
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nephrol. , 17 January 2023
Sec. Blood Purification
Volume 2 - 2022 | https://doi.org/10.3389/fneph.2022.1026874
This article is part of the Research Topic Dialysis and the Age-Friendly Health System Initiative View all 6 articles
The aim of this paper is to illustrate all the clinical epidemiology searches made within the French network REIN to improve CKD stage 4-5 care in older adults. We summarize various studies describing clinical practice, care organization, prognosis and health economics evaluation in order to develop personalized care plans and decision-making tools. In France, for 20 years now, various databases have been mobilized including the national REIN registry which includes all patients receiving dialysis or transplantation. REIN data are indirectly linked to the French administrative healthcare database. They are also pooled with data from the PSPA cohort, a multicenter prospective cohort study of patients aged 75 or over with advanced CKD, monitored for 5 years, and the CKD-REIN clinical-based prospective cohort which included 3033 patients with CKD stage 3-4 from 2013 to 2016. During our various research work, we identified heterogeneous trajectories specific to this growing older population, raising ethical, organizational and economic issues. Renal registries will help clinicians, health providers and policy-makers if suitable decision- making tools are developed and validated.
The care of older people with chronic kidney disease (CKD) is a complex pathway that may benefit from personalized care plans and decision-making tools (1, 2).
Defining the older population is a complex issue in itself (3, 4). The medical literature reflects considerable heterogeneity in the definition of advanced age in research, although the threshold of 65 is most often used. However, medical advances and increased life expectancy are making those aged over 65 a very important part of the general population (5–7). The level of independence and the sheer proportion of this 65+ population are important considerations. Its level of autonomy and role in the active life of society raise the question of shifting the cursor to beyond the age of 75 (8). These debates remind us that the definition of the older population, currently limited to a criterion of chronological age, must evolve to include additional items relating to geriatric syndromes and frailty. For the purpose of this article, the word “older adults” will defined people aged 75 or over unless otherwise specified.
Indeed, older patients at an advanced stage of CKD are very heterogeneous in terms of clinical profile, functional, cognitive and medico-social status with various financial and emotional resources (9, 10). This subpopulation, more often than other CKD patients, presents alterations in these dimensions requiring specific global and personalized care (11). The therapeutic project will be made more complex with respect to renal replacement therapy, in particular a kidney transplant project (12–14). These frail patients will require multidisciplinary care for their kidney disease and their geriatric syndrome in a more medicalized structure. Their risk of death is high and should raise, even more, the question of the benefit of replacement therapy or the most appropriate therapeutic management (15, 16). In this context of the complexity of choosing the right therapeutic project with several options (i.e conservative care, dialysis, kidney transplantation), mixed quantitative and qualitative approaches are desirable to develop better specific support tools and improve the collegial decision-making process (17).
Moreover, the reduced life expectancy of these patients with Stage 4-5 CKD must be taken into account in organizing the care offer, especially access to supportive and end-of-life care (15, 18–20). Finally, the specificities of this population require a careful, though necessary, medico-economic approach (21).
Therefore, there is a definite need to describe and evaluate practices to improve global care and health management in this growing, changing population of older people with CKD (22–24). By providing data and indicators, registries are invaluable tools for following the evolution of care and may highlight variability in medical practices for advanced CKD like dialysis initiation, kidney transplant evaluation, modalities and location of treatment (25, 26). Registry data may also improve health system planning and policy-making by indicating efficient, cost-effective care strategies (27, 28).
The aim of this paper is to illustrate all the clinical epidemiology searches made within the French Renal Epidemiology and Information Network (REIN) over the last 20 years, in order to improve advanced CKD care in the older adults. We summarized various studies describing the clinical practice, care organization, prognosis and health economics evaluations in order to develop and improve personalized care plans and decision-making tools.
In France, the national REIN registry includes all patients receiving renal replacement therapy (RRT) - dialysis or transplantation - for End-Stage Kidney Disease (ESKD) (25, 29). Recording began in 2002 and the registry grew progressively to include the entire country (including overseas territories) in 2012. It aims to be a tool for public health decision support, evaluation and research. Anonymized data are available for researchers on request after validation by the REIN scientific committee. The data collection is ruled with an implicit consent with a drop-out option. Patients are informed on the purpose of the registry and the possible data linkage and data use via publicly available information (poster and welcome booklet in nephrology centers). Since 2019, the intention has been to expand it to patients with Stage 4 and 5 CKD not treated with RRT (30).
REIN data are indirectly linked to the French administrative healthcare database which consists of two main databases: the hospital discharge summaries database and the national health insurance claims database and it covers 98.8% of the French population, over 66 million people, from birth (or immigration) to death (31, 32). Analyzing this database provides insights into the economic burden of CKD and is a means to evaluate care consumption.
REIN was also linked to the PSPA cohort, a multicenter prospective cohort study of patients aged 75 years and older with advanced CKD monitored for 5 years. Details of the study design have been previously published and are summarized hereafter (33). Twenty-four French hospital nephrology centers were involved with an inclusion period of 4 months at each center. Inclusion criteria were age ≥ 75 years, CKD with eGFR < 20 ml/min/1.73m² (calculated by the Modification of Diet in Renal Disease Study equation) and seen by a nephrologist. Exclusion criteria were acute kidney injury and late referral for starting dialysis without any previous nephrology follow-up. The cohort included 573 patients with a median age of 85 years and a median eGFR of 14 ml/min/1.73m². The inclusion questionnaire asked about demographic data, clinical conditions (primary renal disease, comorbidities and disabilities), mobility (walking with or without help), place of abode and biological data. Nephrologists recorded the treatment plan at inclusion and at each subsequent visit during follow-up, depending on changes in their own opinion or the patients’ preference. The dialysis components of the treatment plans reported by the nephrologists were defined as follows (1): ongoing evaluation of the patient’s clinical condition and patient preferences after discussion (“Evaluation”) (2), postponement of the decision about dialysis due to stable clinical condition (“Stable”) (3); decision to start dialysis when it becomes necessary (“Dialysis”) (4), nephrologist’s decision that dialysis is inappropriate (“No Dialysis (Nephrologist)”) or patient’s decision against dialysis (“No Dialysis (Patient)”).
The study was approved by the Ethical Review Board of the research institution (Number 16.04.06), conducted in accordance with international clinical practice guidelines and registered on ClinicalTrials.gov under the number NCT02910908.
The CKD-REIN clinical-based prospective cohort enrolled 3033 patients with CKD stage 3-4 conducted in 40 nephrology outpatient facilities between 2013 and 2016 (34). Follow-up lasted 5 years, including after initiation of renal replacement therapy. The primary objective of the CKD-REIN cohort study was to develop a research platform to address key questions regarding various patient-level factors and biomarkers associated with CKD outcomes, and to assess clinical practices and healthcare system-level determinants of CKD outcomes. The study is registered on ClinicalTrials.gov (Identifier: NCT03381950).
With these datasets we were able to look at different trajectory points: advanced CKD care, transition to dialysis, transplant access, patient survival and overall care consumption (Figure 1). According to the outcome of interest, various statistical tools were used that are briefly described in the various paragraphs and detailed in each published study. Most of them took into account the competing risk of death.
Baseline characteristics of the patients in the PSPA cohort suggest that the older patients referred to nephrologists are probably selected by their general practitioner and/or gerontologists (33). In fact, most of the patients are autonomous in walking and living at home.
These patients had a complex medical regimen with polypharmacy with an average of 9 medications prescribed per day (35). 77.0% of patients had at least one medication classified as a Renally Inappropriate Medication (RIM). At least one Potentially Inappropriate Medication (PIM) was taken by 57.6% of them and 45.5% of patients had at least one medication classified as RIM and PIM. In the PSPA cohort, the prescriptions most frequently requiring reassessment due to potential adverse effects were for proton pump inhibitors and allopurinol. The PIMs for which deprescription is especially important in this population are rilmenidine, long-term benzodiazepines, and anticholinergic drugs such as hydroxyzine. In the overall CKD cohort, anti-gout, cardiovascular agents and antidiabetic agents accounted for most of the inappropriate prescriptions (36). Standardized methodology of conciliation process of drug therapy applicable to these polypathological patients are needed (37, 38).
In this older population, healthcare providers must be more aware of the need to evaluate the benefit-risk ratio of each medication prescribed. Collaborative patient-centered approaches with all patients’ health care professionals must be developed. The development of new medication needs to consider this complex polypharmacy in this growing population.
The estimation of renal function itself appears to be a real challenge in older patients with CKD, mainly for two reasons: the first is that the recognized estimation formulas become inaccurate when renal function is impaired; the second is that most formulas have not been designed for the older population, especially those over 80 years of age (39–42). The Berlin Initiative Study (BIS) has shown that except Cockcroft–Gault equation, existing equations considerably overestimated GFR in older adults. They recommend the use of the BIS2 creatinine- and cystatin C–based equation to estimate GFR in persons aged 70 years or older with normal or mild to moderately reduced kidney function (39). The need for an accurate estimation of renal function is justified, in particular, by the need for dosage adjustment of prescribed drugs. In the study by Laville SM et al., the rate of inappropriate prescriptions in patients with CKD was particularly high, and depended directly on the estimation formula used (36). The CKD-REIN data emphasize the major prevalence of polymedication in patients with CKD: the patients included (median age 71 years, 2/3 over 65 years of age) received a median of 8 different therapeutic classes daily (36). In addition to polymedication, there is a significant risk of inappropriate prescriptions in this population, which is twofold: both with regard to age and the alteration of renal function, which justifies fine adjustments. Thus, half the patients were subject to at least one inappropriate prescription during their follow-up. The risk increased with the progression of CKD. Polymedication was also associated with a greater risk of serious adverse drug reactions, particularly frequent in this population (more than 14%/year), a third of which were considered preventable (43).
Although available and fully covered by the national health insurance for all who need dialysis, only half the older patients with advanced CKD (median eGFR of 14 ml/min) finally start dialysis and only a quarter were alive at 5 years (44) (Figure 2). This poor prognosis confirms the need to consider other perspectives such as quality of life and patients’ end-of-life wishes. However, older patients with very advanced CKD frequently do not articulate explicit decisions about dialysis until very late in their disease course. At baseline the treatment plans were “Stable” for 232 (40%) patients; “Dialysis” for 215 (38%); “No Dialysis (Nephrologist)” for 70 (12%), and 54 (9%) “No Dialysis (Patient)”. These treatment plans that may change over time were associated with their clinical condition and their outcomes (Figure 3). Determining a treatment plan early and re-evaluating it regularly will lead to better organization of patient care, whether it be dialysis or conservative management.
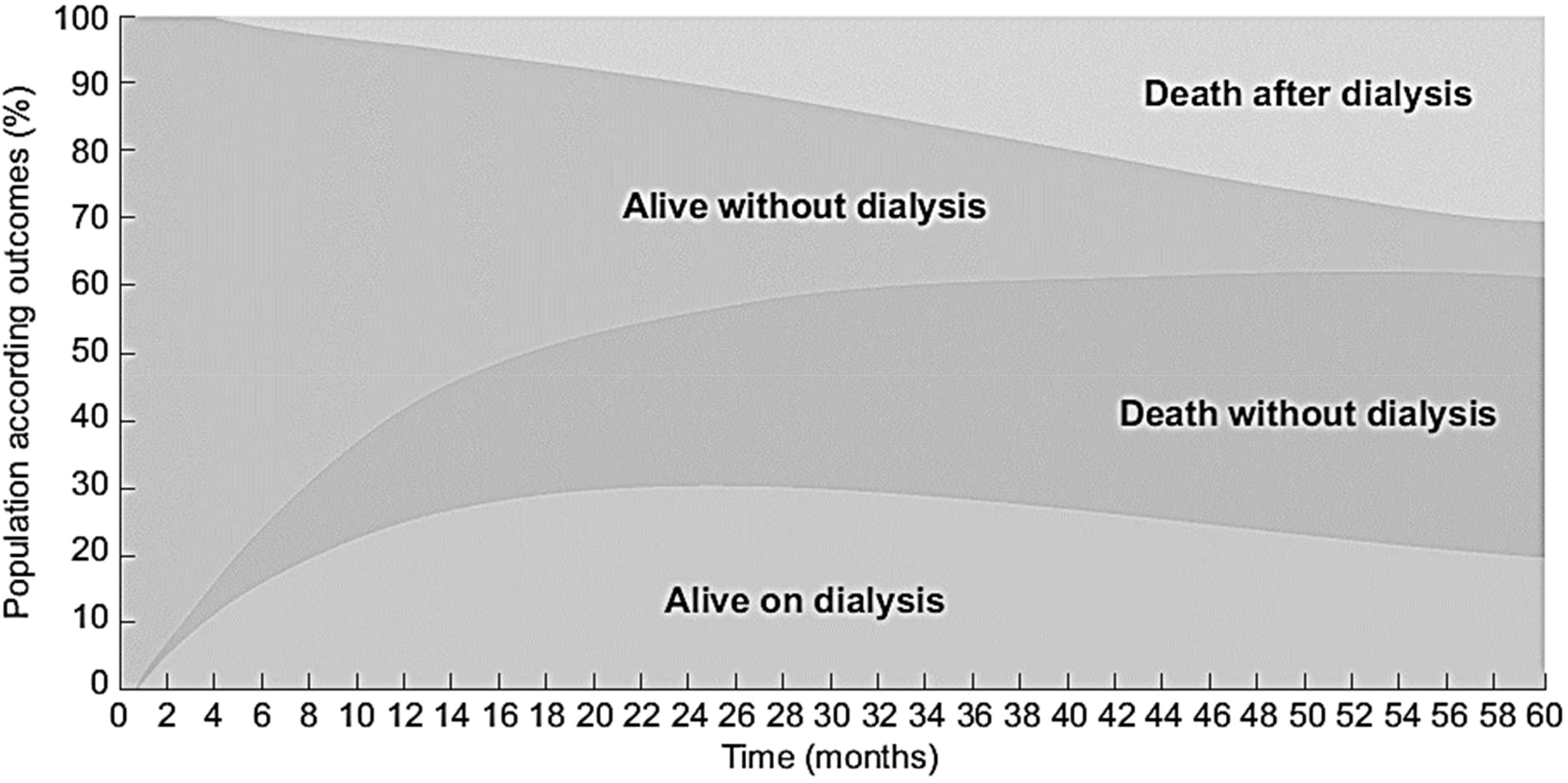
Figure 2 Evolution of the PSPA cohort with outcome according to time since inclusion for 573 CKD patients aged ≥ 75 years with eGFR < 20 ml/min/1.73m2 (source PSPA cohort linked to REIN registry). Adapted from (41, 44).
In order to define a personalized care plan, a central place is given to clear, detailed information on treatment options. However, the data from France confirm that this information is provided very late in the course of CKD (if at all provided), and less often to older patients (Figure 4) (45).
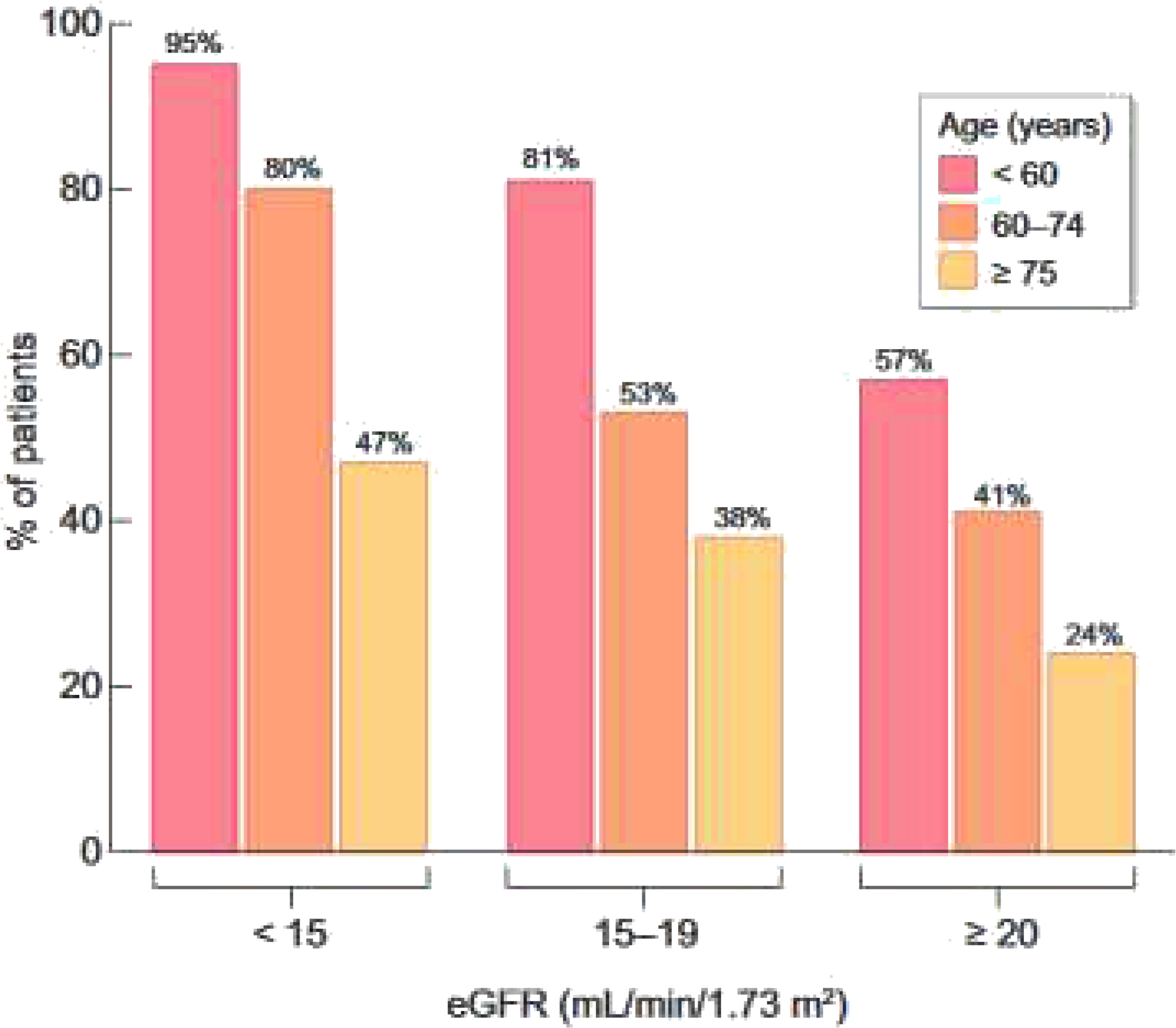
Figure 4 Percentage of patients who reported they have received education about kidney failure treatment options or have discussed them with their doctor, according to age and eGFR level. Adapted from Hamroun et all 2022 (45).
The course of the treatment plan and the patient’s care trajectory upstream are essential information for evaluating dialysis initiation conditions as a quality criterion for medical practice. Because usual score have bad performance in this older population, specific prognosis scores before RRT are need that takes better into account competitive risk between progression to dialysis and death (46–48).
Although more medicalized due to the frequency of comorbidities, older patients are not protected from starting dialysis in an emergency (40% of the 2015 incident patients aged 75 years and over vs. 30% for the total population) (49). All effort should be made to prevent such situation. Patients with a lesser or lack of follow-up with a nephrologist are more likely to start dialysis in emergency, regardless of the frequency of follow-up by a general practitioner (49). Various strategies can be implemented to avoid late referral for specialized nephrology care. Even if early referral does not totally prevent urgent start, a planned approach in which the modality has been chosen prior to the need for dialysis and there is an access ready for use at the initiation of dialysis, is preferable. Many studies have shown poor outcomes of such urgent starters but also the use of temporary vascular access (50, 51).
Among patients aged 75-84 years and 85 years and over, respectively 10.3% and 15.2% die within the first three months (52). This initial high mortality risk is followed by a strong decrease during the first year, but the mean mortality rate rises steadily over time on treatment (Figure 5). At dialysis initiation, 5% of the old patients are unable to walk without help, indirect item chose in the REIN registry to capture frailty. As well, 10% have sign of malnutrition defined as a serum-albumin level of <3 g/dl and/or a BMI of <20 kg/m2. This early mortality should lead to discussion about the complications related to dialysis management as well as the expected benefits in this fragile older population with no improvement on dialysis (15, 53). In this context, developing a prognostic score for early mortality after dialysis initiation can be interesting to inform patients and their relatives and help physicians in the difficult shared decision-making process to define the most appropriate therapeutic choice.
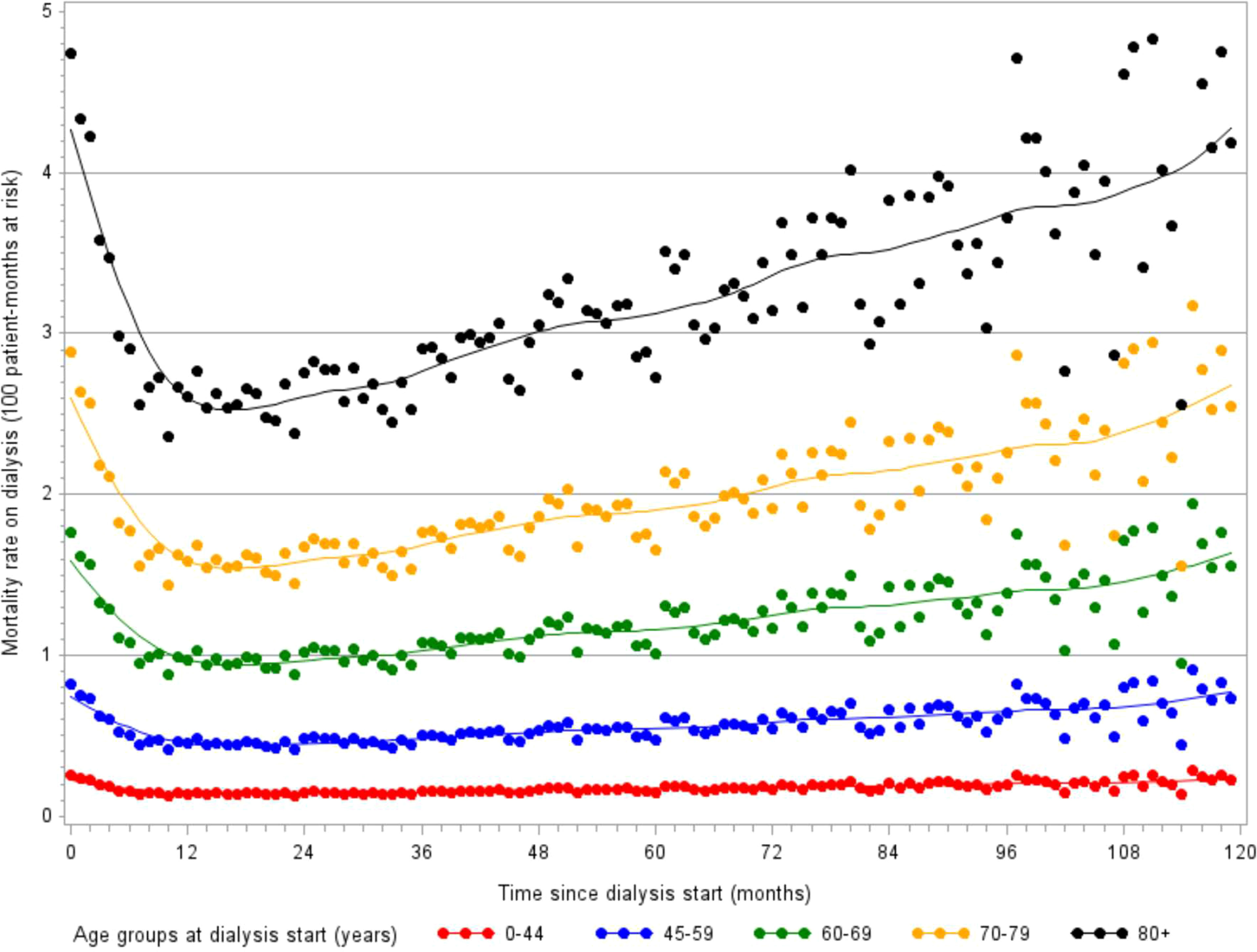
Figure 5 Evolution of monthly mortality rates over 10 years following dialysis initiation, stratified by age (monthly incidence estimated with a Poisson model, source REIN registry).
A prognostic score for early mortality was therefore developed with REIN to make it possible to identify those patients at a low, intermediate or high risk of dying soon after starting dialysis, at the bedside or during a medical visit (54). High-risk patients may benefit from a multidisciplinary approach and more specific geriatric tools to better appreciate their functional status, comorbidities, cognition, mental health status, fatigue, social status and support, nutrition, and presence of other geriatric syndromes (Figure 6). The deployment of such a process should be accompanied by strengthening the cooperation between nephrologists, geriatricians, general practitioners, social workers and physicians trained in palliative care and by improving staff training in ethics and communication. The CKD-REIN cohort has shown that conservative treatment is only occasionally offered to older patients and most of them report that they were not informed of this option (45). In fact, only 28% of nephrologists report proposing this alternative to their patients aged 75+ at that stage of renal failure, and only 5% of patients report having heard of a treatment option without dialysis (Figure 4).

Figure 6 Proposed risk stratification algorithm to screen for, evaluate and decide on an appropriate care strategy for older ESKD patients, according to their level of risk of early death (source REIN registry). Adapted from Couchoud et al., 2015 (54).
The REIN plans to extend data collection to Stage 4 and 5 CKD patients (30). A better understanding of CKD care and patients’ trajectories will enlighten nephrologists about the characteristics of the population selected to start renal replacement therapy. Difference in propensity to accept for dialysis older patients or with poor condition or with higher EGFR may explain geographical variation in ESKD incidence (55, 56). In older patients the median eGFR at dialysis start is at 10.0 vs 8.4 ml/min/1.63m² in patients age lower than 75 years.
As in the general population, the prevalence of fair or poor health status increases with advanced age in dialysis patients (57). However, in CKD and transplanted patients, patients aged 75 years and over do better than those aged 65-74 years old. In men, the prevalence of fair or poor health status in transplanted patients is similar in the 75-and-over age group compared to the general population with same age. Physical and mental health scores are lower in older patients in all populations except for the mental score in transplanted men (Figure 7). In all age group, PCS is decreasing with advanced CKD and dialysis and in a lesser extent in transplanted patients. Like in other studies, these results illustrate the clinical impact of CKD stages on the physical dimensions of Qol, especially in olders (58, 59). Differences are low in MCS score between the general population and the different stage of CKD except in dialysis illustrating the fact that only dialysis affects its mental dimensions. However, some studies have shown that the impact of CKD on Qol is likely multifactorial and partly mediated by co-occurent conditions (60).
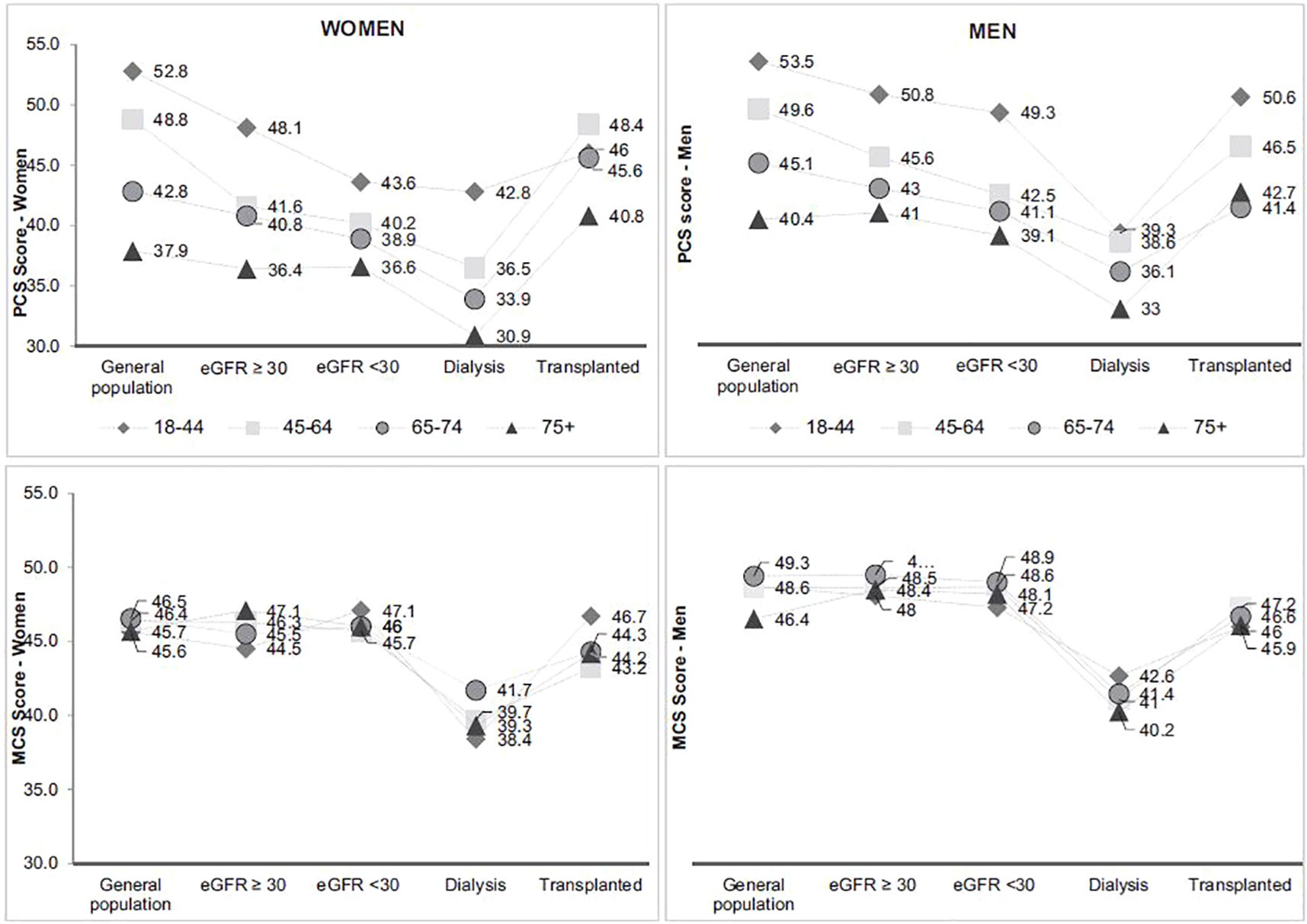
Figure 7 Physical and mental health score according to age and CKD status* (source CKD-REIN, REIN registry). Adapted from Legrand et al., 2020 (57). MCS Mental Component Summary, PCS Physical component Summary. *Gray lines connect the symbols for each age group to facilitate visualization of each group between kidney disease status groups. They do not reflect the course of health-related quality of life across CKD.
Finally, the overriding objective of preserving quality involves adapting treatments to the needs and demands of patients that may evolve over time.
In this older population, variabilities in treatment access can be observed. In a study aimed at identifying factors associated with choosing peritoneal dialysis as a first treatment, we found great variability between districts according to age (61). Higher age is associated with less home-based dialysis and increased mortality rates (62). It is associated with fewer switches to facility-based HD in PD patients but higher rates of switching from home-based HD to facility-based HD. In France, easier access to nursing-assisted care or help from a caregiver/family member for PD rather than home-based HD in older patients may explain this longer survival technique. Despite the lack of data on such practices, one can hypothesize that nursing-assisted PD may allow nephrology teams to keep frail older patients at home “until the end”. Moreover, the quality of life associated with home therapy may play a major role in the older patient’s choice of supportive care. Globally, home dialysis use as first treatment is low in France. While it remains stable around 5.6% for the patients aged lower than 75 years, it is decreasing in older patients (6.1% in 2012, 4.9% in 2020).
Indicators are especially difficult to interpret when the underlying dynamic process is not well understood. Therefore, we have developed a statistical tool to study the 10-year course of incident ESKD patient cohorts and to quantify, by simulations, the impact of various expected changes or new strategies (63). This tool is accessible to all French nephrologists on a secure portal.
Because transplantation may be associated with better Qol, this treatment option should be systematically considered. However to improve kidney transplantation equity, one must empowering clinicians with adequate predictive tools.
The broadening of the indications for transplantation allowed by the use of extended criteria donors has prompted professionals to offer transplants to increasingly older patients. However, despite national and international guidelines, many studies have shown variations in kidney transplant waiting list practices or policies. Some guidelines define age limits whereas others consider that patients should not be deemed ineligible based on their age (64). Indeed, with older patients, the great heterogeneity of prognoses may complicate the careful assessment of eligible patients.
Although available and fully covered by the national health insurance for all who need renal transplantation, in France, less than 2% of older patients (75 years and over) have access to the waiting list for a kidney transplant at 24 months (52). In a study aimed at identifying sources of variation in early kidney transplant waiting list registration in France, older age was associated with a lower probability of registration and greater variability between networks (65). Such lower access to registration for older patients has been shown in many countries (66–68). While the recommendation is to avoid setting a cut-off age limit for eligible older kidney transplant candidates without medical contraindications, it has been observed that there is a higher risk of death in the perioperative and early post-transplant period in this older population compared to wait-listed dialysis patients of similar age and cardiovascular risk (69) (Figure 8). These results lead us to recommend the systematic screening of all older patients for transplantation eligibility. The first screening is followed by a more in-depth assessment that takes into account their comorbidities, frailty index and the balance between the expected benefits and the risk of early death, which the patient must be informed of. This screening should be conducted as early as possible to rapidly identifying suitable candidates, limit the duration of dialysis or enable preemptive transplantation (70).
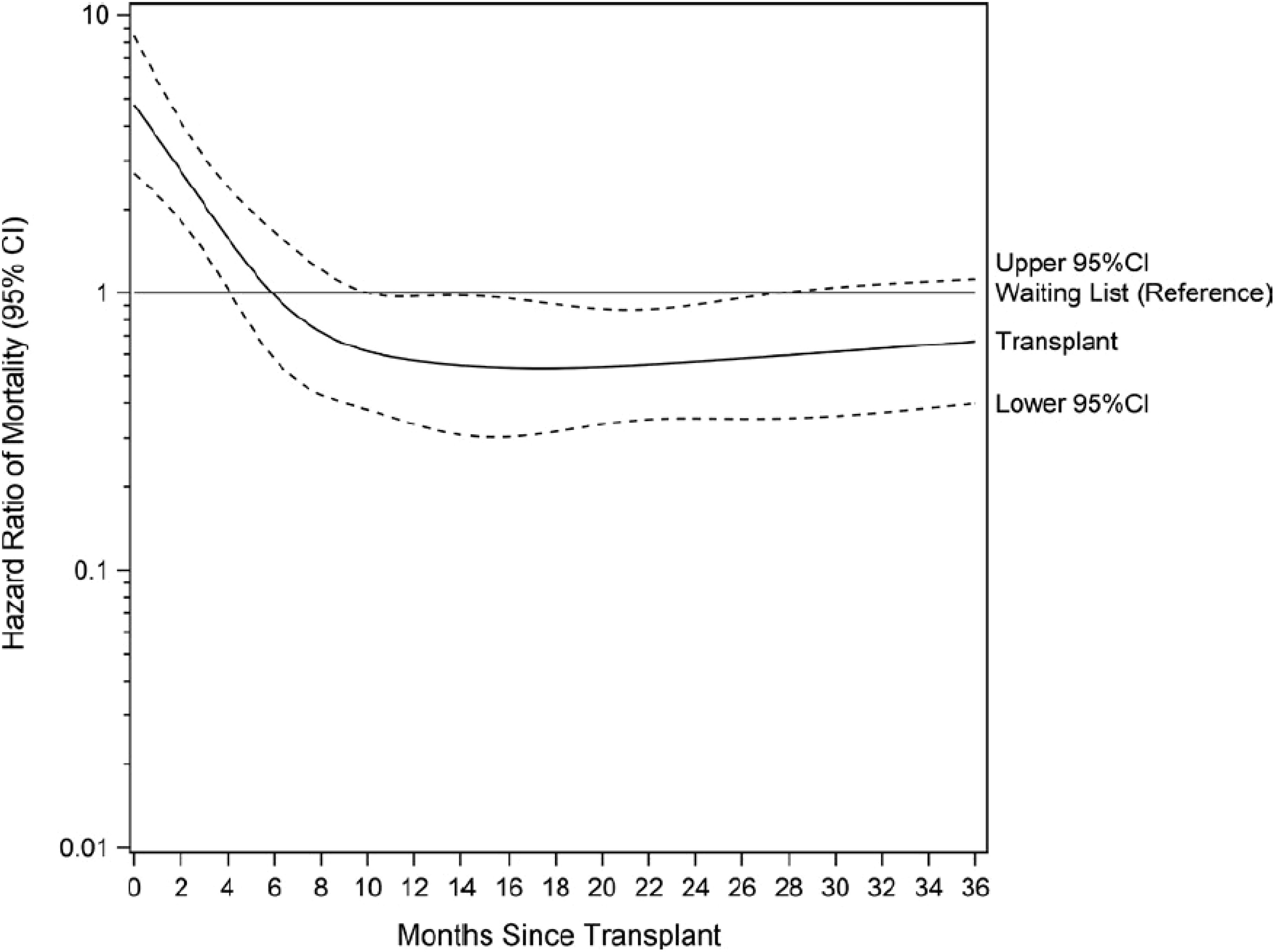
Figure 8 Relative risk of death after renal transplantation in older patients compared to wait-listed patients (source REIN registry). Adapted from Legeai et al., 2018 (69).
Given the median age of 71 years and the heterogeneity of prognoses of the incident cohort arriving at Stage 5 CKD each year, a decision tool capable of identifying those with a good long- term prognosis among all incident CKD patients could be an opportunity to help screening.Indeed, a kidney transplant project should be discussed with the patients and their relatives. An impact study is ongoing in France to evaluate the benefits of using a decision-making score to identify a subgroup of patients with a good long-term prognosis, as early as possible after starting dialysis. This could help nephrologists and older adults at the dialysis center to consider kidney transplant evaluation in collaboration with transplant centers (71).
In 2019, based on hospital database-REIN linkage, we were able to show that hospitalization rates increase with age (Table 1). They represent 1.5 hospitalizations per 1 person-year in patients age 75 years and over and 20% of these are related to cardiovascular disease and cardiovascular events (Table 2) (72).

Table 2 Cause of hospitalization according to age (source REIN registry linked to hospital database).
A health economic evaluation was performed based on the REIN and the National Health Insurance Database, to compare the efficiency of different care strategies. The overall mean monthly cost spent for a stable patient increases with age (27, 73). These higher costs are mainly explained by the higher costs of the dialysis treatment modality: hospital-based HD or nurse- assisted home APD (73). Alternative strategies, like the development of kidney transplantation from deceased donors with perfusion machines, the use of assisted CAPD or out-center HD could be more efficient than the current practices for certain patients (27).
In a French cohort of 51,834 incident ESKD patients, 33% died during the study period, but only 3.6% of them resorted to palliative care-related hospitalization (74). Hospitalizations were classified as being “for palliative care” when the principal diagnosis at discharge was coded as Z51.5 (“Palliative Care” in the International Classification of Diseases, Tenth Revision, Clinical Modification). Hospitalizations classified as being “with palliative care” were defined by at least one night in a bed dedicated to palliative care during hospitalization, independently of the principal diagnosis. Older patients did not have more access to palliative care despite their higher mortality rate (Figure 9).
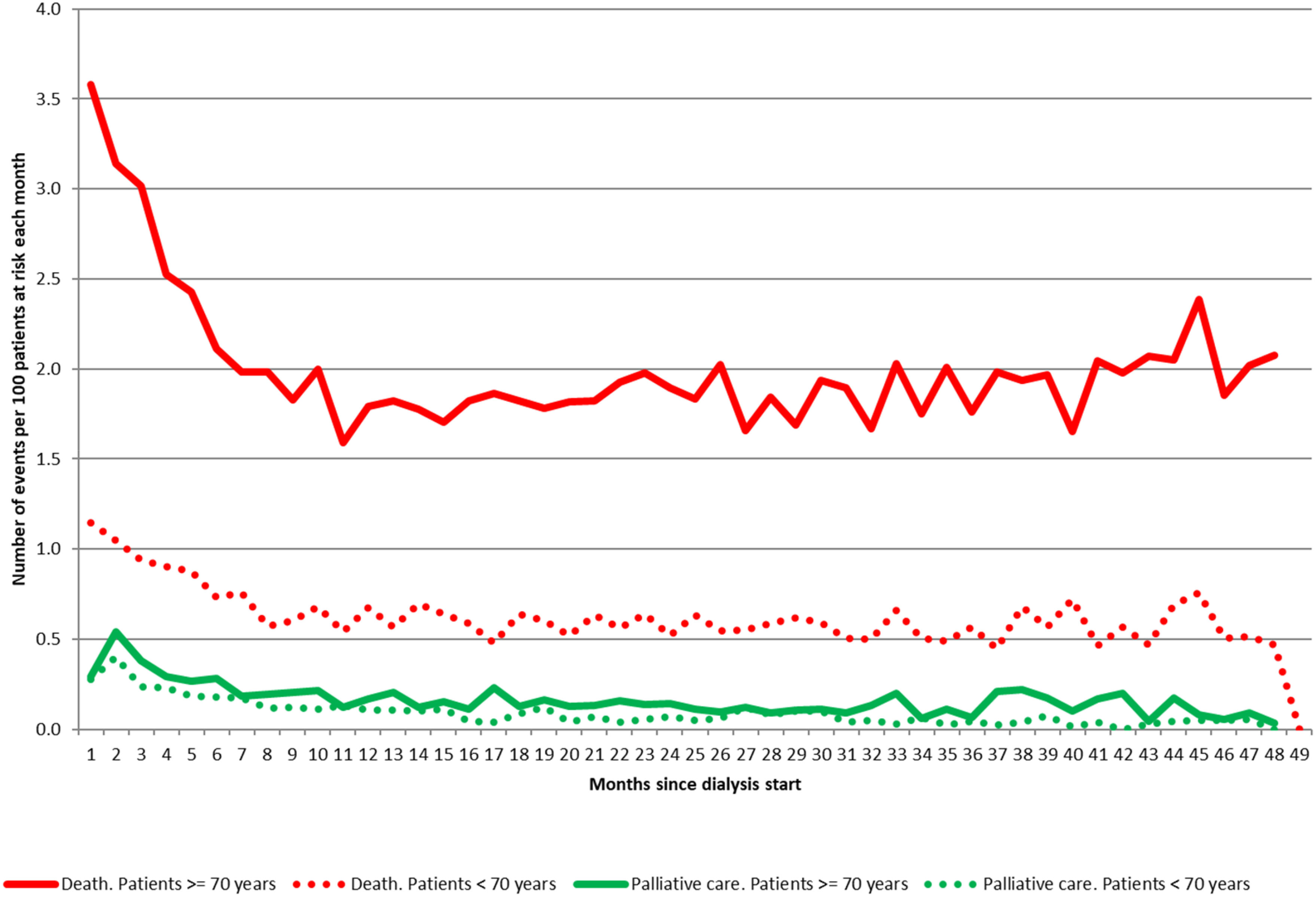
Figure 9 Monthly incidence rates over time of hospitalizations associated with palliative care and death from all causes during the first four years after starting dialysis, according to age (source REIN registry linked to hospital database). Adapted from Couchoud et al., 2017 (74).
In this same cohort, only 10% of the patients who withdrew from dialysis (a situation in which death is expected and predictable) had palliative care-related hospitalization, compared to 43% of hospices used in the US in the same context. Only 19% died at home whereas many people with a terminal illness would prefer to die at home (75). Active cancer was the principal factor associated with the likelihood of palliative care hospitalization. This may reflect the current practice of acceptance in palliative care units or the nephrologists’ perceptions that palliative care is reserved for patients with cancer.
In the REIN registry, when declaring death, it is possible to indicate whether the treatment was discontinued and, if so, the reason why. From 2002 to 2019, 14,600 deaths (19%) occurred after withdrawing dialysis, with a median of 6 days (interquartile range: 3-13) (52).
During these various research works, we identified heterogeneous trajectories specific to this growing aging population, raising ethical, organizational and economic issues Table 3. Therefore, decision-making tools must be developed and validated for this new specific population to help clinicians, health providers and policy-makers.
The transition period between Stage 4 CKD and kidney failure is crucial and has a major impact on the subsequent prognosis (76). It is therefore essential to smooth this transition by better identifying the phenotypes particularly at risk of a poor outcome on renal replacement therapy (77). An important item in the global assessment is frailty and the identification of geriatric syndromes (76, 78–80). Frailty, favored both by age and CKD, is very common in this population and is associated with multiple complications such as falls, dependence, institutionalization, recurrent hospitalizations and death (81). Several tools have been developed to assess it in practice and, in the future, it seems essential to systematically integrate these data into registries (82).
Despite the IDEAL randomized trial in the early 2010s (83), there is currently no clear answer on the right time to begin dialysis, especially in the older adults. Recent developments in the field of epidemiology and the extension of renal registries to patients with Stage 4-5 CKD may provide an answer to this question in the future (84, 85).
The complexity of managing advanced CKD (especially in the older adults) justifies the organization of care around coordinated care pathways (1, 77). In France, an annual flat-rate payment for healthcare structures was introduced in 2019 with the aim of improving the follow-up and support of CKD patients, by combatting disease progression and the occurrence of complications, whilst optimizing the transition to kidney failure around coordinated multi-professional care (https://www.legifrance.gouv.fr/loda/id/JORFTEXT000039138244/).
The particularly high frequency of emergency dialysis initiation on temporary access in older patients has been mentioned above, even in patients under close nephrology follow-up. This potentially refers to an additional option in the choice of therapeutic project: the “Deciding Not to Decide” option (86). This choice is particularly appreciated in the older adults, who prefer to focus on the present to preserve their quality of life at all costs. In order to improve the patient-centered and shared decision-making approach, it would be necessary to go further into the study of what determines the patients’ choice for their care plan. This would allow a better understanding of what is at stake at the time of the decision, especially as the health priorities of caregivers and patients with respect to advanced CKD are not aligned (87).
Kidney registries are well placed to facilitate the large-scale collection of patient-reported outcome measures (PROMs) or patient-reported experience measures (PREMs) to facilitate improvements in health care and organization (88, 89). Such collection may lead to more valuable, person-centered services and enhanced health and wellbeing of people with CKD. Although the quantitative approach does not rule out qualitative studies, registry may help to identify the population to be included in such survey (90).
To guide nephrologists in their daily practice and the patients’ decisional process, prognostic scores have an increasingly important role to play. However, the scores developed have shown limited accuracy in the older adults (47, 48). Furthermore, using mortality as the outcome of choice must be questioned in the older adults: (i) the mortality rate is very high in this population burdened by comorbidities and clinical frailty (2); older patients often place more importance on their quality of life than on life expectancy. Thus, in the coming years, with the help of registries and cohorts of CKD patients, it is important to develop approaches based on quality of life data or relevant proxies such as time spent in hospital. New prognostic scores could thus be developed for an even more patient-centered therapeutic education (91).
From a public health view point, the needs in health care are moving fast and the care offer must adapt to the most vulnerable frail patients. Therefore, registry data, in providing epidemiological knowledge, by describing specific health consumption in detail, are appropriate for better predicting the needs for suitable care arrangements i.e. facility-based, home-based or supportive care. Although an individualized approach is recommended, few studies are available to assess the benefits of alternatives to standard care. By providing real-life data, registers can provide invaluable insights. Individualized care does not rule out differences in practices between professionals that may represent a loss of opportunity for patients. Such evaluations should be made by registries to help health authorities to guarantee the equality of care throughout the territory. Furthermore, in order to offer a real alternative to renal replacement therapies for older frail patients, the deployment of conservative care requires multi-level resources within a coordinated network, supported by significant policy changes (92). Since the end of 2020, an experiment supported by the French national health insurance fund has been implemented to offer home-based medical care programs dedicated to patients undergoing conservative care (https://solidarites-sante.gouv.fr/IMG/pdf/soins_conservateurs_irct_arrete.pdf 2019). This French home-based care network now includes over a hundred patients, and its permanent legal registration is currently under consideration. Thus, CKD registries and cohorts also have an important role to play in monitoring and evaluating both the clinical and economic impact of these types of initiatives.
There is a growing role of registries in randomized controlled trials. The REIN registry is currently conducting a pilot study on the model of the ANZDATA Swift study, a Registry-Based Cluster Randomised Controlled Trial to determine the clinical effectiveness and cost-effectiveness of symptom monitoring with feedback to clinicians and patients compared with standard care in improving quality of life outcomes at 12 months for adults on haemodialysis.
(93). Embedded within a registry, randomized trials capitalize on established and secure data collection system. It is a new way to decrease research cost by avoiding extra collection of information that already exist and are available. In older patients, avoiding extra visits is alos an asset.
The linkage between registry data and health databases is an opportunity to assess expenditures and health care use. In this older population, the medication burden and inappropriate prescription risk are high (35). The linkage between registry data and health databases is an opportunity for in-depth assessment of drug use at “low cost” without the need to go back to the patient or the medical files. Moreover health economics evaluations are necessary for health authorities and health providers to be able to offer sustainable arrangements. As CKD is most often incurable, the most important area of work remains prevention. In order to better identify the target population for this prevention, linking the registry data to health insurance data could eventually allow us to better identify potential CKD patients at an earlier stage. Work on the French health insurance database is currently underway to develop an algorithm to recognize these patients, the first version of which was recently published (94).
Finally, the REIN registry, backed by its expertise and national network, was able to set up epidemiological monitoring of chronic dialysis and/or transplant patients affected by SARS-CoV-2 very quickly (95). A weekly newsletter has been set up (still on-going), sent to all nephrologists, using information transmitted by all the REIN research assistant working with the nephrologists in the regions. These data have shown the usefulness of vaccination (96). It helped the policy-makers identify priority patients for vaccination (97). But unless other sentinel surveillance system (98), it was not a real-time information, due to manual collection. However, it allowed various study that may be useful in the event of a new sanitary crisis. For example, the impacts of kidney transplantation moratorium on the life expectancy of kidney transplantation candidates was simulated. Patients older than 60 were less impacted by KT moratoriums: they had a lower additional time on the waitlist and a lower overmortality than younger patients in each of the three studied populations (99). The role of registers as potential health monitoring tools is greatly underestimated.
All authors contributed to the conception and writing of the manuscript. All authors read and approved the final manuscript.
The REIN registry is coordinated and supported by the French Biomedicine Agency.
The authors wish to thank all REIN registry participants, especially the nephrologists and professionals in charge of data collection and quality control. Dialysis and Transplantation units participating in the registry are listed in the REIN annual report (https://www.agence-biomedecine.fr/Les-chiffres-du-R-E-I-N). We also wish to thank Teresa Sawyers, Medical Writer at the BESPIM, Montpellier University Hospital, for her invaluable help in revising and editing the text.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tonelli M, Wiebe N, Manns BJ, Klarenbach SW, James MT, Ravani P, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system(2018) (Accessed 4 août 2022).
2. Weiss JW, Boyd CM. Managing complexity in older patients with CKD. Clin J Am Soc Nephrol (2017) 12(4):559−61. doi: 10.2215/CJN.02340317
3. Sabharwal S, Wilson H, Reilly P, Gupte CM. Heterogeneity of the definition of elderly age in current orthopaedic research. Springerplus. (2015) 4:516. doi: 10.1186/s40064-015-1307-x
4. Singh S, Bajorek B. Defining « elderly » in clinical practice guidelines for pharmacotherapy. Pharm Pract (Granada). (2014) 12(4):489. doi: 10.4321/S1886-36552014000400007
6. Division UP World population ageing 1950-2050. In: Disponible sur. New-York: UN (2002). Available at: https://digitallibrary.un.org/record/461899.
7. Rudnicka E, Napierała P, Podfigurna A, Męczekalski B, Smolarczyk R, Grymowicz M. The world health organization (WHO) approach to healthy ageing. Maturitas (2020) 139:6−11. doi: 10.1016/j.maturitas.2020.05.018
8. Ouchi Y, Rakugi H, Arai H, Akishita M, Ito H, Toba K, et al. Redefining the elderly as aged 75 years and older: Proposal from the joint committee of Japan gerontological society and the Japan geriatrics society. Geriatr Gerontol Int (2017) 17(7):1045−7. doi: 10.1111/ggi.13118
9. Singh P, Germain MJ, Cohen L, Unruh M. The elderly patient on dialysis: geriatric considerations. NephrolDialTransplant (2013), 1460−2385. doi: 10.1093/ndt/gft246
10. Kallenberg MH, Kleinveld HA, Dekker FW, van Munster BC, Rabelink TJ, van Buren M, et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD-a systematic review. Clin J Am Soc Nephrol (2016) 11(9):1624−39. doi: 10.2215/CJN.13611215
11. Bowling CB, O’Hare AM. Managing older adults with CKD: individualized versus disease-based approaches. AmJKidney Dis (2012) 59(1523−6838(1523−6838 (Electronic):293−302. doi: 10.1053/j.ajkd.2011.08.039
12. Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Semin Nephrol (2009) 29(6):621−35. doi: 10.1016/j.semnephrol.2009.07.011
13. Knoll GA. Kidney transplantation in the older adult. AmJKidney Dis (2013) 61(5):790−7. doi: 10.1053/j.ajkd.2012.08.049
14. McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL. Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc (2014) 62(12):2235−42. doi: 10.1111/jgs.13130
15. Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, et al. Executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int (2015) 88(1523−1755(1523−1755 (Electronic):447−59. doi: 10.1038/ki.2015.110
16. O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, et al. Age affects outcomes in chronic kidney disease. JAmSocNephrol. (2007) 18:2758−65. doi: 10.1681/ASN.2007040422
17. Kalantar-Zadeh K, Wightman A, Liao S. Ensuring choice for people with kidney failure - dialysis, supportive care, and hope. N Engl J Med (2020) 383(2):99−101. doi: 10.1056/NEJMp2001794
18. O’Hare AM, Song MK, Kurella Tamura M, Moss AH. Research priorities for palliative care for older adults with advanced chronic kidney disease. J Palliat Med (2017) 20(5):453−60. doi: 10.1089/jpm.2016.0571
19. Sturgill D, Bear A. Unique palliative care needs of patients with advanced chronic kidney disease - the scope of the problem and several solutions. Clin Med (Lond) (2019) 19(1):26−9. doi: 10.7861/clinmedicine.19-1-26
20. Kurella Tamura M, O’Hare AM, Lin E, Holdsworth LM, Malcolm E, Moss AH. Palliative care disincentives in CKD: Changing policy to improve CKD care. Am J Kidney Dis (2018) 71(6):866−73. doi: 10.1053/j.ajkd.2017.12.017
21. Morton RL, Kurella Tamura M, Coast J, Davison SN. Supportive care: Economic considerations in advanced kidney disease. Clin J Am Soc Nephrol (2016) 11(10):1915−20.doi: 10.2215/CJN.12651115
22. Silver SA, Bell CM, Chertow GM, Shah PS, Shojania K, Wald R, et al. Effectiveness of quality improvement strategies for the management of CKD: A meta-analysis. Clin J Am Soc Nephrol (2017) 12(10):1601−14. doi: 10.2215/CJN.02490317
23. Nakayama T, Imanaka Y, Okuno Y, Kato G, Kuroda T, Goto R, et al. Analysis of the evidence-practice gap to facilitate proper medical care for the elderly: investigation, using databases, of utilization measures for national database of health insurance claims and specific health checkups of Japan (NDB). Environ Health Prev Med 6 juin (2017) 22(1):51. doi: 10.1186/s12199-017-0644-5
24. Fukuma S, Ikenoue T, Shimizu S, Norton EC, Saran R, Yanagita M, et al. Quality of care in chronic kidney disease and incidence of end-stage renal disease in older patients: A cohort study. Med Care (2020) 58(7):625−31. doi: 10.1097/MLR.0000000000001301
25. Caillet A, Mazoué F, Wurtz B, Larre X, Couchoud C, Lassalle M, et al. Which data in the French registry for advanced chronic kidney disease for public health and patient care? Nephrol Ther (2022) 18(4):228−36. doi: 10.1016/j.nephro.2022.01.004
26. Liu FX, Rutherford P, Smoyer-Tomic K, Prichard S, Laplante S. A global overview of renal registries: a systematic review. BMC Nephrol. 19 mars (2015) 16:31. doi: 10.1186/s12882-015-0028-2
27. Couchoud C, Couillerot AL, Dantony E, Elsensohn MH, Labeeuw M, Villar E, et al. Economic impact of a modification of the treatment trajectories of patients with end-stage renal disease. Nephrol DialTransplant (2015) 1460−2385. doi: 10.1093/ndt/gfv300
28. de Vries EF, Rabelink TJ, van den Hout WB. Modelling the cost-effectiveness of delaying end-stage renal disease. Nephron. (2016) 133(2):89−97. doi: 10.1159/000446548
29. Couchoud C, Stengel B, Landais P, Aldigier JC, de CF, Dabot C, et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol DialTransplant (2006) 21(0931−0509(0931−0509 (Print):411−8. doi: 10.1093/ndt/gfi198
30. Vigneau C, Ayav C, Noël N, Gomis S, Glaudet F, Siébert M, et al. Vers une extension du registre REIN aux patients avec une maladie rénale chronique au stade 5 non traités par dialyse ou greffe ? Étude pilote. Néphrologie Thérapeutique (2019) 15(3):143−51. doi: 10.1016/j.nephro.2018.11.010
31. Raffray M, Bayat S, Lassalle M, Couchoud C. Linking disease registries and nationwide healthcare administrative databases: the French renal epidemiology and information network (REIN) insight. BMC Nephrol. déc (2020) 21(1):25. doi: 10.1186/s12882-020-1692-4
32. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merlière Y. French National health insurance information system and the permanent beneficiaries sample. Rev d’Épidémiologie Santé Publique (2010) 58(4):286−90. doi: 10.1016/j.respe.2010.04.005
33. Moranne O, Couchoud C, Vigneau C. Characteristics and treatment course of patients older than 75 years, reaching end-stage renal failure in france. the PSPA study. JGerontolA BiolSciMedSci (2012) 67(1758−535X(1758−535X (Electronic):1394−9. doi: 10.1093/gerona/gls162
34. Stengel B, Combe C, Jacquelinet C, Briancon S, Fouque D, Laville M, et al. The French chronic kidney disease-renal epidemiology and information network (CKD-REIN) cohort study. Nephrol Dial Transplant (2013) 1460−2385(Electronic). doi: 10.1093/ndt/gft388
35. Roux-Marson C, Baranski JB, Fafin C, Exterman G, Vigneau C, Couchoud C, et al. Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease(2020) (Accessed 22 avr 2022).
36. Laville SM, Metzger M, Stengel B, Jacquelinet C, Combe C, Fouque D, et al. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol (2018) 84(12):2811–2823. doi: 10.1111/bcp.13738
37. Alfaro-Lara ER, Vega-Coca MD, Galván-Banqueri M, Nieto-Martín MD, Pérez-Guerrero C, Santos-Ramos B. Metodología de conciliación del tratamiento farmacológico en pacientes pluripatológicos. Atención Primaria (2014) 46(2):89−99. doi: 10.1016/j.aprim.2013.07.002
38. Berthe A, Fronteau C, Le Fur É, Morin C, Huon JF, Rouiller-Furic I, et al. Medication reconciliation: a tool to prevent adverse drug events in geriatrics medicine. Gériatrie Psychol Neuropsychiatrie du Viellissement (2017) 15(1):19−24. doi: 10.1684/pnv.2016.0642
39. Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. AnnInternMed. (2012) 157(1539−3704(1539−3704 (Electronic):471−81. doi: 10.7326/0003-4819-157-7-201210020-00003
40. Pavkov ME, Nelson RG. Estimating GFR in the elderly-new approaches to an old problem. Kidney Int Rep (2019) 4(6):763−5. doi: 10.1016/j.ekir.2019.04.006
41. Raman M, Middleton RJ, Kalra PA, Green D. Estimating renal function in old people: an in-depth review. Int Urol Nephrol (2017) 49(11):1979−88. doi: 10.1007/s11255-017-1682-z
42. Evans M, van Stralen KJ, Schon S, Prutz KG, Stendahl M, Rippe B, et al. Glomerular filtration rate-estimating equations for patients with advanced chronic kidney disease. Nephrol DialTransplant (2013) 28(1460−2385(1460−2385 (Electronic):2518−26. doi: 10.1093/ndt/gft226
43. Laville SM, Gras-Champel V, Moragny J, Metzger M, Jacquelinet C, Combe C, et al. Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol (2020) 15(8):1090−102. doi: 10.2215/CJN.01030120
44. Moranne O, Fafin C, Roche S, Francois M, Allot V, Potier J, et al. Treatment plans and outcomes in elderly patients reaching advanced chronic kidney disease. Nephrol Dial Transplant (2018) 33(12):2182–2191. doi: 10.1093/ndt/gfy046
45. Hamroun A, Speyer E, Ayav C, Combe C, Fouque D, Jacquelinet C, et al. Barriers to conservative care from patients’ and nephrologists’ perspectives: the CKD-REIN study(2022) (Accessed 13 juin 2022).
46. Farrington K, Covic A, Aucella F, Clyne N, de VL, Findlay A, et al. Clinical practice guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR <45 mL/min/1.73 m2). Nephrol DialTransplant (2016) 31(1460−2385(1460−2385 (Electronic):ii1–ii66. doi: 10.1093/ndt/gfw356
47. Prouvot J, Pambrun E, Antoine V, Couchoud C, Vigneau C, Roche S, et al. Low performance of prognostic tools for predicting death before dialysis in older patients with advanced CKD. J Nephrology. (2022) 35(3):993−1004. doi: 10.1007/s40620-021-01180-1
48. Prouvot J, Pambrun E, Couchoud C, Vigneau C, Roche S, Allot V, et al. Low performance of prognostic tools for predicting dialysis in elderly people with advanced CKD. J Nephrol (2021) 34(4):1201−13. doi: 10.1007/s40620-020-00919-6
49. Raffray M, Vigneau C, Couchoud C, Bayat S. Predialysis care trajectories of patients with ESKD starting dialysis in emergency in France. Kidney Int Rep (2021) 6(1):156−67. doi: 10.1016/j.ekir.2020.10.026
50. Michel A, Pladys A, Bayat S, Couchoud C, Hannedouche T, Vigneau C. Deleterious effects of dialysis emergency start, insights from the French REIN registry. BMC Nephrol. (2018) 19(1):233. doi: 10.1186/s12882-018-1036-9
51. Alizada U, Sauleau EA, Krummel T, Moranne O, Kazes I, Couchoud C, et al. Effect of emergency start and central venous catheter on outcomes in incident hemodialysis patients: a prospective observational cohort. J Nephrol (2022) 35(3):977−88. doi: 10.1007/s40620-021-01188-7
52. Couchoud C, Lassalle M. REIN annual report (2019). Available at: https://www.agence-biomedecine.fr/IMG/pdf/rapport_rein_2019_2021-10-14.pdf.
53. Kurella TM, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. NEnglJ Med (2009) 361(1533−4406(1533−4406 (Electronic):1539−47. doi: 10.1056/NEJMoa0904655
54. Couchoud CG, Beuscart JR, Aldigier JC, Brunet PJ, Moranne OP. Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int (2015), (1523−1755 (Electronic)). doi: 10.1038/ki.2015.245
55. Couchoud C, Guihenneuc C, Bayer F, Lemaitre V, Brunet P, Stengel B. Medical practice patterns and socio-economic factors may explain geographical variation of end-stage renal disease incidence. NephrolDialTransplant (2012) 27(1460−2385(1460−2385 (Electronic):2312−22. doi: 10.1093/ndt/gfr639
56. Couchoud C, Guihenneuc C, Bayer F, Stengel B. The timing of dialysis initiation affects the incidence of renal replacement therapy. NephrolDialTransplant (2010) 25(1460−2385(1460−2385 (Electronic):1576−8. doi: 10.1093/ndt/gfp675
57. Legrand K, Speyer E, Stengel B, Frimat L, Ngueyon Sime W, Massy ZA, et al. Perceived health and quality of life in patients with CKD, including those with kidney failure: Findings from national surveys in France. Am J Kidney Dis (2020) 75(6):868−78. doi: 10.1053/j.ajkd.2019.08.026
58. Faulhaber L, Herget-Rosenthal S, Jacobs H, Hoffmann F. Health-related quality of life according to renal function: Results from a nationwide health interview and examination survey. Kidney Blood Press Res (2022) 47(1):13−22. doi: 10.1159/000518668
59. Cruz MC, Andrade C, Urrutia M, Draibe S, Nogueira-Martins LA, de Castro Cintra Sesso R. Quality of life in patients with chronic kidney disease. Clinics. (2011) 66(6):991−5. doi: 10.1590/S1807-59322011000600012
60. Artzi-Medvedik R, Kob R, Fabbietti P, Lattanzio F, Corsonello A, Melzer Y, et al. Impaired kidney function is associated with lower quality of life among community-dwelling older adults : The screening for CKD among older people across Europe (SCOPE) study. BMC Geriatr. 02 (2020) 20(Suppl 1):340. doi: 10.1186/s12877-020-01697-3
61. Couchoud C, Savoye E, Frimat L, Ryckelynck JP, Chalem Y, Verger C. Variability in case mix and peritoneal dialysis selection in fifty-nine French districts. PeritDialInt (2008) 28(0896−8608(0896−8608 (Print):509−17. doi: 10.1177/089686080802800515
62. Couchoud C, Béchade C, Kolko A, Baudoin AC, Bayer F, Rabilloud M, et al. Dialysis-network variability in home dialysis use not explained by patient characteristics: a national registry-based cohort study in France. Nephrol Dial Transplant (2022) 37(10):1962–1973. doi: 10.1093/ndt/gfac055
63. Couchoud C, Dantony E, Elsensohn MH, Villar E, Ecochard R. Modelling treatment trajectories to optimize the organization of renal replacement therapy and public health decision-making. Nephrol Dial Transplant (2013) 1460−2385(Electronic). doi: 10.1093/ndt/gft204
64. Batabyal P, Chapman JR, Wong G, Craig JC, Tong A. Clinical practice guidelines on wait-listing for kidney transplantation: consistent and equitable? Transplantation. (2012) 94(7):703−13. doi: 10.1097/TP.0b013e3182637078
65. Couchoud C, Bayer F, Rabilloud M, Ayav C, Bayat S, Bechade C, et al. Effect of age and care organization on sources of variation in kidney transplant waiting list registration. Am J Transplant. (2021) 21(11):3608−17. doi: 10.1111/ajt.16694
66. Dudley CR, Johnson RJ, Thomas HL, Ravanan R, Ansell D. Factors that influence access to the national renal transplant waiting list. Transplantation (2009) 88(1534−6080(1534−6080 (Electronic):96−102. doi: 10.1097/TP.0b013e3181aa901a
67. Neri L, Gallieni M, Rocca Rey LA, Bertoli SV, Andreucci V, Brancaccio D. Inequalities in transplant waiting list activation across Italian dialysis centers. Am J Nephrol (2013) 37(6):575−85. doi: 10.1159/000351334
68. Schold JD, Meier-Kriesche HU. Comparable barriers to access to kidney transplantation across national lines. Transplantation. (2009) 88(1534−6080(1534−6080 (Electronic):21−2. doi: 10.1097/TP.0b013e3181a9eabe
69. Legeai C, Andrianasolo RM, Moranne O, Snanoudj R, Hourmant M, Bauwens M, et al. Benefits of kidney transplantation for a national cohort of patients aged 70 years and older starting renal replacement therapy. Am J Transplant (2018) 18(11):2695–2707. doi: 10.1111/ajt.15110
70. Segall L, Nistor I, Pascual J, Mucsi I, Guirado L, Higgins R, et al. Criteria for and appropriateness of renal transplantation in elderly patients with end-stage renal disease: A literature review and position statement on behalf of the European renal association-European dialysis and transplant association Descartes working group and European renal best practice. Transplantation. (2016) 100(10):e55−65. doi: 10.1097/TP.0000000000001367
71. Dusseux E, Albano L, Fafin C, Hourmant M, Guerin O, Couchoud C, et al. A simple clinical tool to inform the decision-making process to refer elderly incident dialysis patients for kidney transplant evaluation. Nephrol Dial Transplant (2015) 1523−1755(Electronic). doi: 10.1038/ki.2015.25
72. Mercier D, Lassalle M, Couchoud C. Registre REIN. [Descriptions of hospitalizations during the year 2019 among dialysis patients in France]. Nephrol Ther (2022) 18(6):534–540. doi: 10.1016/j.nephro.2022.02.002
73. Couillerot-Peyrondet AL, Sambuc C, Sainsaulieu Y, Couchoud C, Bongiovanni-Delarozière I. A comprehensive approach to assess the costs of renal replacement therapy for end-stage renal disease in France: the importance of age, diabetes status, and clinical events. Eur J Health Econ (2017) 18(4):459−69. doi: 10.1007/s10198-016-0801-6
74. Couchoud C, Arnaud DB, Lobbedez T, Blanchard S, Chantrel F, Maurizi-Balzan J, et al. Access to and characteristics of palliative care-related hospitalization in the management of end-stage renal disease patients on renal replacement therapy in France. Nephrol (Carlton) (2017) 22(8):598−608. doi: 10.1111/nep.12822
75. Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J PalliatMed. (2000) 3(1096−6218(1096−6218 (Print):287−300. doi: 10.1089/jpm.2000.3.287
76. Lam M, Jassal SV. The concept of frailty in geriatric chronic kidney disease (CKD) patients. Blood Purif. (2015) 39(1−3):50–4. doi: 10.1159/000368952
77. Schaeffner E. Smoothing transition to dialysis to improve early outcomes after dialysis initiation among old and frail adults - a narrative review. Nephrol Dial Transplant (2021) 37(12):2307–2313. doi: 10.1093/ndt/gfab342
78. Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr. (2017) 68:135−42. doi: 10.1016/j.archger.2016.10.007
79. Ostuni M, Musso CG. Usefulness of frailty evaluation for handling chronic kidney disease elderly patients: a review and original proposal. Int Urol Nephrol. (2019) 51(3):461−5. doi: 10.1007/s11255-018-2061-0
80. Nixon AC, Brown J, Brotherton A, Harrison M, Todd J, Brannigan D, et al. Implementation of a frailty screening programme and geriatric assessment service in a nephrology centre: a quality improvement project. J Nephrol. (2021) 34(4):1215−24. doi: 10.1007/s40620-020-00878-y
81. Mei F, Gao Q, Chen F, Zhao L, Shang Y, Hu K, et al. Frailty as a predictor of negative health outcomes in chronic kidney disease: A systematic review and meta-analysis. J Am Med Dir Assoc (2021) 22(3):535−543.e7. doi: 10.1016/j.jamda.2020.09.033
82. Worthen G, Tennankore K. Frailty screening in chronic kidney disease: Current perspectives. Int J Nephrol Renovasc Dis (2019) 12:229−39. doi: 10.2147/IJNRD.S228956
83. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med (2010) 363(1533−4406(1533−4406 (Electronic):609−19. doi: 10.1056/NEJMoa1000552
84. Jager KJ, Åsberg A, Collart F, Couchoud C, Evans M, Finne P, et al. A snapshot of European registries on chronic kidney disease patients not on kidney replacement therapy. Nephrol Dial Transplant (2021) 37(1):8−13. doi: 10.1093/ndt/gfab252
85. Fu EL, Evans M, Carrero JJ, Putter H, Clase CM, Caskey FJ, et al. Timing of dialysis initiation to reduce mortality and cardiovascular events in advanced chronic kidney disease: nationwide cohort study. BMJ. 29 (2021) 375:e066306. doi: 10.1136/bmj-2021-066306
86. Saeed F, Moss AH, Duberstein PR, Fiscella KA. Enabling patient choice: The « deciding not to decide » option for older adults facing dialysis decisions. J Am Soc Nephrol (2022) 33(5):880−2. doi: 10.1681/ASN.2021081143
87. Harrison TG, Tam-Tham H, Hemmelgarn BR, James MT, Sinnarajah A, Thomas CM. Identification and prioritization of quality indicators for conservative kidney management. Am J Kidney Dis (2019) 73(2):174−83. doi: 10.1053/j.ajkd.2018.08.014
88. Ayav C, Couchoud C, Sautenet B, Lobbedez T, Sens F, Moranne O, et al. [Routine collection of perceived health data in the era of payment for quality: Recommendations by the epidemiology and public health commission of the SFNDT]. Nephrol Ther (2020) 16(7):401−7. doi: 10.1016/j.nephro.2020.04.002
89. van der Veer SN, Couchoud C, Morton RL. The role of kidney registries in expediting large-scale collection of patient-reported outcome measures for people with chronic kidney disease. Clin Kidney J (2021) 14(6):1495−503. doi: 10.1093/ckj/sfab061
90. Raffray M, Bayat S, Campéon A, Laude L, Vigneau C. The pre-dialysis care trajectory of chronic kidney disease patients and the start of dialysis in emergency: A mixed method study protocol. Int J Environ Res Public Health (2019) 16(24):5010. doi: 10.3390/ijerph16245010
91. Couchoud C, Hemmelgarn B, Kotanko P, Germain MJ, Moranne O, Davison SN. Supportive care: Time to change our prognostic tools and their use in CKD. Clin J Am Soc Nephrol (2016) 11(10):1892−901. doi: 10.2215/CJN.12631115
92. Diamond LH, Armistead NC, Lupu DE, Moss AH. Steering committee of the coalition for supportive care of kidney patients. recommendations for public policy changes to improve supportive care for seriously ill patients with kidney disease. Am J Kidney Dis (2021) 77(4):529−37. doi: 10.1053/j.ajkd.2020.09.020
93. Greenham L, Bennett PN, Dansie K, Viecelli AK, Jesudason S, Mister R, et al. The symptom monitoring with feedback trial (SWIFT): protocol for a registry-based cluster randomised controlled trial in haemodialysis. Trials. (2022) 23(1):419. doi: 10.1186/s13063-022-06355-0
94. Mansouri I, Raffray M, Lassalle M, de Vathaire F, Fresneau B, Fayech C, et al. An algorithm for identifying chronic kidney disease in the French national health insurance claims database. Nephrol Ther (2022) 18(4):255−62. doi: 10.1016/j.nephro.2022.03.003
95. Lapalu S, Izaaryene G, Honoré N, Couchoud C. Le rôle du registre national REIN en France dans la veille sanitaire des patients en insuffisance rénale chronique terminale infectés par le SARS-CoV-2 : organisation et premières données. Néphrologie Thérapeutique (2021) 17(4):218−25. doi: 10.1016/j.nephro.2020.11.005
96. El Karoui K, Hourmant M, Ayav C, Glowacki F, Couchoud C, Lapidus N, et al. Vaccination and COVID-19 dynamics in dialysis patients. Clin J Am Soc Nephrology. (2022) 17(3):395−402. doi: 10.2215/CJN.10300721
97. Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int (2020) 98(6):1519−29. doi: 10.1016/j.kint.2020.07.042
98. Bellocchio F, Carioni P, Lonati C, Garbelli M, Martínez-Martínez F, Stuard S, et al. Enhanced sentinel surveillance system for COVID-19 outbreak prediction in a Large European dialysis clinics network. Int J Environ Res Public Health 16 sept (2021) 18(18):9739. doi: 10.3390/ijerph18189739
Keywords: elderly, CKD, decision-making, registry, cohort
Citation: Moranne O, Hamroun A and Couchoud C (2023) What does the French REIN registry tell us about Stage 4-5 CKD care in older adults? Front. Nephrol. 2:1026874. doi: 10.3389/fneph.2022.1026874
Received: 24 August 2022; Accepted: 02 December 2022;
Published: 17 January 2023.
Edited by:
Laura Plantinga, Emory University, United StatesReviewed by:
Luca Neri, Fresenius Medical Care - Applied Advanced Analytics, ItalyCopyright © 2023 Moranne, Hamroun and Couchoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cécile Couchoud, Y2VjaWxlLmNvdWNob3VkQGJpb21lZGVjaW5lLmZy
†ORCID: Olivier Moranne, orcid.org/0000-0002-3127-1415
Aghilès Hamroun, orcid.org/0000-0003-4988-206X
Cécile Couchoud, orcid.org/0000-0002-9273-660X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.