
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nephrol. , 12 June 2024
Sec. Kidney Transplantation
Volume 4 - 2024 | https://doi.org/10.3389/fneph.2024.1403096
Introduction: Liver transplant recipients may have pre-formed anti-HLA antibodies directed to mismatched HLA of the liver donor (donor specific antibodies, DSA) or not directed to the liver donor (non-donor specific, non-DSA). We observed the fate of these antibodies (DSA and non-DSA) at 12 months after transplant.
Methods: Patients transplanted between 4/2015 and 12/2018 (N = 216) who had anti-HLA antibody measurements at both transplant and 12 months posttransplant (N = 124) and with DSAs at transplant (N = 31) were considered informative for a paired analysis of the natural history of DSA and non-DSA following liver transplantation.
Results: Class I DSAs and non-DSAs decreased between transplant and 12 months; however, Class I DSAs essentially disappeared by 12 months while Class I non-DSAs did not. Anti-HLA Class II DSAs performed differently. While there was a significant drop in values between transplant and 12 months, these antibodies mostly persisted at a low level.
Discussion: Our study demonstrated a significant difference in the kinetics of DSA compared to non-DSA following liver transplantation, most profoundly for anti-HLA Class I antibodies. Class I DSAs were mostly absent at 12 months while Class II DSAs persisted, although at lower levels. The mechanisms of reduction in anti-HLA antibodies following liver transplantation are not completely understood and were not pursued as a part of this study. This detailed analysis of Class I and Class II DSAs and non-DSAs represents and important study to explore the change in antibodies at one year from liver transplantation.
Patients undergoing liver transplantation may have developed circulating anti-HLA antibodies due to previous blood transfusions, a prior transplant, pregnancy or a combination of these. These preformed anti-HLA antibodies are either against mismatched HLA antigens of the liver donor (donor specific antibodies, DSA) or against other HLA antigens (non-donor specific, non-DSA). In general, donor specific anti-HLA antibodies have no impact on posttransplant outcomes (1–10). Exceptions may occur in second transplants and in patients with a high level of anti-HLA Class II (DP, DQ and DR) antibodies (11–32). Anti-HLA antibodies decrease in amount after liver transplantation (33–36) and Class I DSAs generally disappear (33). In contrast, Class II DSAs are more likely to persist following liver transplantation (35). The absence of consequences of DSA in liver transplantation as compared to kidney or heart transplantation is not fully understood. Frequently cited theories for this phenomenon include a very large capillary bed (100x greater in the liver than in kidneys) expressing HLA Class I antigens (37–39), and secretion of soluble HLA Class I and Class II antigens capable of binding donor specific antibodies (38).
The primary focus of previous studies of anti-HLA antibodies in liver transplant recipients has centered on the potential harm caused by DSAs. This was not the focus of our study although data are presented that confirm previous findings on graft survival. Rather, the primary aim of this study was to observe the fate of all anti-HLA antibodies following liver transplantation. We aimed to determine if there are different patterns of clearance of DSAs compared with non-DSAs and if these patterns suggest mechanisms of anti-HLA antibody clearance. To our knowledge, published accounts of a comparison between non-DSA and DSA in liver transplant recipients are absent in the literature. We assessed the fate, at 12 months after transplant, of anti-HLA antibodies present at transplant, assessing both donor specific and non-donor specific antibodies.
All patients who received a liver transplant at OHSU between April 2015 and December 2018 (N = 216) were considered in this IRB-approved retrospective study. Among those patients, only patients who had anti-HLA antibody measurements at the time of transplant and at 12 months posttransplant were considered informative (N = 124). Thirty-five patients had no testing, 49 patients had no testing at transplant and eight patients had testing at transplant but no testing at 12 months after transplant. Among the 124 patients, we studied patients with DSAs at transplant to fulfill our primary aim for a paired analysis of the natural history of DSAs and non-DSAs. For these thirty-one patients, some of whom had more than one DSA, we studied all DSAs present at transplant. For comparison, we studied up to five non-DSAs for each patient with a DSA. Table 1 lists the MFIs of the 39 Class I DSAs and 37 Class II DSAs at the time of transplant and at 12 months after transplant.
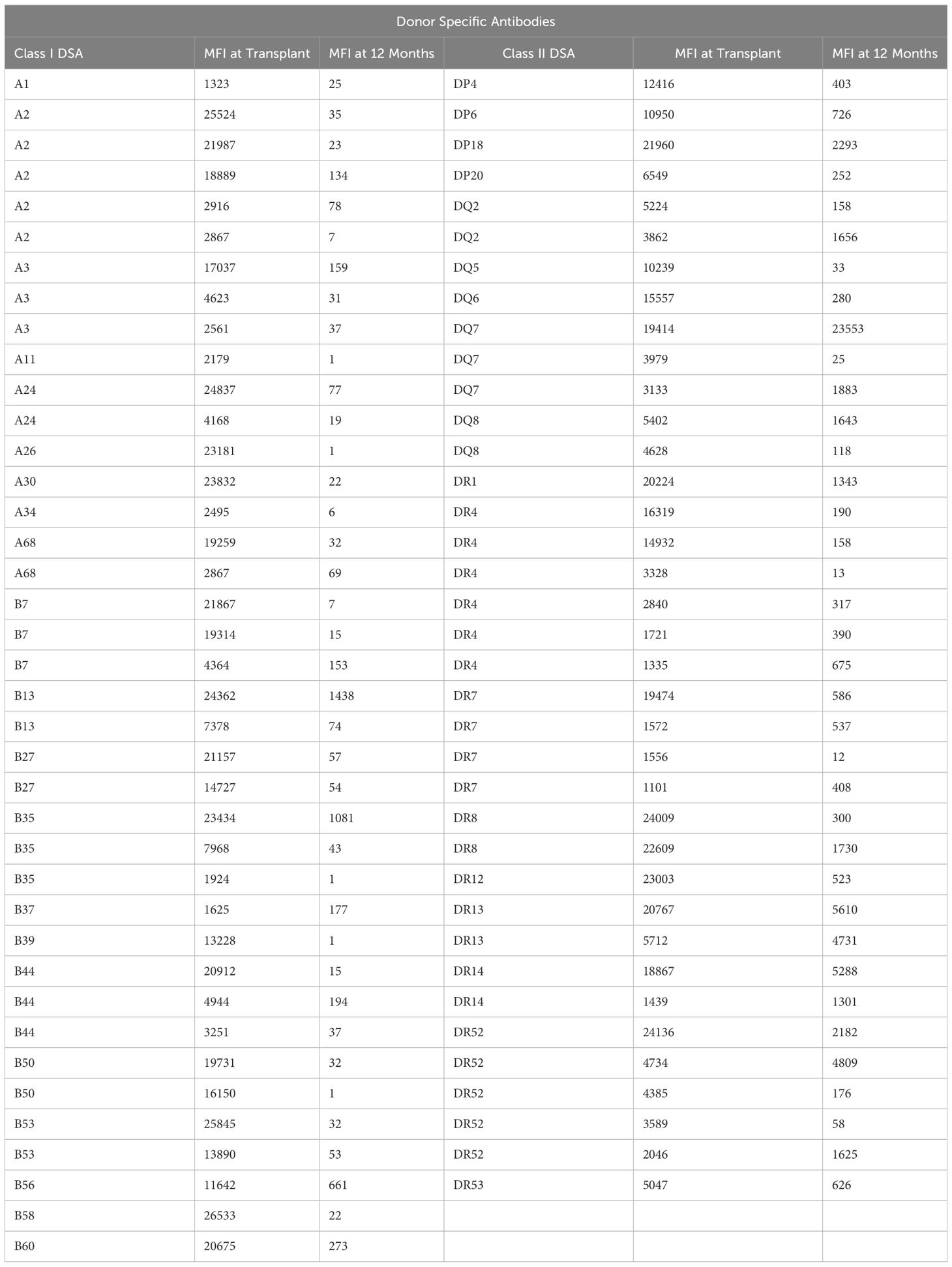
Table 1 MFI of the 39 Class I DSAs and 37 Class II DSAs at transplant and 12 months after transplant.
Table 2 lists the demographics of the patients included in the study. Differences were noted between patients with and without preformed anti-HLA antibodies at the time of transplant.
Molecular HLA typing of donors was performed at HLA-A, B, C, DRB1, DRB3,4,5, DQA1, DQB1 and DPB1 using LinkSeq ABCDRDQDP+ 384 Typing kit (One Lambda Thermo Fisher). Anti-HLA antibody detection was performed on EDTA-treated sera using Class I and II Labscreen Single antigen beads (One Lambda Thermo Fisher) on a Luminex platform. A normalized mean fluorescence intensity (MFI) of >1000 was considered positive for most antigens, while a cutoff of 2000 MFI was used for DPB1 with no common epitope reactivity patterns. MFI is a semi quantitative measure of the level of antibodies present. We considered antibodies to HLA A, B, DP, DQ and DR informative for our analysis. In identifying antibodies to study, we eliminated those considered false positive (cryptic epitope patterns, pan-DR, and common false positive beads not associated with epitopes).
We used a triple therapy immunosuppression regimen including tacrolimus, prednisone and azathioprine. Induction was typically limited to corticosteroids and occasional patients received mycophenolate mofetil instead of azathioprine.
We performed several different analyses using a Wilcoxon paired, non-parametric, two-tailed technique. We analyzed outcomes for changes in MFI between transplant and 12 months for the following: All DSA, all non-DSA, Class I DSA, Class I non-DSA, Class II DSA and Class II non-DSA. Patients served as their own controls when observing changes in DSA and non-DSA MFIs from transplant to 12 months. Percent reductions in MFI, between transplant and 12 months, for both DSA and non-DSA, were calculated and used for comparisons. For those patients who possessed both anti Class I and anti-Class II DSAs at transplant, the reduction in MFI from transplant to 12 months for Class I antibodies and Class II antibodies was also compared.
Thirty-one patients had 76 DSAs identified in the single antigen bead assays performed at transplant. Twenty-two patients had Class I DSAs (N = 39), and twenty patients had Class II DSAs (N = 37). In the same single antigen bead assays performed at transplant, we identified 103 Class I non-DSAs and 78 Class II non-DSAs for comparison. The non-DSAs chosen were those with the highest MFIs, if there were more than five non-DSAs. Eleven patients had both Class I and Class II DSAs.
DSAs and non-DSAs decreased significantly between transplant and 12 months. However, while DSAs mostly disappeared between transplant and 12 months, non-DSAs did not (Figure 1). The difference between DSA and non-DSA was most striking for Class I antibodies. The percent MFI reduction between transplant and 12 months was significantly greater for the Class I DSAs compared with the non-DSAs (Figure 2). Class II DSAs performed differently from Class I DSAs between transplant and 12 months. While there still was a significant drop in the MFI values between transplant and 12 months, these antibodies mostly persisted at a low level and the percent reduction of Class II DSA was not significantly different from the percent reduction of Class II non-DSA (Figure 3). The percent reduction for Class I DSA was significantly greater than for Class II DSA (Figure 4). Among the 11 patients with both Class I and II DSAs, the percent reduction in Class I was also significantly greater than for Class II.
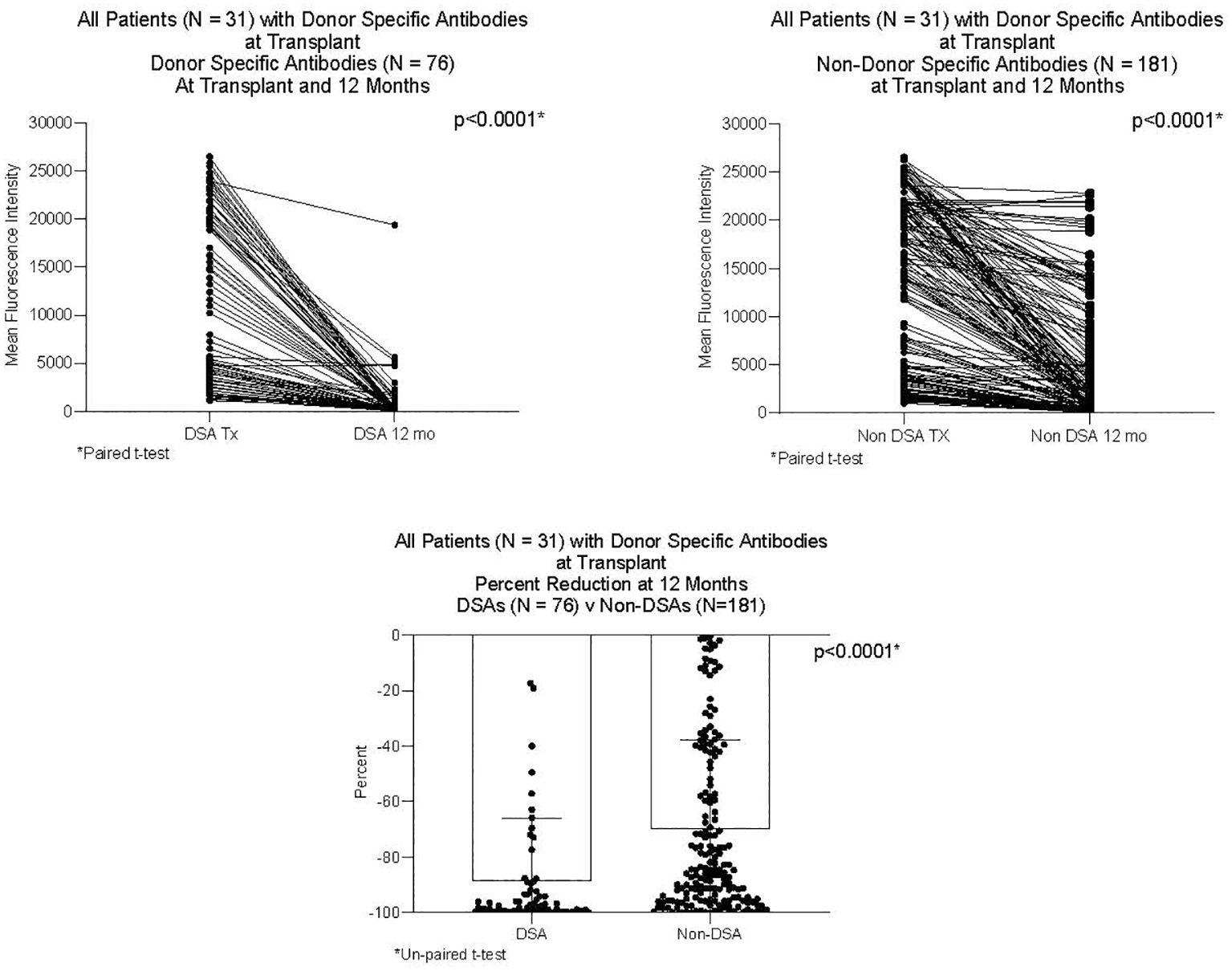
Figure 1 MFI of DSAs and non-DSAs at transplant and 12 months after transplant. Thirty-one patients had DSAs. The Figure compares seventy-six DSAs and 181 non-DSAs from those patients. Each line represents the value of an individual antibody at transplant and again at 12 months. There was a significant reduction in MFI by 12 months for both DSAs and non-DSAs, although the percent reduction for DSAs was significantly greater than for non-DSAs.
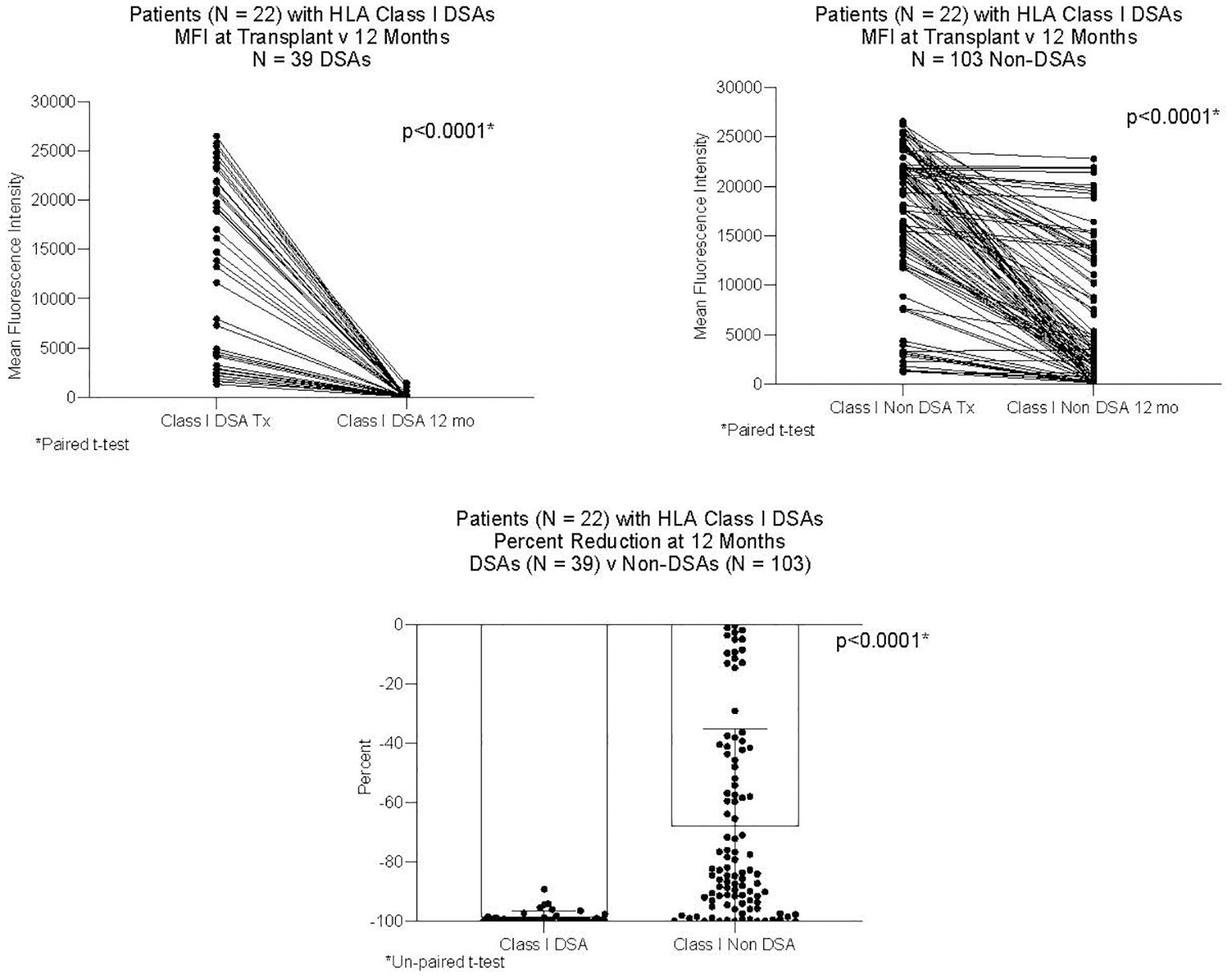
Figure 2 MFI of Class I DSAs and Class I non-DSAs at transplant and 12 months after transplant. Twenty-two patients had Class I DSAs. The Figure compares 39 DSAs and 103 non-DSAs from those patients. Each line represents the value of an individual antibody at transplant and again at 12 months. There was a significant reduction in MFI by 12 months for both DSAs and non-DSAs, although the percent reduction for DSAs was significantly greater than for non-DSAs.
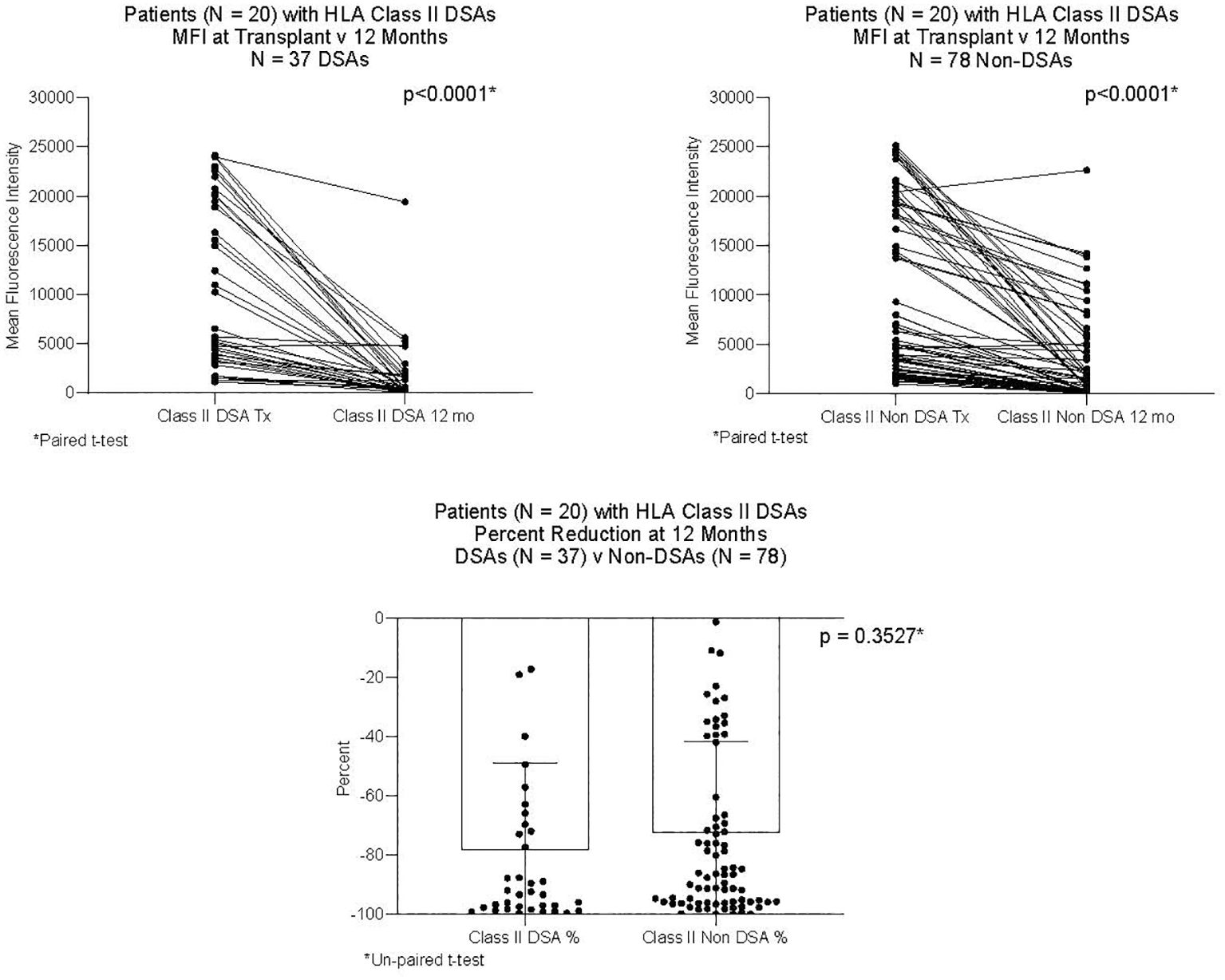
Figure 3 MFI of Class II DSAs and Class II non-DSAs at transplant and 12 months after transplant. Twenty patients had Class II DSAs at transplant. The Figure compares 37 DSAs and 78 non-DSAs from those patients. Each line represents the value of an individual antibody at transplant and again at 12 months. There was a significant reduction in MFI by 12 months for both DSAs and non-DSAs, although the percent reduction for DSAs was not different from that of non-DSAs.
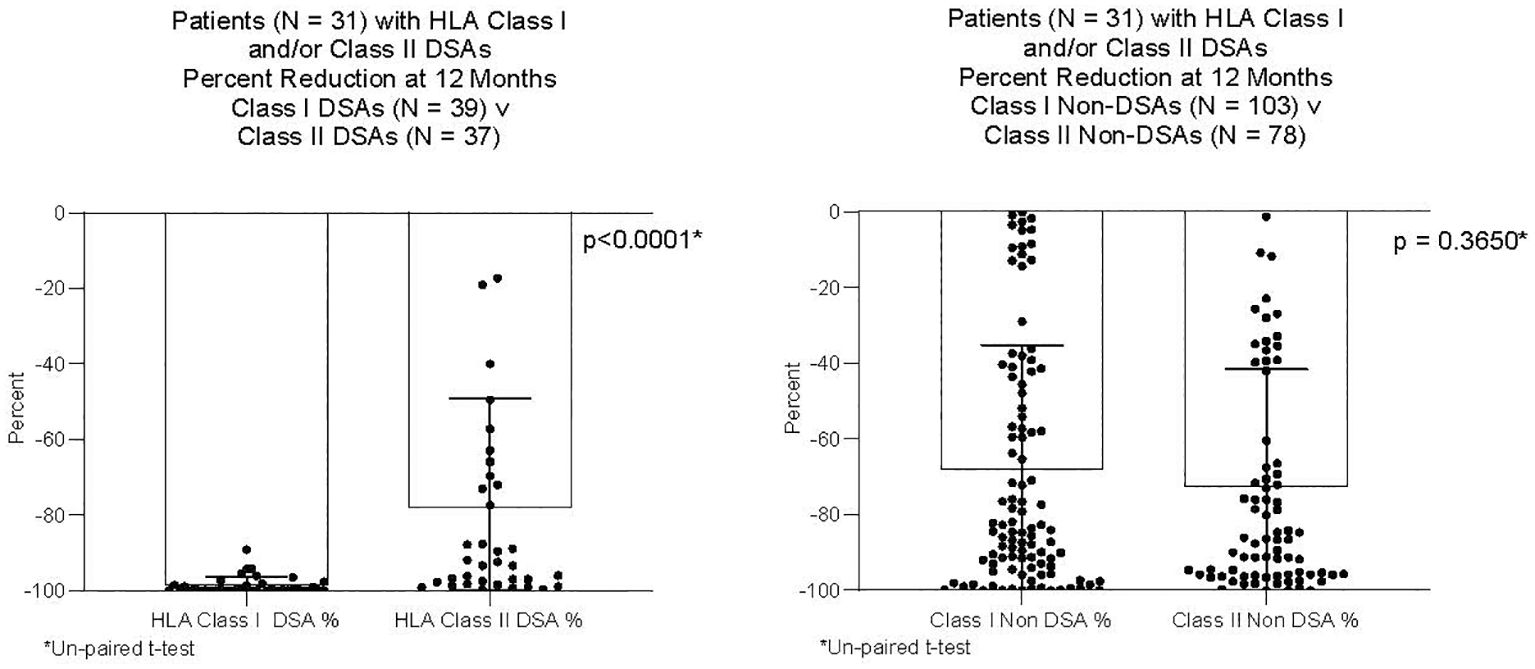
Figure 4 Percent reduction in MFI from transplant to 12 months after transplant. Thirty-one patients had Class I and/or Class II DSAs. The Figure compares 39 Class I DSAs to 37 Class II DSAs from those patients; and, 103 Class I non-DSAs to 78 Class II non-DSAs from those patients. The percent reduction was significantly greater for Class I DSAs compared to Class II DSAs. There was no difference in percent reduction between Class I non-DSAs and Class II non-DSAs.
Although it was not the primary focus of this study to analyze graft survival in relation to the presence of anti-HLA DSAs, we found that graft survival of patients with DSAs at transplant was similar to that of patients without DSAs at transplant (Figure 5). For this analysis, we studied only patients who survived the first year. Twelve of the 136 patients tested for the presence of antibodies at transplant failed before one year. Six patients died with function and six had graft failure. Four patients did not have anti HLA antibodies. Eight patients had anti HLA antibodies but none of these had DSAs.
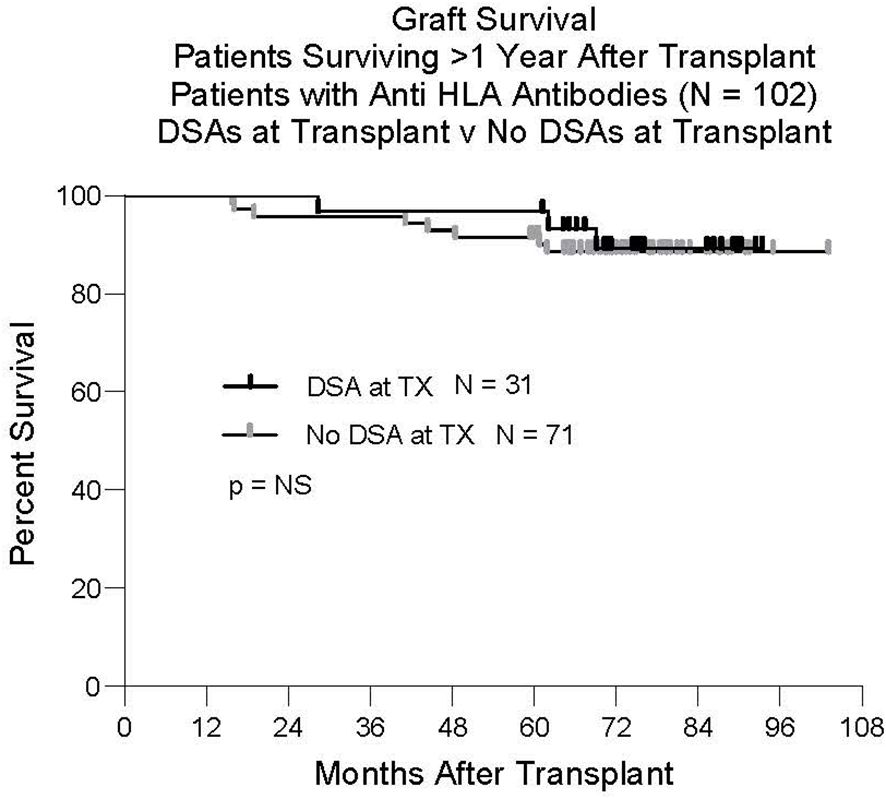
Figure 5 Graft survival. One-hundred-two patients had anti HLA antibodies at transplant. Thirty-one had Class I and/or Class II DSAs. Seventy-one did not have DSAs at transplant. There was no difference in outcome between these groups.
There are several overviews in the published literature documenting that DSAs tend to disappear after liver transplant (40–43). Using established DSA monitoring assays (44, 45), our study demonstrated a significant difference in the fate of DSAs compared to non-DSAs following liver transplantation. We used patients as their own controls when comparing the reduction in DSA to the reduction in non-DSA. The difference between DSA and non-DSA was most striking for anti-HLA Class I antibodies. There was also a significance difference between Class I and Class II DSAs. Class I DSA was mostly absent at 12 months while Class II DSA persisted, although at lower levels. The differential outcomes of Class I DSAs and Class II DSAs have been previously described (33) but not with the detailed comparison to non-DSAs and the large cohort present in this study.
The mechanisms of reduction in anti-HLA antibodies following liver transplantation are not completely understood and were not pursued as a part of this study. A reduction in non-DSA following liver transplantation is likely due to clearance of antibodies in concert with a natural decrease in the production of antibodies, enhanced by the use of immunosuppression. The further reduction in DSA is likely to be related to the liver allograft itself. While no mechanism has been proven, authors have speculated that the liver’s large capillary bed (21 square meters) contributes to the disappearance or reduction in DSA (37–39). HLA molecules expressed on endothelial cells in this large capillary bed could potentially bind donor specific anti-HLA antibodies. If so, this would have to occur without activating complement or propagating other inflammatory processes that could damage the liver. Another previously mentioned mechanism is the secretion of cell free HLA molecules by the liver that could bind donor specific antibodies in the circulation. The differential handling of DSA versus non-DSA also raises a possibility of tolerance induction. However, anti-Class I DSAs disappear in nearly all patients while tolerance (the ability to remove immunosuppression) occurs in only 10–15% of liver transplant recipients (46).
The reasons for the differential handling of anti-Class II antibodies compared with anti-Class I are not clear. If one implicates the binding and removal of antibodies by liver tissue as the primary mechanism, the absence of expression of Class II on most liver cells could explain the persistence of anti-Class II DSAs.
The strength of this observational study derives primarily from the large number of patients who had high titer donor specific antibodies at the time of transplant and who also had high titer non-donor specific antibodies. This study was only possible because it had been previously determined that DSAs have almost no adverse consequences in liver transplantation (confirmed by our analysis in the present study) and therefore DSAs are not assessed and avoided prior liver transplantation. Indeed, pre transplant crossmatches are only performed in liver transplantation when patients are considered for multi-visceral or other multi-organ transplants that include the liver or a retransplant is considered after graft failure from severe antibody mediated rejection. This is not true for other organs, in which donors with HLA antigens against which a recipient has antibodies are avoided because of their demonstrated adverse effects on outcomes (42).
A weakness of this study is the fact that it is retrospective and observational only. No attempt was made to determine the mechanisms of antibody reduction observed. Binding of antibodies to the endothelia of the large capillary beds of livers would require serial liver biopsies. The presence of secreted HLA molecules in liver transplant recipients and their potential for binding anti-HLA antibodies could be studied in the future. Donor-recipient interactions relative to gender, blood type, and age were also not considered for their impact on DSA and non-DSA expression. The cause of liver failure, while described as a demographic feature, was not analyzed as a predictor of de novo DSA or non-DSA. For instance, those patients with PSC or PBC as an indication for liver transplantation – both of which are considered on the auto-immune spectrum - may have a propensity for forming de novo antibodies.
This was a detailed assessment of DSA and non-DSA expression against both Class I and Class II HLA that explored the change in antibodies at one year from liver transplantation. A more complete picture of this temporal change is crucial as a first step toward understanding how the effects of antibody exposure to the liver graft evolves and should provide direction for future investigations. These efforts are particularly important as the transplant community develops a more nuanced understanding of AMR in liver transplantation.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Institution Review Board, Oregon Health & Science University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a retrospective study of data collected for clinical reasons and no PHI is included.
DN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CE: Formal analysis, Writing – review & editing. WN: Formal analysis, Writing – review & editing. RE: Data curation, Methodology, Writing – review & editing. CS: Data curation, Formal analysis, Methodology, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ciszek M, Foroncewicz B, Mucha K, Zochowska D, Ziarkiewicz-Wroblewska B, Krawczyk M, et al. Anti-HLA and anti-MICA antibodies in liver transplant recipients: effect on long-term graft survival. Clin Dev Immunol. (2013) 2013:1–5. doi: 10.1155/2013/828201
2. Donaldson PT, Thomson LJ, Heads A, Underhill JA, Vaughan RW, Rolando N, et al. IgG donor-specific crossmatches are not associated with graft rejection or poor graft survival after liver transplantation. Assess by cytotoxic Flow cytom Transplant. (1995) 60:1016–23. doi: 10.1097/00007890-199511000-00024
3. Grabhorn E, Binder TMC, Obrecht D, Brinkert F, Lehnhardt A, Herden U, et al. Long-term clinical relevance of de novo donor-specific antibodies after pediatric liver transplantation. Transplantation. (2015) 99:1876–81. doi: 10.1097/TP.0000000000000638
4. Grant L, Tujios S, Singal AG. Outcomes of simultaneous liver-kidney transplantation: implications for patient selection. Curr Opin Organ Transplant. (2018) 23:264–70. doi: 10.1097/MOT.0000000000000501
5. Hirata Y, Yoshizawa A, Egawa H, Ueda D, Okamoto S, Okajima H, et al. Impact of antibodies that react with liver tissue and donor-specific anti-HLA antibodies in pediatric idiopathic posttransplantation hepatitis. Transplantation. (2017) 101:1074–83. doi: 10.1097/TP.0000000000001653
6. Kim H, Yi N-J, Song EY, Lee K, Lee KW, Lee HW, et al. Preformed donor-specific antibodies do not affect the 1-year allograft survival in living donor liver transplantation. Clin Transplant. (2018) 32:e13244. doi: 10.1111/ctr.13244
7. Leca N, Warner P, Bakthavatsalam R, Nelson K, Halldorson J, Rayhill S, et al. Outcomes of simultaneous liver and kidney transplantation in relation to a high level of preformed donor-specific antibodies. Transplantation. (2013) 96:914–8. doi: 10.1097/TP.0b013e3182a192f5
8. Paterno F, Girnita A, Brailey P, Witte D, Wang J, Cuffy MC, et al. Successful simultaneous liver-kidney transplantation in the presence of multiple high-titered class I and II antidonor HLA antibodies. Transplant Direct. (2016) 2:e121. doi: 10.1097/TXD.0000000000000633
9. Perera MT, Silva MA, Murphy N, Briggs D, Mirza DF, Neil DH. Influence of preformed donor-specific antibodies and C4d on early liver allograft function. Scand J Gastroenterol. (2013) 48:1444–51. doi: 10.3109/00365521.2013.845795
10. Xu Q, Shrum B, Leckie S, Skaro A, McAlister VC. The impact of alloantibodies directed against the second donor on long-term outcomes of repeat liver transplantation. Hepatobil Surg Nutr. (2019) 8:246–52. doi: 10.21037/hbsn.2019.01.14
11. Castillo-Rama M, Castro MJ, Bernardo I, Meneu-Diaz JC, Elola-Olaso AM, Calleja-Antolin SM, et al. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: role of human leukocyte antigen compatibility. Liver Transpl. (2008) 14:554–62. doi: 10.1002/lt.21408
12. Del Bello A, Congy-Jolivet N, Danjoux M, Muscari F, Lavayssiere L, Esposito L, et al. De novo donor-specific anti-HLA antibodies mediated rejection in liver-transplant patients. Transpl Int. (2015) 28:1371–82. doi: 10.1111/tri.12654
13. Couchonnal E, Rivet C, Ducreux S, Dumortier J, Bosch A, Boillot O, et al. Deleterious impact of C3d-binding donor-specific anti-HLA antibodies after pediatric liver transplantation. Transpl Immunol. (2017) 45:8–14. doi: 10.1016/j.trim.2017.08.001
14. den Dulk AC, Shi X, Verhoeven CJ, Dubbeld J, Class FHJ, Wolterbeek R, et al. Donor-specific anti-HLA antibodies are not associated with nonanastomotic biliary strictures but both are independent risk factors for graft loss after liver transplantation. Clin Transplant. (2018) 32:e13163. doi: 10.1111/ctr.13163
15. Feng S, Demetris AJ, Spain KM, Kanaparthi S, Burrell BE, Ekong UD, et al. Five-year histological and serological follow-up of operationally tolerant pediatric liver transplant recipients enrolled in WISP-R. Hepatology. (2017) 65:647–60. doi: 10.1002/hep.28681
16. Iacob S, Cicinnati VR, Lindemann M, Heinemann FM, Radtke A, Kaiser GM, et al. Donor-specific anti-HLA antibodies and endothelial C4d deposition-association with chronic liver allograft failure. Transplantation. (2015) 99:1869–75. doi: 10.1097/TP.0000000000000613
17. Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients: de novo DSA in liver transplantation. Am J Transplant. (2013) 13(6):1541–8. doi: 10.1002/ajt.12212
18. Legaz I, Boix F, López M, Alfaro R, Galian JA, Llorente S, et al. Influence of preformed antibodies in liver transplantation. J Clin Med. (2020) 9(3):708–22. doi: 10.3390/jcm9030708
19. Leonard GR, Shike H, Uemura T, Gaspari JL, Ruggiero FM, Shah RA, et al. Liver transplantation with a strongly positive crossmatch: case study and literature review. Liver Transpl. (2013) 19:1001–10. doi: 10.1002/lt.23694
20. Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. (2011) 11:500–10. doi: 10.1111/j.1600-6143.2010.03414.x
21. Musat AI, Pigott CM, Ellis TM, Agni RM, Leverson GE, Powell AJ, et al. Pretransplant donor-specific anti-HLA antibodies as predictors of early allograft rejection in ABO-compatible liver transplantation. Liver Transpl. (2013) 19:1132–41. doi: 10.1002/lt.23707
22. Neau-Cransac M, Le Bail B, Guidicelli G, Visentin J, Moreau K, Quinart A, et al. Evolution of serum and intra-graft donor-specific anti-HLA antibodies in a patient with two consecutive liver transplantations. Transpl Immunol. (2015) 33:58–62. doi: 10.1016/j.trim.2015.08.002
23. O’Leary JG, Gebel HM, Ruiz R, Bray RA, Marr JD, Zhou XJ, et al. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant. (2013) 13:954–60. doi: 10.1111/ajt.12147
24. O’Leary JG, Kaneku H, Susskind BM, Jennings LW, Neri MA, Davis GL, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant. Am J Transplant. (2011) 11:1868–76. doi: 10.1111/j.1600-6143.2011.03593.x
25. O’Leary JG, Kaneku H, Jennings LW, Banuelos N, Susskind BM, Terasaki PI, et al. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. (2013) 19:973–80. doi: 10.1002/lt.23687
26. O’Leary JG, Klintmalm GB. Impact of donor-specific antibodies on results of liver transplantation. Curr Opin Organ Transplant. (2013) 18:279–84. doi: 10.1097/MOT.0b013e3283614a10
27. Shindoh J, Sugawara Y, Tamura S, Kaneko J, Yamashiki N, Hasegawa K, et al. Living donor liver transplantation for patients immunized against human leukocyte antigen. J Hepatobil Pancreat Sci. (2013) 20:279–85. doi: 10.1007/s00534–012-0511–0
28. Süsal C, Wettstein D, Döhler B, Morath C, Ruhenstroth A, Scherer S, et al. Association of kidney graft loss with de novo produced donor-specific and non-donor-specific HLA antibodies detected by single antigen testing. Transplantation. (2015) 99:1976–80. doi: 10.1097/TP.0000000000000672
29. Tamura K, Tohyama T, Watanabe J, Nakamura T, Ueno Y, Inoue H, et al. Preformed donor-specific antibodies are associated with 90-day mortality in living-donor liver transplantation. Hepatol Res. (2019) 49:929–41. doi: 10.1111/hepr.13352
30. Waki K, Sugawara Y, Mizuta K, Taniguchi M, Ozawa M, Hirata M, et al. Predicting operational tolerance in pediatric living-donor liver transplantation by absence of HLA antibodies. Transplant J. (2013) 95:177–83. doi: 10.1097/TP.0b013e3182782fef
31. Wozniak LJ, Hickey MJ, Venick RS, Vargas JH, Framer DG, Busuttil RW, et al. Donor-specific HLA antibodies are associated with late allograft dysfunction after pediatric liver transplantation. Transplantation. (2015) 99:1416–22. doi: 10.1097/TP.0000000000000796
32. Wozniak LJ, Naini BV, Hickey MJ, Bhattacharyya S, Reed EF, Busuttil RW, et al. Acute antibody-mediated rejection in ABO-compatible pediatric liver transplant recipients: case series and review of the literature. Pediatr Transplant. (2017) 21:e12791. doi: 10.1111/petr.12791
33. Dar W, Agarwal A, Watkins C, Gebel HM, Bray RA, Kokko KE, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. (2011) 11:841–7. doi: 10.1111/j.1600-6143.2011.03467.x
34. Lai C, Newman A, Mawson J, Abou-Daher, Watson N, Majumdar A, et al. Rapid reduction of high-level pre-formed donor-specific antibodies after simultaneous liver-kidney transplantation: a report of two cases. BMC Nephrol. (2020) 21:47. doi: 10.1186/s12882-020-01714-y
35. Taner T, Gandhi MJ, Sanderson SO, Poterrucha CR, De Foey SR, Stegall MD, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. (2012) 12:1504–10. doi: 10.1111/j.1600-6143.2012.03995.x
36. Tokodai K, Kawagishi N, Miyagi S, Nakanishi C, Hara Y, Fujuo A, et al. The significance of screening for HLA antibodies in the long-term follow-up of pediatric liver transplant recipients. Transplant Proc. (2016) 48:1139–41. doi: 10.1016/j.transproceed.2015.12.081
37. Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. (1988) 8:449–54. doi: 10.1002/hep.1840080302
38. O’Leary JG, Demetris AJ, Friedman LS, Gebel HM, Halloran PF, Kirk AD, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. (2014) 14:779–87. doi: 10.1111/ajt.12667
39. Steinhoff G, Wonigeit K, Pichlmayr R. Analysis of sequential changes in major histocompatibility complex expression in human liver grafts after transplantation. Transplantation. (1988) 45:394–401. doi: 10.1097/00007890–198802000–00030
40. Cheng EY. The role of humoral alloreactivity in liver transplantation: lessons learned and new perspectives. J Immunol Res. (2017) 2017:3234906. doi: 10.1155/2017/3234906
41. Demetris AJ, Zeevi A, O’Leary JG. ABO-compatible liver allograft antibody-mediated rejection: an update. Curr Opin Organ Transplant. (2015) 20:314–24. doi: 10.1097/MOT.0000000000000194
42. Everly MJ. Update on alloantibodies in solid organ transplantation. Clin Transpl. (2014), 125–9.
43. Vandevoorde K, Ducreux S, Bosch A, Guillaud O, Hervieu V, Chambon-Augoyard C, et al. Prevalence, risk factors, and impact of donor-specific alloantibodies after adult liver transplantation. Liver Transpl. (2018) 24:1091–100. doi: 10.1002/lt.25177
44. Monteiro F, Rodrigues H, Kalil J, Castro MC, Panajotopoulos N, Paredes M, et al. Pre- and posttransplant monitoring of alloantibodies by complement-dependent cytotoxicity and luminex methodologies in liver transplantation. Transplant Proc. (2012) 44:2411–2. doi: 10.1016/j.transproceed.2012.07.005
45. Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant. (2013) 13:1859–70. doi: 10.1111/ajt.12287
Keywords: liver transplant, anti HLA antibodies, donor specific antibodies, HLA class I and class II typing, liver graft survival
Citation: Norman DJ, Enestvedt CK, Naugler WE, Erhan R and Shaut CA (2024) The fate of anti-HLA antibodies following liver transplantation. Front. Nephrol. 4:1403096. doi: 10.3389/fneph.2024.1403096
Received: 18 March 2024; Accepted: 13 May 2024;
Published: 12 June 2024.
Edited by:
Masoud Sadeghi, Islamic Azad University, IranReviewed by:
Ilias Doxiadis, University Hospital Leipzig, GermanyCopyright © 2024 Norman, Enestvedt, Naugler, Erhan and Shaut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Douglas J. Norman, bm9ybWFuZEBvaHN1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.