- 1Department of Medicine, Division of Nephrology, Loma Linda University School of Medicine, Loma Linda, CA, United States
- 2Department of Medicine, Division of Nephrology, University of Wisconsin, Madison, WI, United States
- 3Department of Medicine, Division of Kidney Diseases and Hypertension, Zucker School of Medicine at Hofstra/Northwell, Northwell Health, Great Neck, NY, United States
- 4Department of Medicine, Division of Nephrology, University of Cincinnati College of Medicine, Cincinnati, OH, United States
- 5Department of Medicine, Division of Nephrology, Advocate Christ Medical Center, University of Illinois at Chicago, Oak Lawn, IL, United States
Lupus nephritis (LN), a severe complication of systemic lupus erythematosus (SLE), leads to significant kidney inflammation and damage and drastically increases mortality risk. Predominantly impacting women in their reproductive years, LN poses specific risks during pregnancy, including pre-eclampsia, growth restrictions, stillbirth, and preterm delivery, exacerbated by lupus activity, specific antibodies, and pre-existing conditions like hypertension. Effective management of LN during pregnancy is crucial and involves carefully balancing disease control with the safety of the fetus. This includes pre-conception counseling and a multidisciplinary approach among specialists to navigate the complexities LN patients face during pregnancy, such as distinguishing LN flare-ups from pregnancy-induced conditions. This review focuses on exploring the complex dynamics between pregnancy and LN, emphasizing the management difficulties and the heightened risks pregnant women with LN encounter.
Introduction
Lupus nephritis (LN) is a severe manifestation of systemic lupus erythematosus (SLE), characterized by inflammation and kidney damage. Prevalence rates in SLE patients range from 20% to 65%, with LN associated with higher mortality compared to the general population (1–3). It predominantly affects women in their reproductive years, presenting unique challenges during pregnancy (4). Pregnant women with LN are at increased risk for complications like pre-eclampsia, intrauterine growth restriction, stillbirth, and preterm delivery (5). The risk is further amplified by factors such as lupus activity, the presence of specific antibodies (like Antiphospholipid antibody (APA), anti-SSA/Ro, and anti-SSB/La), and pre-existing conditions like hypertension (6). The differentiation between an LN flare and pregnancy-induced hypertension or proteinuria can be challenging but is crucial for appropriate management.
Lupus nephritis involves the deposition of immune complexes in the kidneys, triggering a cascade of inflammatory responses, including local complement activation, leukocyte recruitment, and cytokine signaling, leading to glomerular and tubulointerstitial injury. This inflammatory process leads to kidney damage presenting as proteinuria, acute kidney injury, chronic kidney disease (CKD), and eventually, in some cases, end-stage kidney disease (ESKD).
The progression of LN to ESKD is influenced by various factors, including the initial nephron number (determined at birth), episodes of nephron loss (during LN flare), and the rate of nephron loss beyond physiological aging (ongoing inflammation due to LN) or in some cases concomitant uncontrolled diabetes mellitus, and/or hypertension (6). Flares of LN are episodes of acute kidney injury that result in irreversible nephron loss, shortening the kidney lifespan. Persistent or smoldering LN, even with induction therapy, accelerates the annual rate of nephron loss, hastening the onset of ESKD (7, 8).
Effective management of LN during pregnancy is pivotal to minimizing maternal and fetal risks. Tailoring treatment strategies that balance disease control and fetal safety is imperative. Pre-conception counseling and multidisciplinary care involving rheumatologists, nephrologists, and obstetricians are vital to optimize outcomes. Monitoring and managing blood pressure, kidney function, and lupus activity throughout pregnancy and postpartum safeguard maternal and fetal health (Table 1). The management strategy should also consider the potential impact of medications on the fetus and adjust them accordingly to ensure safety while maintaining disease control.
This review aims to comprehensively explore the complex interplay between pregnancy and LN, shedding light on the increased risks and challenges encountered by pregnant women with LN.
Preconception counseling and risk assessment for women with lupus nephritis
Preconception counseling
Preconception counseling is pivotal for women with LN to navigate through the complexities of pregnancy safely. Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus (PROMISSE) study findings indicated that LN relapse rate of 7.8% of patients in complete remission and 21% of those in partial remission during pregnancy (9). Current evidence has shown that SLE patients without LN flare in the pre-conception period have a small risk of relapse during pregnancy (10–12). The reduction in SLE flare rates during pregnancy may be attributed to thorough pre-pregnancy counseling, diligent management, and continuous monitoring, which includes adjusting medications for maternal and fetal safety and identifying signs of disease activity early. This leads to the sustained use of suitable medications like hydroxychloroquine, effectively lowering flare risks (13). The optimal time for a woman with LN to conceive is generally during a period of stable disease remission, typically sustained for at least 6 months (14). Specifically, Attia et al. observed that only 12.5% of patients with inactive LN in the six months preceding conception experienced LN relapse during pregnancy. In contrast, this incidence was significantly higher (81.3%) in patients with active LN (15). However, the time between LN flare remission and the conception period could be shorter if the disease flare is mild, but a careful and individualized assessment is crucial.
Assessing disease activity and organ involvement
Before conception, a thorough evaluation of disease activity and organ involvement is imperative. This involves:
● Disease Activity: Ensuring SLE and LN are in remission or low activity. The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) is a concise tool that assesses SLE activity over the previous ten days, incorporating clinical symptoms and laboratory findings such as skin rashes, arthritis, kidney function, neurological symptoms, blood abnormalities, and immunological markers (16) (Table 2). It offers a global score that reflects disease severity, proving to be an excellent, easy-to-use method with strong psychometric properties for evaluating disease activity (18). Higher scores indicate more active disease, guiding treatment decisions and monitoring progression or remission effectively. This score is essential in pregnancy, as it can help determine the risk level for pregnant patients with SLE and LN. A SLEDAI score of 4 or more or a history of LN six months before conception has been shown to predict adverse maternal outcomes (19).
● Kidney Function: Monitoring serum creatinine levels (Normal range: 0.6 to 1.1 mg/dL) urinalysis with urine sediment, and spot urine protein/creatinine ratio (A ratio of less than 0.2 mg/mg or <200 mg/g are considered normal).
● Other Organ Involvement: In addition to renal involvement, LN frequently affects other organ systems, notably the cardiovascular, neuropsychiatric, and pulmonary systems. To accurately assess and document the extent of tissue damage in these systems, specific diagnostic approaches are recommended:
● Pulmonary assessment: The utilization of chest X-ray, High resonance computed topography, pulmonary angiography, and pulmonary function tests is crucial in identifying conditions such as pulmonary hypertension, fibrosis, and pleural fibrosis.
● Cardiovascular assessment: Echocardiography serves as the primary tool for detecting abnormalities, including valvular diseases and pericarditis. In cases where there is suspicion or evidence of cardiovascular disease, further investigation through angiography may be warranted.
● Neuropsychiatric assessment: Paying attention to the patient’s current medications, particularly those targeting psychological or neurological symptoms. Active seizures and their treatment status are of particular concern, requiring thorough evaluation and management.
● Laboratory Testing: Including anti-Ro/SSA, anti-La/SSB antibodies, Complete blood count, liver function tests, anti-dsDNA antibodies, and complement levels.
● Antiphospholipid Antibodies: Evaluating for IgG and IgM anticardiolipin (aCL) antibodies, IgG and IgM anti-beta2 glycoprotein I (anti-beta2GPI) antibodies, and lupus anticoagulant (LA) especially if there is a history of thrombotic events or pregnancy-related complications.
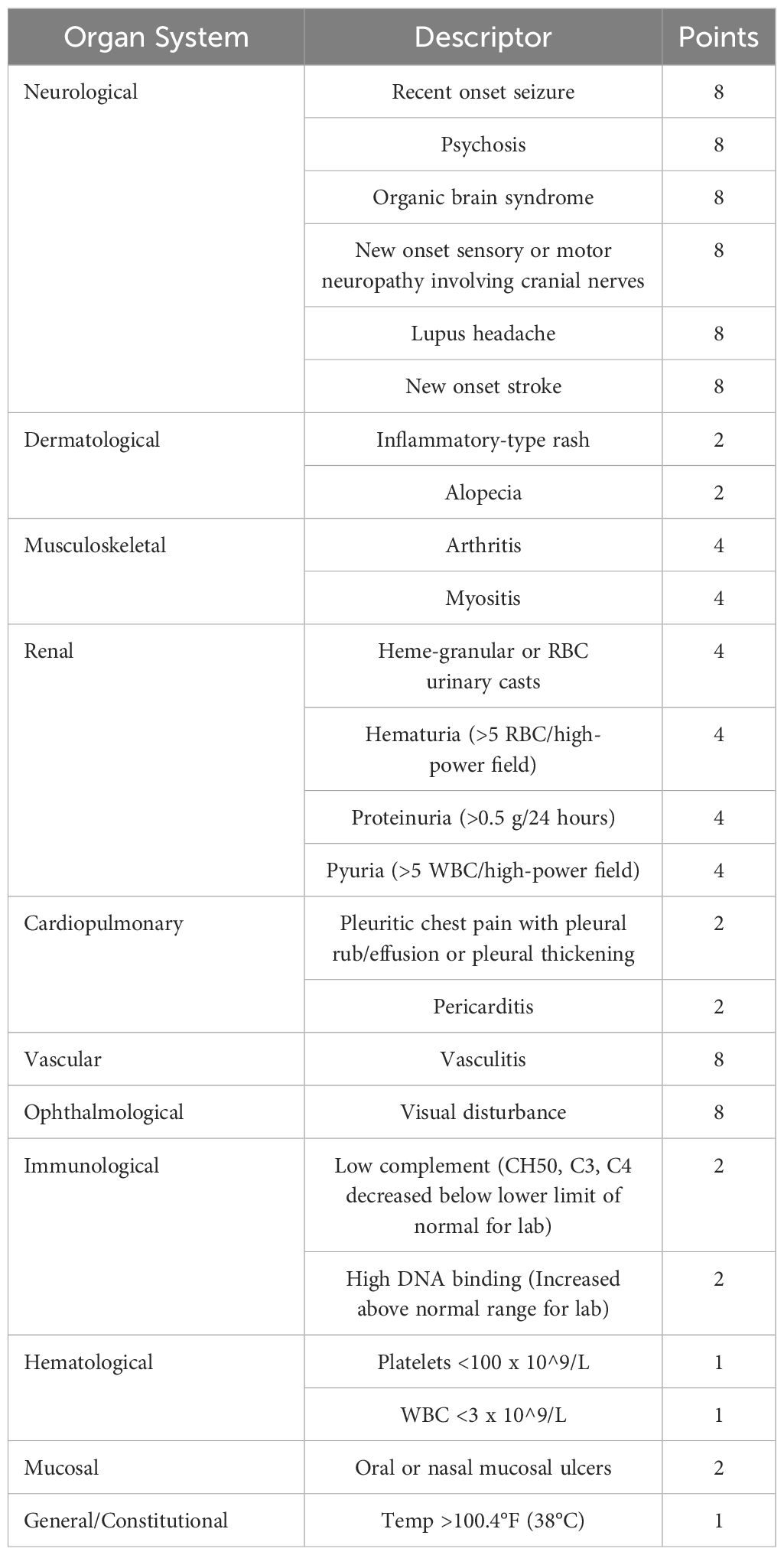
Table 2 SLEDAI-2K score descriptors grouped by organ systems (17).
Identifying potential risks and complications
Identifying and discussing potential risks and complications during pregnancy is crucial. This includes:
● LN Flares: Understanding that LN flares can occur during pregnancy and postpartum.
● Preeclampsia: Women with LN have an increased risk of developing preeclampsia and should be monitored closely.
● Fetal Risks: Discuss risks like preterm birth, intrauterine growth restriction, and neonatal lupus, especially if anti-Ro/SSA and/or anti-La/SSB antibodies are present.
● Medication Management: Ensuring medications are adjusted to be safe during pregnancy and breastfeeding while maintaining disease control.
Medication management in preconception and during pregnancy
Lupus nephritis management involves various medications to control disease activity and protect kidney function. Pregnancy necessitates a careful review of medications due to potential risks to the fetus. Medications should be adjusted to those that are safe during pregnancy while still effectively managing LN. Adjustments to medication regimens should be individualized, considering both the mother’s health and fetal safety. Active disease management during pregnancy might require modifying treatment plans while minimizing risks. A brief overview of the medications commonly utilized in the treatment of SLE and LN is provided in the following sections (Figure 1).
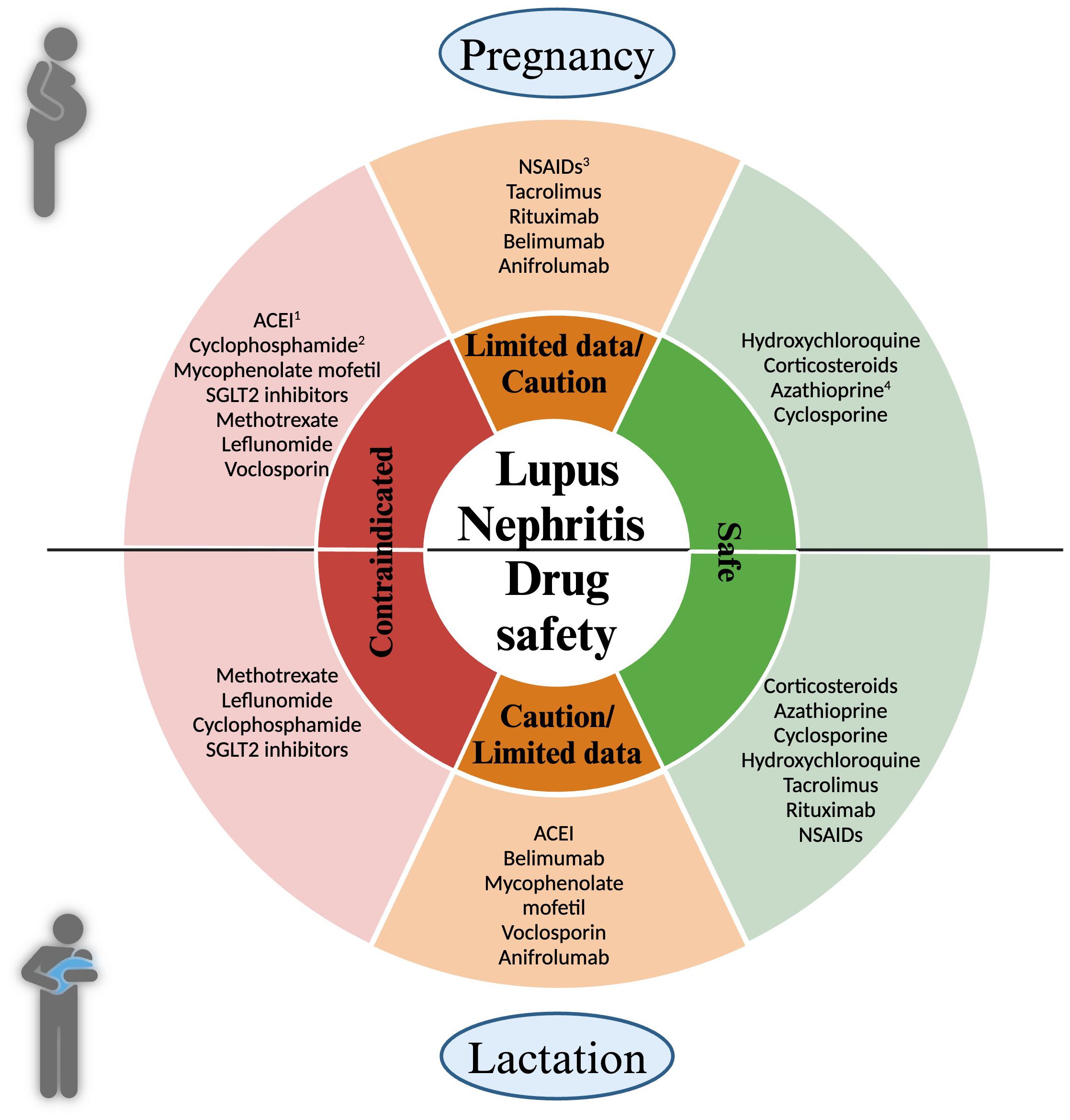
Figure 1 Medication Safety in Pregnancy and Lactation for Lupus Nephritis Patients. This figure delineates the safety of medications used in managing lupus nephritis during pregnancy (top half) and lactation (bottom half). Medications are categorized into three distinct groups: Green: safe; Orange: drugs requiring caution or with limited; Red: contraindicated. During pregnancy: 1. ACEI (Angiotensin-converting enzyme inhibitor): Contraindicated in the second and third trimester due to fetal renal effects; 2. Cyclophosphamide: Its use may be justified in severe relapses in the 2nd and 3rd trimester, 3. NSAIDs (Nonsteroidal Anti-inflammatory Drugs): Caution in the first trimester for women having difficulty conceiving; Increased risk of oligohydramnios after 20 weeks; 4. Azathioprine: Doses should not exceed 2 mg/kg/day.
Recommended during pregnancy
Hydroxychloroquine
Hydroxychloroquine (HCQ) effectively reduces the risk of SLE during pregnancy, as demonstrated in multiple studies (20–23). Hydroxychloroquine also enhances renal remission rates and lowers the risk of renal flares in lupus nephritis patients. Studies have shown that patients with Class V lupus nephritis on HCQ have significantly higher rates of complete renal remission than those without HCQ (24).
This was supported by further evidence indicating that antimalarial treatment more than doubled the complete remission rates in Class V nephritis patients (25). Additionally, HCQ was associated with a 60% higher chance of achieving complete response in patients with proliferative forms of the disease (26). A retrospective cohort study also found an increased likelihood of renal recovery among lupus nephritis patients with renal impairment on HCQ (27). Moreover, a study on patients with quiescent lupus demonstrated that continuing HCQ reduced the risk of renal flares over three years (28).
However, while traditionally considered safe during pregnancy, a study revealed a slight elevation in the risk of congenital anomalies associated with a daily HCQ dosage exceeding 400 mg in the first trimester. However, this correlation did not emerge when the dosage remained below 400 mg (29). Additionally, there’s potential evidence for reducing the incidence of congenital heart block among at-risk fetuses from mothers with anti-Ro/SSA and anti-La/SSB antibodies when exposed to HCQ (30). In addition to the previously discussed studies, recent research has further reinforced the favorable impact of hydroxychloroquine (HCQ) on pregnancy outcomes in women with lupus nephritis. The study by Balevic et al. demonstrated that maintaining HCQ adherence significantly reduced the odds of preterm birth, highlighting the importance of medication compliance (31). Additionally, findings from Rahman et al. underscored the broader benefits of HCQ, revealing improved neonatal outcomes and a reduction in adverse pregnancy outcomes and antenatal complications such as hypertension and diabetes (32). Together, these studies provide compelling evidence for the continued use of HCQ in pregnant individuals with lupus nephritis to optimize both maternal and neonatal health.
Low-dose aspirin
In the management of SLE during pregnancy, the initiation of low-dose aspirin around the 12th week of gestation is recommended to minimize the risk of preeclampsia and its complications, irrespective of the presence of antiphospholipid antibodies (aPLs). This approach, endorsed by the United States Preventive Services Task Force, is particularly crucial for pregnant women at high risk for preeclampsia, a category that includes those with SLE (33).
Recent studies further contribute to understanding aspirin’s role during pregnancy in SLE patients. A comprehensive analysis by Tani et al., involving 216 pregnancies, found that while initiating low-dose aspirin did not significantly alter overall complication rates, it showed promise in potentially lowering the frequency of pre-eclampsia (34). Additionally, Zhang et al.’s study demonstrated that combining hydroxychloroquine with aspirin significantly improved pregnancy outcomes, suggesting aspirin as a valuable adjunctive therapy for pregnant women with SLE (34, 35).
Selective use allowed during pregnancy
Nonsteroidal anti-inflammatory drugs
NSAIDs pose considerations during pregnancy, with evidence showing no link to congenital anomalies (36–38). However, uncertainty persists regarding their association with spontaneous abortion in the first trimester (39, 40). Caution is advised beyond 20 weeks due to a small risk of oligohydramnios (41).
The FDA recommends the lowest NSAID dose between 20 and 30 weeks, with avoidance after 30 weeks to prevent complications, including premature closure of the ductus arteriosus (42, 43).
Generally, NSAIDs, including Cox-1 and Cox-2 inhibitors, are discouraged in the third trimester due to heightened risks of adverse outcomes like ductus arteriosus closure.
In the postpartum period, the use of NSAIDs does not lead to an increase in blood pressure in women who have experienced hypertensive disorders during pregnancy (44, 45).
Glucocorticoids
Glucocorticoids may be cautiously used during pregnancy to manage lupus nephritis flares if clinically appropriate (46, 47). It is widely acknowledged that treating severe diseases may warrant higher doses. Inflammation resulting from unmanaged autoimmune conditions can be more detrimental to the health of both mother and fetus than the impact of high-dose steroids (45). A study by Shimada et al. showed that an even lower dose of glucocorticoid (mean dose of 6.5 mg/day) during pregnancy was strongly linked to adverse pregnancy outcomes (48). This emphasizes the importance of carefully managing glucocorticoid use in SLE patients during pregnancy to mitigate these risks.
Azathioprine
Azathioprine (AZA) is considered a suitable option during pregnancy when benefits outweigh potential risks. Comprehensive studies have not shown a significant correlation between AZA use during pregnancy and the occurrence of congenital anomalies (49, 50). It is essential to note that AZA is compatible with pregnancy (5), but caution should be exercised to ensure that doses do not exceed 2 mg/kg/day. Despite these considerations, AZA is generally recommended to be continued during pregnancy.
Cyclosporine
Preliminary data indicate that children who were exposed to cyclosporine in utero have normal kidney function. However, its administration during pregnancy should be limited to situations where the maternal benefit outweighs potential risks to the fetus (51, 52). Classified as FDA category C, cyclosporine may be considered an acceptable alternative in specific circumstances where the potential benefits justify the potential risks. Its use should be carefully evaluated, particularly in autoimmune diseases refractory to conventional treatment (53).
Tacrolimus
Tacrolimus, employed in both induction and maintenance therapy for lupus nephritis, has demonstrated effectiveness during pregnancy (54). A recently published case series reported successful management of lupus nephritis flares with tacrolimus in pregnant individuals. In another study involving 54 deliveries in SLE patients, comparable pregnancy outcomes were observed between those exposed to tacrolimus and those who were not (55).
Despite the limited number of fetuses exposed, a definitive causal link between tacrolimus use and congenital anomalies has not been established. Reports on using tacrolimus to treat lupus nephritis during pregnancy are limited, but a small study indicated that mothers on tacrolimus had healthy newborns (56).
Antihypertensive medications
Methyldopa, nifedipine, and labetalol are safe antihypertensive options in pregnant patients (57, 58). In contrast, the use of angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers is unequivocally contraindicated during pregnancy due to the increased risk of adverse pregnancy outcomes associated with their administration (59). While diuretics may be considered with caution, careful and deliberate use is imperative.
Selective use with caution in pregnancy
Biologic medications
The use of biologic medications, like rituximab, during pregnancy is constrained by limited data. Given that IgG does not significantly cross the placenta until the 15th week of gestation, these medications can be continued through conception. In cases of maintenance treatment, rituximab is often strategically administered just before conception or in the first trimester (60, 61). Notably, for severe lupus nephritis flares, rituximab is increasingly considered, with the RITUXILUP study suggesting a potential role as a steroid-sparing agent (62). Additionally, it should not be used in the third trimester due to the risk of neonatal B cell depletion (63). However, caution is advised, and further research is warranted to assess risks and benefits comprehensively.
Belimumab, a novel BAFF inhibitor recently introduced, has undergone studies during pregnancy. Current research has not indicated an increased risk of fetal anomalies or severe infection (64–66). Nevertheless, cautious monitoring is advised, and additional data are required to validate its safety in pregnant patients fully.
Contraindicated in pregnancy
Cyclophosphamide
is associated with fetal loss and congenital malformations. Therefore, it is recommended to refrain from its use during the first 10 weeks of gestation, a period marked by heightened fetal susceptibility to teratogens (67). However, in life-threatening clinical scenarios, this medication has been employed during later stages of pregnancy (68).
Mycophenolate mofetil
Avoidance of this medication is imperative during pregnancy due to its association with increased risks of first-trimester pregnancy loss and a heightened incidence of congenital malformations (69).
To mitigate these risks, it is recommended to discontinue mycophenolate at least 6 weeks before conception (70, 71). Alternatively, azathioprine or tacrolimus can be substituted for mycophenolate before and during pregnancy. Another viable option involves using glucocorticoids at the minimal effective dose for controlling disease activity. Ideally, this transition in medication should be implemented four to six months before the intended conception, ensuring a proactive approach to managing potential adverse outcomes.
Methotrexate
Administering methotrexate before and during pregnancy carries the potential for lasting consequences on fetal growth (72). To avert adverse effects, it is advised to cease methotrexate use within the window of one to three months preceding conception.
Leflunomide
It is cautioned to be avoided during pregnancy based on the results of preclinical reproductive studies, although its definitive teratogenicity in humans remains uncertain. For those desiring conception while on this medication, a cholestyramine washout is recommended until blood levels reach undetectable concentrations (73, 74).
Voclosporin
The utilization of voclosporin in pregnant women is discouraged due to its formulation containing alcohol. The presence of alcohol during pregnancy is correlated with potential adverse effects on fetal development, including abnormalities in the central nervous system and potential compromise of intellectual development (75).
Anifrolumab
Current data is limited, making it inadequate for a comprehensive assessment of potential drug-related risks, including significant congenital abnormalities, spontaneous pregnancy loss, or unfavorable consequences for both maternal and fetal health (75).
SGLT2 inhibitors
The role of SGLT2 inhibitors in managing LN presents a nuanced picture. These medications have shown renoprotective effects in lupus models, suggesting potential as non-immunosuppressive agents to enhance renal function in autoimmune kidney diseases like LN (76).
A phase I/II trial indicated that dapagliflozin, an SGLT2 inhibitor, has an acceptable safety profile when used as an add-on therapy in adult SLE patients. However, it did not effectively reduce disease activity or proteinuria in LN patients (77). Their use in pregnancy is cautioned against due to associated risks such as dilation of renal pelvis and tubules, congenital anomalies, and a higher incidence of miscarriages (78). The recommendation is to avoid these drugs during pregnancy and lactation, especially considering the availability of safer alternatives.
Management during pregnancy
The care of pregnant individuals with LN necessitates a coordinated effort among a rheumatologist, nephrologist, and an obstetrician well-versed in managing high-risk pregnancies. A comprehensive strategy for overseeing pregnant women with LN, along with addressing active LN flare-ups during pregnancy, is outlined in the subsequent sections.
Monitoring LN activity
We recommend closely monitoring LN activity during pregnancy, necessitating regular assessments by a nephrologist every trimester. For individuals with active SLE, a higher frequency of evaluations is advised. The structured schedule for monitoring comprises the following key elements:
Initial evaluation
This initial evaluation, as recommended by prior studies (79, 80), should encompass the following investigations:
● Physical examination and vital signs measurement.
● Complete blood count (CBC).
● Kidney function tests (serum creatinine, urinalysis, spot urine protein/creatinine ratio).
● Liver function tests.
● Anti-Ro/SSA and anti-La/SSB antibodies, if not previously assessed.
● Complement (CH50, or C3 and C4).
● Anti-double-stranded DNA (dsDNA) antibodies.
● Antiphospholipid antibodies (lupus anticoagulant [LA], IgG and IgM anticardiolipin [aCL] antibodies, and IgG and IgM anti-beta2 glycoprotein [anti-beta2GPI] antibodies).
During pregnancy, specific physiological alterations can coincide with manifestations of active LN, posing challenges in differentiation. For instance, common laboratory indicators seen in a typical pregnancy involve an increased erythrocyte sedimentation rate (ESR) and proteinuria. Notably, there is an increase in protein excretion during normal pregnancy; however, it should not exceed 300 mg/24 hours. To differentiate between an LN flare, preeclampsia, and routine pregnancy-related alterations in later stages, a baseline 24-hour urine collection or urine protein to creatinine ratio proves valuable, especially after stopping ACE-I/ARB.
Consequently, interpreting laboratory results necessitates a comprehensive consideration of the clinical context. Women exhibiting increased serologic activity without accompanying symptoms require vigilant monitoring. It is not recommended to initiate immunosuppressants based solely on serologic findings.
Laboratory assessments during pregnancy
Throughout pregnancy in individuals with lupus nephritis, routine laboratory assessments are pivotal for monitoring maternal and fetal well-being. A comprehensive evaluation, including a physical examination with blood pressure measurement, is essential. Regular intervals necessitate the performance of the following laboratory tests:
● Complete Blood Count:
● Creatinine Levels
● Urinalysis with Examination of Urinary Sediment
● Spot Urine Protein/Creatinine Ratio or 24-hour Urine Collection
In cases where active disease is evident or there is a history of prior alterations in associated levels during a flare, additional specific laboratory tests become imperative:
● Anti-dsDNA Antibodies
● Complement Levels (CH50, or C3 and C4): Recent research has identified low C4 levels at preconception as an independent risk factor for SLE flare during pregnancy (81). Low C3 levels have also been shown to be a potential risk factor for preterm delivery in SLE patients (82). This highlights the importance of monitoring the absolute levels of complement components C3 and C4 and their variations over time (81, 82). Understanding that C3/C4 levels naturally increase during pregnancy is crucial. Therefore, baseline levels should be established early in pregnancy. Significant changes in these levels, even if they fall within the normal range for non-pregnant individuals, may indicate a potential flare of SLE. It is crucial to emphasize that the clinical presentation must drive the decision to request additional laboratory assessments, such as assessments for liver function and serum uric acid. The frequency of laboratory testing is personalized and contingent upon the level of disease activity. Ideally, stable patients need testing every trimester. Conversely, individuals with active disease need more frequent testing. Importantly, regular monitoring every 4-8 weeks is recommended due to the utility of observing trends in these parameters over time, aiding in detecting and diagnosing both SLE flare-ups and pre-eclampsia (83).
Maternal-fetal monitoring
Maternal-fetal monitoring strategies during pregnancy lack a universally optimal schedule. Increased monitoring frequency is warranted for women with SLE in conjunction with routine prenatal care; the monitoring protocol includes:
● A first-trimester ultrasound estimates the delivery date, with a fetal anatomic survey around 18 weeks of gestation (84).
● Ultrasound examinations in the third trimester are utilized to evaluate fetal growth and identify placental insufficiency, with the scheduling, often every four weeks, tailored based on the health of the mother and fetus (84). In cases where there is a suspicion of growth restriction or placental insufficiency, more frequent assessments, including doppler velocimetry, are advised. Furthermore, for late-onset intrauterine growth restriction (IUGR) identified after 34 weeks, signs such as a reduced abdominal circumference, a slowdown in growth velocity, or a low cerebroplacental ratio on doppler studies may signal a higher risk of adverse perinatal outcomes.
● Fetal monitoring, which includes nonstress tests and/or biophysical profiles, is typically recommended for most women with lupus during the last four to six weeks of pregnancy. Surveillance strategies are customized according to continuous fetal and maternal health assessments.
● It is highly recommended that patients who tested positive for anti-Ro/SSA and/or anti-La/SSB antibodies undergo vigilant screening for congenital heart block (85). Women with anti-Ro antibodies linked to neonatal lupus and risk of congenital heart block (ranging from 0.7% to 2%), especially when antibody levels are high, require additional fetal surveillance. The EULAR guidelines recommend fetal echocardiography for suspected cases of fetal dysrhythmia or myocarditis, particularly in those who are positive for Ro/SSA or La/SSB autoantibodies (85).
Management of LN flares during pregnancy
The diagnosis of lupus nephritis flare during pregnancy necessitates the initiation of therapy (86). A severe lupus nephritis flare, particularly in a young woman early in her pregnancy, should catalyze deliberations regarding therapeutic abortion. Although medical termination of pregnancy might be the safest option to manage lupus optimally, many women, particularly later in gestation or when it may represent their sole opportunity for pregnancy, opt against abortion. Treating LN flare as soon as possible becomes the sensible pathway (84, 87).
Recommended therapeutic approach
A first-line strategy typically involves steroids. Lupus nephritis usually warrants the addition of immunosuppressive treatment to steroids. A conventional approach is starting azathioprine up to 2mg/kg/day. The utility of calcineurin agent tacrolimus is beneficial due to its steroid-sparing capacity, rapid reduction of proteinuria due to off-target effects on podocyte stabilization, and favorable tolerability. Treatments involving tacrolimus have reported favorable pregnancy outcomes (84, 88).
Furthermore, women with nephrotic range proteinuria should be prescribed prophylactic low-molecular-weight heparin to mitigate venous thromboembolism risk. Tight monitoring in the first six months after giving birth is crucial due to the elevated risk of an LN flare-up (88).
During the later stages of pregnancy, cyclophosphamide could potentially be used to treat severe cases of lupus or LN (89). Nevertheless, the decision to use this treatment involves careful consideration of the risks associated with premature delivery and the subsequent separate management of the health of both mother and baby.
Preeclampsia
Enhanced monitoring is essential for these patients, especially when symptoms such as hypertension, proteinuria, or end-organ dysfunction emerge after the 20th week of pregnancy, signaling the possible onset of preeclampsia. The occurrence of severe, early-onset fetal growth restriction further signifies an increased risk of developing preeclampsia (90). Although preeclampsia that arises later in pregnancy can typically be managed with a watchful waiting approach, early detection during pre- and peri-viable stages is critical to facilitate prompt delivery and prevent complications in the mother.
The likelihood of developing preeclampsia in individuals with SLE varies from 16 to 30 percent. Low-dose aspirin therapy between the 12th and 16th weeks of pregnancy can decrease the absolute risk of preeclampsia by about 10 percent for those at higher risk, including all patients with SLE (33).
Preeclampsia versus lupus nephritis
Pre-eclampsia is a severe multisystem disorder of pregnancy characterized by high blood pressure after 20 weeks of gestation, accompanied by signs like proteinuria and maternal or fetal complications (91). Although numerous risk factors for pre-eclampsia have been identified, their ability to predict the condition is generally limited, even when combined. Lupus, especially in its active form with lupus nephritis, significantly increases the risk of pre-eclampsia (92, 93).
Flares of lupus nephritis, while potentially being the initial presentation of lupus, remain relatively rare, particularly in those devoid of prior or inactive nephritis at pregnancy onset. Distinguishing preeclampsia from lupus nephritis or a lupus flare poses a considerable challenge (Table 3). During pregnancy, flares of lupus nephritis may resemble preeclampsia, showcasing heightened proteinuria, hypertension, thrombocytopenia, and declining renal function (94). Simultaneous occurrences of active lupus nephritis and preeclampsia are also plausible. If a woman shows signs of increasing proteinuria, hypertension, and deteriorating kidney function early in pregnancy, especially if she has a history of lupus nephritis, this strongly indicates the likelihood of a lupus nephritis flare-up (84, 94).
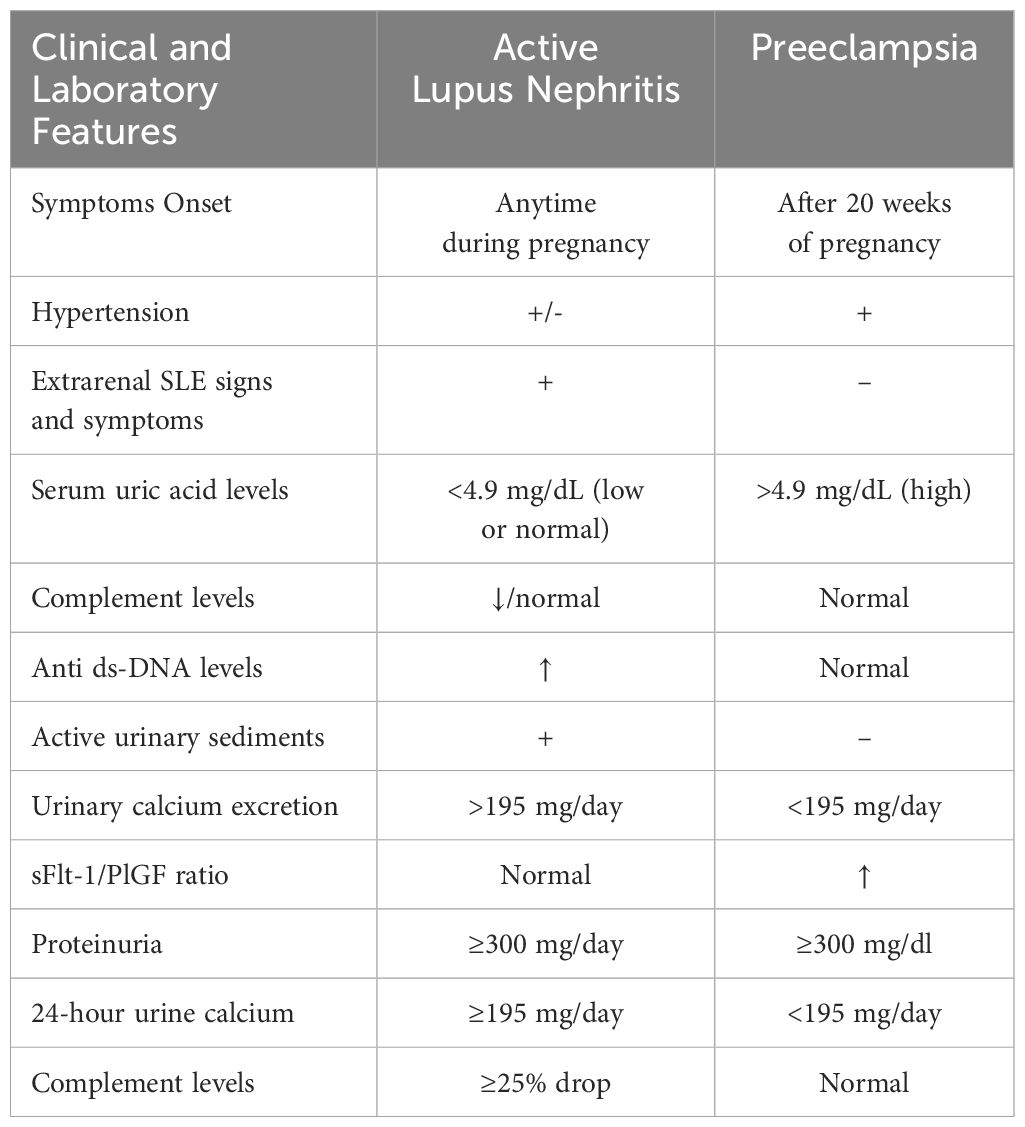
Table 3 Key clinical indicators differentiating preeclampsia from lupus nephritis in pregnancy (94–96).
The task of differentiating lupus nephritis from preeclampsia, especially from the late second trimester, becomes markedly complex, as both can manifest with proteinuria, hypertension, generalized symptoms, thrombocytopenia, and kidney impairment. Diagnostic confirmation often leans heavily on historical data and is augmented by suitable investigations. Complement levels should typically be normal or elevated, given the pregnancy’s state of acute phase response. A decrement, even within the normal range, alongside elevating dsDNA antibody levels, should raise alarms regarding lupus activity. A higher soluble fms-like tyrosine kinase-1 (sFLT-1) to placental growth factor (PIGF) ratio in the third trimester was particularly noted in women with preeclampsia compared to those with chronic kidney disease (97). This indicates the possible clinical benefit of assessing the sFlt-1/PIGF ratio in cases of new-onset lupus nephritis and other forms of glomerulonephritis that are associated with hypertension. However, it is essential to note that this diagnostic modality is not yet widely available for routine clinical use.
If there is a notable and unexpected increase in proteinuria that could potentially impact immunosuppressive management, and the woman is in the early or middle stages of the second trimester, a kidney biopsy might be performed, bearing in mind that pregnancy does not increase biopsy risks.
However, consideration must be given to the potential post-biopsy bleeding and the permissible duration for delaying anticoagulation in a pregnant woman with substantial proteinuria and possibly anti-phospholipid antibodies, thus increasing the risk for venous thromboembolism. If thromboembolism risks exceed the diagnostic benefits, biopsy should be forsaken. A biopsy is unnecessary if serology aligns with an LN flare and previous nephritis is documented.
Delivery and postpartum care
For patients with lupus nephritis, it is crucial to provide advice regarding the likelihood of delivery before 37 weeks and the increased risk of operative delivery if it is very early. The delivery timing should be meticulously determined based on a thorough maternal and fetal well-being evaluation. It is imperative to note that a nephritis flare is not an indication to proceed with delivery.
Postpartum monitoring is imperative, recognizing that many disease flares and thromboembolic events manifest within the initial six months post-delivery (98). Breastfeeding is encouraged; however, a personalized discussion regarding the safety of medications during lactation is crucial. Individual risk assessments are necessary, particularly for premature or ill infants who may face heightened vulnerability to certain medications. Hydroxychloroquine (HCQ), cyclosporine, tacrolimus, prednisone, and azathioprine are considered safe for use during breastfeeding. On the other hand, mycophenolate mofetil, leflunomide methotrexate, and cyclophosphamide are deemed unsafe for breastfeeding mothers. Biologics, except for NFα inhibitors, seem to be safe and effective without a significant maternal or fetal risk and are not recommended during pregnancy and breastfeeding due to insufficient data (99, 100).
Management of pregnancy after kidney transplant
Pregnancy in women with LN who have undergone a kidney transplant is a feasible option despite presenting specific challenges that necessitate careful preparation. These women can achieve pregnancies that do not jeopardize their transplant’s health, though they do face a higher likelihood of experiencing preeclampsia and miscarriages (101). However, with diligent care and oversight, the health outcomes for both the mother and child can match those of transplant recipients without LN (102). It’s essential to begin preparing for pregnancy between one and two years after the transplant to ensure the kidney is functioning stably without major issues like significant proteinuria, recurrence of LN, uncontrolled hypertension, episodes of rejection, or other graft complications (103). A crucial part of this preparation involves adjusting immunosuppressive treatment, notably discontinuing mycophenolate mofetil before conception to mitigate its risks and potentially switching to azathioprine, a less risky antimetabolite, when necessary (103).
Risk of cardiovascular complication
Although the risk of cardiovascular complications in patients with lupus nephritis during pregnancy has been less studied, recent research indicates that pregnant patients with SLE face increased risks of acute cardiovascular complications, including peripartum cardiomyopathy, heart failure, arrhythmias, stroke, and venous thromboembolism, during delivery hospitalization (104).
Additionally, these patients are at a higher risk of developing hypertension later and are nearly four times more likely to encounter subclinical cardiovascular diseases in the future, particularly those who have had adverse pregnancy outcomes like pre-eclampsia (105).
Given these risks, it is crucial for women with SLE and lupus nephritis to receive thorough counseling about the potential for acute cardiovascular complications during their delivery hospital stay. This counseling should address not only common comorbidities such as hypertension, diabetes, obesity, and dyslipidemia but also the systemic inflammatory state typical of SLE. Implementing this comprehensive approach is essential to effectively mitigate both immediate and long-term cardiovascular risks for pregnant patients with lupus nephritis.
Renal replacement therapy
In the management of pregnant women with lupus nephritis and significant renal impairment undergoing renal replacement therapy, several critical interventions are essential to ensure both maternal and fetal well-being. To improve clearance and reduce volume fluctuations, it is recommended that hemodialysis sessions be increased in frequency and intensity, with five to seven sessions per week and a minimum of 24 hours per week (106). This approach has been shown to improve outcomes significantly, reducing pre-dialysis urea levels to below 20 mmol/L, thus mitigating the risk of uremic complications that could affect both mother and child (106, 107).
Ensuring adequate erythropoiesis and managing anemia are equally crucial; increased doses of erythropoiesis-stimulating agents may be required to maintain hemoglobin levels within the desired range, thereby supporting proper fetal growth and reducing risks associated with anemia, such as low birth weight and premature birth (108).
Nutritional management tailored to the needs of renal impairment also plays a pivotal role. High-protein diets are necessary to meet the increased metabolic demands during pregnancy, and adjustments in vitamin and mineral intake, such as calcium and phosphate, are vital (106, 109). Careful selection of phosphate binders like calcium carbonate, which is deemed safe during pregnancy, helps manage serum phosphate levels without exposing the fetus to potential harm (106).
Additionally, daily monitoring and adjustments of fluid balance, electrolytes, and dialysis parameters are necessary to address the physiological changes during pregnancy (106, 107). These measures help prevent complications such as hypercalcemia or metabolic alkalosis, which could otherwise lead to significant maternal and fetal health issues. Thus, a multidisciplinary approach involving nephrologists, obstetricians, and dieticians is imperative to optimize outcomes for both the mother and the developing fetus.
Conclusions
Pregnancy-induced changes hold the potential to trigger LN flares, impacting maternal health. To optimize pregnancy outcomes for patients with SLE and lupus nephritis, it is crucial to plan conception during periods of disease stability and to ensure the use of medications that are safe during pregnancy. This scenario poses a significant challenge for nephrologists and rheumatologists due to the heightened risk of adverse perinatal outcomes and complications related to pregnancy-induced hypertension. Future efforts in the management of lupus nephritis during pregnancy should emphasize the development of personalized management strategies, underscoring the pressing need for more clinical trials to better understand the safety and efficacy of current SLE treatments during this critical period.
Author contributions
ZG: Conceptualization, Data curation, Validation, Writing – original draft, Writing – review & editing. TS: Supervision, Validation, Writing – original draft, Writing – review & editing. KJ: Validation, Writing – original draft, Writing – review & editing. SS: Validation, Writing – original draft, Writing – review & editing. EL: Validation, Writing – original draft, Writing – review & editing. AA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. SN: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor DG declared a past co-authorship with the author KJ.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hocaoğlu M, Valenzuela-Almada MO, Dabit JY, Osei-Onomah SA, Chevet B, Giblon RE, et al. Incidence, prevalence, and mortality of lupus nephritis: A population-based study over four decades using the lupus midwest network. Arthritis Rheumatol. (2023) 75:567–73. doi: 10.1002/art.42375
2. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Med (Baltimore). (1999) 78:167–75. doi: 10.1097/00005792-199905000-00003
3. Bastian HM, Roseman JM, McGwin G Jr., Alarcón GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. (2002) 11:152–60. doi: 10.1191/0961203302lu158oa
4. Seligman VA, Lum RF, Olson JL, Li H, Criswell LA. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am J Med. (2002) 112:726–9. doi: 10.1016/S0002-9343(02)01118-X
5. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse MEB, Lockshin MD, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken). (2020) 72:461–88. doi: 10.1002/acr.24130
6. Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. (2020) 6:7. doi: 10.1038/s41572-019-0141-9
7. Brenner BM, Charlton J, Luyckx V, Manfellotto D, Perico N, Remuzzi G, et al. The impact of kidney development on the life course: A consensus document for action. Nephron. (2017) 136:3–49. doi: 10.1159/000457967
8. Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. Chronic kidney disease. Nat Rev Dis Primers. (2017) 3:17088. doi: 10.1038/nrdp.2017.88
9. Buyon JP, Kim MY, Guerra MM, Lu S, Reeves E, Petri M, et al. Kidney outcomes and risk factors for nephritis (Flare/de novo) in a multiethnic cohort of pregnant patients with lupus. Clin J Am Soc Nephrol. (2017) 12:940–6. doi: 10.2215/cjn.11431116
10. Davis-Porada J, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, et al. Low frequency of flares during pregnancy and post-partum in stable lupus patients. Arthritis Res Ther. (2020) 22:52. doi: 10.1186/s13075-020-2139-9
11. Teng YKO, Bredewold EOW, Rabelink TJ, Huizinga TWJ, Eikenboom HCJ, Limper M, et al. An evidence-based approach to pre-pregnancy counselling for patients with systemic lupus erythematosus. Rheumatology. (2018) 57:1707–20. doi: 10.1093/rheumatology/kex374
12. Götestam Skorpen C, Lydersen S, Gilboe IM, Skomsvoll JF, Salvesen K, Palm Ø, et al. Disease activity during pregnancy and the first year postpartum in women with systemic lupus erythematosus. Arthritis Care Res (Hoboken). (2017) 69:1201–8. doi: 10.1002/acr.23102
13. Shaharir SS, Mohamed Said MS, Mohd R, Abdul Cader R, Mustafar R, Abdul Rahman R. Predictors of SLE relapse in pregnancy and post-partum among multi-ethnic patients in Malaysia. PloS One. (2019) 14:e0222343. doi: 10.1371/journal.pone.0222343
14. Stanhope TJ, White WM, Moder KG, Smyth A, Garovic VD. Obstetric nephrology: lupus and lupus nephritis in pregnancy. Clin J Am Soc Nephrol. (2012) 7:2089–99. doi: 10.2215/cjn.12441211
15. Attia DH, Mokbel A, Haggag HM, Naeem N. Pregnancy outcome in women with active and inactive lupus nephritis: A prospective cohort study. Lupus. (2019) 28:806–17. doi: 10.1177/0961203319846650
16. Mikdashi J, Nived O. Measuring disease activity in adults with systemic lupus erythematosus: the challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res Ther. (2015) 17:183. doi: 10.1186/s13075-015-0702-6
17. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29:288–91.
18. Lattanzi B, Consolaro A, Solari N, Ruperto N, Martini A, Ravelli A. Measures of disease activity and damage in pediatric systemic lupus erythematosus: British Isles Lupus Assessment Group (BILAG), European Consensus Lupus Activity Measurement (ECLAM), Systemic Lupus Activity Measure (SLAM), Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), Physician's Global Assessment of Disease Activity (MD Global), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC/ACR DI; SDI). Arthritis Care Res (Hoboken). (2011) 63 Suppl 11:S112–7. doi: 10.1002/acr.20623
19. Kwok LW, Tam LS, Zhu T, Leung YY, Li E. Predictors of maternal and fetal outcomes in pregnancies of patients with systemic lupus erythematosus. Lupus. (2011) 20:829–36. doi: 10.1177/0961203310397967
20. Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheumatol. (2006) 54:3640–7. doi: 10.1002/art.22159
21. Sperber K, Hom C, Chao CP, Shapiro D, Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. (2009) 7:9. doi: 10.1186/1546-0096-7-9
22. Dima A, Jurcut C, Chasset F, Felten R, Arnaud L. Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge. Ther Adv Musculoskeletal Dis. (2022) 14:1759720X211073001. doi: 10.1177/1759720x211073001
23. Levy RA, Vilela VS, Cataldo MJ, Ramos RC, Duarte JL, Tura BR, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. (2001) 10:401–4. doi: 10.1191/096120301678646137
24. Kasitanon N, Fine DM, Haas M, Magder LS, Petri M. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus. (2006) 15:366–70. doi: 10.1191/0961203306lu2313oa
25. Mejía-Vilet JM, Córdova-Sánchez BM, Uribe-Uribe NO, Correa-Rotter R. Immunosuppressive treatment for pure membranous lupus nephropathy in a Hispanic population. Clin Rheumatol. (2016) 35:2219–27. doi: 10.1007/s10067-016-3366-y
26. Galindo-Izquierdo M, Rodriguez-Almaraz E, Pego-Reigosa JM, López-Longo FJ, Calvo-Alén J, Olivé A, et al. Characterization of patients with lupus nephritis included in a large cohort from the spanish society of rheumatology registry of patients with systemic lupus erythematosus (RELESSER). Med (Baltimore). (2016) 95:e2891. doi: 10.1097/md.0000000000002891
27. Lee JS, Oh JS, Kim YG, Lee CK, Yoo B, Hong S. Recovery of renal function in patients with lupus nephritis and reduced renal function: the beneficial effect of hydroxychloroquine. Lupus. (2020) 29:52–7. doi: 10.1177/0961203319890007
28. Tsakonas E, Joseph L, Esdaile JM, Choquette D, Senécal JL, Cividino A, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. Lupus. (1998) 7:80–5. doi: 10.1191/096120398678919778
29. Huybrechts KF, Bateman BT, Zhu Y, Straub L, Mogun H, Kim SC, et al. Hydroxychloroquine early in pregnancy and risk of birth defects. Am J Obstet Gynecol. (2021) 224:290.e1–.e22. doi: 10.1016/j.ajog.2020.09.007
30. Izmirly P, Saxena A, Buyon JP. Progress in the pathogenesis and treatment of cardiac manifestations of neonatal lupus. Curr Opin Rheumatol. (2017) 29:467–72. doi: 10.1097/bor.0000000000000414
31. Balevic SJ, Weiner D, Clowse MEB, Eudy AM, Maharaj AR, Hornik CP, et al. Hydroxychloroquine PK and exposure-response in pregnancies with lupus: the importance of adherence for neonatal outcomes. Lupus Sci Med. (2022) 9:e000602. doi: 10.1136/lupus-2021-000602
32. Abd Rahman R, Min Tun K, Kamisan Atan I, Mohamed Said MS, Mustafar R, Zainuddin AA. New benefits of hydroxychloroquine in pregnant women with systemic lupus erythematosus: A retrospective study in a tertiary centre. Rev Bras Ginecol Obstet. (2020) 42:705–11. doi: 10.1055/s-0040-1715140
33. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: US preventive services task force recommendation statement. Jama. (2021) 326:1186–91. doi: 10.1001/jama.2021.14781
34. Tani C, Zucchi D, Haase I, Gerosa M, Larosa M, Cavagna L, et al. Impact of low-dose acetylsalicylic acid on pregnancy outcome in systemic lupus erythematosus: results from a multicentre study. Lupus Sci Med. (2022) 9:e000714. doi: 10.1136/lupus-2022-000714
35. Zhang N, Zhang H-X, Li Y-W, Li Y. Benefits of hydroxychloroquine combined with low-dose aspirin on pregnancy outcomes and serum cytokines in pregnant women with systemic lupus erythematosus. Drugs R&D. (2023) 23:35–42. doi: 10.1007/s40268-022-00408-0
36. Nezvalová-Henriksen K, Spigset O, Nordeng H. Effects of ibuprofen, diclofenac, naproxen, and piroxicam on the course of pregnancy and pregnancy outcome: a prospective cohort study. BJOG: Int J Obstetrics Gynaecology. (2013) 120:948–59. doi: 10.1111/1471-0528.12192
37. Kozer E, Nikfar S, Costei A, Boskovic R, Nulman I, Koren G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J obstetrics gynecology. (2002) 187:1623–30. doi: 10.1067/mob.2002.127376
38. Cleary BJ, Källén B. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defects Res Part A: Clin Mol Teratology. (2009) 85:647–54. doi: 10.1002/bdra.20583
39. Chan L, Yuen P. Risk of miscarriage in pregnant users of NSAIDs: more information is needed to be able to interpret study's results. BMJ: Br Med J. (2001) 322:1365. doi: 10.1136/bmj.322.7298.1365
40. Nakhai-Pour HR, Broy P, Sheehy O, Bérard A. Use of nonaspirin nonsteroidal anti-inflammatory drugs during pregnancy and the risk of spontaneous abortion. Cmaj. (2011) 183:1713–20. doi: 10.1503/cmaj.110454
41. Abou-Ghannam G, Usta IM, Nassar AH. Indomethacin in pregnancy: applications and safety. Am J Perinatology. (2011) 29:175–86. doi: 10.1055/s-0031-1284227
42. Koren G, Florescu A, Costei AM, Boskovic R, Moretti ME. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Ann Pharmacotherapy. (2006) 40:824–9. doi: 10.1345/aph.1G428
43. Takahashi Y, Roman C, Chemtob S, Tse MM, Lin E, Heymann MA, et al. Cyclooxygenase-2 inhibitors constrict the fetal lamb ductus arteriosus both in vitro and in vivo. Am J physiology-Regulatory Integr Comp Physiol. (2000) 278:R1496–R505. doi: 10.1152/ajpregu.2000.278.6.R1496
44. Bellos I, Pergialiotis V, Antsaklis A, Loutradis D, Daskalakis G. Safety of non-steroidal anti-inflammatory drugs in postpartum period in women with hypertensive disorders of pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2020) 56:329–39. doi: 10.1002/uog.21997
45. Premkumar A, Ayala NK, Miller CH, Grobman WA, Miller ES. Postpartum NSAID use and adverse outcomes among women with hypertensive disorders of pregnancy: A systematic review and meta-analysis. Am J Perinatol. (2021) 38:1–9. doi: 10.1055/s-0040-1713180
46. Kuriya B, Hernández-Díaz S, Liu J, Bermas BL, Daniel G, Solomon DH. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. (2011) 63:721–8. doi: 10.1002/acr.20422
47. Buttgereit F, Straub RH, Wehling M, Burmester GR. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis rheumatism. (2004) 50:3408–17. doi: 10.1002/art.20583
48. Shimada H, Wakiya R, Kanenishi K, Miyatake N, Nakashima S, Mansour MMF, et al. Preterm birth is strongly affected by the glucocorticoid dose during pregnancy in women complicated by systemic lupus erythematosus. Arthritis Res Ther. (2022) 24:10. doi: 10.1186/s13075-021-02699-1
49. Coelho J, Beaugerie L, Colombel JF, Hébuterne X, Lerebours E, Lémann M, et al. Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME Study. Gut. (2011) 60:198–203. doi: 10.1136/gut.2010.222893
50. Goldstein LH, Dolinsky G, Greenberg R, Schaefer C, Cohen-Kerem R, Diav-Citrin O, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res Part A: Clin Mol Teratology. (2007) 79:696–701. doi: 10.1002/bdra.20399
51. Chambers CD, Tutuncu ZN, Johnson D, Jones KL. Human pregnancy safety for agents used to treat rheumatoid arthritis: adequacy of available information and strategies for developing post-marketing data. Arthritis Res Ther. (2006) 8:1–10. doi: 10.1186/ar1977
52. Oz BB, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis1. Transplantation. (2001) 71:1051–5. doi: 10.1097/00007890-200104270-00006
53. Paziana K, Del Monaco M, Cardonick E, Moritz M, Keller M, Smith B, et al. Ciclosporin use during pregnancy. Drug Saf. (2013) 36:279–94. doi: 10.1007/s40264-013-0034-x
54. Ponticelli C, Reggiani F, Moroni G. Old and new calcineurin inhibitors in lupus nephritis. J Clin Med. (2021) 10:4832. doi: 10.3390/jcm10214832
55. Ichinose K, Sato S, Kitajima Y, Horai Y, Fujikawa K, Umeda M, et al. The efficacy of adjunct tacrolimus treatment in pregnancy outcomes in patients with systemic lupus erythematosus. Lupus. (2018) 27:1312–20. doi: 10.1177/0961203318770536
56. Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrology: CJASN. (2013) 8:563. doi: 10.2215/CJN.06400612
57. Tuffnell D, Jankowicz D, Lindow S, Lyons G, Mason G, Russell I, et al. Outcomes of severe pre-eclampsia/eclampsia in Yorkshire 1999/2003. BJOG: Int J Obstetrics Gynaecology. (2005) 112:875–80. doi: 10.1111/j.1471-0528.2005.00565.x
58. Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet. (2019) 394:1011–21. doi: 10.1016/s0140-6736(19)31282-6
59. Buawangpong N, Teekachunhatean S, Koonrungsesomboon N. Adverse pregnancy outcomes associated with first-trimester exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: A systematic review and meta-analysis. Pharmacol Res Perspectives. (2020) 8:e00644. doi: 10.1002/prp2.644
60. Wiles K, Lightstone L. Glomerular disease in women. Kidney Int Rep. (2018) 3:258–70. doi: 10.1016/j.ekir.2018.01.010
61. Gleeson S, Lightstone L. Glomerular disease and pregnancy. Adv Chronic Kidney Disease. (2020) 27:469–76. doi: 10.1053/j.ackd.2020.08.001
62. Condon MB, Ashby D, Pepper RJ, Cook HT, Levy JB, Griffith M, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann rheumatic diseases. (2013) 72:1280–6. doi: 10.1136/annrheumdis-2012-202844
63. Klink DT, van Elburg RM, Schreurs MW, van Well GT. Rituximab administration in third trimester of pregnancy suppresses neonatal B-cell development. Clin Dev Immunol. (2008) 2008:271363. doi: 10.1155/2008/271363
64. Kraaij T, Huizinga TW, Rabelink TJ, Teng YO. Belimumab after rituximab as maintenance therapy in lupus nephritis. Rheumatology. (2014) 53:2122–4. doi: 10.1093/rheumatology/keu369
65. Mysler EF, Spindler AJ, Guzman R, Bijl M, Jayne D, Furie RA, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheumatism. (2013) 65:2368–79. doi: 10.1002/art.38037
66. Skorpen CG, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann rheumatic diseases. (2016) 75:795–810. doi: 10.1136/annrheumdis-2015-208840
67. Vaux KK, Kahole NC, Jones KL. Cyclophosphamide, methotrexate, and cytarabine embropathy: is apoptosis the common pathway? Birth Defects Res Part A: Clin Mol Teratology. (2003) 67:403–8. doi: 10.1002/bdra.10060
68. Sammaritano LR. Management of systemic lupus erythematosus during pregnancy. Annu Rev Med. (2017) 68:271–85. doi: 10.1146/annurev-med-042915-102658
69. Peterson EA, Lynton J, Bernard A, Santillan MK, Bettendorf B. Rheumatologic medication use during pregnancy. Obstet Gynecol. (2020) 135:1161–76. doi: 10.1097/aog.0000000000003755
70. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant. (2002) 17 Suppl 4:50–5. doi: 10.1093/ndt/17.suppl_4.50
72. Rajaei E, Shahbazian N, Rezaeeyan H, Mohammadi AK, Hesam S, Zayeri ZD. The effect of lupus disease on the pregnant women and embryos: a retrospective study from 2010 to 2014. Clin Rheumatol. (2019) 38:3211–5. doi: 10.1007/s10067-019-04682-3
73. Brent RL. Teratogen update: Reproductive risks of leflunomide(Arava™); A pyrimidine synthesis inhibitor: counseling women taking leflunomide before or during pregnancy and men taking leflunomide who are contemplating fathering a child. Teratology. (2001) 63:106–12. doi: 10.1002/(ISSN)1096-9926
74. Chambers CD, Johnson DL, Robinson LK, Braddock SR, Xu R, Lopez-Jimenez J, et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheumatol. (2010) 62:1494–503. doi: 10.1002/art.27358
75. Zucchi D, Fischer-Betz R, Tani C. Pregnancy in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. (2023) 16:101860. doi: 10.1016/j.berh.2023.101860
76. Zhao XY, Li SS, He YX, Yan LJ, Lv F, Liang QM, et al. SGLT2 inhibitors alleviated podocyte damage in lupus nephritis by decreasing inflammation and enhancing autophagy. Ann Rheum Dis. (2023) 82:1328–40. doi: 10.1136/ard-2023-224242
77. Wang H, Li T, Sun F, Liu Z, Zhang D, Teng X, et al. Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: a phase I/II trial. RMD Open. (2022) 8:e002686. doi: 10.1136/rmdopen-2022-002686
78. Muller DRP, Stenvers DJ, Malekzadeh A, Holleman F, Painter RC, Siegelaar SE. Effects of GLP-1 agonists and SGLT2 inhibitors during pregnancy and lactation on offspring outcomes: a systematic review of the evidence. Front Endocrinol (Lausanne). (2023) 14:1215356. doi: 10.3389/fendo.2023.1215356
79. Petri M. The Hopkins Lupus Pregnancy Center: ten key issues in management. Rheum Dis Clin North Am. (2007) 33:227–35. doi: 10.1016/j.rdc.2007.01.003
80. Khamashta MA. Systemic lupus erythematosus and pregnancy. Best Pract Res Clin Rheumatol. (2006) 20:685–94. doi: 10.1016/j.berh.2006.04.003
81. Crisafulli F, Andreoli L, Zucchi D, Reggia R, Gerardi MC, Lini D, et al. Variations of C3 and C4 before and during pregnancy in systemic lupus erythematosus: association with disease flares and obstetric outcomes. J Rheumatol. (2023) 50:1296–301. doi: 10.3899/jrheum.2022-1135
82. Hiramatsu Y, Isoda K, Kotani T, Nakamura E, Wada Y, Fujiki Y, et al. Pre-pregnancy serum complement C3 level is a predictor of preterm birth for pregnancies with systemic lupus erythematosus. Arthritis Res Ther. (2021) 23:140. doi: 10.1186/s13075-021-02522-x
83. Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. (2017) 76:476–85. doi: 10.1136/annrheumdis-2016-209770
84. Dao KH, Bermas BL. Systemic lupus erythematosus management in pregnancy. Int J Womens Health. (2022) 14:199–211. doi: 10.2147/ijwh.S282604
85. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. (2020) 79:713–23. doi: 10.1136/annrheumdis-2020-216924
86. Knight CL, Nelson-Piercy C. Management of systemic lupus erythematosus during pregnancy: challenges and solutions. Open Access Rheumatol. (2017) 9:37–53. doi: 10.2147/oarrr.S87828
87. Lightstone L, Hladunewich MA. Lupus nephritis and pregnancy: concerns and management. Semin Nephrol. (2017) 37:347–53. doi: 10.1016/j.semnephrol.2017.05.006
88. Fakhouri F, Schwotzer N, Cabiddu G, Barratt J, Legardeur H, Garovic V, et al. Glomerular diseases in pregnancy: pragmatic recommendations for clinical management. Kidney Int. (2023) 103:264–81. doi: 10.1016/j.kint.2022.10.029
89. Ponticelli C, Moroni G. Immunosuppression in pregnant women with systemic lupus erythematosus. Expert Rev Clin Immunol. (2015) 11:549–52. doi: 10.1586/1744666x.2015.1033404
90. Takahashi M, Makino S, Oguma K, Imai H, Takamizu A, Koizumi A, et al. Fetal growth restriction as the initial finding of preeclampsia is a clinical predictor of maternal and neonatal prognoses: a single-center retrospective study. BMC Pregnancy Childbirth. (2021) 21:678. doi: 10.1186/s12884-021-04152-2
91. Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynecology Obstetrics. (2019) 145:1–33. doi: 10.1002/ijgo.12802
92. Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. (2008) 199:127.e1–6. doi: 10.1016/j.ajog.2008.03.012
93. Wu J, Ma J, Zhang WH, Di W. Management and outcomes of pregnancy with or without lupus nephritis: a systematic review and meta-analysis. Ther Clin Risk Manage. (2018) 14:885–901. doi: 10.2147/tcrm.S160760
94. Yamamoto Y, Aoki S. Systemic lupus erythematosus: strategies to improve pregnancy outcomes. Int J Womens Health. (2016) 8:265–72. doi: 10.2147/ijwh.S90157
95. Hirashima C, Ogoyama M, Abe M, Shiraishi S, Sugase T, Niki T, et al. Clinical usefulness of serum levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio to rule out preeclampsia in women with new-onset lupus nephritis during pregnancy. CEN Case Rep. (2019) 8:95–100. doi: 10.1007/s13730-018-0373-7
96. Tan B, So PN, Krishnan A, Carriazo S, Bahamonde JR, Lamech TM, et al. Approach to pregnancy in patients with lupus nephritis. Kidney Med. (2023) 5:100724. doi: 10.1016/j.xkme.2023.100724
97. Leaños-Miranda A, Graciela Nolasco-Leaños A, Ismael Carrillo-Juárez R, José Molina-Pérez C, Janet Sillas-Pardo L, Manuel Jiménez-Trejo L, et al. Usefulness of the sFlt-1/PlGF (Soluble fms-Like Tyrosine Kinase-1/Placental Growth Factor) Ratio in Diagnosis or Misdiagnosis in Women With Clinical Diagnosis of Preeclampsia. Hypertension. (2020) 76:892–900. doi: 10.1161/hypertensionaha.120.15552
98. Mouyis M. Postnatal care of woman with rheumatic diseases. Adv Ther. (2020) 37:3723–31. doi: 10.1007/s12325-020-01448-1
99. Development BMNIoCHaH. Drugs and lactation database (LactMed®). Bethesda, MD, United States: National Library of Medicine (US) (2023).
100. Puchner A, Gröchenig HP, Sautner J, Helmy-Bader Y, Juch H, Reinisch S, et al. Immunosuppressives and biologics during pregnancy and lactation : A consensus report issued by the Austrian Societies of Gastroenterology and Hepatology and Rheumatology and Rehabilitation. Wien Klin Wochenschr. (2019) 131:29–44. doi: 10.1007/s00508-019-1448-y
101. Campise M, Giglio E, Trespidi L, Messa P, Moroni G. Pregnancies in women receiving renal transplant for lupus nephritis: description of nine pregnancies and review of the literature. Lupus. (2015) 24:1210–3. doi: 10.1177/0961203315583542
102. McGrory CH, McCloskey LJ, DeHoratius RJ, Dunn SR, Moritz MJ, Armenti VT. Pregnancy outcomes in female renal recipients: a comparison of systemic lupus erythematosus with other diagnoses. Am J Transplantation. (2003) 3:35–42. doi: 10.1034/j.1600-6143.2003.30107.x
103. Wong T, Goral S. Lupus nephritis and kidney transplantation: where are we today? Adv Chronic Kidney Dis. (2019) 26:313–22. doi: 10.1053/j.ackd.2019.08.007
104. Zahid S, Mohamed MS, Wassif H, Nazir NT, Khan SS, Michos ED. Analysis of cardiovascular complications during delivery admissions among patients with systemic lupus erythematosus, 2004-2019. JAMA Netw Open. (2022) 5:e2243388. doi: 10.1001/jamanetworkopen.2022.43388
105. Lin P, Rhew E, Ness RB, Peaceman A, Dyer A, McPherson D, et al. Adverse pregnancy outcomes and subsequent risk of cardiovascular disease in women with systemic lupus erythematosus. Lupus Sci Med. (2014) 1:e000024. doi: 10.1136/lupus-2014-000024
106. Ribeiro CI, Silva N. Pregnancy and dialysis. J Bras Nefrol. (2020) 42:349–56. doi: 10.1590/2175-8239-jbn-2020-0028
107. Chou CY, Ting IW, Lin TH, Lee CN. Pregnancy in patients on chronic dialysis: a single center experience and combined analysis of reported results. Eur J Obstet Gynecol Reprod Biol. (2008) 136:165–70. doi: 10.1016/j.ejogrb.2007.01.017
108. Nadeau-Fredette AC, Hladunewich M, Hui D, Keunen J, Chan CT. End-stage renal disease and pregnancy. Adv Chronic Kidney Dis. (2013) 20:246–52. doi: 10.1053/j.ackd.2013.01.010
Keywords: lupus nephritis, pregnancy, management of pregnancy, pre-conception counseling, multidisciplinary management
Citation: Gholizadeh Ghozloujeh Z, Singh T, Jhaveri KD, Shah S, Lerma E, Abdipour A and Norouzi S (2024) Lupus nephritis: management challenges during pregnancy. Front. Nephrol. 4:1390783. doi: 10.3389/fneph.2024.1390783
Received: 23 February 2024; Accepted: 15 May 2024;
Published: 04 June 2024.
Edited by:
Duvuru Geetha, Johns Hopkins Medicine, United StatesReviewed by:
Dhanunjay Mukhi, University of Pennsylvania, United StatesEnrique Morales, CSUR complex glomerular pathology, Spain
Copyright © 2024 Gholizadeh Ghozloujeh, Singh, Jhaveri, Shah, Lerma, Abdipour and Norouzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sayna Norouzi, U25vcm91emlAbGx1LmVkdQ==
 Zohreh Gholizadeh Ghozloujeh
Zohreh Gholizadeh Ghozloujeh Tripti Singh
Tripti Singh Kenar D. Jhaveri
Kenar D. Jhaveri Silvi Shah4
Silvi Shah4 Edgar Lerma
Edgar Lerma