
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nephrol., 16 July 2024
Sec. Kidney Transplantation
Volume 4 - 2024 | https://doi.org/10.3389/fneph.2024.1385447
Background: In kidney transplant (KT) patients, magnesium (Mg2+) deficiency is widespread. It is often encountered early after KT, may persist longer, and is frequently promoted by calcineurin inhibitors (CNIs) and tubular leakage. Studies demonstrated an association between post-KT hypomagnesemia and allograft dysfunction. The concentration of the active form, the ionized Mg2+ (iMg2+), is not measured clinically, and total Mg2+ (tMg2+) and iMg2+ correlations are conflicting. We assess the cross-sectional prevalence of hypomagnesemia in KT patients. The correlation of demographic and anthropometric parameters was also studied.
Methods: A prospective, single-center analysis of KT patients was conducted at the University Hospital of Bern, Switzerland (March 2023–August 2023). Blood samples were collected at least twice for the majority of patients. tMg2+ has been quantified from a plasma sample at the Clinical Chemistry Department of the University Hospital of Bern. The PRIME® ES analyzer (Nova Biomedical, USA) provided results for iMg2+. The following co-variables were considered: age, comorbidities, kidney disease, KT history, estimated glomerular filtration rate (eGFR), and treatment (including Mg2+ supplementation and immunosuppression).
Results: A total of 208 measurements in 104 patients were performed [once in 9/104 patients (8.7%), twice in 86/104 (82.7%), and three times in 9/104 (8.7%)]. Compared to that in healthy volunteers (51 measurements in 51 participants), mean iMg2+ was significantly lower in KT patients {KT: 0.46 mmol/L [interquartile range (IQR): 0.40–0.50], volunteers: 0.57 mmol/L (IQR 0.54–0.61), p < 0.01}. Overall, iMg2+ and tMg2+ showed strong category agreement (r2 = 0.93, p < 0.01). In linear regression, low iMg2+ correlated with CNI exposure. For 110/208 measurements (52.9%), a reduced iMg2+ (cutoff: 0.42 mmol/L) was shown. In 58/208 (27.9%), both values were reduced, and 52/208 (25%) had isolated reduced iMg2+. In principal component analysis, patients with isolated low iMg2+ clustered with patients with low iMg2+ and tMg2+.
Conclusion: iMg2+ and tMg2+ were strongly correlated. A substantial proportion of patients show isolated low iMg2+. Currently, it is unclear if these patients suffer from Mg2+ deficiency.
Magnesium (Mg2+) ranks as the fourth most abundant cation in the human body, playing a crucial role in various cellular and biological processes (1). Hypomagnesemia, characterized by depletion of Mg2+ in the blood, is mainly due to conditions that promote renal or gastrointestinal Mg2+ wasting and is responsible for cardiac, neurological, and neuromuscular disorders. Mg2+ deficit, often overlooked compared to other electrolyte disorders, raises substantial health concerns and is frequently encountered in clinical settings, affecting as much as 30% of the elderly population (2). Hypomagnesemia has been linked to cardiovascular risk and is commonly associated with unfavorable clinical outcomes and higher mortality (3–7).
Dysmagnesemia has also been linked to unfavorable outcomes in individuals undergoing dialysis and those with chronic kidney disease (CKD), acting as a potential predictor for both mortality and the deterioration of renal function in CKD patients (1, 7). In kidney transplant (KT) patients, hypomagnesemia is widespread, often encountered in the first weeks after KT, and frequently promoted by calcineurin inhibitors (CNIs), other concomitant medications (i.e., thiazide-type and loop diuretics and pump proton inhibitors), and tubular leakage (1). In more than 20% of KT patients, hypomagnesemia persists for several years after KT (6). Several studies have demonstrated that pre- and post-KT hypomagnesemia is independently associated with immune dysfunction, thus likely promoting opportunistic infections (8, 9). KT patients experiencing hypomagnesemia may also be prone to impaired glucose metabolism, increasing their susceptibility to the development of diabetes mellitus (6, 10). Moreover, associations between low Mg2+ levels and a more rapid decline in renal function, increased risk of graft loss (1, 6, 11), and major cardiovascular outcomes have been reported (7). This underscores the importance of paying more attention to abnormal Mg2+ values in clinical practice within this patient population.
Clinical laboratories typically measure total Mg2+ (tMg2+), which represents the sum of both the protein-bound (approximately 20%–30%) and the free and biologically active form of Mg2+ (approximately 60%–70%), also referred to as ionized magnesium (iMg2+). Even though protein-bound and free Mg2+ fractions are usually in a steady state changing in similar directions, they may also change independently from each other with complex buffering and compartment-shift mechanisms involved. Dysproteinemias may change bound Mg2+ fractions without necessary changes in free Mg2+. Acid–base disorders may affect Mg2+ binding affinities, resulting in altered iMg2+ fraction, while tMg2+ level remains unchanged (12, 13). Evidence suggests that tMg2+ may not adequately reflect a patient’s Mg2+ state, underestimating the prevalence of clinically relevant hypomagnesemia (14–16). Earlier studies have shown discrepancy and poor correlation between tMg2+ and iMg2+. Favoring iMg2+ over tMg2+ could enhance the detection of hypomagnesemia, enabling more accurate identification of patients in need of supplementation and mitigating the risk of toxicity (14–18). However, there is still a lack of comprehensive understanding regarding the clinical utility and the subsequent impact on therapeutic interventions. Consequently, the measurement of iMg2+ remains uncommon as a routine test in most clinical settings.
The study aims to prospectively investigate plasma tMg2+ and iMg2+ levels in both healthy subjects and a cohort of KT patients and to define 1) the cross-sectional prevalence of hypomagnesemia and 2) correlate this state with clinical, demographic, and anthropometric parameters.
This study was conducted between March 16, 2023, and August 11, 2023, at the University Hospital of Bern, Switzerland. The study is a prospective, single-center exploratory study, designed to investigate Mg2+ levels in KT patients compared with a group of healthy volunteers. The study protocol was approved by the Medical Ethics Committee of the study centers (Project-ID 2022-01953), and informed consent was obtained from all the participants. Participants (KT and volunteers) were free to take part and were recruited in the environment of the primary investigator and project partners.
tMg2+ was measured in venous whole-blood samples. Blood samples were collected at two instances for KT patients at least 2 weeks apart and less than 6 months apart and at one instance for volunteers’ controls. tMg2+ has been quantified at the Clinical Chemistry Department of the University Hospital of Bern. The PRIME® ES analyzer (Nova Biomedical, Waltham, MA, USA) provided results for iMg2+. The tubes were not centrifuged, all analyses were performed within 6 hours of sample collection after preparation and calibration of the device, and the results were pH corrected. In volunteer participants, only iMg2+ was always analyzed; tMg2+ was analyzed on a case-by-case basis if the medical situation required a larger blood sample for another reason. iMg2+ concentrations were not used for clinical decision-making purposes.
KT patients’ data (including kidney disease characteristics, cardiovascular risk factors and/or disease, and medication) were collected from the clinical information platform, and laboratory analyses were extracted via the Insel Data Science Center (IDSC). The IDSC is the IT organizational unit of Insel Gruppe AG for the collection, provision, and use of digital data for scientific purposes. Investigators utilized filled questionnaires to gather medical data. A medical visit, conducted on the day of inclusion, was performed for all participants. During this visit, anthropometric measurements and blood pressure readings were collected for both groups. Additionally, a second visit, scheduled at least 2 weeks apart, was exclusively designated for KT patients.
The primary endpoint of the study was the prevalence of hypomagnesemia in KT patients, defined by repeated serum iMg2+ levels below reference values. Lower reference values were defined as 2 standard deviations (SDs) below mean tMg2+ and iMg2+ among volunteers, and the reference ranges were as follows: serum tMg2+ 0.66 mmol/L and serum iMg2+ 0.42 mmol/L. It is important to acknowledge that the standard reference ranges for Mg2+ exhibit significant variation (3%–6%) due to differences in analytical methods, as well as variations within individual subjects and between different subjects (16). Secondary endpoints include the cross-sectional prevalence of hypomagnesemia, identification of subgroups with isolated low iMg2+ but normal tMg2+, and the correlation of iMg2+ levels with various baseline factors [age, gender, delayed graft function (DGF), KT history, estimated glomerular filtration rate (eGFR) given in mL/min/1.73 m2, cardiovascular risk factors or disease, and medications].
Participants were excluded from the analysis if they 1) were aged ≤18 years at the inclusion, 2) were pregnant or breastfeeding, and 3) were unable to understand and sign the informed consent form. Overall, no such patients were excluded from the primary endpoint analysis.
Neither patients nor the public was involved in the design, conduct, or analysis of this study.
The sample size was based on a priori calculations with a mean expected difference between iMg2+ in KT and volunteers of 0.10 mmol/L (0.45 vs. 0.55 +/− 0.15 mmol/L) and an enrollment ratio of 2:1. A minimal sample size of 78 participants (52 KT and 26 volunteers) was calculated with an alpha error of 0.05 and power of 0.8. Descriptive statistics are presented as median [interquartile range (IQR)] or as mean ± SD for continuous variables, and as numbers and percentages for categorical variables. Univariate logistic regression was performed for the dichotomous secondary endpoint of normal iMg2+ and low iMg2+ for the following dependent parameters: age, gender, KT history (years), eGFR, blood pressure, history of DGF, Mg2+ supplementation, CNI treatment, presence of cardiovascular risk factors [diabetes mellitus, hypertension, dyslipidemia, obesity, or obstructive sleep apnea (OSA)], and presence of cardiovascular disease (ischemic or hypertensive cardiomyopathy). Subsequently, a multivariate logistic regression analyzed normal and low iMg2+ for all factors listed above. Results are shown as odds ratio (OR) and confidence intervals (95% CIs). Correlation between iMg2+ and tMg2+ was established using linear regression and the strength of the correlation with Pearson’s coefficient (r2). Principal component analysis (PCA) was used for KT participants and was performed using the following parameters: iMg2+, gender, age, KT history, eGFR, Mg2+ supplementation, and CNI treatment. Dichotomous parameters were recoded as 0 for absent and 1 for present. Dimension 1 vs. Dimension 2 was depicted. Each point represents one patient. Color code represents patient group: normal iMg2+, normal tMg2+, isolated low iMg2+, low iMg2+, and low tMg2+. Statistical analyses were performed using R statistical software (version 4.0.3) and R Studio (version 1.3.1093). A two-tailed p < 0.05 was considered statistically significant.
A total of 208 measurements in 104 KT patients [once in 9/104 patients (8.7%), twice in 86/104 (82.7%), and three times in 9/104 (8.7%)] and 51 measurements in 51 volunteers were performed. The selection procedure is summarized in Supplementary Figure 1. Demographic data and clinical characteristics of participants (KT and volunteers) are shown in Tables 1 and 2A, B. The majority of KT participants were Caucasian men with an average age of 60 years (IQR 50–70). In 87% of instances, participants experienced hypertension, with dyslipidemia and diabetes affecting 41% and 30%, respectively. KT participants had undergone KT for an average of 5.1 years, with an interquartile range of 0.8 to 13.6 years. In 21% of cases, they had experienced DGF, and the mean eGFR at the study entry was 42 mL/min/1.73 m2. Additionally, 45% of KT participants received cyclosporine therapy and 31% tacrolimus therapy. In contrast, the majority of volunteers consisted of Caucasian women, accounting for 63% of the group, with an average age of 51 (IQR 38–59). In both groups, a small percentage of individuals were on Mg2+ replacement therapy—specifically, 19% in the KT group and 12% in the volunteer group.
Compared to that in volunteers, mean iMg2+ was significantly lower in KT patients [KT: 0.46 mmol/L (IQR 0.40–0.50), volunteers: 0.57 mmol/L (IQR 0.54–0.61), p < 0.01] (Figure 1). For KT patients, the association between tMg2+ and iMg2+ is depicted in a scatter plot in Figure 2. Overall, iMg2+ and tMg2+ showed strong category agreement (r2 = 0.93, p < 0.01). Dashed lines delimit the reference range values for iMg2+ and tMg2+. For 110/208 measurements (52.9%), a reduced iMg2+ was shown. In 58/208 (27.9%), both values were reduced, and 52/208 (25%) had isolated reduced iMg2+. In principal component analysis, patients with isolated low iMg2+ clustered with patients with reduced tMg2+ and not with patients with normal Mg2+ (Figure 3). Considering the well-known association between CNI use and serum Mg2+ levels (16, 19), and the potential risks related to hypomagnesemia in KT patients, the association between Mg2+ values and the use of CNI was investigated. In logistic regression, low iMg2+ correlated with CNI exposure. No other correlating factors were identified, notably excluding factors such as eGFR, KT history, or the antecedent of prior DGF (Table 3).
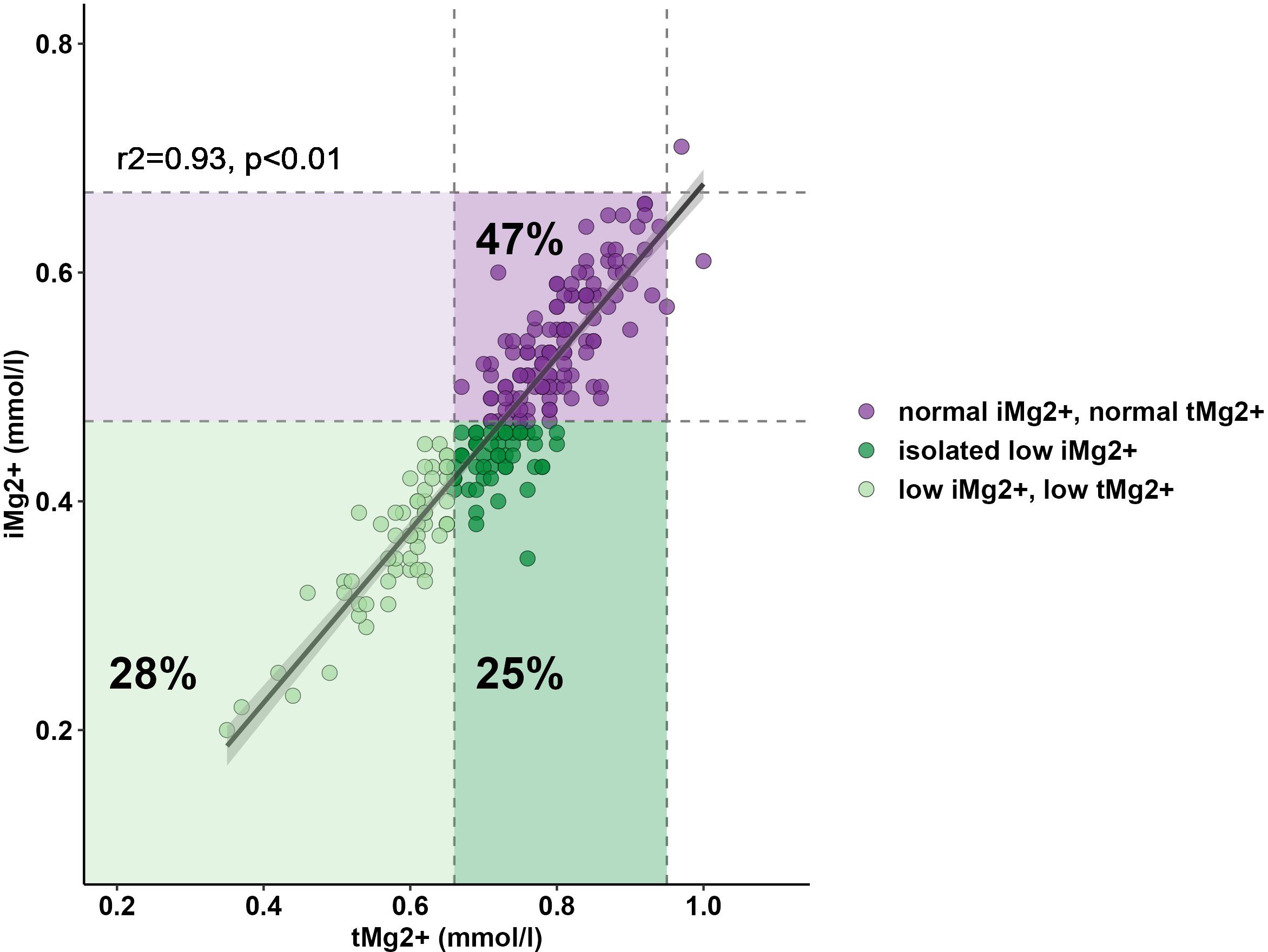
Figure 2 Relationship between serum tMg2+ and iMg2+. Lower reference ranges of tMg2+ (0.66 mmol/L) and iMg2+ (0.42 mmol/L) are shown and delimited with dashed lines.
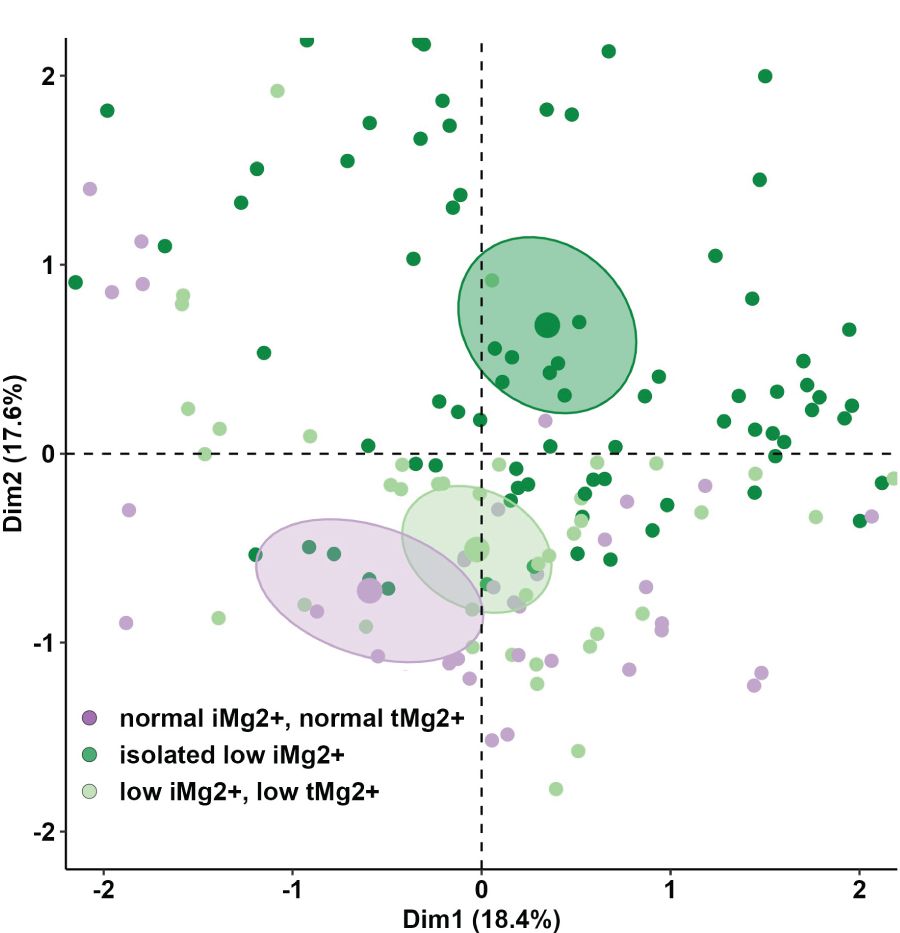
Figure 3 Principal component analysis (PCA) of anthropometric and demographics parameters and lab values of KT participants between the different three groups (normal iMg2+ and normal tMg2+, isolated low iMg2+, low iMg2+ and low tMg2+).
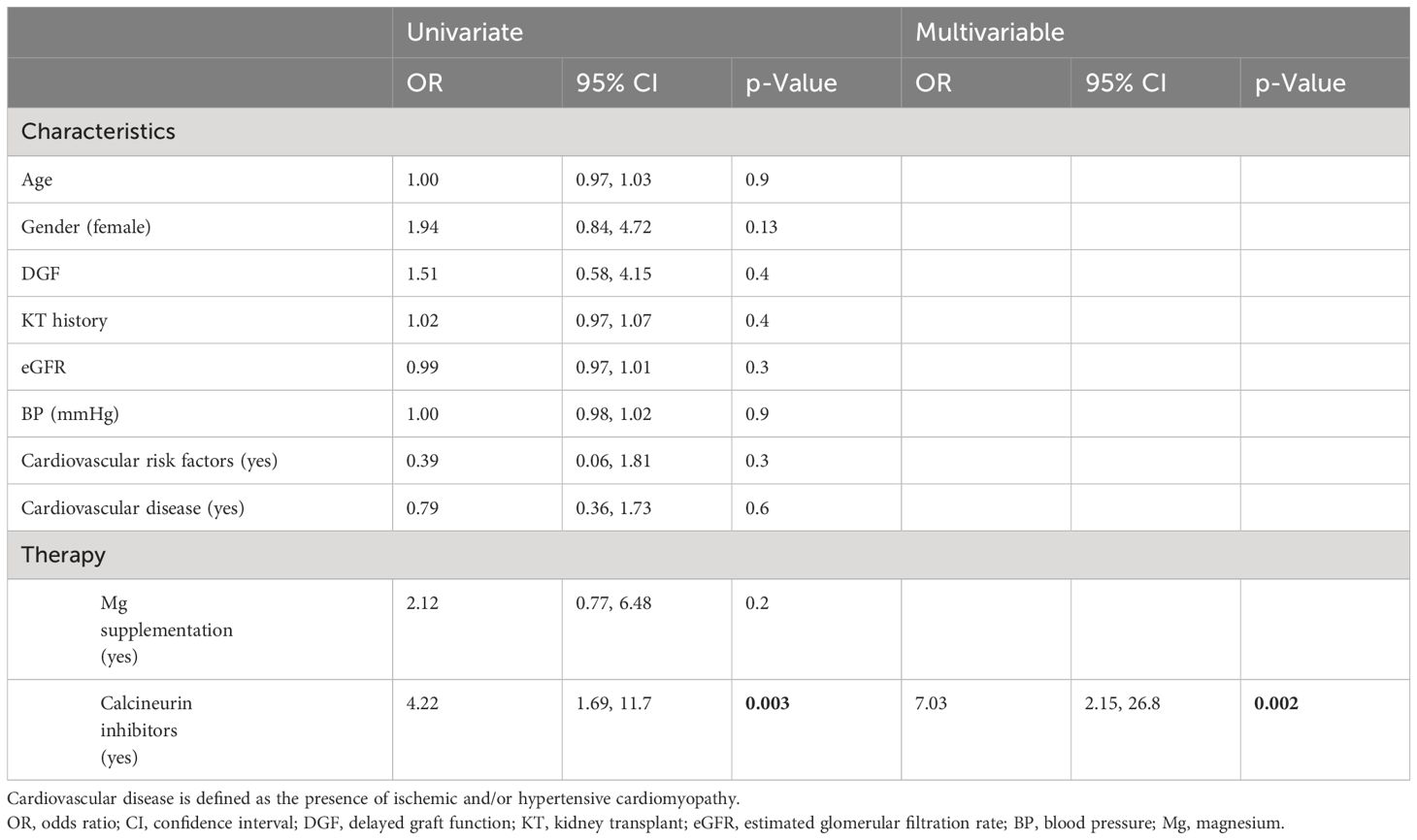
Table 3 Univariate and multivariable logistic regression for the dichotomous secondary endpoint of normal iMg2+ and low iMg2+.
In this study, we investigated the add-on value of assessing serum iMg2+ compared to tMg2+ in detecting KT patients with pathological Mg2+ levels. The ultimate goal was to establish the cross-sectional prevalence of hypomagnesemia and examine its correlation with various clinical, demographic, and anthropometric parameters.
In a study sample including 208 measurements, we found that hypomagnesemia was frequent, with a prevalence of 52.9% (reduced iMg2+ and tMg2+). The notable high percentage contrasts with findings in other studies (20, 21), likely attributable to variations in methodology and the accepted Mg2+ cutoff criteria. This highlights the importance of determining the best measurement method and universal reference values. We also proved a strong correlation between iMg2+ and tMg2+ concentrations. These results have also been found in previous studies (16, 19). Most of our KT patients had CKD with moderate renal failure, and normo-magnesemia was expected. The high prevalence of hypomagnesemia in our study can also be explained by the use of CNI (76% of KT patients received cyclosporine or tacrolimus therapy). Indeed, after analysis of various factors, only the use of CNI correlated statistically significantly with hypomagnesemia. CNI induces hypomagnesemia through urinary Mg2+ wasting by downregulating the renal expression of the epidermal growth factor (EGF) and the Mg2+ channel Transient Receptor Potential Pelastatin 6 (TRMP6) in the distal collecting tubule (1, 22). The incidence of tacrolimus- or cyclosporine-induced hypomagnesemia has been studied in-depth, and these common side effects are now well known in the medical world (20, 21, 23).
Studies demonstrated that hypomagnesemia is associated with unfavorable outcomes and mortality (21, 22). Favoring iMg2+ over tMg2+ could enhance the detection of hypomagnesemia, enabling more accurate identification of patients in need of supplementation. Previous studies have investigated the sensitivity and correlations of measurements of iMg2+ compared to tMg2+ in detecting hypomagnesemia (24, 25). Our study suggests that tMg2+ could represent the active form of Mg2+ and be sufficient to detect hypomagnesemia (strong category agreement and correlation between iMg2+ and tMg2+). In contrast, the PCA illustrated that a significant proportion of patients otherwise considered normo-magnesemic on the basis of tMg2+ can be reclassified as hypomagnesemic if considering only iMg2+. This seems particularly true for patients with short KT follow-up (Supplementary Figure 2). The misclassification could have clinical and therapeutic implications. Non-treated hypomagnesemia is associated with poor outcomes, including allograft function and mortality (26, 27). On the contrary, the administration of Mg2+ supplements is not without side effects. Furthermore, there is a lack of well-established knowledge regarding the influence of substitution therapy notably, on iMg2+ levels, and the appropriate timing for its initiation. While some studies (28) indicate that Mg2+ supplementation leads to an elevation in tMg2+, Rooney et al. demonstrated that Mg2+ substitution did not produce a statistically significant alteration in iMg2+ concentrations (29). These inquiries merit additional investigation.
Our study also has some limitations. First, although extensive in terms of parameters evaluated, it is a prospective and single-center study. Moreover, the study sample was small, limiting the generalizability of our results. In addition, the lack of standardized iMg2+ reference thresholds, inter-personal variability, and relation to the device used is a further obstacle to the generalization of the results. However, the inclusion of a control group allowed us to better define the reference value of Mg2+ and not base it solely on the already known literature, which we further specify is variable and not well defined. Second, although correlations with drugs have been studied, only analyses with immunosuppressant and Mg2+ supplements have been carried out. Exploring additional variables would have been valuable, particularly investigating the usage of other commonly prescribed drugs within the KT population. This could include diuretics and pump proton inhibitors, both known to contribute to urinary Mg2+ losses. Additionally, concerning the use of CNI, we were unable to investigate whether there was a therapy-dependent dose effect on the occurrence of hypomagnesemia. Finally, we could not capture the untoward clinical consequences of the reported ionized hypomagnesemia, such as allograft or cardiac outcomes. The percentage of participants on Mg2+ replacement therapy was small, and we did not study the potential beneficial or damaging effect of supplementation.
In conclusion, the determination of iMg2+ as a standard of care could be useful in recognizing false normo-magnesemic patients, particularly in high-risk populations such as KT patients. Currently, it is unclear if these patients suffer from Mg2+ deficiency and if supplementation is indicated. Further prospective interventional trials are needed.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Medical Ethics Committee of the study centers (Project-ID 2022-01953). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. SS: Conceptualization, Investigation, Validation, Writing – review & editing. UH: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing. BV: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing. DS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Nova Biomedical provided the machine and supplies to perform this study. However, they played no role in any part of the study design and data analysis, nor were they involved in any part of the drafting of the manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneph.2024.1385447/full#supplementary-material
Supplementary Figure 1 | Cohort analyzed. KT : Kidney Transplant.
Supplementary Figure 2 | Percentage of KT participants with normal iMg2+ and normal tMg2+, isolated low iMg2+, low iMg2+ and low tMg2+ according to KT history (0-1 year, 1-3 years and > 3 years).
1. Garnier AS, Duveau A, Planchais M, Subra JF, Sayegh J, Augusto JF. Serum magnesium after kidney transplantation: A systematic review. Nutrients. (2018) 10(6):729. doi: 10.3390/nu10060729
2. Schimatschek HF, Rempis R. Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes Res. (2001) 14:283–90.
3. Dyckner T, Wester PO. Effect of magnesium on blood pressure. Br Med J (Clin Res Ed). (1983) 286:1847–9. doi: 10.1136/bmj.286.6381.1847
4. Li L, Streja E, Rhee CM, Mehrothra R, Soohoo M, Brunelli SM, et al. Hypomagnesemia and mortality in incident hemodialysis patients. Am J Kidney Dis. (2015) 66:1047–55. doi: 10.1053/j.ajkd.2015.05.024
5. Upala S, Jaruvongvanich V, Wijarnpreecha K, Sanguankeo A. Hypomagnesemia and mortality in patients admitted to intensive care unit: a systematic review and meta-analysis. QJM. (2016) 109:453–9. doi: 10.1093/qjmed/hcw048
6. Van Laecke S, Van Biesen W. Hypomagnesaemia in kidney transplantation. Transplant Rev (Orlando). (2015) 29:154–60. doi: 10.1016/j.trre.2015.05.002
7. Lahav I, Steinmetz T, Molcho M, Lev N, Agur T, Nesher E, et al. The association between exposure to low magnesium blood levels after renal transplantation and cardiovascular morbidity and mortality. Front Med (Lausanne). (2021) 8:690273. doi: 10.3389/fmed.2021.690273
8. Dominguez LJ, Veronese N, Guerrero-Romero F, Barbagallo M. Magnesium in infectious diseases in older people. Nutrients. (2021) 13(1):180. doi: 10.3390/nu13010180
9. Odler B, Deak AT, Pregartner G, Riedl R, Bozic J, Trummer C, et al. Hypomagnesemia is a risk factor for infections after kidney transplantation: A retrospective cohort analysis. Nutrients. (2021) 13(4):1296. doi: 10.3390/nu13041296
10. Huang JW, Famure O, Li Y, Kim SJ. Hypomagnesemia and the risk of new-onset diabetes mellitus after kidney transplantation. J Am Soc Nephrol. (2016) 27:1793–800. doi: 10.1681/ASN.2015040391
11. Mazzola BL, Vannini SD, Truttmann AC, von Vigier RO, Wermuth, Ferrari P, et al. Long-term calcineurin inhibition and magnesium balance after renal transplantation. Transpl Int. (2003) 16:76–81. doi: 10.1111/j.1432-2277.2003.tb00267.x
12. Wang S, McDonnell EH, Sedor FA, Toffaletti JG. pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med. (2002) 126:947–50. doi: 10.5858/2002-126-0947-PEOMOI
13. Dimeski G, Treacy O. The influence of albumin and pH on total and ionized calcium and magnesium. Point Care. (2018) 17:123–6. doi: 10.1097/POC.0000000000000173
14. Ordak M, Maj-Zurawska M, Matsumoto H, Bujalska-Zadrozny, Kieres-Salomonski, Nasierowski T, et al. Ionized magnesium in plasma and erythrocytes for the assessment of low magnesium status in alcohol dependent patients. Drug Alcohol Depend. (2017) 178:271–6. doi: 10.1016/j.drugalcdep.2017.04.035
15. Barbagallo M, Di Bella G, Brucato V, D'Angelo D, Damiani P, Monteverde A, et al. Serum ionized magnesium in diabetic older persons. Metabolism. (2014) 63:502–9. doi: 10.1016/j.metabol.2013.12.003
16. Saha H, Harmoinen A, Nisula M, Pasternack A. Serum ionized versus total magnesium in patients with chronic renal disease. Nephron. (1998) 80:149–52. doi: 10.1159/000045158
17. Dent A, Selvaratnam R. Measuring magnesium - Physiological, clinical and analytical perspectives. Clin Biochem. (2022) 105–106:1–15. doi: 10.1016/j.clinbiochem.2022.04.001
18. Hutten TJA, Sikma MA, Stokwielder RH, Wesseling M, Hoefer IE, Tiel Groenestege WM. Ionized and not total magnesium as a discriminating biomarker for hypomagnesaemia in continuous venovenous hemofiltration patients. Nephrol Dial Transplant. (2021) 36:742–3. doi: 10.1093/ndt/gfaa330
19. Hasson DC, Mohan S, Rose JE, Merrill KA, Goldstein SL, Benoit SW, et al. Ionized magnesium correlates with total blood magnesium in pediatric patients following kidney transplant. Ann Lab Med. (2024) 44:21–8. doi: 10.3343/alm.2024.44.1.21
20. Rodrigues N, Santana A, Guerra J, Neves M, Nascimento C, Gonçalves J, et al. Serum magnesium and related factors in long-term renal transplant recipients: an observational study. Transplant Proc. (2017) 49:799–802. doi: 10.1016/j.transproceed.2017.01.070
21. Holzmacher R, Kendziorski C, Michael Hofman R, Jaffery J, Becker B, Djamali A. Low serum magnesium is associated with decreased graft survival in patients with chronic cyclosporin nephrotoxicity. Nephrol Dial Transpl. (2005) 20:1456–62. doi: 10.1093/ndt/gfh831
22. Stefanelli LF, Alessi M, Bertoldi G, Rossato V, Di Vico V, Nalesso F, et al. Calcineurin-inhibitor-induced hypomagnesemia in kidney transplant patients: A monocentric comparative study between sucrosomial magnesium and magnesium pidolate supplementation. J Clin Med. (2023) 12(3):752. doi: 10.3390/jcm12030752
23. Navaneethan SD, Sankarasubbaiyan S, Gross MD, Jeevanantham V, Monk RD. Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant Proc. (2006) 38:1320–2. doi: 10.1016/j.transproceed.2006.02.077
24. Koch SM, Warters RD, Mehlhorn U. The simultaneous measurement of ionized and total calcium and ionized and total magnesium in intensive care unit patients. J Crit Care. (2002) 17:203–5. doi: 10.1053/jcrc.2002.35813
25. Greenway DC, Hindmarsh JT, Wang J, Khodadeen JA, Hébert PC. Reference interval for whole blood ionized magnesium in a healthy population and the stability of ionized magnesium under varied laboratory conditions. Clin Biochem. (1996) 29:515–20. doi: 10.1016/S0009-9120(96)00091-4
26. Soliman HM, Mercan D, Lobo SS, Mélot C, Vincent JL. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med. (2003) 31:1082–7. doi: 10.1097/01.CCM.0000060867.17556.A0
27. Escuela MP, Guerra M, Añón JM, Martínez-Vizcaíno V, Zapatero MD, García-Jalón A, et al. Total and ionized serum magnesium in critically ill patients. Intensive Care Med. (2005) 31:151–6. doi: 10.1007/s00134-004-2508-x
28. Lutsey PL, Chen LY, Eaton A, Jaeb M, Rudser KD, Neaton JD, et al. A pilot randomized trial of oral magnesium supplementation on supraventricular arrhythmias. Nutrients. (2018) 10(7):884. doi: 10.3390/nu10070884
Keywords: magnesium, total magnesium, ionized magnesium, hypomagnesemia, kidney transplant
Citation: Bocchi F, Schietzel S, Huynh-Do U, Vogt B and Sidler D (2024) Magnesium matters: unveiling hidden risks in kidney transplant patients through total and ionized magnesium profiling. Front. Nephrol. 4:1385447. doi: 10.3389/fneph.2024.1385447
Received: 12 February 2024; Accepted: 02 July 2024;
Published: 16 July 2024.
Edited by:
Phuong-Thu Pham, University of California, Los Angeles, United StatesReviewed by:
Gaurav Gupta, Virginia Commonwealth University, United StatesCopyright © 2024 Bocchi, Schietzel, Huynh-Do, Vogt and Sidler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Bocchi, ZmVkZXJpY2Fib2NjaGkxNkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.