- 1The Institute of Basic and Biomedical Sciences, Levy Mwanawasa Medical University, Lusaka, Zambia
- 2Department of Nephrology, Nephrology Vanderbilt O’Brien Center for Kidney Disease, Nashville, TN, United States
- 3Tennessee Valley Health Systems (TVHS), Veterans Affairs, Nashville, TN, United States
- 4University of Alabama at Birmingham, Birmingham, AL, United States
- 5Department of Nephrology, Nephrology Vanderbilt Institute for Global Health (VIGH), Nashville, TN, United States
- 6Department of Global Health, Boston University School of Public Health, Boston, MA, United States
- 7Health Economics and Epidemiology Research Office, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 8Department of Epidemiology, Boston University School of Public Health, Boston, MA, United States
- 9Center for Primary Care Research, School of Medicine, University of Zambia, Lusaka, Zambia
- 10Department of Internal Medicine, University of Zambia, Lusaka, Zambia
- 11Department of Physiological Sciences, Mulungushi University School of Medicine and Health Sciences, Livingstone, Zambia
- 12Department of Health Policy, Vanderbilt University Medical Center (VUMC), Nashville, TN, United States
- 13Department of Medicine, Vanderbilt University Medical Center (VUMC), Nashville, TN, United States
Introduction: Antiretroviral therapy (ART) increases the life expectancy of persons living with HIV (PLWH), but not without potentially serious adverse effects. Tenofovir disoproxil fumarate (TDF) can cause nephrotoxicity, manifesting as acute kidney injury (AKI) that may persist after treatment discontinuation. Kidney injury biomarkers such as kidney injury molecule-1 (KIM-1), retinol-binding protein-4 (RBP-4), interleukin-18 (IL-18), and neutrophil gelatinase-associated lipocalin (NGAL) can aid early diagnosis and predict TDF-associated nephrotoxicity. This study aimed to determine whether the change from baseline in urine KIM-1 (δKIM-1) and NGAL (δNGAL) following 2 weeks of TDF use could predict subclinical TDF-associated nephrotoxicity before the overt manifestation as acute kidney disease after 3 months.
Methods: A prospective cohort study of 205 PLWH was conducted at the Adult Center for Infectious Disease Research (AIDC) in Lusaka, Zambia. ART-naïve PLWH who were starting treatment with TDF with intact kidney function [estimated glomerular filtration rate (eGFR)> 60 mL/min/1.73m2] were followed at initiation, 2 weeks, and approximately 3 months to determine the incidence of TDF-associated nephrotoxicity. We measured urine KIM-1 and NGAL at baseline and after 2 weeks of treatment to determine if it predicted subclinical nephrotoxicity. The presence of TDF-associated nephrotoxicity was defined according to the established acute kidney disease and disorders criteria (AKD) as having either 1) one or more episodes of eGFR< 60ml/min/1.73m2 within 3 months, 2) a reduction in eGFR of greater than 35% (from baseline) within 3 months, and/or 3) an increase in serum creatinine of more than 50% (from baseline) within 3 months.
Results: The incidence of TDF-associated nephrotoxicity was 22%. Baseline eGFR, creatinine, age, female sex, and BMI predicted the risk of overt TDF-associated nephrotoxicity. The median baseline KIM-1-to-creatinine and NGAL-1-to-creatinine ratios of the participants who developed overt TDF-associated nephrotoxicity and those who did not were not significantly different. However, every 1 pg/mg increase in δKIM-1 was associated with a 41% higher risk of TDF-associated nephrotoxicity. No association was observed with δNGAL.
Conclusions: The incidence of TDF-associated nephrotoxicity was high. Change in KIM-1 level within 2 weeks of the initiation of TDF treatment predicted subclinical TDF-associated nephrotoxicity before overt manifestation as acute kidney disease while δNGAL within the same period did not predict subclinical TDF-associated nephrotoxicity.
1 Introduction
Sub-Saharan Africa (SSA) remains the epicenter of the HIV/AIDS pandemic, with more than 70% of the global HIV burden and 52% of HIV-related deaths (1). The widescale implementation of antiretroviral therapy (ART) has ameliorated the impact of HIV-associated morbidity and mortality, but not without ART-associated adverse drug events, some of which can be serious (2–4). Tenofovir disoproxil fumarate (TDF), a leading ART agent that is increasingly used for HIV prevention and chronic hepatitis B virus treatment, can uncommonly lead to nephrotoxicity that may manifest as acute kidney injury (AKI) associated with proximal tubular damage and can progress to chronic kidney disease (CKD) and/or end-stage kidney disease (ESKD) requiring dialysis (5, 6). AKI causes significant morbidity and mortality (7); it is a risk factor for a myriad of complications, including CKD, ESKD and cardiovascular disease (8, 9). The risk of these complications remains high, even following treatment discontinuation or dose modification of the causative agent (8–12). In SSA, the outcomes of ESKD are very poor; therefore, preventing TDF-related complications is of paramount importance.
TDF causes proximal tubular damage by drug-transporter interactions; specifically, the drug accumulates and inhibits mitochondrial DNA (mDNA) polymerase-γ, electron transport chain (ETC) complexes, depletes mitochondria, and reduces cell survival (13–16). Unfortunately, monitoring of kidney health entails measuring serum creatinine levels, which often detects kidney injury late in its course, often at a late stage when the damage is irreversible (17). Before creatinine levels rise, several blood and urine biomarkers may play a role in recognizing early kidney tubular injury, including interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) (18–21), N-acetyl-β-D-glycosaminidase (NAG) (22), liver-type fatty acid-binding protein (L-FABP) (23–25), retinol-binding protein-4 (RBP-4), and β2-microglobulin (26). This study assessed the value of urine KIM-1 and NGAL for the early detection of TDF-associated nephrotoxicity because the two biomarkers are produced in the proximal renal tubular cells which TDF nephrotoxicity primarily affects. For example, the blood/urine concentration of KIM1, a type 1 transmembrane protein of 60-90 KD molecular weight with an immunoglobulin and mucin domain, increases following kidney tubular injury (27). NGAL, in turn, is expressed in proximal tubular cells as a 135 KD barrel-shaped gelatinase heterodimer of the lipocalin family with the ability to bind lipophilic molecules and be sensitive to kidney injury (28).
There is a paucity of knowledge about the diagnostic value of KIM-1 and NGAL in detecting subclinical nephrotoxicity among Africans living with HIV within the early stages of treatment with TDF before overt manifestation as acute kidney disease and this study aims to close that knowledge gap. We hypothesized that the change in urine KIM-1 and urine NGAL levels can indicate subclinical nephrotoxicity after 2 weeks of exposure to TDF, since these two biomarkers are highly expressed in the proximal tubular cells, the primary site for TDF-associated kidney injury.
2 Methods
This was a prospective observational cohort study of 205 persons living with HIV (PLWH) who were recruited at the start of their TDF-based therapy and followed for a maximum period of 4 months at the Adult Infectious Disease Research Centre (AIDC), Zambia’s largest HIV referral and treatment center. Participants were ART-naïve adults (18+ years) who were initiating TDF-based ART under standard treatment conditions and had intact/preserved kidney function at enrollment [estimated glomerular filtration rate (eGFR)≥ 60 mL/min/1.73m2 based on serum creatinine with eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation]. Clinicians initiated the participants’ treatment and treated them according to the Zambian National ART treatment guidelines (24) and their expert clinical discretion to minimize the risk of kidney injury. In addition to 300mg TDF, ART contained 300mg lamivudine (3TC) and 600mg efavirenz (EFV) in a fixed-dose combination pill taken once daily. After ART was started, eGFR was re-assessed at 2 weeks and 3 months. None of the participants were reported to have been taking any specific nephrotoxic drugs.
The primary outcome was the incidence of TDF-associated nephrotoxicity after 3 months of TDF-based ART defined according to the acute kidney disease and disorders (AKD) criteria (29) as having either 1) eGFR< 60ml/min/1.73m2 at 3 months, 2) a reduction in eGFR of greater than 35% within 3 months, or 3) increase in serum creatinine of more than 50% from baseline at 3 months. In this study, participants were lost to follow-up (LTFU) if they did not attend their scheduled 3-month visit and were not traced during contact tracing. Contact tracing involved contacting the participants using the contact number they provided. If they were unreachable after four attempts weekly in the fourth month of follow-up and no kidney function data was found for the third visit in their electronic health record (EHR), they were deemed to be LTFU.
2.1 Participant recruitment
Participants were recruited and followed up starting on 24 September 2018 and ending on 16 September 2019. A REDCap (30) questionnaire was used in the interviews to collect baseline demographics and the participants’ medical history. The interview collected information on their history of concomitant traditional medicine/herb use, non-steroidal autoinflammatory drug (NSAID) use, lifestyle choices such as smoking and alcohol use, and history of comorbidities. Comorbidities included a known history of diabetes, hypertension, chronic hepatitis B or C, and/or tuberculosis, which were based on medical records and the SmartCare system. SmartCare (31) is the national EHR system for HIV deployed by the Zambian Ministry of Health in collaboration with the U.S. Centers for Disease Control and Prevention (CDC).
2.2 Biosample analyses
Blood and urine samples were collected on the day of recruitment and at 2 weeks (+/-3 days) of treatment and only a blood sample was collected after 3 months (+/-1 month) of treatment. Approximately 4 mL of blood was collected in vacutainers containing ethylenediaminetetraacetic acid (EDTA) and potassium oxalate, and in plain vacutainers. A sterile container was used for the collection of approximately 12 mL of urine. CD4+ analysis was conducted within 2 hours on whole blood while the remaining blood was centrifuged, and the plasma or serum was stored at 2 0C until testing within 72 hours of collection. Urine was stored at -80 0C for less than 7 days until biomarker testing. The laboratory tests conducted on stored samples included viral load, serum and urine creatinine, serum sodium, serum potassium, serum and urine phosphate, blood urea nitrogen, serum glucose, and urine albumin. Urine KIM-1 and NGAL were analyzed using a commercial enzyme-linked immunosorbent assay (ELISA) from Elabscience (Wuhan, China) with reagent codes for human KIM-1 (E-EL-H0186) and human NGAL (E-EL-H0096). The calibrators, controls, and the samples for KIM-1 and NGAL were measured in duplicate and then the mean of the two measurements was used as the final concentration. The coefficient of variation for both biomarkers was less than 2%. The CD4+ cell count was analyzed using a Becton Dickinson (BD) FACS Calibur (BD. Biosciences, Erembodegem, Belgium), and viral load was analyzed using the COBAS1 Ampliprep/COBAS1 Taqman 48 HIV-1 Tests version 2 (Roche Diagnostics Corporation, Indianapolis, Indiana, USA) and a Hologic Panther (Hologic, Massachusetts, USA). The remaining tests were conducted on a Beckman Coulter AU480 chemistry analyzer (Beckman Coulter, Midrand, South Africa).
2.3 Data management and analysis
Study data were exported from REDCap to Stata 15 (StataCorp LCC., College Station, Texas, USA) for analysis (28, 29). Categorical variables were summarized using frequencies and percentages. The difference in proportions was computed using Pearson’s chi-square test or Fischer’s exact test. The normality of distribution for continuous variables was tested using the Shapiro–Wilk test. Median and interquartile ranges summarized the continuous variables. The difference between the two medians was tested by the Wilcoxon rank-sum test. The likelihood ratio test was used to test the assumption that introducing the two biomarkers (δKIM-1 and δNGAL) into the constrained model changed the prediction of nephrotoxicity. The baseline predictors fitted in the prognostic model included age, sex, BMI, urine albumin-to-creatinine ratio, CD4+ count, mean arterial pressure (MAP), viremia, serum creatinine, eGFR, and the biomarkers δKIM-1 and δNGAL. Data reduction was conducted using subject knowledge from the literature search, and hierarchical variable clustering eliminated collinearity and redundant variables. The Spearman index was used to select age, sex, BMI, baseline eGFR, baseline serum creatinine, mean arterial pressure, CD4+ count, viremia (HIV viral load > 1000 copies/mL), aviremia (HIV viral load >50 copies/mL but <1000 copies/mL), and the log-transformed urine albumin-to-creatinine ratio as covariates with predictive potential which were included in the final model. The logistic regression model was used to determine the association between the baseline predictors and TDF-associated nephrotoxicity. The assumption of linearity, heteroskedasticity, and association were tested. The model’s chi-square likelihood ratio was used to determine discrimination. The area under the curve (AUC) was drawn to determine the accuracy (linear prediction) of the model score and the sensitivity and specificity of the model were determined. Since logistic regression is based on mean least squares, outliers that can influence the ability of the model to accurately predict were eliminated by determining participants with high influential patterns [delta beta (dbeta) influence statistic > 0.2 and delta chi-square influence statistics (dx2) > 4].
A study of PLWH with viremia (cases) and aviremia (controls) with one control(s) per case was designed. Prior data indicated that the KIM-1 concentration increases by 0.20 in aviremia after 1 year of TDF use (21). If the true increase in KIM-1 in viremia is 0.43, we needed to study a minimum of 81 persons initiating TDF with viremia and 81 persons with aviremia to be able to reject the null hypothesis that the change in KIM-1 between persons with viremia and aviremia is equal with 80% power and α of 0.05 with a 10% adjustment for loss to follow-up. The continuity-corrected chi-squared statistic or Fisher’s exact test formula was used.
2.4 Ethics approval and consent to participate
The University of Zambia Biomedical Research Committee (UNZABREC) reviewed and granted ethical approval (Ref: 043-08-18) and the National Health Research Authority (NHRA) also granted permission to conduct the study.
3 Results
A total of 205 participants were studied of which two were not followed because they declined to provide blood and urine samples even though their demographics were collected, and the participants’ subsequent kidney outcomes were imputed in the analyses using Multiple Imputations by Chained Monte Carlo Equations (MICE). More details about the analyses can be found in our previous publication (32). Out of a total of 205 participants and 638 person-months, 45 (22%) developed nephrotoxicity during follow-up for an incidence rate of 70 cases per 1,000 person-months. The baseline clinical and laboratory characteristics of the participants stratified by TDF-associated nephrotoxicity are shown in Table 1. There were no differences in nephrotoxicity by sex, cigarette smoking, alcohol use, herb intake, viremia, baseline eGFR, or baseline serum creatinine between those who did and did not develop nephrotoxicity. None of the participants self-reported or had a documented history of comorbidities including diabetes mellitus, kidney disease, chronic diarrhea, hypertension, hepatitis B, hepatitis C, and tuberculosis. Furthermore, there were no statistically significant differences between the two groups of participants in median age, sex-stratified BMI, mean arterial pressure (MAP), duration of follow-up, viral load, CD4+ count, serum creatinine, fasting blood glucose (FBG), eGFR, serum cholesterol, urine creatinine, urine albumin-to-creatinine ratio, Kidney Disease: Improving Global Outcomes (KDIGO) stage of kidney dysfunction, and urine phosphate-to-creatinine ratio (Table 1).
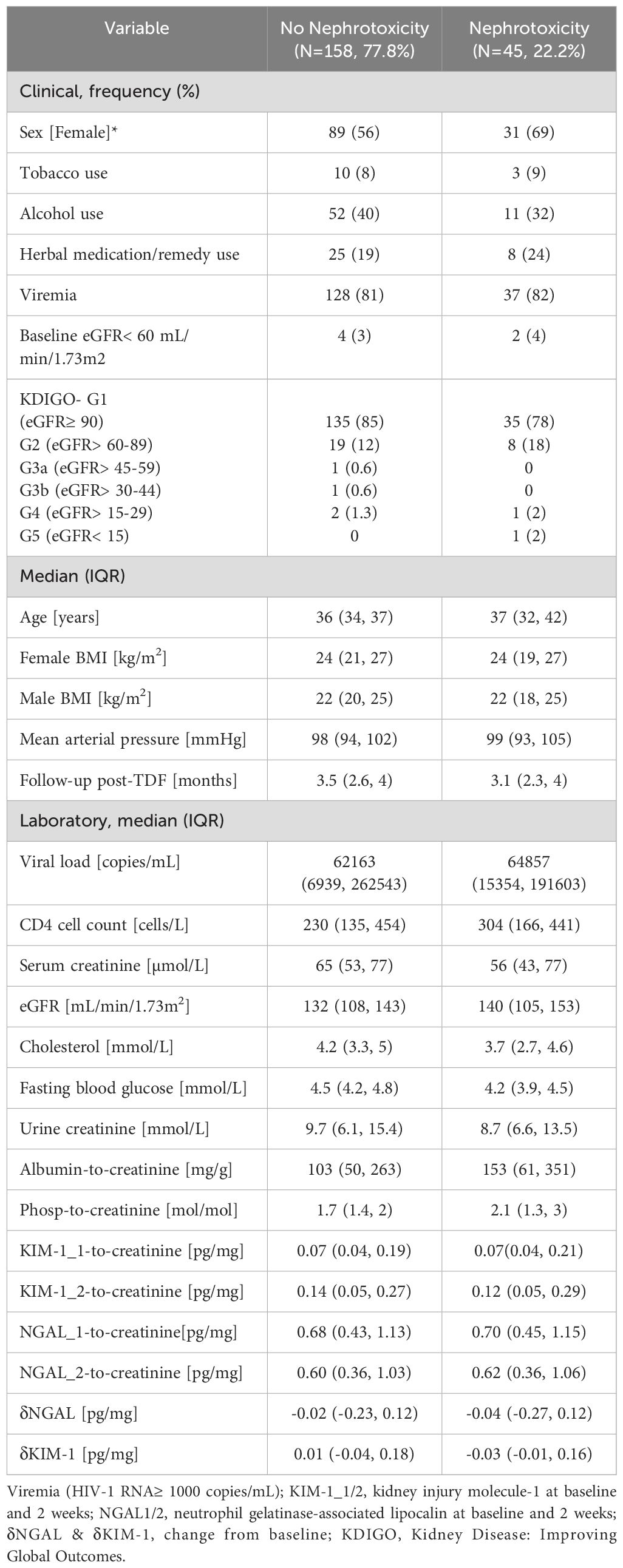
Table 1. Comparison of baseline clinical and laboratory characteristics stratified by TDF-associated nephrotoxicity.
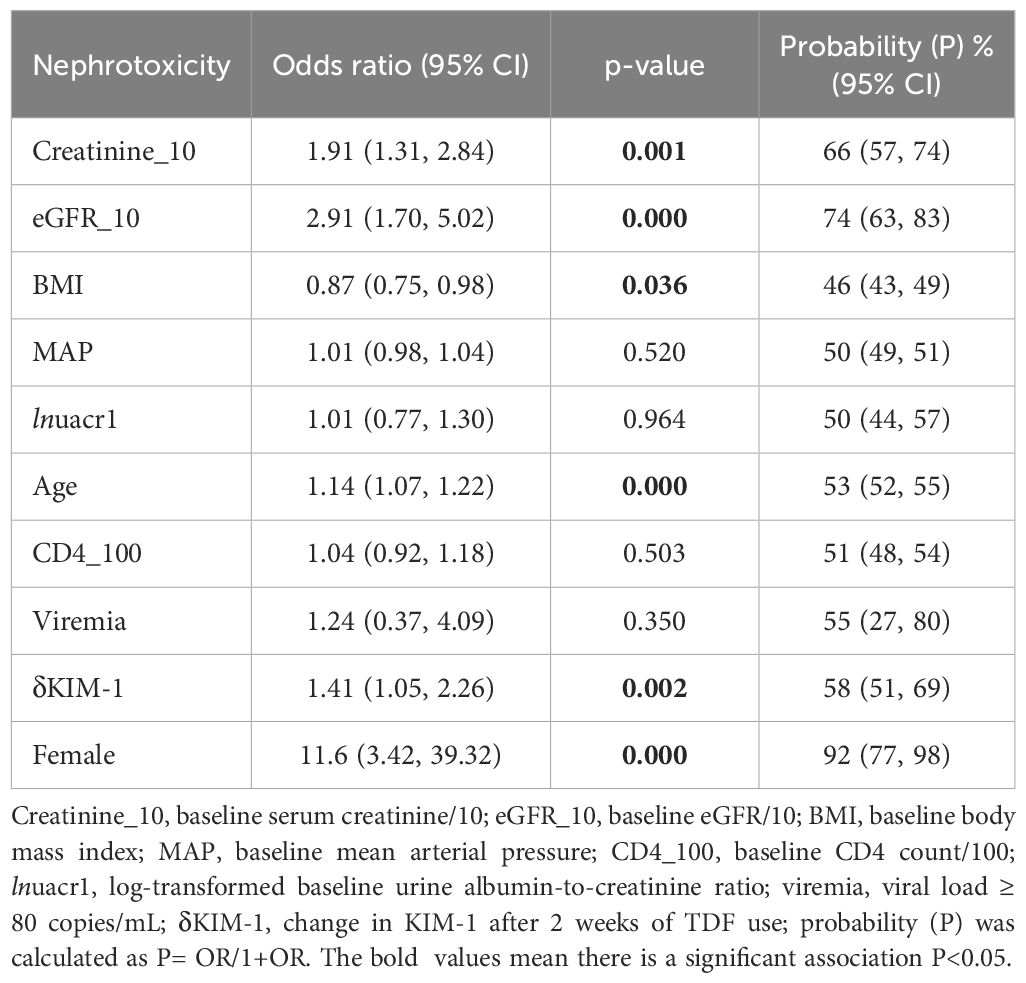
Upon eliminating the four participants with high influential patterns (dbeta influence statistic > 0.2 and dx2 > 4) the model showed that every pg/mg increase in the difference between the baseline and 2-week concentration of urine KIM-1 (δKIM-1) was associated with a probability of 58% (95%CI 51, 69%) to develop TDF-associated nephrotoxicity. Furthermore, every 10 µmol/L increase in the baseline serum creatinine (creatinine_10pt) was associated with a 66% (95%CI 57, 74%) increase in the probability of developing TDF-associated nephrotoxicity. Furthermore, every 10 mL/min/1.73m2 increase in the baseline eGFR (egfr_10pt) was associated with a 74% (95%CI 63, 83%) increase in the probability of developing TDF-associated nephrotoxicity while every 1-year increase in age of the participant was associated with a 53% (95%CI 52, 55%) increase in the probability of developing TDF-associated nephrotoxicity. Every kg/m2 increase in BMI was associated with a 46% (95%CI 43, 49%) decrease in the probability of developing TDF-associated nephrotoxicity. Finally, compared to men, being a woman was associated with a 92% (95%CI 77, 98%) increase in the probability of developing TDF-associated nephrotoxicity (Table 2). However, baseline MAP, urine albumin-to-creatinine ratio (lnuacr1), CD4 (CD4_100pt), and viremia were not associated with a risk of nephrotoxicity (Table 2).
Fitting the model with δKIM-1 and δNGAL showed an improvement compared to another model we published previously (32) which did not have the biomarkers; a statistically significant improvement in the Akaike Information Criterion (AIC) was observed, p= 0.001. However, when the biomarkers δKIM-1 and δNGAL were introduced into the nested model one at a time, the improvement in model showed that only δKIM-1 improved the model, p= 0.001; δNGAL showed no significant change to the AIC, p= 0.15.
3.1 Model diagnostics and performance
The Hosmer–Lemeshow goodness-of-fit test for model calibration resulted in p = 0.16, suggesting that the data fitted well to the model. The model’s c-statistic obtained from the AUC resulted in a model discrimination of 0.81. Testing the model classification resulted in a positive predictive value (PPV) of 69% and a negative predictive value (NPV) of 85%. The sensitivity of the model was 30% while the specificity was 97%. Furthermore, the model correctly classified 84% of the participants.
3.2 Sensitivity analysis for urine KIM-1-to-creatinine for the probability of nephrotoxicity
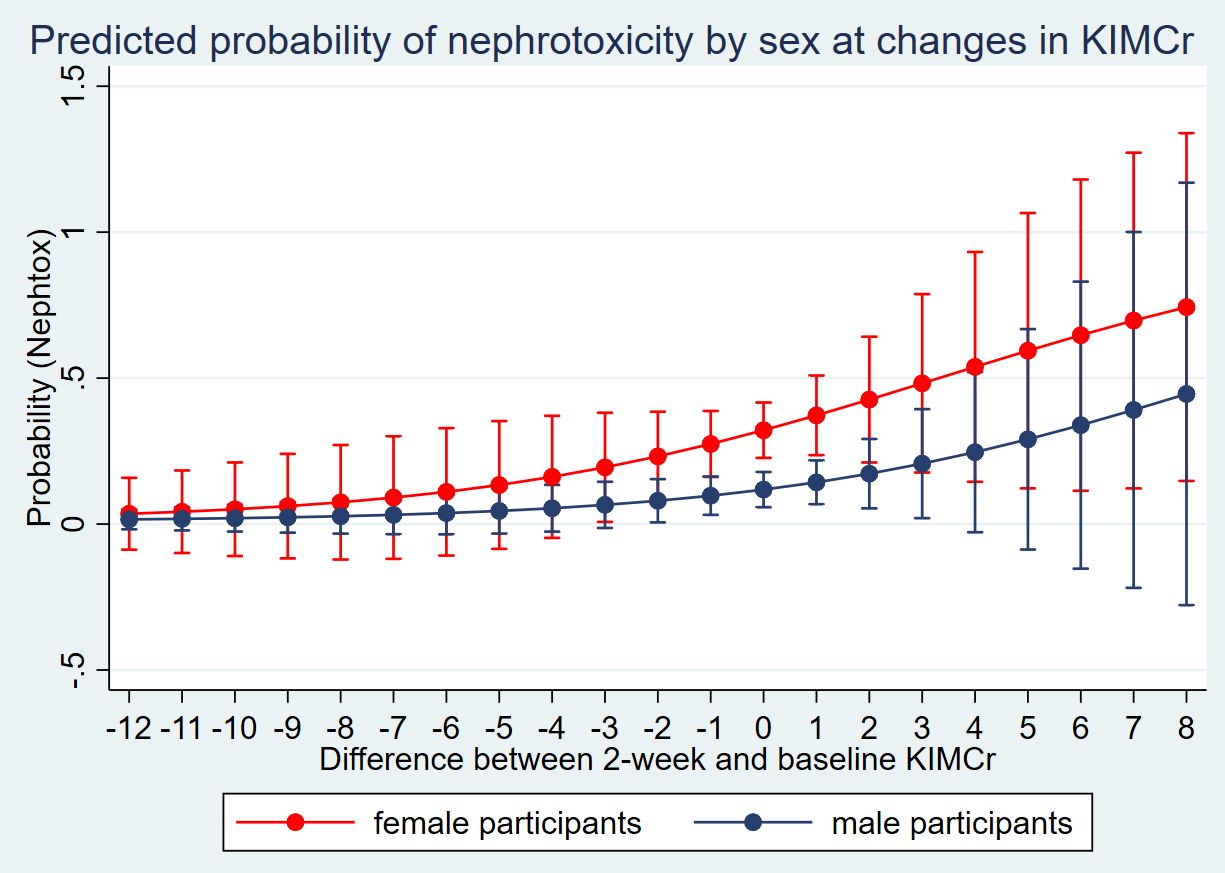
Further analysis of the 45 participants who developed nephrotoxicity based on our criteria showed that 15 (33%) had an eGFR reduction below 60mL/min/1.73, 32 (71%) had an eGFR decrease of 35%, while 36 (80%) had a serum creatinine increase of 50% from baseline; implying that some met more than one criterion. Analysis of the sensitivity of a unit change in the 2-week urine KIM-1-to-creatinine to predict the probability of nephrotoxicity amongst men and women showed higher marginal probabilities amongst women (Figure 1).
4 Discussion
In a cohort of adults living with HIV in Zambia with no or minimal kidney dysfunction at the start of TDF-based ART according to standard treatment initiation criteria, we measured the incidence of TDF-associated nephrotoxicity within 3 months and sought to identify the associated urine, blood biomarker, and demographic factors. We found that the baseline serum creatinine, eGFR, BMI, age, and change in urine KIM-1 within 2 weeks of TDF initiation predicted the risk of TDF-associated nephrotoxicity, which built upon evidence from previous studies. These data support the use of change in the concentration of KIM-1 within 2 weeks of initiating a TDF-based regimen to predict subclinical TDF-associated nephrotoxicity before overt manifestation at 3 months and could be used in clinical care to stratify the patients’ risk before initiating treatment with TDF. This could be invaluable, especially in low- and middle-income countries (LMICs) where there are no readily available options for treating end-stage kidney dysfunction such as chronic dialysis and kidney transplant which may result from concomitant TDF-associated nephrotoxicity.
A plethora of studies have linked various factors to the risk of TDF-associated nephrotoxicity including female sex, advanced age, pre-existing kidney insufficiency, unsuppressed HIV viral load, protease inhibitor-containing regimens (32–34), co-infection with hepatitis B or C, concomitant TDF use, comorbidities such as hypertension and diabetes, and advanced HIV clinical stage (WHO stages 3 or 4) (35–37). Our previous study (32) showed that treatment with a TDF-based regimen caused nephrotoxicity that manifested within the first 4 months among PLWH who initiated treatment with intact/preserved kidney function. There is no prognostic model for Zambia and most LMICs to estimate the risk of PLWH developing TDF-associated nephrotoxicity if treated. Consequently, anecdotal clinical/laboratory assessments are used to decide who initiates TDF-based therapy. The ability of these assessments to stratify patients by risk of nephrotoxicity is questionable considering the high cases of kidney disease reported in this population (32). Our study derived a prognostic model using routine clinical/laboratory data from patients at TDF initiation and discovered that no significant difference existed between the clinical/laboratory characteristics of those who developed kidney injury and those who did not. Finding no significant difference in the clinical/laboratory characteristics on which the current treatment algorithm is based [initiating TDF for PLWH with eGFR> 60 mL/min/1.73m2 (38)] exposed the futility of these assessments which are not based on found evidence. This algorithm based on clinical/laboratory assessments is pointless for kidney injury risk stratification as, for instance, it misclassifies approximately 87% of patients at risk of TDF-associated nephrotoxicity as being safe (32).
The primary aim of this study was to determine if including the change in the concentration of two biomarkers (KIM-1 and NGAL) within 2 weeks of initiating TDF-based therapy can predict subclinical nephrotoxicity before overt manifestation. The findings in this study showed that an increase in the difference between the baseline and the 2-week concentration of KIM-1-to-creatinine was associated with an increased probability of TDF-associated nephrotoxicity. This finding concurred with reports elsewhere that KIM-1 levels can be an early signal for kidney injury (19, 21, 25, 39). However, our finding that KIM-1 detected subclinical injury within 2 weeks of starting treatment with TDF was new considering that many studies measured the biomarker much later in the course of treatment, usually at a time when it has stabilized (18, 21, 40, 41). Furthermore, this study found that NGAL does not detect subclinical injury within 2 weeks of TDF use; this was also unique compared to reports by others that claimed NGAL could be used for the early detection of proximal tubular injury (42–44) but agreed with findings elsewhere (21) where the biomarker was assessed after 1 year of TDF therapy. The differences between our findings and other reports may be due to different times of assessing the change in the biomarker with our study assessing the change earlier in the treatment course.
The key findings of this study are that KIM-1 can predict subclinical TDF-associated nephrotoxicity within 2 weeks of initiating treatment for HIV infection while NGAL cannot. This suggests that using KIM-1 may augment risk stratification to prevent TDF-associated nephrotoxicity, especially among those predicted to have low/borderline risk of nephrotoxicity using the prognostic model we published previously (32). It should be underscored that most LMICs, such as Zambia, do not have options such as chronic dialysis and kidney transplant to effectively treat the ESKD that may result from TDF-associated nephrotoxicity, therefore, investing time in deriving and validating prognostic models that can predict and effectively discriminate patients by risk is crucial. Given that studies have linked nephrotoxicity to many kidney-related (45–49) and non-kidney-related complications (11, 48, 50–52) and that victims of kidney injury remain at high risk of complications even after removing the cause of injury (8, 9, 11), the findings of this study are important.
Finally, consistent with other reports (13, 32, 53, 54), this study also found that the baseline serum creatinine, eGFR, BMI, age, and being female were associated with the risk of developing nephrotoxicity. Advancing age increased the risk of TDF-associated nephrotoxicity, concurring with reports that an aging kidney suffers nephrosclerosis, which disturbs the transcriptomics, hemodynamics, and physiological behavior of the kidney, thereby impairing its response to injury (55, 56). The finding that women compared to men have a higher probability of developing TDF-associated nephrotoxicity is consistent with findings from South Africa and the USA (33, 57, 58); however, the pathophysiology of this difference remains unclear. Therefore, close monitoring of patients is crucial regardless of sex to mitigate the risk of kidney complications. Furthermore, on the one hand, an increase in serum creatinine (59, 60) was associated with an increased risk of TDF-associated nephrotoxicity consistent with findings elsewhere which showed that having pre-existing kidney impairment (61) increased the risk of TDF-associated nephrotoxicity (62, 63). On the other hand, paradoxically, an increase in baseline eGFR, which corresponds with better kidney function at baseline, seemed to be associated with a higher risk of TDF-associated nephrotoxicity (64, 65). This could be due to most participants initiating TDF with preserved kidney function entailing lower serum creatinine and high baseline eGFR with medians of 132 mL/min/1.73m2 and 140 mL/min/1.73m2, respectively. Extremely high and extremely low values are prone to regression to the mean. Additionally, since TDF typically significantly reduces kidney function within the first 3-6 months of therapy and the AKD criteria flagged nephrotoxicity when there is a 50% increase in creatinine, a 35% decrease in eGFR, or eGFR goes below 60mL/min/1.73m2, it is more likely that those with an elevated baseline eGFR level fulfilled any of the above AKD criteria than those around the mean. This study also found that an increase in BMI was inversely associated with the risk of TDF-associated nephrotoxicity. This was contrary to other studies (66, 67) that found a direct association. This was probably because our study was conducted among PLWH who are more likely to have a low BMI.
The strengths of this study were that it leveraged routine clinical biomarkers that are done among PLWH and included an enzyme-linked immunosorbent assay-based biomarker which is not difficult to test for and can be determined within 3 hours to improve the prediction of subclinical TDF-associated nephrotoxicity. Furthermore, these findings are unique because to our knowledge, no study has attempted to assess the changes in these biomarkers following short-term exposure to TDF and determined how this would forecast the risk of adverse kidney outcomes downstream. The limitation of this study is that the prognostic model needs external validation before use and might need optimization for other excluded populations such as children and adolescents.
5 Conclusions
In conclusion, the change in the KIM-1-to-creatinine ratio that follows 2 weeks of initiating treatment with TDF-based therapy can predict subclinical nephrotoxicity before overt manifestation 4 months later as TDF-associated nephrotoxicity. Using a prognostic model is a realistic and cost-effective way of preventing TDF-associated nephrotoxicity among PLWH, and these results build upon our previous study (32) that built a model using routine clinical and laboratory data; including kidney injury sensitive biomarkers to improve prediction was the next realistic step. One may worry about the cost implication and complexity of including such biomarkers, however, KIM-1 is a convenient ELISA-based biomarker that can be tested within 3 hours for approximately $10 per test. Considering the huge cost of treating ESKD and the threat to life it poses, including such a biomarker may be more cost-effective. However, it must be emphasized that there is a need to externally validate these prognostic models before integration into clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The University of Zambia Biomedical Research Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. ES: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. CW: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. AB: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. MP: Validation, Visualization, Writing – review & editing. MV: Data curation, Supervision, Validation, Writing – original draft, Writing – review & editing. SM: Conceptualization, Validation, Visualization, Writing – review & editing. MA: Data curation, Supervision, Validation, Funding acquisition, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research reported in this publication was supported by the Fogarty International Center and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award D43 TW009744 (FC and SM) and R21TW012635 (SM), the American Heart Association Award Number 24IVPHA1297559 https://doi.org/10.58275/AHA.24IVPHA1297559.pc.gr.193866 (SM) and the International Federation of Clinical Chemistry and Laboratory Medicine-(IFCC)’s Task Force on Outcome Studies in Laboratory Medicine (TF-OSLM) (SM). ES was supported by the Vanderbilt O’Brien Kidney Center P30-DK114809. AB was supported by the National Institute of Diabetes and Digestive and Kidney (NIDDK) grant 1K01DK116929-01A1.
Acknowledgments
Special gratitude to Dr Douglas C. Heimburger for the support in securing funding for the research.
Conflict of interest
ES reports consulting for Akebia, Inc., a lecture honorarium from the Da Vita Physician Leadership Conference, royalties as an author for UpToDate, and serving on the editorial board for the Clinical Journal of the American Society of Nephrology but that does not have any ability to conflict with the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the American Heart Association.
References
1. UNAIDS. Global HIV & AIDS statistics — 2021 fact sheet (2021). Geneva, Switzerland (Accessed 07/02/2022).
2. Silva BF, Peixoto G, da Luz SR, Moraes S, Peres SB. Adverse effects of chronic treatment with the Main subclasses of highly active antiretroviral therapy: a systematic review. HIV Med. (2019) 20:429–38. doi: 10.1111/hiv.2019.20.issue-7
3. Chowta MN, Kamath P, Ramapuram JT, Shenoy KA, Hadigal S. Evaluation of adverse drug reaction profile of drugs used as first-line antiretroviral therapy. Interdiscip Perspect Infect Dis. (2018), 8095609. doi: 10.1155/2018/8095609
4. Louis M, Cottenet J, Salmon-Rousseau A, Blot M, Bonnot PH, Rebibou JM, et al. Prevalence and incidence of kidney diseases leading to hospital admission in people living with HIV in France: an observational nationwide study. BMJ Open. (2019) 9:e029211. doi: 10.1136/bmjopen-2019-029211
5. Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Nino MD, Izquierdo MC, Poveda J, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. (2011), 354908. doi: 10.1155/2011/354908
6. Del Palacio M, Romero S, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. (2012) 14:179–87.
7. Fabian J, Maher HA, Clark C, Naicker S, Becker P, Venter WD, et al. Morbidity and mortality of black HIV-positive patients with end-stage kidney disease receiving chronic haemodialysis in South Africa. S Afr Med J. (2015) 105:110–4. doi: 10.7196/SAMJ.8369
8. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. (2014) 371:58–66. doi: 10.1056/NEJMra1214243
9. Tsagalis G, Akrivos T, Alevizaki M, Manios E, Stamatellopoulos K, Laggouranis A, et al. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant. (2009) 24:194–200. doi: 10.1093/ndt/gfn471
10. Jose S, Hamzah L, Campbell LJ, Hill T, Fisher M, Leen C, et al. Incomplete reversibility of estimated glomerular filtration rate decline following tenofovir disoproxil fumarate exposure. J Infect Dis. (2014) 210:363–73. doi: 10.1093/infdis/jiu107
11. Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. (2014) 9:448–56. doi: 10.2215/CJN.02440213
12. Matłosz B, Kowalska JD, Bąkowska E, Firląg-Burkacka E, Vassilenko A, Horban A. Discontinuation of tenofovir due to nephrotoxicity: insight into 12 years of clinical practice. Przegl Epidemiol. (2019) 73:249–55. doi: 10.32394/pe.73.24
13. Hall AM. Update on tenofovir toxicity in the kidney. Pediatr Nephrol. (2013) 28:1011–23. doi: 10.1007/s00467-012-2269-7
14. Milián L, Peris JE, Gandía P, Andújar I, Pallardó L, Górriz JL, et al. Tenofovir-induced toxicity in renal proximal tubular epithelial cells: involvement of mitochondria. Aids. (2017) 31:1679–84. doi: 10.1097/QAD.0000000000001572
15. Ramamoorthy H, Abraham P, Isaac B. Mitochondrial dysfunction and electron transport chain complex defect in a rat model of tenofovir disoproxil fumarate nephrotoxicity. J Biochem Mol Toxicol. (2014) 28:246–55. doi: 10.1002/jbt.2014.28.issue-6
16. Ramamoorthy H, Abraham P, Isaac B, Selvakumar D. Mitochondrial pathway of apoptosis and necrosis contribute to tenofovir disoproxil fumarate-induced renal damage in rats. Hum Exp Toxicol. (2019) 38:288–302. doi: 10.1177/0960327118802619
17. Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for biomarker evaluation. Clin Trials. (2013) 10:696–700. doi: 10.1177/1740774513497540
18. Ascher SB, Scherzer R, Estrella MM, Zhang WR, Muiru AN, Jotwani V, et al. Association of urinary biomarkers of kidney injury with estimated GFR decline in HIV-infected individuals following tenofovir disoproxil fumarate initiation. Clin J Am Soc Nephrol. (2018) 13:1321–9. doi: 10.2215/CJN.01700218
19. Jotwani V, Scherzer R, Estrella MM, Jacobson LP, Witt MD, Palella F, et al. Association of HIV infection with biomarkers of kidney injury and fibrosis in the Multicenter AIDS Cohort Study. Antivir Ther. (2017) 22:421–9. doi: 10.3851/IMP3124
20. Malhotra R, Katz R, Jotwani V, Ambrosius WT, Raphael KL, Haley W, et al. Urine markers of kidney tubule cell injury and kidney function decline in SPRINT trial participants with CKD. Clin J Am Soc Nephrol. (2020) 15:349–58. doi: 10.2215/CJN.02780319
21. Zhang WR, Scherzer R, Estrella MM, Ascher SB, Muiru A, Jotwani V, et al. Tenofovir disoproxil fumarate initiation and changes in urinary biomarker concentrations among HIV-infected men and women. AIDS (London England). (2019) 33:723–33. doi: 10.1097/QAD.0000000000002114
22. Oboho I, Abraham AG, Benning L, Anastos K, Sharma A, Young M, et al. Tenofovir use and urinary biomarkers among HIV-infected women in the Women’s Interagency HIV Study (WIHS). J Acquir Immune Defic Syndr. (2013) 62:388–95. doi: 10.1097/QAI.0b013e31828175c9
23. Fiseha T, Gebreweld A. Urinary markers of tubular injury in HIV-infected patients. Biochem Res International. (2016) p:1501785. doi: 10.1155/2016/1501785
24. Hikasa S, Shimabukuro S, Hideta K, Higasa S, Sawada A, Tokugawa T, et al. Utility of urinary liver-type fatty acid-binding protein as a predictor of renal dysfunction in Japanese patients with HIV receiving tenofovir disoproxil fumarate with low urinary β2 microglobulin levels: a retrospective observational study. J Pharm Health Care Sci. (2019) 5:12. doi: 10.1186/s40780-019-0140-8
25. Scherzer R, Lin H, Abraham A, Thiessen-Philbrook H, Parikh CR, Bennett M, et al. Use of urine biomarker-derived clusters to predict the risk of chronic kidney disease and all-cause mortality in HIV-infected women. Nephrol Dial Transplant. (2016) 31:1478–85. doi: 10.1093/ndt/gfv426
26. Chan A, Park L, Collins LF, Cooper C, Saag M, Dieterich D, et al. Correlation between tenofovir drug levels and the renal biomarkers RBP-4 and ß2M in the ION-4 study cohort. Open Forum Infect Dis. (2019) 6:ofy273. doi: 10.1093/ofid/ofy273
27. Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquired Immune Deficiency Syndromes. (2012) 61:565–73. doi: 10.1097/QAI.0b013e3182737706
28. Sola-Del Valle DA, Mohan S, Cheng J-T, Paragas NA, Sise ME, D'Agati V, et al. Urinary NGAL is a useful clinical biomarker of HIV-associated nephropathy. Nephrology Dialysis Transplantation: Off Publ Eur Dialysis Transplant Assoc Eur Renal Assoc. (2011) 26:2387–90. doi: 10.1093/ndt/gfr258
29. Barry R, James MT. Guidelines for classification of acute kidney diseases and disorders. Nephron. (2015) 131:221–6. doi: 10.1159/000441425
30. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J BioMed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
31. Muyunda G. Zambia leads the way in SmartCare electronic health records system a benefit to both providers and patients, 2011. (2018) (Zambia: UNZA Repository).
32. Chabala FW, et al. Prognostic model for nephrotoxicity among HIV-positive Zambian adults receiving tenofovir disoproxil fumarate-based antiretroviral therapy. PloS One. (2021) 16:e0252768. doi: 10.1371/journal.pone.0252768
33. Ojeh BV, Abah IO, Ugoagwu P, Agaba PA, Agbaji OO, Gyang SS. Incidence and predictors of tenofovir disoproxil fumarate-induced renal impairment in HIV infected Nigerian patients. Germs. (2018) 8:67–76. doi: 10.18683/germs.2018.1133
34. Lee JE, Lee S, Song SH, Kwak IS, Lee SH. Incidence and risk factors for tenofovir-associated nephrotoxicity among human immunodeficiency virus-infected patients in Korea. Korean J Internal Med. (2019) 34:409–17. doi: 10.3904/kjim.2016.418
35. Kaboré NF, Poda A, Zoungrana J, Da O, Ciaffi L, Semdé A, et al. Chronic kidney disease and HIV in the era of antiretroviral treatment: findings from a 10-year cohort study in a west African setting. BMC Nephrol. (2019) 20:155. doi: 10.1186/s12882-019-1335-9
36. George JA, Brandenburg J-T, Fabian J, Crowther NJ, Agongo G, Alberts M, et al. Kidney damage and associated risk factors in rural and urban sub-Saharan Africa (AWI-Gen): a cross-sectional population study. Lancet Glob Health. (2019) 7:e1632–43. doi: 10.1016/S2214-109X(19)30443-7
37. Muiru AN, et al. Kidney disease risk factors associate with urine biomarkers concentrations in HIV-positive persons; a cross-sectional study. BMC Nephrol. (2019) 20:4. doi: 10.1186/s12882-018-1192-y
38. Health, M.O. Zambia consolidated guidelines for prevention and treatment of HIV, Vol. 1. (2020) (Zambia: Ministry of health).
39. Torregrosa I, Montoliu C, Urios A, Andres-Costa MJ, Gimenez-Garzo C, Juan I, et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels. (2015) 30:703–11. doi: 10.1007/s00380-014-0538-z
40. Eltounali SA, Moodley J, Naicker T. Role of kidney biomarkers [Kidney injury molecule-1, Calbindin, Interleukin-18 and Monocyte chemoattractant protein-1] in HIV associated pre-eclampsia. Hypertension Pregnancy. (2017) 36:288–94. doi: 10.1080/10641955.2017.1385793
41. Peralta C, Scherzer R, Grunfeld C, Abraham A, Tien P, Devarajan P, et al. Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS). HIV Med. (2014) 15:291–300. doi: 10.1111/hiv.2014.15.issue-5
42. Kaucsar T, Godo M, Revesz C, Kovacs M, Mocsai A, Kiss N, et al. Urine/plasma neutrophil gelatinase associated lipocalin ratio is a sensitive and specific marker of subclinical acute kidney injury in mice. PloS One. (2016) 11:e0148043. doi: 10.1371/journal.pone.0148043
43. Palazzuoli A, Ruocco G, Pellegrini M, Gori De C, Castillo Del G, Franci B, et al. Comparison of neutrophil gelatinase-associated lipocalin versus B-type natriuretic peptide and cystatin C to predict early acute kidney injury and outcome in patients with acute heart failure. Am J Cardiol. (2015) 116:104–11. doi: 10.1016/j.amjcard.2015.03.043
44. Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, et al. Urinary, plasma, and serum biomarkers’ Utility for predicting acute kidney injury associated with cardiac surgery in adults: A meta-analysis. Am J Kidney Dis. (2015) 66:993–1005. doi: 10.1053/j.ajkd.2015.06.018
45. Asar Ö, Ritchie J, Kalra PA, Diggle PJ. Short-term and long-term effects of acute kidney injury in chronic kidney disease patients: A longitudinal analysis. Biometrical J. (2016) 58:1552–66. doi: 10.1002/bimj.201500270
46. Ball J. What are the long-term outcomes after acute, severe kidney injury and what should we be doing about them?*. Crit Care Med. (2017) 45:136–7. doi: 10.1097/CCM.0000000000002116
47. Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis. (2008) 15. doi: 10.1053/j.ackd.2008.04.009
48. Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int. (2010) 78:478–85. doi: 10.1038/ki.2010.171
49. Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med. (2016) 67:293–307. doi: 10.1146/annurev-med-050214-013407
50. Bedford M, Stevens PE, Wheeler TWK, Farmer CKT. What is the real impact of acute kidney injury? BMC Nephrol. (2014) 15:95–5. doi: 10.1186/1471-2369-15-95
51. Bwakura-Dangarembizi M, et al. Risk factors for postdischarge mortality following hospitalization for severe acute malnutrition in Zimbabwe and Zambia. Am J Clin Nutr. (2021) 113:665–74. doi: 10.1093/ajcn/nqaa346
52. Campos P, Ortiz A, Soto K. HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J. (2016) 9:772–81. doi: 10.1093/ckj/sfw104
53. Baxi SM, Scherzer R, Greenblatt RM, Minkoff H, Sharma A, Cohen M, et al. Higher tenofovir exposure is associated with longitudinal declines in kidney function in women living with HIV. AIDS. (2016) 30:609–18. doi: 10.1097/QAD.0000000000000958
54. Overton ET, Nurutdinova D, Freeman J, Seyfried W, Mondy KE, et al. Factors associated with renal dysfunction within an urban HIV-infected cohort in the era of highly active antiretroviral therapy. HIV Med. (2009) 10. doi: 10.1111/j.1468-1293.2009.00693.x
55. O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. (2017) 28:407–20. doi: 10.1681/ASN.2015121308
56. Nadkarni GN, Konstantinidis I, Wyatt CM. HIV and the aging kidney. Curr Opin HIV AIDS. (2014) 9. doi: 10.1097/COH.0000000000000067
57. Ryom L, Mocroft A, Lundgren J. HIV therapies and the kidney: some good, some not so good? Curr HIV/AIDS Rep. (2012) 9. doi: 10.1007/s11904-012-0110-3
58. Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. (2016) 3:e23–32. doi: 10.1016/S2352-3018(15)00211-8
59. Boswell MT, Rossouw TM. Approach to acute kidney injury in HIV-infected patients in South Africa. South Afr J HIV Med. (2017) 18:714. doi: 10.4102/sajhivmed.v18i1.714
60. Okpa HO, Bisong EM, Enang OE, Effa EE, Monjok E, Essien EJ. Predictors of chronic kidney disease among HIV-infected patients on highly active antiretroviral therapy at the University of Calabar Teaching Hospital, Calabar, South-South Nigeria. HIV AIDS (Auckl). (2019) 11:61–7. doi: 10.2147/HIV.S189802
61. Sutton SS, Magagnoli J, Hardin JW, Hsu L-I, Beaubrun A, Majethia S. Association of tenofovir disoproxil fumarate exposure with chronic kidney disease and osteoporotic fracture in US veterans with HIV. Curr Med Res Opin. (2020) 36:1635–42. doi: 10.1080/03007995.2020.1816538
62. Gilbert A, Robertson L, Heron JE, Chadban S, Ndhlovu C, Dahwa RF, et al. Risk factors for development of acute kidney injury in hospitalised adults in Zimbabwe. PloS One. (2020) 15:e0241229. doi: 10.1371/journal.pone.0241229
63. Mulenga L, Musonda P, Mwango A, Vinikoor MJ, Davies MA, Mweemba A, et al. Effect of baseline renal function on tenofovir-containing antiretroviral therapy outcomes in Zambia. Clin Infect Dis. (2014) 58:1473–80. doi: 10.1093/cid/ciu117
64. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. (2010) 51:496–505. doi: 10.1086/655681
65. Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. Aids. (2012) 26:867–75. doi: 10.1097/QAD.0b013e328351f68f
66. Soto GJ, Frank AJ, Christiani DC, Gong MN. Body mass index and acute kidney injury in the acute respiratory distress syndrome. Crit Care Med. (2012) 40:2601–8. doi: 10.1097/CCM.0b013e3182591ed9
Keywords: kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, acute kidney injury, HIV, tenofovir disoproxil fumarate-associated nephrotoxicity, Zambia
Citation: Chabala FW, Siew ED, Wester CW, Brennan AT, Phiri MM, Vinikoor MJ, Masenga SK and Aliyu MH (2025) Urine kidney injury molecule-1 predicts subclinical kidney disease among persons living with HIV initiating tenofovir disoproxil fumarate-based ART in Zambia. Front. Nephrol. 4:1468409. doi: 10.3389/fneph.2024.1468409
Received: 22 July 2024; Accepted: 29 November 2024;
Published: 06 January 2025.
Edited by:
Gert Mayer, Innsbruck Medical University, AustriaReviewed by:
Xavier Fulladosa, Bellvitge University Hospital, SpainEtienne Macedo, University of California, San Diego, United States
Copyright © 2025 Chabala, Siew, Wester, Brennan, Phiri, Vinikoor, Masenga and Aliyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sepiso K. Masenga, c2VwaXNvbWFzZW5nYUBnbWFpbC5jb20=; Freeman W. Chabala, d2FnYWRmcmVlbWFuODZAZ21haWwuY29t
 Freeman W. Chabala
Freeman W. Chabala Edward D. Siew2,3,4
Edward D. Siew2,3,4 Alana T. Brennan
Alana T. Brennan Sepiso K. Masenga
Sepiso K. Masenga