor being associated with up to seven-fold higher risk for infection occurrence (8). Additional risk factors for severe COVID-19 in these patients include a higher prevalence of hypertension and diminished kidney function (9, 10). Both GDs and COVID-19 are associated with a hypercoagulable state and thrombotic events causing high morbidity and mortality (11). Additionally, chronic immunosuppression, a frequent aspect of GD treatment, has been linked to a reduced response to SARS-CoV-2 vaccines and therefore might influence their effectiveness in preventing severe COVID-19. In this regard, patients with GDs have a weakened immunity due to disease activity and/or treatments than healthy subjects, and the third dose of the SARS-CoV-2 mRNA vaccine was shown to effectively increase the humoral immune response against SARS-CoV-2 and, to a lesser extent, the cellular response against SARS-CoV-2 (12). Furthermore, there is an added concern that COVID-19 may exacerbate GDs or cause acute kidney injury and increase the risk of kidney failure (13, 14). Relapses during the course of GDs are known to occur anyway, in different rates across histopathological types, i.e., 50%–75% in minimal change disease (MCD), one-third of cases in patients with membranous nephropathy (MN), and 30%–50% of cases in patients with pauci-immune glomerulonephritis. Taken all together, these patients are a particularly vulnerable group in the global COVID-19 pandemic, and information on how the illness affects their health position and GD activity is critical for their management.
We sought to investigate the clinical features of SARS-CoV-2 infection in patients with previously diagnosed GDs and evaluate the probability of associated GD reactivation in an actively monitored cohort of patients, throughout an observation period that spanned over the COVID-19 pandemic.
Materials and methods
Study design and inclusion criteria
This study was a multicenter retrospective observational study that included 10 centers from all over the Greek territory. Patients were eligible to be included in the study if they had biopsy-proven GD of autoimmune origin and positive polymerase chain reaction (PCR) test for SARS-CoV-2 infection after GD diagnosis. The included patients were studied retrospectively. Patients who received the GD diagnosis prior to SARS-CoV-2 vaccination or infection or had reached ESKD were excluded. Histopathological diagnosis of GD might be IgA nephropathy (IgAN), MN, MCD, primary focal segmental glomerulosclerosis (FSGS), lupus nephritis (LN), pauci-immune glomerulonephritis, IgA vasculitis, fibrillary glomerulonephritis, and C3 glomerulonephritis. Of these, podocytopathies are called the ones in which podocyte injury drives proteinuria and progressive kidney disease. They include MCD and FSGS. We recorded demographics; histopathological diagnoses; past medical history including history of hypertension, diabetes, or cardiovascular disease; immunosuppressive regimens and outcome of GDs; and information regarding vaccination against SARS-CoV-2, i.e., vaccine type and total number of doses administered. In terms of the SARS-CoV-2 infection, we recorded the reason for testing, i.e., symptoms versus exposure, as well as the type of symptoms (fever, myalgias, arthralgias, rhinitis, pharyngitis, anosmia, ageusia, fatigue, cough, dyspnea, respiratory failure). Information regarding hospitalization requirement; oxygen supplementation or mechanical ventilation; specific therapy for COVID-19 including glucocorticoids, remdesivir, or nirmatrelvir; and final outcome (full recovery, post-acute sequelae, death) was collected. The time interval between the diagnostic kidney biopsy and the diagnostic PCR test was captured. Patients with GDs and documented SARS-CoV-2 infection were managed with adjustments to immunosuppressants and other medication regimens at the clinician’s discretion (15, 16). The time period of observation was between the positive PCR test for COVID-19 to the last visit in the glomerular clinic.
Written informed consent from the participants or legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Characteristics of GD
Histopathological diagnosis and related immunosuppressive regimens were recorded (cyclophosphamide, glucocorticoids, mycophenolate mofetil, azathioprine, rituximab). Immunosuppressive therapy was considered from the time of GD diagnosis to the end of follow-up. The first outcome of the GD, i.e., after initial therapy, was recorded in all patients, which might be remission or active disease (patients with ESKD were excluded by study design). The patients’ GD activity status was assessed in every visit to the glomerular clinic before and after SARS-CoV-2 vaccination and infection up to the end of follow-up. Patients who experienced an alteration in activity status from remission to active disease were considered to have a GD relapse. In this regard, time to relapse was estimated counting from the time of SARS-CoV-2 infection to the documentation of GD relapse. Data collection prompted research coordinators to review medical records in each institution back to the time of SARS-CoV-2 infection in order to collect all related information. The included patients were required to have all information available regarding histopathological diagnosis, immunosuppressive treatment, and GD outcomes dating back to the diagnostic kidney biopsy. Definitions of GDs and related outcomes, including disease relapse, followed the ones provided by the Kidney Diseases Improving Global Outcomes (17). Activity status alteration might include change from active disease to remission or change from remission status to active disease, i.e., relapse. End-stage kidney disease was defined as the requirement of life-long maintenance dialysis. Acute dialysis was defined as the need for dialysis for less than 3 months. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (18). Laboratory measurements with hematologic and renal indexes were performed throughout the follow-up time, including labs obtained during the closest visit to the glomerular clinic prior to infection (baseline laboratory measurements), the first visit after infection, and at the end of follow-up.
Statistical analysis
The statistical analysis was performed using the R 4.0.4 packages “survival” (19) and “survminer” (20). A two-sided p-value of ≤0.05 determined statistical significance. We visually assessed the normality of continuous variable distributions by constructing and examining histograms. Variables found to be normally distributed were described using their mean and standard deviation, and the Student’s t-test was used for comparisons. The median and interquartile range were reported for variables that were not normally distributed, and the non-parametric Mann–Whitney U test was used for comparisons. When comparing categorical variables across different groups, we used the chi-square test or Fisher’s exact test if the assumptions of the chi-square test were not met. For the outcome of relapse-free survival, Kaplan–Meier curves were constructed and compared using the non-parametric log-rank test.
Results
Description of the study population
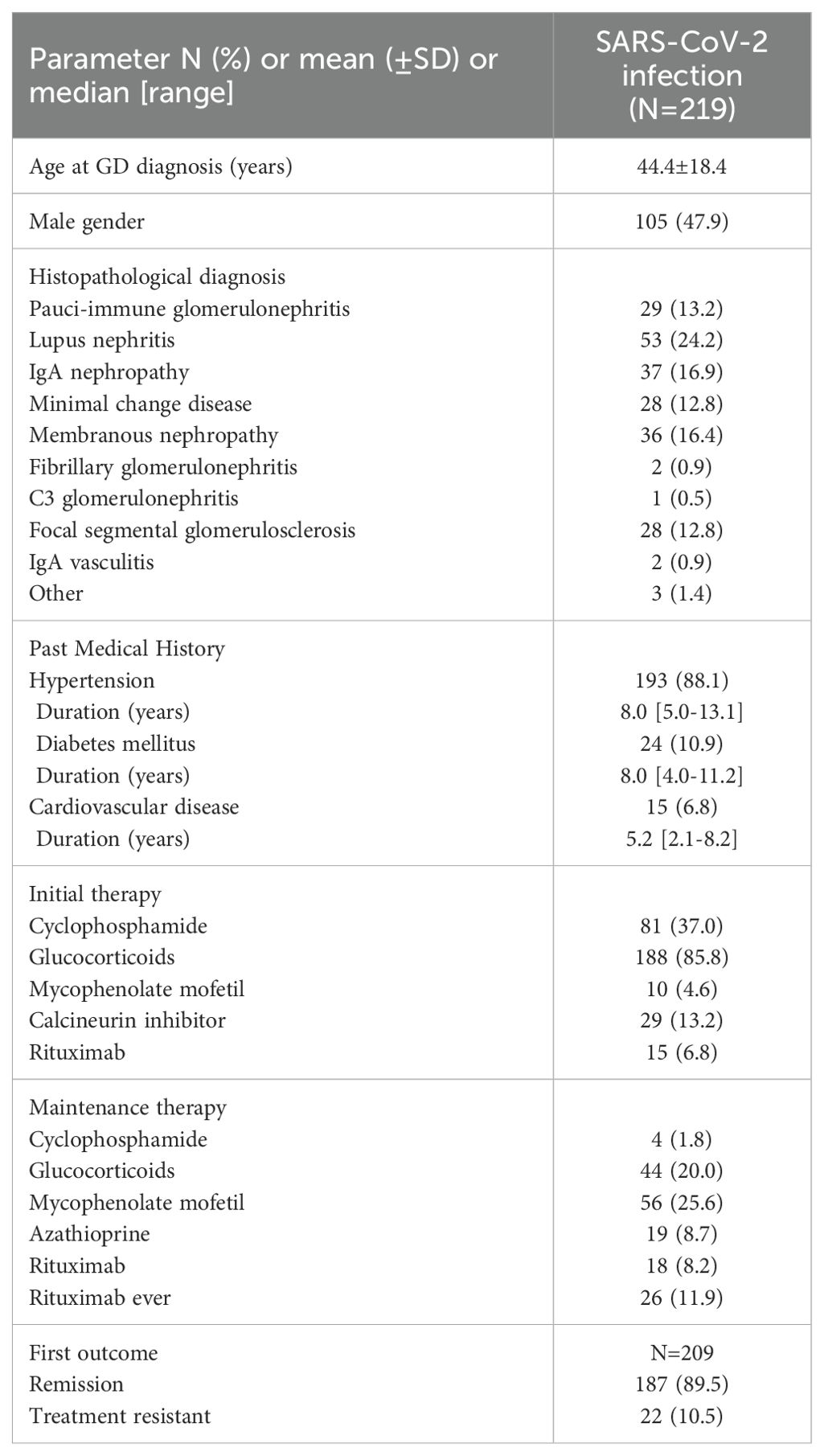
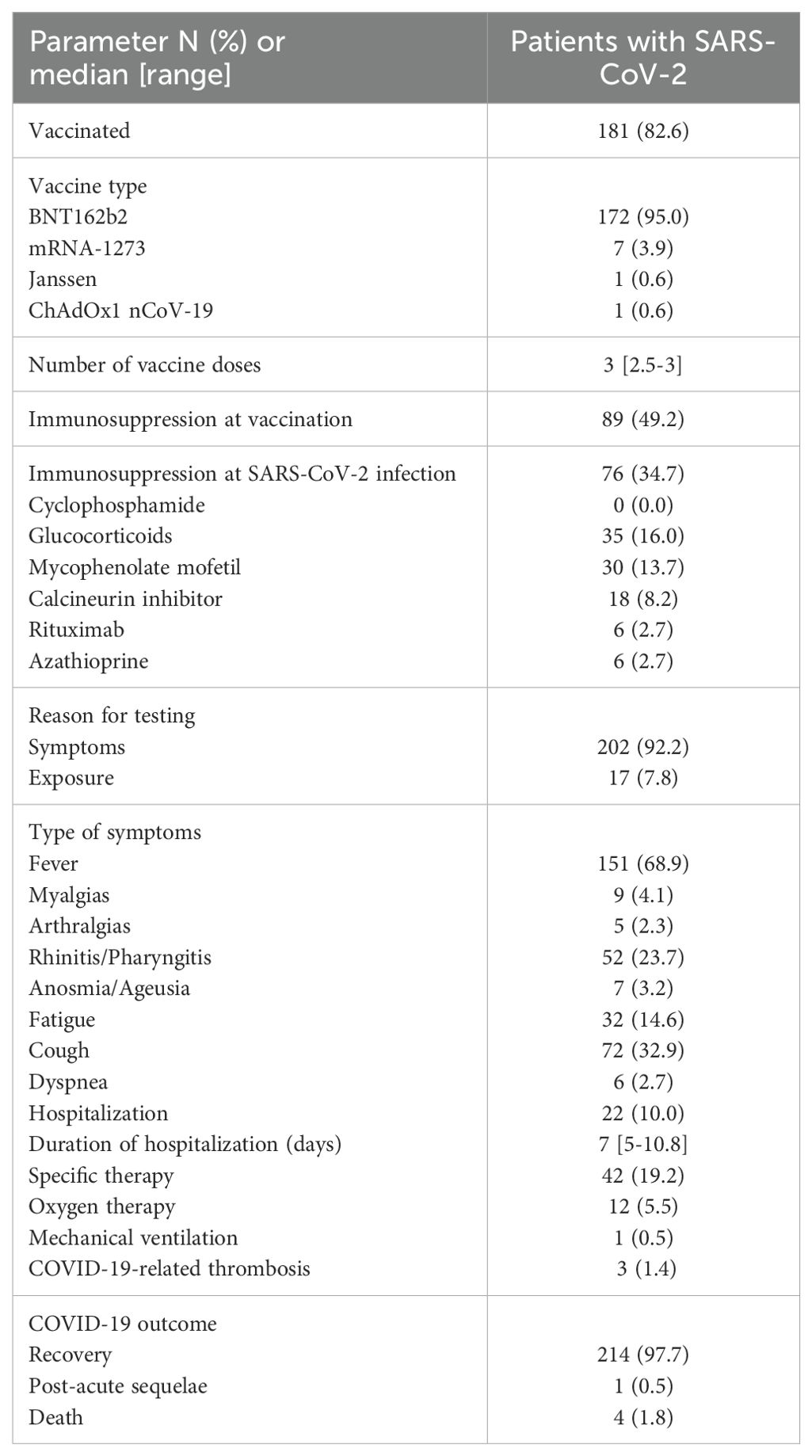
A total of 219 patients with a history of biopsy-proven GD and positive PCR test for SARS-CoV-2 were included in the study. All patients who received a GD diagnosis after SARS-CoV-2 vaccination or after infection were excluded (n = 64). The mean age of the study population was 44.4 ± 18.4 years and 105 (47.9%) were men. The major types of GDs were represented in this cohort as shown in Table 1. The vast majority of patients (88.1%) had a past medical history of hypertension, diabetes (10.9%), or cardiovascular disease (6.8%). Overall, 188 (85.8%) of the included patients had received at least one course of glucocorticoids as initial therapy for GD, while 81 (37%) had also received cyclophosphamide, 10 (4.6%) mycophenolate mofetil, 29 (13.2%) calcineurin inhibitors, and 15 (6.8%) rituximab. Most patients (89.5%) had achieved remission as a result of initial therapy. In total, 181 patients (82.6%) were vaccinated against SARS-CoV-2 with a median of 3 doses (range: 2.5–3). The BNT162b2 vaccine was the most commonly administered (95%), while 49.2% of the patients were on immunosuppressive therapy of any kind at vaccination. No symptom or sign of GD reactivation was observed in 181 (96.3%) of the patients, who were vaccinated against SARS-CoV-2 by the time of SARS-CoV-2 infection diagnosis. Seven patients experienced a GD relapse following vaccination (six patients with MCD and one patient with pauci-immune glomerulonephritis), which was treated accordingly, and all achieved remission prior to COVID-19 diagnosis, while 76 patients (34.7%) were on immunosuppressive therapy at the same time (17).
Clinical course of SARS-CoV-2 infection
The largest portion of patients (92.2%) underwent testing for SARS-CoV-2 infection due to symptoms (versus 7.8% after exposure). The most frequently reported symptom was fever (68.9%), among various other clinical manifestations (Table 1), while 10% of cases overall required admission to hospital for a median of 7 days (range: 5–10.8). Among the admitted patients, 5.5% required oxygen supplementation, but only one needed mechanical ventilation. Generally, patients (97.7%) experienced full recovery from SARS-CoV-2 infection, four patients died (1.8%) following complications of COVID-19, and one patient reported long COVID-19 sequela. In total, laboratory measurements including renal and hematologic indexes were similar before and after SARS-CoV-2 infection. Parameters related to COVID-19 and related outcomes in patients with GDs are shown in Table 2.
SARS-CoV-2 infection and GD activity
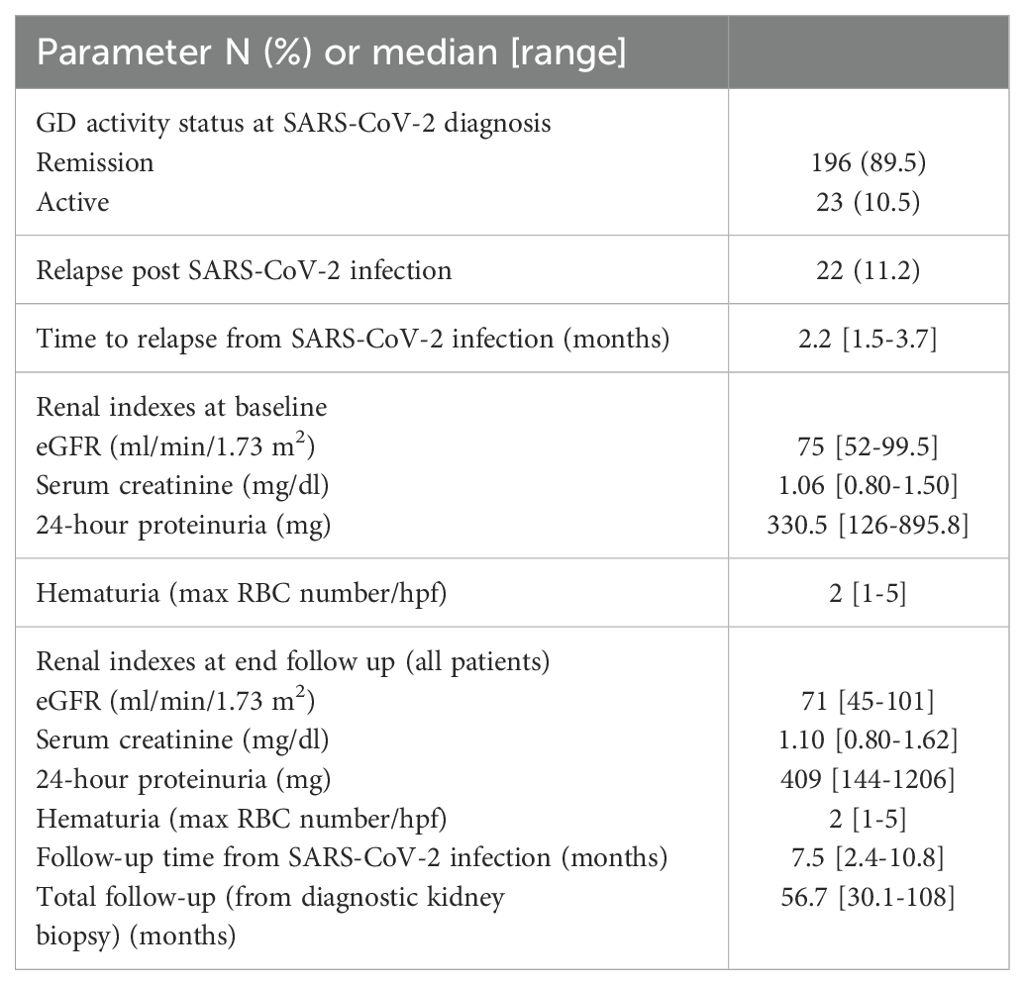
During the visit prior to SARS-CoV-2 infection, 196 (89.5%) patients were in sustained remission for GD, while 23 (10.5%) had still active disease. In 22 patients (11.2%), an alteration of the GD activity status, i.e., from remission to active disease (i.e., GD relapse), was documented, within a median time of 2.2 months (range: 1.5–3.7) from the diagnostic SARS-CoV-2 test. Renal parameters and outcomes of GD post-SARS-CoV-2 infection are shown in Table 3. Among relapsers, there were six patients with pauci-immune glomerulonephritis (27.2%), two (9%) with renal lupus (one with lupus podocytopathy), eight (36.4%) with MCD, four (18.2%) with FSGS, one (4.5%) with IgAN, and one (4.5%) with MN. Notably, 14 (63.65%) of the relapsing patients had podocytopathies as primary disease and developed full-blown nephrotic syndrome upon relapse. The mean age of the patients who relapsed after COVID-19 was 49.2 years (±14.9), and there were 10 (45.5%) male patients. All relapsers had been vaccinated against SARS-CoV-2, and all except one were tested for COVID-19 due to symptoms (one due to exposure). Two of the relapsers required hospitalization for COVID-19. A comparison of baseline renal indexes between patients who relapsed after COVID-19 versus those who did not revealed that there was no difference in 24-h proteinuria, renal function, and microscopic hematuria (Table 4). However, at the time of GD relapse, patients with podocytopathies had significantly higher 24-h proteinuria versus the corresponding values at baseline (p = 0.0001), while serum creatinine did not differ. The relapse-free survival differed significantly by the histopathological type of GD (Figure 1) with MCD being the most frequently relapsing GD post-COVID-19. Yet, the relapse-free survival after COVID-19 was significantly shorter for patients with MCD, pauci-immune glomerulonephritis, and FSGS compared to patients with IgAN. Notably, seven (31.8%) patients who experienced a GD relapse post-COVID-19 had also documented GD reactivation after SARS-CoV-2 vaccination. There was no difference in the relapse-free survival post-COVID-19 based on the history of SARS-CoV-2 vaccination.
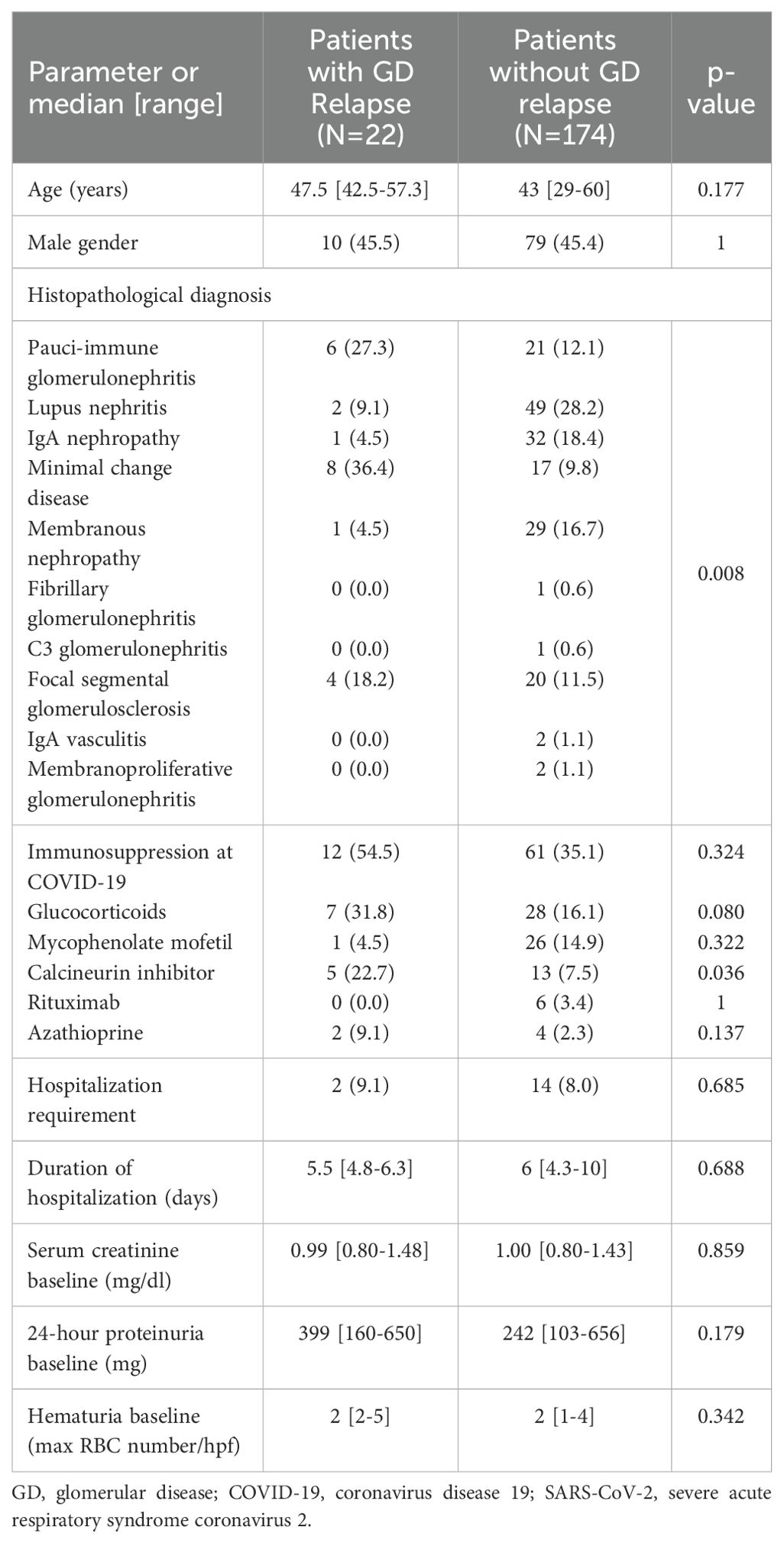
Table 4. Comparison of patients who experienced re-activation of the GD post COVID-19 and those who did not.
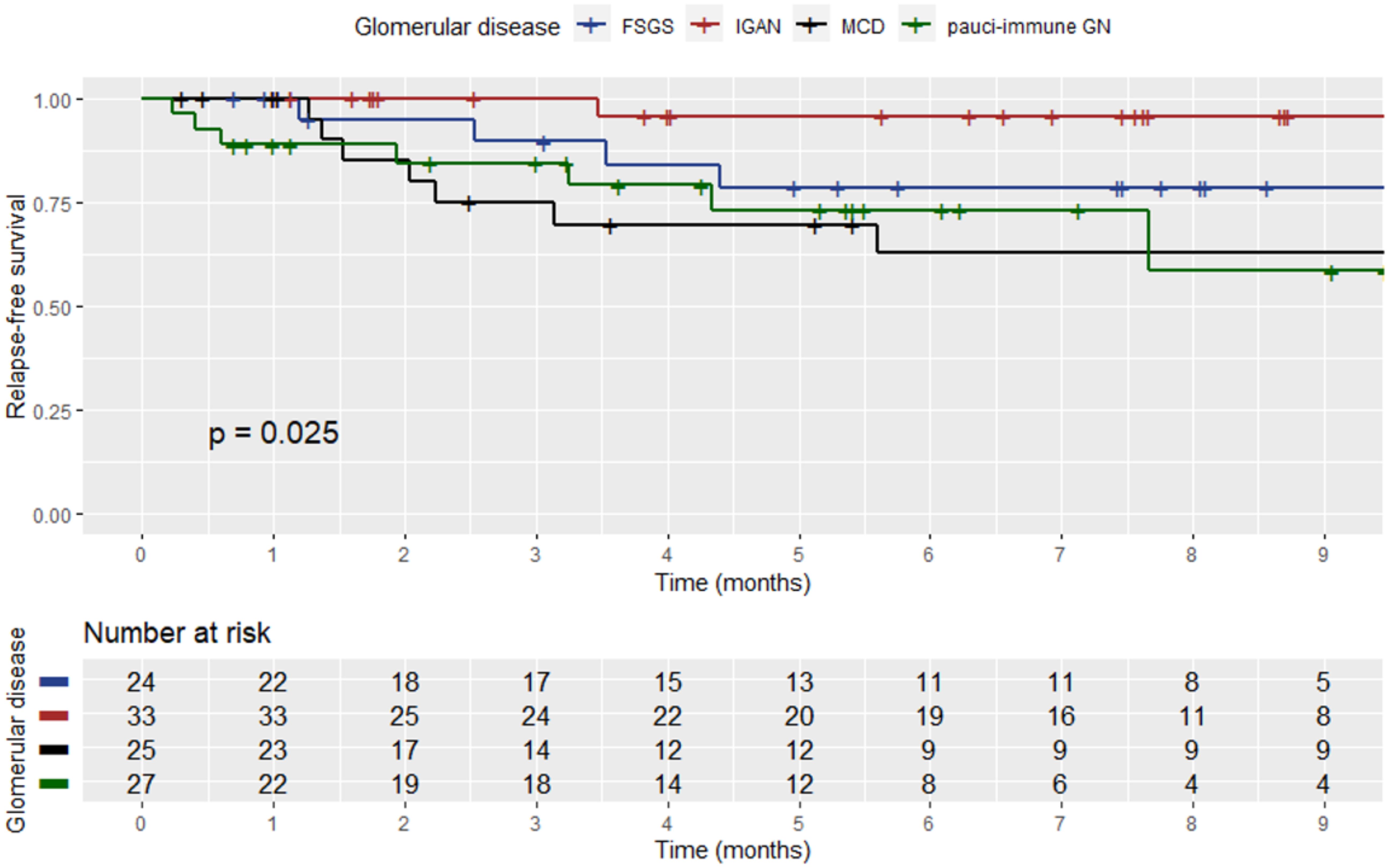
Figure 1. Kaplan–Meier curve showing the relapse-free survival post-SARS-CoV-2 infection among patients who experienced a relapse based on the histopathological type of GD.
Discussion
This study investigated the clinical picture and outcome of COVID-19 in patients with GDs, as well as its impact, on the probability of disease reactivation. Most patients in this cohort underwent SARS-CoV-2 testing due to symptoms, while 34.7% were on immunosuppressive therapy at the time of diagnosis. The most frequent symptom of COVID-19 was fever, while 10% of the total (n = 219) required hospitalization for approximately 1 week. Nearly 98% of individuals experienced complete recovery from COVID-19 and 1.8% died due to related complications. Thus, the vast majority of patients in our cohort had symptomatic but uncomplicated COVID-19. Many patients with SARS-CoV-2 virus infection have various comorbidities. Their presence in the background of coronavirus tends to worsen the course of the disease and increase the risk of unfavorable outcomes (4). In this cohort of patients with biopsy-proven GDs, the clinical manifestations of COVID-19 were similar to the general population, in agreement with the findings previously reported (21). The United Kingdom’s ICHNT Renal COVID-19 group with systematic SARS-CoV-2 serology screening results in 493 patients with primary GD, vasculitis, and systemic lupus erythematosus prior to December 2020 reported that 19% of all cases required hospitalization and 7% resulted in death, pointing to substantial vulnerability of these patients. However, these rates were higher before the widespread use of SARS-CoV-2 vaccination (22). Generally, the clinical features of COVID-19 among patients with autoimmune disorders are variable but are not known to be different than patients without these underlying diseases. There are insufficient data from the literature to determine whether the type of autoimmune disease or the intensity of immunosuppressive therapy influences the clinical presentation of COVID-19. A systematic review investigating the impact of comorbidities found that patients with certain co-existing diseases are at a higher risk for severe courses of COVID-19 and unfavorable outcomes (23). These include heart diseases, immunosuppression, kidney diseases, metabolic syndrome, and hematologic diseases. Unmodified factors like older age and male sex also present an increased risk for severe events (23). Patients with GDs especially those on immunosuppressive therapy represent a population similar to kidney transplant recipients, who may also have some degree of renal impairment combined with a compromised immune system secondary to chronic immunosuppression. However, a meta-analysis, which compared the clinical outcome of COVID-19 in solid-organ transplant recipients, who typically receive long-term immunosuppression, to the one of the general population, found a higher risk of intensive care unit admission and occurrence of acute kidney injury but no difference in the 30-day mortality rate (24). Furthermore, we found that, among patients who had achieved long-term GD remission, 11.2% experienced documented disease reactivation, which occurred within 2.2 (range: 1.5–3.7) months after COVID-19. At first sight, the relapse rates after COVID-19 seem to be lower than the ones expected without this infection. However, it is of note that the time interval in which they have occurred is considerably shorter. Specifically, the relapse-free survival after COVID-19 was significantly shorter for patients with MCD, pauci-immune glomerulonephritis, and FSGS compared to patients with IgAN. Hence, SARS-CoV-2 infection appears to have a significant impact on the clinical course of GDs, associated with an increased probability of GD relapse in certain histopathological types. Nonetheless, COVID-19 induces a hyperimmune response in some patients which may hypothetically trigger a GD flare. Multiple mechanisms have been proposed for AKI, proteinuria, and hematuria in the setting of COVID-19. The cytokine storm induced may trigger common post-viral glomerular diseases such as IgAN, pauci-immune vasculitides, and anti-GBM that have also been described in COVID-19 patients. Also, direct viral invasion of glomerular structures is hypothesized to cause podocytopathy due to the affinity of the virus to angiotensin-converting enzymes. For decades, it has been observed that non-COVID viral infections are implicated in the relapse of nephrotic syndrome in pediatric patients and IgAN (25). While the mechanism of disease flare is not fully understood, it is thought to be due to a dysregulated immune response to infection. Patients may also be particularly vulnerable to a flare-up in the setting of withholding some of their immunosuppressive medication to allow a host immune response to the virus (26). For instance, MCD for 10%–15% of primary nephrotic syndrome cases in adults (27) while 30%–62% of patients experience a single relapse and up to 39% have frequent relapses (28, 29). Although the pathogenesis of MCD remains poorly understood, there is compelling evidence to suggest that it involves both immune system impairment and podocyte dysfunction. It is considered to involve intrinsic and extrinsic factors in a “long-chain” cascade reaction (30). It may be triggered by immunological stimuli, such as viral infection, immunization, allergens (31, 32), or chronic viral infection (33, 34). In children, a prospective two-winter study found that exacerbation and relapses of childhood nephrotic syndrome are temporally related to upper respiratory virus infections (35). The association of respiratory tract infections with the occurrence, relapse, and aggravation of nephrotic syndrome has been confirmed for over two decades (36). Moreover, human immunodeficiency virus (HIV) infection is a well-known cause of renal disease (37) and the most common cause of glomerular injury in these patients (38), triggered by the direct infection of glomerular epithelial cells and tubular epithelial cells by HIV (39). Likewise, the pathogenesis of pauci-immune vasculitides is thought to involve an interplay of environmental and epigenetic factors in genetically susceptible individuals (40). Seasonal and geographic variations in incidence provide insight into the potential role of environmental exposures in these diseases (41). Falk et al. and others found a seasonal variation in symptom onset, which was statistically higher than expected in winter and lower than expected in summer in patients with renal involvement (42–44). Moreover, Kemma et al. showed a seasonal influence on the risk of relapse at a rise of antineutrophil cytoplasmic antibodies (ANCA) in vasculitis patients with renal involvement (45). Specifically, ANCA rises, which occurred during the fall season, were more frequently followed by a relapse than ANCA rises occurring during other seasons (45). Although an ANCA rise might be directly pathogenic, this potential was considered unlikely, since fewer than half of the patients in whom an ANCA rise occurs will relapse within a year. Thus, a second hit is probably required (46, 47). In relation to this in a mouse model of MPO-ANCA, it has been demonstrated that proinflammatory stimuli of infectious origin and ANCA act synergistically to induce glomerulonephritis, which is in agreement with the hypothesis that patients at increased risk of a respiratory tract infection during the following month more frequently relapse and that antibiotic maintenance therapy did not protect against relapse after an ANCA rise (48).
With respect to the treatment effect, although it appears from this study that calcineurin inhibitor (CNI) therapy may be associated with an increased probability of relapse, this is an epiphenomenon and not a real effect, related to the primary disease and not the actual treatment, i.e., the CNI. Many patients with podocytopathies, i.e., MCD and FSGS (who frequently are on CNIs, especially after relapsing disease), experienced a relapse after COVID-19.
The limitations of this study pertain to its retrospective design and the fact that information regarding COVID-19 identification and clinical features relied on the review of medical records and patients’ reports in each institution. The lack of B-cell status in patients receiving rituximab and the vaccine immunity status in some of them are also limitations. However, associations with GD reactivation may be underrepresented due to potentially missing relapsing episodes related to the relatively short follow-up period after COVID-19. Our study design required participants to have had serum and urine laboratory tests performed before and after COVID-19, which precluded the inclusion of patients who did not have all tests available or may be biased toward those with more severe GDs who had more frequent monitoring.
In conclusion, according to our results, SARS-CoV-2 infection appears to have a symptomatic and sporadically complicated sequence in patients with GDs. It appears that COVID-19 may have a significant impact on the clinical course of GD, associated with an increased probability of relapse in certain histopathological types. However, some of these relapses may be related to the infection, whereas some of them may be coincidental. In favor of the relation to COVID-19 is the timing of relapse, i.e., occurring within a few weeks of the infection. Understanding the interactions between SARS-CoV-2 and GDs is key to the successful management of both COVID-19 and GD in the long term, which poses unique challenges, especially with regard to immunosuppression planning.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SL: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. ED: Data curation, Writing – review & editing. SM: Data curation, Writing – review & editing. KK: Data curation, Writing – review & editing. MP: Data curation, Writing – review & editing. DGa: Data curation, Writing – review & editing. IBe: Data curation, Formal analysis, Methodology, Software, Writing – original draft. AS: Data curation, Writing – review & editing. PK: Data curation, Writing – review & editing. VL: Data curation, Writing – review & editing. CM: Data curation, Writing – review & editing. DGo: Data curation, Writing – review & editing. SF: Data curation, Writing – review & editing. AV: Data curation, Writing – review & editing. PV: Data curation, Writing – review & editing. EA: Data curation, Writing – review & editing. GM: Data curation, Writing – review & editing. SP: Data curation, Writing – review & editing. IBo: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate covid-19. N Engl J Med. (2020) 383:1757–66. doi: 10.1056/NEJMcp2009249
2. Leung K, Wu JT, Liu D, Leung GM. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. (2020) 395:1382–93. doi: 10.1016/S0140-6736(20)30746-7
3. Saadoun D, Vieira M, Vautier M, Baraliakos X, Andreica I, da Silva JAP, et al. SARS-CoV-2 outbreak in immune-mediated inflammatory diseases: the Euro-COVIMID multicentre cross-sectional study. Lancet Rheumatol. (2021) 3:e481–8. doi: 10.1016/S2665-9913(21)00112-0
4. Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. (2020) 98:20–6. doi: 10.1016/j.kint.2020.04.030
5. Dickinson BL. Unraveling the immunopathogenesis of glomerular disease. Clin Immunol. (2016) 169:89–97. doi: 10.1016/j.clim.2016.06.011
6. Obriscă B, Vornicu A, Jurubită R, Achim C, Bobeică R, Andronesi A, et al. Corticosteroids are the major contributors to the risk for serious infections in autoimmune disorders with severe renal involvement. Clin Rheumatol. (2021) 40:3285–97. doi: 10.1007/s10067-021-05646-2
7. Glenn DA, Henderson CD, O’Shaughnessy M, Hu Y, Bomback A, Gibson K, et al. Infection- related acute care events among patients with glomerular disease. Clin J Am Soc Nephrol. (2020) 15:1749–61. doi: 10.2215/CJN.05900420
8. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. (2021) 80:384–91. doi: 10.1136/annrheumdis-2020-218946
9. Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, march 2020-march 2021. Prev Chronic Dis. (2021) 18:E66. doi: 10.5888/pcd18.210123
10. Ko JY, Danielson ML, Town M, Derado G, Greenlund KJ, Kirley PD, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. (2021) 72:e695–703. doi: 10.1093/cid/ciaa1419
11. Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood. (2020) 136:381–3. doi: 10.1182/blood.2020007335
12. Cremoni M, Benzaken S, Zorzi K, Fernandez C, Esnault V, Barrière J, et al. Humoral and cellular responses after a third dose of SARS-CoV-2 mRNA vaccine in patients with glomerular disease. Nephrol Dialysis Transplant. (2023) 38:249–51. doi: 10.1093/ndt/gfac294
13. Hsu CM, Gupta S, Tighiouart H, Goyal N, Faugno AJ, Tariq A, et al. Kidney recovery and death in critically ill patients with COVID-19-associated acute kidney injury treated with dialysis: the STOP-COVID cohort study. Am J Kidney Dis. (2022) 79:404–416.e1.
14. Nasr SH, Kopp JB. COVID-19-associated collapsing glomerulopathy: an emerging entity. Kidney Int Rep. (2020) 5:759–61. doi: 10.1016/j.ekir.2020.04.030
15. Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, et al. American college of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 3. Arthritis Rheumatol. (2021) 73:e1–12. doi: 10.1002/art.41596
16. Anders HJ, Bruchfeld A, Fernandez Juarez GM, Floege J, Goumenos D, Turkmen K, et al. Recommendations for the management of patients with immune-mediated kidney disease during the severe acute respiratory syndrome coronavirus 2 pandemic. Nephrol Dial Transplant. (2020) 35:920–5. doi: 10.1093/ndt/gfaa112
17. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100:S1–276.
18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
19. Therneau T. A package for survival analysis in R. Copyright 2000 Mayo Foundation for Medical Education and Research. (2024).
20. Kassambra A, Kosinski M, Biecek P. Package “survminer” Type package title drawing survival curves using “ggplot2.”2021. (2021).
21. Wang CS, Doma R, Westbrook AL, Johnson J, Anderson EJ, Greenbaum LA, et al. Vaccine attitudes and COVID-19 vaccine intention among parents of children with kidney disease or primary hypertension. Am J Kidney Dis. (2023) 81:25–35.e1. doi: 10.1053/j.ajkd.2022.04.011
22. Turner-Stokes T, Jiang E, Johnson N, Khakhria K, Kong E, Cairns T, et al. Serologic screening for coronavirus disease 2019 in patients with glomerular disease. Kidney Int Rep. (2021) 6:1402–6. doi: 10.1016/j.ekir.2021.02.006
23. Eidininkienė M, Cesarskaja J, Talačkaitė S, Traškaitė-Juškevičienė V, Macas A. Mini review: co-existing diseases and COVID-19-A one way ticket? Int J Environ Res Public Health. (2022) 19:4.
24. Gatti M, Rinaldi M, Bussini L, Bonazzetti C, Pascale R, Pasquini Z, et al. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect. (2022) 28:1057–65. doi: 10.1016/j.cmi.2022.02.039
25. Takahashi S, Wada N, Murakami H, Funaki S, Inagaki T, Harada K, et al. Triggers of relapse in steroid-dependent and frequently relapsing nephrotic syndrome. Pediatr Nephrol. (2007) 22:232–6. doi: 10.1007/s00467-006-0316-y
26. Shimmel A, Shaikhouni S, Mariani L. Current understanding of clinical manifestations of COVID-19 in glomerular disease. Glomerular Dis. (2021) 1:250–64. doi: 10.1159/000518276
28. Nakayama M, Katafuchi R, Yanase T, Ikeda K, Tanaka H, Fujimi S. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis. (2002) 39:503–12. doi: 10.1053/ajkd.2002.31400
29. Korbet SM, Schwartz MM, Lewis EJ. Minimal-change glomerulopathy of adulthood. Am J Nephrol. (1988) 8:291–7. doi: 10.1159/000167603
30. Zhang H, Wang Z, Dong L, Guo Y, Wu J, Zhai S. New insight into the pathogenesis of minimal change nephrotic syndrome: Role of the persistence of respiratory tract virus in immune disorders. Autoimmun Rev. (2016) 15:632–7. doi: 10.1016/j.autrev.2016.02.007
31. Arrestier R, Satie AP, Zhang SY, Plaisier E, Isnard-Bagnis C, Gatault P, et al. Minimal change nephrotic syndrome in patients infected with human immunodeficiency virus: a retrospective study of 8 cases. BMC Nephrol. (2018) 19:331. doi: 10.1186/s12882-018-1132-x
32. Sahali D, Sendeyo K, Mangier M, Audard V, Zhang SY, Lang P, et al. Immunopathogenesis of idiopathic nephrotic syndrome with relapse. Semin Immunopathol. (2014) 36:421–9. doi: 10.1007/s00281-013-0415-3
33. Aoyama M, Sugimoto T, Yokono T, Sakaguchi M, Deji N, Uzu T, et al. Minimal-change nephropathy and chronic hepatitis C infection: coincidental or associated? Nephrol Dial Transplant. (2007) 22:1479–80.
34. Zhou TB, Jiang ZP. Is there an association of hepatitis B virus infection with minimal change disease of nephrotic syndrome? A clinical observational report. Ren Fail. (2015) 37:459–61. doi: 10.3109/0886022X.2014.1001711
35. MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr. (1986) 108:378–82. doi: 10.1016/S0022-3476(86)80876-9
37. Cohen SD, Kopp JB, Kimmel PL. Kidney diseases associated with human immunodeficiency virus infection. N Engl J Med. (2017) 377:2363–74. doi: 10.1056/NEJMra1508467
38. Rosenberg AZ, Naicker S, Winkler CA, Kopp JB. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. (2015) 11:150–60. doi: 10.1038/nrneph.2015.9
39. Ross MJ. Advances in the pathogenesis of HIV-associated kidney diseases. Kidney Int. (2014) 86:266–74. doi: 10.1038/ki.2014.167
40. Scott J, Hartnett J, Mockler D, Little MA. Environmental risk factors associated with ANCA associated vasculitis: A systematic mapping review. Autoimmun Rev. (2020) 19:102660. doi: 10.1016/j.autrev.2020.102660
41. Katz G, Wallace ZS. Environmental triggers for vasculitis. Rheum Dis Clin North Am. (2022) 48:875–90. doi: 10.1016/j.rdc.2022.06.008
43. Draibe J, Rodó X, Fulladosa X, Martínez-Valenzuela L, Diaz-Encarnación M, Santos L, et al. Seasonal variations in the onset of positive and negative renal ANCA-associated vasculitis in Spain. Clin Kidney J. (2018) 11:468–73. doi: 10.1093/ckj/sfx127
44. Carruthers DM, Watts RA, Symmons DP, Scott DG. Wegener’s granulomatosis–increased incidence or increased recognition? Br J Rheumatol. (1996) 35:142–5.
45. Kemna MJ, Damoiseaux J, Austen J, Winkens B, Peters J, van Paassen P, et al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol. (2015) 26:537–42. doi: 10.1681/ASN.2013111233
46. Cohen Tervaert JW, Damoiseaux J. Antineutrophil cytoplasmic autoantibodies: how are they detected and what is their use for diagnosis, classification and follow-up? Clin Rev Allergy Immunol. (2012) 43:211–9.
47. Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol. (2005) 167:47–58. doi: 10.1016/S0002-9440(10)62952-5
Keywords: COVID-19, glomerulonephritis, outcome, podocytopathies, relapse
Citation: Lionaki S, Dounousi E, Marinaki S, Kantartzi K, Papasotiriou M, Galitsiou D, Bellos I, Sardeli A, Kalogeropoulos P, Liakopoulos V, Mpintas C, Goumenos D, Flouda S, Venetsanopoulou A, Voulgari P, Andronikidi E, Moustakas G, Panagoutsos S and Boletis I (2024) The clinical course of SARS-CoV-2 infection in patients with glomerular diseases and evaluation of the subsequent risk of relapse. Front. Nephrol. 4:1472294. doi: 10.3389/fneph.2024.1472294
Received: 29 July 2024; Accepted: 12 September 2024;
Published: 21 October 2024.
Edited by:
Xuefei Tian, Yale University, United StatesReviewed by:
Anthony Michael Valeri, Columbia University, United StatesLouise Oni, University of Liverpool, United Kingdom
Copyright © 2024 Lionaki, Dounousi, Marinaki, Kantartzi, Papasotiriou, Galitsiou, Bellos, Sardeli, Kalogeropoulos, Liakopoulos, Mpintas, Goumenos, Flouda, Venetsanopoulou, Voulgari, Andronikidi, Moustakas, Panagoutsos and Boletis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophia Lionaki, c29waGlhbEBtZWQudW9hLmdy
 Sophia Lionaki
Sophia Lionaki Evangelia Dounousi2
Evangelia Dounousi2 Smaragdi Marinaki
Smaragdi Marinaki Konstantia Kantartzi
Konstantia Kantartzi Marios Papasotiriou
Marios Papasotiriou Ioannis Bellos
Ioannis Bellos Aggeliki Sardeli
Aggeliki Sardeli Vassilios Liakopoulos
Vassilios Liakopoulos Sophia Flouda
Sophia Flouda Aliki Venetsanopoulou
Aliki Venetsanopoulou Stylianos Panagoutsos
Stylianos Panagoutsos Ioannis Boletis
Ioannis Boletis