- 1Department of Pharmacy Practice and Science, University of Kentucky College of Pharmacy, Lexington, KY, United States
- 2Department of Pharmacy Services, University of Kentucky HealthCare, Lexington, KY, United States
- 3Division of Nephrology, Bone & Mineral Metabolism, Department of Internal Medicine, University of Kentucky College of Medicine, Lexington, KY, United States
Introduction: The combination of vancomycin/piperacillin-tazobactam is associated with increases in serum creatinine compared to other antibiotic combinations in the treatment of infections for hospitalized patients. However, the available literature is limited to the study of incident acute kidney injury (AKI). The combination has not been evaluated in patients with AKI already present and the degree to which the trajectory of AKI is influenced by this combination is unknown.
Methods: This was a single center, retrospective cohort study of adult patients with sepsis and AKI present on admission prescribed a combination of vancomycin with either piperacillin-tazobactam or cefepime within the first 3 days of admission. The primary outcome was maximum serum creatinine observed within days 2-7 of the hospital stay. Subsequent kidney outcomes were evaluated at one week and hospital discharge.
Results: Of 480 patients with sepsis and AKI who met inclusion criteria, 288 (60%) received vancomycin/piperacillin-tazobactam, and 192 (40%) received vancomycin/cefepime. Patients were well-matched on clinical factors, including severity of illness, stage of AKI, exposure to other nephrotoxins, and durations of antimicrobial therapy. There were no differences in AKI trajectory during the first week as assessed by maximum serum creatinine (2.1 (1.4-3.5) mg/dl vs. 2.1 (1.4-3.0) mg/dl; p=0.459) and AKI progression (24.0% vs. 23.4%; p=0.895). No differences were observed with other kidney related outcomes, including the need for dialysis (14.6% vs. 13.0%; p=0.628) or major adverse kidney events at hospital discharge (48.3% vs. 47.9%; p=0.941).
Conclusions: In patients with sepsis and AKI, the combination of vancomycin/piperacillin-tazobactam compared to vancomycin/cefepime was not associated with higher serum creatinine values or AKI progression in the week following ICU admission.
Introduction
In recent years, several observational studies have suggested the combination of vancomycin and piperacillin-tazobactam is associated with increased acute kidney injury (AKI) compared to the combination of vancomycin with other beta-lactam antibiotics such as cefepime or meropenem (1–3). The entirety of data available involve incident AKI; thus patients with AKI are often excluded. However, vancomycin combined with a beta-lactam is a common empiric antibiotic prescription for patients with sepsis, and up to 42% of patients with sepsis will present with concurrent AKI, often termed sepsis-associated AKI (SA-AKI) (4). Thus, consideration to potential nephrotoxicity from these antibiotic combinations is a common clinical challenge when caring for patients with SA-AKI.
These data appear to be impacting clinical practice, with a recent survey indicating a majority of clinicians are less likely to recommend combination therapy with vancomycin and piperacillin-tazobactam in a patient with preexisting AKI (5). The downstream effects of alternative combination regimens in SA-AKI include concerns with neurotoxicity (cefepime), lack of anaerobic coverage (cefepime), and antibiotic stewardship (meropenem) (6). Further, kidney function at discharge for patients with SA-AKI is predictive of long-term kidney complications (7), and it is unknown how the potential nephrotoxicity of these different antibiotic combinations may impact the progression, or resolution, of SA-AKI out to hospital discharge. Accordingly, we sought to evaluate the progression of kidney injury in SA-AKI patients prescribed the combination of vancomycin and piperacillin-tazobactam compared to vancomycin and cefepime.
Materials and methods
Study design
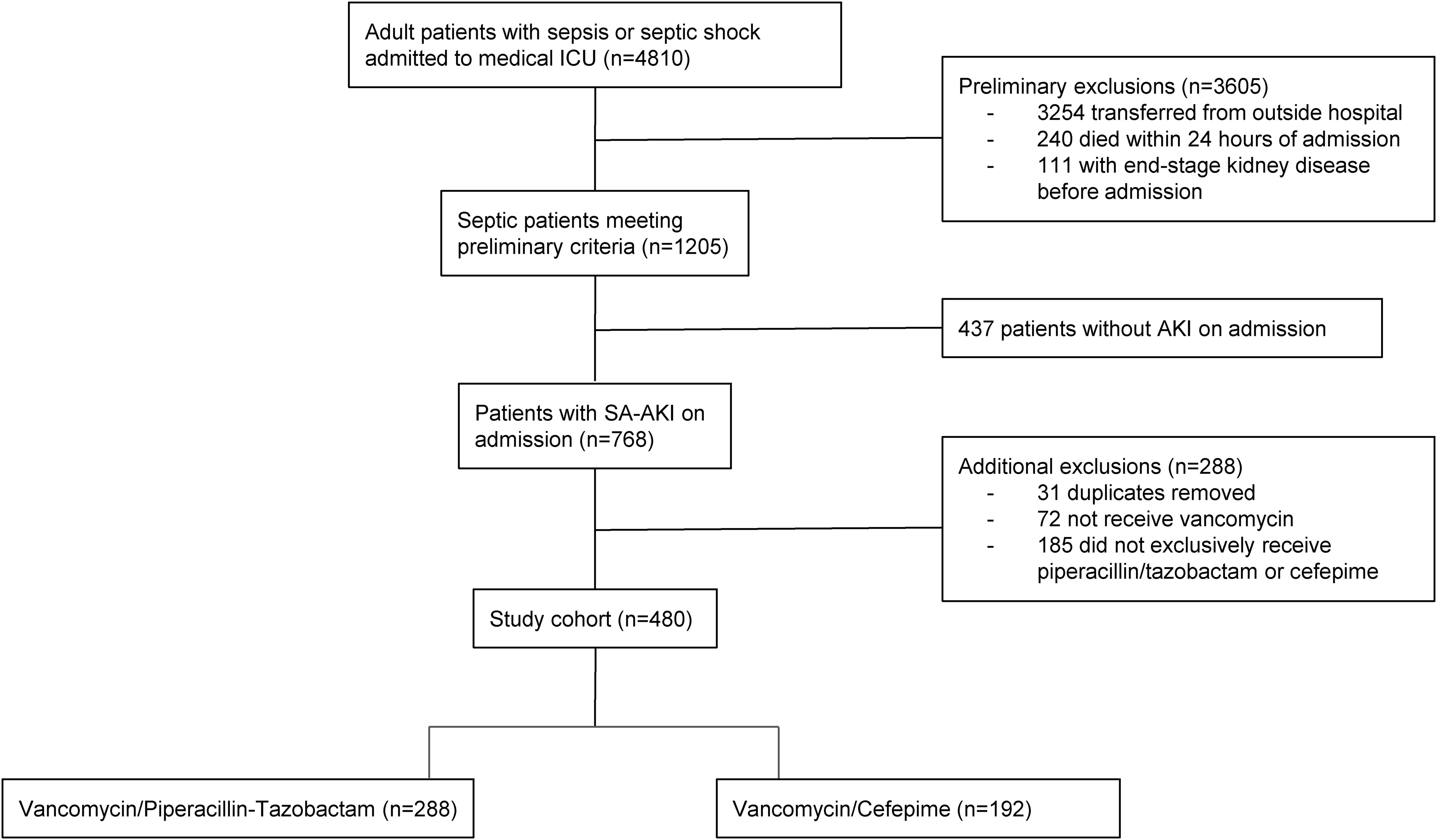
This was a single center, retrospective cohort study of critically ill adult patients admitted to the medical intensive care unit (ICU) of a tertiary care hospital with SA-AKI over a 7.5-year period (January 1, 2013-July 31, 2020). Patients were initially considered if they were ≥ 18 years of age and presented with both sepsis (including septic shock) and AKI on admission. Patients with sepsis or septic shock were identified based on International Classification of Diseases (ICD)-9 and ICD-10 codes, while AKI was classified as stage 1 AKI or higher using the serum creatinine component of the Kidney Disease: Improving Global Outcomes (KDIGO) criteria (8). Exclusion criteria included: transfer from an outside hospital, death within 24 hours of admission, or end-stage kidney disease. If multiple ICU admissions were identified during the time period, only the first ICU encounter was included in the analysis.
Patient demographics, comorbidities, and severity of illness measures were extracted from the Enterprise Data Warehouse at the University of Kentucky. Comorbidities were identified based on ICD-9 and -10 codes and severity of illness using the Sequential Organ Failure Assessment (SOFA) score, which was calculated electronically upon data extraction (9). Baseline kidney function was assessed using the most recent serum creatinine in the six months prior to admission, when available. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate estimated glomerular filtration rate (eGFR) (10).
Patients were classified into two groups based on their receipt of vancomycin in combination with either piperacillin-tazobactam or cefepime within the first 72 hours of ICU admission. Given that vancomycin is often dosed intermittently in the setting of AKI, vancomycin exposure was defined as one or more doses of vancomycin received on day 1 or 2 of ICU admission. Further, patients must have received at least 2 consecutive days (within the first 72 hours) of piperacillin-tazobactam or cefepime to be classified into those respective groups. In order to strictly define the exposure of interest, patients were excluded if they received more than one beta-lactam antibiotic within the first 72 hours of ICU admission. Antibiotic prescribing was at the discretion of the treating medical team; no formal protocols existed to direct selection of either piperacillin-tazobactam or cefepime over the other during the time period evaluated. Due to the minimal number of patients that received solely the combination of vancomycin and meropenem, we excluded patients who were given this combination in order to focus the comparison on piperacillin/tazobactam and cefepime combination therapy with vancomycin.
The primary outcome compared between groups was the maximum serum creatinine value between days 2 and 7. This outcome was selected to evaluate the patient’s kidney function within the first week of ICU admission for as long as possible, given the competing event of mortality common in sepsis research. Secondary outcomes included AKI progression within the first 7 days (defined as an advancement in KDIGO stage from admission), composite of Acute Kidney Disease (AKD) based on the Acute Disease Quality Initiative consensus definition (11) and death within 7 days, requirement for kidney replacement therapy (KRT) within 7 days, ICU mortality, ICU and hospital length of stay, and Major Adverse Kidney Events (MAKE) assessed at hospital discharge, a composite of death, need for KRT, or decrease in eGFR ≥25% from baseline (12). The study was approved by the Institutional Review Board (IRB) at the University of Kentucky with patient follow-up for all outcomes truncated at discharge. The methods and findings are reported per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies (13).
Statistical analysis
Our prior work in this population suggested a maximum serum creatinine of 3 ± 2 mg/dl in the first week for critically ill SA-AKI patients. Assuming that the combination of vancomycin and piperacillin-tazobactam would result in a 20% higher maximum serum creatinine in the first week compared to the combination of vancomycin and cefepime, 176 patents in each group (n=352 total) would be necessary for 80% power with α=0.05.
Categorical data are presented as counts along with percentages and compared using a chi-square test. Continuous data are presented using medians (interquartile range) and compared between groups using the Wilcoxon rank-sum test. In those cases where no baseline serum creatinine was documented prior to admission, multiple imputations were conducted using SAS 9.4 Proc MI (SAS Institute Inc) including the variables age, sex, race, diabetes, and hypertension, full conditional specification, with 50 imputed data sets and the average creatinine value from the 50 imputations was used to assess AKI staging for inclusion in the cohort and further classification (14, 15). A sensitivity analysis of maximum serum creatinine and AKI progression was conducted in the subset of patients with a documented (non-imputed) baseline serum creatinine to evaluate the potential influence of imputation on the findings.
Results
From January 2013 to July 2020, 4,810 patients were admitted to the medical ICU with sepsis. Following the subsequent inclusion and exclusion criteria applied (Figure 1), 480 patients with SA-AKI were included for final evaluation with exclusive receipt of either combination vancomycin/piperacillin-tazobactam (n=288) or vancomycin/cefepime (n=192) within the first 72 hours of ICU admission.
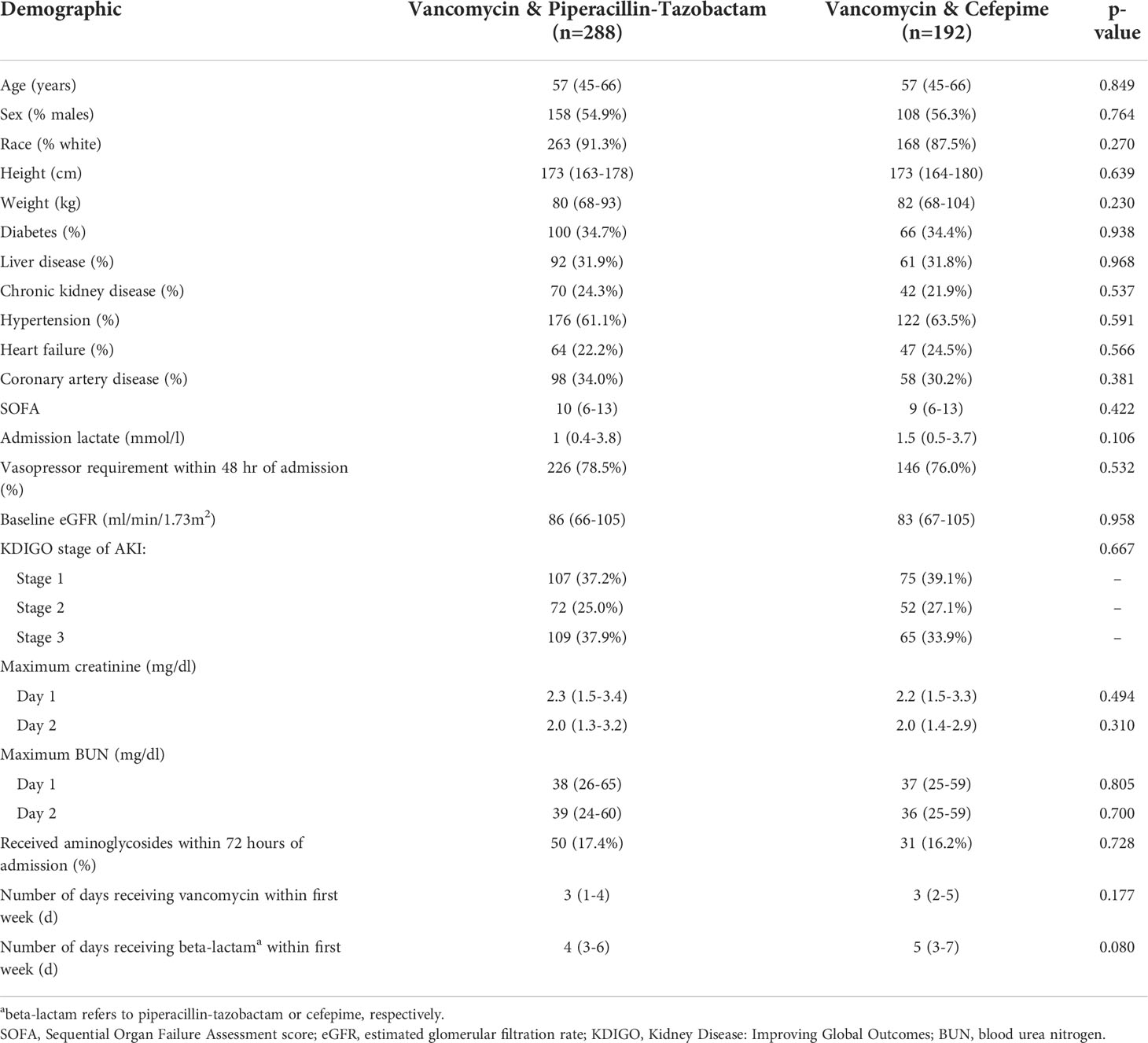
Patient demographics are shown in Table 1. The two cohorts were well-matched at baseline without any significant differences in terms of demographics, comorbidities, or severity of illness. The cohort was predominantly Caucasian, with slightly more males than females, and with a typical comorbidity pattern observed in medically critically ill patients. A documented baseline creatinine was available for 230/480 (47.9%) of patients in the cohort with a similar degree of missingness between groups (p=0.709). Acuity of illness was high as demonstrated by SOFA scores and with over three-quarters of the cohort exhibiting a requirement for vasopressors within 48 hours of ICU admission. Importantly, serum creatinine, blood urea nitrogen, and KDIGO AKI stage were similar between groups during the first 2 days of admission. A similar number of patients received concurrent aminoglycosides in each group. The duration of antimicrobial therapy, assessed within the first 7 days, was similar between the two groups.
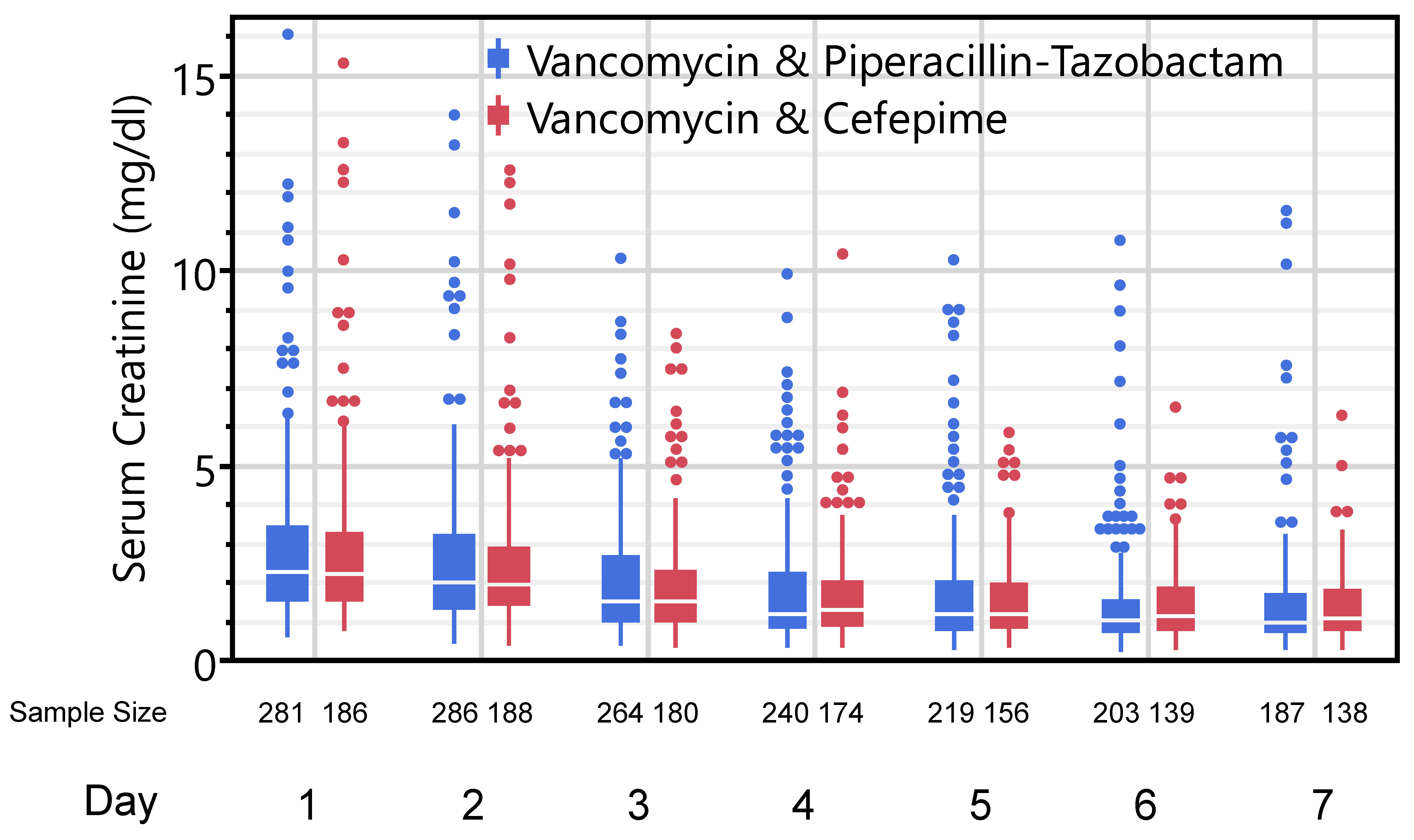
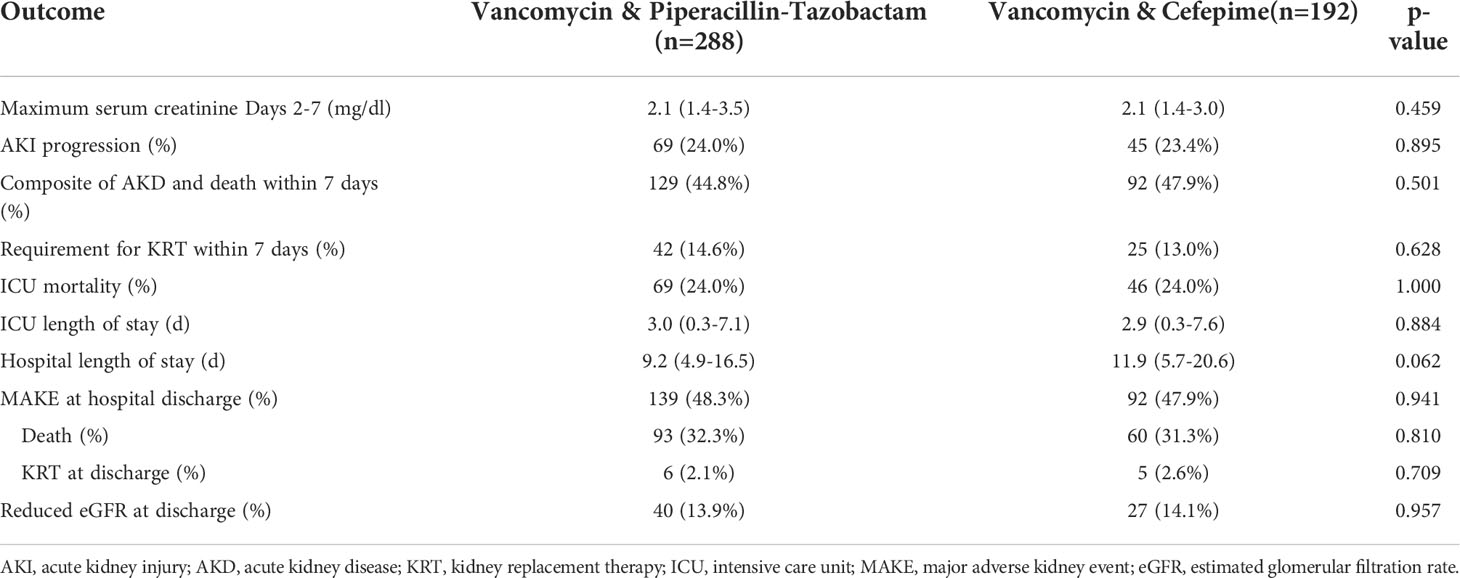
The primary outcome of maximum serum creatinine observed on days 2-7 was similar between groups: 2.1 (1.4-3.5) mg/dl vs. 2.1 (1.4-3.0) mg/dl in the vancomycin/piperacillin-tazobactam and vancomycin-cefepime groups, respectively (p=0.459). Daily serum creatinine values between the two groups over the course of the first week are displayed in Figure 2. There was also no difference in AKI progression to the next KDIGO stage in the first week between the two groups: 24.0% vs. 23.4% (p=0.895). Patients in both groups exhibited similar lengths of stay and ICU mortality. Finally, there were no differences in kidney-specific secondary outcomes between the two groups including the composite of death or AKD, requirement for KRT within 7 days, or MAKE assessed at discharge. Complete secondary outcomes are reported in Table 2. In sensitivity analyses only considering those cases with a documented (non-imputed) baseline serum creatinine, there were no differences in maximum creatinine (2.1 (1.3-3.4) vs. 2.0 (1.5-2.8); p=0.775) or AKI progression (23.5% vs. 28.7%; p=0.375) in the vancomycin/piperacillin-tazobactam and vancomycin-cefepime groups, respectively.
Discussion
Our study observed no difference in kidney-specific or other secondary outcomes, including the maximum serum creatinine in the first week and AKI progression, between vancomycin/piperacillin-tazobactam and vancomycin/cefepime combination therapy in patients with SA-AKI. This cohort is unique as the first to evaluate these combinations’ potential for nephrotoxicity in the setting of existing AKI. Prior literature studying the potential nephrotoxicity of vancomycin/piperacillin-tazobactam has excluded AKI at baseline (i.e. the start date of vancomycin/piperacillin-tazobactam prescription) in order to evaluate incident or new AKI while on therapy (1, 16). While this is necessary to study a particular question about the potential nephrotoxicity of vancomycin/piperacillin-tazobactam (i.e. incident AKI), we also suggest that it excludes a large proportion of patients prescribed this combination in clinical practice. We studied a population in sepsis that is universally exposed to antibiotic therapy by nature of the condition but also commonly presents with AKI in over 40% of cases (4).
While vancomycin is a well-documented nephrotoxin warranting therapeutic drug monitoring (17, 18), recent data have consistently shown an association between the combination of vancomycin/piperacillin-tazobactam (compared to vancomycin combined with other beta-lactam antibiotics) and AKI assessed using serum creatinine (1, 16). Precise mechanisms for this observation continue to be debated, particularly given that piperacillin-tazobactam is not commonly considered a nephrotoxin as monotherapy. Most of the AKI assessments suggesting nephrotoxicity from the combination of vancomycin/piperacillin-tazobactam conducted in retrospective observational studies are based on serum creatinine. In a secondary analysis of the Sapphire study, kidney stress markers, specifically the product of tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding-protein 7 (IGFBP7), were higher in patients receiving the combination of vancomycin/piperacillin-tazobactam compared to either agent alone (19, 20). However, debate remains over the specific mechanisms of nephrotoxicity from the combination, and whether the phenomenon observed is due to impaired tubular secretion of creatinine, or pseudo-nephrotoxicity, without any injury to the kidney (21).
Both piperacillin and tazobactam are substrates of organic anion transporter (OAT) 1 and 3 and may compete with serum creatinine secretion from the basolateral side of the nephron (22, 23). There are also data that vancomycin may reduce the expression of these OATs, subsequently decreasing creatinine secretion and increasing serum creatinine concentrations (24). OAT1 and 3 are downregulated in AKI, presumed to be the case in our cohort of patients with SA-AKI, which may alter the interplay of piperacillin-tazobactam, vancomycin, and creatinine secretion if the pseudo-nephrotoxicity hypothesis is true (25). While this is hypothesis-generating only as we did not measure OATs, translational rat models have shown no decrease in GFR or urinary biomarkers of kidney injury when vancomycin and piperacillin-tazobactam are used together (26). Regardless of the potential mechanism for a creatinine elevation without kidney injury, we did not observe any impact on kidney function in the subsequent week following antibiotic initiation, nor at hospital discharge, as assessed with multiple endpoints.
The combination of vancomycin and piperacillin-tazobactam is one of the most commonly used empiric regimens for sepsis in hospitalized patients (27). Importantly, the number of studies published on the potential nephrotoxicity of vancomycin and piperacillin-tazobactam have influenced prescribing patterns, which may have downstream implications. In a survey of infectious disease and critical care pharmacists, 89% of respondents reported a belief that the combination of vancomycin and piperacillin-tazobactam resulted in an increased probability of developing AKI compared with vancomycin alone or in combination with other beta-lactam antibiotics (5). Of those respondents, 64% reported they less frequently recommend the combination of vancomycin and piperacillin-tazobactam in a patient with existing AKI (5). This would appear to complicate the antibiotic selection for patients with sepsis and AKI. Clinically, alternative regimens would include vancomycin in combination with either cefepime or meropenem, which each have issues of their own. Cefepime is associated with encephalopathy and neurotoxicity in 3-23% of patients, particularly in the setting of reduced kidney function; nearly 90% of patients with neurotoxicity attributed to cefepime have reduced kidney function (6, 28–33). Cefepime also does not provide anaerobic coverage as may be desired in empiric antibiotic therapy, requiring additional antibiotic exposure which may increase cost, volume intake, and nursing time. Meropenem as empiric therapy for sepsis carries significant antimicrobial stewardship concerns due to the potential development of resistance to precious antibiotic resources (34). Although each combination undoubtedly comes with some set of risks and benefit, our study adds additional data for the clinician to consider, and shows similar kidney outcomes in patients with SA-AKI and suggests the combination of vancomycin/piperacillin-tazobactam may not need to be avoided in this population in attempts to spare kidney function.
This study contains several unique strengths. It is the first to our knowledge to address the issue of vancomycin/piperacillin-tazobactam nephrotoxicity in the setting of existing kidney injury, a scenario where combination antibiotic therapy is routinely utilized when treating patients with sepsis and AKI. As noted, prior evaluations of the potential nephrotoxicity of vancomycin and piperacillin-tazobactam have excluded AKI patients, including studies from different time periods conducted at our center (35, 36), which makes the AKI patient population extremely under-represented in this line of inquiry regarding potential nephrotoxicity of antibiotic combinations. Second, we used strict beta-lactam inclusion definitions, which resulted in included patients having unique and well-defined antibiotic combinations, without overlapping beta-lactam antibiotics to confound the study. Third, we evaluated several different kidney endpoints at various time intervals in order to evaluate safety of this combination in patients presenting with AKI.
This study is also subject to several limitations. First, the single center, retrospective nature limits the sample size and potential generalizability to other centers. Second, we did not measure GFR nor any notable biomarkers of kidney injury or function to phenotype AKI; our results were solely based on serum creatinine, which has well-identified limitations (37). We also did not capture urine output which may have differentiated the KDIGO grade transition independently of serum creatinine. Third, we limited our study to critically ill patients, and the combination of vancomycin and piperacillin-tazobactam may have differential effects on kidney function in critically ill vs. non-critically ill patients (1). Fourth, we were unable to quantify exposure, including dosing and therapeutic drug monitoring for all drugs in question, which may play a role in assessing any toxicity. Fifth, we did not conduct a time-to-event analysis to a meaningful event such as AKI progression or recovery, although we also acknowledge that death likely represents a significant competing event in this SA-AKI population for this type of approach. Such an approach, if carefully considered, may be beneficial in future work. Finally, although the groups appeared to be well-balanced, we cannot eliminate residual confounding in a study of this nature.
Conclusion
In patients with SA-AKI, the combination of vancomycin/piperacillin-tazobactam compared to vancomycin/cefepime was not associated with higher serum creatinine values or AKI progression in the week following ICU admission. Other kidney-related outcomes measured out to hospital discharge were similar between groups. This suggests use of vancomycin/piperacillin-tazobactam during existing SA-AKI may need not necessarily be avoided for nephrotoxicity concerns when considering the safety profiles and limitations of alternatives.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by University of Kentucky Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
KW and AF are responsible for developing the study question and design, MB contributed to data acquisition and cleaning, AF is responsible for statistical analysis, KW and AF wrote the initial draft of the manuscript, and MB, MTB, JA, and PA provided critical insights and analysis. All authors have read and approve of the final version of the manuscript.
Funding
AF is supported by grant K23DK128562. The project described was supported by the NIH National Center for Advancing Translational Sciences through grant number UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: A systematic review and meta-analysis. Crit Care Med (2018) 46(1):12–20. doi: 10.1097/CCM.0000000000002769
2. Bellos I, Karageorgiou V, Pergialiotis V, Perrea DN. Acute kidney injury following the concurrent administration of antipseudomonal β-lactams and vancomycin: a network meta-analysis. Clin Microbiol Infect (2020) 26(6):696–705. doi: 10.1016/j.cmi.2020.03.019
3. Mellen CK, Ryba JE, Rindone JP. Does piperacillin-tazobactam increase the risk of nephrotoxicity when used with vancomycin: A meta-analysis of observational trials. Curr Drug Saf. (2017) 12(1):62–6. doi: 10.2174/1574886311666161024164859
4. Bagshaw SM, George C, Bellomo R, Committee ADM. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care (2008) 12(2):R47. doi: 10.1186/cc6863
5. Hammond DA, Trivedi AP, Esmero VM, Hamadeh L, Heikkinen B, Tan C, et al. Pharmacists' perceptions and practice changes resulting from emergence of the vancomycin-piperacillin/tazobactam nephrotoxicity phenomenon. J Am Coll Clin Pharm (2020) 3(2):410–6. doi: 10.1002/jac5.1166
6. Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care (2017) 21(1):276. doi: 10.1186/s13054-017-1856-1
7. Flannery AH, Li X, Delozier NL, Toto RD, Moe OW, Yee J, et al. Sepsis-associated acute kidney disease and long-term kidney outcomes. Kidney Med (2021) 3(4):507–14.e1. doi: 10.1016/j.xkme.2021.02.007
8. Kidney Disease: Improving Global Outcomes (KDIGO), Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney inter. Suppl (2012) 2:1–138. doi: 10.1038/kisup.2012.6
9. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med (1996) 22(7):707–10. doi: 10.1007/BF01709751
10. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
11. Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. (2017) 13(4):241–57. doi: 10.1038/nrneph.2017.2
12. Billings F, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract (2014) 127(1-4):89–93. doi: 10.1159/000363725
13. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med (2007) 147(8):573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
14. Laszczyńska O, Severo M, Correia S, Azevedo A. Estimation of missing baseline serum creatinine for acute kidney injury diagnosis in hospitalized patients. Nephron. (2021) 145(2):123–32. doi: 10.1159/000512080
15. Siew ED, Peterson JF, Eden SK, Moons KG, Ikizler TA, Matheny ME. Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin J Am Soc Nephrol. (2013) 8(1):10–8. doi: 10.2215/CJN.00200112
16. Alshehri AM, Alzahrani MY, Abujamal MA, Abdalla MH, Alowais SA, Alfayez OM, et al. Comparative risk of acute kidney injury following concurrent administration of vancomycin with Piperacillin/Tazobactam or meropenem: A systematic review and meta-analysis of observational studies. Antibiotics (Basel). (2022) 11(4):526. doi: 10.3390/antibiotics11040526
17. Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther (2017) 102(3):459–69. doi: 10.1002/cpt.726
18. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother (2013) 57(2):734–44. doi: 10.1128/AAC.01568-12
19. Kane-Gill SL, Ostermann M, Shi J, Joyce EL, Kellum JA. Evaluating renal stress using pharmacokinetic urinary biomarker data in critically ill patients receiving vancomycin and/or piperacillin-tazobactam: A secondary analysis of the multicenter sapphire study. Drug Saf. (2019) 42(10):1149–55. doi: 10.1007/s40264-019-00846-x
20. Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care (2013) 17(1):R25. doi: 10.1186/cc12503
21. Avedissian SN, Pais GM, Liu J, Rhodes NJ, Scheetz MH. Piperacillin-tazobactam added to vancomycin increases risk for acute kidney injury: Fact or fiction? Clin Infect Dis (2020) 71(2):426–32. doi: 10.1093/cid/ciz1189
22. Komuro M, Maeda T, Kakuo H, Matsushita H, Shimada J. Inhibition of the renal excretion of tazobactam by piperacillin. J Antimicrob Chemother (1994) 34(4):555–64. doi: 10.1093/jac/34.4.555
23. Wen S, Wang C, Duan Y, Huo X, Meng Q, Liu Z, et al. OAT1 and OAT3 also mediate the drug-drug interaction between piperacillin and tazobactam. Int J Pharm (2018) 537(1-2):172–82. doi: 10.1016/j.ijpharm.2017.12.037
24. Wen S, Wang C, Huo X, Meng Q, Liu Z, Yang S, et al. JBP485 attenuates vancomycin-induced nephrotoxicity by regulating the expressions of organic anion transporter (Oat) 1, Oat3, organic cation transporter 2 (Oct2), multidrug resistance-associated protein 2 (Mrp2) and p-glycoprotein (P-gp) in rats. Toxicol Lett (2018) 295:195–204. doi: 10.1016/j.toxlet.2018.06.1220
25. Schneider R, Sauvant C, Betz B, Otremba M, Fischer D, Holzinger H, et al. Downregulation of organic anion transporters OAT1 and OAT3 correlates with impaired secretion of para-aminohippurate after ischemic acute renal failure in rats. Am J Physiol Renal Physiol (2007) 292(5):F1599–605. doi: 10.1152/ajprenal.00473.2006
26. Chang J, Pais GM, Valdez K, Marianski S, Barreto EF, Scheetz MH. Glomerular function and urinary biomarker changes between vancomycin and vancomycin plus piperacillin-tazobactam in a translational rat model. Antimicrob Agents Chemother (2022) 66(3):e0213221. doi: 10.1128/aac.02132-21
27. Magill SS, O'Leary E, Ray SM, Kainer MA, Evans C, Bamberg WM, et al. Antimicrobial use in US hospitals: Comparison of results from emerging infections program prevalence surveys, 2015 and 2011. Clin Infect Dis (2021) 72(10):1784–92. doi: 10.1093/cid/ciaa373
28. Boschung-Pasquier L, Atkinson A, Kastner LK, Banholzer S, Haschke M, Buetti N, et al. Cefepime neurotoxicity: thresholds and risk factors. a retrospective cohort study. Clin Microbiol Infect (2020) 26(3):333–9. doi: 10.1016/j.cmi.2019.06.028
29. Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care (2013) 17(6):R264. doi: 10.1186/cc13094
30. Huwyler T, Lenggenhager L, Abbas M, Ing Lorenzini K, Hughes S, Huttner B, et al. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect (2017) 23(7):454–9. doi: 10.1016/j.cmi.2017.01.005
31. Singh TD, O'Horo JC, Day CN, Mandrekar J, Rabinstein AA. Cefepime is associated with acute encephalopathy in critically ill patients: A retrospective case-control study. Neurocrit Care (2020) 33(3):695–700. doi: 10.1007/s12028-020-01035-w
32. Haddad NA, Schreier DJ, Fugate JE, Gajic O, Hocker SE, Ice CJ, et al. Incidence and predictive factors associated with beta-lactam neurotoxicity in the critically ill: A retrospective cohort study. Neurocrit Care (2022) 37(1):73–80. doi: 10.1007/s12028-022-01442-1
33. Appa AA, Jain R, Rakita RM, Hakimian S, Pottinger PS. Characterizing cefepime neurotoxicity: A systematic review. Open Forum Infect Dis (2017) 4(4):ofx170. doi: 10.1093/ofid/ofx170
34. Ong DS, Jongerden IP, Buiting AG, Leverstein-van Hall MA, Speelberg B, Kesecioglu J, et al. Antibiotic exposure and resistance development in pseudomonas aeruginosa and enterobacter species in intensive care units. Crit Care Med (2011) 39(11):2458–63. doi: 10.1097/CCM.0b013e318225756d
35. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: A retrospective cohort analysis. J Hosp Med (2017) 12(2):77–82. doi: 10.12788/jhm.2684
36. Rutter WC, Cox JN, Martin CA, Burgess DR, Burgess DS. Nephrotoxicity during vancomycin therapy in combination with piperacillin-tazobactam or cefepime. Antimicrob Agents Chemother (2017) 61(2):e02089-16. doi: 10.1128/AAC.02089-16
Keywords: vancomycin, piperacillin, tazobactam, nephrotoxicity, acute kidney injury, sepsis
Citation: Whitenack K, Behal ML, Thompson Bastin ML, Aycinena JC, Adams PM and Flannery AH (2022) Progression of kidney injury with the combination of vancomycin and piperacillin-tazobactam or cefepime in sepsis-associated acute kidney injury. Front. Nephrol. 2:995358. doi: 10.3389/fneph.2022.995358
Received: 15 July 2022; Accepted: 06 October 2022;
Published: 20 October 2022.
Edited by:
Rolando Claure-Del Granado, Hospital Obrero No 2 - CNS, BoliviaReviewed by:
Calvin Meaney, University at Buffalo, United StatesJonathan Samuel Chávez-Iñiguez, University of Guadalajara, Mexico
Copyright © 2022 Whitenack, Behal, Thompson Bastin, Aycinena, Adams and Flannery. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander H. Flannery, YWxleC5mbGFubmVyeUB1a3kuZWR1
 Kaylee Whitenack1
Kaylee Whitenack1 Paul M. Adams
Paul M. Adams Alexander H. Flannery
Alexander H. Flannery