
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Nephrol., 19 June 2023
Sec. Cardionephrology
Volume 3 - 2023 | https://doi.org/10.3389/fneph.2023.1194989
This article is part of the Research TopicKidney and Heart Cross-talkView all 10 articles
A correction has been applied to this article in:
Corrigendum: Case report: Multisystem inflammatory syndrome in children with associated proximal tubular injury
 Silvia Maria Orsi1
Silvia Maria Orsi1 Carlotta Pepino1
Carlotta Pepino1 Lisa Rossoni1
Lisa Rossoni1 Margherita Serafino2
Margherita Serafino2 Roberta Caorsi3
Roberta Caorsi3 Stefano Volpi3
Stefano Volpi3 Serena Palmeri1,3
Serena Palmeri1,3 Alessandro Faragli4
Alessandro Faragli4 Francesca Lugani5,6
Francesca Lugani5,6 Carolina Bigatti5,6
Carolina Bigatti5,6 Gian Marco Ghiggeri5,6
Gian Marco Ghiggeri5,6 Enrico Eugenio Verrina5,7
Enrico Eugenio Verrina5,7 Edoardo La Porta5,7*†
Edoardo La Porta5,7*† Andrea Angeletti5,6
Andrea Angeletti5,6Introduction: SARS-CoV-2 infection in the pediatric population can be associated with a multiorgan inflammatory syndrome called children’s multisystem inflammatory syndrome (MIS-C). The kidneys can be affected by a broad spectrum of possible injuries, whose pathogenetic mechanisms are still unclear.
We report the case of a 5-year-old boy with severe cardiac involvement in the context of MIS-C. After two weeks of hospitalization, an abdominal ultrasound showed massive bladder “debris”, followed by the onset of normoglycemic glycosuria. Over time, there was a progressive increase in glycosuria, and the presence of a mat of amorphous phosphate crystals was evidenced on urinary sediment. Together with the findings of hypo-uricemia, increased urinary uric acid, and globally increased urinary amino acids, a clinical picture of kidney proximal tubular damage with secondary Fanconi-like syndrome took shape.
Discussion: This case report describes the case of a patient with MIS-C with cardiac and kidney involvement characterized by proximal tubular damage, which slowly improved but still persisted at the 8-month follow-up. The pathogenesis of the damage is unclear and probably multifactorial.
Kidney dysfunction is a common consequence of SARS-CoV-2 infection (1, 2), having been reported in adults and, to a lesser degree, in children. Kidney consequences of COVID-19 can include a broad spectrum of damages, ranging from acute kidney injury (AKI) with glomerular or tubular injury to mild proteinuria and/or hematuria (3).
In children, SARS-CoV-2 infection can manifest as a multisystem inflammatory syndrome (MIS-C) that typically occurs 3–6 weeks after mild or asymptomatic COVID-19 disease (4). This rare disorder is characterized by a hyperinflammatory state with a range of clinical presentations that can involve multiple organs with a generalized increase in inflammatory biomarkers, such as C-reactive protein (CRP), ferritin, D-dimer, and lactate dehydrogenase (5, 6). Common manifestations include fever, rash, abdominal pain, and gastrointestinal symptoms, mimicking appendicitis in some children (4). Cardiological involvement is characterized by diminished left ventricular systolic function with or without coronary artery abnormalities, fluid overload or hypotension, and an increase in pro-brain natriuretic peptide and cardiac enzymes. The kidneys can be affected by a broad spectrum of possible injuries; the incidence of AKI ranges from 10% to 46% (7) and its pathogenetic mechanisms are still unclear and probably multifactorial (8, 9).
Here, we report a case of MIS-C with peculiar nephro-urological involvement and ultra-sonographic features characterized by proximal tubule dysfunction.
A previously healthy 5-year-old boy was admitted to hospital in February 2022 due to fever lasting for 6 days with spikes up to 40°C, accompanied by vomiting, diarrhea, and intense abdominal pain. Because of suspected intestinal adenitis with neutrophilic leukocytosis and markedly increased CRP (26 mg/dL n.l. < 0.46), the patient underwent a laparoscopic appendicectomy that showed an uninjured appendix. Considering the recent paucisymptomatic SARS-CoV-2 infection confirmed by an antigenic pharyngo-nasal swab test and the persistent fever unresponsive to antibiotics, further investigations were carried out. Blood tests revealed the presence of a hypochloremic metabolic alkalosis and increased blood urea nitrogen (max value 60mg/dl), troponin I, and NT-Pro-BNP, while other parameters were normal, including urine examination (see Table 1). On admission, Creatinine was 0.4 mg/dL. It then progressively decreased and stabilized at 0.2-0.25 mg/dL, together with the decrease in blood urea nitrogen values. The echocardiogram demonstrated diffuse hypokinesia with a reduced left ventricular ejection fraction (LVEF 35%), a right ventricle with volumetric overload, and a left coronary artery ectasia. The case was suggestive of MIS-C; therefore, steroids (methylprednisolone 30mg/kg), immunoglobulins (IGIV 1gr/kg), and immunosuppressive therapy with anti-IL-1 receptor antagonist (Anakinra 200mg twice daily) were started. In addition, supportive therapy with furosemide was administered and, on day 14, an ACE inhibitor drug (enalapril 0.05mg/kg) was added due to persisting high values of arterial blood pressure. Finally, anticoagulant therapy with heparin (2000 IU/day) was started and was later replaced by anti-platelet therapy (aspirin 75 mg/day) on day 11 (see Figure 1).
The therapy was well tolerated, and progressive clinical, laboratory, and instrumental improvements were observed. In particular, the echocardiograms showed a progressive normalization of LVEF and the left coronary artery (Figure 1).
On day 18, during an abdominal ultrasound performed as surgery follow-up, the presence of bladder debris was discovered (Figures 2A, B), despite the absence of other signs of urinary tract infection.
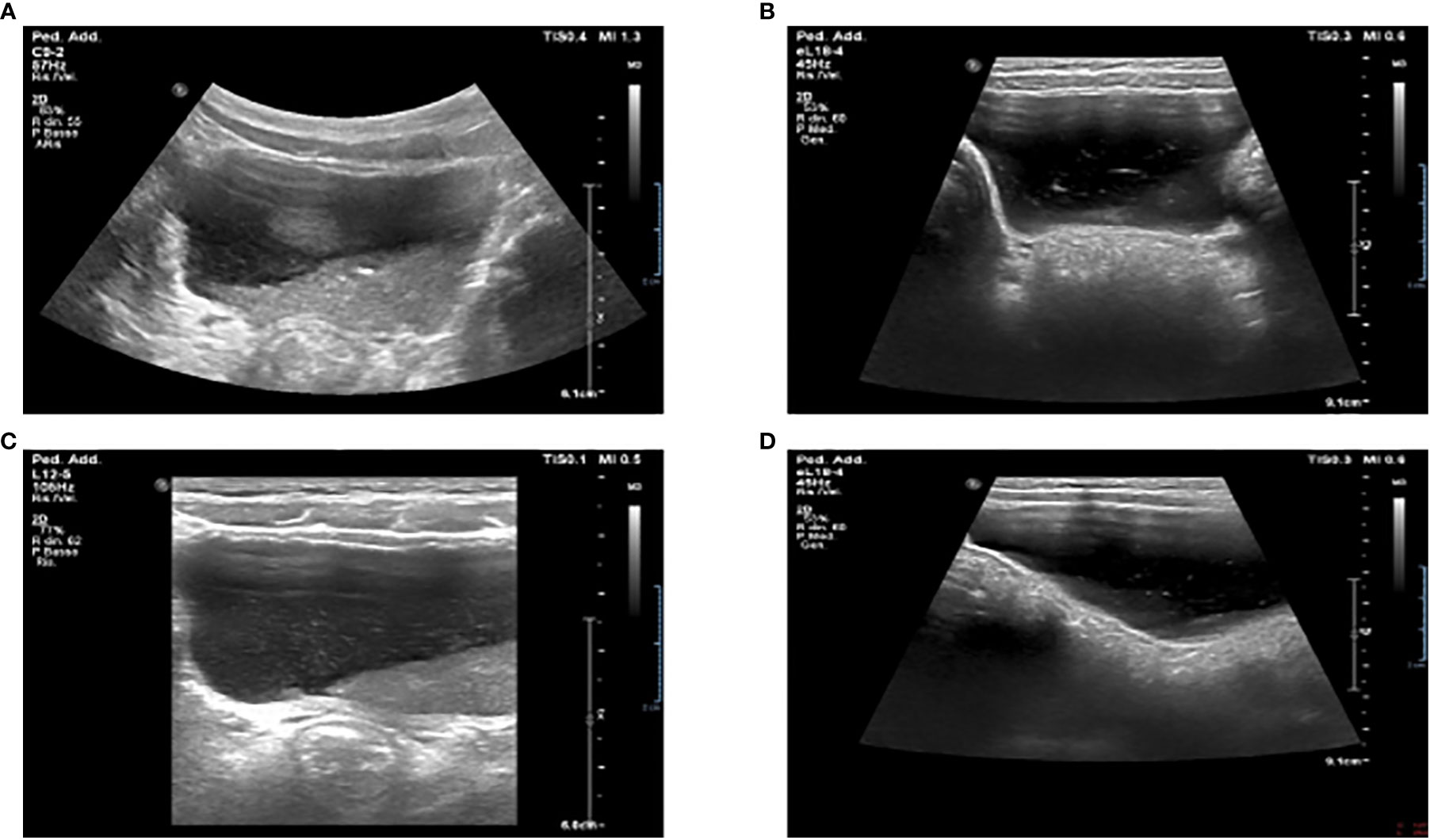
Figure 2 Ultrasound examination showing massive bladder debris in a 5-year-old child affected by MIS-C (A, B). First incidental finding of massive sediment and floating bladder debris at abdominal ultrasound examination. (C, D). Reduction of the debris after X days at ultrasound control examination.
Thus, more examinations were performed. They showed a persistent normal renal function with normoglycemic glycosuria, confirmed by the urinary sediment and the 24-hour urine collection (glucose 1.1 g/24h n.l < 0.1), together with hypercalciuria (rCaU/CrU 0.66 n.l < 0.2) and mild proteinuria (0.18 g/24h n.l. < 0.15). In addition, the urinary sediment reported numerous amorphous phosphate crystals. Due to suspicions of Fanconi syndrome, further tests were performed: uricemia had decreased (1,6 mg/dl n.l. > 2) and hyperuricosuria was found together with a significant increase in all urinary amino acids excreted (see Table 1). During the ultrasound examination performed on day 29, a sharp decrease of multiple echoes of hyperechogenic corpuscular material in suspension was reported, with no morphological abnormalities of the kidneys and urinary tract (Figures 2C, D).
Thus, after a few days, the patient was discharged in good condition, with normal echocardiography and urinary exams. They were given instructions to continue with oral steroid therapy and antiplatelet and antihypertensive therapy, which were later stopped due to the normalization of blood pressure values. At the 8-month follow-up, no bladder debris was present upon ultrasound examination, while urinalysis showed an improvement of persistent proximal renal tubule damage characterized by increased amino acids, sodium chloride, and uric acid excretion.
We have described the case of a child diagnosed with MIS-C with severe cardiac involvement, who experienced alterations in urinalysis consisting of normo-glycemic glycosuria, amino-aciduria, hyperuricosuria, and the presence of amorphous phosphate crystals. These findings, together with decreased uricemia, are consistent with proximal tubule damage, more precisely, a Fanconi-like syndrome tubulopathy that persists over months. MIS-C is thought to be an exaggerated immune response to SARS-CoV-2 infection, but the exact pathogenesis of multiorgan dysfunction is still unknown. Very few studies have described the incidence and characteristics of renal complications in MIS-C. AKI is frequently reported in children diagnosed with this disorder (7–10), but no studies have specifically reported acute tubular involvement.
The pathophysiology of renal dysfunction seems to be multifactorial. Hemodynamic, iatrogenic, viral, or immune-mediated causes could have all contributed to the development of both pre-renal and renal parenchymal effects (7). Firstly, the reduced cardiac ejection fraction and the capillary leakage due to the inflammatory state both lead to kidney hypoperfusion and consequent ischemic damage, ischemic tubular damage. Secondly, our patient experienced a subclinical AKI, with increased values of blood urea nitrogen; creatinine, albeit not enough to meet the KDIGO (Kidney Disease Improving Global Outcomes) criteria for AKI; and elevated serum urea/creatinine ratio. Moreover, the contribution of drug toxicity to kidney injury cannot be excluded. Diuretics and iACE could have also contributed to glomerular hypoperfusion and consequent pre-renal damage. However, iatrogenic kidney damage due to steroids, IL-1 receptor antagonists, or immunoglobulins is very unlikely and can be excluded. Finally, a possible contribution of immune overactivation or a direct kidney tropism of the SARS-CoV-2 virus leading to tubular injury and podocytopathy cannot be ruled out. SARS-CoV-2 virus is suggested to reach proximal tubule and podocytes through spike (S) glycoprotein and ACE-2 receptor binding, and the consequent transmembrane serine proteases (TMPRSSs) action, which facilitates membrane fusion (7, 11–13). In the human kidney, ACE-2 and TMPRSSs are expressed in the nephron and demonstrate high tropism, primarily in the proximal tubule apical membrane, along with other proteases necessary for SARS-CoV-2 (14, 15). The development of a hyperinflammatory state with similar aspects to cytokine release syndrome has been hypothesized, with a possible crucial role of IL-6 IL-2R (7) in worsening renal function.
Proximal tubule dysfunction in adults was investigated in a recent study by A. Werion et al. (12). They showed that the dysfunction occurs early during the course of SARS-CoV-2 infection, and it is characterized by low molecular weight proteinuria, defective handling of uric acid and phosphate, and aminoaciduria. Normoglycemic glycosuria was not evidenced in their cohort of patients. Moreover, the aminoaciduria they detected in 46% of patients tested was limited to neutral amino acids, while in our patient, a generalized aminoaciduria was found.
Our patient did not present a clinical AKI, but the results obtained are coherent with an alteration of the proximal renal tubular structure. Because we chose not to perform a kidney biopsy, the exact etiology of the damage is unknown, and we can only speculate on the possible causes behind it. The abovementioned presence of bladder debris could be associated with a concomitant urinary tract infection (16), a clinical condition that was ruled out in our patient. Nevertheless, the significance of bladder debris in the alteration of urine analysis is still unclear and deserves further study (17). In our case, we ruled out the presence of urinary tract infection. Thus, the significance of bladder debris is attributable to an aspecific urine alteration with a massive presence of phosphate crystals.
Subclinical AKI, defined as the presence of kidney dysfunction not meeting the criteria for AKI, has been described in SARS-CoV-2 infection. Even if clinical data are still poorly known, especially in MIS-C, it seems to be correlated to a more severe course of the disease (18). Our patient experienced a subclinical AKI by presenting, at admission, serum creatinine values that were double those when discharged. These persisted for 10 days before starting to decrease. A few days after reaching the peak of serum creatine, abdominal US was performed with the incidental evidence of bladder debris and urinary alterations. Unfortunately, no urine examinations were performed before this because there was no suspicion of nephron-urological involvement. We, therefore, cannot determine exactly when kidney tubular injury arose.
Increasing evidence suggests that subclinical AKI and urinary alterations are clinically significant and independently associated with adverse outcomes (19, 20). It also underlines the important role of urinary biomarkers and urinary analysis in the recognition of precocious subclinical and clinical AKI (21, 22).
Regardless of the etiopathology of the tubular injury, this illustrative case aims to emphasize the relevance of urinary examinations in this clinical setting, with the ultimate goal of aiding the early recognition of clinical and subclinical AKI.
The primary goals of therapy in MIS-C are to reduce systemic inflammation, to give hemodynamic support in cases of cardiac dysfunction, and to treat singular organ involvement. Most widely used pharmacologic approaches consider IVIG and steroids, anakinra (IL-R1 antagonist), infliximab (TNF-alfa blocker), or tocilizumab (IL-6 antagonist) in cases of persistent inflammatory state and poor response to first-line therapy (23). If renal damage is present, the therapy is based, at first, on renal supportive care, optimizing hemodynamics through infusive therapy or diuretics, depending on the volemic status of the patient. Critical cases could require kidney replacement therapy (7).
In conclusion, we suggest considering possible renal involvement in cases of MIS-C, and in particular, assessing renal function and performing frequent urine tests in order to recognize AKI and dysfunction of the kidney proximal tubule as early as possible.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
SO and EP contributed to the conception of the study and wrote the paper. MS, AA, GMG, CP, SV, SP, AF, FL, CB and EV reviewed the manuscript and contributed to the final draft. All authors contributed to the article and approved the submitted version.
The APC was supported by Italian Ministry of Health “Ricerca Corrente” Annual Program 2023. The funder had no role in the preparation, review, or approval of the manuscript nor in the decision to submit the manuscript for publication.
The authors GMG declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. La Porta E, Baiardi P, Fassina L, Faragli A, Perna S, Tovagliari F. The role of kidney dysfunction in COVID-19 and the influence of age. Sci Rep (2022) 12(1):8650. doi: 10.1038/s41598-022-12652-0
2. Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int (2020) 97(5):824–8. doi: 10.1016/j.kint.2020.03.001
3. Serafinelli J, Mastrangelo A, Morello W, Cerioni VF, Salim A, Nebuloni M, et al. Kidney involvement and histological findings in two pediatric COVID-19 patients. Pediatr Nephrol (2021) 36(11):3789–93. doi: 10.1007/s00467-021-05212-7
4. Gottlieb M, Bridwell R, Ravera J, Long B. Multisystem inflammatory syndrome in children with COVID-19. Am J Emergency Med (2021) 49:148–52. doi: 10.1016/j.ajem.2021.05.076
5. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
6. Centers for disease control and prevention: multisystem inflammatory syndrome (MIS-c): health department–reported cases of multisystem inflammatory syndrome (MIS-c) in the united states (2020). Available at: https://www.cdc.gov/mis/mis-c/hcp_cstecdc/index.html.
7. Sethi SK, Rana A, Adnani H, McCulloch M, Alhasan K, Sultana A, et al. Kidney involvement in multisystem inflammatory syndrome in children: a pediatric nephrologist’s perspective. Clin Kidney J (2021) 14(9):2000–11. doi: 10.1093/ckj/sfab073
8. Tripathi AK, Pilania RK, Bhatt GC, Atlani M, Kumar A, Malik S. Acute kidney injury following multisystem inflammatory syndrome associated with SARS-CoV-2 infection in children: a systematic review and meta-analysis. Pediatr Nephrol (2023) 38(2):357–70. doi: 10.1007/s00467-022-05701-3
9. Grewal MK, Gregory MJ, Jain A, Mohammad D, Cashen K, Ang JY, et al. Acute kidney injury in pediatric acute SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-c): is there a difference? Front Pediatr (2021) 9:692256. doi: 10.3389/fped.2021.692256
10. Lipton M, Mahajan RG, Kavanagh C, Shen CL, Batal I, Dogra S, et al. Acute kidney injury in COVID-19-associated multisystem inflammatory syndrome in children (MIS-c). Kidney360 (2021) 2:611–8. doi: 10.34067/KID.0005372020
11. Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. COVID-19 and ACE2 in cardiovascular, lung, and kidney working group. acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol (2020) 31(7):1380–3. doi: 10.1681/ASN.2020040419
12. Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int (2020) 98(5):1296–307. doi: 10.1016/j.kint.2020.07.019
13. Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med (2020) 46:1114–6. doi: 10.1007/s00134-020-06026-1
14. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019- nCoV infection. Front Med (2020) 14:185–92. doi: 10.1007/s11684-020-0754-0
15. Rahmani W, Chung H, Sinha S, Bui-Marinos MP, Arora R, Jaffer A. Attenuation of SARS-CoV-2 infection by losartan in human kidney organoids. IScience (2022) 25(2):103818. doi: 10.1016/j.isci.2022
16. McQuaid JW, Kurtz MP, Logvinenko T, Nelson CP. Bladder debris on renal and bladder ultrasound: a significant predictor of positive urine culture. J Pediatr Urol (2017) 385:e1–385.e5. doi: 10.1016/j.jpurol.2017.04.020
17. Cheng SN, Phelps A. Correlating the sonographic finding of echogenic debris in the bladder lumen with urinalysis. J Ultrasound Med (2016) 35(7):1533–40. doi: 10.7863/ultra.15.09024
18. Silva-Aguiar RP, Teixeira DE, Peres RAS, Peruchetti DB, Gomes CP, Schmaier AH. Subclinical acute kidney injury in COVID-19: possible mechanisms and future perspectives. Int J Mol Sci (2022) 23(22):14193. doi: 10.3390/ijms232214193
19. Fang F, Hu X, Dai X, Wang S, Bai Z, Chen J. Subclinical acute kidney injury is associated with adverse outcomes in critically ill neonates and children. Crit Care (2018) 22:256. doi: 10.1186/s13054-018-2193-8
20. Zou C, Wang C, Lu L. Advances in the study of subclinical AKI biomarkers. Front Physiol (2022) 13:960059. doi: 10.3389/fphys.2022.960059
21. Perazella MA, Cosa S. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol (2010) 5(3):402–8. doi: 10.2215/CJN.06960909
22. Li N, Zhou WJ. Association between urine microscopy and severe acute kidney injury in critically ill patients following non-cardiac surgery: a prospective cohort study. Ann Palliative Med (2022) 22(7):2327–37. doi: 10.21037/apm-21-3085
23. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol (2022) 74(4):e1–e20. doi: 10.1002/art.42062
Keywords: MIS-C multisystem inflammatory syndrome in children, proximal tubule injury, bladder debris, Fanconi syndrome, kidney injury, SARS – CoV – 2
Citation: Orsi SM, Pepino C, Rossoni L, Serafino M, Caorsi R, Volpi S, Palmeri S, Faragli A, Lugani F, Ghiggeri GM, Marco G, Verrina EE, La Porta E and Angeletti A (2023) Case Report: Multisystem inflammatory syndrome in children with associated proximal tubular injury. Front. Nephrol. 3:1194989. doi: 10.3389/fneph.2023.1194989
Received: 27 March 2023; Accepted: 30 May 2023;
Published: 19 June 2023.
Edited by:
Sara Samoni, Sant’Anna School of Advanced Studies, ItalyReviewed by:
Mariadelina Simeoni, University of Campania Luigi Vanvitelli, ItalyCopyright © 2023 Orsi, Pepino, Rossoni, Serafino, Caorsi, Volpi, Palmeri, Faragli, Lugani, Bigatti, Ghiggeri, Verrina, La Porta and Angeletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edoardo La Porta, ZWRvYXJkb2xhcG9ydGFAZ2FzbGluaS5vcmc=
†ORCID: Edoardo La Porta, orcid.org/0000-0003-2368-1139
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.