- 1Department of Nephrology, Hitachi General Hospital, Hitachi, Japan
- 2Department of Nephrology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 3Department of Nephrology, Mito Kyodo General Hospital, Mito, Japan
- 4Department of Nephrology, Mito Saiseikai General Hospital, Mito, Japan
- 5Department of Nephrology, Ushiku Aiwa Hospital, Ushiku, Japan
- 6Department of Nephrology, Tokyo Medical University Kasumigaura Hospital, Ami, Japan
- 7Department of Nephrology, JA Toride Medical Center, Toride, Japan
- 8Department of Nephrology, Ibaraki Prefectural Central Hospital, Kasama, Japan
Background: Immune dysfunction in hemodialysis patients is partially due to NK cell impairment. Ligands for NK activating receptors such as NKG2D expressed on cancer cells are involved in NK cell dysfunction and can lead to cancer development.
Methods: A cohort with 370 patients who started hemodialysis (HD) was investigated. Serum levels of soluble NKG2D ligands were measured. Cancer history was defined as any cancer diagnosis at induction and hospitalization and death due to cancer during 2-year follow-up.
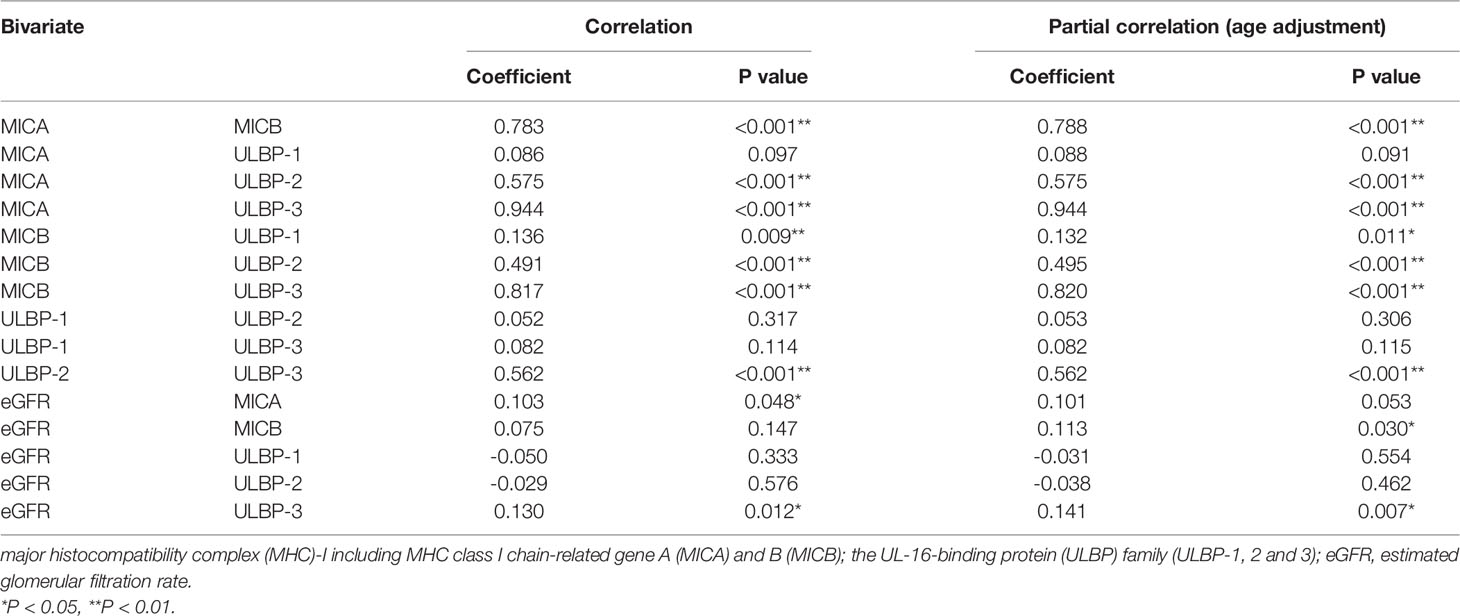
Results: Sixty-two patients with and 308 patients without a cancer history showed mostly comparable biochemical parameters and uremic status at HD induction. Soluble MICB, ULBP-1, and ULBP-2 were detected in sera from most patients starting HD rather than MICA, the most representative NKG2D ligand. Measured NKG2D ligands, except for ULBP-1, were strongly correlated with each other. Correlations between NKG2D ligands and renal function were significant but modest in patients starting HD. Cancer history did not have any impact on levels of soluble NKG2D ligands.
Discussion: Even though this investigation lacked a control cohort and serial measurement of parameters, expression patterns of NKG2D ligands were comprehensively described, and the significance of cancer in patients starting HD was elucidated for the first time. Elevated levels of soluble NKG2D ligands occurred potentially due to complex mechanisms of oxidative stress, with insufficient metabolism and excretion in a uremic milieu, but they might mask the significance of elevations in serum levels of soluble NKG2DLs in patients with a cancer history.
Introduction
Cancer is the one of the leading reasons for death in hemodialysis (HD) patients. In epidemiological studies, patients requiring replacement therapy are reported to have an increased cancer risk (1). Several lines of evidence have demonstrated that the immune system protects the host against cancer development (2–5). For instance, reduced kidney function is considered to alter the immune response, which potentially contributes to the development of cancer (6, 7). Dysfunction of natural killer (NK) cells is responsible for patients being considered immune-compromised in end-stage kidney disease (ESKD), especially HD patients (8). In the human body, it has been found that a large number of cancer cells can be produced even in the healthy state, and these cells have the potential to develop into hematopoietic and solid organ malignancies (9). Immune cells, mainly NK cells, are eradicated each time cancer cells are formed through cell-mediated immunity to tumors (2, 3). To eliminate tumor cells differentially, NK cells must recognize and distinguish healthy “self” cells from abnormal “non-self” cells via receptor-ligand interactions at the cell plasma membrane (8, 10). By activation signals through their interactions, NK cells become capable of recognizing and eliminating malignant cells. This mechanism is called immunosurveillance (4, 5). However, there is currently sparse evidence for molecular mechanisms linking uremic status and tumor genesis in HD patients.
Abnormal cells such as cancer cells and stressed cells express ligands for activating NK cell receptors and induce NK cytotoxic activities (11). As a representative immunoreceptor, natural killer cell receptor G2D (NKG2D), which is predominantly expressed on NK cells, activates NK cells via NKG2D ligands (NKG2DLs) differentially expressed on tumor cells, but not on normal cells, which implies that NKG2D plays an important role in immunosurveillance (12). This made NKG2D one of the most intensively studied immunoreceptors of the past decades (13). Animal studies of in vivo cancer models strongly suggest that activated NKG2D is involved in anti-cancer immune responses (4, 5). In these models, NKG2DLs expressed on tumor cells and bound to NKG2D can activate NK cells to kill tumor cells by means of perforin-based cytotoxicity (4, 5). Upon NKG2D engagement, adoptor proteins such as DNAX-activating Protein 10 are phosphorylated and recruits both the p85 subunit of the phosphatidylinisitol-3-kinase and a Grb2-Vav1 intermediating NK activation (10). Therfore, a pronouced expression of NKG2DLs renders susceptibility to NK cell cytotoxicity.
In humans, NKG2DLs comprise two members of the major histocompatibility complex (MHC)-I, including MHC class I chain-related genes A (MICA) and B (MICB) and members of the UL-16-binding protein (ULBP) family (from ULBP-1 to 6). MICA and MICB are broadly expressed by epithelial tumors, melanom and various hematopoietic malignancies and expression of mouse NKG2DL is up-regulated during tumorgenesis (11, 14–17). At the same time, MICA molecules are released from tumor cells in a soluble form (18). Elevated levels of soluble MICA (sMICA) were found in sera of patients with various hematopoietic and non-hematopoietic cancers (18, 19). Several experiments suggested that MICA is shed from the surface of tumor cells by proper metalloproteases such as “a disintegrin and metalloproteinase (ADAM)” family: ADAM 10 and ADAM17 (19, 20). These five ligand proteins were investigated in leukemia and other cancers, and they showed significantly elevated levels with a few exceptions (14, 21–23). It is also known that cancer prognosis is related to concentration of soluble NKG2DLs, which could be used as novel prognosis markers, and suggested differences in the clinical significance of individual soluble NKG2DLs (24–26).
In the uremic milieu, patients with ESKD have both an increased membrane-bound NKG2DL, MICA, on oxidative stressed monocytes and increased soluble MICA in sera compared to healthy donors, suggesting that renal dysfunction upregulates MICA (27). The enhanced expression of MICA was proven to be dependent on excessive reactive oxygen species (ROS) and uremic serum in in vitro analyses (27). Though changes in NKG2DL levels are likely to be involved in the immune deficiency of ESKD patients, clinical evidence to show alteration of NK cell activation affecting cancer development and prognosis in ESKD patients is still not established. To examine the clinical implications of links between the expression and functions of NK cell receptors and cancer development, the levels of various soluble NKG2DLs in serum were examined in a cohort of patients starting HD.
Methods
Study Protocol and Ethics
The study was performed as part of the prospective ongoing Ibaraki Dialysis Initiation Cohort Study (iDIC) project. Other details, such as the participants’ areas of residence will be reported elsewhere (Tawara T. et al, in preparation). Briefly, the inclusion criteria of this study were: consecutive patients starting dialysis at each participating institute from January 2013 to December 2015; and patients who needed maintenance dialysis at each participating institute. This study was conducted according to the guidelines of the Declaration of Helsinki and was granted ethics approval by the relevant institutional review board (the main institute being the University of Tsukuba Hospital, H24-116, UMIN: 000010806). Based on the written informed consent, clinical information and laboratory data were collected at study entry via a uniform questionnaire prepared by dialysis physicians. The data included information about age, sex, body mass index (BMI), systolic blood pressure, diastolic blood pressure, and serological testing for serum urea nitrogen, creatinine, urea acid, beta-2 microglobulin, and albumin concentration, hemoglobin, serum lipid status, and hemoglobin A1c. This cohort lacked information regarding chronic viral infections, including hepatitis B, hepatitis C, and human immunodeficiency virus. Information on death and hospitalization events with cause specificity in the subsequent two years was obtained from dialysis physicians. This investigation was limited to 370 cases who had sufficient serum samples in the original cohort.
Sample Measurement
The concentrations of soluble NKG2DLs for which highly specific antibodies are available were comprehensively analyzed (28). Serum concentrations of soluble human MICA, MICB, ULBP-1, ULBP-2, and ULBP-3 were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit system (R&D systems, Minneapolis, MN). ELISA was performed in accordance with the manufacturer’s protocol. Brifly, plates (Nunc-Immuno Plate; NUNC, Roskilde, Denmark) were coated with 4 μg ml−1 of capture antibodies in phosphate-buffered saline (PBS) overnight at 4°C, washed with 0.05% Tween 20 in PBS and blocked with 3% bovine serum albumin (BSA) in PBS before serially diluted sera samples and standard proteins were added. After conditioning, each serum sample was appropriately diluted from 1:10 to 1:1000. After 18 h of incubation at 4°C, biotinylated detection antibodies was added and incubated for 2 h at room temperature. The plates were washed, and then streptavidin–horseradish peroxidase conjugate was added for NKG2D ligands detection. After incubation for 1 h at room temperature, ABTS peroxidase substrate (506601, SeraCare Life Sciences, Milford, MA, USA) was added and absorbance was measured with a microplate reader (Bio-Rad, Hercules, CA, USA) at 405 nm. The sensitivities of the ELISAs in our laboratory were 62.5 pg/mL, 156.3 pg/mL, 93.8 pg/mL, 62.5 pg/mL, and 125.0 pg/mL, respectively, according to the data sheets. Undetected concentrations, that is those below the lower limit, were considered and analyzed as 0 ng/mL. Other biochemical parameters were evaluated as routine clinical examinations in each facility.
Statistical Analysis
Patients with a prior history of cancer and/or death or hospitalization due to cancer during follow-up were considered to have a cancer history. Categorical variables are presented as percentages, and continuous variables are presented as means and standard deviations in Table 1. Contingency was assessed by the chi-squared test. A p value <0.05 was considered significant. Spearman correlation and partial correlation tests were performed to assess relationships between soluble NKG2DL levels and eGFR. Statistical analyses and graphical presentation were performed using SPSS version 24 and GraphPad Prism version 6, as appropriate.
Results
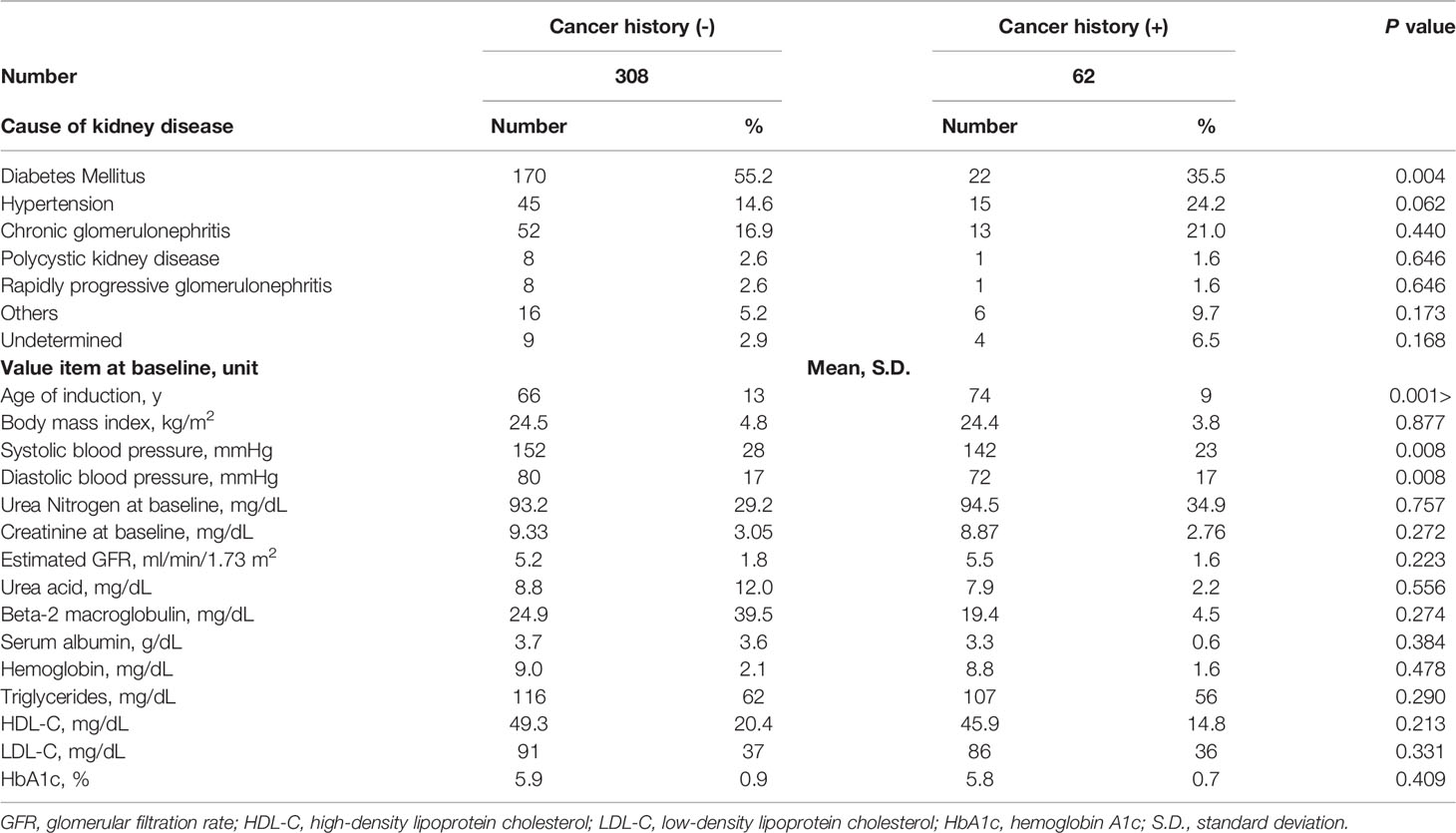
Table 1 presents the background data of the patients with and without a cancer history. The mean age of the 62 patients with a cancer history, including 54 patients with known cancer at induction and 14 hospitalizations and 8 deaths (composed of 3 cases with urethral cancer, and one case each of pancreatic cancer, rectal cancer, multiple myeloma, breast cancer, and unknown origin cancer) by including duplications, was significantly higher than that of the 308 patients without a cancer history. With respect to the causes of the patients’ renal diseases, diabetes mellitus was more common in patients without a cancer history, and higher age was more common in those with a cancer history. The two groups showed mostly comparable biochemical parameters and uremic status at dialysis induction.
Detectable sample numbers of MICA, MICB, ULBP-1, ULBP-2, and ULBP-3 were 130, 368, 365, 368, and 86, respectively (Figure 1). Of the NKG2D ligands in this study cohort, MICA, MICB, ULBP-2, and ULBP-3, but not ULBP-1, were strongly correlated each other (Table 2 and Figure 2), and age-adjustment by partial correlation testing did not affect the relationships (Table 2). Figure 3 shows that the correlations between NKG2D ligands and residual renal function were significant, but extremely modest in patients starting HD (coefficients on age-adjusted partial correlation tests: MICA 0.103, ULBP-1 0.130).
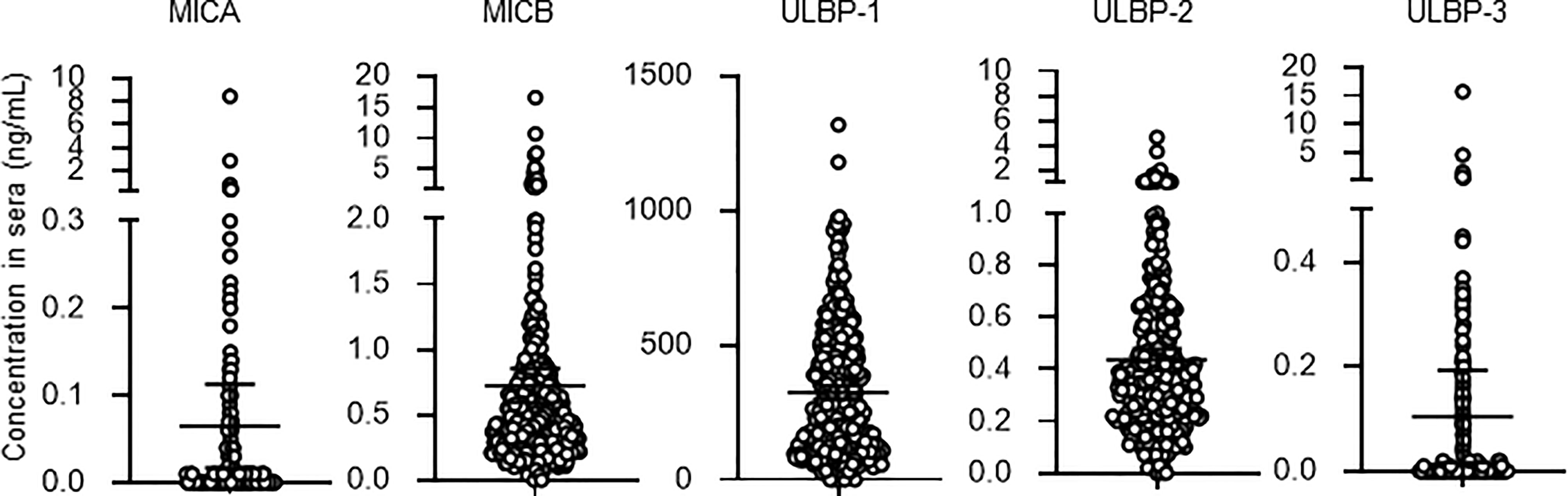
Figure 1 Serum concentrations of soluble NKG2D ligands in incident hemodialysis patients. Serum levels of soluble forms of major histocompatibility complex (MHC)-I including MHC class I chain-related genes A (MICA) and B (MICB) and members of the UL-16-binding protein (ULBP) family from 370 patients of the study cohort. Undetected concentrations, that this those that were below the lower limit, were considered and analyzed as 0 mg/dL. Median and 95% confidence intervals are presented as bars and error values.
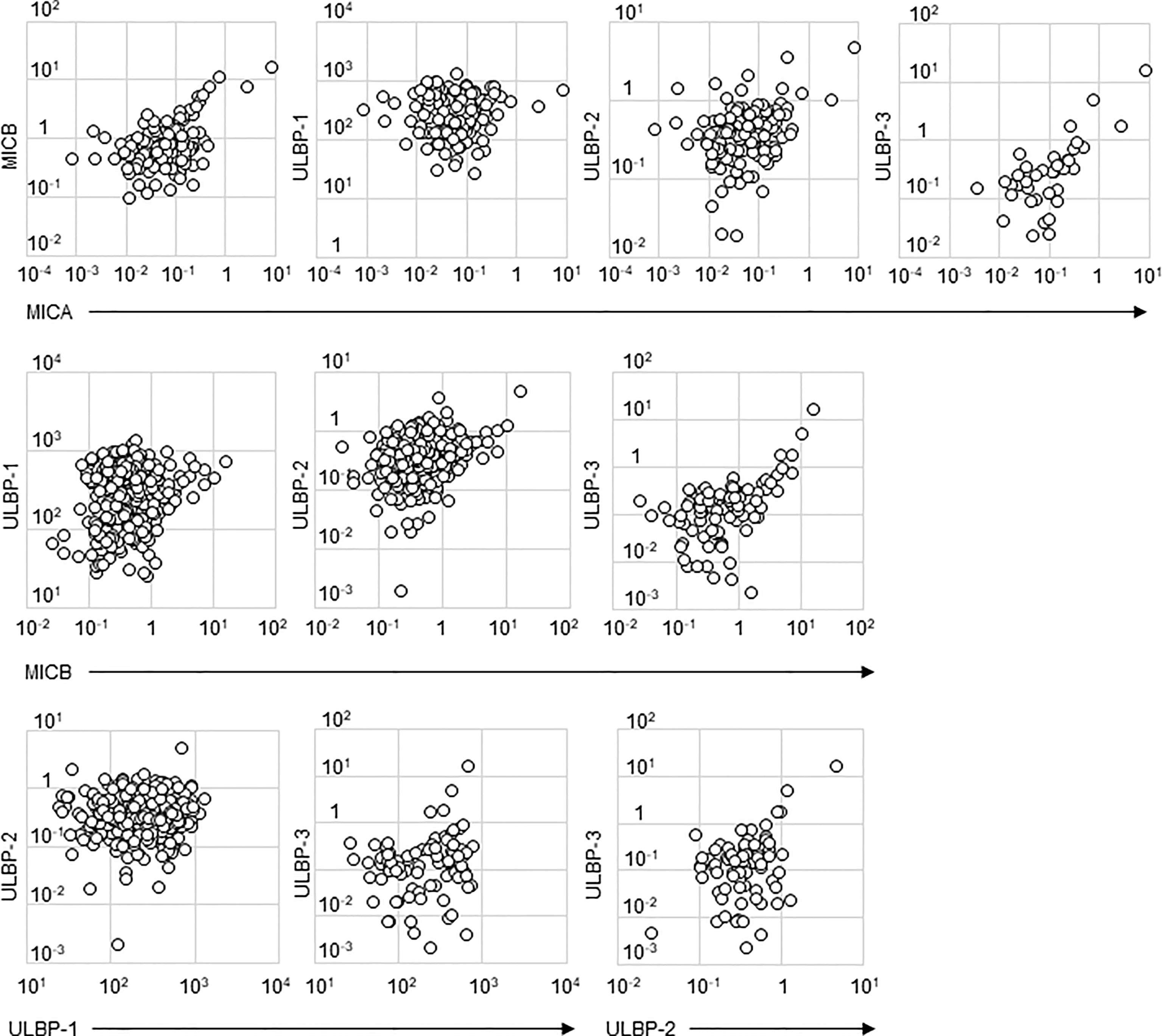
Figure 2 Scatter plots of soluble NKG2D ligand levels in incident hemodialysis patients. Serum levels of two types of NKG2D ligands investigated in this study are plotted in each figure panel. Logarithms are used for the axes. All the concentrations of NKG2D were shown as ng/mL. Their correlation was assessed and the results were shown in Table 2. major histocompatibility complex (MHC)-I including MHC class I chain-related gene A (MICA) and B (MICB); the UL-16-binding protein (ULBP) family (ULBP-1, 2 and 3).
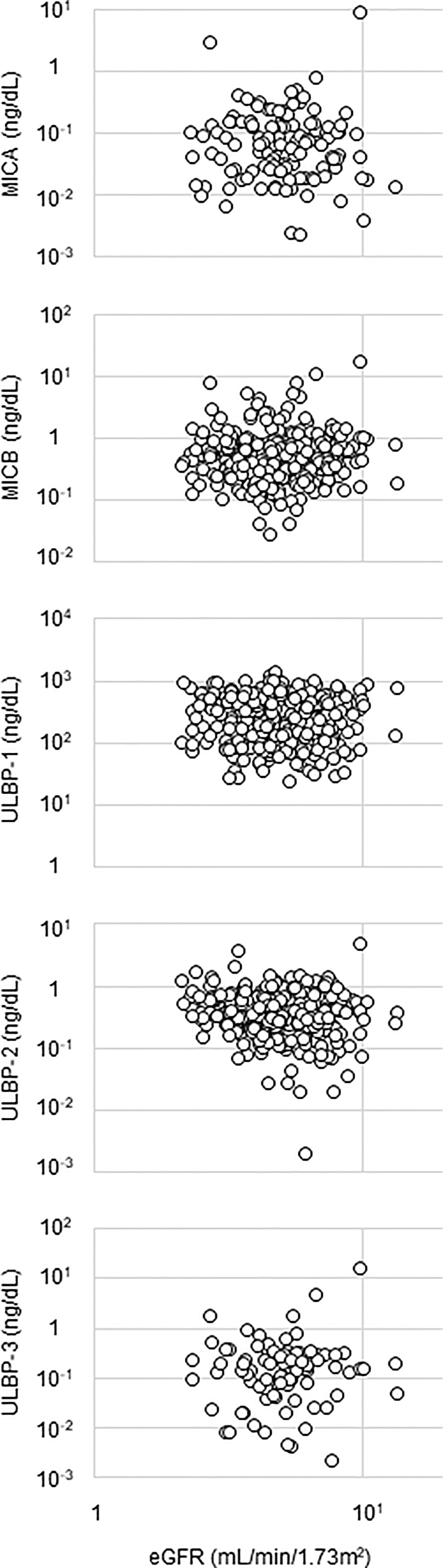
Figure 3 Relationship between NKG2D ligands and renal function. Serum level of a NKG2D ligand and estimated glomerular filtration ratio (eGFR) are plotted in each figure panel. Logarithms are used for the axes. Their correlation was assessed and the results were shown in Table 2. major histocompatibility complex (MHC)-I including MHC class I chain-related gene A (MICA) and B (MICB); the UL-16-binding protein (ULBP) family (ULBP-1, 2 and 3).
Mean serum levels (95% confidence intervals) of soluble NKG2DLs in 308 incident HD patients without a cancer history were 0.072 (0.015-0.130) ng/mL, 0.742 (0.593-0.891) ng/mL, 327 (300-342) ng/mL, 0.444 (0.396-0491) ng/mL, and 0.118 (0.012-0.224) ng/mL, respectively in MICA, MICB, ULBP-1, ULBP-2, and ULBP-3. Those in 62 incident HD patients with a cancer history were 0.026 (0.008-0.044) ng/mL, 0.628 (0.392-0.863) ng/mL, 319 (265-373) ng/mL, 0.398 (0.333-0.464) ng/mL, and 0.039 (0.010-0.067) ng/mL, respectively in MICA, MICB, ULBP-1, ULBP-2, and ULBP-3 (Figure 4). Collectively, there were no differences in levels of soluble NKG2D ligands between the presence and absence of a cancer history in this study cohort with two-year follow-up (Figure 4).
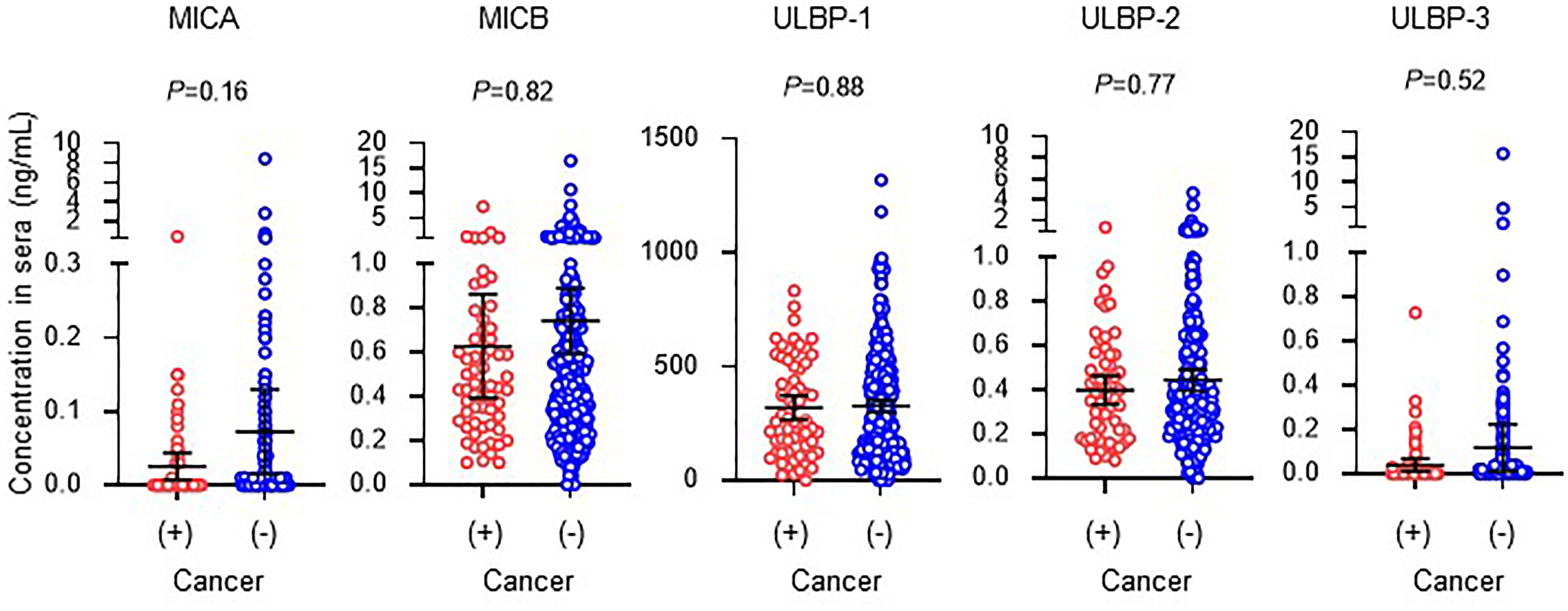
Figure 4 Serum concentrations of soluble NKG2D ligands in incident hemodialysis patients with or without a cancer history. Serum levels of soluble forms of major histocompatibility complex (MHC)-I including MHC class I chain-related genes A (MICA) and B (MICB) and members of the UL-16-binding protein (ULBP) family. Undetected concentrations, that this those that were below the lower limit, were considered and analyzed as 0 mg/dL. Patients with a prior history of cancer and death or hospitalization due to cancer during follow-up were considered to have a cancer history. Median and 95% confidence intervals are presented as bars and error values. Differences in median values between with and without a cancer history are compared by the Mann-Whitney U test.
Discussion
In this study, soluble NKG2DL concentrations were examined in sera from patients starting HD. Mechanisms of production of soluble NKG2DLs are generally considered to be the regulation of their release by various processes, including protease-mediated cleavage (29). Particularly in the uremic state, Peraldi et al. characterized NKG2D and MICA in healthy control and uremic patients, and they concluded that oxidative stress-related NKG2D downregulation and up-regulation of MICA protein may be involved in the immune deficiency of ESKD patients (27). Though this study sufficiently demonstrated biological dysfunction of NK cells in HD patients, little supporting clinical implication was reported. Serum levels of NKG2DLs vary with regard to regulation of their expressions, surface attachment, and affinities to NKG2D, suggesting differential roles in NK cell biology (21). The axis of NKG2D and its ligands had been well investigated and most established in the context of cancer immunosurveillance (21). In particular, soluble MICA levels, but also other soluble NKG2DLs, have been analyzed in a multitude of different malignancies (22). For example, in one large-sized study (205 leukemia patients and 30 healthy controls), there were significantly elevated levels of soluble MICA, MICB, ULBP-1, ULBP-2 and ULBP-3 in all leukemia entities except soluble ULBP-2 in chronic myeloid leukemia (CML), soluble ULBP-3 in chronic lymphocytic leukemia (CLL), and soluble ULBP-1 in acute myeloid leukemia (AML) (28). This suggests that the differences in the functional properties of NKG2DLs may provide an explanation as to why the various NKG2DLs differ in their correlations with diseases. Therefore, expressions of five NKG2DLs were comprehensively studied in 370 patients starting HD, and the clinical impact on cancer history, namely, past history of cancer, and/or hospitalization and death due to cancer was examined in two-year follow-up.
One of the main findings of the present study was that, rather than MICA, soluble MICB, ULBP-1, and ULBP-2 were detected in sera from most patients starting HD. Levels of sMICA in healthy donors were undetectable or in the very low value range, whereas pre-therapeutic serum levels in patients with various malignancies were significantly higher (30). Regarding soluble NKG2DLs other than sMICA, neither sULBP-1 nor sULBP-3 levels were detectable in the blood samples of healthy controls. Similarly, much lower levels of sMICB and sULBP-2 were seen in healthy controls compared to CLL patients (31). Though the present study cohort did not contain healthy controls or patients with normal renal function, one can assume that detectable levels of NKG2DLs are likely to occur due to renal dysfunction. In addition to oxidative stress in the uremic milieu, insufficient metabolism and excretion should be considered as causes of elevated levels of NKG2DLs in incident HD patients (32). In this study, eGFR was significantly but very modestly correlated only with MICB and ULBP-3. Thus, one cannot conclude whether reduced renal function is linked with detectable levels of serum sNKG2DLs, possibly due to the narrow range of eGFR in the study population (eGFR 5.3 ± 1.8, ranging from 2.1 to 13.7 ml/min/1.73 m2).
A pathological rationale for the soluble form is to down-modulate cell surface expression of ligand protein on tumor cells. In addition, soluble MICA can cause the downregulation of surface expression of NKG2D receptors by promoting their internalization and degradation, leading to reduced immune responses against tumors (33). These pathways contribute to escape from immune surveillance by host NK cell immunity and elevation in soluble NKG2DLs considered to result in progression of malignant disease and metastasis in vivo. Therefore, soluble NKG2DLs levels are expected to be markers of tumor progression and prognosis, because soluble MICA and ULBP-2 levels distinguish significantly between benign and malignant disease, both in solid organ cancers and in hematopoietic cancers (22, 31). However, based on the present investigation, these soluble NKG2DLs seemed to fail to distinguish patients with a cancer history in the incident HD cohort. This might be mainly caused by the effect of renal dysfunction and excessive oxidative stress on masking elevation of sNKG2DLs produced by tumor cells in HD patients. However, further examinations are needed to characterize the links between renal dysfunction and elevations in soluble NKG2DL levels in sera of HD patients.
Most previous laboratory investigations did not provide any clinical implications in ESKD patients. In fact, compared to previous studies, a major drawback the present investigation is the lack of a control group and lack of cell surface expression analyses of NKG2DLs. Nevertheless, the present research has novelty from the perspective of exploring clinical significance in an incident HD cohort, though no difference was seen between the presence and absence of a cancer history. Recently, Dendle et al. reported that reduced NK cytotoxic activity assessed in vitro is associated with NK cell-related infectious complications in ESKD (34). We first assessed cancer, another NK cell-related clinical complication (35, 36), as a clinical outcome in an ESKD cohort. Further clinical research on the relationship between NK cell function and development of cancer in dialysis patients is desirable.
As limitations, in this cohort study, biomarkers were only available at baseline at the time of starting HD. Therefore, changes in levels of soluble NKG2DLs could not be examined, so it is not possible to determine whether there was new onset of cancer during follow-up or there was pre-existing cancer at baseline. Another problem is the absence of a control group; therefore, correlations between parameters and soluble NKG2D levels were examined at baseline only in a cross-sectional manner. Because a cancer history was determined based on a uniform questionnaire prepared by physicians, reporting bias is considerable. Moreover, this cohort had no information regarding chronic viral infections, which may have an effect on expression levels of NKG2D and NKG2DLs and may mask the significance of elevations in serum levels of soluble NKG2DLs in patients with a cancer history.
In summary, serum levels of soluble NKG2DLs were examined in 370 incident HD patients to explore the clinical implications of links between expression of NK cell receptor function and cancer development. MICB, ULBP-1, and ULBP-2, but not MICA and ULBP-3, were detected in sera from most patients starting HD, and it was difficult to discriminate between the presence and absence of a cancer history using the levels of soluble NKG2DLs. This difficulty may have occurred potentially due to the complex mechanisms of oxidative stress, insufficient metabolism, and excretion in the uremic milieu.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available on request to steering committee of iDIC study. Requests to access these datasets should be directed to KY, https://nephtsukuba.wixsite.com/nephrology-tsukuba/idic.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Tsukuba Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The corresponding author (KN) designed this research and was involved in measurements of samples and data analyses with supervision by JU and TT. The chief investigators of the iDIC study (KY), the officer of the research center (JU), and investigators (IE, TI, MK, YM, and HK) comprised the study’s steering committee. Steering committee members contributed to patient entries, design of the study, data analysis, and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This article was supported, in part, by JSPS Grant No. 18KK0431, 19K17729 and by the Japanese Association of Dialysis Physicians Grant No. 2020-3.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study would not have been possible without the generous support of the nephrologists and dialysis physicians in Ibaraki prefecture, Japan. The authors would also like to thank Rie Kikko for technical assistance.
References
1. Matas AJ, Simmons RL, Kjellstrand CM, Buselmeier TJ, Najarian JS. Increased Incidence of Malignancy During Chronic Renal Failure. Lancet (1975) 1(7912):883–6. doi: 10.1016/S0140-6736(75)91684-0
2. Dunn GP, Old LJ, Schreiber RD. The Three Es of Cancer Immunoediting. Annu Rev Immunol (2004) 22(1):329–60. doi: 10.1146/annurev.immunol.22.012703.104803
3. Swann JB, Smyth MJ. Immune Surveillance of Tumors. J Clin Invest (2007) 117(5):1137–46. doi: 10.1172/JCI31405
4. Cerwenka A, Baron JL, Lanier LL. Ectopic Expression of Retinoic Acid Early Inducible-1 Gene (RAE-1) Permits Natural Killer Cell-Mediated Rejection of a MHC Class I-Bearing Tumor In Vivo. Proc Natl Acad Sci USA (2001) 98(20):11521–6. doi: 10.1073/pnas.201238598
5. Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 Ligands of the NKG2D Receptor Stimulate Tumour Immunity. Nature (2001) 413(6852):165–71. doi: 10.1038/35093109
6. Kurts C, Panzer U, Anders H-J, Rees AJ. The Immune System and Kidney Disease: Basic Concepts and Clinical Implications. Nat Rev Immunol (2013) 13(10):738–53. doi: 10.1038/nri3523
7. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-Induced Cancer: Crosstalk Between Tumours, Immune Cells and Microorganisms. Nat Rev Cancer (2013) 13(11):759–71. doi: 10.1038/nrc3611
8. Nagai K. Dysfunction of Natural Killer Cells in End-Stage Kidney Disease on Hemodialysis. Ren Replace Ther (2021) 78(1):1–7. doi: 10.1186/s41100-021-00324-0
9. Jakóbisiak M, Lasek W, Gołąb J. Natural Mechanisms Protecting Against Cancer. Immunol Lett (2003) 90(2):103–22. doi: 10.1016/j.imlet.2003.08.005
10. Bauer S, Groh V, Wu J, Steinle A, Phillips Joseph H, Lanier Lewis L, et al. Activation of NK Cells and T Cells by NKG2D, a Receptor for Stress-Inducible MICA. Science (1999) 285(5428):727–9. doi: 10.1126/science.285.5428.727
11. Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T Cell Autoreactivity by Anomalous Expression of NKG2D and its MIC Ligands in Rheumatoid Arthritis. Proc Natl Acad Sci USA (2003) 100(16):9452–7. doi: 10.1073/pnas.1632807100
12. Vivier E, Nunès Jacques A, Vély F. Natural Killer Cell Signaling Pathways. Science (2004) 306(5701):1517–9. doi: 10.1126/science.1103478
13. Eagle RA, Trowsdale J. Promiscuity and the Single Receptor: NKG2D. Nat Rev Immunol (2007) 7(9):737–44. doi: 10.1038/nri2144
14. Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee H-G, et al. Functional Expression and Release of Ligands for the Activating Immunoreceptor NKG2D in Leukemia. Blood (2003) 102(4):1389–96. doi: 10.1182/blood-2003-01-0019
15. Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of Cutaneous Malignancy by Gammadelta T Cells. Science (2001) 294(5542):605–9. doi: 10.1126/science.1063916
16. Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, et al. Expression and Role of MICA and MICB in Human Hepatocellular Carcinomas and Their Regulation by Retinoic Acid. Int J Cancer (2003) 104(3):354–61. doi: 10.1002/ijc.10966
17. Vetter CS, Groh V, thor Straten P, Spies T, Bröcker EB, Becker JC. Expression of Stress-Induced MHC Class I Related Chain Molecules on Human Melanoma. J Invest Dermatol (2002) 118(4):600–5. doi: 10.1046/j.1523-1747.2002.01700.x
18. Groh V, Wu J, Yee C, Spies T. Tumour-Derived Soluble MIC Ligands Impair Expression of NKG2D and T-Cell Activation. Nature (2002) 419(6908):734–8. doi: 10.1038/nature01112
19. Salih HR, Rammensee H-G, Steinle A. Cutting Edge: Down-Regulation of MICA on Human Tumors by Proteolytic Shedding. J Immunol (2002) 169(8):4098. doi: 10.4049/jimmunol.169.8.4098
20. Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, et al. Tumor-Associated MICA is Shed by ADAM Proteases. Cancer Res (2008) 68(15):6368–76. doi: 10.1158/0008-5472.CAN-07-6768
21. Champsaur M, Lanier LL. Effect of NKG2D Ligand Expression on Host Immune Responses. Immunol Rev (2010) 235(1):267–85. doi: 10.1111/j.0105-2896.2010.00893.x
22. Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D Ligands: Prevalence, Release, and Functional Impact. Front Bioscience-Landmark (2008) 13(9):3448–56. doi: 10.2741/2939
23. Waldhauer I, Steinle A. Proteolytic Release of Soluble UL16-Binding Protein 2 From Tumor Cells. Cancer Res (2006) 66(5):2520. doi: 10.1158/0008-5472.CAN-05-2520
24. Kegasawa T, Tatsumi T, Yoshioka T, Suda T, Ikezawa K, Nakabori T, et al. Soluble UL16-Binding Protein 2 is Associated With a Poor Prognosis in Pancreatic Cancer Patients. Biochem Biophys Res Commun (2019) 517(1):84–8. doi: 10.1016/j.bbrc.2019.07.020
25. Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, et al. Differential Clinical Significance of Individual NKG2D Ligands in Melanoma: Soluble ULBP2 as an Indicator of Poor Prognosis Superior to S100B. Clin Cancer Res (2009) 15(16):5208. doi: 10.1158/1078-0432.CCR-09-0886
26. Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICB in Malignant Diseases: Analysis of Diagnostic Significance and Correlation With Soluble MICA. Cancer Immunol Immunother (2006) 55(12):1584–9. doi: 10.1007/s00262-006-0167-1
27. Peraldi M-N, Berrou J, Dulphy N, Seidowsky A, Haas P, Boissel N, et al. Oxidative Stress Mediates a Reduced Expression of the Activating Receptor NKG2D in NK Cells From End-Stage Renal Disease Patients. J Immunol (2009) 182(3):1696. doi: 10.4049/jimmunol.182.3.1696
28. Hilpert J, Grosse-Hovest L, Grünebach F, Buechele C, Nuebling T, Raum T, et al. Comprehensive Analysis of NKG2D Ligand Expression and Release in Leukemia: Implications for NKG2D-Mediated NK Cell Responses. J Immunol (2012) 189(3):1360. doi: 10.4049/jimmunol.1200796
29. Zingoni A, Molfetta R, Fionda C, Soriani A, Paolini R, Cippitelli M, et al. NKG2D and Its Ligands: "One for All, All for One". Front Immunol (2018) 9:476–. doi: 10.3389/fimmu.2018.00476
30. Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in Malignant Diseases. Int J Cancer (2006) 118(3):684–7. doi: 10.1002/ijc.21382
31. Nückel H, Switala M, Sellmann L, Horn PA, Dürig J, Dührsen U, et al. The Prognostic Significance of Soluble NKG2D Ligands in B-Cell Chronic Lymphocytic Leukemia. Leukemia (2010) 24(6):1152–9. doi: 10.1038/leu.2010.74
32. Holdenrieder S, Eichhorn P, Beuers U, Samtleben W, Stieber P, Nagel D, et al. Soluble NKG2D Ligands in Hepatic Autoimmune Diseases and in Benign Diseases Involved in Marker Metabolism. Anticancer Res (2007) 27(4A):2041.
33. Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, et al. Sustained Localized Expression of Ligand for the Activating NKG2D Receptor Impairs Natural Cytotoxicity In Vivo and Reduces Tumor Immunosurveillance. Nat Immunol (2005) 6(9):928–37. doi: 10.1038/ni1239
34. Dendle C, Gan P-Y, Polkinghorne KR, Ngui J, Stuart RL, Kanellis J, et al. Natural Killer Cell Function Predicts Severe Infection in Kidney Transplant Recipients. Am J Transplant (2019) 19(1):166–77. doi: 10.1111/ajt.14900
35. Orange JS. Natural Killer Cell Deficiency. J Allergy Clin Immunol (2013) 132(3):515–25. doi: 10.1016/j.jaci.2013.07.020
Keywords: natural killer cell (NK cells), end-stage kidney disease (ESKD), natural killer cell receptor G2D (NKG2D), cancer, MHC class I chain-related genes A (MICA), MHC class I chain-related genes B (MICB), UL-16-binding protein (ULBP) family
Citation: Nagai K, Tawara T, Usui J, Ebihara I, Ishizu T, Kobayashi M, Maeda Y, Kobayashi H and Yamagata K (2022) Levels of Soluble NKG2D Ligands and Cancer History in Patients Starting Hemodialysis. Front. Nephrol. 2:875207. doi: 10.3389/fneph.2022.875207
Received: 13 February 2022; Accepted: 28 February 2022;
Published: 22 March 2022.
Edited by:
Ming Yi, Huazhong University of Science and Technology, ChinaReviewed by:
Younghoon Kim, LG Chem, South KoreaRana A. Youness, University of Hertfordshire, United Kingdom
Copyright © 2022 Nagai, Tawara, Usui, Ebihara, Ishizu, Kobayashi, Maeda, Kobayashi and Yamagata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kei Nagai, a25hZ2FpQG1kLnRzdWt1YmEuYWMuanA=
 Kei Nagai
Kei Nagai Takashi Tawara3
Takashi Tawara3 Kunihiro Yamagata
Kunihiro Yamagata