
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Nephrol., 29 July 2024
Sec. Clinical Research in Nephrology
Volume 4 - 2024 | https://doi.org/10.3389/fneph.2024.1409098
 Shigekazu Kurihara1
Shigekazu Kurihara1 Naoki Sawa1
Naoki Sawa1 Keiichi Sumida1
Keiichi Sumida1 Daisuke Ikuma1
Daisuke Ikuma1 Yuki Oba1
Yuki Oba1 Hiroki Mizuno1
Hiroki Mizuno1 Akinari Sekine1
Akinari Sekine1 Masayuki Yamanouchi1
Masayuki Yamanouchi1 Eiko Hasegawa1
Eiko Hasegawa1 Tatsuya Suwabe1
Tatsuya Suwabe1 Shinji Urakami2
Shinji Urakami2 Kei Kono3
Kei Kono3 Keiichi Kinowaki3
Keiichi Kinowaki3 Kenichi Ohashi3,4
Kenichi Ohashi3,4 Yutaka Yamaguchi5
Yutaka Yamaguchi5 Yoshifumi Ubara1*
Yoshifumi Ubara1*A kidney biopsy was performed in a 64-year-old woman with type 2 diabetes mellitus and less than 1 g of proteinuria who rapidly progressed to end-stage renal failure after approximately 2 years of treatment with two dipeptidyl peptidase 4 (DPP-4) inhibitors for type 2 diabetes mellitus. The biopsy revealed not only a coincidental diagnosis of renal cell carcinoma, which was not evident on pre-biopsy computed tomography, but also severe thrombotic microangiopathy (TMA)-like glomerular endothelial cell damage in the noncancerous areas. These results suggest that DPP4 inhibitors may have been involved in two kidney diseases.
Kidney biopsies were performed to investigate the cause of proteinuria, hematuria, and renal dysfunction. However, they are usually only conducted after diagnostic imaging tests, such as ultrasound and computed tomography (CT) have confirmed the absence of morphological abnormalities, such as renal tumors. Consequently, renal tumors are usually not diagnosed by biopsy. The Japanese Guidebook on Kidney Biopsy, which was developed from the results of a survey on the indications for kidney biopsy, describes the use of biopsy for diagnosing renal tumors. Some reports describe the incidental diagnosis of neoplasms, including intravascular lymphoma, by kidney biopsy; however, reports of incidental diagnosis of renal cancer are scarce (1–3).
Here, we report a case in which renal cancer was incidentally diagnosed by kidney biopsy performed to investigate the pathogenesis of rapid decline in renal function in a patient with type 2 diabetes mellitus (T2D).
A 64-year-old woman with progressive renal impairment was admitted to our hospital. She was diagnosed with T2D at the age of 45 years and maintained good control of blood glucose with diet therapy only. A right adrenal tumor was found at the age of 46 years and was diagnosed as nonfunctional.
Fourteen months before the current admission, the laboratory findings were as follows: serum creatinine (Cre), 1.2 mg/dL; hemoglobin A1c (HbA1c), 8.5%; urinary protein, 0.3 g/gCre; and erythrocytes in the urinary sediment, more than 30 per high power field. Sitagliptin, a dipeptidyl peptidase 4 (DPP4) inhibitor, was administered at a dose of 25 mg to treat hyperglycemia, and the HbA1c level subsequently decreased to 6.1%. Eight months later, sitagliptin was discontinued because renal function had declined, as shown by a Cre value of 2.2 mg/dL. The subsequent progression of renal function decline was slow (Figure 1).This patient was admitted to investigate the cause of kidney impairment.
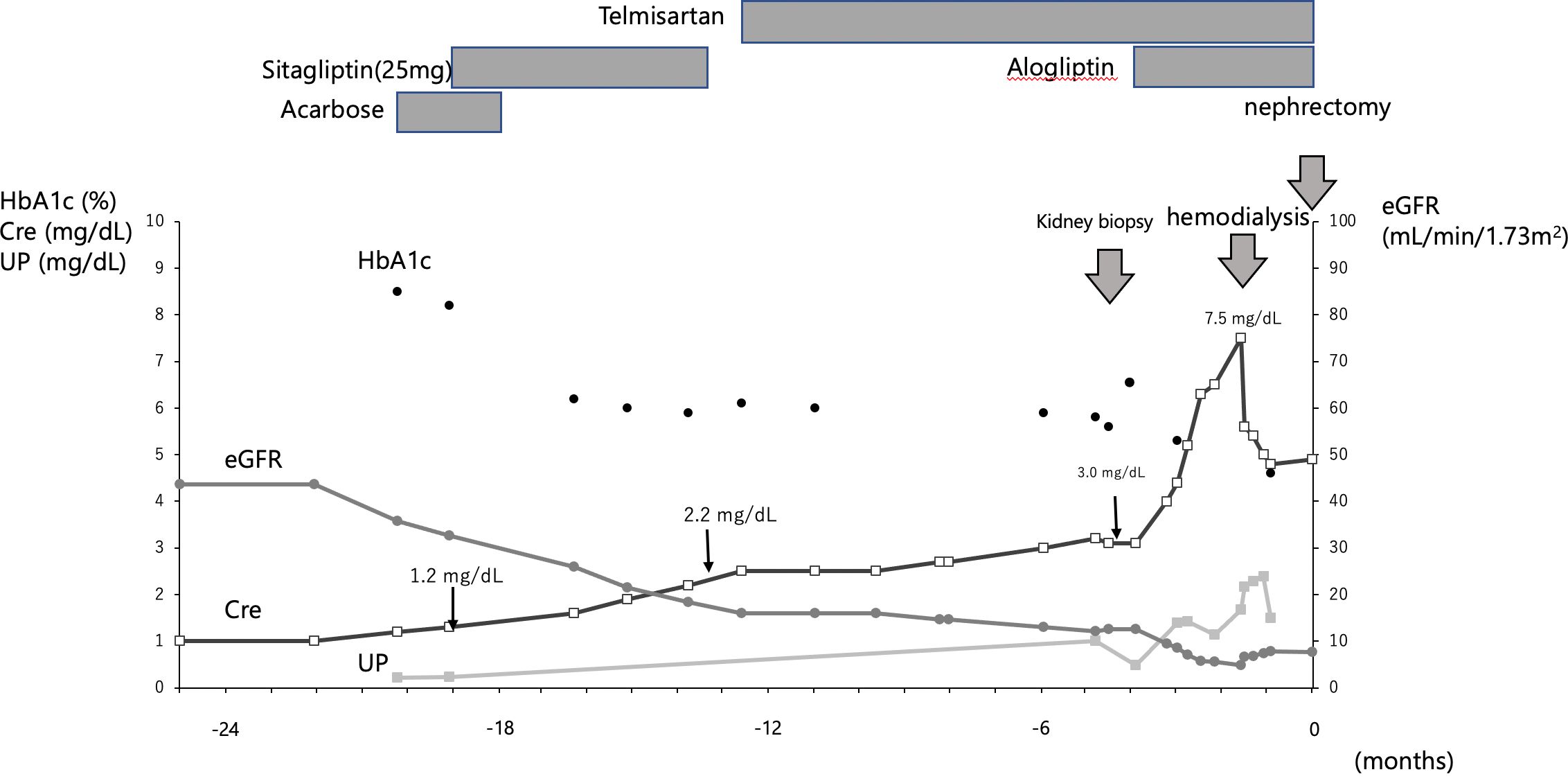
Figure 1 Clinical course. The figure shows the clinical course up to nephrectomy. The timing of kidney biopsy, hemodialysis and nephrectomy is indicated by thick arrows. Sitagliptin was given at Cre 1.2 mg/dL and was stopped when Cre fell to 2.2 mg/dL; alogliptin was started at Cre 3.0 mg/dL; hemodialysis was started when Cre reached 7.5 mg/dL.
On admission, the patient was 147 cm tall and weighed 45.7 kg. Her blood pressure was 155/66 mmHg, heart rate was 82 beats/min, and body temperature was 36.4°C. The heart and breath sounds were normal; however, edema was present in the lower extremities. Funduscopic examinations revealed no retinopathy.
The results of blood chemistry tests were as follows: serum albumin, 3.5 g/dL; serum creatinine, 3.0 mg/dL, estimated glomerular filtration rate, 17.6 ml/min/1.73 m2; C-reactive protein, less than 0.1 mg/dL; immunoglobulin G (IgG), 1105 mg/dL; IgA, 301 mg/dL; IgM, 54.0 mg/dL; total complement activity (assessed as CH50), 57 U/mL; antinuclear antibody, negative; fasting plasma glucose, 97 mg/dL; HbA1c, 6.0%; glycoalbumin, 16.8%; total cholesterol, 170 mg/dL; and triglycerides, 121 mg/dL. The urinary protein excretion was 0.84 g/day, and the urinary sediment contained more than 90 erythrocytes per high-power field. Ultrasonic examination revealed that the maximum diameter of both kidneys was 10 cm, no atrophy was observed, and no renal tumor was detected. The right kidney was then biopsied.
Five specimens were obtained by kidney biopsy. Light microscopic examination of the three specimens revealed global sclerosis in four out of 23 glomeruli. Tubulointerstitial fibrosis and atrophy with thickening of the tubular basement membrane were observed in approximately 70% of the cortical area (Figures 2A, B). Many of the preserved glomeruli showed fibrotic thickening of the basement membrane of the Bowman’s capsule (Figure 2C). The mesangium matrix was obscure. Duplication of the glomerular basement membrane (GBM) (Figure 2C) and endothelial cell proliferation were observed (Figure 2D). Arteriolar hyalinosis was mild, and fibroelastosis of the interlobular arteries was mild-to-moderate. Immunofluorescence microscopy revealed linear fluorescence of IgG along the glomerular basement membrane (GBM), Bowman’s capsule, and tubular basement membrane (Figure 2E), but staining for IgA, IgM, C3, and C1q was negative. Electron microscopy revealed endothelial cell proliferation with mesangiolysis and subendothelial edema. The GBM was thickened to a width of 400–500 nm (Figure 2F); however, electron-dense deposits were not observed. According to Tervaert’s pathologic classification of diabetic nephropathy(DN) (4), the glomerular classification was Class IIa (with mild mesangial expansion),interstitial lesions IFTA score 3(>50%), interstitial inflammation score 1, vascular lesions arteriolar hyalinosis score 1, and arteriosclerosis score 1. In this case, the glomerular lesions were mild, but the interstitial lesions were moderate to severe. The glomerular lesions in this case were characterized by a high degree of thrombotic microangiopathy (TMA)-like endothelial cell damage, consistent with glomerular microangiopathy. Although the glomerular lesions associated with diabetic nephropathy were mild, advanced interstitial lesions and TMA-like glomerular microangiopathy may have been related to the renal prognosis in this case. This TMA-like lesion is not usually observed in diabetic nephropathy. This is similar to drug-induced nephropathy caused by recent anticancer drugs (5), which will be discussed later.
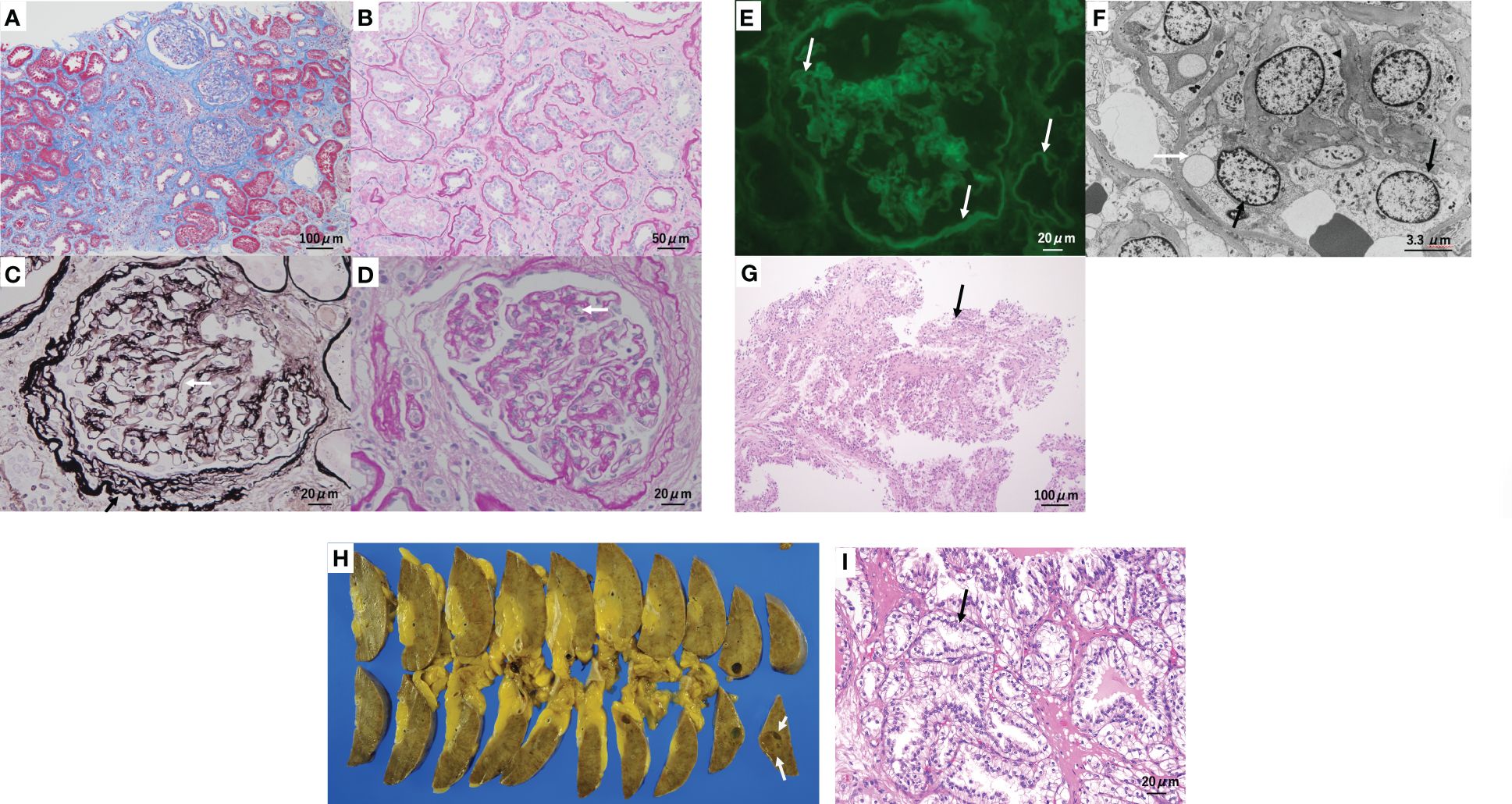
Figure 2 Kidney biopsy and surgical specimen. (A, B) Tubulointerstitial fibrosis and tubular atrophy are observed in approximately 70% of the cortical area. (A) Masson trichrome staining. (B) Periodic acid–Schiff (PAS) staining. (C) Many of the preserved glomeruli show prominent fibrotic thickening (black arrow) of the glomerular basement membrane (GBM) of Bowman’s capsule and duplication (white arrow) of the GBM. Periodic acid methenamine silver and Masson staining. (D) Glomerular endothelial cell proliferation (arrow) was observed. PAS staining. (E) Immunofluorescence microscopy reveals linear fluorescence of immunoglobulin G (arrow) along the GBM, Bowman’s capsule, and tubular basement membrane. (F) Electron microscopy reveals endothelial cell proliferation (black arrow) with mesangiolysis (arrowhead) and subendothelial edema (white arrow). The GBM thickened to a width of 400–500 nm. (G) Two biopsy specimens showed clear cell renal cell carcinoma(arrow). Hematoxylin and eosin staining: original magnification ×200. (H) split section of the surgical specimen. A tumor(arrow), 9–10 mm in size, was found in the surgical specimen in the lower pole of the right kidney. (I) Histology also confirmed clear cell renal cell carcinoma(arrow). Hematoxylin and eosin staining; original magnification ×400.
Two biopsy specimens showed clear cell renal cell carcinoma (RCC), characterized by proximal tubular cell-like tumor cells with pale or acidophilic cytoplasms (Figure 2G). The border between the cancerous and non-cancerous areas was clearly defined by a fibrous capsule.
After kidney biopsy, the spread of the tumor was re-examined using contrast-enhanced CT. A hypervascular stain, 8 mm in size, was observed in the lower pole of the right kidney (Figure 3). Magnetic resonance imaging (MRI) T2-weighted imaging showed a mass, 9 mm in size, that had a higher intensity than the renal parenchyma and was located in the lower pole of the right kidney. MRI also confirmed the presence of a right adrenal tumor, which was still the same size even after 20 years. Dehydroepiandrosterone sulfate, adrenocorticotropic hormone, aldosterone, adrenaline, noradrenaline, dopamine, and renin were all within their respective reference ranges.
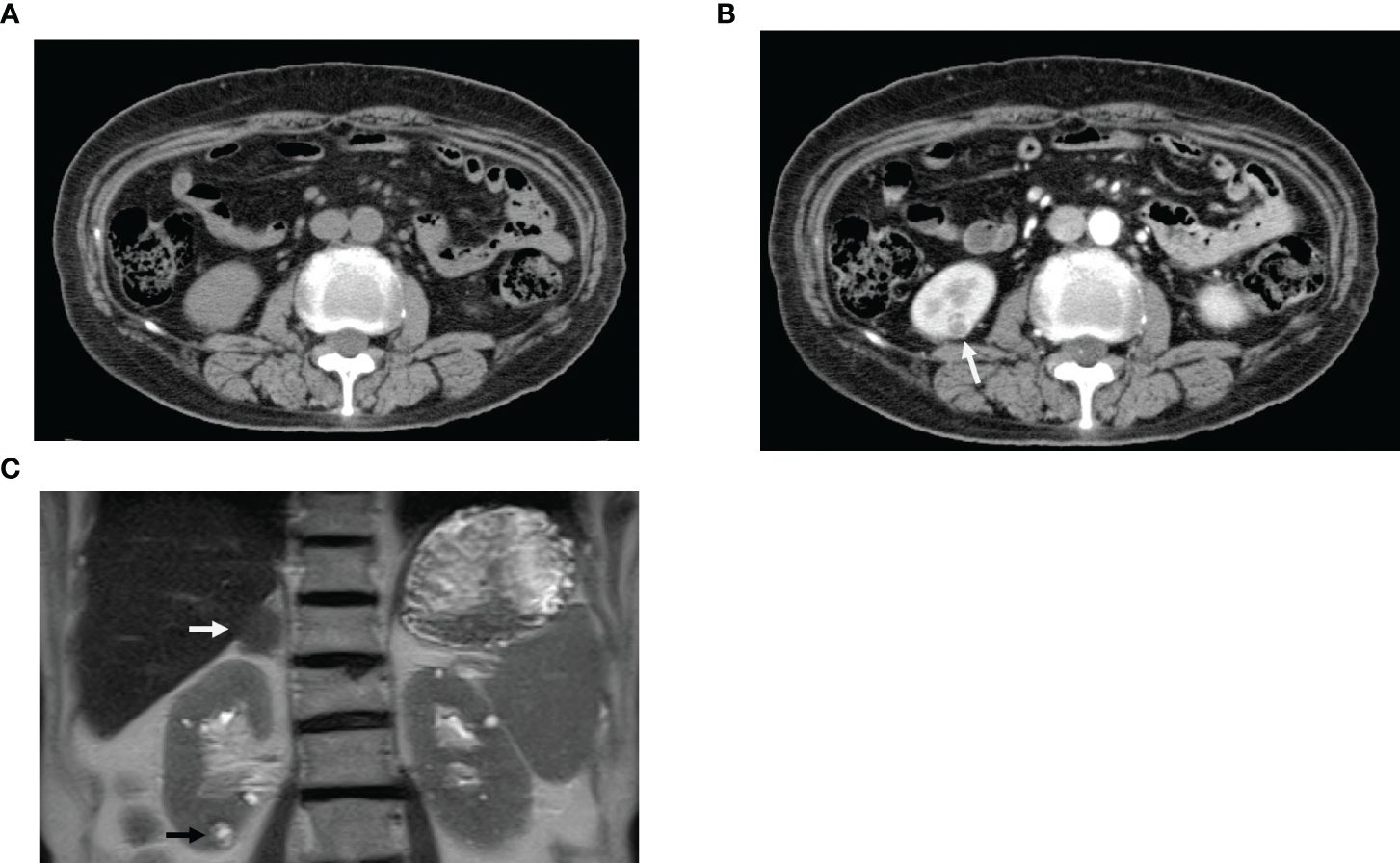
Figure 3 Diagnostic imaging. (A) Computed tomography (CT) scan before contrast enhancement. (B) CT scan immediately after contrast enhancement; white arrow indicates hypervascular staining (Equivalent to renal cancer). (C) Magnetic resonance imaging; Black arrow indicates mass, 9 mm in size (Equivalent to renal cancer) in the lower pole of the right kidney. White arrow confirmed the presence of a right adrenal tumor.
After kidney biopsy, HbA1c began to rise again and another DPP-4 inhibitor, alogliptin, was administered. Alogliptin, a DPP4 inhibitor, was started, and the decline in renal function progressed to Cre 7.5 mg/dL; hemodialysis was initiated for end-stage renal failure. Two months later, the patient underwent a radical laparoscopic right nephrectomy and adenectomy. No drugs were used that might contribute to TMA-like lesions, except for DPP4 inhibitors (Figure 1).
The surgical specimen contained a tumor, 9 mm in size, in the lower pole of the right kidney (Figure 2H). Histological examination revealed clear cell RCC. The size of the tumor cell nucleus was equivalent to that of the nucleus of the surrounding tubules (grade 1, Fuhrman grade 1, INFα, pT1a) (Figure 2I). TNM classification was cT1aN0M0. The non-cancerous tissue of the resected specimen consisted of highly fibrotic interstitial lesions and sclerotic glomeruli corresponding to end-stage renal failure. The pathophysiology of the development of renal failure in a rather short period of time is noteworthy. No invasion of the surrounding veins or lymph vessels was observed. The adrenal gland showed adrenocortical adenoma.
This article contains two elements: First, the RCC revealed by kidney biopsy, and second, the rapid progression of renal dysfunction in the context of noncancerous renal tissue.
This was the first case of incidental diagnosis of RCC on kidney biopsy at our hospital; however, from 1985 to April 2022, we performed 7497 kidney biopsies for pathological diagnosis of kidney disease, including allogeneic transplants (this number does not include biopsies of renal tumors identified by diagnostic imaging before biopsy).
We searched for reports of similar cases in which a malignancy was incidentally diagnosed on kidney biopsy. Pankhurst et al. reported that among 11880 kidney biopsies, including allogeneic transplants, performed from 1982 through 2003, 25 identified accidental neoplasms. Of the 25 cases, two were clear cell RCC, one was in situ carcinoma in a collecting duct, and the remaining 22 were papillary neoplasms (6). Sperati et al. reported a case of papillary RCC (Fuhrman grade 3) that was incidentally diagnosed by percutaneous biopsy. The diameter of papillary RCC was 1.1 cm. At their institution, 9330 kidney biopsies were performed between 1984 and 2008, and a total of three cases (0.03%) had evidence of possible malignancy with unremarkable kidney imaging (results of kidney biopsy of transplanted kidneys were excluded). The other two cases involved a 51-year-old man with tubulopapillary hyperplasia and a 47-year-old woman with chromophobe RCC associated with fibrillary glomerulonephritis (7). Jamis-Dow CA examined the limits of detection of renal cancer on CT and ultrasound scans by analyzing a database of 21 patients with von Hippel-Lindau disease or hereditary papillary renal cancer. Contrast-enhanced CT and ultrasound detection rates were 47% and 0% for lesions of 0–5 mm, respectively, and 60% and 21% for lesions of 5–10 mm (8). O’Connor et al. reported that renal cancers < 3 cm are often difficult to diagnose using unenhanced CT (9).
Looking back at the clinical course of this case, it is clear that glycemic control improved after sitagliptin administration, but renal function progressively deteriorated. After discontinuation of sitagliptin, the progression of renal function loss slowed, but the patient rapidly progressed to end-stage renal failure following the administration of alogliptin. This suggests that DPP4 inhibitors may contribute to the progression of renal failure. To answer this question, we began with a detailed review of kidney biopsy results.
Regarding the causes of renal dysfunction, we searched for articles discussing the association between the general prognosis of diabetic nephropathy and renal histology findings. We found that Mise et al. reported a better 10-year prognosis for class IIa in Tervaert’s pathological classification of diabetic nephropathy than for classes IIb, III, and IV (10). If a diagnosis of class IIa of Tervaert’s pathological classification is made, the decline in renal function progresses slowly. However, renal function declined in the present case; therefore, we considered the possible causes. Kidney biopsy showed fewer glomeruli with total nodular sclerosis and more damage to glomeruli that were spared from sclerosis compared to the progression of loss of renal function. Therefore, we focused on the presence of TMA-like lesions in the preserved glomeruli. Eremina et al. reported that patients treated with bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF)A, had TMA-like glomerular lesions characterized by glomerular endothelial cell damage. They explained that VEGF released from podocytes protects endothelial cells, but the drug suppresses VEGF, resulting in endothelial cell damage. They also reported that the drug also induces hypertension, which worsens the glomerular lesions. The glomerular lesions described by Eremina et al. are similar to those described by us in the present study and may therefore represent a common type of drug-induced glomerular lesion, although the drugs used are different and the mechanisms are different (5); therefore, we reviewed our case in the context of drug-induced nephropathy. The clinical course of the patient showed progressive deterioration of renal function after the use of the two DPP-4 inhibitors. Suenaga et al. recently reported glomerular lesions of TMA-like glomerular microangiopathy as well as bullous pemphigoid after DPP4 inhibitor treatment in type 2 diabetic patients (11, 12). In terms of the mechanism, the following article does not discuss direct glomerular damage, but may be of interest as endothelial regeneration of blood vessels may lead to glomerular endothelial damage. Brenner et al. reported that sitagliptin, a DPP-4 inhibitor, prevents the degradation of the chemokine SDF-1α and improves the recruitment of regenerative circulating CXCR4+ progenitor cells that mediate local endothelial cell proliferation without adversely affecting the structure of the vessel wall, using a mouse carotid artery injury model, and it can promote endothelial regeneration after acute endothelial injury (13). DPP-4 inhibitors may induce endothelial cell proliferation in the glomerulus, leading to TMA-like lesions.
DPP-4 inhibitors are effective in controlling blood glucose levels, have a low risk of hypoglycemia, are well tolerated in patients with T2D and diabetic kidney disease (DKD), and are expected to reduce the progression of diabetic nephropathy. Some studies reported significant reductions in albuminuria or less progression of albuminuria, while others reported no significant efficacy in inhibiting renal progression of DKD. We were unable to find any publications on the long-term renal prognosis associated with DPP4 inhibitors (14, 15). Although the glomerular lesions in this case were mild according to the diabetic nephropathy criteria based on mesangial matrix expansion, TMA-like lesions in the glomeruli and severe interstitial lesions, which were not included in the diabetic nephropathy criteria, were clearly involved in the renal damage in this case, suggesting that DPP4 inhibitors were involved in renal damage via a mechanism different from that of the conventional mechanism.
It is possible that the DPP4 inhibitor may have contributed to the progressive decline in renal function in this case despite good glycemic control during DPP4 inhibitor treatment. In our case, renal cancer was found incidentally after sitagliptin treatment. The relationship between this drug and the development of renal cancer is not yet clear as this is the only case of this drug. However, the following article on DPP4 inhibitors and malignant tumors is considered an interesting pathogenesis. Yang et al. studied human and mouse breast cancer cell lines and DPP4 inhibitor-treated mouse allograft models. They reported that DPP-4 inhibitors may promote cancer progression via induction of the CXCL12/CXCR4/mTOR axis, which is also important for vascular damage in cancer tissue. They did not discuss renal lesions, but DPP4 inhibitors and renal cancer may be considered in the future (16). Akashi et al. suggested that DPP4 inhibitors may improve vascular endothelial function and heal impaired vessels by increasing the number of circulating endothelial progenitor cells (EPCs). In this case, the mild arteriohyalinosis in spite of the history of DM may have contributed to the prevention of small renal arteries by DPP4 inhibitors, whereas in the glomeruli, it was speculated that the increase in the number of endothelial cells may have caused TMA-like lesions (17).
In conclusion, we encountered a case of renal cancer less than 1 cm in size that was incidentally diagnosed by kidney biopsy. Nephrologists usually perform kidney biopsy to investigate the cause of proteinuria, hematuria, or renal function loss and are not aware of malignant tumors. It is important to consider the possibility of microscopic renal cancer, although this is less common. The noncancerous renal area is a TMA-like lesion associated with DPP4 inhibitor administration, which may contribute to the rapid decline in renal function. The patient already had hematuria before starting sitagliptin. This could suggest that renal cancer may have been an inducer of TMA-like lesions under DPP4-inhibitor treatment. We should not conclude from this single case report that DPP4-inhibitors are associated with TMA-like lesions or renal cancer. However, awareness of these conditions may be helpful in interpreting similar cases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Toranomon Hospital institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YU: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Conceptualization, Writing – original draft. NS: Conceptualization, Writing – review & editing. KS: Investigation, Writing – review & editing. DI: Data curation, Writing – review & editing. YO: Conceptualization, Writing – review & editing. HM: Validation, Writing – review & editing. AS: Formal analysis, Writing – review & editing. MY: Data curation, Writing – review & editing. EH: Data curation, Writing – review & editing. TS: Data curation, Writing – review & editing. SU: Supervision, Writing – review & editing. KKo: Data curation, Writing – review & editing. KKi: Data curation, Writing – review & editing. KO: Supervision, Writing – review & editing. YY: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We are grateful to all past and present members of our center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
T2D, type 2 diabetes; RCC, renal cell carcinoma; CT, computed tomography; DPP-4, dipeptidyl peptidase 4; MRI, magnetic resonance imaging.
1. Ubara Y, Kawaguchi T, Nagasawa T, Miura K, Katsuno T, Morikawa T, et al. Kidney biopsy guidebook 2020 in Japan. Clin Exp Nephrol. (2021) 25:325–64. doi: 10.1007/s10157-020-01986-6
2. Sawa N, Ubara Y, Katori H, Hoshino J, Suwabe T, Tagami T, et al. Renal intravascular large B-cell lymphoma localized only within peritubular capillaries. Rep case. Intern Med. (2007) 46:657–62. doi: 10.2169/internalmedicine.46.6333
3. Hasegawa J, Hoshino J, Suwabe T, Hayami N, Sumida K, Mise K, et al. Characteristics of intravascular large B-cell lymphoma limited to the glomerular capillaries: A case report. Case Rep Nephrol Dial. (2015) 5:173–9. doi: 10.1159/000437296
4. Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Renal Pathology Society. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. (2010) 21:556–63. doi: 10.1681/ASN.2010010010
5. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. (2008) 358:1129–36. doi: 10.1056/NEJMoa0707330
6. Pankhurst T, Howie AJ, Adu D, Wallace DM, Lipkin GW. Incidental neoplasms in renal biopsies. Nephrol Dial Transplant. (2006) 21:64–9. doi: 10.1093/ndt/gfi149
7. Sperati CJ, Alachkar N, Rodriguez R, Haas M, Choi MJ. Incidental discovery of a renal cell carcinoma on native kidney biopsy. Am J Kidney Dis. (2010) 56:175–80. doi: 10.1053/j.ajkd.2009.08.018
8. Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM. Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. (1996) 198:785–8. doi: 10.1148/radiology.198.3.8628872
9. O'Connor SD, Silverman SG, Cochon LR, Khorasani RK. Renal cancer at unenhanced CT: imaging features, detection rates, and outcomes. Abdom Radiol (NY). (2018) 43:1756–63. doi: 10.1007/s00261-017-1376-0
10. Mise K, Hoshino J, Ubara Y, Sumida K, Hiramatsu R, Hasegawa E, et al. Renal prognosis a long time after renal biopsy on patients with diabetic nephropathy. Nephrol Dial Transplant. (2014) 29:109–18. doi: 10.1093/ndt/gft349
11. Suenaga A, Sawa N, Oba Y, Ikuma D, Sekine A, Yamanouchi M, et al. A case of bullous pemphigoid and renal disease after dipeptidyl peptidase 4 inhibitor administration. CEN Case Rep. (2023) 6. doi: 10.1007/s13730-023-00835-1
12. Suenaga A, Sawa N, Oba Y, Ikuma D, Sekine A, Hasegawa E, et al. Dipeptidyl peptidase-4 inhibitor-related renal disease. J Diabetes Complications. (2023) 37:108590. doi: 10.1016/j.jdiacomp.2023.108590
13. Brenner C, Kränkel N, Kühlenthal S, Israel L, Remm F, Fischer C, et al. Franz WM.Short-term inhibition of DPP-4 enhances endothelial regeneration after acute arterial injury via enhanced recruitment of circulating progenitor cells. Int J Cardiol. (2014) 177:266–75. doi: 10.1016/j.ijcard.2014.09.016
14. Coppolino G, Leporini C, Rivoli L, Ursini F, di Paola ED, Cernaro V, et al. Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol Res. (2018) 129:274–94. doi: 10.1016/j.phrs.2017.12.001
15. Daza-Arnedo R, Rico-Fontalvo JE, Pájaro-Galvis N, Leal-Martínez V, Abuabara-Franco E, Raad-Sarabia M, et al. Dipeptidyl peptidase-4 inhibitors and diabetic kidney disease: A narrative review. Kidney Med. (2021) 3:1065–73. doi: 10.1016/j.xkme.2021.07.007
16. Yang F, Takagaki Y, Yoshitomi Y, Ikeda T, Li J, Kitada M, et al. Inhibition of dipeptidyl peptidase-4 accelerates epithelial-mesenchymal transition and breast cancer metastasis via the CXCL12/CXCR4/mTOR axis. Cancer Res. (2019) 79:735–46. doi: 10.1158/0008-5472.CAN-18-0620
17. Akashi N, Umemoto T, Yamada H, Fujiwara T, Yamamoto K, Taniguchi Y, et al. Teneligliptin, a DPP-4 inhibitor, improves vascular endothelial function via divergent actions including changes in circulating endothelial progenitor cells. Diabetes Metab Syndr Obes. (2023) 16:1043–54. doi: 10.2147/DMSO.S403125
Keywords: kidney biopsy, renal cell carcinoma, dipeptidyl peptidase (DPP) 4 inhibitors, thrombotic microangiopathy (TMA)-like lesion, end-stage renal failure
Citation: Kurihara S, Sawa N, Sumida K, Ikuma D, Oba Y, Mizuno H, Sekine A, Yamanouchi M, Hasegawa E, Suwabe T, Urakami S, Kono K, Kinowaki K, Ohashi K, Yamaguchi Y and Ubara Y (2024) A case report of dipeptidyl peptidase 4 inhibitor-related kidney disease combined with renal cancer. Front. Nephrol. 4:1409098. doi: 10.3389/fneph.2024.1409098
Received: 23 April 2024; Accepted: 24 June 2024;
Published: 29 July 2024.
Edited by:
Vikram Sabapathy, University of Virginia, United StatesReviewed by:
Yoshimasa Aso, Dokkyo Medical University, JapanCopyright © 2024 Kurihara, Sawa, Sumida, Ikuma, Oba, Mizuno, Sekine, Yamanouchi, Hasegawa, Suwabe, Urakami, Kono, Kinowaki, Ohashi, Yamaguchi and Ubara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshifumi Ubara, dWJhcmFAdG9yYW5vbW9uLmdyLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.